Abstract
While the cerebellum plays a critical role in motor coordination and control no studies have investigated its involvement in idiopathic mobility impairment in community-dwelling elderly. In this study we tested the hypothesis that structural changes in the cerebellar peduncles not detected by conventional magnetic resonance imaging are associated with reduced mobility performance. The analysis involved eighty-five subjects (age range: 75–90 years) who had no clinical signs of cerebellar dysfunction. Based on the short physical performance battery (SPPB) score, we defined mobility status of the subjects in the study as normal (score 11–12, n = 26), intermediate (score 9–10, n = 27) or impaired (score < 9, n = 32). We acquired diffusion tensor imaging data to obtain indices of white matter integrity: fractional anisotropy (FA), mean diffusivity (MD), axial diffusivity (AD) and radial diffusivity (RD). Using a parcellation atlas, regional indices within the superior, middle, and inferior cerebellar peduncles (ICP, MCP, SCP) were calculated and their associations with mobility performance were analyzed. Subjects with impaired mobility showed reduced FA and AD values in the ICP and SCP but not in the MCP. The ICP-FA, ICP-AD and SCP-FA indices showed a significant association with the SPPB score. We also observed significant correlation between ICP-FA and walk time (r = − 0.311, p = 0.004), as well as between SCP-AD and self-paced maximum walking velocity (r = 0.385, p = 0.003) and usual walking velocity (r = 0.400, p = 0.002). In logistic regression analysis ICP-FA and ICP-AD together explained 51% of the variability in the mobility status of a sample comprising the normal and impaired subgroups, and correctly classified more than three-quarters of those subjects. Our findings suggest that presence of microstructural damage, likely axonal, in afferent and efferent connections of the cerebellum contributes to the deterioration of motor performance in older people.
Keywords: Aging, Mobility, Cerebellar peduncles, Diffusion tensor imaging
Highlights
-
•
DTI study of the cerebellar peduncles and mobility in elderly.
-
•
Fractional anisotropy and axial diffusivity of inferior peduncle predict mobility.
-
•
Decreased anisotropy in the peduncles in the absence of T2 lesions.
-
•
Findings likely reflect axonal degeneration of proprioceptive afferent fibers.
-
•
Abnormalities in infratentorial white matter are novel findings in the field.
1. Introduction
Mobility impairment is a common disabling clinical problem which occurs with aging and increases the risk of falls, injuries and even death (Tinetti et al., 1988). Although there are known neurologic (e.g., Parkinson's disease, stroke, peripheral neuropathy), non-neurologic (e.g., musculoskeletal, joint diseases) and pharmacological (Leipzig et al., 1999) causes of impaired mobility, in a significant fraction of older individuals the underlying etiology remains unclear (idiopathic). In these subjects an association between gait impairment and the extent of brain white matter (WM) damage, visible as T2-weighted white matter hyperintensities (WMH) upon upon on magnetic resonance imaging (MRI), has been reported (Baezner et al., 2008; Baloh and Vinters, 1995; Briley et al., 1997; Camicioli et al., 1999; Guttmann et al., 2000; Masdeu et al., 1989; Sachdev et al., 2005; Starr et al., 2003; Wakefield et al., 2010). Such abnormalities are a common finding in the elderly population and are thought to represent cerebro-microvascular damage (Pantoni, 2010) including abnormal permeability of the blood brain barrier (Wardlaw et al., 2003).
While lesions in the brain stem have been reported and analyzed in association with mobility (Starr et al., 2003) imaging studies have thus far focused on the supratentorial white matter where WMHs mostly occur. In addition, subjects enrolled in these studies showed no clinical or MRI signs of cerebellar and brainstem pathology.
The cerebellar peduncles are the communication portals to and from the cerebellum and therefore critical components of the neural network underpinning balance and coordination in voluntary motor activity.
Diffusion tensor imaging (DTI), an established advanced in vivo imaging technique that enables the characterization of anisotropic water diffusion in white matter fibers (Pierpaoli et al., 1996), has shown sensitivity to white matter changes in areas not affected by focal WMH on T2-weighted images (Werring et al., 1999) and is therefore well-suited to probe the microstructural integrity of these previously unexplored but potentially informative regions. The DTI parameters reflecting WM structural integrity are the following: fractional anisotropy (FA), a sensitive but not specific index of overall white matter tracts' integrity; mean diffusivity (MD), a general indicator of tissue water accumulation; axial diffusivity (AD), representing diffusivity parallel to the fiber tracts and an index regarded as a more specific marker for axonal injury; and radial diffusivity (RD), a parameter representing diffusivity perpendicular to the fiber tracts and considered more specific for damage to the myelin sheath. To date, a clear interpretation of the relationship between these indices and the underlying microstructural tissue properties is still lacking and therefore it requires caution. However, there is wide evidence in DTI literature (Alexander et al., 2007) that WM damage, which leads to increase in water molecule diffusion, generally is reflected in local decrease of FA and increase of MD (O'Donnell and Westin, 2011). The AD and RD indices can further help to distinguish between axonal degeneration (decrease of AD) or myelin loss (increase in RD) as primary mechanism of damage to the WM tracts (Budde et al., 2007; Song et al., 2002).
Cerebellum plays a major role in voluntary motor control and its three peduncles are a relevant and anatomically well-defined target for assessing with DTI the integrity of critical parts of the neural networks, i.e. afferent and efferent connections, involving the cerebellum. The cerebellar peduncles have been investigated using DTI in various clinical conditions affecting motor functions such as ataxia (Alcauter et al., 2011; Della Nave et al., 2011), Parkinsonian syndromes (Nicoletti et al., 2006, 2008) and multiple sclerosis (Anderson et al., 2011). However, to our knowledge there are no similar studies on the idiopathic mobility impairment in community-dwelling older subjects.
In this report we describe the findings of a cross-sectional study we undertook with the goal of assessing the relationship between poor performance in mobility tests and reduced microstructural integrity of the cerebellar peduncles.
2. Methods
2.1. Subjects and study design
The subjects included in this analysis represent a subset from a cohort of ninety-nine elderly subjects enrolled in a 4-year prospective study on the relationship between brain changes, cardiovascular risk factors and mobility on which we have reported previously (Moscufo et al., 2011; Wakefield et al., 2010; White et al., 2011). Data for the present cross-sectional analysis included eighty-five subjects who had DTI data of sufficient quality acquired at baseline. Recruitment methods and eligibility criteria have been described in Wakefield et al. (2010). Subjects were included if they were 75 to 90 years old and were enrolled according to a balanced 3 × 3 matrix based on age (75–79, 80–84, ≥ 85) and mobility (short physical performance battery (Guralnik et al., 1994), SPPB scores: 11–12 = normal; 9–10 = intermediate; < 9 = impaired). Briefly, the following exclusion criteria were applied: systemic conditions (e.g., severe arthritis) or neurologic disease (e.g., neuropathy, Parkinson's disease) compromising mobility, medication impairing motor function, cognitive impairment (Mini-Mental State Examination, MMSE < 24), corrected distance vision < 20/70, unstable cardiovascular disease (e.g., myocardial infarction within 6 months, unstable angina), pulmonary disease requiring oxygen, inability to walk 10 m independently in < 50 s, and evidence of cerebral infarction or intracranial mass lesions on MRI. All the subjects were evaluated with a battery of mobility performance tests and MRI of the brain. The following variables, as indicators of cerebrovascular risk, were included in our analyses: history of hypertension and/or diabetes mellitus, average 24-h systolic (SBP) and diastolic blood pressure (DBP), and serum lipoproteins (total, HDL, LDL cholesterol). The 24-h ambulatory blood pressure monitoring was conducted with the Oscar II BP device (Suntech Medical Instruments, Morrisville, NC) and obtained every 15 min from 6 AM to 10 PM and every 30 min from 10 PM to 6 AM (Campbell et al., 2010); the data were analyzed as previously described (White et al., 2011). Neurologic examination was performed on each subject by the senior investigator (LW) in order to determine the presence of diseases compromising mobility (study exclusion criteria). The exam included evaluation of sensory function (touch, pin position sense and vibration) and motor function (strength, tone, coordinated/rapid alternating hand movements, finger to nose, heel-knee-shin tests and observation of gait/balance). Although minor findings were occasionally encountered, there was no consistent clinical evidence of cerebellar dysfunction. The review board of the involved institutions approved the study protocol, which included a written informed consent.
