Abstract
Brentuximab vedotin is an antibody-drug conjugate (ADC) that selectively delivers monomethyl auristatin E (MMAE) into CD30-expressing cells. This study evaluated the CYP3A-mediated drug-drug interaction potential of brentuximab vedotin and the excretion of MMAE. Two 21-day cycles of brentuximab vedotin (1.2 or 1.8 mg/kg intravenously) were administered to 56 patients with CD30-positive hematologic malignancies. Each patient also received either a sensitive CYP3A substrate (midazolam), an effective inducer (rifampin), or a strong inhibitor (ketoconazole). Brentuximab vedotin did not affect midazolam exposures. ADC exposures were unaffected by concomitant rifampin or ketoconazole; however, MMAE exposures were lower with rifampin and higher with ketoconazole. The short-term safety profile of brentuximab vedotin in this study was generally consistent with historic clinical observations. The most common adverse events were nausea, fatigue, diarrhea, headache, pyrexia, and neutropenia. Over a 1-week period, ~23.5% of intact MMAE was recovered after administration of brentuximab vedotin; all other species were below the limit of quantitation. The primary excretion route is via feces (median 72% of the recovered MMAE). These results suggest that brentuximab vedotin (1.8 mg/kg) and MMAE are neither inhibitors nor inducers of CYP3A; however, MMAE is a substrate of CYP3A.
Keywords: Clinical Pharmacology, Clinical Trials, Biotechnology, Oncology, Pharmacokinetics and Drug Metabolism
INTRODUCTION
Antibody-drug conjugates (ADCs), consisting of an antibody, a cytotoxic agent, and a stable linker, are an important modality to increase the therapeutic index of potent cytotoxic agents. Conjugation to the antibody results in a longer apparent half-life and altered biodistribution of the cytotoxic agent.1,2 Stable in circulation, the ADC releases the cytotoxic agent after internalization into antigen-expressing cells, resulting in targeted delivery to tumors while sparing normal tissues exposure to high systemic concentrations of the cytotoxic agent.2,3
Brentuximab vedotin (ADCETRIS®) is an ADC directed to human CD30, a cell-surface protein commonly found on Hodgkin lymphoma (HL) and non-Hodgkin lymphomas such as anaplastic large cell lymphoma (ALCL), but generally absent on normal cells except for activated T and B cells.4,5 The microtubule-disrupting cytotoxic agent monomethyl auristatin E (MMAE) is conjugated to the anti-CD30 antibody cAC106,7 through a protease-cleavable linker.8 After internalization into CD30-positive cells, lysosomal proteases release MMAE, which induces cell cycle arrest and apotosis.9,10 In a murine xenograft model, brentuximab vedotin achieved intratumor MMAE concentrations 1 to 2 orders of magnitude higher than an equimolar dose of unconjugated MMAE,1 demonstrating the value of targeted delivery.
In pivotal clinical trials, objective response rates of 75% and 86% were observed for patients with relapsed/refractory HL and relapsed/refractory systemic ALCL, respectively,11,12 in conjunction with an acceptable safety profile when 1.8 mg/kg brentuximab vedotin was administered intravenously every 3 weeks for up to 16 cycles. Brentuximab vedotin pharmacokinetics (PK) were characterized by monitoring total antibody (TAb, the sum of ADC and cAC10), ADC, and released MMAE.13–15 The PK of all 3 analytes are approximately dose proportional in the therapeutic dose range. Peak concentrations typically occurred at the end of infusion for ADC and approximately 2 to 3 days postdose for MMAE. TAb had the greatest exposure and a PK profile similar to that of the ADC. MMAE exposures decreased after the first dose, and were ~2000-fold lower than those of the ADC on a mass basis.15 Much higher exposures of other ADCs relative to their associated small molecule components have also been reported,16–19 with the relative exposure ratio likely driven by clearance rates and drug-linker stability of the individual ADCs.
While brentuximab vedotin is an antibody-based therapeutic and drug-drug interactions (DDIs) involving antibodies are typically limited,20,21 the cytotoxic agent portion of an ADC can be subject to metabolism-based DDIs. Nonclinical data suggested that MMAE is a substrate of CYP3A and P-glycoprotein (P-gp), and that MMAE is a potential inhibitor of CYP3A but not other CYP isoforms.22 As CYP3A is involved in the metabolism of most small molecule drugs, understanding the potential for CYP3A-based DDIs is important to provide guidance for the administration of brentuximab vedotin to patients with concomitant medications. This report summarizes the results of a clinical study designed to evaluate the CYP3A-mediated DDI potential of brentuximab vedotin using a prototypical substrate and modulators of CYP3A. The primary route of excretion of MMAE in patients with CD30-positive hematologic malignancies was also determined.
METHODS
Patients
Patients were required to have measurable, histologically-confirmed, CD30-positive hematologic malignancies. Patients with relapsed, refractory, or progressive disease following at least 1 prior systemic chemotherapy regimen, as well as treatment-naïve patients unable to tolerate intensive or potentially curative regimens, could be eligible. Patients were excluded from study participation if they had allogeneic stem cell transplant within 100 days or autologous stem cell transplant within 4 weeks prior to first dose of study drug; concurrent therapy with corticosteroids ≥20 mg/day prednisone equivalent; or active systemic viral, bacterial, or fungal infection requiring antimicrobial therapy within 2 weeks prior to first dose of study drug. Patients were not eligible for study participation if they had current primary cutaneous ALCL, New York Heart Association Class III or IV congestive heart failure, or active acute or chronic graft-versus-host disease; or if they required hemodialysis or chronic ambulatory peritoneal dialysis. Patients were also excluded if they had absolute neutrophil count <1000/μL, platelets <50,000/μL, serum bilirubin or creatinine >1.5 times the upper limit of normal, alanine aminotransferase (ALT) or aspartate aminotransferase (AST) >2.5 times the upper limit of normal, or Eastern Cooperative Oncology Group (ECOG) performance status >1. Effective inducers and strong inhibitors of CYP3A were prohibited beginning 4 weeks before the first dose of study drug and continuing throughout the study. Written informed consent was obtained from all patients prior to any study-specific procedures, in accordance with the Declaration of Helsinki.
Study Design
This phase 1 study (ClinicalTrials.gov NCT01026415) was conducted at 7 clinical sites in the United States. The protocol was reviewed and approved by the local Institutional Review Boards at City of Hope National Medical Center (Duarte, CA), Healthcare Corporation of America (HCA)-HealthONE (Glendale, CO), New York University Medical Center (New York, NY), St. Francis Hospital and Health Center (Beech Grove, IN), and Wayne State University (Detroit, MI), and by the Western Institutional Review Board (Olympia, WA).
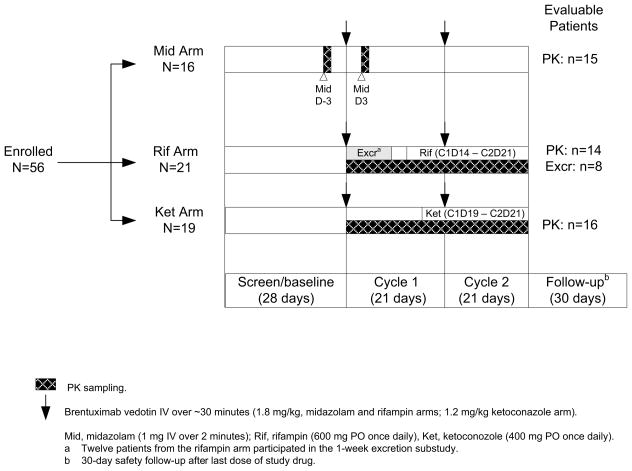
The study used an open label, parallel arm, one-sequence crossover design (Figure 1). The primary objectives were to assess the effect of brentuximab vedotin on the PK of a sensitive CYP3A substrate (midazolam); to assess the effect of a CYP3A inducer (rifampin) on the PK of brentuximab vedotin (ADC and MMAE); to assess the effect of a CYP3A inhibitor (ketoconazole) on the PK of brentuximab vedotin (ADC and MMAE); and to determine the primary route of excretion of MMAE. Secondary objectives were to assess safety and tolerability, to assess the incidence of antitherapeutic antibodies (ATA), and to identify the excreted metabolite(s) of MMAE. Efficacy was not evaluated in this 2-cycle study.
