Abstract
Background
Abnormal posture (AP) is often seen in Parkinson's disease (PD), and marked forms known as dropped head syndrome and camptocormia encumber daily living activities. Unlike other motor disabilities such as bradykinesia or muscular rigidity, AP is not always improved but rather deteriorated by PD medication.
Purpose
To clarify factors associated with neck and thoracolumbar AP.
Methods
Neck flexion (NF) and thoracolumbar (TL) angles were measured in 216 consecutive PD patients and 175 elderly healthy controls. The differences in NF and TL angles between PD patients and controls were designated as ΔNFA and ΔTLA, respectively. The association of ΔNFA or ΔTLA and predictable factors such as age, sex, duration of PD, Hoehn Yahr (H–Y) stage, Unified Parkinson's Disease Rating Scale Part 3 (UPDRS-3), daily dose of dopamine agonists, and comorbid orthopedic spinal lesions was investigated in PD patients. Patients were divided into quartiles according to ΔNFA or ΔTLA. The association between predictable factors and ΔNFA or ΔTLA was estimated as odds ratio (OR), comparing with the lowest quartile as the reference by multivariate regression analysis.
Results
Compared with controls, distributions of all three posture angles were significantly shifted rightward in PD patients. Although there were no difference in UPDRS-3 scores in the quartiles of ΔNFA, the highest quartile was associated with H–Y stage ≥3 [OR 2.99, 95% confidence interval (CI) 1.33–6.70, p = 0.008] after adjustment for age, sex and comorbid orthopedic spinal lesions. The highest quartile of ΔTLA was associated with comorbid orthopedic spinal lesions [OR 5.83 (1.42–23.8), p = 0.014], and UPDRS-3 score [OR 3.04 (1.80–5.15)/10 points, p<0.0001].
Conclusion
Thoraco-lumbar AP was associated with UPDRS-3 scores and orthopedic spinal lesions, and in contrast, neck AP was not associated with these factors, suggesting that they had different pathomechanisms.
Introduction
Abnormal posture (AP) is a frequent complication of Parkinson's disease (PD), and severe AP such as dropped head syndrome and camptocormia (marked bending of thoracolumbar spine) encumber the activities of daily living. To explore the pathogenesis of AP, the following issues should be addressed: disease specificity, definition, coexistence of two or more types of AP, and relationship to other extrapyramidal motor disturbances of PD. Bending posture, including a marked form called bent spine syndrome, is seen in elderly people without PD, suggesting that AP could occur in people without PD [1]. Therefore, comparison of body angles between PD patients and elderly healthy people is required. A consistent definition of AP including dropped head syndrome or camptocormia has not been established because the distribution of body angles has not been studied, and cut-off points between normal postures and AP cannot be set. Although various types of AP, anterocollis (dropped head), scoliosis, and camptocormia have been reported in patients with PD, it is unclear whether they are related to each other. A combination of two of more APs, such as dropped head and camptocormia, can be observed simultaneously; however, it has not been elucidated whether they are caused by the same pathological process or occur coincidentally. AP is thought to be an extrapyramidal sign because it can appear in untreated patients, as described in the historic paper by James Parkinson [2], and many clinical factors regarding severity of PD have been identified as risk factors for camptocormia [3], [4], [5], [6]. However, recent case reports indicate the association of dopaminergic replacement therapy, especially by dopamine agonists, and anterocollis [7], [8], [9], [10], [11], [12] or Pisa syndrome [13], [14], [15], [16], suggesting a drug-related phenomenon. Camptocormia is thought to be caused by muscular rigidity [17], [18] or axial dystonia [5], [19], [20], at least in the early stages of PD. In addition, myopathy [21], [22] and myositis [23] in the relevant muscles are also thought to be involved in AP. As described above, the precise mechanisms of AP have not been elucidated, and identification of factors that are associated with body angles is required to explore the pathogenesis of AP.
