Abstract
The development of influenza A virus (IAV) vaccines capable of inducing cytotoxic CD8 T cell responses could potentially provide superior, long-term protection against multiple, heterologous strains of IAV. While prior studies have demonstrated the effectiveness of baculovirus-derived virus-like particle (VLP) vaccination in generating antibody-mediated protection, what role CD8 T cell immunity plays in overall VLPmediated protection is less understood. Herein we demonstrate that intranasal vaccination of mice with a VLP containing the hemagglutinin (HA) and matrix 1 (M1) proteins of influenza virus A/PR/8/34 leads to a significant increase in HA533-specific CD8 T cells in the lungs and protection following subsequent homologous challenge with IAV. VLP-mediated protection was significantly reduced by CD8 T cell depletion, indicating a critical role for CD8 T cells in contributing to protective immunity. Importantly, our results show VLP-vaccine induced CD8 T cell mediated protection is not limited to homologous IAV strains. VLP vaccination leads to an increase in protection following heterosubtypic challenge with a strain of IAV that avoids vaccine-induced neutralizing antibodies but contains conserved, immunodominant CD8 T cell epitopes. Overall, our results demonstrate the ability of influenza protein-containing VLPs to prime IAV-specific CD8 T cell responses, which contribute to protection from homo- and heterosubtypic influenza A virus infections. These results further suggest that vaccination strategies focused on the development of cross-protective CD8 T cell responses may contribute to the development of “universal” IAV vaccines.
Keywords: Influenza virus, CD8 T cell, lung, vaccination
Introduction
Influenza A virus (IAV) causes significant seasonal illness, leading to approximately 200,000 hospitalizations and 36,000 deaths annually in the United States during non-pandemic years (1, 2). This high rate of severe illness, along with the constant threat of a pandemic influenza outbreak, has renewed interest in developing novel influenza vaccination strategies. Following primary IAV infection, the development of a cytotoxic, influenza-specific CD8 T cell response is important in terminating the acute infection and contributes to long-term immunity (3, 4). The current influenza subunit vaccine leads to the production of influenza neutralizing antibodies (5). However, whether a robust influenza-specific CD8 T cell response is generated following vaccination remains unclear (5, 6). Importantly, as the virus drifts, neutralizing antibodies induced by either previous vaccination or IAV infection can rapidly become ineffective due to constant antibody-mediated selective pressure on IAV’s hemagglutinin (HA) and neuraminidase (NA) (7). Conversely, CD8 T cell responses to IAV are often generated against influenza-derived proteins/epitopes that are more highly conserved and are thus able to contribute to heterosubtypic protection (8, 9). Therefore, the development of an IAV vaccine capable of inducing a cytotoxic CD8 T cell response could potentially provide superior, long-term protection against multiple, heterologous strains of IAV (10, 11).
Recently, influenza virus-like particles (VLPs) have been developed from recombinant baculoviruses containing influenza virus proteins such as HA and/or NA on the surface and matrix (M1) as a core of the VLP. Influenza VLPs resemble influenza virions in both size and morphology (12). These influenza VLPs are an attractive vaccination candidate as they are replication deficient (i.e. they contain no IAV genomic material) and therefore could be administered to the young and elderly populations that exhibit the highest risk for complications from seasonal influenza virus infection. Influenza VLPs induce potent antibody responses and provide heterosubtypic cross-protection from morbidity and mortality during lethal IAV challenge following a single, intranasal (i.n.) treatment (12–17). However, the immune component mediating heterosubtypic protection following influenza VLP vaccination remains unknown. Given this cross-protection, VLP-induced CD8 T cell responses may contribute to protection from subsequent infections. Indeed, one in vitro study has highlighted the ability of dendritic cells pulsed with influenza VLPs to stimulate human influenza-specific CD8 T cells (17). However, whether i.n. administered influenza VLPs can induce influenza-specific CD8 T cell responses in vivo remains unknown. Further, the role of VLP-induced CD8 T cell immunity in mediating protection following homo- and heterosubtypic IAV challenges remains to be elucidated.
Herein we examine the development and contribution of influenza VLP-induced CD8 T cells to IAV immunity following a single, i.n. vaccination with VLPs containing HA and M1 of A/PR/8/34. Our findings demonstrate a small, but significant increase in HA533-specific CD8 T cells immediately following influenza VLP administration, which is sustained for at least one month. Our results also indicate HA533-specific CD8 T cells primed by influenza VLP vaccination are increased in the lungs upon subsequent IAV challenge. These vaccine-induced CD8 T cells are crucial in providing protection from mortality during lethal, homologous IAV challenge as mice depleted of CD8 T cells 30 days following vaccination succumb to the challenge infection. Further support for vaccine-induced CD8 T cells in mediating protection is demonstrated by the ability of VLP-induced, HA533-specific CD8 T cells to aid in protection from high-dose, heterosubtypic IAV challenge. Together, our findings highlight the potential use of influenza VLPs to induce effective, cross-protective CD8 T cells that can contribute to protective immunity during particularly severe seasonal and pandemic outbreaks of influenza virus infections.
Materials and Methods
Mice
Six- to eight-week-old wild type (WT) BALB/c mice were obtained from The National Cancer Institute (Frederick, MD). Clone 4 (CL-4) CD90.1+ mice containing TCR transgenic T cells specific for the HA533/HA529 epitope of A/PR/8/34 and A/Japan/305/57, respectively, were obtained from The Jackson Laboratory (Bar Harbor, ME). All mice were housed and maintained in the specific pathogen-free animal care facility at the University of Iowa (Iowa City, IA). All experiments were performed in accordance with regulatory standards and guidelines and were approved by the Institutional Animal Care and Use Committee of the University of Iowa.
Virus-like particle vaccination
VLPs containing HA and M1 of mouse-adapted A/PR/8/34 were produced as described previously (12). Mice were anesthetized with isoflorane and administered 2.5 µg of VLPs in phosphate buffered saline (PBS) or 50 µl PBS (as a control) i.n..
Influenza virus infection
Mouse-adapted (ma) influenza A viruses A/PR/8/34 (maH1N1), A/HK/1/68 (maH3N2) and A/Japan/305/57 (maH2N2) were prepared from stocks as described previously (18). Mice were anesthetized with isoflurane and infected i.n. with 5.5×104 tissue culture infectious units (TCIU) of virus. For day 8 analysis, mice were challenged with a dose 5.5×103 TCIU of A/PR/8/34.
MHC I Tetramers
MHC class I tetramers HA533–541 (H-2K(d)/IYSTVASSL) and NP147–155 (H-2K(d)/TYQRTRALV) were obtained from the National Institute of Allergy and Infectious Disease MHC Tetramer Core Facility (Atlanta, GA).
Surface Staining
Single-cell suspensions of lungs, lung-draining LN (dLN, i.e. mediastinal and peribronchial), and spleens were prepared by pressing the tissues through wire mesh screens and plating 1×106 cells/well in a 96-well plate and blocked with 2 µl rat serum in FACS buffer for 30 min at 4°C.
Following blocking, cells were incubated with FACS buffer containing rat antimouse CD8α conjugated to FITC (53–6.7), rat anti-mouse CD3ɛ conjugated to PerCP Cy5.5 (145–2C11) purchased from BD, and HA533 or NP147 tetramers conjugated to APC and PE for 1 h at 4°C. Cells were then fixed in FACS Lysis Buffer (BD) per manufacturer’s instructions and resuspended in PBS. Data was acquired on a BD FACSCanto II and analyzed with FlowJo software (TreeStar, Inc.).
Intracellular Cytokine Staining
Intracellular cytokine staining was performed as previously described(19). Briefly, 5×105 cells from lung single-cell suspensions were incubated for 6 hs with HA533–541 or NP147–155 peptides in complete media containing rhIL-2 (Novartis) and brefeldin A (Sigma). Following incubation, cells were surface stained with anti-mouse CD8α and anti-mouse CD3ɛ monoclonal antibodies (mAb). Following fixation, cells were permeablized by incubation for 30 min at 4°C in FACS Buffer containing .5% saponin (ACROS) and subsequently stained with rat anti-mouse IFNγ mAb (XMG1.2) conjugated to APC (BD) for 30 min at 4°C in FACS Buffer containing .5% saponin. Data was acquired on a BD FACSCanto II and analyzed with FlowJo software (TreeStar, Inc.).