Figure 1.
Study design and patient evaluability.
Each treatment arm was to enroll approximately 12 evaluable patients, and treatment assignment was planned to be carried out sequentially beginning with the midazolam arm, then the rifampin arm, then the ketoconazole arm. In most cases, treatment arm assignment was as planned. However, due to rapid enrollment, the 3 treatment arms were not enrolled in discrete stages. Determination of patient evaluability was ongoing during the study, and when a previous treatment arm was found to need another patient because of non-evaluability, the next available patient was assigned to that treatment arm as a replacement patient.
The excretion analysis was conducted in a subset of patients from the rifampin arm, with a target of 6 excretion-evaluable patients. After initial treatment with brentuximab vedotin (1.8 mg/kg) and prior to administration of rifampin, all urine and fecal samples were collected for 1 week (24-hour pools of each) in an in-patient setting, and the concentrations of MMAE and related metabolites in the samples were determined.
Study Treatment
Brentuximab vedotin was administered on Day 1 of two 21-day cycles as an IV infusion over ~30 minutes (1.8 mg/kg in the midazolam and rifampin arms; 1.2 mg/kg in the ketoconazole arm). Patients received a maximum of 2 cycles of brentuximab vedotin on this study; however, those with clinical benefit and free from unacceptable toxicity could receive extended treatment in a separate trial.
Based on their assigned treatment arm, patients also received midazolam, rifampin, or ketoconazole. Concomitant midazolam (1 mg) was administered intravenously over a period of at least 2 minutes on Day -3 and Day 3 of Cycle 1. Concomitant rifampin (600 mg) was taken orally once daily from Cycle 1, Day 14 through Cycle 2, Day 21. Concomitant ketoconazole (400 mg) was taken orally once daily from Cycle 1, Day 19 through Cycle 2, Day 21.
Patient compliance with administration of brentuximab vedotin and midazolam was well controlled, as both were administered intravenously at the study site. Compliance with rifampin and ketoconazole was assessed at each scheduled clinic visit by review of patient diaries and reconciliation with unused tablets or empty bottles. Additionally, home healthcare nursing services were provided to help ensure compliance with the PK blood collection schedule, assess compliance with oral medication administration, and reinforce the importance of compliance with the protocol.
Safety Assessments
Safety assessments included evaluation of adverse events, routine hematology and serum chemistry tests (conducted at local laboratories), and assessment of ATA (conducted at a central laboratory). Adverse events were summarized using the Medical Dictionary for Regulatory Activities (MedDRA), version 13.0. Peripheral neuropathy events were defined by a standardized MedDRA query (SMQ). Adverse events and laboratory results were graded using the National Cancer Institute’s Common Terminology Criteria for Adverse Events, version 3.0.
Sample Collection and Handling
In the midazolam arm, blood samples for midazolam analysis were collected prior to each midazolam infusion, within 1 minute after the end of infusion, at 15, 30, and 45 minutes; and at 1, 1.5, 2, 3, 4, 6, 8, 12, 16, and 24 hours postdose. Blood samples for brentuximab vedotin analysis were collected prior to each dose of midazolam as well as prior to and at the end of infusion for the first dose of brentuximab vedotin.
In the rifampin and ketoconazole arms, blood samples for brentuximab vedotin analysis were collected for both treatment cycles. MMAE samples were collected predose; at the end of infusion; at 2, 4, 8, 12, 24, and 36 hours; and at 2, 3, 4, 7, 10, 14, 17, and 21 days post-infusion. Blood samples for ADC and TAb assays were collected predose; at the end of infusion; at 2, 4, and 36 hours; and at 3, 7, 14, 17, and 21 days post-infusion. Blood samples for trough rifampin and ketoconazole analysis were collected prior to each dose of brentuximab vedotin, 14 days (rifampin) or 19 days (ketoconazole) post-infusion in cycle 1, and 7, 14, and 21 days post-infusion in cycle 2.
In all 3 treatment arms, blood samples for ATA analysis were collected on Day 1 of each treatment cycle prior to brentuximab vedotin administration, and at the end-of-treatment visit. ADC, TAb, and ATA were measured in serum and MMAE was measured in plasma, using samples processed within 1 hour of collection.
In addition to blood samples, urine and fecal samples were collected from patients who participated in the excretion portion of the study. All urine and fecal samples were collected into separate containers and refrigerated within 30 minutes. At the end of each 24-hour period, urine samples were pooled and mixed thoroughly, the total volume was recorded, and aliquots were frozen. All fecal samples for each 24-hour period also were frozen; prior to analysis, combined 24-hour fecal samples were weighed and homogenized in water. Both urine and fecal samples were shipped frozen to a central laboratory for evaluation.
Bioanalytical Methods
All analytes were measured using validated assays at Covance, Inc. (Chantilly, VA and Madison, WI). ADC concentrations in serum were measured with an enzyme-linked immunosorbent assay (ELISA) in a sandwich format, using a monoclonal anti-MMAE antibody for capture and a monoclonal anti-idiotypic-cAC10 (anti-ID30, specific for the antigen-combining site of brentuximab vedotin) for detection. TAb concentrations in serum were measured with an ELISA immunoassay in a bridging format, using anti-ID30 for both capture and detection. The limits of quantitation for both assays were 12.5 to 400 ng/mL. Concentrations of unconjugated MMAE were measured in plasma, urine, and fecal homogenates by liquid chromatography and tandem mass spectrometry (LC-MS/MS). Free MMAE and the internal standard D8-MMAE were extracted from the biological matrix by solid-phase extraction (plasma) or liquid-liquid extraction (urine and fecal homogenates). Extracts were separated on a 50 × 3 mm Betasil silica-100 or Aquasil C18 (Thermo Scientific, Waltham, MA) column using gradients containing 0.1% formic acid in water and acetonitrile. Detection was on an API5000 (Sciex, Framingham, MA) with multiple reaction monitoring transitions of 718 to 686 (MMAE) and 726 to 694 (D8-MMAE). The limits of quantitation were 0.025 to 1 ng/mL for plasma, 0.1 to 50 ng/mL for urine, and 5 to 2500 ng/g for feces. Metabolites of MMAE were identified by LC-MS/MS of neat and 10-fold concentrated urine and feces liquid-liquid extracts, with structures confirmed by parent ion fragmentation and high resolution mass analysis. ATA in serum was detected with an electrochemiluminescence assay that had a sensitivity of 4 ng/mL anti-brentuximab vedotin monoclonal antibody and drug tolerance of 3125 ng/mL brentuximab vedotin. ATA titer and specificity were evaluated for samples that were positive in the screening assay, and ATA was subsequently confirmed by pre-exposure to saturating concentrations of brentuximab vedotin before assay. Midazolam in plasma was analyzed by LC-MS/MS; the lower limit of quantitation was 0.1 ng/mL.