In the present study, three body angles, neck flexion (NF), fore-bent (FB) and lateral-bent (LB) angles were surveyed in PD patients and in healthy controls. The distribution of the body angles was compared between PD patients and controls. Clinical characteristics were investigated by the quartiles of body angle difference from the mean angle in the controls. The association of predictable variables was investigated using multinomial regression models. With reference to the lowest quartile of body angles, odds ratios (ORs) of predictable variables were estimated using multivariate multinomial regression.
Materials and Methods
Participants
From March to July 2010, we enrolled 221 consecutive patients with PD who were able to keep a standing position without any assistance, and were treated at the Center for Parkinson's Disease and Related Disorders, Utano National Hospital, Kyoto, Japan. A diagnosis of PD was made according to Steps 1 and 2 of the United Kingdom Parkinson's Disease Society Brain Bank Clinical Diagnostic Criteria [24]. AP can be seen in multiple system atrophy (MSA) [25] or progressive supranuclear palsy (PSP) [26]; therefore, patients with the possibility of MSA or PSP were excluded. Patients with possible or probable MSA according to the second consensus statement on MSA diagnosis [27] were excluded, and in addition, 1.5 Tesla brain magnetic resonance imaging (MRI) was performed in all individuals to rule out the possibility of MSA. Those with atrophy or signal changes in the putamen or middle cerebellar peduncles, atrophy of the cerebellum, or cross sign of the pons were excluded. Patients with possible or probable PSP according to the clinical criteria of the National Institute for Neurological Diseases and Stroke/Society for Progressive Supranuclear Palsy [28] were excluded, and those with atrophy of the midbrain tegmentum were excluded using brain MRI. Patients who had undergone deep brain stimulation were excluded because it could be related to AP [29], [30].
As healthy elderly controls, we recruited people registered with the Employment Service Center for Elderly People in Kyoto (a public registration center for small jobs or volunteers in the community) and spouses of PD patients who gave informed consent for the study. Posture angles were measured using the same procedures as for the patients.
The study was approved by the National Hospital Organization Utano Hospital Review Board, and all eligible individuals were informed of the purpose and methods of the study, and all of them provided informed consent. According to the comment from the committee, written consent was not required but oral consent was obtained because of the observational nature of the study and the photographs were not recognizable. The study investigators documented the oral consent of the participants in their medical records, and the committee approved the procedure to document oral consent.
Measurement of NF, FB and LB angles
NF, FB and LB angles were measured on photographs of the lateral and back views of the participants in an upright position (in an “ON” period if the participants had motor fluctuations). On lateral view photographs, the angle between the two crossing lines—the line connecting the external acoustic foramen and the acromion, and the line connecting the acromion and the grater trochanter—was defined as the NF angle. Similarly, FB angle was the angle between the line connecting the acromion and the greater trochanter and a vertical line. On back view photographs, the angle between the line connecting the posterior process of the seventh cervical vertebra and that of the fifth lumbar vertebra and a vertical line was defined as the LB angle (Figure S1). NF and FB angles toward flexion and extension were expressed as positive and negative values, respectively, and LB angle was an absolute value because bending toward left or right sides occurred by chance.
Data collection
Clinical data including age, sex, duration of PD, PD onset age, initial symptoms (tremor-dominant or not), Hoehn and Yahr (H–Y) stage, history of psychosis, and history of agonist-related AP were collected as possible predictors of AP. History of agonist-related AP was defined when AP occurred after dose escalation of dopamine agonists and recovered after discontinuation or de-escalation. In addition, scores on the Unified Parkinson's Disease Rating Scale Part 3 (UPDRS-3) were collected. Comorbid orthopedic spinal lesions (compression fracture of the vertebrae, disc hernia, or spondylolisthesis of the vertebrae) were diagnosed on plain X-ray or MRI performed in all participants within 6 months before the acquisition of body angle photographs. Information about regular physical exercise prior to entry was extracted from clinical records.
Daily doses of dopamine agonists and use of selegiline and amantadine at entry were also recorded. L-Dopa dose was calculated using the following formula: 1.0× regular levodopa dose or 1.25×regular levodopa dose if taking entacapone. The dose of dopamine agonists was calculated as the levodopa equivalent dose (LDED) according to the formula: LDED (mg) = pramipexole (mg) ×67 + ropinirole (mg) ×25 + pergolide (μg) ×67 + cabergoline (mg) ×67 + bromocriptine (mg) ×10 + talipexole (mg) ×67.