2.2. Mobility assessment
Mobility assessment was carried out at the Balance and Gait Evaluation Laboratory, University of Connecticut Health Center, by trained expert investigators (Panzer et al., 2011). SPPB, Tinetti and mobility lab testing were done on different days for most subjects. Instruction and demonstration were provided prior to testing and subjects practiced and rested as needed then performed the requested tasks. The assessment included the SPPB (Guralnik et al., 1994), usual walking velocity (velocity, meters/second) and self-paced maximum walking velocity (SPMV, meters/second) (Panzer et al., 2011), as well as Tinetti gait and balance (Tinetti, 1986). The SPPB is a composite score representing the quartile distribution (worse = 1, best = 4) of the mobility performance on the following three timed sub-scores: time to walk a course of 2.5 m (walk time, seconds); five chair rises from an erect sitting position on an unpadded armless chair set at 41 cm (average height in the community) with arms crossed below the sternum to a stand up position with knees and hips fully extended; standing balance consisted in side-by-side, semi-tandem, and tandem stands for ten seconds each. On the basis of the total SPPB score, subjects were assigned to the following three categories defining mobility status: normal (SPPB score = 11–12, n = 26), intermediate (SPPB score = 9–10, n = 27) or impaired (SPPB score < 9, n = 32). To obtain the gait velocity, subjects were asked to walk at preferred (‘usual’—performed twice) or as-fast-as possible (‘max’—once) pace. The test was performed from a static start on a force-measuring platform (AMTI, Waltham, MA; sample rate 200 Hz) on an enclosed walkway (out and back, 8.1 m total, with turn). Average velocity was calculated for each performance and the faster of the two preferred pace performances (usual velocity) and the single self-paced max pace (SPMV) values were used for the analyses (Panzer et al., 2011) (for methodology review see Graham et al. (2008)). Tinetti gait and balance scores are from the Performance-Oriented Mobility Assessment (Tinetti, 1986). Tinetti gait testing included initiation of gait, step height, step length, step symmetry, step continuity, path deviation, trunk stability, walk stance, and turning while walking. Each item scored 1 if normal or 0 if abnormal. Tinetti balance testing included sitting balance, arising from chair, immediate standing, standing balance, balance with eyes closed, turning balance, neck turning, back extension, one-leg standing, push test, reaching up, bending down, and sitting down. Each item scored 1 (normal) or 0 (abnormal) with some items having an additional scored intermediate classification, i.e., 'adaptive'. For SPPB, usual velocity, SPMV, Tinetti gait and Tinetti balance better mobility performance corresponds to higher value. For walk time (seconds) the relationship is reversed, i.e. the better mobility performance takes fewer seconds.
2.3. Magnetic resonance imaging (MRI) and image analysis
Brain MR images for the subjects included in this study were acquired over a 12-month period on a 3-Tesla Siemens Allegra scanner (Erlangen, Germany) following mobility assessment (average time interval between mobility testing and MRI: 52 ± 30 days). Parameters of the conventional sequences were: 3D-T1-weighted Magnetization Prepared Rapid Gradient Echo (MPRAGE) (176 contiguous 1-mm thick axial slices, relaxation time/echo time (TR/TE) = 2500/2.74 ms, TI = 900 ms, matrix size = 256 × 208, nominal in-plane pixel dimensions = 1 mm × 1 mm); 3D-Fast Spin Echo (T2) (176 contiguous 1-mm thick sagittal slices, TR/TE = 2500/353 ms, matrix size = 256 × 220, nominal in-plane pixel dimensions = 1 mm × 1 mm), and 3D-Fluid Attenuated Inversion Recovery (FLAIR) (128 contiguous 1.3-mm thick sagittal slices, TR/TE = 6000/353 ms, TI = 2200 ms, matrix size = 256 × 208, nominal in-plane pixel dimensions = 1 mm × 1 mm). Pre-processing included correction of magnetic field-related signal inhomogeneities (Sled et al., 1998) and linear affine registration of FLAIR and T2 images to the MPRAGE images (Jenkinson and Smith, 2001). DTI was performed using a standard twice-refocused EPI sequence with TR/TE = 5800/87 ms, FOV = 20 cm, acquisition and reconstruction matrices = 128 × 96 and 128 × 128, diffusion sensitizing orientations in 12 directions with one B0, and 8 averages for each direction at b = 1000 s/mm2. Forty-five contiguous axial slices with 3 mm section thickness were acquired. DTI data were checked for excessive background noise, motion and other artifacts; significant artifacts resulted in subject exclusion. FSL software (MMRIB software library (www.fmrib.ox.ac.uk/fsl)) was used for standard analysis including motion and eddy current corrections. All DTI images were co-registered to the B0 image, with gradient directions corrected for the applied rotation. To enhance reproducibility and ensure overlap of narrow white matter (WM) structures between subjects, Tract Based Spatial Statistics (TBSS) (Smith et al., 2006) were utilized. This approach defines a WM skeleton from the DTI data (one for the whole study population, using all data) by finding continuous tracts of local FA maxima. Each subject's FA, MD, AD and RD maps were then propagated to this skeleton to ensure that narrow WM tracts, which may not precisely overlap after whole brain co-registration, are projected to the same WM skeleton. To reproducibly and efficiently identify the cerebellar peduncles we used the white matter parcellation atlas (WMPA) from the International Consortium of Brain Mapping (Mori et al., 2008). The DTI maps and WM skeleton from the TBSS method were aligned to the WMPA by means of affine registration (12 degrees of freedom). The FA, MD, AD, and RD in the cerebellar peduncles for each subject were obtained by using the WMPA and the WM skeleton as masks to select only the skeleton WM pixels within the three peduncles (Fig. 1). These regional indices were expressed as mean values. Total WMH burden and the brain parenchymal fraction (BPF) were determined as previously described and expressed as percentage of the intracranial volume (Moscufo et al., 2011).
Fig. 1.
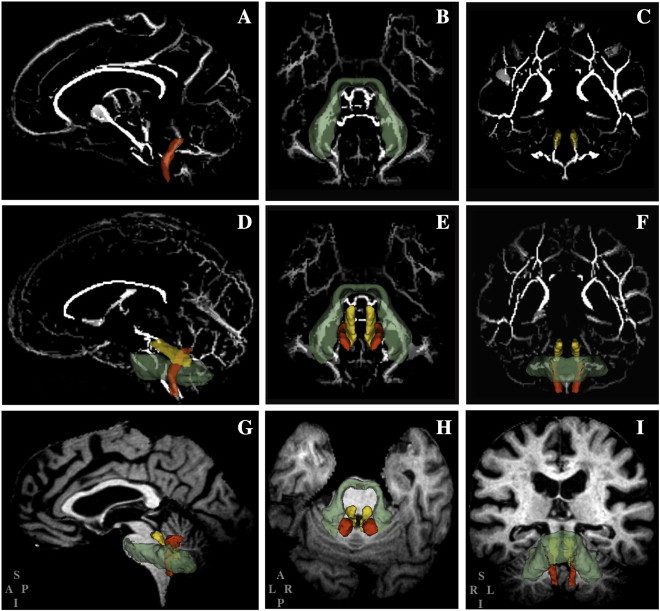
The figure illustrates the location of the inferior (red), middle (green) and superior (yellow) cerebellar peduncles. The 3D model reconstructions of the peduncles are superimposed on the standard DTI-FA (fractional anisotropy) skeleton map (A–F) or on the structural T1-weighted magnetic resonance images of a study subject (G–I). The cerebellar peduncles are shown individually in A–C and all together in D–I. Columns left to right show sagittal, axial and coronal orientations, respectively. Models of the peduncles are made transparent to show layering of the models as well as part of the skeleton from which average values were calculated from the respective fractional anisotropy (FA), mean diffusivity (MD), axial diffusivity (AD), and radial diffusivity (RD) maps. The lettering at bottom left of the panels G, H and I, indicate the orientation of the corresponding panels in the column (S: superior; A: anterior; P: posterior; I: inferior; L: left; R: right).