Purification and adoptive transfer of CL-4 T cells
Single-cell suspensions of spleens from CL-4 CD90.1+ mice (containing TCR transgenic T cells specific for the HA533/HA529 epitope of A/PR/8/34 and A/Japan/305/57, respectively) were labeled with CD8α microbeads (Miltenyi) and purified using LS columns according to manufacturer’s instructions (Miltenyi). 5×105 purified donor CD90.1+/CD8α+ CL-4 cells were labeled with 2.5 µM CFSE (Invitrogen) and adoptively transferred i.v. into BALB/c CD90.2+ host mice (20). Mice were vaccinated with VLPs 24 hs later. Three days following VLP vaccination, dLN were collected and proliferation was determined by analyzing CFSE dilution in CD3ɛ+/CD8α+/CD90.1+ cells.
CD8α Depletion
An anti-CD8α depletion strategy described to reduce splenic IAV-specific memory CD8 T cells, but allow naïve T cell repopulation, was modified to ensure depletion of HA533-specific memory CD8 T cells within the lungs and dLN (21). Briefly, mice were treated with anti-CD8α monoclonal antibody (clone 2.43) i.p. (250 µg) and i.n. (100 µg) 30 days following VLP vaccination or sublethal (0.1 LD50) A/PR/8/34 challenge. The repopulation of naïve CD8 T cells in the spleen, lungs and dLN was monitored for 14 weeks. In subsequent experiments, all memory CD8 T cell depleted mice were used 14 weeks after anti-CD8α Ab treatment (i.e. a time point when naïve CD8 T cells had reestablished and stabilized). Control mice were given equivalent volumes of PBS i.n. and i.p.
Pulmonary Virus Titer
Lungs were harvested on days 2, 4, 6 and 8 post infection, homogenized and snap frozen. Pulmonary virus titers were determined as previously described (22). Briefly, 5×105 Madin-Darby canine kidney (MDCK) cells in Iscove’s complete media containing 50 µg/ml gentamicin (Gibco), 100 U/ml penicillin/100 µg/ml streptomycin (Gibco) were infected with 10-fold serial dilutions of lung homogenates, as well as stock influenza A virus (A/PR/8/34) as a control, and incubated at 37°C. Twenty-four hours later, media was removed from all wells and replaced with Iscove’s complete media containing 0.0002% TPCK-Trypsin (Worthington), 50 µg/ml gentamicin (Gibco), and 100 U/ml penicillin/100 µg/ml streptomycin (Gibco). Three days later, 0.5% chicken red blood cells (Colorado Veterinary Products) were mixed 1:1 with supernatant from each of the cell culture wells and agglutination was measured after incubating for 1 h at room temperature. Tissue culture infectious units were subsequently calculated using the Reed-Muench method.
Measurement of airway resistance
A whole body plethysmograph (Buxco Electronics) was used to measure enhanced pause (Penh), a measure of airway resistance, during respiration (23). Penh values for each mouse were recorded daily following IAV infection. Day 0 baseline Penh values were recorded prior to infection.
Statistical Analysis
Data were compiled in graphical format using Prism software (Graphpad Software, San Diego, CA). Error bars represent the SEM. Statistical significance was determined by using unpaired, two-tailed Student’s t tests, logrank tests or ANOVAs followed by Bonferroni post test.
Results
Influenza VLP vaccination leads to an increase of HA533-specific CD8 T cells in the lungs following vaccination
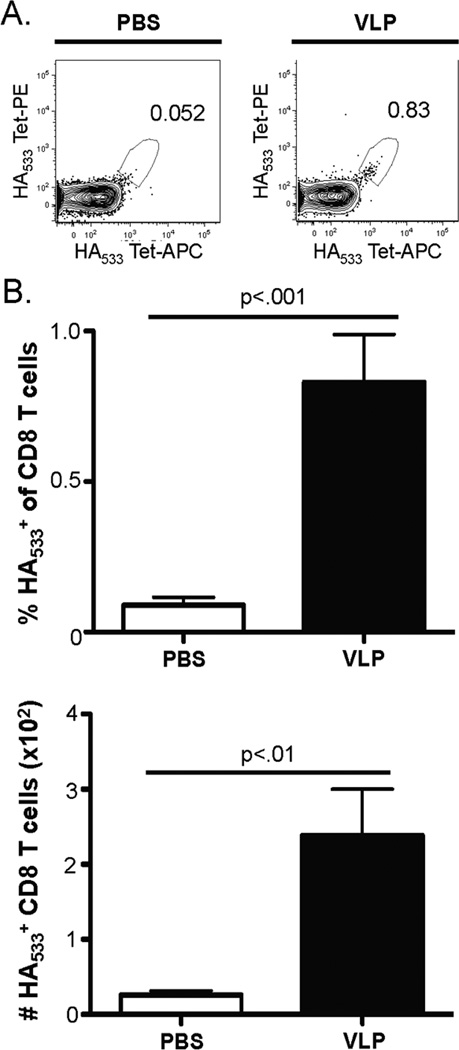
We first determined whether vaccine-specific CD8 T cells accumulate in the lungs 7 and 14 days following a single, i.n. VLP vaccination. Given the expected low frequency of antigen-specific CD8 T cells present at baseline and following vaccination, we employed a dual tetramer labeling strategy to identify influenza-specific CD8 T cells that had expanded following vaccination (Fig. 1A) (24–26). No change in the frequency or numbers of influenza-specific lung CD8 T cells was observed on day 7 post vaccination (data not shown). The frequency and number of HA533-specific CD8 T cells is significantly increased in the lungs on day 14 post vaccination compared to mice that were given PBS i.n. as a control (Fig. 1A & B). Further, this expansion on day 14 post i.n. vaccination was specific for the IAV proteins contained within the VLPs as no change in the NP147-specific CD8 T cell population was observed when comparing vaccinated mice to controls (data not shown). (Note: IAV nucleocapsidprotein (NP) is not present in the VLP preparation utilized in these studies.) We also observed no changes in the total number of CD8 T cells present in the lungs at day 7 or 14 following VLP vaccination (Fig. S1 and data not shown). In the dLN, we observed no increase in the frequency or total numbers of HA533-specific CD8 T cells in the dLN at days 7 or 14 following vaccination (data not shown). Together, the results above indicate the expansion of HA533-specific CD8 T cells was specific to the HA protein contained within the VLP and not an unknown, non-specific effect of influenza VLP vaccination on total, or influenza-specific, CD8 T cell expansion.
Figure 1. VLP vaccination boosts the HA533+ CD8 T cell response in the lungs.
BALB/c mice were treated i.n. with 2.5 µg VLPs or 50 µl PBS as a control. On day 14 post vaccination, lungs were harvested and the frequency and total numbers of HA533+ CD8 T cells were determined by flow cytometry. A) Representative tetramer gating of CD8α+/CD3ɛ+ lung samples on day 14 post VLP vaccination or PBS administration. B) Cumulative frequency and total numbers of HA533+ CD8 T cells. The data are the mean values ± SEM of two independent experiments (n=9 mice/group). Data were analyzed using Student’s t test.