Definition of body angle difference between PD and controls
The differences in NF angle (ΔNFA) and TL angle (ΔTLA) between PD patients and healthy controls were evaluated according to the following formulae:
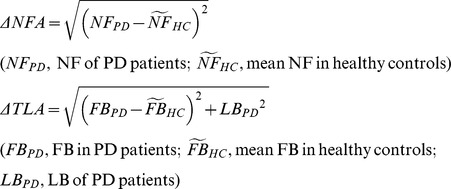 |
In addition FB angle (ΔFBA) was also investigated because there may be quite different mechanisms in lateral bending and antero-posterior bending APs.
Variables
Age, duration, onset age, daily dose of L-Dopa, dopamine agonists, and UPDRS-3 scores were regarded as scale variables. Sex, initial symptoms, H–Y stage (1–2 vs. 3–4), history of psychosis, history of agonist-related AP, orthopedic spinal lesions, and use/non-use of selegiline and amantadine were regarded as dichotomous variables.
Statistical analysis
NF, FB and LB angles were compared between patients with PD and healthy elderly controls by Mann–Whitney U test because of non-Gaussian distributions. ΔNFA and ΔTLA were divided into quartiles. The association of predictable variables and ΔNFA or ΔTLA was statistically analyzed using multivariate multinomial regression models. The association was estimated as OR, compared with the lowest quartile as the reference. After adjustment for age, sex, and presence of orthopedic spinal lesions, suitable predictable variables were selected by forward stepwise likelihood test. A value of p<0.05 was considered statistically significant. Statistical analyses were performed using the statistical software program IBM SPSS Statistics version 19.0. Results are presented as mean (standard deviation; SD).
Results
PD patients and elderly healthy controls
As controls, 91 men and 84 women aged 70.0 (7.1) and 69.7 (8.2) years, respectively, gave informed consent and their body angles were measured. In the controls, NF angle was 21.1° (10.6) in men and 11.8° (10.5) in women, and FB angle was –5.5° (4.0) in men and –3.8° (3.3) in women. There were significant differences in NF and FB angles between male and female controls (p<0.001 and p = 0.005). Due to significant sex differences in the angles in the controls, ΔNFA, ΔTLA and ΔFBA were calculated after separation by sex.
Among 221 enrolled patients with PD, five were eliminated because of deep brain stimulation (n = 2) and missing data (n = 3) and the remaining 216 patients were analyzed. In 216 PD patients (83 men and 133 women) with a mean age of 69.0 (9.0) years, PD duration was 8.0 (4.9) years, and onset age was 61.0 (9.7) years. Ninety-nine patients (45.8%) had tremor-dominant onset, and 116 (53.7%) were H–Y 3 or 4 and UPDRS-3 score was 20.6 (10.8). Orthopedic spinal lesions on X-ray or MRI were found in 41 (19.0%) patients. Sixty-one patients (28.2%) experienced psychosis and 22 (10.2%) had a history of agonist-related AP. Mean daily dose of L-Dopa and dopamine agonists (LDED) was 392.7 (187) mg and 103.7 (135.3) mg, respectively. Mean NF, FB and LB angle was 22.9° (14.9), 3.9° (10.0) and 3.2° (3.5) degrees, respectively.