2.4. Statistical analysis
Statistical analysis was performed using SPSS v.13 (SPSS Inc., Chicago, Illinois). Normal distribution of the data was assessed with Kolmogorov–Smirnov Test. Since most of the mobility variables had a non-normal distribution, all statistical analyses were performed using non-parametric tests. Differences in DTI metrics between the three mobility groups (i.e., normal, intermediate, impaired) were analyzed by Kruskal–Wallis or Mann–Whitney tests as indicated. We measured association by calculating the Spearman's rho correlation coefficient. In order to assess predictive value we performed logistic regression as follows. In univariate logistic regression the DTI indices for each peduncle and the other variables (age, gender, BMI, BPF, WMH, 24 h-SBP, 24 h-DBP) were tested individually as potential explanatory variables of the categorical response variable representing normal or impaired mobility (binary values assigned: 0 and 1, respectively). In order to maximize the predictive power of the test we focused on the two subgroups with clear functional differences and thus subjects falling in the intermediate mobility group with an SPPB score = 9–10 were excluded from the logistic regression analysis. In a subsequent step the variables that had a significant predictive value in univariate testing above were entered together in a multivariate forward stepwise logistic regression model and the best predictor/s of mobility status was identified. The significance threshold for statistical testing was ≤ 0.05. However, to minimize the identification of false positive associations (type I error) when multiple comparisons were performed, a lower p-value was calculated according to the Bonferroni correction method, i.e. we divided the p-value above (0.05) by the number of tests that included the variable being tested.
3. Results
Study subjects' characteristics are summarized in Table 1 for the entire cohort (n = 85) and for the SPPB-based mobility groups, i.e. normal (n = 26), intermediate (n = 27) and impaired (n = 32). While the impaired group showed on average higher BMI, larger WMH burden, more frequent diabetes and hypertension, and lower MMSE and BPF, the difference among the groups was statistically significant only for BPF. Mobility characteristics are reported in Table 2. The DTI metrics of the peduncles and their differences among the mobility groups are illustrated in Fig. 2. A statistically significant difference in the mean values of the DTI parameters among the three peduncles was noted, particularly the lower ICP-FA and ICP-AD values compared to those of the SCP. Both FA and AD indices of ICP and SCP were lower (p ≤ 0.007) in the impaired than in the normal mobility group. Consistent with this finding decreased FA and AD (both indicative of damaged WM) correlated with poorer mobility (i.e. SPPB, as well as individual mobility measures such as walk time, SPMV, usual velocity and Tinetti balance, Table 3). Correlation analysis results with the three SPPB sub-scores, i.e. walk time, chair rise, and standing balance, show that subjects who performed poorly in these mobility tests (lower score) are more likely to have lower AD values (indicative of WM damage) in ICP and SCP (Supplementary Table 1).
Table 1.
Characteristics of the study subjects.
| Mobility Groups |
|||||
|---|---|---|---|---|---|
| All subjects (n = 85) |
Normal (n = 26) |
Intermediate (n = 27) |
Impaired (n = 32) |
p | |
| Age (years) | 83 ± 4 | 82 ± 4 | 84 ± 4 | 84 ± 4 | 0.09a |
| Gender (F/M) | 47/38 | 14/12 | 15/12 | 18/14 | 0.98b |
| BMI (kg/m2) | 26 ± 4 | 24 ± 3 | 26 ± 3 | 27 ± 5 | 0.12a |
| Hypertension, n (%) | 59 (69%) | 17 (65%) | 16 (59%) | 26 (81%) | 0.16b |
| 24 h-SBP (mm Hg) | 131.5 ± 13.1 | 132.3 ± 12 | 131.4 ± 14.6 | 130.8 ± 13 | 0.91a |
| 24 h-DBP (mm Hg) | 67.5 ± 7.1 | 67.8 ± 7.1 | 67 ± 5.9 | 67.7 ± 8.2 | 0.97a |
| Diabetes, n (%) | 7 (8.2%) | 1 (1.2%) | 1 (1.2%) | 5 (5.9%) | 0.16b |
| Total cholesterol (mg/dL) | 196.6 ± 40.4 | 192.7 ± 35.9 | 202 ± 38.7 | 195.7 ± 47.1 | 0.81a |
| LDL (mg/dL) | 123.8 ± 36 | 121.1 ± 31.1 | 126.9 ± 35.2 | 123.7 ± 42.4 | 0.63a |
| HDL (mg/dL) | 56.4 ± 15.3 | 56.5 ± 12.8 | 61.2 ± 16.3 | 52 ± 16.1 | 0.10a |
| MMSE | 28 ± 1 | 29 ± 1 | 29 ± 2 | 28 ± 1 | 0.09a |
| WMH (% ICC) | 0.97 ± .89 | 0.84 ± .85 | 0.76 ± .53 | 1.25 ± 1.09 | 0.19a |
| BPF (% ICC) | 70.7 ± 3.4 | 72.3 ± 3.4 | 70.6 ± 3.6 | 69.6 ± 2.9 | 0.008a |
Values are expressed as mean ± standard deviation.
Abbreviations: body mass index (BMI), systolic blood pressure (SPB), diastolic blood pressure (DPB), low-density lipoprotein cholesterol (LDL), high-density lipoprotein cholesterol (HDL), mini-mental state examination (MMSE), white matter hyperintensities (WMH), intracranial cavity volume (ICC), brain parenchymal fraction (BPF).
Group differences:
Kruskal-Wallis test.
Chi-squared test.
Table 2.
Mobility characteristics of the study population.
| Mobility Groups |
|||||
|---|---|---|---|---|---|
| All subjects (n = 85) |
Normal (n = 26) |
Intermediate (n = 27) |
Impaired (n = 32) |
p | |
| SPPB (score) | 8.95 ± 2.29 | 11.23 ± 0.43 | 9.67 ± 0.48 | 6.50 ± 1.70 | NA |
| WS (score) | 3.40 ± 0.76 | 3.96 ± 0.20 | 3.70 ± 0.47 | 2.69 ± 0.69 | < 10− 6 |
| CR (score) | 2.15 ± 1.13 | 3.27 ± 0.45 | 2.30 ± 0.78 | 1.13 ± 0.79 | < 10− 6 |
| SB (score) | 3.39 ± 1.01 | 3.96 ± 0.20 | 3.67 ± 0.56 | 2.69 ± 1.28 | < 10− 6 |
| Walk time (s) | 3.25 ± 1.16 | 2.58 ± 0.343 | 2.99 ± 0.38 | 4.01 ± 1.56 | < 10− 6 |
| Velocity (m/s) | 0.66 ± 0.16 | 0.77 ± 0.13 | 0.69 ± 0.13 | 0.52 ± 0.11 | 2 × 10− 6 |
| SPMV (m/s) | 0.71 ± 0.17 | 0.83 ± 0.13 | 0.74 ± 0.15 | 0.56 ± 0.11 | 3 × 10− 6 |
| Tinetti gait (score) | 11.23 ± 1.26 | 11.73 ± 0.55 | 11.26 ± 1.73 | 10.65 ± 1.09 | 0.001 |
| Tinetti balance (score) | 14.95 ± 1.54 | 15.68 ± 0.72 | 15.21 ± 1.18 | 13.95 ± 1.94 | 0.001 |
Values are expressed as mean ± standard deviation.