Pharmacokinetic and Statistical Analyses
PK parameters (AUC, Cmax, Tmax, and t1/2) were estimated by noncompartmental analysis. WinNonlin 5.2 Enterprise Edition (Pharsight, Mountain View, CA) was used for all pharmacokinetic calculations. Drug interactions were evaluated by summarizing geometric mean ratios (GMRs) of the AUC and Cmax with 90% confidence intervals (CIs). The GMR and 90% CIs were derived from log-transformed PK parameters and back-transformed for presentation.
An exploratory analysis was performed to estimate the period effect on MMAE PK. An analysis of covariance (ANCOVA) model was developed that combined data from this study and the initial brentuximab vedotin clinical trial.13 Covariates including sex, weight, and baseline albumin were considered in the model; baseline albumin was included in the model. Key assumptions for the model were that the GMR of Cycle 2 to Cycle 1 is independent of brentuximab vedotin dose level, with or without rifampin/ketoconazole; the error variance-covariance is the same across studies and dose levels; and the errors are normally distributed.
Descriptive statistics were used to summarize continuous variables. Geometric mean and the coefficient of variation for the geometric mean (CV) were provided for selected PK parameters. Frequencies and percentages were used to summarize categorical variables. Summary statistics and ANCOVA results were calculated using SAS® version 9.2 (SAS Institute Inc, Cary, NC).
Evaluability Criteria
The PK analyses included all PK-evaluable patients and the excretion analyses included all excretion-evaluable patients. For determination of evaluability, doses of brentuximab vedotin and midazolam within 10% of the intended dose level were considered acceptable, prohibited medications were manually identified, and missing PK samples and missing rifampin/ketoconazole doses were manually reviewed to assess the potential impact on PK results. Patients were replaced if they were not evaluable.
Evaluable patients in the midazolam arm received the first dose of brentuximab vedotin and both doses of midazolam at the intended dose levels, had no protocol-prohibited medications during the evaluation period (study entry through Cycle 1, Day 4), and had adequate samples available for estimation of PK parameters. If a patient met all the evaluability criteria and if the AUC0-∞ ratio and/or Cmax ratio for midazolam was estimable, the patient was included in the analysis.
Similarly, evaluable patients in the rifampin and ketoconazole arms received both doses of brentuximab vedotin at the intended dose level, received sufficient doses of rifampin/ketoconazole for PK evaluation, had no protocol-prohibited medications during the evaluation period (study entry through Cycle 2, Day 22), and had adequate PK samples available for estimation of PK parameters. In the rifampin and ketoconazole arms, if a patient met all the evaluability criteria and if the AUC0-∞ ratio and/or Cmax ratio for MMAE and/or ADC was estimable, the patient was included in the analyses.
Evaluable patients for the excretion portion of the study received the first dose of brentuximab vedotin at the intended dose level, did not receive any protocol-prohibited medications during the evaluation period (study entry through Cycle 1, Day 8), and provided sufficient matched urine and fecal samples for evaluation. In order for a patient to be included in the excretion analysis, matched urine and fecal samples were required for all collection days.
RESULTS
Patients
Data were collected from December 2009 to June 2010. Fifty-six patients enrolled, received at least 1 dose of study drug, and were evaluated for safety. For the study population overall (N = 56), the median age of patients was 33.5 years (range, 16 to 71 years), 59% were male, and 86% were White. Patients entering the study were generally ambulatory and able to perform normal activities without assistance, as indicated by an ECOG performance status of 0 (57%) or 1 (43%). Fifty-two patients (93%) had relapsed/refractory HL, 3 patients (5%) had relapsed/refractory systemic ALCL, and 1 patient (2%) had a relapsed/refractory peripheral T-cell lymphoma not otherwise specified. Median time from initial diagnosis to initial dose of brentuximab vedotin was 38 months (range, 9 to 162). Patients had received a median of 3 prior systemic chemotherapy regimens (range, 1 to 13), and 43 patients (77%) had received prior autologous and/or allogeneic hematopoietic stem cell transplant.
Forty-five patients were evaluable for PK: 15 in the midazolam arm, 14 in the rifampin arm, and 16 in the ketoconazole arm and 8 patients in the rifampin arm were evaluable for the excretion analysis (Figure 1). Eleven patients were not evaluable for PK due to inadequate PK samples (n=4); insufficient doses of rifampin or ketoconazole (n=4); use of prohibited concomitant medications (n=2); and early discontinuation due to an adverse event, resulting in no criteria being met (n=1). Four of 12 patients who participated in the excretion portion of the study were not evaluable due to inadequate urine and fecal samples. In general, demographics and baseline characteristics for the evaluable patients (Table 1) were similar to those of all enrolled patients in the corresponding treatment arm.
Table 1.
Demographic and baseline characteristics (evaluable patients)
| PK Evaluable
|
Excretion Evaluablea (n = 8) | |||
|---|---|---|---|---|
| Mid (n = 15) | Rif (n = 14) | Ket (n = 16) | ||
| Age, median (range), years | 39.0 (16,b 68) | 37.0 (24, 71) | 29.0 (23, 46) | |
| Gender, n (%) | ||||
| Male | 10 (67) | 10 (71) | 9 (56) | 5 (63) |
| Female | 5 (33) | 4 (29) | 7 (44) | 3 (38) |
| Race, n (%) | ||||
| White | 12 (80) | 13 (93) | 13 (81) | 7 (88) |
| Black or African American | 2 (13) | 0 | 2 (13) | 0 |
| Asian | 1 (7) | 1 (7) | 0 | 1 (13) |
| Other | 0 | 0 | 1 (6) | 0 |
| ECOG Status, n (%) | ||||
| 0 | 10 (67) | 7 (50) | 6 (38) | 3 (38) |
| 1 | 5 (33) | 7 (50) | 10 (63) | 5 (63) |
| Renal function | ||||
| Creatinine clearance, median (range), mL/min | 142 (85, 281) | 127 (60, 185) | 124 (62, 211) | 134 (101, 185) |
| Hepatic function | ||||
| ALT, median (range), U/L | 21 (7, 70) | 22 (12, 47) | 21 (7, 87) | 21 (12, 47) |
| AST, median (range), U/L | 19 (9, 55) | 23 (15, 54) | 25 (10, 73) | 22 (15, 54) |
| Disease diagnosis, n (%) | ||||
| Hodgkin lymphoma | 14 (93) | 12 (86) | 15 (94) | 7 (88) |
| Anaplastic large cell lymphomac | 0 | 2 (14) | 1 (6) | 1 (13) |
| Peripheral T-cell lymphoma NOS | 1 (7) | 0 | 0 | 0 |
| Duration of disease, median (range), monthsd | 33.0 (10, 162) | 35.5 (9, 145) | 50.0 (10, 159) | 39.0 (13, 145) |
| Prior Therapies | ||||
| Systemic chemotherapy, n (%) | 15 (100) | 14 (100) | 16 (100) | 8 (100) |
| Median (min, max) number of regimense | 3.0 (2, 8) | 3.0 (1, 5) | 5.0 (2, 13) | 3.5 (1, 5) |
| Radiotherapy, n (%) | 9 (60) | 8 (57) | 12 (75) | 4 (50) |
| Prior hematopoietic stem cell transplant, n (%) | 12 (80) | 8 (57) | 13 (81) | 6 (75) |
| Autologous only, n (%)f | 8 (67) | 6 (75) | 9 (69) | 4 (67) |
| Allogeneic only, n (%)f | 0 | 1 (13) | 2 (15) | 1 (17) |
| Both autologous and allogeneic, n (%)f | 4 (33) | 1 (13) | 2 (15) | 1 (17) |
Subset of patients enrolled in the rifampin arm.