Distributions of body angles in PD patients and controls
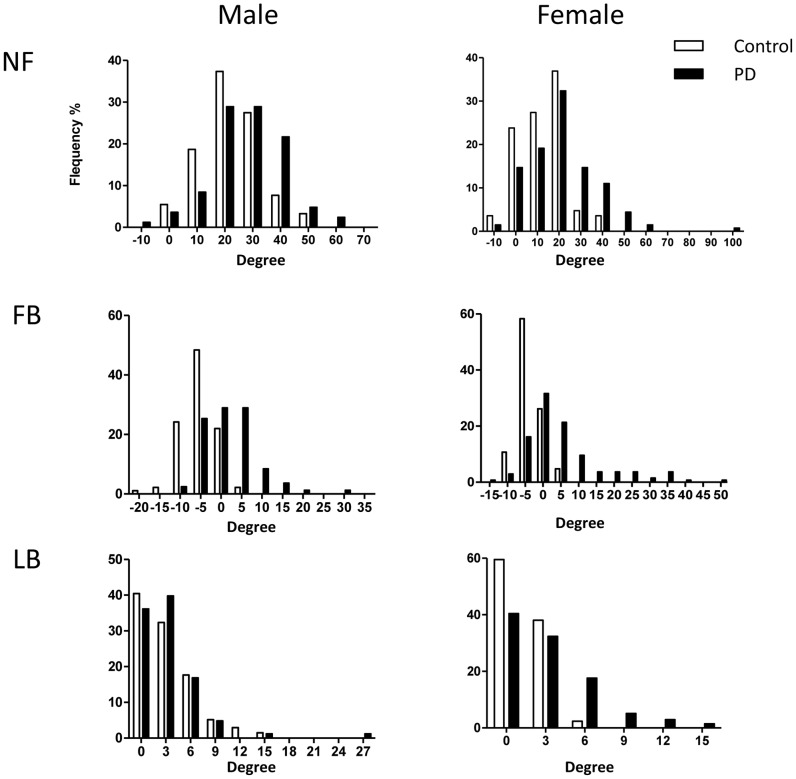
Distribution of NF, FB and LB angles in patients with PD and healthy controls is shown by sex in Figure 1. The distribution shifted rightward in the PD patients compared with the controls. All three posture angles in PD patients were significantly larger than in the controls in men and women (male NF, p = 0.0003; male FB, p<0.0001; male LB, p<0.0001; female NF, p = 0.0001; female FB, p<0.0001; female LB, p<0.0001). It was difficult to establish a cut-off point for AP because of the continuous distribution of the body angles in patients with PD.
Figure 1. Surveyed posture angles in healthy controls (n = 175) and patients with PD (n = 216).
NF angle and FB angle were distributed in a bell shape. They were shifted rightward in PD patients compared with healthy controls.
Factors associated with body angles in PD
There was no correlation between ΔNFA and ΔTLA (Pearson's correlation 0.066, p = 0.336). The demographic data by the quartiles of ΔNFA or ΔTLA are shown in Table 1 and Table 2. As for NFA (Table 1), H–Y and history of agonist-related AP differed significantly between the quartiles. Interestingly mean UPDRS-3 scores were very similar in the quartiles of ΔNFA as shown in Table 1, suggesting that severity of neck flexion AP were independent of overall motor disability.
Table 1. Demographic data by quartiles of neck-flexion angle.
| 1st quartile | 2nd quartile | 3rd quartile | 4th quartile | |||||||||||||||
| n = 60 | n = 50 | n = 50 | n = 56 | |||||||||||||||
| (0.1–4.9) | (5.1–9.9) | (10.2–16.9) | (17.2–83.2) | p | ||||||||||||||
| Age (Y), mean (SEM) | 69.4 | ( | 1.1 | ) | 68.1 | ( | 1.4 | ) | 67.8 | ( | 1.3 | ) | 70.3 | ( | 1.1 | ) | 0.45 | |
| Sex, n (%) | Female Male | 40 20 | ( ( | 66.7% 33.3% | ) ) | 25 25 | ( ( | 50.0% 50.0% | ) ) | 32 18 | ( ( | 64.0% 36.0% | ) ) | 36 20 | ( ( | 64.3% 35.7% | ) ) | 0.285 |
| Duration (Y), mean (SEM) | 7.7 | ( | 0.7 | ) | 7.2 | ( | 0.7 | ) | 8.3 | ( | 0.6 | ) | 8.6 | ( | 0.6 | ) | 0.44 | |