Group comparison: p-values (Kruskal–Wallis test) indicate level of significance among the three mobility groups.
Abbreviations: short physical performance battery (SPPB); walk time (WS); chair rise (CR); standing balance (SB); velocity and self-paced maximum velocity (SPMV) are expressed in meters/second (m/s); walk time represents the time to walk a distance of 2.5 m; WS, CR and SB are the SPPB sub-scores.
Fig. 2.
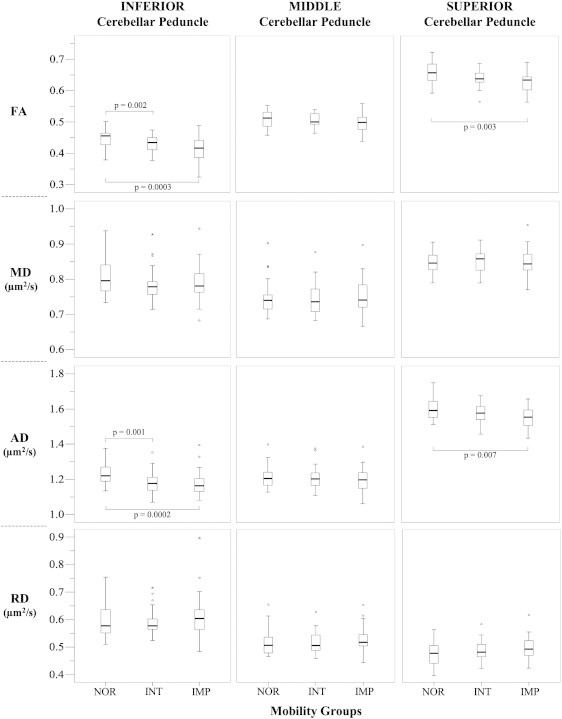
Boxplots show the values and distributions of fractional anisotropy (FA), mean diffusivity (MD), axial diffusivity (AD) and radial diffusivity (RD) in the inferior, middle and superior cerebellar peduncles in the normal (NOR: SPPB = 11–12, n = 26), intermediate (INT: SPPB = 9–10, n = 27) and impaired (IMP: SPPB < 9, n = 32) mobility groups. The p-values of post-hoc comparison analysis (Mann–Whitney) between the two specified groups are reported. Thick lines inside the boxes indicate the median value. The whiskers indicate the top and bottom quartiles; circles are outliers; stars are extreme outliers. FA is a normalized index with values between 0 and 1; MD, AD, and RD are expressed as μm2/s.
Table 3.
Correlation of diffusion tensor imaging (DTI) indices of the cerebellar peduncles with mobility performance.
| Peduncle DTI-index | SPPB | Walk time | SPMV | Velocity | Tinetti gait | Tinetti balance |
|---|---|---|---|---|---|---|
| ICP-FA |
0.415 (0.00008) |
− 0.311 (0.004) |
0.263 (0.044) |
0.260 (0.047) |
0.108 (0.410) |
0.287 (0.024) |
| ICP-MD | 0.097 (0.376) |
− 0.041 (0.711) |
0.031 (0.816) |
0.091 (0.494) |
− 0.043 (0.789) |
− 0.043 (0.784) |
| ICP-AD |
0.408 (0.0001) |
− 0.269 (0.013) |
0.252 (0.050) |
0.299 (0.021) |
0.024 (0.855) |
0.111 (0.390) |
| ICP-RD | − 0.115 (0.297) |
0.108 (0.327) |
− 0.165 (0.212) |
− 0.118 (0.375) |
− 0.078 (0.550) |
− 0.198 (0.124) |
| MCP-FA | 0.182 (0.095) |
− 0.227 (0.036) |
0.262 (0.045) |
0.264 (0.043) |
0.048 (0.714) |
0.117 (0.367) |
| MCP-MD | − 0.034 (0.757) |
0.050 (0.652) |
− 0.062 (0.642) |
− 0.053 (0.692) |
0.057 (0.664) |
− 0.055 (0.669) |
| MCP-AD | 0.081 (0.461) |
− 0.100 (0.364) |
0.117 (0.376) |
0.116 (0.383) |
0.125 (0.338) |
0.051 (0.696) |
| MCP-RD | − 0.146 (0.184) |
0.179 (0.101) |
− 0.248 (0.058) |
− 0.242 (0.065) |
− 00.16 (0.903) |
− 0.154 (0.233) |
| SCP-FA |
0.306 (0.004) |
− 0.253 (0.019) |
0.289 (0.026) |
0.273 (0.036) |
0.147 (0.258) |
0.161 (0.212) |
| SCP-MD | 0.016 (0.882) |
− 0.073 (0.509) |
0.107 (0.420) |
0.120 (0.365) |
0.035 (0.789) |
0.046 (0.722) |
| SCP-AD |
0.271 (0.012) |
− 0.291 (0.007) |
0.385 (0.003) |
0.400 (0.002) |
0.222 (0.085) |
0.272 (0.032) |
| SCP-RD | − 0.201 (0.066) |
0.146 (0.184) |
− 0.120 (0.365) |
− 0.093 (0.484) |
− 0.097 (0.459) |
− 0.081 (0.531) |
Associations are expressed as Spearman's rho (p). Statistical significance is indicated in bold (p ≤ 0.05) and in italic (p ≤ 0.004, after Bonferroni correction for multiple comparisons: 0.05/12). Abbreviations: self-paced maximum velocity (SPMV); short physical performance battery (SPPB); inferior cerebellar peduncles (ICP); middle cerebellar peduncles (MCP); superior cerebellar peduncles (SCP); fractional anisotropy (FA); mean diffusivity (MD); axial diffusivity (AD); radial diffusivity (RD). Velocity and SPMV expressed in m/s; walk time: seconds to walk a distance of 2.5 m.
To uncover clues regarding possible cause-effect mechanisms, we tested the relationship of DTI markers with age, as a factor known to influence DTI characteristics (Inano et al., 2011), and available indicators of cardiovascular risk, i.e. cholesterol, blood pressure, or neurodegenerative risk, i.e. brain atrophy (BPF), WMH burden. The results are shown in Table 4. We found that age is consistently related with the FA, MD and RD indices in the three peduncles. However, only the correlation with ICP-RD remained significant after Bonferroni correction for multiple testing. No relationship with levels of serum lipoproteins was observed. While both 24-h average SBP and DBP measurements showed some degree of association with DTI metrics of the cerebellar peduncles, DBP showed slightly more widespread correlations of which the most significant were those with ICP-RD, MCP-MD and MCP-AD. BPF showed the strongest correlation with the peduncles' FA and to a lower extent with RD in ICP, MCP and SCP; and with AD in ICP and SCP. We found that WMH burden correlated weakly with FA, MD and RD in all three peduncles (Table 4). WMH burden correlated with SPPB walk time (r = 0.385, p = 0.0003) and Tinetti gait (r = − 0.381, p = 0.002).
Table 4.
