Abstract
Background
Biliary complications after liver transplant are a frequent source of morbidity. However, little recent mortality data exists related to endoscopic management of these complications.
Aims
To determine the effect of endoscopic retrograde cholangiopancreatography (ERCP) utilization and biliary complications on patient and graft survival after liver transplantation.
Methods
This study was a retrospective cohort study at the University of North Carolina Hospitals from 2004 to 2007. One hundred thirty-two consecutive liver transplant patients were included. Recipient, donor, and clinical data were extracted from electronic resources. The main outcome measurements were all-cause mortality and graft failure.
Results
Of 132 transplants, 59 (45%) required ERCP post transplant, and 49 (37%) were found to have a biliary complication by ERCP. The 1-year patient survival for those treated by ERCP with a biliary complication was 90% compared with 81% in those without a biliary complication [P = 0.018; unadjusted hazard ratio (HR) = 0.32; 95% confidence interval (CI) 0.11–0.93]. The 1-year graft survival in those with and without a biliary complication was 94% and 73%, respectively (P < 0.001; unadjusted HR 0.19; 95% CI 0.07–0.56). This effect on patient and graft survival persisted after multivariate analysis. Similar results were seen for ERCP utilization alone, and when early deaths within the first 30 days were excluded.
Conclusions
Patients who underwent ERCP for a biliary complication post liver transplantation had better overall and graft survival than patients who did not have an ERCP. Biliary complications and ERCP utilization are common after liver transplant, but they do not confer excess mortality.
Keywords: Liver transplantation, Endoscopic retrograde cholangiopancreatography, ERCP, Biliary complication, Mortality
Introduction
Biliary complications after liver transplant are common, having been reported in 6–51% of cases [1-3]. These complications, which include anastomotic strictures, ischemic-type biliary lesions, choledocholithiasis or bile casts, bile leaks, and cholangitis, are a major source of morbidity following transplant. The degree of mortality associated with these problems, however, is less well established. Initial series investigating this included surgical management strategies used at the beginning of the liver transplant era, and found a mortality rate of 6.5–44% attributable to biliary complications over 12 months [4-11]. Because these studies predate the era of frequent utilization of endoscopic retrograde cholangiopancreatography (ERCP) in the management of post-transplant biliary complications, they may no longer be applicable to current clinical practice.
When biliary complications occur after liver transplantation in the current era, ERCP is now the first-line therapeutic intervention, and surgical correction of biliary complications is now typically an option of last resort [12]. ERCP allows effective treatment of anastomotic or ischemic strictures via stenting programs, clearance of debris from bile ducts, and treatment of bile leaks and cholangitis [13]. However, there are tangible risks to this procedure, including cardiopulmonary complications from sedation, bleeding, infection, perforation, and pancreatitis, which may counteract the presumed benefits [14].
It was our clinical impression that increasing numbers of ERCPs were being performed post transplant at our center, but that the benefits of this strategy were not well established. Therefore, the aim of this study is to determine the effect of ERCP utilization and biliary complications on patient and graft survival post liver transplantation. We hypothesized that the need for ERCP post transplant and the development of biliary complications would be a significant source of graft failure as well as increased patient morbidity and mortality.
Methods
Consecutive adult (recipient age ≥18 years) liver transplants performed at the University of North Carolina (UNC) Hospitals between 27 March 2004 and 4 December 2007 were identified via UNC’s transplant database, TransChart (TransChart, LLC, Dublin, OH). All data were analyzed over this same time period plus an additional 5-month follow-up period through 4 May 2008 to allow for adequate follow-up time to capture ERCP. ERCP procedure data were obtained through our electronic procedure database, ProvationMD (Provation Medical, Minneapolis, MN). Further data were obtained via the United Network of Organ Sharing (UNOS) database and from electronic patient medical records where appropriate. Data collection was performed after approval from the University of North Carolina Institutional Review Board.
After determining which patients underwent ERCP and quantifying the number of procedures they required during the study timeframe, the presence and type of biliary complications were recorded. These included: bile leak or biloma, intra- or extrahepatic biliary stricture from any cause, and obstructing stone or bile cast/sludge. Therapeutic maneuvers during the ERCP were also recorded.