| Onset age(Y) | 61.8 | ( | 1.3 | ) | 60.9 | ( | 1.4 | ) | 59.5 | ( | 1.5 | ) | 61.7 | ( | 1.2 | ) | 0.61 | |
| Initial symptoms | Tremor dominant Others | 25 35 | ( ( | 41.7% 58.3% | ) ) | 25 25 | ( ( | 50.0% 50.0% | ) ) | 22 28 | ( ( | 44.0% 56.0% | ) ) | 27 29 | ( ( | 48.2% 51.8% | ) ) | 0.81 |
| H-Y | 1, 2 | 32 | ( | 53.3% | ) | 30 | ( | 60.0% | ) | 22 | ( | 44.0% | ) | 16 | ( | 28.6% | ) | 0.007 |
| 3, 4 | 28 | ( | 46.7% | ) | 20 | ( | 40.0% | ) | 28 | ( | 56.0% | ) | 40 | ( | 71.4% | ) | ||
| UPDRS-3, mean (SEM) | 20.3 | ( | 1.6 | ) | 21.1 | ( | 1.6 | ) | 20.7 | ( | 1.6 | ) | 20.4 | ( | 1.0 | ) | 0.98 | |
| Orthopedic spinal lesions | Yes No | 12 48 | ( ( | 20.0% 80.0% | )) | 9 41 | ( ( | 18.0% 82.0% | ) ) | 8 42 | ( ( | 16.0% 84.0% | ) ) | 12 44 | ( ( | 21.4% 78.6% | ) ) | 0.90 |
| History of psychosis | Yes No | 18 42 | ( ( | 30.0% 70.0% | ) ) | 10 40 | ( ( | 20.0% 80.0% | ) ) | 14 36 | ( ( | 28.0% 72.0% | ) ) | 19 37 | ( ( | 33.9% 66.1% | ) ) | 0.45 |
| Agonist-related history | Yes No | 9 51 | ( ( | 15.0% 85.0% | ) ) | 2 48 | ( ( | 4.0% 96.0% | ) ) | 2 48 | ( ( | 4.0% 96.0% | ) ) | 9 47 | ( ( | 16.1% 83.9% | ) ) | 0.0498 |
| Dopa, mean (SEM) | mg/day | 384 | ( | 27.1 | ) | 375 | ( | 27 | ) | 402 | ( | 27 | ) | 411 | ( | 22 | ) | 0.75 |
| Agonist LDED, mean (SEM) | mg/day | 104 | ( | 20 | ) | 105 | ( | 18 | ) | 111 | ( | 21 | ) | 96 | ( | 15 | ) | 0.95 |
| Dopa + Agonist, mean (SEM) | mg/day | 488 | ( | 32 | ) | 480 | ( | 31 | ) | 512 | ( | 35 | ) | 506 | ( | 26 | ) | 0.87 |
| Selegiline Use | Use No use | 23 37 | ( ( | 38.3% 61.7% | ) ) | 23 27 | ( ( | 46.0% 54.0% | ) ) | 20 30 | ( ( | 40.0% 60.0% | ) ) | 29 27 | ( ( | 51.8% 48.2% | ) ) | 0.46 |
| Amantadine Use | Use No use | 19 41 | ( ( | 31.7% 68.3% | ) ) | 7 43 | ( ( | 14.0% 86.0% | ) ) | 12 38 | ( ( | 24.0% 76.0% | ) ) | 15 41 | ( ( | 26.8% 73.2% | ) ) | 0.19 |
| Rehabilitation | Yes No | 9 51 | ( ( | 15.0% 85.0% | ) ) | 6 44 | ( ( | 12.0% 88.0% | ) ) | 8 42 | ( ( | 16.0% 84.0% | ) ) | 11 45 | ( ( | 19.6% 80.4% | ) ) | 0.75 |
p was calculated by ANOVA(scale variables) or by Pearson Chi square test (categorical variables).