Correlations of diffusion tensor imaging (DTI) indices of the cerebellar peduncles with cardiovascular and neurodegenerative risk factors.
| Peduncle DTI-index | Age | Total cholesterol | LDL | HDL | 24 h-SBP | 24 h-DBP | BPF | WMH |
|---|---|---|---|---|---|---|---|---|
| ICP-FA |
− 0.300 (0.005) |
0.082 (0.527) |
0.138 (0.286) |
0.178 (0.167) |
− 0.124 (0.274) |
− 0.236 (0.036) |
0.542 (0.000001) |
− 0.212 (0.052) |
| ICP-MD |
0.300 (0.005) |
− 0.067 (0.603) |
− 0.084 (0.517) |
− 0.057 (0.658) |
0.176 (0.122) |
0.285 (0.011) |
− 0.117 (0.288) |
0.220 (0.043) |
| ICP-AD | 0.119 (0.279) |
− 0.030 (0.818) |
− 0.016 (0.904) |
0.081 (0.532) |
0.134 (0.238) |
0.145 (0.202) |
0.221 (0.042) |
0.068 (0.534) |
| ICP-RD |
0.317 (0.003) |
− 0.035 (0.790) |
− 0.101 (0.436) |
− 0.170 (0.186) |
0.210 (0.063) |
0.330 (0.003) |
− 0.316 (0.003) |
0.256 (0.018) |
| MCP-FA |
− 0.213 (0.05) |
− 0.056 (0.666) |
− 0.011 (0.930) |
0.061 (0.636) |
− 0.100 (0.382) |
− 0.091 (0.427) |
0.453 (0.00001) |
− 0.264 (0.015) |
| MCP-MD |
0.232 (0.033) |
0.202 (0.115) |
0.126 (0.329) |
− 0.057 (0.660) |
0.270 (0.016) |
0.358 (0.001) |
− 0.111 (0.310) |
0.125 (0.255) |
| MCP-AD | 0.136 (0.214) |
0.210 (0.101) |
0.147 (0.253) |
0.012 (0.927) |
0.249 (0.027) |
0.389 (0.0004) |
0.061 (0.579) |
− 0.022 (0.843) |
| MCP-RD |
0.267 (0.014) |
0.187 (0.145) |
0.112 (0.386) |
− 0.110 (0.395) |
0.246 (0.029) |
0.283 (0.011) |
− 0.264 (0.014) |
0.237 (0.029) |
| SCP-FA |
− 0.282 (0.009) |
0.157 (0.223) |
0.062 (0.632) |
0.228 (0.074) |
− 0.135 (0.235) |
− 0.013 (0.909) |
0.439 (0.00003) |
− 0.282 (0.009) |
| SCP-MD |
0.228 (0.036) |
− 0.045 (0.728) |
0.054 (0.676) |
− 0.182 (0.157) |
0.219 (0.052) |
0.249 (0.027) |
− 0.022 (0.843) |
0.210 (0.054) |
| SCP-AD | − 0.040 (0.713) |
0.087 (0.500) |
0.078 (0.546) |
0.114 (0.377) |
0.058 (0.611) |
0.227 (0.044) |
0.328 (0.002) |
− 0.037 (0.739) |
| SCP-RD |
0.289 (0.007) |
− 0.145 (0.262) |
− 0.031 (0.810) |
− 0.251 (0.049) |
0.187 (0.100) |
0.134 (0.241) |
− 0.289 (0.007) |
0.323 (0.003) |
Data are expressed as Spearman's rho (p). Statistical significance is indicated in bold (p ≤ 0.05) and in italic (p ≤ 0.004, after Bonferroni correction for multiple comparisons: 0.05/12). Abbreviations: body mass index (BMI), systolic blood pressure (SPB), diastolic blood pressure (DPB), low-density lipoprotein cholesterol (LDL), high-density lipoprotein cholesterol (HDL), white matter hyperintensities (WMH), brain parenchymal fraction (BPF); inferior cerebellar peduncles (ICP); middle cerebellar peduncles (MCP); superior cerebellar peduncles (SCP); fractional anisotropy (FA); mean diffusivity (MD); axial diffusivity (AD); radial diffusivity (RD).
Presence of WM lesions in the brain stem has been reported in a previous study on mobility and MR T2-weighted brain WM abnormalities in a sample of elderly subjects 78–79 years of age (Starr et al., 2003). We confirmed the absence of focal morphological abnormalities in the peduncles of the subjects included in this study by careful review of the structural MR images (performed by MC, a neurologist with image analysis expertise). However, fourteen subjects presented with MR signal inhomogeneities in the pontine areas neighboring the superior and middle cerebellar peduncles (ten and four subjects, respectively). In addition one subject showed small bilateral slight hyperintensity in the SCP. These fifteen subjects did not cluster in a particular mobility group (five in the normal, six in the intermediate and four in the impaired group) and exclusion from the analysis did not significantly change the observed correlation results.
To estimate the ability of the DTI indices to predict mobility status we performed a logistic regression analysis using normal versus impaired status as categorical response variable. For this analysis we included only subjects in the normal (SPPB score = 11–12) and impaired (SPPB score < 9) mobility groups (see Methods). Age, BMI, BPF, WMH burden, and blood pressure were also analyzed as factors potentially relevant in mobility performance. When we tested the variables individually we found that age, BMI, BPF, ICP-FA, ICP-AD, SCP-FA, SCP-AD and SCP-RD were significant explanatory variables of mobility status (Table 5). To identify the best predictor/s we included all the significant variables above in a multivariate logistic regression model analyzed with the forward stepwise method. The ICP-FA and ICP-AD were the only two variables retained in the final model. Together they explained 51% of the variability in the mobility status (Nagelkerke-r2 = 0.510) (Table 5) and resulted in correct overall classification of 77.6% of the subjects in the two mobility groups.
Table 5.
Logistic Regression — variables tested as predictors of mobility status (normal = 0 or impaired = 1).
| Variable | χ2 (p) | Nagelkerke pseudo-r2 |
|---|---|---|
| Age | 3.73 (0.05) | 0.08 |
| Gender | 0.03 (0.86) | 0.001 |
| BMI | 5.59 (0.02) | 0.12 |
| 24 h-SBP | 0.187 (0.67) | 0.005 |
| 24 h-DBP | 4 · 10− 4 (0.98) | 0.00001 |
| WMH | 2.57 (0.11) | 0.06 |
| BPF | 9.59 (0.005) | 0.20 |
| ICP-FA | 13.39 (0.0003) | 0.28 |
| ICP-MD | 0.30 (0.58) | 0.01 |
| ICP-AD | 10.74 (0.001) | 0.23 |
| ICP-RD | 0.62 (0.43) | 0.01 |
| MCP-FA | 3.55 (0.06) | 0.08 |
| MCP-MD | 0.025 (0.88) | 0.001 |
| MCP-AD | 1.04 (0.31) | 0.02 |
| MCP-RD | 0.94 (0.33) | 0.02 |
| SCP-FA | 11.38 (0.001) | 0.24 |
| SCP-MD | 0.025 (0.87) | 0.001 |
| SCP-AD | 9.76 (0.002) | 0.21 |
| SCP-RD | 4.33 (0.04) | 0.10 |
| Multivariate model (forward stepwise method) Variables included: age, BMI, BPF, ICP-FA, ICP-AD, SCP-FA, SCP-AD, SCP-RD | ||
| ICP-FA + ICP-AD | 27.5 (10− 6) | 0.51 |
Variables individually tested in univariate logistic regression (top). The variables with chi-square (χ2) values reaching statistical significance (p ≤ 0.05), highlighted in bold, were all included in the multivariate model (bottom). ICP-FA and ICP-AD were the only variables retained in the final model.
Abbreviations: body mass index (BMI), systolic blood pressure (SPB), diastolic blood pressure (DPB), white matter hyperintensities (WMH), brain parenchymal fraction (BPF); inferior cerebellar peduncles (ICP); middle cerebellar peduncles (MCP); superior cerebellar peduncles (SCP); fractional anisotropy (FA); mean diffusivity (MD); axial diffusivity (AD); radial diffusivity (RD).
4. Discussion
We investigated the relationship between mobility performance and indices of microstructural integrity of the cerebellar peduncles in elderly subjects who had no clinical evidence of cerebellar dysfunction. We found that impaired mobility associated with decreased FA and AD within the inferior and superior peduncles. These findings likely reflect the presence of microstructural alterations in tracts connecting the cerebellum with structures that participate in motor control (i.e. spinal cord, vestibular system, and thalamus). It is plausible that these alterations decrease the efficiency of processing and integration of sensory and motor information by the cerebellum.