Influenza VLP vaccination leads to proliferation of HA533-specific CD8 T cells in dLN
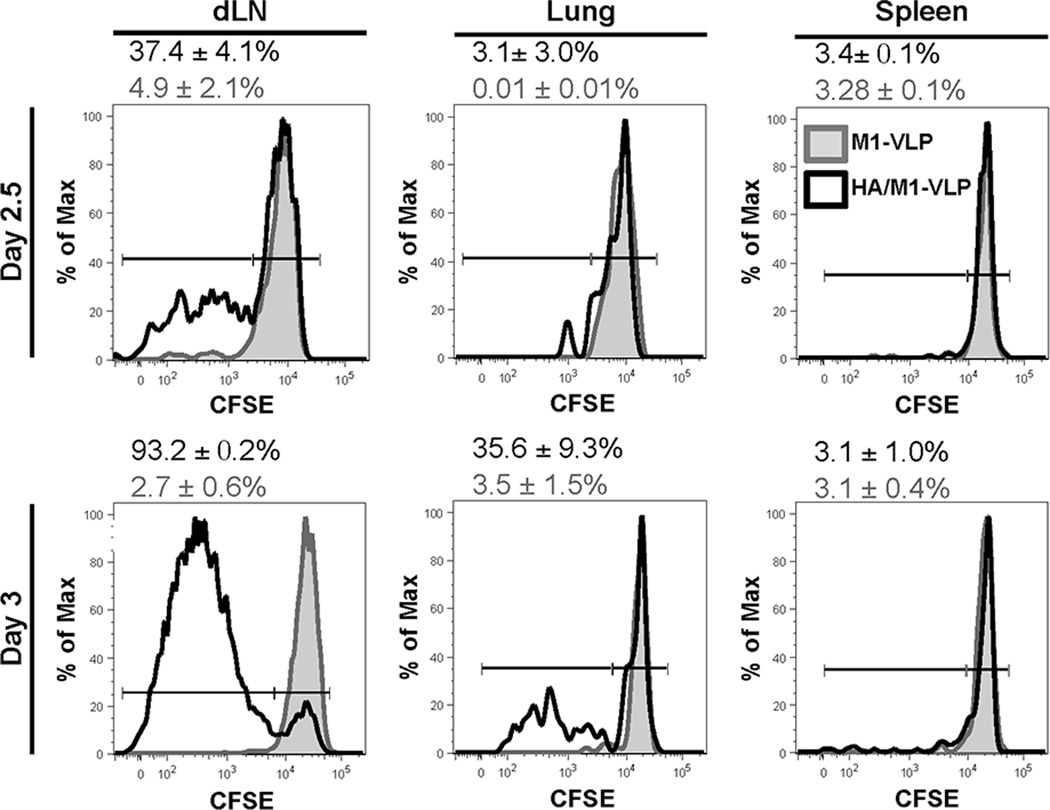
Given that we observed an increase in HA533-specific CD8 T cells only in the lungs, and not dLN, following VLP vaccination, we next sought to determine the location where these T cells were primed. During infection with influenza virus, naïve CD8 T cells are primed in dLN and then migrate to the site of infection (i.e. lungs) to kill virally infected cells (19, 27–30). While the dLN is the initial site of expansion, activated CD8 T cells continue to proliferate in the lung during IAV infection (31). Thus, analysis of dLN at day 7 post vaccination may have been too late to observe vaccine-induced CD8 T cell expansion (20). Further, recent studies have demonstrated that influenza-specific CD8 T cells can be primed and expand in the lungs of mice lacking LN, spleen, and Peyer’s patches when challenged with IAV (32). Therefore, whether HA533-specific CD8 T cells were activated and expanded in lung-draining lymph nodes or the lung tissue was determined. To monitor this expansion, 5×105 CFSE-labeled, naïve CL-4, CD90.1+ CD8 T cells (transgenic CD8 T cells specific for the HA533 epitope) were adoptively transferred into host WT (CD90.2+) mice. The host mice were then vaccinated 24 hs later with either HA/M1 containing VLPs or a control VLP containing only M1. At 2.5 and 3 days following vaccination, the dLN, lungs, and spleens were harvested and analyzed to determine CL-4 proliferation. Compared to M1-VLP controls (in which we observed limited CFSE dilution in donor CL-4 cells), HA/M1-VLP vaccination induced substantial proliferation of HA533-specific CD8 T cells in the dLN by day 2.5 post vaccination (Fig. 2, left column). On day 3 post vaccination a greater frequency of CL-4 cells were CFSElo/- in the dLN of HA/M1-VLP versus M1-VLP treated mice. Further, limited divided CL-4 cells were present in the lungs, but not in the spleen at day 2.5 post vaccination (Fig. 2, top row). However, by day 3, divided CL-4 cells had begun to accumulate in the lungs of HA/M1-VLP vaccinated mice (Fig. 2, bottom, center plot), suggesting these cells are trafficking to the site of vaccination. These results imply that activation and early division of influenza VLP-specific CD8 T cells initially occurs in dLN following i.n. influenza HA/M1-VLP administration, and that these influenza-specific cells subsequently migrate to the lung tissue.
Figure 2. VLP treatment leads to proliferation of IAV-specific CD8 T cells in dLN.
CD90.2+ BALB/c mice were given 5×105 MACs purified, CFSE-labeled CL-4 CD90.1+ cells i.v. and treated i.n. with 2.5 µg of VLP (black line) or PBS (not shown) or 2.5 µg of VLP containing only M1 (shown-shaded grey) 24 hours later. 2.5 and 3 days following vaccination, lung-draining lymph nodes, lungs and spleens were collected and analyzed via flow cytometry for proliferation (i.e. CFSE dilution). Data are representative of two independent experiments (n=3-6 mice pooled/group).
HA533-specific CD8 T cells are increased in dLN and lungs following lethal IAV challenge 30 days post HA/M1-VLP vaccination
Following infection with influenza virus, IAV-specific, long-lived memory CD8 T cells are generated and can reside in both the local lung tissue and in secondary lymphoid organs (33–41). These local, tissue-resident memory cells are key in early (days 0–3 p.i.) control of viral infection, as they are present at the site of initial antigen encounter and are able to rapidly respond to mediate protective immunity (35, 36). At later times following infection (day 5 p.i. and beyond), memory T cells that reside in draining LN during the steady state predominate the response. These LN-resident memory CD8 T cells have a greater proliferative and cytotoxic capacity compared to local, tissue-resident CD8 T cells (38).
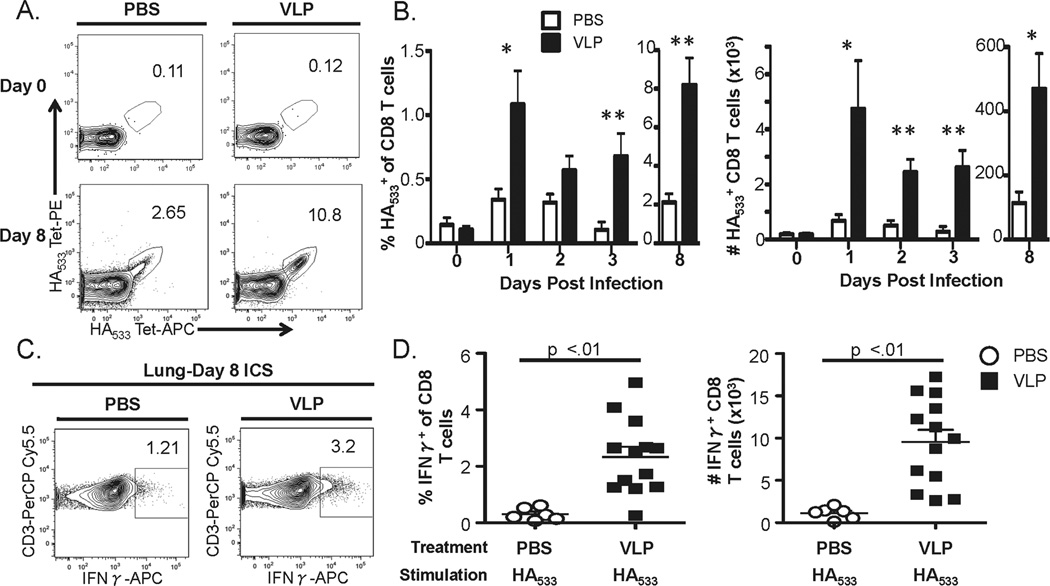
To determine whether CD8 VLP-induced T cell-mediated protection occurs following challenge, we examined the frequency and numbers of the influenza-specific CD8 T cell in both the lungs and dLN prior to, and at various times post, challenge. In the dLN, we observed no significant difference between VLP-vaccinated or control mice in the frequency or numbers of HA533-specific CD8 T cells at any of the times analyzed post challenge (data not shown). Similarly, at 30 days following VLP vaccination (i.e. day 0 of challenge), no difference in the number of HA533-specific CD8 T cells present in the lungs was detected between the 2 groups (Fig. 3A/B). However, as early as one day following lethal IAV challenge we observed a significant increase in HA533-specific CD8 T cells in the lungs that continued through at least day 8 post challenge (Fig. 3). In contrast, we observed no significant differences in the NP147-specific CD8 T cell response (i.e. an epitope not contained within the VLPs) in the lungs and dLN of VLP vaccinated vs. non-VLP primed mice (Fig. S2 and data not shown).
Figure 3. Kinetics of the HA533-specific CD8 T cell response primed by VLP vaccination following lethal IAV challenge.