Table 2. Demographic data by quartiles of thoracolumbar angle.
| 1st quartile | 2nd quartile | 3rd quartile | 4th quartile | |||||||||||||||
| n = 56 | n = 51 | n = 52 | n = 57 | |||||||||||||||
| (0.2–3.9) | (4.0–7.1) | (7.4–11.2) | (11.5–54) | p | ||||||||||||||
| Age (Y), mean (SEM) | 66.7 | ( | 1.2 | ) | 66.6 | ( | 1.5 | ) | 71.1 | ( | 1.2 | ) | 71.4 | ( | 1.0 | ) | 0.003 | |
| Sex, n (%) | Female Male | 35 21 | ( ( | 62.5% 37.5% | ) ) | 30 21 | ( ( | 58.8% 41.2% | ) ) | 29 23 | ( ( | 55.8% 44.2% | ) ) | 39 18 | ( ( | 68.4% 31.6% | ) ) | 0.561 |
| Duration (Y), mean (SEM) | 6.9 | ( | 0.5 | ) | 7.1 | ( | 0.6 | ) | 8.2 | ( | 0.6 | ) | 9.5 | ( | 0.8 | ) | 0.021 | |
| Onset age(Y) | 59.8 | ( | 1.3 | ) | 59.5 | ( | 1.4 | ) | 62.9 | ( | 1.4 | ) | 61.9 | ( | 1.1 | ) | 0.21 | |
| Initial symptoms | Tremor dominant Others | 29 27 | ( ( | 51.8% 48.2% | ) ) | 24 27 | ( ( | 47.1% 52.9% | ) ) | 25 27 | ( ( | 48.1% 51.9% | ) ) | 21 36 | ( ( | 36.8% 63.2% | ) ) | 0.42 |
| H-Y | 1, 2 3, 4 | 32 24 | ( ( | 57.1% 42.9% | ) ) | 31 20 | ( ( | 60.8% 39.2% | ) ) | 23 29 | ( ( | 44.2% 55.8% | ) ) | 14 43 | ( ( | 24.6% 75.4% | ) ) | 0.0005 |
| UPDRS-3, mean (SEM) | 16.2 | ( | 1.2 | ) | 18.7 | ( | 1.4 | ) | 19.7 | ( | 1.1 | ) | 27.4 | ( | 1.7 | ) | <0.0001 | |
| Orthopedic spinal lesions | Yes No | 3 53 | ( ( | 5.4% 94.6% | ) ) | 7 44 | ( ( | 13.7% 86.3% | ) ) | 13 39 | ( ( | 25.0% 75.0% | ) ) | 18 39 | ( ( | 31.6% 68.4% | ) ) | 0.002 |
| History of psychosis | Yes No | 10 46 | ( ( | 17.9% 82.1% | ) ) | 11 40 | ( ( | 21.6% 78.4% | ) ) | 13 39 | ( ( | 25.0% 75.0% | ) ) | 27 30 | ( ( | 47.4% 52.6% | ) ) | 0.002 |
| Agonist-related history | Yes No | 2 54 | ( ( | 3.6% 96.4% | ) ) | 3 48 | ( ( | 5.9% 94.1% | ) ) | 9 43 | ( ( | 17.3% 82.7% | ) ) | 8 49 | ( ( | 14.0% 86.0% | ) ) | 0.06 |
| Dopa, mean (SEM) | mg/day | 340 | ( | 25 | ) | 337 | ( | 26 | ) | 456 | ( | 23 | ) | 437 | ( | 25 | ) | 0.0003 |
| Agonist LDED, mean (SEM) | mg/day | 115 | ( | 18 | ) | 136 | ( | 23 | ) | 120 | ( | 20 | ) | 49 | ( | 8 | ) | 0.003 |
| Dopa + Agonist, mean (SEM) | mg/day | 454 | ( | 32 | ) | 473 | ( | 37 | ) | 576 | ( | 28 | ) | 486 | ( | 25 | ) | 0.029 |
| Selegiline Use | Use No use | 23 33 | ( ( | 41.1% 58.9% | )) | 25 26 | ( ( | 49.0% 51.0% | ) ) | 25 27 | ( ( | 48.1% 51.9% | ) ) | 22 35 | ( ( | 38.6% 61.4% | ) ) | 0.63 |
| Amantadine Use | Use No use | 8 48 | ( ( | 14.3% 85.7% | ) ) | 11 40 | ( ( | 21.6% 78.4% | ) ) | 18 34 | ( ( | 34.6% 65.4% | ) ) | 16 41 | ( ( | 28.1% 71.9% | ) ) | 0.08 |
| Rehabilitation | Yes No | 8 48 | ( ( | 14.3% 85.7% | ) ) | 4 47 | ( ( | 7.8% 92.2% | ) ) | 8 44 | ( ( | 15.4% 84.6% | ) ) | 14 43 | ( ( | 24.6% 75.4% | ) ) | 0.12 |
p was calculated by ANOVA(scale variables) or by Pearson Chi square test (categorical variables).