The ICP includes both cerebellar efferent and afferent fibers to and from the vestibular nuclei (vestibulocerebellar tracts) that carry information about eye movements, and the orientation of head and body as well as afferent spinal fibers (spinocerebellar tracts) carrying ipsilateral proprioceptive information important for posture, locomotion and muscle tone control (Thach and Bastian, 2004). The MCP contains exclusively tracts from the pontine nuclei to the cerebellum conveying information from the cerebral cortex, mostly motor and somatosensory areas. The SCP is constituted predominantly by efferent fibers (pontocerebellar tracts) carrying information related to skilled limb movements (Fitzgerald, 1992) and, in small proportion, by afferent vestibulocerebellar tracts from the inferior midbrain colliculi and spinocerebellar fibers from the spinal cord, involved in reflex eye movements and locomotion, respectively (Kandel et al., 2000; Nieuwenhuys et al., 1981). The results in supplementary Table 1 showing that the most highly significant correlations are observed between SCP and walk score and between ICP and chair rise score seem consistent with the above physiological description. The complex network architecture likely relates to the differences in the DTI indices of the peduncles, particularly between the ICP and SCP, we observed in the whole study sample (Fig. 2). While we did not investigate further this result being outside the scope of our analysis, we interpret it as being related to the presence of fanning fibers and/or axon-to-myelin ratio differences among the three peduncles (Stieltjes et al., 2001). The coexistence of both efferent and afferent type of fibers with various bend and trajectories within the inferior and superior peduncles is consistent with this explanation.
While our interpretation is limited by the fact that afferent and efferent projections cannot be distinguished on DTI, the finding that ICP-FA and ICP-AD indices are the best predictors of impaired mobility among the variables tested suggests that our study population is more sensitive to alterations in spinal proprioceptive afferents rather than to alterations in the SCP pontocerebellar efferents. None of the DTI indices in the MCP showed significant correlation with mobility performance. One may speculate from these findings that mobility is relatively less sensitive to microstructural damage in the pontocerebellar connections of the MCP due to the functional compensatory reserve potential provided by the abundance of fibers in this peduncle (Tomasch, 1969).
The novelty of our observations is in the detection of changes in brain white matter regions that appear normal in conventional T2-weighted MRI and that were not analyzed previously in elderly with idiopathic mobility impairment. Cerebellar dysfunction in the study subjects was excluded by neurological examination at enrollment. The impact of macroscopic damage in the brainstem and peduncles on motor functions is also unlikely since we confirmed the absence of focal abnormalities in the peduncles on structural MR images. The findings therefore support a link between decreased mobility and microstructural damage in the cerebellar peduncles. Previous studies on inherited cerebellar disorders in subjects with spinocerebellar or Friedreich's ataxia have reported decreased FA in the ICP and/or SCP (Alcauter et al., 2011; Della Nave et al., 2008; Mandelli et al., 2007) thus showing that DTI metrics can reveal diffuse changes in the cerebellar peduncles that have evident clinical impact on motor skills.
The pathophysiological significance of the DTI indices is still uncertain and we are cautious in deciphering the findings from our study. Evidence from DTI literature and results from human studies help in the interpretation of the different DTI indices and the implications for underlying white matter pathology. The FA parameter is a sensitive but not specific index of white matter tracts' overall integrity reflecting one or several factors including axonal degeneration, myelin damage and extracellular/intracellular water distribution (O'Donnell and Westin, 2011). The AD index is regarded as a more specific marker for axonal injury (Budde et al., 2007; Song et al., 2002). RD is considered more specific for damage to the myelin sheath (Song et al., 2002) while the MD is a general indicator of tissue water accumulation (Alexander et al., 2007). Based on the above criteria we interpret the observed associations of impaired mobility with FA and AD, but not with MD or RD, as supporting axonal damage rather than other types of fiber injury as the underlying mechanism. The association of mobility impairment with decreased axonal integrity index is independent from the effect of age. In fact, no correlation between age and AD was observed. In contrast, we found that age correlated with the other DTI markers, particularly RD, consistent with evidence from other studies showing that normal aging is predominantly associated with reduced myelin integrity (Inano et al., 2011; Salat et al., 2005).
As seen with age, cerebellar peduncles AD was not related to supratentorial WMH burden, which is only minimally related to the other DTI characteristics of the peduncles. This evidence suggests that the observed AD reduction in the cerebellar peduncles and the supratentorial WMH burden reflect different underlying mechanisms. This is further supported by the lack or weak correlation of both FA and AD in ICP and SCP with cardiovascular risk factors. Therefore while WMH is thought to reflect cerebral small vessel disease (Pantoni, 2010; Wardlaw et al., 2003) the axonal damage we observed in the ICP and SCP likely results from a mechanism distinct from the one that leads to cerebral WMH. In this regard the strong association of the peduncles' FA with brain atrophy suggests that at least in part the observed changes may occur through a neurodegenerative mechanism. Future longitudinal analysis or, alternatively, neuropathology studies could help in shedding light on this particular aspect.
We acknowledge limitations to our study. These include the relatively small sample size that may limit the general applicability of the findings, the descriptive nature of the cross-sectional analysis, and the limitations related to image processing. In that regard, reduced accuracy could have potentially resulted from the relatively small white matter regions we investigated due to the effort of the skeletonized-WM approach to exclude non-WM pixels, and from the calculation of specific regional indices after warping of individual brain to standard atlas. Since we have undertaken this study with a focus on these three regions we did not perform a comprehensive analysis with a voxel-wise whole-brain approach. While we observed some degree of regional specificity between mobility performance and the micro-structural integrity of the cerebellar peduncles (i.e., ICP > SCP > MCP) our findings do not exclude similar relationship with other tract/s. Previous works demonstrated an association of mobility impairment with specific supratentorial WM regions (Bhadelia et al., 2009; de Laat et al., 2011; Della Nave et al., 2007; Moscufo et al., 2011; Srikanth et al., 2010) and therefore it is likely that associations with other relevant tracts will be observed in a whole-brain analysis on a larger sample with sufficient statistical power. Additional results from a limited correlation analysis (supplementary Tables 2 and 3) showed that also the corticospinal tracts and cerebral peduncles, two other tracts in the brain stem, correlated with measures of mobility performance. Future work should dissect in more detail the issue of specificity and interrelationship of different WM tracts with respect to mobility impairment in the elderly. Finally, we acknowledge that given the co-existence of different fiber bundles within the ICP and SCP we cannot distinguish if the observed DTI changes result from one or all the contributing fascicles, e.g. spino- or vestibulo-cerebellar tracts in the ICP.
In conclusion the study findings confirm that DTI, a sensitive in vivo non-invasive medical imaging tool, can detect clinically relevant alterations in the microstructure of infratentorial white matter. Importantly, damage to the cerebellar peduncles should be considered a potential pathogenic mechanism in older individuals with idiopathic mobility impairment.
Acknowledgments
Study supported by the National Institute on Aging — 2R01AG22092-06A1; University of Connecticut Health Center General Clinical Research Center grant M01 RR06192; NIH 5 P41 RR13218. We thank Julie Schmidt for her contribution to recruitment and study coordination and Dorothy Wakefield for her assistance with data management.
Footnotes
This is an open-access article distributed under the terms of the Creative Commons Attribution-NonCommercial-ShareAlike License, which permits non-commercial use, distribution, and reproduction in any medium, provided the original author and source are credited.
Appendix A. Supplementary data
Supplementary tables.