BALB/c mice were treated i.n. with 2.5 µg VLPs or 50 µl PBS as a control. Thirty days later all mice were infected with 5 LD50 of A/PR/8/34 except for mice that were to be analyzed on day 8 post infection, which were instead challenged with 1 LD50 dose to ensure the survival of controls animals at this time point. A and B) Lungs were harvested on the indicated time points prior to (Day 0) or post infection and HA533-specific CD8α+/CD3ɛ+ cells were identified by flow cytometry with HA533-tetramers. A) Representative tetramer gating of CD8α+/ CD3ɛ+ gated lung samples on days 0 and 8 post IAV challenge. B) The frequency and total numbers of HA533-specific CD8α+/CD3ɛ+ cells. C and D) Lungs were harvested on day 8 p.i. and single-cell homogenates and HA533 cells were measured by ICS. C) Representative IFNγ gating of CD8α+/ CD3ɛ+ gated lungs on day 8 post IAV challenge. D) The frequency and total numbers of IFNγ+ CD3+CD8+ T cells. The data are the mean values ± SEM from three independent experiments (n=6-13 mice/group for lungs). Data were analyzed using a two-way ANOVA followed by Bonferroni post test. *=p<.05, **=p<.01
HA533-specific CD8 T cells generated during VLP vaccination provide protection during lethal IAV challenge
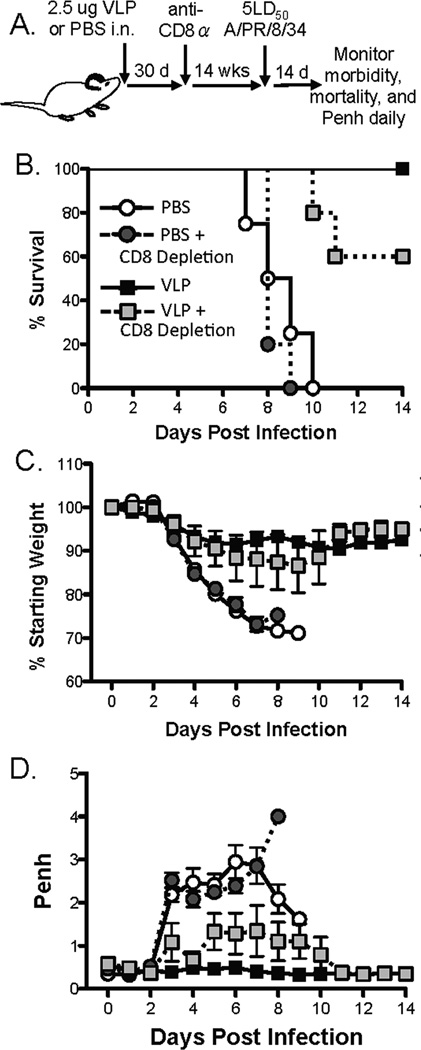
Influenza-specific CD8 T cells generated following IAV challenge are crucial in clearance of virus from the host during primary IAV infections (3, 4). However, neutralizing -H1 antibody responses are important in providing sterilizing immunity following subsequent homologous IAV challenge (42, 43). To evaluate whether CD8 T cells primed during influenza VLP vaccination were required for protection against subsequent lethal influenza virus infection, we first determined the efficiency of anti-CD8α depletion in a model generating a robust CD8 T cell response. We challenged mice with a sublethal dose of IAV, depleted CD8α+ cells 30 days later, and monitored the total and HA533-specific CD8 T cell response. Forty-eight hours following depletion virtually no CD8 T cells remained in the blood, dLN, lungs, or spleen of mice (Fig. S3). Fourteen weeks following CD8α depletion total CD8 T cell recovery had plateaued in the blood, dLN, lungs, and spleen of mice (Fig. S3). Further, when HA533-specific CD8 T cells were examined, we observed that anti-CD8α administration likewise depleted these cells. However, there was no significant increase in the HA533-specific cells within the recovery of the total CD8 T cell population, suggesting that IAV-induced CD8 T cell immunity had been ablated (Fig. S4).
After determining the efficiency of our depletion strategy, HA/M1-VLP-vaccinated mice were challenged 14 weeks following CD8 T cell depletion with a lethal dose of A/PR/8/34. While mice that were vaccinated and challenged without CD8 depletion survived following lethal IAV challenge, only 60% survival was observed when vaccinated mice were depleted of CD8 T cells and then lethally challenged with IAV (Fig. 4B). Further, we observed a slight, but not significant, increase in weight loss and Penh in vaccinated mice that had been depleted of CD8 T cells and allowed to recover total CD8 T cell numbers prior to lethal challenge compared to mice that were vaccinated and challenged with IAV (Fig. 4C & 4D). These data indicate that HA533-specific CD8 T cells generated following VLP vaccination are required to mediate complete protection from lethal IAV challenge. It is likely that the remaining protection observed after removal of memory CD8 T cells is due to the IAV-specific antibody previously demonstrated to be induced following VLP vaccination (16).
Figure 4. CD8 T cells primed by VLP vaccination are required for complete protection during IAV infection.
A) BALB/c mice were treated i.n with 2.5 µg VLP in 50 µl PBS or 50 µl PBS as a control. Thirty days later mice were treated i.n. and i.p. with anti-CD8α. Fourteen weeks later mice were challenged with 5LD50 A/PR/8/34. (B) Mortality, (C) morbidity, and (D) airway resistance was monitored daily for 14 days (n=4-5 mice/group).
Influenza VLPs provide protection from high dose, heterosubtypic IAV challenge
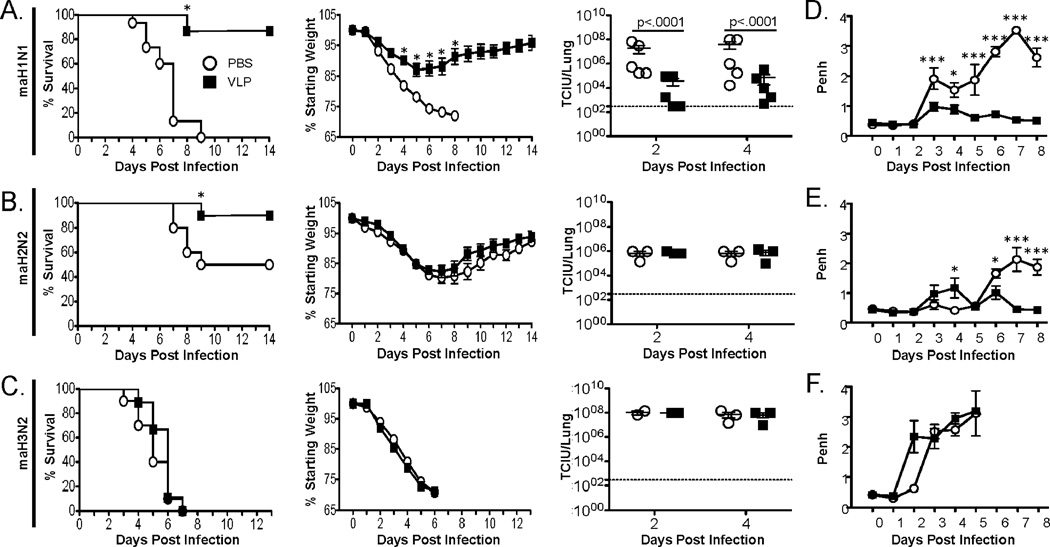
Influenza-specific CD8 T cells have been demonstrated to provide cross-protection against heterosubtypic strains of influenza virus in cases where previously generated neutralizing antibodies may not be able to control IAV infection (11, 44, 45). To determine whether CD8 T cells primed following i.n. influenza VLP administration could mediate a similar protection from heterosubtypic IAV challenge, groups of mice were vaccinated with HA/M1-VLPs and challenged 30 days later with homo- or hetero-subtypic strains of IAV. When mice were challenged with a lethal dose of homosubtypic mouse-adapted (ma) H1N1 (A/PR/8/34) following influenza VLP vaccination, 86.6% of the mice survived the challenge and had significantly reduced morbidity compared to PBS treated controls (Fig. 5A). We also observed significantly lower virus titers on days 2 and 4 post IAV infection in mice that were administered influenza VLPs i.n. compared to PBS-treated controls (Fig. 5A). To determine whether influenza VLP vaccine-induced CD8 T cells play a role in mediating protection during heterosubtypic IAV infection, we next challenged mice vaccinated i.n. with VLPs containing HA and M1 from A/PR/8/34 with maH2N2 (A/Japan/305/57). Challenge with A/Japan/305/57 avoids neutralizing antibody responses directed against HA, but a modified, cross-reactive version of the HA533 epitope is still present within A/Japan/305/57 (Table 1) (46). Following challenge with maH2N2, 50% of control mice survived infection while a significantly greater frequency of mice (90%) survived infection when they were given A/PR/8/34 HA/M1-VLPs 30 days prior to challenge (Fig. 5B). Interestingly, despite this difference in lethality we observed no difference in morbidity or virus titer at days 2 and 4 post infection (Fig. 5B). To ensure these results were specific to the conserved CD8 T cell response generated by vaccination and not due to a highly conserved component of the immune response or another non-specific effect mediated by VLP vaccination, we also challenged HA/M1 (H1) vaccinated mice with maH3N2 (A/HK/1/68). Challenge with A/HK/1/68 avoids neutralizing antibody responses along with the majority of memory CD4 and CD8 T cell responses that could have been generated following i.n. VLP administration specific for HA and M1 of A/PR/8/34 (8). Further, the HA533 epitope is altered in the maH3N2 strain such that the peptide is no longer recognized by CD8 T cells (Table 1) (47). As expected, all of the vaccinated and control mice succumbed to infection, and we observed no difference in weight loss or virus titer in the lungs of these mice (Fig 5C).