Exceptions were granted for enrollment of patients ≤ 18 years of age.
No ALCL patient was ALK positive.
Time from initial diagnosis to first dose of brentuximab vedotin.
Excludes stem cell mobilization therapy.
Number of patients (percent of patients with any prior stem cell transplant).
Of the 56 patients treated with brentuximab vedotin, 54 (96%) received both doses of brentuximab vedotin and completed the study per protocol. Two patients discontinued treatment early, 1 due to disease progression and 1 due to Grade 5 (fatal) adverse events. Fifty patients (89%) went on to receive extended treatment in a separate trial. In the midazolam arm, all 16 patients received both midazolam doses as planned. Three of 21 patients in the rifampin arm and 2 of 19 patients in the ketoconazole arm were not evaluable for PK because they did not receive sufficient doses of rifampin or ketoconazole.
Effect of brentuximab vedotin (MMAE) on midazolam PK
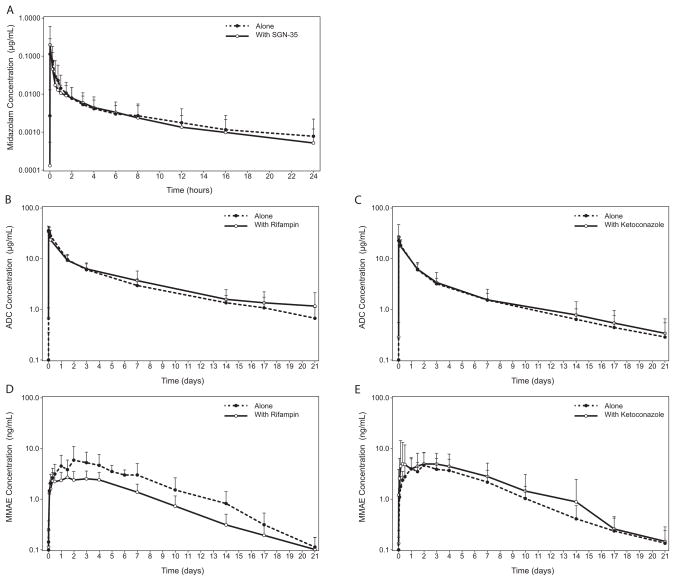
The effect of brentuximab vedotin on the PK of midazolam was evaluated by comparing the midazolam PK parameters between midazolam administered alone vs. 2 days following brentuximab vedotin infusion (the expected Tmax for MMAE) (Table 2 and Figure 2). The midazolam AUC0-∞ GMR (midazolam + brentuximab vedotin/midazolam alone) and the corresponding 90% CI were 0.94 and 0.81–1.10 and the Cmax GMR and corresponding 90% CI were 1.15 and 0.76–1.74. The midazolam Tmax was similar with and without brentuximab vedotin, as was the t1/2.
Table 2.
Effects of brentuximab vedotin on midazolam PK parameters and effects of rifampin and ketoconazole on brentuximab vedotin PK parameters
| Evaluation | Geometric Mean (% CV)a
|
|||
|---|---|---|---|---|
| Analyte | ||||
| PK Parameter | n | Alone | Combination | GMR (90% CI) |
| Midazolam With or Without Brentuximab Vedotin | ||||
| Midazolam | ||||
| AUC0-∞ (hr·μg/mL) | 15 | 0.079 (92) | 0.074 (74) | 0.94 (0.81–1.10)b |
| Cmax (μg/mL) | 14 | 0.073 (116) | 0.084 (176) | 1.15 (0.76–1.74)b |
| Tmax (hr) | 14 | 0.050 (0.00, 0.27)a | 0.042 (0.03, 0.28)a | — |
| t1/2 (hr) | 15 | 6.40 (48) | 5.69 (38) | — |
|
| ||||
| Brentuximab Vedotin With or Without Rifampin | ||||
| ADC | ||||
| AUC0-∞ (d·μg/mL) | 11 | 89.84 (25) | 93.40 (42) | 1.04 (0.87–1.24)c |
| Cmax (μg/mL) | 11 | 36.74 (34) | 34.05 (32) | 0.93 (0.81–1.06)c |
| Tmax (d) | 11 | 0.024 (0.02, 0.09)a | 0.024 (0.02, 0.08)a | — |
| t1/2 (d) | 11 | 5.87 (66) | 6.73 (58) | — |
| MMAE | ||||
| AUC0-∞ (d·ng/mL) | 14 | 40.06 (53) | 21.54 (38) | 0.54 (0.43–0.68)c |
| Cmax (ng/mL) | 14 | 4.98 (67) | 2.80 (36) | 0.56 (0.42–0.76)c |
| Tmax (d) | 14 | 3.00 (0.99, 5.01)a | 1.49 (0.33, 4.02)a | — |
| t1/2 (d) | 14 | 3.71 (19) | 3.66 (17) | — |
|
| ||||
| Brentuximab Vedotin With or Without Ketoconazole | ||||
| ADC | ||||
| AUC0-∞ (d·μg/mL) | 11 | 52.77 (28) | 56.26 (40) | 1.07 (0.95–1.19)d |
| Cmax (μg/mL) | 16 | 22.57 (23) | 22.38 (70) | 0.99 (0.75–1.31)d |
| Tmax (d) | 16 | 0.02 (0.02, 0.17)a | 0.02 (0.02, 0.25)a | — |
| t1/2 (d) | 11 | 5.70 (33) | 5.45 (31) | — |
| MMAE | ||||
| AUC0-∞ (d·ng/mL) | 14 | 26.65 (71) | 35.72 (126) | 1.34 (0.98–1.84)d |
| Cmax (ng/mL) | 16 | 4.11 (71) | 5.13 (114) | 1.25 (0.90–1.72)d |
| Tmax (d) | 16 | 1.97 (0.97, 6.99)a | 2.45 (0.22, 4.06)a | — |
| t1/2 (d) | 14 | 3.06 (13) | 3.47 (66) | — |
GMR, geometric mean ratio; CI, confidence interval; CV, coefficient of variation.
Geometric mean (%CV) presented for AUC0-∞, Cmax, and t1/2. Median (range) presented for Tmax.
Geometric mean ratio of midazolam + brentuximab vedotin/midazolam alone.
Geometric mean ratio of brentuximab vedotin with rifampin/brentuximab vedotin alone.
Geometric mean ratio of brentuximab vedotin with ketoconazole/brentuximab vedotin alone.
Figure 2.
Effects of brentuximab vedotin on midazolam (A), rifampin on ADC (B), ketoconazole on ADC (C), rifampin on MMAE (D), and ketoconazole on MMAE (E) concentration-time profiles. Data are presented as mean (SD).
Effect of rifampin on brentuximab vedotin and MMAE PK
The effect of rifampin on the PK of brentuximab vedotin was evaluated by comparing ADC and MMAE exposures between brentuximab vedotin alone vs. coadministered with a daily dose of rifampin (Table 2 and Figure 2).
For ADC, the AUC0-∞ GMR (brentuximab vedotin with rifampin/brentuximab vedotin alone) and the corresponding 90% CI were 1.04 and 0.87–1.24 and the Cmax GMR and corresponding 90% CI were 0.93 and 0.81–1.06. The ADC Tmax was similar with and without rifampin, as was the t1/2.