The main outcomes of the study were all-cause mortality and graft failure. Vital status was determined by review of electronic medical records, and cause of death was determined through independent chart review by two investigators (A.S.B. and C.B.M.); discrepancies were adjudicated by all of the investigators. Graft failure was defined as progressive hepatic dysfunction requiring retransplant or leading to patient death.
Other factors of interest, as well as potential confounders, included recipient demographics and clinical information [cause of liver failure and model for end-stage liver disease (MELD) score at transplant], donor characteristics (age, donor type, and donor cause of death), and operative parameters (warm and cold ischemia time).
Statistical analyses were performed using Stata (version 9.2; Statacorp, College Station, TX). Descriptive statistics were used to characterize the study subjects. For bivariate analysis, chi-square was used for categorical variables and Student’s t-test was used for continuous variables. Kaplan–Meier survival analysis was performed, censoring patients at time of death, time of graft failure, or end of study period, to determine 1- and 3-year patient and graft survival. Multivariate analysis with Cox proportional-hazards modeling was used to calculate hazard ratios (HR) adjusted for potential confounding factors. These included recipient race, sex, age, MELD score, indication for transplant, warm and cold ischemia time, donor age, donor sex, and type of donor (cadaveric or donation after cardiac death). In order to fully characterize the findings, all analyses were performed for both outcomes, as well as for ERCP status, presence of a biliary complication, and vital status. Missing data were minimal in our data set, but subjects with missing data points were excluded from bivariate and multivariate analysis.
Results
Patient, Donor, and Transplant Characteristics
A total of 132 transplants were performed during the study period, 12 (9%) of which were retransplants (Table 1). Mean age at transplant was 53 years, 69% of recipients were male, and 73% were Caucasian. Mean MELD score at transplant was 25. The leading indication for transplant was chronic hepatitis C infection (20%), followed by cryptogenic cirrhosis (17%), comorbid hepatitis C and alcohol (12%), and alcohol alone (11%). For analysis, the etiologies of liver disease were put into four major groups: acute hepatic failure (AHF, 2%), chronic viral hepatitis (hepatitis B, C, and alcohol plus hepatitis C, 39%), alcohol-induced liver disease (ETOH, 11%), and others including cryptogenic cirrhosis and nonalcoholic fatty liver disease (48%).
Table 1.
Characteristics of the study population and bivariate analysis of patients who received ERCP versus those who did not
| Total population (n = 132) | Any ERCP (n = 59) | No ERCP (n = 73) | P for any ERCP versus no ERCP | |
|---|---|---|---|---|
| Recipient characteristics | ||||
| Mean age at transplant (years) | 52.8 ± 8.9 | 53.3 ± 8.6 | 52.4 ± 9.2 | 0.54 |
| Male, n (%) | 91 (69) | 42 (71) | 49 (67) | 0.62 |
| Caucasian, n (%) | 96 (73) | 40 (78) | 56 (76) | 0.25 |
| Indication for transplant, n (%) | ||||
| AHF | 3 (2) | 2 (3) | 1 (1) | |
| Viral | 51 (39) | 22 (37) | 29 (40) | 0.50 |
| ETOH | 15 (11) | 9 (15) | 6 (8) | |
| Other | 63 (48) | 26 (44) | 37 (51) | |
| MELD score | 24.8 ± 7.1 | 25.7 ± 7.3 | 24.0 ± 7.0 | 0.16 |
| Donor characteristics | ||||
| Male donor, n (%) | 74 (60) | 36 (64) | 38 (57) | 0.39 |