Figure 5. VLP vaccination leads to protection from mortality during heterosubtypic IAV infection.
BALB/c mice were treated i.n with 2.5 µg VLP or 50 µl PBS as a control. Thirty days later mice were challenged with 5 LD50 PR/8 (A, maH1N1) or equivalent TCIU (~5.5×104 TCIU/mouse) doses of A/Japan/305/57 (B, maH2N2) or A/HK/1/68 (C, maH3N2) and morbidity (second column) and mortality (first column) were measured daily for 14 days. Lung homogenates (n=3/group) were also collected on days 2 and 4 post infection to determine viral titers (third column). Airway resistance following challenge with (D) maH1N1, (E) maH2N2, or (F) maH3N2 was measured using a whole body plethysmograph daily for 8 days [n=6 (maH1N1 and maH2N2) =3 (maH3N2) mice/group]. The morbidity and mortality data are pooled from three independent experiments (n=10–15 mice/group). Titer data were analyzed using Student’s t-test. Morbidity data were analyzed using a two-way ANOVA followed by Bonferroni post test. Mortality data were analyzed using logrank test. *=p<.05, **=p<.01, ***=p<.001
Table I.
The HA533 Epitope
| IAV Strain | Epitope | Amino Acid Sequence |
|---|---|---|
| A/PR/8/34 (maH1N1) | HA533–541 | IYSTVASSL |
| A/JAPAN/305/57 (maH2N2) | HA529–537 | IYATVAGSL |
| A/HK/1/168 (maH3N2) | None (aa 533-541 of HA) | ISFAISCFL |
Bold residues differ from the A/PR/8/34 aa sequence
To further investigate differences that could be accounting for the survival of influenza VLP vaccinated mice challenged with maH2N2 even though no early difference in viral titer was detected, we challenged mice and monitored their airway resistance daily for 8 days using a whole body plethysmograph. We observed significantly higher Penh values in mice that were not vaccinated and challenged with maH1N1 compared to vaccinated mice on days 3–8 post infection (Fig. 5D). In contrast, we observed no difference in Penh when mice were challenged with maH3N2 following i.n. VLP vaccination or PBS administration (Fig. 5F, bottom right panel). When mice were challenged with maH2N2 following VLP vaccination, a significant reduction in Penh was observed starting on day 6 post infection compared to PBS-treated controls (Fig. 5E). This reduction in Penh correlates with the period of time in which unvaccinated mice challenged with maH2N2 succumb to infection (Fig. 5B).
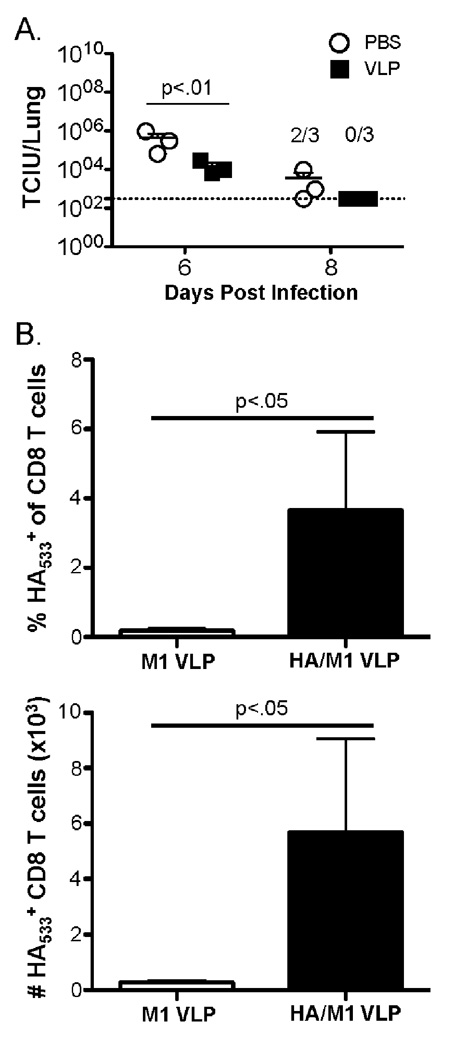
As Penh was significantly reduced at day 6 post heterosubtypic challenge in H1 influenza VLP vaccinated mice we also determined lung viral titers at days 6 and 8 post infection. At both days 6 and 8 post heterosubtypic IAV challenge virus titers were significantly reduced in mice that had been vaccinated compared to PBS controls (Fig. 6A). The lack of early control of infection, but reduced Penh and virus titers at day 6 following infection suggests early antibody-mediated clearance of virus is not leading to increased survival in HA/M1-VLP vaccinated mice challenged with maH2N2. Given this, we sought to determine whether A/PR/8/34 HA/M1-VLP vaccination lead to a significant increase in protective HA533-specific CD8 T cells following A/Japan/305/57 challenge. We detected no significant difference in HA533-specific CD8 T cells in the lungs on day 2 post infection between mice that were vaccinated with HA/M1-VLPs or control, M1-VLPs (data not shown). This finding correlates with the lack of difference in Penh and virus titers observed at day 2 post challenge. We did, however, observe a significant increase in both the frequency and number of HA533-specific CD8 T cells in the lungs of HA/M1-VLPs vs. M1-VLP vaccinated mice at day 6 post challenge (Fig. 6B). Together, the results from figures 5 and 6 strongly suggest the cross-reactive HA533-specific CD8 T cells primed by HA/M1-VLP vaccination are key to mediating protection during maH2N2 (A/Japan/305/57) challenge.
Figure 6. VLP vaccination leads to a reduction in virus titer and significant increase in HA533-specific CD8 T cells at later time points following heterosubtypic maH2N2 challenge.
BALB/c mice were treated i.n with 2.5 µg VLP or 50 µl PBS as a control. Thirty days later mice were challenged with ~5.5×104 TCIU A/Japan/305/57. A) Mice were sacrificed and lungs (n=3/group) were collected on days 6 and 8 post infection to determine viral titers. 2/3 and 0/3 indicate the number of mice with detectable IAV titers in the lungs at day 8 post infection. B) Lungs were harvested at day 6 post A/Japan/305/57 infection and tetramer specific CD8α+/CD3ɛ+ cells were identified by flow cytometry (n=4 mice/group). Data were analyzed using Student’s t test.
Discussion
Vaccination continues to be the best defense against influenza virus infection. However, whether current subunit vaccination strategies induce influenza-specific CD8 T cell responses remains unclear (17). Our findings suggest HA533-specific CD8 T cells generated following influenza VLP vaccination are important in providing full protection during lethal IAV challenge, as mice depleted of CD8 T cells displayed reduced survival and increased airway resistance compared to non-depleted, vaccinated mice (Fig. 4). While a characterization of the full breadth of the immune response generated following influenza VLP vaccination has not been completed, recent work has demonstrated the ability of influenza VLPs to generate antibodies specific to the stalk domain of A/PR/8/34 HA (48). These HA stalk antibodies were found to broadly neutralize group 1 and 2 influenza virus and have been suggested as a universal influenza vaccination candidate (49, 50). However, reduced airway resistance and virus titers following maH2N2 heterosubtypic challenge in HA/M1-VLP vaccinated mice does not occur until day 6 p.i., when we detected significantly greater HA533-specific CD8 T cell responses in the lungs (Figs. 5B & 6). This delayed protection and lack of reduction in early viral titers suggests stalk-specific antibody-mediated protection is likely not responsible for the protection observed following VLP-vaccination and heterosubtypic maH2N2 challenge. These findings, along with a loss of protection observed during homologous IAV challenge when mice have been vaccinated and subsequently depleted of IAV-reactive CD8 T cells (Fig. 4), suggests investigation of IAV vaccine candidates that elicit CD8 T cell responses will also be important in developing broadly cross-reactive IAV vaccines.