References
- Alcauter S., Barrios F.A., Diaz R., Fernandez-Ruiz J. Gray and white matter alterations in spinocerebellar ataxia type 7: an in vivo DTI and VBM study. NeuroImage. 2011;55:1–7. doi: 10.1016/j.neuroimage.2010.12.014. [DOI] [PubMed] [Google Scholar]
- Alexander A.L., Lee J.E., Lazar M., Field A.S. Diffusion tensor imaging of the brain. Neurotherapeutics. 2007;4:316–329. doi: 10.1016/j.nurt.2007.05.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anderson V.M., Wheeler-Kingshott C.A., Abdel-Aziz K., Miller D.H., Toosy A., Thompson A.J., Ciccarelli O. A comprehensive assessment of cerebellar damage in multiple sclerosis using diffusion tractography and volumetric analysis. Multiple Sclerosis. 2011;17:1079–1087. doi: 10.1177/1352458511403528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baezner H., Blahak C., Poggesi A., Pantoni L., Inzitari D., Chabriat H., Erkinjuntti T., Fazekas F., Ferro J.M., Langhorne P., O'Brien J., Scheltens P., Visser M.C., Wahlund L.O., Waldemar G., Wallin A., Hennerici M.G. Association of gait and balance disorders with age-related white matter changes: the LADIS study. Neurology. 2008;70:935–942. doi: 10.1212/01.wnl.0000305959.46197.e6. [DOI] [PubMed] [Google Scholar]
- Baloh R.W., Vinters H.V. White matter lesions and disequilibrium in older people. II. Clinicopathologic correlation. Archives of Neurology. 1995;52:975–981. doi: 10.1001/archneur.1995.00540340067014. [DOI] [PubMed] [Google Scholar]
- Bhadelia R.A., Price L.L., Tedesco K.L., Scott T., Qiu W.Q., Patz S., Folstein M., Rosenberg I., Caplan L.R., Bergethon P. Diffusion tensor imaging, white matter lesions, the corpus callosum, and gait in the elderly. Stroke. 2009;40:3816–3820. doi: 10.1161/STROKEAHA.109.564765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Briley D.P., Wasay M., Sergent S., Thomas S. Cerebral white matter changes (leukoaraiosis), stroke, and gait disturbance. Journal of the American Geriatrics Society. 1997;45:1434–1438. doi: 10.1111/j.1532-5415.1997.tb03192.x. [DOI] [PubMed] [Google Scholar]
- Budde M.D., Kim J.H., Liang H.F., Schmidt R.E., Russell J.H., Cross A.H., Song S.K. Toward accurate diagnosis of white matter pathology using diffusion tensor imaging. Magnetic Resonance in Medicine. 2007;57:688–695. doi: 10.1002/mrm.21200. [DOI] [PubMed] [Google Scholar]
- Camicioli R., Moore M.M., Sexton G., Howieson D.B., Kaye J.A. Age-related brain changes associated with motor function in healthy older people. Journal of the American Geriatrics Society. 1999;47:330–334. doi: 10.1111/j.1532-5415.1999.tb02997.x. [DOI] [PubMed] [Google Scholar]
- Campbell P., Ghuman N., Wakefield D., Wolfson L., White W.B. Long-term reproducibility of ambulatory blood pressure is superior to office blood pressure in the very elderly. Journal of Human Hypertension. 2010;24:749–754. doi: 10.1038/jhh.2010.8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Laat K.F., Tuladhar A.M., van Norden A.G., Norris D.G., Zwiers M.P., de Leeuw F.E. Loss of white matter integrity is associated with gait disorders in cerebral small vessel disease. Brain. 2011;134:73–83. doi: 10.1093/brain/awq343. [DOI] [PubMed] [Google Scholar]
- Della Nave R., Foresti S., Pratesi A., Ginestroni A., Inzitari M., Salvadori E., Giannelli M., Diciotti S., Inzitari D., Mascalchi M. Whole-brain histogram and voxel-based analyses of diffusion tensor imaging in patients with leukoaraiosis: correlation with motor and cognitive impairment. AJNR. American Journal of Neuroradiology. 2007;28:1313–1319. doi: 10.3174/ajnr.A0555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Della Nave R., Ginestroni A., Tessa C., Salvatore E., Bartolomei I., Salvi F., Dotti M.T., De Michele G., Piacentini S., Mascalchi M. Brain white matter tracts degeneration in Friedreich ataxia. An in vivo MRI study using tract-based spatial statistics and voxel-based morphometry. NeuroImage. 2008;40:19–25. doi: 10.1016/j.neuroimage.2007.11.050. [DOI] [PubMed] [Google Scholar]
- Della Nave R., Ginestroni A., Diciotti S., Salvatore E., Soricelli A., Mascalchi M. Axial diffusivity is increased in the degenerating superior cerebellar peduncles of Friedreich's ataxia. Neuroradiology. 2011;53:367–372. doi: 10.1007/s00234-010-0807-1. [DOI] [PubMed] [Google Scholar]
- Fitzgerald M.J.T. Bailliere Tindall; 1992. Neuroanatomy: Basic and Clinical. [Google Scholar]
- Graham J.E., Ostir G.V., Kuo Y.F., Fisher S.R., Ottenbacher K.J. Relationship between test methodology and mean velocity in timed walk tests: a review. Archives of Physical Medicine and Rehabilitation. 2008;89:865–872. doi: 10.1016/j.apmr.2007.11.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guralnik J.M., Simonsick E.M., Ferrucci L., Glynn R.J., Berkman L.F., Blazer D.G., Scherr P.A., Wallace R.B. A short physical performance battery assessing lower extremity function: association with self-reported disability and prediction of mortality and nursing home admission. Journal of Gerontology. 1994;49:M85–M94. doi: 10.1093/geronj/49.2.m85. [DOI] [PubMed] [Google Scholar]
- Guttmann C.R., Benson R., Warfield S.K., Wei X., Anderson M.C., Hall C.B., Abu-Hasaballah K., Mugler J.P., III, Wolfson L. White matter abnormalities in mobility-impaired older persons. Neurology. 2000;54:1277–1283. doi: 10.1212/wnl.54.6.1277. [DOI] [PubMed] [Google Scholar]
- Inano S., Takao H., Hayashi N., Abe O., Ohtomo K. Effects of age and gender on white matter integrity. AJNR. American Journal of Neuroradiology. 2011;32:2103–2109. doi: 10.3174/ajnr.A2785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jenkinson M., Smith S. A global optimisation method for robust affine registration of brain images. Medical Image Analysis. 2001;5:143–156. doi: 10.1016/s1361-8415(01)00036-6. [DOI] [PubMed] [Google Scholar]
- Kandel Eric R., Schwartz James H., Jessell Thomas M. McGraw-Hill; 2000. Principles of Neural Science. [Google Scholar]
- Leipzig R.M., Cumming R.G., Tinetti M.E. Drugs and falls in older people: a systematic review and meta-analysis: I. Psychotropic drugs. Journal of the American Geriatrics Society. 1999;47:30–39. doi: 10.1111/j.1532-5415.1999.tb01898.x. [DOI] [PubMed] [Google Scholar]
- Mandelli M.L., De Simone T., Minati L., Bruzzone M.G., Mariotti C., Fancellu R., Savoiardo M., Grisoli M. Diffusion tensor imaging of spinocerebellar ataxias types 1 and 2. AJNR. American Journal of Neuroradiology. 2007;28:1996–2000. doi: 10.3174/ajnr.A0716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Masdeu J.C., Wolfson L., Lantos G., Tobin J.N., Grober E., Whipple R., Amerman P. Brain white-matter changes in the elderly prone to falling. Archives of Neurology. 1989;46:1292–1296. doi: 10.1001/archneur.1989.00520480034016. [DOI] [PubMed] [Google Scholar]