| Donor age (years) | 35.7 ± 15.0 | 35.1 ± 14.8 | 36.1 ± 15.3 | 0.72 |
| Donor type | ||||
| Cadaveric | 108 (82) | 61 (83) | 47 (80) | 0.50 |
| DCD | 23 (17) | 11 (15) | 12 (20) | |
| Unknown | 1 (1) | 1 (1) | 0 (0) | |
| Donor cause of death, n (%) | ||||
| Anoxia | 9 (7) | 5 (9) | 4 (6) | |
| Cerebral vascular accident (CVA) | 48 (39) | 22 (39) | 26 (38) | 0.91 |
| Trauma | 62 (50) | 27 (48) | 35 (51) | |
| Unknown | 5 (4) | 2 (4) | 3 (4) | |
| Operative characteristics | ||||
| Warm ischemia time, min | 40.5 ± 10.8 | 40.5 ± 12.9 | 40.5 ± 8.8 | 0.99 |
| Cold ischemia time, min | 419.3 ± 121.3 | 430 ± 122 | 411 ± 121 | 0.38 |
| Required retransplant, n (%) | 12 (9) | 0 (0) | 12 (16) | 0.001 |
| Survival | ||||
| Graft survival, % | ||||
| 1 year | 81 | 92.8 | 69.3 | <0.0001 |
| 3 years | 74 | 90.4 | 60.8 | |
| Patient survival, % | ||||
| 1 year | 85 | 92.8 | 77.5 | 0.01 |
| 3 years | 81 | 90.4 | 74.7 | |
| Patient cause of death, n (%) | ||||
| Graft failure/1* liver disease | 2 | 1 (20) | 1 (6) | |
| Infection/sepsis | 2 | 0 (0) | 2 (11) | |
| Cardiac | 4 | 0 (0) | 4 (22) | 0.34 |
| Biliary complication | 4 | 2 (40) | 2 (11) | |
| Other | 11 | 2 (40) | 9 (50) |
Mean donor age was 36 years, and 60% were male. Of donors, 82% were traditional cadaveric donors and 17% were donations after cardiac death (DCD). No living-donor liver transplants were performed during the study period. Mean cold ischemic time was 419 min (7 h), and mean warm ischemia time was 41 min. Overall 1- and 3-year graft survival was 81% and 74%, respectively. Overall 1- and 3-year patient survival was 85% and 81%, respectively.
ERCP Utilization and Effect on Survival
A total of 59 (45%) of the transplant recipients underwent ERCP post transplant, and 44 (33%) had more than one ERCP. The maximum number of ERCPs performed on a single patient during the study timeframe was ten. Overall, 5 ERCPs (8%) occurred within 1 week of transplant, 12 (20%) within 2 weeks, and 23 (39%) within 1 month after transplant. There was no difference in the number of ERCPs performed per year, and all procedures were performed by the same cohort of four highly experienced biliary endoscopists during the study period.
Patients who required an ERCP had comparable demographic, clinical, donor, and operative characteristics to those who did not (Table 1). While there were no differences in cause of death between the two groups, all of the patients who required retransplant came from the group of patients who did not receive an ERCP (12 versus 0, P = 0.001).
Survival times also differed between groups. For those who underwent any ERCP, the 1- and 3-year patient survival was 93% and 90%, respectively, compared with 78 and 75% (P < 0.001) in the no-ERCP group (unadjusted HR = 0.31, 95% CI 0.11–0.82). The Kaplan–Meier survival curve is shown in Fig. 1. This effect on patient survival persisted after adjusting for potential recipient, operative, and donor confounders (HR = 0.21, 95% CI 0.06–0.72, P = 0.013). Similarly, the 1- and 3-year graft survival in the any-ERCP group was 93% and 90%, respectively, compared with 69% and 61% (P < 0.001) in the no-ERCP group (unadjusted HR = 0.19; 95% CI 0.07–0.49, P = 0.001). This effect also persisted after multivariate analysis (HR = 0.20, 95% CI 0.06–0.68, P = 0.010). The hazard ratio was similar for patients who underwent multiple ERCPs, but did not reach statistical significance due to a smaller sample size (Table 2).
Fig. 1.
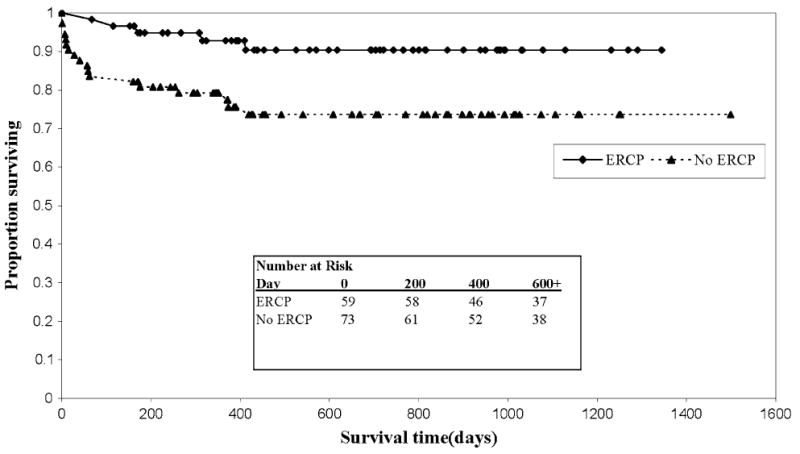
Kaplan–Meier survival estimates by ERCP
Table 2.