For MMAE, the AUC0-∞ GMR and the corresponding 90% CI were 0.54 and 0.43–0.68 and the Cmax GMR and corresponding 90% CI were 0.56 and 0.42–0.76. The median MMAE Tmax was reduced in the presence of concomitant rifampin (3.00 [range, 0.99–5.01] alone vs. 1.49 [range, 1.33–4.02] in combination); however, the ranges overlapped extensively. The MMAE t1/2 was similar with and without rifampin.
Because MMAE exposures decreased after multiple doses,15 to approximately 50% to 80% relative to the first dose (data on file), an exploratory analysis that was not prespecified in the statistical analysis plan was performed to adjust for the period effect on the PK of MMAE. Using a model-based approach, the adjusted GMR (90% CI) for MMAE AUC0-∞ was 0.69 (0.49, 0.98) with concomitant rifampin.
Effect of ketoconazole on brentuximab vedotin and MMAE PK
The effect of ketoconazole on the PK of brentuximab vedotin was evaluated by comparing the brentuximab vedotin PK parameters between brentuximab vedotin alone vs. coadministered with a daily dose of ketoconazole (Table 2 and Figure 2).
For ADC, the AUC0-∞ GMR (brentuximab vedotin with ketoconazole/brentuximab vedotin alone) and the corresponding 90% CI were 1.07 and 0.95–1.19; the Cmax GMR and corresponding 90% CI were 0.99 and 0.75–1.31. The ADC Tmax and t1/2 were similar with and without concomitant ketoconazole.
For MMAE, the AUC0-∞ GMR and the corresponding 90% CI were 1.34 and 0.98–1.84 and the Cmax GMR and corresponding 90% CI were 1.25 and 0.90–1.72. The MMAE Tmax and t1/2 were similar with and without concomitant ketoconazole.
Using a model based-approach to account for the MMAE period effect, the adjusted GMR (90% CI) for MMAE AUC0-∞ was 1.73 (1.22, 2.46) with concomitant ketoconazole.
Effect of ATA on brentuximab vedotin PK
Five of 14 PK-evaluable patients in the rifampin arm and 8 of 16 PK-evaluable patients in the ketoconazole arm had at least 1 positive postbaseline ATA result. An analysis was performed to determine whether exclusion of these patients would affect the GMR analyses for brentuximab vedotin. For both treatment arms, the GMRs with and without patients with positive postbaseline ATA results were generally consistent (data not shown). As expected, variability was higher when these patients were excluded, due to the smaller numbers of patients.
Safety
During the 2 treatment cycles of this study, 53 of 56 patients (95%) experienced at least 1 treatment-emergent adverse event. The most commonly reported treatment-emergent adverse events were nausea (30%); fatigue (29%); diarrhea, headache, and pyrexia (23% each); and neutropenia (20%). A total of 22 patients (39%) in the study experienced at least 1 adverse event ≥ Grade 3. One post-allogeneic transplant patient died due to intracranial hemorrhage, CMV reactivation, and pancytopenia complicated by Streptococcus pneumoniae sepsis and pneumonia, and concurrent multisystem organ failure after 1 dose of brentuximab vedotin. The clinical significance of CMV reactivation was unclear. Seven percent of patients had a most severe adverse event of Grade 4 during the study (neutropenia and/or thrombocytopenia), and 30% had a most severe adverse event of Grade 3. The most common adverse events ≥ Grade 3 were neutropenia (18%) and anemia (7%). No other adverse events ≥ Grade 3 were observed in more than 2 patients each.
Pre-existing Grade 1 peripheral neuropathy was reported for 27% of patients and treatment-emergent peripheral neuropathy occurred in 18% of patients during the 2 treatment cycles of this study. With one exception, Grade 1 gait disturbance, all treatment-emergent peripheral neuropathy was sensory in nature. One patient’s pre-existing peripheral neuropathy worsened to Grade 2 during the study, all other peripheral neuropathy was Grade 1 (asymptomatic).
Laboratory abnormalities with baseline to postbaseline changes from Grade 1 or 2 to ≥ Grade 3 were most commonly observed for decreased absolute neutrophil count (16 patients, 29%); low white blood cell count (7 patients, 13%); elevated ALT (6 patients, 11%); and for low hemoglobin, low lymphocyte counts, and low platelet counts (5 patients, 9% each).
Although the study was not designed to quantitatively evaluate differences between treatment arms, some numeric differences were noted. For example, the incidence of adverse events ≥ Grade 3 during the study was higher in the rifampin and ketoconazole arms (9 patients, 43% and 9 patients, 47%, respectively) compared to the midazolam arm (4 patients, 25%). Additionally, the incidence of gastrointestinal disorders (e.g., nausea, diarrhea, and vomiting) was higher in the ketoconazole arm (13 patients, 68%) compared to the midazolam and rifampin arms (7 patients, 44% and 10 patients, 48%, respectively). Post hoc analyses were performed to determine whether these observations were potentially associated with co-administration of rifampin or ketoconazole (Table 3).
Table 3.
Most common adverse events (occurring in ≥ 15% of the total population)
| Mid
|
Rifa
|
Ketb
|
Total
|
|||||
|---|---|---|---|---|---|---|---|---|
| Both Cycles (n = 16) n (%) |
Cycle 1c (N = 21) n (%) |
Cycle 2c (N = 20) n (%) |
Both Cycles (n = 21) n (%) |
Pre-ketc (N = 19) n (%) |
Post-ketc (N = 19) n (%) |
Both Cycles (n = 19) n (%) |
Both Cycles (N = 56) n (%) |
|
| Any event | 14 (88) | 18 (86) | 17 (85) | 20 (95) | 16 (84) | 16 (84) | 19 (100) | 53 (95) |
| Preferred term | ||||||||
| Nausea | 2 (13) | 6 (29) | 2 (10) | 6 (29) | 5 (26) | 5 (26) | 9 (47) | 17 (30) |
| Fatigue | 3 (19) | 1 (5) | 4 (20) | 5 (24) | 4 (21) | 5 (26) | 8 (42) | 16 (29) |
| Diarrhea | 3 (19) | 2 (10) | 3 (15) | 4 (19) | 1 (5) | 6 (32) | 6 (32) | 13 (23) |
| Headache | 4 (25) | 3 (14) | 2 (10) | 5 (24) | 0 | 4 (21) | 4 (21) | 13 (23) |
| Pyrexia | 0 | 6 (29) | 0 | 6 (29) | 6 (32) | 2 (11) | 7 (37) | 13 (23) |
| Neutropenia | 1 (6) | 6 (29) | 5 (25) | 7 (33) | 0 | 3 (16) | 3 (16) | 11 (20) |
| Vomiting | 2 (13) | 2 (10) | 1 (5) | 2 (10 | 3 (16) | 6 (32) | 6 (32) | 10 (18) |
| Pruritis | 1 (6) | 3 (14) | 2 (10) | 4 (19) | 1 (5) | 3 (16) | 4 (21) | 9 (16) |
A by-cycle analysis was performed, as maximal induction of CYP3A by rifampin was anticipated to occur prior to the beginning of Cycle 2.
A pre- vs. post-initiation of ketoconazole dosing analysis was performed because inhibition of CYP3A by the reversible inhibitor ketoconazole is concentration dependent; maximal inhibition of CYP3A by ketoconazole was anticipated to occur after the first dose of ketoconazole was administered.
Patients could be counted in both time periods.