Our study demonstrates influenza VLPs drive proliferation of naïve, HA533-specific CD8 T cells in dLN as early as 2.5 days post vaccination (Fig. 2). While influenza VLP were administered herein without adjuvant, recent publications have highlighted the wide range of baculovirus and Sf9 host cell proteins present within the VLPs (48, 51). Both wild type baculovirus, and baculovirus-derived influenza VLPs administered i.n. led to similar mRNA upregulation of IFNβ and Mx1 in the lungs 6 hs post administration (48). While this indicates that baculovirus particles alone prime the system to respond, our results still suggest the protective effect we observe is specific to the influenza proteins present in the virus-like particles. HA/M1-VLPs induced proliferation of transgenic, transferred HA533-specific CD8 T cells (CL-4 cells) in dLN, while influenza VLPs containing only M1 did not (Fig. 2).
The ability of influenza VLP vaccination to prime CD8 T cell responses that play a role in protection during lethal and heterosubtypic IAV infections is surprising, especially considering these virus-like particles are non-replicative. During IAV infection, LN resident CD8α+ dendritic cells (DCs) and migratory CD103+ DCs (the known cross-presenting DC populations) are the primary DC subsets responsible for presentation of IAV-antigens to CD8 T cells within dLN (27–29, 52). While acquisition of antigen by these DC subsets can occur via direct (infection) or indirect (cross-presentation or trogocytosis) methods, evidence has demonstrated that cross-presentation alone is sufficient to induce functional CD8 T cell responses during IAV infection in vivo (53–55). Our results support the idea that cross-presentation alone is sufficient to induce functional and protective CD8 T cell responses following influenza VLP vaccination as the influenza VLPs utilized in these studies have no internal, IAV genomic material and can not replicate. Interestingly, DC activation and cross-presenting ability has been linked to type I interferon responses. Natural influenza virus infection is known to induce type I IFN within the lungs, and type I IFN has been well documented to boost the ability of DCs to activate naïve, influenza-specific CD8 T cell responses (56–60). At this time it remains unclear which mechanisms “license” cross-presentation during VLP-vaccination (61). Whether experimental induction of type I interferon at the time of influenza VLP vaccination would improve the ability of DCs in activating IAV-specific CD8 T cell responses remains to be elucidated. Furthermore, in the future it will be important to determine the APC subsets (presumably CD103+ DCs) priming this VLP-specific CD8 T cell response, and whether these APCs are similar to those responsible for activating CD8 T cells during IAV infection and live attenuated influenza vaccination. Determining the differences in the ability of DC subsets to regulate CD8 T cell responses following different methods of i.n. IAV vaccination will allow for the development of IAV vaccines that are better able to induce broadly cross-protective CD8 T cell responses.
Vaccines that generate broadly cross-protective CD8 T cell responses may aid in the development of human IAV vaccines in the future. Phase I and II clinical trials testing a baculovirus derived VLP containing HA, NA and M1 of A/California/04/2009 (H1N1) and A/Indonesia/05/2005 (H5N1) independently have demonstrated these vaccines to be safe and elicit antibody responses (62, 63). Whether these vaccinations elicit CD8 T cell responses in humans, however, remains unknown. The Immune Epitope Database and Analysis Resource identifies 456 positive T cell epitopes within the M1 protein of various IAV strains contained in the database (iedb.org). Therefore, in humans the M1 and HA proteins may elicit cross-reactive CD8 T cell populations, which could mediate protection from homo- and heterologous IAV infection in addition to HA and M1 specific antibodies. In contrast to humans, there are no identified T cell epitopes contained within the M1 protein of A/PR/8/34 in BALB/c mice (iedb.org). Given this, it is not surprising that M1 VLPs alone did not mediate protection from lethality in our BALB/c murine model (not shown). In the future it will be important to determine in human trials whether CD8 T cell responses are generated in response to VLP vaccination in addition to antibody titers, and whether these CD8 T cells are capable of killing virally infected cells.
In summary, the results presented herein demonstrate for the first time that i.n. vaccination with influenza VLPs containing HA and M1 leads to proliferation of HA533-specific CD8 T cells in dLN and subsequent accumulation of these T cells in the lungs. These HA533-specific CD8 T cells rapidly accumulate in the lungs following IAV challenge and are crucial in mediating complete protection from IAV challenge. Furthermore, influenza VLP vaccination induces heterosubtypic protection correlated with a late (day 6 p.i. and beyond) decrease in airway resistance and Penh, and a significant increase vaccine-induced CD8 T cell responses. These results support continued investigation into influenza vaccines capable of generating protective CD8 T cell responses, and the potential use of influenza VLPs as a cross-protective IAV vaccine.
Supplementary Material
Acknowledgements
We thank Dr. Jae-Min Song for technical assistance. We would also like to than Drs. John Harty, Steven Varga, and Thomas Waldschmidt for their critical evaluation of this manuscript.
Abbreviations used in this manuscript
- CL-4
clone-4
- DC
dendritic cell
- dLN
lung-draining lymph node
- HA
hemagglutinin
- IAV
influenza A virus
- i.n.
intranasal
- M1
matrix 1 protein
- ma
mouse-adapted
- NP
nucleocapsidprotein
- PBS
phosphate buffered saline
- Penh
enhanced pause
- TCIU
tissue culture infectious units
- VLP
virus-like particle
Footnotes
References
- 1.Thompson WW, Shay DK, Weintraub E, Brammer L, Bridges CB, Cox NJ, Fukuda K. Influenza-associated hospitalizations in the United States. JAMA. 2004;292:1333–1340. doi: 10.1001/jama.292.11.1333. [DOI] [PubMed] [Google Scholar]
- 2.Thompson WW, Shay DK, Weintraub E, Brammer L, Cox N, Anderson LJ, Fukuda K. Mortality associated with influenza and respiratory syncytial virus in the United States. JAMA. 2003;289:179–186. doi: 10.1001/jama.289.2.179. [DOI] [PubMed] [Google Scholar]
- 3.Topham DJ, Tripp RA, Doherty PC. CD8+ T cells clear influenza virus by perforin or Fas-dependent processes. J. Immunol. 1997;159:5197–5200. [PubMed] [Google Scholar]
- 4.Bender BS, Croghan T, Zhang L, Small PA., Jr Transgenic mice lacking class I major histocompatibility complex-restricted T cells have delayed viral clearance and increased mortality after influenza virus challenge. J. Exp. Med. 1992;175:1143–1145. doi: 10.1084/jem.175.4.1143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bodewes R, Fraaij PL, Kreijtz JH, Geelhoed-Mieras MM, Fouchier RA, Osterhaus AD, Rimmelzwaan GF. Annual influenza vaccination affects the development of heterosubtypic immunity. Vaccine. 2012;30:7407–7410. doi: 10.1016/j.vaccine.2012.04.086. [DOI] [PubMed] [Google Scholar]
- 6.Combadiere B, Vogt A, Mahe B, Costagliola D, Hadam S, Bonduelle O, Sterry W, Staszewski S, Schaefer H, Werf Svan der, Katlama C, Autran B, Blume-Peytavi U. Preferential amplification of CD8 effector-T cells after transcutaneous application of an inactivated influenza vaccine: a randomized phase I trial. PLoS One. 2010;5:e10818. doi: 10.1371/journal.pone.0010818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Blackburne BP, Hay AJ, Goldstein RA. Changing selective pressure during antigenic changes in human influenza H3. PLoS Pathog. 2008;4:e1000058. doi: 10.1371/journal.ppat.1000058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Effros RB, Doherty PC, Gerhard W, Bennink J. Generation of both cross-reactive and virus-specific T-cell populations after immunization with serologically distinct influenza A viruses. J. Exp. Med. 1977;145:557–568. doi: 10.1084/jem.145.3.557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Yap KL, Ada GL, McKenzie IF. Transfer of specific cytotoxic T lymphocytes protects mice inoculated with influenza virus. Nature. 1978;273:238–239. doi: 10.1038/273238a0. [DOI] [PubMed] [Google Scholar]