Crude and adjusted hazard ratios for graft failure and death
| Hazard ratio (95% CI)
|
||||
|---|---|---|---|---|
| Graft failure | P | Death | P | |
| Risk of graft failure and death, unadjusted (crude) model (n = 132) | ||||
| Any ERCP | 0.19 (0.07–0.49) | 0.001 | 0.31 (0.11–0.82) | 0.019 |
| >1 ERCP | 0.24 (0.08–0.69) | 0.008 | 0.39 (0.13–1.14) | 0.086 |
| Biliary complication | 0.19 (0.07–0.56) | 0.002 | 0.32 (0.11–0.93) | 0.037 |
| Risk of graft failure and death, full model* (n = 122) | ||||
| Any ERCP | 0.20 (0.06–0.68) | 0.010 | 0.21 (0.06–0.72) | 0.013 |
| >1 ERCP | 0.33 (0.9–1.20) | 0.092 | 0.34 (0.9– 1.26) | 0.11 |
| Biliary complication | 0.19 (0.05–0.74) | 0.017 | 0.18 (0.04–0.72) | 0.018 |
Adjusted for age at transplant, sex, race, MELD score, liver–kidney transplant, need for retransplant, indication for transplant, warm ischemia time, cold ischemia time, type of donor, donor age, and donor cause of death
Biliary Complications and Effect on Survival
A total of 49 (37%) of patients had a biliary complication identified by ERCP at some point after transplant. Extrahepatic strictures were the most common finding on first ERCP, detected in 25 (42%) of cases, followed by bile leaks in 13 (22%) and stones/debris in 5 (8%). Ten (17%) of the initial ERCPs were interpreted as normal post-transplant anatomy and did not require specific action. The most common intervention was stent placement, occurring in 39 (66%) of cases, followed by sphincterotomy in 7 (12%) cases and balloon sweep in 3 (5%) cases. There were no differences in patient or graft survival based on either ERCP finding or maneuver.
Patients who had a biliary complication treated by ERCP had generally comparable demographic, clinical, donor, and operative characteristics to those who did not (Table 3). There were no differences in cause of death between the two groups. Notable differences, however, were that more patients who received organs from male donors (72% versus 53%, P = 0.04) had biliary complications, and none of the patients who required retransplant had biliary complications (0 versus 12, P = 0.005).
Table 3.
Bivariate analysis of patients who had a biliary complication versus those who did not
| Biliary complication (n = 49) | No biliary complication (n = 83) | P | |
|---|---|---|---|
| Recipient characteristics | |||
| Mean age at transplant (years) | 53.3 ± 8.5 | 52.5 ± 9.2 | 0.63 |
| Male, n (%) | 35 (71) | 56 (67) | 0.64 |
| Caucasian, n (%) | 17 (35) | 19 (23) | 0.14 |
| Indication for transplant, n (%) | |||
| AHF | 2 (4) | 1 (1) | |
| Viral | 19 (39) | 32 (39) | 0.31 |
| ETOH | 8 (16) | 7 (8) | |
| Other | 20 (41) | 43 (52) | |
| MELD score | 26.0 ± 7.1 | 24.0 ± 7.1 | 0.12 |
| Donor characteristics | |||
| Male donor, n (%) | 33 (72) | 41 (53) | 0.04 |
| Donor age (years) | 34.8 ± 14.7 | 36.2 ± 15.3 | 0.61 |
| Donor type | |||
| Cadaveric | 39 (80) | 69 (83) | |
| DCD | 10 (20) | 13 (16) | 0.60 |
| Unknown | 0 (0) | 1 (1) | |
| Donor cause of death, n (%) | |||
| Anoxia | 4 (9) | 5 (6) | |
| CVA | 14 (30) | 34 (44) | 0.54 |
| Trauma | 26 (57) | 36 (46) | |
| Unknown | 2 (4) | 3 (4) | |
| Operative characteristics | |||
| Warm ischemia time, min | 41 ± 14 | 40 ± 9 | 0.66 |
| Cold ischemia time, min | 421 ± 122 | 418 ± 122 | 0.89 |