In Table 2, age, disease duration, H–Y, UPDRS-3, orthopedic spinal lesions, history of psychosis and daily dose of L-Dopa and dopamine agonists were differed significantly between the quartile of ΔTLA (Table 2). These data were very similar in analysis of ΔFBA (Table S1).
There was strong multicollinearity between age and PD onset age; therefore, PD onset age was excluded. L-Dopa+agonist dose was eliminated from predictable variables because there was multicollinearity between L-Dopa dose and L-Dopa+agonist dose, (Figure S2). All the other predictive variables were incorporated into the multivariate multinomial regression models. The model showed that the highest quartile ΔNFA was significantly associated with H–Y 3 or 4 and the ORs were 2.99 [95% confidence interval (CI) 1.33–6.70, p = 0.008] with reference to the lowest quartile (Table 3). In contrast to ΔNFA, the highest quartile ΔTLA was associated with orthopedic spinal lesions [OR 5.83 (1.42–23.8), p = 0.014], and UPDRS-3 score [OR 3.04 (1.80–5.15)/10 points, p<0.0001] (Table 4). These data were similar in analysis of ΔFBA, but highest quartile was negatively associated with daily dose of agonists (Table S2).
Table 3. Odds ratios of factors for quartiles of neck flexion angle.
| 2nd quartile | 3rd quartile | 4th quartile | ||||||||||||||||||||
| Predictable variables | OR (95% CI) | p | OR (95% CI) | p | OR (95% CI) | p | ||||||||||||||||
| Age | /Year | 0.88 | ( | 0.56 | – | 1.38 | ) | 0.57 | 0.77 | ( | 0.49 | – | 1.22 | ) | 0.27 | 0.97 | ( | 0.61 | – | 1.53 | ) | 0.89 |
| Sex | Male Female (Ref) | 2.01 1 | ( | 0.92 | – | 4.35 | ) | 0.08 | 1.08 1 | ( | 0.49 | – | 2.39 | ) | 0.85 | 1.07 1 | ( | 0.49 | – | 2.34 | ) | 0.87 |
| Orthopedic spinal lesions | Yes No (Ref) | 1.07 1 | ( | 0.38 | – | 2.98 | ) | 0.90 | 0.77 1 | ( | 0.27 | – | 2.18 | ) | 0.62 | 0.84 1 | ( | 0.32 | – | 2.20 | ) | 0.72 |
| Hoehn-Yahr | III, IV I, II (Ref) | 0.79 1 | ( | 0.35 | – | 1.78 | ) | 0.57 | 1.73 1 | ( | 0.78 | – | 3.88 | ) | 0.18 | 2.99 | ( | 1.33 | – | 6.7 | ) | 0.008 |
| 1 | ||||||||||||||||||||||
| The reference category is: 1st quartile. | ||||||||||||||||||||||
predictable variables: forced entry (age, sex, and orthopedic lesions), forward stepwise likelihood ratio test (duration of PD, H-Y, history of psychosis, history of agonist-related AP, UPDRS-3, Dopa dose, DA agonist dose, amantadine use, selegiline use, and rehabilitation).