- 10.Brown LE, Kelso A. Prospects for an influenza vaccine that induces cross-protective cytotoxic T lymphocytes. Immunol. Cell Biol. 2009;87:300–308. doi: 10.1038/icb.2009.16. [DOI] [PubMed] [Google Scholar]
- 11.Hillaire ML, Osterhaus AD, Rimmelzwaan GF. Induction of virus-specific cytotoxic T lymphocytes as a basis for the development of broadly protective influenza vaccines. J. Biomed. Biotechnol. 2011;2011:939860. doi: 10.1155/2011/939860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Quan FS, Huang C, Compans RW, Kang SM. Virus-like particle vaccine induces protective immunity against homologous and heterologous strains of influenza virus. J. Virol. 2007;81:3514–3524. doi: 10.1128/JVI.02052-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bright RA, Carter DM, Daniluk S, Toapanta FR, Ahmad A, Gavrilov V, Massare M, Pushko P, Mytle N, Rowe T, Smith G, Ross TM. Influenza virus-like particles elicit broader immune responses than whole virion inactivated influenza virus or recombinant hemagglutinin. Vaccine. 2007;25:3871–3878. doi: 10.1016/j.vaccine.2007.01.106. [DOI] [PubMed] [Google Scholar]
- 14.Quan FS, Kim MC, Lee BJ, Song JM, Compans RW, Kang SM. Influenza M1 VLPs containing neuraminidase induce heterosubtypic cross-protection. Virology. 2012;430:127–135. doi: 10.1016/j.virol.2012.05.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Quan FS, Steinhauer D, Huang C, Ross TM, Compans RW, Kang SM. A bivalent influenza VLP vaccine confers complete inhibition of virus replication in lungs. Vaccine. 2008;26:3352–3361. doi: 10.1016/j.vaccine.2008.03.055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Quan FS, Yoo DG, Song JM, Clements JD, Compans RW, Kang SM. Kinetics of immune responses to influenza virus-like particles and dose-dependence of protection with a single vaccination. J. Virol. 2009;83:4489–4497. doi: 10.1128/JVI.02035-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Song H, Wittman V, Byers A, Tapia T, Zhou B, Warren W, Heaton P, Connolly K. In vitro stimulation of human influenza-specific CD8+ T cells by dendritic cells pulsed with an influenza virus-like particle (VLP) vaccine. Vaccine. 2010;28:5524–5532. doi: 10.1016/j.vaccine.2010.06.044. [DOI] [PubMed] [Google Scholar]
- 18.Legge KL, Braciale TJ. Lymph node dendritic cells control CD8+ T cell responses through regulated FasL expression. Immunity. 2005;23:649–659. doi: 10.1016/j.immuni.2005.11.006. [DOI] [PubMed] [Google Scholar]
- 19.Legge KL, Braciale TJ. Accelerated migration of respiratory dendritic cells to the regional lymph nodes is limited to the early phase of pulmonary infection. Immunity. 2003;18:265–277. doi: 10.1016/s1074-7613(03)00023-2. [DOI] [PubMed] [Google Scholar]
- 20.McGill J, Van Rooijen N, Legge KL. Protective influenza-specific CD8 T cell responses require interactions with dendritic cells in the lungs. J. Exp. Med. 2008;205:1635–1646. doi: 10.1084/jem.20080314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Wlodarczyk MF, Kraft AR, Chen HD, Kenney LL, Selin LK. Anti-IFN-gamma and peptide-tolerization therapies inhibit acute lung injury induced by cross-reactive influenza A-specific memory T cells. J. Immunol. 2013;190:2736–2746. doi: 10.4049/jimmunol.1201936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Langlois RA, Meyerholz DK, Coleman RA, Cook RT, Waldschmidt TJ, Legge KL. Oseltamivir treatment prevents the increased influenza virus disease severity and lethality occurring in chronic ethanol consuming mice. Alcohol. Clin. Exp. Res. 2010;34:1425–1431. doi: 10.1111/j.1530-0277.2010.01226.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Castilow EM, Legge KL, Varga SM. Cutting edge: Eosinophils do not contribute to respiratory syncytial virus vaccine-enhanced disease. J. Immunol. 2008;181:6692–6696. doi: 10.4049/jimmunol.181.10.6692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Stetson DB, Mohrs M, Mallet-Designe V, Teyton L, Locksley RM. Rapid expansion and IL-4 expression by Leishmania-specific naive helper T cells in vivo. Immunity. 2002;17:191–200. doi: 10.1016/s1074-7613(02)00363-1. [DOI] [PubMed] [Google Scholar]
- 25.Moon JJ, Chu HH, Pepper M, McSorley SJ, Jameson SC, Kedl RM, Jenkins MK. Naive CD4(+) T cell frequency varies for different epitopes and predicts repertoire diversity and response magnitude. Immunity. 2007;27:203–213. doi: 10.1016/j.immuni.2007.07.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Obar JJ, Khanna KM, Lefrancois L. Endogenous naive CD8+ T cell precursor frequency regulates primary and memory responses to infection. Immunity. 2008;28:859–869. doi: 10.1016/j.immuni.2008.04.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Belz GT, Smith CM, Kleinert L, Reading P, Brooks A, Shortman K, Carbone FR, Heath WR. Distinct migrating and nonmigrating dendritic cell populations are involved in MHC class I-restricted antigen presentation after lung infection with virus. Proc. Natl. Acad. Sci. U.S.A. 2004;101:8670–8675. doi: 10.1073/pnas.0402644101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.GeurtsvanKessel CH, Willart MA, van Rijt LS, Muskens F, Kool M, Baas C, Thielemans K, Bennett C, Clausen BE, Hoogsteden HC, Osterhaus AD, Rimmelzwaan GF, Lambrecht BN. Clearance of influenza virus from the lung depends on migratory langerin+CD11b- but not plasmacytoid dendritic cells. J. Exp. Med. 2008;205:1621–1634. doi: 10.1084/jem.20071365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kim TS, Braciale TJ. Respiratory dendritic cell subsets differ in their capacity to support the induction of virus-specific cytotoxic CD8+ T cell responses. PLoS One. 2009;4:e4204. doi: 10.1371/journal.pone.0004204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Brincks EL, Katewa A, Kucaba TA, Griffith TS, Legge KL. CD8 T cells utilize TRAIL to control influenza virus infection. J. Immunol. 2008;181:4918–4925. doi: 10.4049/jimmunol.181.7.4918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.McGill J, Legge KL. Cutting edge: contribution of lung-resident T cell proliferation to the overall magnitude of the antigen-specific CD8 T cell response in the lungs following murine influenza virus infection. J. Immunol. 2009;183:4177–4181. doi: 10.4049/jimmunol.0901109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Moyron-Quiroz JE, Rangel-Moreno J, Kusser K, Hartson L, Sprague F, Goodrich S, Woodland DL, Lund FE, Randall TD. Role of inducible bronchus associated lymphoid tissue (iBALT) in respiratory immunity. Nat. Med. 2004;10:927–934. doi: 10.1038/nm1091. [DOI] [PubMed] [Google Scholar]
- 33.Marshall DR, Turner SJ, Belz GT, Wingo S, Andreansky S, Sangster MY, Riberdy JM, Liu T, Tan M, Doherty PC. Measuring the diaspora for virus-specific CD8+ T cells. Proc. Natl. Acad. Sci. U.S.A. 2001;98:6313–6318. doi: 10.1073/pnas.101132698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kohlmeier JE, Miller SC, Woodland DL. Cutting edge: Antigen is not required for the activation and maintenance of virus-specific memory CD8+ T cells in the lung airways. J. Immunol. 2007;178:4721–4725. doi: 10.4049/jimmunol.178.8.4721. [DOI] [PubMed] [Google Scholar]
- 35.Hogan RJ, Usherwood EJ, Zhong W, Roberts AA, Dutton RW, Harmsen AG, Woodland DL. Activated antigen-specific CD8+ T cells persist in the lungs following recovery from respiratory virus infections. J. Immunol. 2001;166:1813–1822. doi: 10.4049/jimmunol.166.3.1813. [DOI] [PubMed] [Google Scholar]