| Required retransplant, n (%) | 0 | 12 (14) | 0.005 |
| Survival | |||
| Graft survival, % | |||
| 1 year | 93.5 | 73.4 | 0.0007 |
| 3 years | 90.6 | 63.9 | |
| Patient survival, % | |||
| 1 year | 93.5 | 80.5 | 0.03 |
| 3 years | 90.6 | 75.5 | |
| Patient cause of death, n (%) | |||
| Graft failure/1* liver disease | 1 (25) | 1 (5) | |
| Infection/sepsis | 0 (0) | 2 (11) | |
| Cardiac | 0 (0) | 4 (21) | 0.18 |
| Biliary complication | 2 (50) | 2 (11) | |
| Other | 1 (25) | 10 (53) |
There were also survival differences based on the presence of biliary complications treated by ERCP. For those who had any biliary complication, the 1- and 3-year patient survival was 94% and 91%, respectively, compared with 81% and 76% (P = 0.03) in the no-biliary-complication group (unadjusted HR = 0.32; 95% CI 0.11–0.93, P = 0.037). This effect on patient survival persisted after adjusting for potential recipient, operative, and donor confounders (HR = 0.18; 95% CI 0.04–0.72, P = 0.018). The Kaplan–Meier survival curve is shown in Fig. 2. Similarly, the 1- and 3-year graft survival in the biliary complication group was 94% and 91%, respectively, compared with 73% and 64% (P = 0.007) in the no-biliary-complication group (unadjusted HR = 0.19; 95% CI 0.07–0.56, P = 0.002). This effect also persisted after multivariate analysis (HR = 0.19, 95% CI 0.05–0.74, P = 0.017). Excluding death and graft loss in the first 30 days after transplant did not change the trend demonstrated in the primary analysis (data not shown).
Fig. 2.
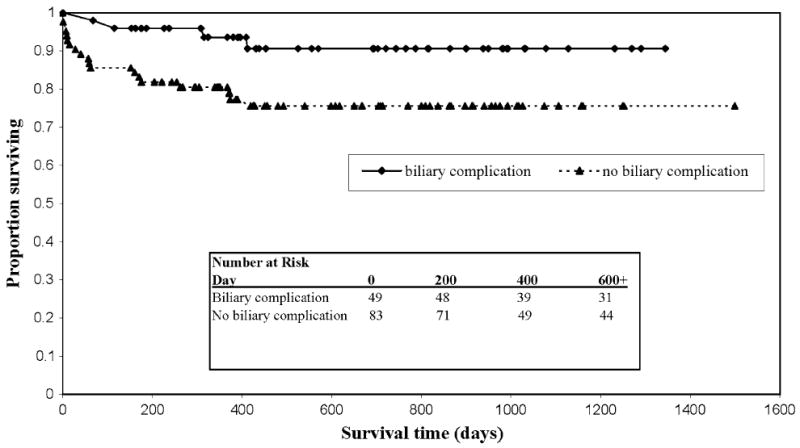
Kaplan–Meier survival estimates by biliary complication
A total of 30 patients had a percutaneous transhepatic cholangiogram (PTC) during the study period. There were no significant differences in demographic, clinical, donor, and operative characteristics between those who did and did not receive a PTC. Sixteen of these 30 (53%) patients also had an ERCP. The majority (60%) of the PTCs were performed on patients transplanted during the first half of the study period, and only one was performed on a patient transplanted in the final 6 months of the study period. There was no difference in survival between the group of patients who received PTC (HR = 1.15; CI 0.45–2.9, P = 0.77) and those who did not.
Vital Status Analysis
Bivariate analysis was performed to further investigate differences between the study patients who lived and those who died (Table 4). Overall, there were 23 (17%) deaths during the study period. Patients transplanted at older age (56 years versus 52 years, P = 0.04) were more likely to die. Patients who died tended to have longer cold ischemia times (465 versus 410 min, P = 0.052). The main cause of death was the “other” category, which included 11 (48%) deaths attributed to graft versus host disease, trauma, stroke, pulmonary complications, and unknown causes of death. Four (17%) of those who died succumbed to cardiac complications, and an equal number died of biliary complications, primarily progressive intrahepatic vanishing ducts and cholangitis. On multivariate modeling, the only independent predictors of remaining alive during the study period were having an ERCP, experiencing a biliary complication, younger age at transplant, and lower MELD score.
Table 4.