For the rifampin arm, no difference was observed in the incidence of adverse events overall between Cycles 1 and 2 (18 patients, 86% in Cycle 1 vs. 17 patients, 85% in Cycle 2), and the incidence of adverse events ≥ Grade 3 was similar during Cycles 1 and 2 (6 patients, 29% vs. 4 patients, 20%, respectively).
For the ketoconazole arm, the overall incidence of adverse events was 84% (16 patients) both pre- and post-ketoconazole; the incidence of adverse events ≥ Grade 3 was higher after ketoconazole dosing began (4 patients, 21% before vs. 7 patients, 37% after). Anemia (n=2; 1 patient pre- and 1 patient post-ketoconazole) and neutropenia (n=3 patients; all post-ketoconazole) were the only events ≥ Grade 3 reported for more than 1 patient in the ketoconazole arm. Investigation of gastrointestinal disorders showed that the incidence of diarrhea (1 patient, 5% pre- vs. 6 patients, 32% post-) and vomiting (3 patients, 16% pre- vs. 6 patients, 32% post-) increased after initiation of ketoconazole dosing, but the incidence of nausea did not (5 patients, 26% both pre- and post-ketoconazole) (Table 3). No consistent pattern was observed pre- vs. post-initiation of ketoconazole dosing for adverse events commonly observed in clinical studies of brentuximab vedotin, including peripheral neuropathy events (2 patients, 11% before vs. 1 patient, 5% after), elevated ALT (1patient, 5% both before and after), elevated AST (1 patient, 5% before vs. 2 patients, 11% after), neutropenia (0 patients, 0% before vs. 3 patients, 16% after), fatigue (4 patients, 21% before vs. 5 patients, 26% after), pyrexia (6 patients, 32% before vs. 2 patients, 11% after), and nausea (5 patients, 26% both before and after). No clear contributing factors to the numeric differences were identified.
Eighteen patients were free of confirmed ATA at baseline but tested positive at one or both postbaseline timepoints. With one exception, the titers of all confirmed positive ATA results in this study were ≤ 125. One patient had ATA titers of 3125 prior to the second dose of brentuximab vedotin and 125 at the end of treatment. This patient’s second dose was interrupted due to Grade 2 events of dyspnea, back pain, and chills, and Grade 1 hypertension. Four other patients had their second brentuximab vedotin infusion interrupted by infusion-related reactions: 3 patients were negative at baseline but had positive ATA titers at both postbaseline timepoints and 1 patient was negative for ATA at all 3 timepoints.
Excretion
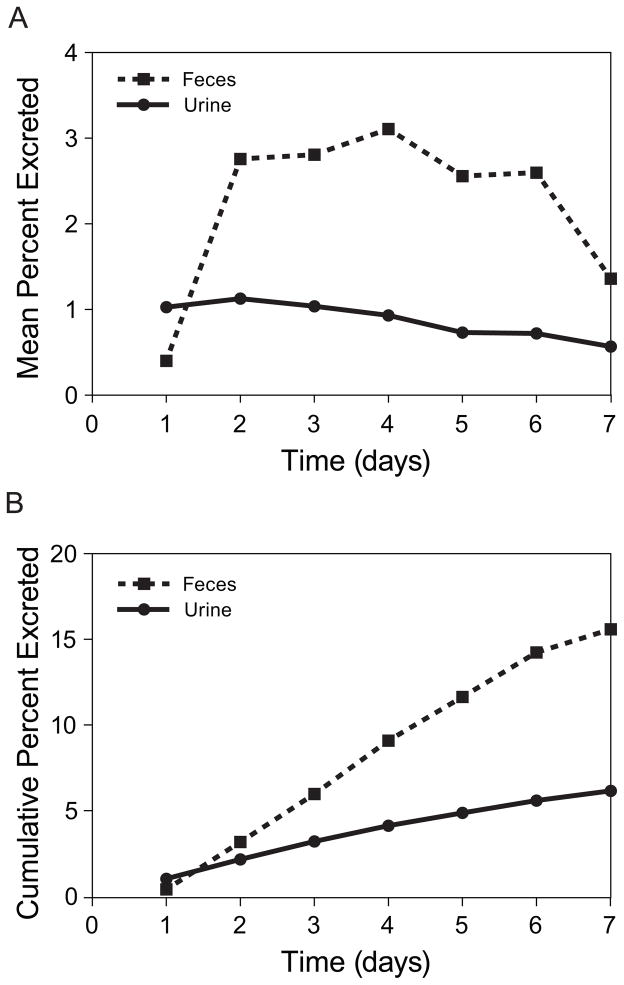
Approximately 23.5% of the MMAE received during a brentuximab vedotin infusion was recovered in both urine and feces over a 1-week period. Of the MMAE recovered, the median percentage of MMAE excreted in feces was 72% (range, 59% to 77%), with the remainder excreted in urine. Figure 3 presents the mean and cumulative excretion of MMAE over time.
Figure 3.
Mean (A) and cumulative (B) excretion of MMAE over time.
MMAE metabolites in urine and feces were identified by tandem and high resolution mass spectrometry. MMAE was the only observed species in unconcentrated urine and feces liquid-liquid extracts. However, after concentrating the liquid-liquid extracts ten-fold, 8 metabolites of MMAE were detected. The metabolites and metabolic pathways are shown in Supplemental Figure S1.
DISCUSSION
This report describes the results of a clinical trial that evaluated the CYP3A-mediated drug interaction potential of brentuximab vedotin. As CYP3A is one of the major enzymes in drug metabolism, characterizing this interaction is essential to providing guidance for the administration of brentuximab vedotin to patients with concomitant medications. Additionally, the primary route of excretion of MMAE in humans was determined.
Challenges that are both similar to and distinct from those associated with traditional antibody or small molecule drug development are encountered in the development of an ADC. In terms of DDIs, the ADC is not expected to be subject to P450 hepatic metabolism, similar to other antibody-based therapeutics;23 however, the small molecule component of an ADC can be metabolized.24 Because of the formation-limited kinetics, the apparent half-life of the cytotoxic agent is on the order of days following administration of an ADC. This long apparent half-life complicates the clinical assessment of DDI potential and necessitates continued dosing of a DDI perpetrator on the order of weeks, as performed in this study, as opposed to a few days for the development of small molecule drugs.
Although in vitro studies suggested that MMAE does not induce CYP3A, the potential for inhibition of CYP3A was identified at concentrations several orders of magnitude higher than those achieved clinically, and MMAE was a time-dependent inhibitor. Thus, the potential for brentuximab vedotin (MMAE) to modulate CYP3A was evaluated using midazolam as a probe substrate. A low dose of midazolam was used in this study to minimize the risk of substantial sedation and amnesia that may be seen in the presence of a CYP3A inhibitor.25,26 Brentuximab vedotin did not affect the AUC0-∞ of midazolam when midazolam was administered at the approximate Tmax for MMAE: the 90% CI for the AUC0-∞ GMR was within bioequivalence bounds (0.80, 1.25). The 90% CI for the Cmax GMR extended past both the upper and lower bioequivalence bounds, thus the Cmax variability does not exclude the possibility of an interaction; however, the point estimate for the Cmax GMR was close to 1. These results support the conclusion that brentuximab vedotin, administered at a dose of 1.8 mg/kg, and resultant MMAE levels neither inhibit nor induce CYP3A.