- Mori S., Oishi K., Jiang H., Jiang L., Li X., Akhter K., Hua K., Faria A.V., Mahmood A., Woods R., Toga A.W., Pike G.B., Neto P.R., Evans A., Zhang J., Huang H., Miller M.I., van Zijl P., Mazziotta J. Stereotaxic white matter atlas based on diffusion tensor imaging in an ICBM template. NeuroImage. 2008;40:570–582. doi: 10.1016/j.neuroimage.2007.12.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moscufo N., Guttmann C.R., Meier D., Csapo I., Hildenbrand P.G., Healy B.C., Schmidt J.A., Wolfson L. Brain regional lesion burden and impaired mobility in the elderly. Neurobiology of Aging. 2011;32:646–654. doi: 10.1016/j.neurobiolaging.2009.04.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nicoletti G., Lodi R., Condino F., Tonon C., Fera F., Malucelli E., Manners D., Zappia M., Morgante L., Barone P., Barbiroli B., Quattrone A. Apparent diffusion coefficient measurements of the middle cerebellar peduncle differentiate the Parkinson variant of MSA from Parkinson's disease and progressive supranuclear palsy. Brain. 2006;129:2679–2687. doi: 10.1093/brain/awl166. [DOI] [PubMed] [Google Scholar]
- Nicoletti G., Tonon C., Lodi R., Condino F., Manners D., Malucelli E., Morelli M., Novellino F., Paglionico S., Lanza P., Messina D., Barone P., Morgante L., Zappia M., Barbiroli B., Quattrone A. Apparent diffusion coefficient of the superior cerebellar peduncle differentiates progressive supranuclear palsy from Parkinson's disease. Movement Disorders. 2008;23:2370–2376. doi: 10.1002/mds.22279. [DOI] [PubMed] [Google Scholar]
- Nieuwenhuys R., Voogd J., van Huijzen C. Springer-Verlag; Berlin, Heidelberg, New York: 1981. The Human Central Nervous System. A Synopsis and Atlas. [Google Scholar]
- O'Donnell L.J., Westin C.F. An introduction to diffusion tensor image analysis. Neurosurgery Clinics of North America. 2011;22:185–196. doi: 10.1016/j.nec.2010.12.004. (viii) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pantoni L. Cerebral small vessel disease: from pathogenesis and clinical characteristics to therapeutic challenges. Lancet Neurology. 2010;9:689–701. doi: 10.1016/S1474-4422(10)70104-6. [DOI] [PubMed] [Google Scholar]
- Panzer V.P., Wakefield D.B., Hall C.B., Wolfson L.I. Mobility assessment: sensitivity and specificity of measurement sets in older adults. Archives of Physical Medicine and Rehabilitation. 2011;92:905–912. doi: 10.1016/j.apmr.2011.01.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pierpaoli C., Jezzard P., Basser P.J., Barnett A., Di Chiro G. Diffusion tensor MR imaging of the human brain. Radiology. 1996;201:637–648. doi: 10.1148/radiology.201.3.8939209. [DOI] [PubMed] [Google Scholar]
- Sachdev P.S., Wen W., Christensen H., Jorm A.F. White matter hyperintensities are related to physical disability and poor motor function. Journal of Neurology, Neurosurgery & Psychiatry. 2005;76:362–367. doi: 10.1136/jnnp.2004.042945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salat D.H., Tuch D.S., Greve D.N., van der Kouwe A.J., Hevelone N.D., Zaleta A.K., Rosen B.R., Fischl B., Corkin S., Rosas H.D., Dale A.M. Age-related alterations in white matter microstructure measured by diffusion tensor imaging. Neurobiology of Aging. 2005;26:1215–1227. doi: 10.1016/j.neurobiolaging.2004.09.017. [DOI] [PubMed] [Google Scholar]
- Sled J.G., Zijdenbos A.P., Evans A.C. A nonparametric method for automatic correction of intensity nonuniformity in MRI data. IEEE Transactions on Medical Imaging. 1998;17:87–97. doi: 10.1109/42.668698. [DOI] [PubMed] [Google Scholar]
- Smith S.M., Jenkinson M., Johansen-Berg H., Rueckert D., Nichols T.E., Mackay C.E., Watkins K.E., Ciccarelli O., Cader M.Z., Matthews P.M., Behrens T.E. Tract-based spatial statistics: voxelwise analysis of multi-subject diffusion data. NeuroImage. 2006;31:1487–1505. doi: 10.1016/j.neuroimage.2006.02.024. [DOI] [PubMed] [Google Scholar]
- Song S.K., Sun S.W., Ramsbottom M.J., Chang C., Russell J., Cross A.H. Dysmyelination revealed through MRI as increased radial (but unchanged axial) diffusion of water. NeuroImage. 2002;17:1429–1436. doi: 10.1006/nimg.2002.1267. [DOI] [PubMed] [Google Scholar]
- Srikanth V., Phan T.G., Chen J., Beare R., Stapleton J.M., Reutens D.C. The location of white matter lesions and gait—a voxel-based study. Annals of Neurology. 2010;67:265–269. doi: 10.1002/ana.21826. [DOI] [PubMed] [Google Scholar]
- Starr J.M., Leaper S.A., Murray A.D., Lemmon H.A., Staff R.T., Deary I.J., Whalley L.J. Brain white matter lesions detected by magnetic resonance [correction of resonance] imaging are associated with balance and gait speed. Journal of Neurology, Neurosurgery & Psychiatry. 2003;74:94–98. doi: 10.1136/jnnp.74.1.94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stieltjes B., Kaufmann W.E., van Zijl P.C., Fredericksen K., Pearlson G.D., Solaiyappan M., Mori S. Diffusion tensor imaging and axonal tracking in the human brainstem. NeuroImage. 2001;14:723–735. doi: 10.1006/nimg.2001.0861. [DOI] [PubMed] [Google Scholar]
- Thach W.T., Bastian A.J. Role of the cerebellum in the control and adaptation of gait in health and disease. Progress in Brain Research. 2004;143:353–366. doi: 10.1016/s0079-6123(03)43034-3. [DOI] [PubMed] [Google Scholar]
- Tinetti M.E. Performance-oriented assessment of mobility problems in elderly patients. Journal of the American Geriatrics Society. 1986;34:119–126. doi: 10.1111/j.1532-5415.1986.tb05480.x. [DOI] [PubMed] [Google Scholar]
- Tinetti M.E., Speechley M., Ginter S.F. Risk factors for falls among elderly persons living in the community. The New England Journal of Medicine. 1988;319:1701–1707. doi: 10.1056/NEJM198812293192604. [DOI] [PubMed] [Google Scholar]
- Tomasch J. The numerical capacity of the human cortico-pontocerebellar system. Brain Research. 1969;13:476–484. doi: 10.1016/0006-8993(69)90261-3. [DOI] [PubMed] [Google Scholar]
- Wakefield D.B., Moscufo N., Guttmann C.R., Kuchel G.A., Kaplan R.F., Pearlson G., Wolfson L. White matter hyperintensities predict functional decline in voiding, mobility, and cognition in older adults. Journal of the American Geriatrics Society. 2010;58:275–281. doi: 10.1111/j.1532-5415.2009.02699.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wardlaw J.M., Sandercock P.A., Dennis M.S., Starr J. Is breakdown of the blood-brain barrier responsible for lacunar stroke, leukoaraiosis, and dementia? Stroke. 2003;34:806–812. doi: 10.1161/01.STR.0000058480.77236.B3. [DOI] [PubMed] [Google Scholar]
- Werring D.J., Clark C.A., Barker G.J., Thompson A.J., Miller D.H. Diffusion tensor imaging of lesions and normal-appearing white matter in multiple sclerosis. Neurology. 1999;52:1626–1632. doi: 10.1212/wnl.52.8.1626. [DOI] [PubMed] [Google Scholar]
- White W.B., Wolfson L., Wakefield D.B., Hall C.B., Campbell P., Moscufo N., Schmidt J., Kaplan R.F., Pearlson G., Guttmann C.R. Average daily blood pressure, not office blood pressure, is associated with progression of cerebrovascular disease and cognitive decline in older people. Circulation. 2011;124:2312–2319. doi: 10.1161/CIRCULATIONAHA.111.037036. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary tables.