Bivariate analysis of those patients who lived versus those who died
| Alive (n = 109) | Deceased (n = 23) | P | |
|---|---|---|---|
| Recipient characteristics | |||
| Age at transplant, mean ± SD (years) | 52.1 ± 9.4 | 56.3 ± 5.6 | 0.04 |
| Male, n (%) | 77 (71) | 14 (61) | 0.36 |
| Caucasian, n (%) | 78 (72) | 18 (78) | 0.51 |
| Indication for transplant, n (%) | |||
| AHF | 2 (2) | 1 (4) | 0.82 |
| Viral | 43 (39) | 8 (35) | |
| ETOH | 13 (12) | 2 (9) | |
| Other | 51 (47) | 12 (52) | |
| MELD score | 24.3 ± 6.8 | 26.9 ± 8.3 | 0.11 |
| Donor characteristics | |||
| Male donor, n (%) | 62 (61) | 12 (57) | 0.76 |
| Donor age (years) | 35.7 ± 15.0 | 35.6 ± 15.7 | 0.98 |
| Donor type | |||
| Cadaveric | 86 (79) | 22 (96) | |
| DCD | 22 (20) | 1 (4) | 0.17 |
| Unknown | 1 (1) | 0 (0) | |
| Donor cause of death, n (%) | |||
| Anoxia | 6 (6) | 3 (14) | |
| CVA | 44 (43) | 4 (19) | 0.17 |
| Trauma | 49 (48) | 13 (62) | |
| Unknown | 4 (4) | 1 (5) | |
| Operative characteristics | |||
| Warm ischemia time, min | 41 ± 11 | 40 ± 7 | 0.70 |
| Cold ischemia time, min | 410 ± 120 | 465 ± 120 | 0.052 |
| Any ERCP, n (%) | 54 (50) | 5 (22) | 0.015 |
| Finding at first ERCP | |||
| Nothing/normal | 9 (17) | 1 (20) | |
| Intrahepatic stricture(s) | 1 (2) | 0 (0) | |
| Stone | 5 (9) | 0 (0) | 0.92 |
| Bile leak | 12 (22) | 1 (20) | |
| Extrahepatic stricture(s) | 23 (43) | 2 (40) | |
| Other | 4 (7) | 1 (20) | |
| Maneuver at first ERCP | |||
| None | 8 (14) | 2 (40) | |
| Sphincterotomy | 7 (13) | 0 (0) | |
| Balloon sweep | 3 (6) | 0 (0) | 0.45 |
| Stent placement | 36 (67) | 3 (60) | |
| Other | 0 (0) | 1 (20) | |
| Multiple ERCP, n (%) | 40 (37) | 4 (17) | 0.07 |
| Biliary complication, n (%) | 45 (41) | 4 (17) | 0.03 |
| Required retransplant, n (%) | 8 (7) | 4 (17) | 0.13 |
| Patient cause of death | |||
| Graft Failure/1* liver disease | 2 (9) | ||
| Infection/sepsis | 2 (9) | ||
| Cardiac | N/A | 4 (17) | N/A |
| Biliary complication | 4 (17) | ||
| Other | 11 (48) |
Discussion
Because biliary complications are common after liver transplantation and often require treatment with repeated invasive endoscopic procedures, this study aimed to determine the effect of ERCP utilization and the presence of biliary complications on patient and graft survival post liver transplantation. To our surprise, and in contrast to the original hypothesis, we found that patients who required ERCP after transplantation had better survival than those who did not, and that this also held for patients found to have biliary complications after transplant.
What might explain this somewhat counterintuitive result? In the literature, multiple publications and abstracts allude to the significant morbidity and mortality attributable to biliary complications after liver transplant. Biliary complications are reported to occur in 6–51% of liver transplants [1-3], and we found that at our center, 45% of patients underwent at least one ERCP, and 36% had a biliary complication defined as a bile leak, stricture, stone, or “other” finding. Thus, biliary morbidity was as common in our center as cited in the literature.
While the morbidity is well established, it appears that most data attributing mortality to biliary complications predate the era of routine endoscopic management. Specifically, Kuo et al. cite a biliary complication rate of 25% with an associated 1-year mortality of 43.5% [11], and Stratta et al. report 76% actuarial 1-year survival among patients with biliary complications [5]. Because of these data, Ororio, et al. were among the first to advocate nonoperative management of biliary complications through endoscopic and interventional radiographic approaches [12]. Since then, other authors have advocated frequent and aggressive use of endoscopic management of biliary complication [15-17], yet there have been few reports delineating the mortality related to biliary complications. Those reports that do include mortality data, such as those of Rizk et al. and Pfau et al., cite no difference between biliary sources of mortality and other causes [18, 19].