CYP3A is the primary pathway for the hepatic metabolism of MMAE. Thus, the effect of an effective inducer (rifampin) and a strong inhibitor (ketoconazole) were evaluated. When rifampin was coadministered with brentuximab vedotin, the 90% CI for both the AUC0-∞ and Cmax GMRs were within bioequivalence bounds for ADC. MMAE exposures were ~46% lower when rifampin was coadministered, with the upper 90% CI bounds for the AUC0-∞ and Cmax GMRs being less than 1. When ketoconazole was coadministered with brentuximab vedotin, the 90% CI for AUC0-∞ GMR of ADC was within bioequivalence bounds whereas that of the Cmax GMR extended past both bounds, suggesting greater variability; however, both GMRs for ADC were close to 1. MMAE exposures were ~34% higher when ketoconazole was coadministered, with the upper 90% CI bounds for the AUC0-∞ and Cmax GMRs being greater than 1. An exploratory analysis of the period effect on MMAE PK, based on the reduced concentration of MMAE in later cycles compared to Cycle 1, estimated that coadministration of ketoconazole has the potential to increase MMAE AUC0-∞ by ~73% in Cycle 1. Since MMAE levels decrease to 80% or less following Cycle 1, the increase in MMAE levels for later cycles is expected to be ~38% or less relative to Cycle 1 MMAE levels in the absence of concomitant ketoconazole.
In addition to modulating CYP3A, rifampin and ketoconazole can modulate the expression of P-gp, and Rifampin is a known inducer of P-gp and ketoconazole has been associated with P-gp inhibition.27 MMAE was identified as a P-gp substrate in in vitro studies, and so the observed results in this study cannot exclude a potential role of P-gp in altering MMAE clearance. The contribution of P-gp modulation on the disposition of MMAE relative to that of CYP3A modulation is unknown.
Taken together, results from the rifampin and ketoconazole arms indicate that the ADC was not affected by an effective CYP3A inducer or a strong CYP3A inhibitor, and that ADC exposures were consistent between Cycles 1 and 2. In addition, the data suggest that MMAE is a substrate of CYP3A. Thus, patients who are receiving strong CYP3A inhibitors concomitantly with brentuximab vedotin should be closely monitored for adverse reactions.
Results of safety evaluations from this study are generally consistent with those of other brentuximab vedotin clinical studies.11–14 The most commonly reported treatment-emergent adverse events were nausea, fatigue, diarrhea, headache, pyrexia, and neutropenia. Treatment-emergent peripheral neuropathy, reported for 18% of patients during the 2 treatment cycles of this study, was predominantly sensory and Grade 1 (asymptomatic). Four of 5 patients whose second brentuximab vedotin infusion was interrupted by infusion-related reactions had positive postbaseline ATA results. A single patient discontinued treatment due to an adverse event. Fifty-four patients (96%) completed the study per protocol, and 89% went on to receive extended treatment with brentuximab vedotin in a separate study.
Although the study was not designed to evaluate differences between treatment arms, post hoc analyses suggested that numeric differences in safety observations across treatment arms were unlikely to be due to interaction of brentuximab vedotin with midazolam, rifampin, or ketoconazole. The higher incidence of vomiting and diarrhea observed in the ketoconazole arm may have been attributable, at least in part, to individual factors such as pre-existing gastrointestinal problems and to concomitant use of ketoconazole and other potentially contributory medications. No consistent patterns were detected for adverse events commonly observed in clinical studies of brentuximab vedotin, and no clear contributing factors were identified. This 2-cycle study did not address the safety of long-term administration of CYP3A modulators during extended therapy with brentuximab vedotin; however, the observed short-term safety profile was generally consistent with historic clinical observations.
Mass balance was not achieved in the excretion portion of this study. Based on the data collected, the primary route of excretion of MMAE in humans is via feces (median 72% of total excreta), which is consistent with preclinical results in a rat excretion study. MMAE was the predominant species detected in excreta and MMAE metabolites were only detected upon 10-fold concentration of the samples. Thus, it is likely that the concentration of MMAE in excreta is at least 10-fold higher than that of its metabolites. This study characterized the CYP3A DDI profile of brentuximab vedotin and MMAE. Taken together, the results suggest that MMAE is neither an inhibitor nor inducer of CYP3A when brentuximab vedotin is administered to humans at a dose of 1.8 mg/kg; however, MMAE is a substrate of CYP3A and potentially a substrate of P-gp. Results of this study may also apply to other MMAE-based ADCs that achieve circulating concentrations of both ADC and unconjugated MMAE similar to those observed for brentuximab vedotin. Although the brentuximab vedotin safety profile did not appear to be appreciably affected by concomitant administration of rifampin or ketoconazole, patients who are receiving strong CYP3A inhibitors concomitantly with brentuximab vedotin should be closely monitored for adverse reactions. The primary route of excretion of MMAE in humans is via feces.
Supplementary Material
Acknowledgments
Research funding for the study was provided by Seattle Genetics, Inc., and Millennium: The Takeda Oncology Company. The authors wish to acknowledge Edmund Ng and Emmanuel Mohandoss for statistical guidance and Roberta Connelly for medical writing assistance, all under the sponsorship of Seattle Genetics, Inc. Author Robert Chen is a NCI K12 career development award recipient. Author Ajay Gopal is a Scholar in Clinical Research for the Leukemia and Lymphoma Society.
Footnotes
Presented in part as a poster at the American Society for Clinical Pharmacology and Therapeutics (ASCPT) Annual Meeting, March 14–17, 2012; Washington, DC.
AUTHORSHIP
T.H.H., L.E.G., S.C.A., and C.M.L contributed to the conception and design of the trial. A.K.G., R.R., A.G., R.C., J.V.M., M.C., and O.A.O. collected clinical data. T.H.H., L.E.G., and S.C.A. interpreted the data and wrote the first draft of the manuscript, which was finalized and approved by all authors.
Financial Disclosures: This study was sponsored by Seattle Genetics, Inc., and Millennium: The Takeda Oncology Company. Research funding for the study was provided by Seattle Genetics, Inc. to the institutions of A.K.G., R.R., A.G., R.C., J.V.M., M.C., and O.A.O. A.K.G. has been a consultant to and received honoraria from Seattle Genetics, Inc., and has been on the speakers’ bureau for Takeda. R.R. has been on the speakers’ bureau for Seattle Genetics, Inc. A.G. has been on the speakers’ bureau for Millennium and has been an advisory/scientific board member for Seattle Genetics, Inc., Millennium, Celgene, Johnson and Johnson, Pharmacyclics, and Pfizer. R.C. has been a consultant to, been on the speakers’ bureau for, and received travel expenses from Seattle Genetics, Inc. J.V.M. has been on the speakers’ bureau for Seattle Genetics, Inc., Millennium, and Celgene, and has been an advisory/scientific board member for Celgene. O.A.O. has been a consultant to Seattle Genetics, Inc., Millennium, Allos Therapeutics, Celgene, and Purdue Pharma and has received research funding/grants from Millennium, Allos Therapeutics, and Merck. T.H.H., L.E.G., and S.C.A. are employed by and have equity ownership in Seattle Genetics, Inc. C.M.L. was employed by Seattle Genetics, Inc. at the time the work was performed and has equity ownership in Seattle Genetics, Inc.