- 36.Roberts AD, Woodland DL. Cutting edge: effector memory CD8+ T cells play a prominent role in recall responses to secondary viral infection in the lung. J. Immunol. 2004;172:6533–6537. doi: 10.4049/jimmunol.172.11.6533. [DOI] [PubMed] [Google Scholar]
- 37.Sallusto F, Lenig D, Forster R, Lipp M, Lanzavecchia A. Two subsets of memory T lymphocytes with distinct homing potentials and effector functions. Nature. 1999;401:708–712. doi: 10.1038/44385. [DOI] [PubMed] [Google Scholar]
- 38.Wherry EJ, Teichgraber V, Becker TC, Masopust D, Kaech SM, Antia R, von Andrian UH, Ahmed R. Lineage relationship and protective immunity of memory CD8 T cell subsets. Nat. Immunol. 2003;4:225–234. doi: 10.1038/ni889. [DOI] [PubMed] [Google Scholar]
- 39.Kaech SM, Ahmed R. Memory CD8+ T cell differentiation: initial antigen encounter triggers a developmental program in naive cells. Nat. Immunol. 2001;2:415–422. doi: 10.1038/87720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Topham DJ, Castrucci MR, Wingo FS, Belz GT, Doherty PC. The role of antigen in the localization of naive, acutely activated, and memory CD8(+) T cells to the lung during influenza pneumonia. J. Immunol. 2001;167:6983–6990. doi: 10.4049/jimmunol.167.12.6983. [DOI] [PubMed] [Google Scholar]
- 41.Yewdell JW, Bennink JR. Immunodominance in major histocompatibility complex class I-restricted T lymphocyte responses. Annu. Rev. Immunol. 1999;17:51–88. doi: 10.1146/annurev.immunol.17.1.51. [DOI] [PubMed] [Google Scholar]
- 42.Virelizier JL. Host defenses against influenza virus: the role of anti-hemagglutinin antibody. J. Immunol. 1975;115:434–439. [PubMed] [Google Scholar]
- 43.Krause JC, Tsibane T, Tumpey TM, Huffman CJ, Albrecht R, Blum DL, Ramos I, Fernandez-Sesma A, Edwards KM, Garcia-Sastre A, Basler CF, Crowe JE., Jr Human monoclonal antibodies to pandemic 1957 H2N2 and pandemic 1968 H3N2 influenza viruses. J. Virol. 2012;86:6334–6340. doi: 10.1128/JVI.07158-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Epstein SL, Price GE. Cross-protective immunity to influenza A viruses. Expert Rev. Vaccines. 2010;9:1325–1341. doi: 10.1586/erv.10.123. [DOI] [PubMed] [Google Scholar]
- 45.Grebe KM, Yewdell JW, Bennink JR. Heterosubtypic immunity to influenza A virus: where do we stand? Microbes Infect. 2008;10:1024–1029. doi: 10.1016/j.micinf.2008.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Ream RM, Sun J, Braciale TJ. Stimulation of naive CD8+ T cells by a variant viral epitope induces activation and enhanced apoptosis. J. Immunol. 2010;184:2401–2409. doi: 10.4049/jimmunol.0902448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Hufford MM, Kim TS, Sun J, Braciale TJ. Antiviral CD8+ T cell effector activities in situ are regulated by target cell type. J Exp. Med. 2011;208:167–180. doi: 10.1084/jem.20101850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Margine I, Martinez-Gil L, Chou YY, Krammer F. Residual baculovirus in insect cell-derived influenza virus-like particle preparations enhances immunogenicity. PLoS One. 2012;7:e51559. doi: 10.1371/journal.pone.0051559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Corti D, Voss J, Gamblin SJ, Codoni G, Macagno A, Jarrossay D, Vachieri SG, Pinna D, Minola A, Vanzetta F, Silacci C, Fernandez-Rodriguez BM, Agatic G, Bianchi S, Giacchetto-Sasselli I, Calder L, Sallusto F, Collins P, Haire LF, Temperton N, Langedijk JP, Skehel JJ, Lanzavecchia A. A neutralizing antibody selected from plasma cells that binds to group 1 and group 2 influenza A hemagglutinins. Science. 2011;333:850–856. doi: 10.1126/science.1205669. [DOI] [PubMed] [Google Scholar]
- 50.Ekiert DC, Friesen RH, Bhabha G, Kwaks T, Jongeneelen M, Yu W, Ophorst C, Cox F, Korse HJ, Brandenburg B, Vogels R, Brakenhoff JP, Kompier R, Koldijk MH, Cornelissen LA, Poon LL, Peiris M, Koudstaal W, Wilson IA, Goudsmit J. A highly conserved neutralizing epitope on group 2 influenza A viruses. Science. 2011;333:843–850. doi: 10.1126/science.1204839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Song JM, Choi CW, Kwon SO, Compans RW, Kang SM, Kim SI. Proteomic characterization of influenza H5N1 virus-like particles and their protective immunogenicity. J Proteome Res. 2011;10:3450–3459. doi: 10.1021/pr200086v. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Ballesteros-Tato A, Leon B, Lund FE, Randall TD. Temporal changes in dendritic cell subsets, cross-priming and costimulation via CD70 control CD8(+) T cell responses to influenza. Nat. Immunol. 2010;11:216–224. doi: 10.1038/ni.1838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Langlois RA, Varble A, Chua MA, Garcia-Sastre A, Tenoever BR. Hematopoietic-specific targeting of influenza A virus reveals replication requirements for induction of antiviral immune responses. Proc. Natl. Acad. Sci. U.S.A. 2012;109:12117–12122. doi: 10.1073/pnas.1206039109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.den Haan JM, Lehar SM, Bevan MJ. CD8(+) but not CD8(-) dendritic cells cross-prime cytotoxic T cells in vivo. J. Exp. Med. 2000;192:1685–1696. doi: 10.1084/jem.192.12.1685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Norbury CC, Princiotta MF, Bacik I, Brutkiewicz RR, Wood P, Elliott T, Bennink JR, Yewdell JW. Multiple antigen-specific processing pathways for activating naive CD8+ T cells in vivo. J. Immunol. 2001;166:4355–4362. doi: 10.4049/jimmunol.166.7.4355. [DOI] [PubMed] [Google Scholar]
- 56.Cella M, Salio M, Sakakibara Y, Langen H, Julkunen I, Lanzavecchia A. Maturation, activation, and protection of dendritic cells induced by double-stranded RNA. J. Exp. Med. 1999;189:821–829. doi: 10.1084/jem.189.5.821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Lopez CB, Garcia-Sastre A, Williams BR, Moran TM. Type I interferon induction pathway, but not released interferon, participates in the maturation of dendritic cells induced by negative-strand RNA viruses. J. Infect. Dis. 2003;187:1126–1136. doi: 10.1086/368381. [DOI] [PubMed] [Google Scholar]
- 58.Moltedo B, Li W, Yount JS, Moran TM. Unique type I interferon responses determine the functional fate of migratory lung dendritic cells during influenza virus infection. PLoS Pathog. 2011;7:e1002345. doi: 10.1371/journal.ppat.1002345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Beignon AS, Skoberne M, Bhardwaj N. Type I interferons promote cross-priming: more functions for old cytokines. Nat. Immunol. 2003;4:939–941. doi: 10.1038/ni1003-939. [DOI] [PubMed] [Google Scholar]
- 60.Diamond MS, Kinder M, Matsushita H, Mashayekhi M, Dunn GP, Archambault JM, Lee H, Arthur CD, White JM, Kalinke U, Murphy KM, Schreiber RD. Type I interferon is selectively required by dendritic cells for immune rejection of tumors. J. Exp. Med. 2011;208:1989–2003. doi: 10.1084/jem.20101158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Dresch C, Leverrier Y, Marvel J, Shortman K. Development of antigen cross-presentation capacity in dendritic cells. Trends Immunol. 2012;33:381–388. doi: 10.1016/j.it.2012.04.009. [DOI] [PubMed] [Google Scholar]
- 62.Khurana S, Wu J, Verma N, Verma S, Raghunandan R, Manischewitz J, King LR, Kpamegan E, Pincus S, Smith G, Glenn G, Golding H. H5N1 virus-like particle vaccine elicits cross-reactive neutralizing antibodies that preferentially bind to the oligomeric form of influenza virus hemagglutinin in humans. J. Virol. 2011;85:10945–10954. doi: 10.1128/JVI.05406-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Lopez-Macias C, Ferat-Osorio E, Tenorio-Calvo A, Isibasi A, Talavera J, Arteaga-Ruiz O, Arriaga-Pizano L, Hickman SP, Allende M, Lenhard K, Pincus S, Connolly K, Raghunandan R, Smith G, Glenn G. Safety and immunogenicity of a virus-like particle pandemic influenza A (H1N1) 2009 vaccine in a blinded, randomized, placebo-controlled trial of adults in Mexico. Vaccine. 2011;29:7826–7834. doi: 10.1016/j.vaccine.2011.07.099. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.