Could our finding that post-transplant ERCP was independently associated with better patient and graft survival represent an epiphenomenon? This is unlikely with such strongly protective hazard ratios in the 0.2–0.3 range. We believe that selection bias is similarly unlikely. While it is theoretically possible that those who received ERCP may not have been as sick as those who did not, this is not borne out in practice. At our center, ERCP are performed in the operating room under general anesthesia, or at the bedside in the Surgical Intensive Care Unit when indicated. Indeed, the first ERCP in this series was performed just 5 days post transplant, and no patients were denied a diagnostic or therapeutic procedure due to comorbidities.
Even when excluding death and graft loss in the first 30 days after transplant, the point estimate for the hazard ratio associated with biliary complications remains similar. This adjustment is overcompensation for selection bias, however. While it excludes early deaths and patients who theoretically may have been too sick to undergo a procedure, it also excludes 39% of our population who did undergo a procedure and ignores those who received benefit from aggressive early endoscopic management of operative complications.
We also perform ERCP on patients with prior hepaticojejunostomy or Roux en-Y anastomoses via traditional or single-balloon methods [20]. Given the richness of our data sources, misclassification bias is also unlikely. Furthermore, that the observed relationships hold after adjusting for multiple confounding factors lends strength to the finding.
The interpretation of these results may in fact be relatively simple. In the current era of aggressive management of biliary complications via ERCP, biliary complications are common, but not life-threatening. Moreover, undergoing therapeutic ERCP to treat biliary complications, even if this is required on multiple occasions, may attenuate the adverse effects that such complications have on patient and graft survival. Conversely, complications that are not amenable to ERCP, such as hepatic artery thrombosis, chronic rejection, or infections in the setting of profound immunosuppression, confer a risk that is not easily mitigated by an endoscopic intervention.
In conclusion, this retrospective cohort study of post-liver-transplant patients shows that patients who received an ERCP for endoscopic management of a biliary complication had lower rates of graft failure and death than those who did not have an ERCP. The implications of these results are that, in the era of endoscopic management, biliary complications are a substantial source of morbidity post transplant, but they do not confer excess mortality, likely because these complications can be effectively treated by ERCP and do not portend the same mortality as other complications associated with liver transplant.
Acknowledgments
This research was supported, in part, by a grant from the National Institutes of Health T32 DK07634.
Abbreviations
- ERCP
Endoscopic retrograde cholangiopancreatography
- OLT
Orthotopic liver transplant
- HR
Hazard ratio
- UNOS
United Network of Organ Sharing
- MELD
Model for end-stage liver disease
- AHF
Acute hepatic failure
Footnotes
Conflicts of interest None
References
- 1.Verdonk RC, Buis CI, Porte RJ, Haagsma EB. Biliary complications after liver transplantation: A review. Scand J Gastroenterol Suppl. 2006:89–101. doi: 10.1080/00365520600664375. [DOI] [PubMed] [Google Scholar]
- 2.Tung BY, Kimmey MB. Biliary complications of orthotopic liver transplantation. Dig Dis. 1999;17:133–144. doi: 10.1159/000016918. [DOI] [PubMed] [Google Scholar]
- 3.Thuluvath PJ, Atassi T, Lee J. An endoscopic approach to biliary complications following orthotopic liver transplantation. Liver Int. 2003;23:156–162. doi: 10.1034/j.1600-0676.2003.00823.x. [DOI] [PubMed] [Google Scholar]
- 4.Klein AS, Savader S, Burdick JF, et al. Reduction of morbidity and mortality from biliary complications after liver transplantation. Hepatology. 1991;14:818–823. doi: 10.1002/hep.1840140513. [DOI] [PubMed] [Google Scholar]