References
- 1.Alley S, Oflazoglu E, Mechthild J, et al. Antibody-targeted delivery of auristatin using SGN-35 leads to one to two logs greater intratumoral auristatin concentrations than non-targeted therapy [abstract] AACR Meeting Abstracts. 2009;2009:3234. [Google Scholar]
- 2.Alley SC, Okeley NM, Senter PD. Antibody-drug conjugates: targeted drug delivery for cancer. Curr Opin Chem Biol. 2010;14:529–537. doi: 10.1016/j.cbpa.2010.06.170. [DOI] [PubMed] [Google Scholar]
- 3.Ducry L, Stump B. Antibody-drug conjugates: linking cytotoxic payloads to monoclonal antibodies. Bioconjug Chem. 2010;21:5–13. doi: 10.1021/bc9002019. [DOI] [PubMed] [Google Scholar]
- 4.Chiarle R, Podda A, Prolla G, Gong J, Thorbecker GJ, Inghirami G. CD30 in normal and neoplastic cells. Clin Immunol. 1999;90:157–164. doi: 10.1006/clim.1998.4636. [DOI] [PubMed] [Google Scholar]
- 5.Durkop H, Latza U, Hummel M, Eitelbach F, Seed B, Stein H. Molecular cloning and expression of a new member of the nerve growth factor receptor family that is characteristic for Hodgkin’s disease. Cell. 1992;68:421–427. doi: 10.1016/0092-8674(92)90180-k. [DOI] [PubMed] [Google Scholar]
- 6.Bartlett NL, Younes A, Carabasi MH, et al. A phase 1 multidose study of SGN-30 immunotherapy in patients with refractory or recurrent CD30+ hematologic malignancies. Blood. 2008;111:1848–1854. doi: 10.1182/blood-2008-01-127118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Wahl AF, Klussman K, Thompson JD, et al. The anti-CD30 monoclonal antibody SGN-30 promotes growth arrest and DNA fragmentation in vitro and affects antitumor activity in models of Hodgkin’s disease. Cancer Res. 2002;62:3736–3742. [PubMed] [Google Scholar]
- 8.Doronina SO, Toki BE, Torgov MY, et al. Development of potent monoclonal antibody auristatin conjugates for cancer therapy. Nat Biotechnol. 2003;21:778–784. doi: 10.1038/nbt832. [DOI] [PubMed] [Google Scholar]
- 9.Francisco JA, Cerveny CG, Meyer DL, et al. cAC10-vcMMAE, an anti-CD30-monomethyl auristatin E conjugate with potent and selective antitumor activity. Blood. 2003;102:1458–1465. doi: 10.1182/blood-2003-01-0039. [DOI] [PubMed] [Google Scholar]
- 10.Okeley NM, Miyamoto JB, Zhang X, et al. Intracellular activation of SGN-35, a potent anti-CD30 antibody-drug conjugate. Clin Cancer Res. 2010;16:888–897. doi: 10.1158/1078-0432.CCR-09-2069. [DOI] [PubMed] [Google Scholar]
- 11.Pro B, Advani R, Brice P, et al. Brentuximab vedotin (SGN-35) in patients with relapsed or refractory systemic anaplastic large cell lymphoma: results of a phase II study. J Clin Oncol. 2012;30:2190–2196. doi: 10.1200/JCO.2011.38.0402. [DOI] [PubMed] [Google Scholar]
- 12.Younes A, Gopal AK, Smith SE, et al. Results of a pivotal phase II study of brentuximab vedotin for patients with relapsed or refractory Hodgkin’s lymphoma. J Clin Oncol. 2012;30:2183–9. doi: 10.1200/JCO.2011.38.0410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Younes A, Bartlett NL, Leonard JP, et al. Brentuximab vedotin (SGN-35) for relapsed CD30-positive lymphomas. N Engl J Med. 2010;363(19):1812–21. doi: 10.1056/NEJMoa1002965. [DOI] [PubMed] [Google Scholar]
- 14.Fanale MA, Forero-Torres A, Rosenblatt JD, et al. A phase I weekly dosing study of brentuximab vedotin in patients with relapsed/refractory CD30-positive hematologic malignancies. Clin Cancer Res. 2012;18:248–255. doi: 10.1158/1078-0432.CCR-11-1425. [DOI] [PubMed] [Google Scholar]
- 15.Han TH, Kennedy D, Hayes S, Lynch CM. The pharmacokinetics of brentuximab vedotin (SGN-35), an antibody-drug conjugate (ADC) [abstract] Clin Pharmacol Ther. 2012;91(suppl 1):PII-1. [Google Scholar]
- 16.Dowell JA, Korth-Bradley J, Liu H, King SP, Berger MS. Pharmacokinetics of gemtuzumab ozogamicin, an antibody-targeted chemotherapy agent for the treatment of patients with acute myeloid leukemia in first relapse. J Clin Pharmacol. 2001;41:1206–1214. doi: 10.1177/00912700122012751. [DOI] [PubMed] [Google Scholar]
- 17.Girish S, Gupta M, Wang B, et al. Clinical pharmacology of trastuzumab emtansine (T-DM1): an antibody-drug conjugate in development for the treatment of HER2-positive cancer. Cancer Chemother Pharmacol. 2012;69:1229–1240. doi: 10.1007/s00280-011-1817-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lapusan S, Vidriales MB, Thomas X, et al. Phase I studies of AVE9633, an anti-CD33 antibody-maytansinoid conjugate. in adult patients with relapsed/refractory acute myeloid leukemia. Invest New Drugs. 2012;30:1121–1131. doi: 10.1007/s10637-011-9670-0. [DOI] [PubMed] [Google Scholar]
- 19.Shen BQ, Xu K, Liu L, et al. Conjugation site modulates the in vivo stability and therapeutic activity of antibody-drug conjugates. Nat Biotechnol. 2012;30:184–189. doi: 10.1038/nbt.2108. [DOI] [PubMed] [Google Scholar]
- 20.Huang SM, Zhao H, Lee JI, et al. Therapeutic protein-drug interactions and implications for drug development. Clin Pharmacol Ther. 2010;87:497–503. doi: 10.1038/clpt.2009.308. [DOI] [PubMed] [Google Scholar]
- 21.Seitz KH, Zhou H. Pharmacokinetic drug-drug interaction potentials for therapeutic monoclonal antibodies: reality check. J Clin Pharmacol. 2007;47:1104–1118. doi: 10.1177/0091270007306958. [DOI] [PubMed] [Google Scholar]
- 22.ADCETRIS® [package insert] Bothell, Wa: Seattle Genetics, Inc; Jan, 2012. [Google Scholar]
- 23.Mahmood I, Green MD. Drug interaction studies of therapeutic proteins or monoclonal antibodies. J Clin Pharmacol. 2007;47:1540–1554. doi: 10.1177/0091270007308616. [DOI] [PubMed] [Google Scholar]
- 24.Lu D, Krop I, Modi S, et al. Pharmacokinetics (PK) of Trastuzumab-DM1 (T-DM1) and Paclitaxel (T) in Patients with HER2-Positive Locally Advanced or Metastatic Breast Cancer (MBC) Previously Treated with a Trastuzumab-Containing Regimen [abstract] Cancer Res. 2010;70(24 suppl):P3-14-22. [Google Scholar]
- 25.Olkkola KT, Aranko K, Luurila H, et al. A potentially hazardous interaction between erythromycin and midazolam. Clin Pharmacol Ther. 1993;53:298–305. doi: 10.1038/clpt.1993.25. [DOI] [PubMed] [Google Scholar]
- 26.Olkkola KT, Backman JT, Neuvonen PJ. Midazolam should be avoided in patients receiving the systemic antimycotics ketoconazole or itraconazole. Clin Pharmacol Ther. 1994;55:481–485. doi: 10.1038/clpt.1994.60. [DOI] [PubMed] [Google Scholar]
- 27.Lin JH. Drug-drug interaction mediated by inhibition and induction of P-glycoprotein. Adv Drug Deliv Rev. 2003;55:53–81. doi: 10.1016/s0169-409x(02)00171-0. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.