- 5.Stratta RJ, Wood RP, Langnas AN, et al. Diagnosis and treatment of biliary tract complications after orthotopic liver transplantation. Surgery. 1989;106:675–683. discussion 683-674. [PubMed] [Google Scholar]
- 6.Lopez RR, Benner KG, Ivancev K, Keeffe EB, Deveney CW, Pinson CW. Management of biliary complications after liver transplantation. Am J Surg. 1992;163:519–524. doi: 10.1016/0002-9610(92)90401-c. [DOI] [PubMed] [Google Scholar]
- 7.Greif F, Bronsther OL, Van Thiel DH, et al. The incidence, timing, and management of biliary tract complications after orthotopic liver transplantation. Ann Surg. 1994;219:40–45. doi: 10.1097/00000658-199401000-00007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lerut J, Gordon RD, Iwatsuki S, et al. Biliary tract complications in human orthotopic liver transplantation. Transplantation. 1987;43:47–51. doi: 10.1097/00007890-198701000-00011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lemmer ER, Spearman CW, Krige JE, et al. The management of biliary complications following orthotopic liver transplantation. S Afr J Surg. 1997;35:77–81. [PubMed] [Google Scholar]
- 10.Davidson BR, Rai R, Nandy A, Doctor N, Burroughs A, Rolles K. Results of choledochojejunostomy in the treatment of biliary complications after liver transplantation in the era of nonsurgical therapies. Liver Transpl. 2000;6:201–206. doi: 10.1002/lt.500060215. [DOI] [PubMed] [Google Scholar]
- 11.Kuo PC, Lewis WD, Stokes K, Pleskow D, Simpson MA, Jenkins RL. A comparison of operation, endoscopic retrograde cholangiopancreatography, and percutaneous transhepatic cholangiography in biliary complications after hepatic transplantation. J Am Coll Surg. 1994;179:177–181. [PubMed] [Google Scholar]
- 12.Osorio RW, Freise CE, Stock PG, et al. Nonoperative management of biliary leaks after orthotopic liver transplantation. Transplantation. 1993;55:1074–1077. doi: 10.1097/00007890-199305000-00026. [DOI] [PubMed] [Google Scholar]
- 13.Johnston TD, Reddy KS, Khan TT, Ranjan D. Ercp in the management of early versus late biliary leaks after liver transplantation. Int Surg. 2006;91:301–305. [PubMed] [Google Scholar]
- 14.Fogel EL, Sherman S, Park SH, McHenry L, Lehman GA. Therapeutic biliary endoscopy. Endoscopy. 2003;35:156–163. doi: 10.1055/s-2003-37011. [DOI] [PubMed] [Google Scholar]
- 15.Sanna C, Saracco GM, Reggio D, et al. Endoscopic retrograde cholangiopancreatography in patients with biliary complications after orthotopic liver transplantation: Outcomes and complications. Transplant Proc. 2009;41:1319–1321. doi: 10.1016/j.transproceed.2009.03.086. [DOI] [PubMed] [Google Scholar]
- 16.Tabibian JH, Asham EH, Goldstein L, et al. Endoscopic treatment with multiple stents for post-liver-transplantation nonanastomotic biliary strictures. Gastrointest Endosc. 2009;69:1236–1243. doi: 10.1016/j.gie.2008.09.057. [DOI] [PubMed] [Google Scholar]
- 17.Pasha SF, Harrison ME, Das A, et al. Endoscopic treatment of anastomotic biliary strictures after deceased donor liver transplantation: Outcomes after maximal stent therapy. Gastrointest Endosc. 2007;66:44–51. doi: 10.1016/j.gie.2007.02.017. [DOI] [PubMed] [Google Scholar]
- 18.Rizk RS, McVicar JP, Emond MJ, et al. Endoscopic management of biliary strictures in liver transplant recipients: Effect on patient and graft survival. Gastrointest Endosc. 1998;47:128–135. doi: 10.1016/s0016-5107(98)70344-x. [DOI] [PubMed] [Google Scholar]
- 19.Pfau PR, Kochman ML, Lewis JD, et al. Endoscopic management of postoperative biliary complications in orthotopic liver transplantation. Gastrointest Endosc. 2000;52:55–63. doi: 10.1067/mge.2000.106687. [DOI] [PubMed] [Google Scholar]
- 20.Dellon ES, Kohn GP, Morgan DR, Grimm IS. Endoscopic retrograde cholangiopancreatography with single-balloon enteroscopy is feasible in patients with a prior roux-en-y anastomosis. Dig Dis Sci. 2008 doi: 10.1007/s10620-008-0538-x. [DOI] [PubMed] [Google Scholar]