Table 4. Odds ratios of factors for quartiles of thoracolumbar angle.
| 2nd quartile | 3rd quartile | 4th quartile | ||||||||||||||||||||
| Predictable variables | OR (95% CI) | p | OR (95% CI) | p | OR (95% CI) | p | ||||||||||||||||
| Age | /Year | 0.94 | ( | 0.59 | – | 1.49 | ) | 0.79 | 1.58 | ( | 0.94 | – | 2.66 | ) | 0.08 | 1.01 | ( | 0.60 | – | 1.71 | ) | 0.96 |
| Sex | Female | 0.94 | ( | 0.41 | – | 2.18 | ) | 0.892 | 1.09 | ( | 0.46 | – | 2.57 | ) | 0.84 | 1.78 | ( | 0.72 | – | 4.40 | ) | 0.21 |
| Male (Ref) | 1 | 1 | 1 | |||||||||||||||||||
| Orthopedic spinal lesions | Yes | 3.41 | ( | 0.78 | – | 14.8 | ) | 0.102 | 3.58 | ( | 0.89 | – | 14.4 | ) | 0.073 | 5.83 | ( | 1.42 | – | 23.8 | ) | 0.014 |
| No (Ref) | 1 | 1 | 1 | |||||||||||||||||||
| UPDRS–3 | /10 points | 1.65 | ( | 0.99 | – | 2.77 | ) | 0.06 | 1.40 | ( | 0.84 | – | 2.34 | ) | 0.20 | 3.04 | ( | 1.80 | – | 5.15 | ) | <0.0001 |
| Dopa daily dose | /100mg | 0.91 | ( | 0.72 | – | 1.15 | ) | 0.44 | 1.33 | ( | 1.03 | – | 1.71 | ) | 0.03 | 1.07 | ( | 0.83 | – | 1.39 | ) | 0.59 |
| Agonist daily dose | /100mg LDED | 1.21 | ( | 0.90 | – | 1.63 | ) | 0.21 | 1.20 | ( | 0.88 | – | 1.63 | ) | 0.25 | 0.64 | ( | 0.39 | - | 1.07 | ) | 0.09 |
| The reference category is: 1st quartile. | ||||||||||||||||||||||
predictable variables: forced entry (age, sex, and orthopedic lesions), forward stepwise likelihood ratio test (duration of PD, H-Y, history of psychosis, history of agonist-related AP, UPDRS-3, Dopa dose, DA agonist dose, amantadine use, selegiline use, and rehabilitation).
Discussion
All of the NF, FB and LB angles in PD were significantly larger than those in healthy controls. Distributions of NF and FB angles were bell-shaped both in PD patients and controls, and were shifted to the right in PD patients (Figure 1). Although distribution of LB angle was not bell-shaped because of absolute values, it was also shifted rightward in patients with PD. These data demonstrate that there is no distinct threshold of body angles for AP, but rather suggest the possibility that the truncal body angles are shifted in most PD patients. Although extremely abnormal postures such as dropped head syndrome or camptocormia are rare, AP could be a common problem in PD. These observations suggest that right-shifted body angle in PD is related to extrapyramidal symptoms such as axial rigidity or dystonia, which is reflected in impaired perception of body angles [31], [32], [33].
Previous studies have identified many clinical factors as risk factors for camptocormia, such as duration of PD [3], age [3], H–Y stage [3], [4], UPDRS-3 [3], [4], [5], axial rigidity [6], levodopa daily dose [4], levodopa treatment duration [3], dementia [3], autonomic dysfunction [4], and history of vertebral surgery [3]. The present study revealed that ΔTLA was associated with comorbid orthopedic spinal lesions and UPDRS-3 score. In contrast to ΔNFA, ΔTLA was associated with UPDRS-3 scores after adjustment comorbid orthopedic spine lesions, suggesting that thoracolumbar AP might be a part of motor disturbance of PD.
There are no reports of risk of neck AP such as anterocollis or dropped head in PD. In the present study, ΔNFA was associated only with H–Y stage ≥3. As shown in Table 1 there were no difference in UPDRS-3 scores between quartiles of ΔNFA. These data suggest that neck AP would be associated with postural reflex disturbance but not with overall motor disturbance of PD and patients with neck flexion AP may have relatively mild motor symptoms in limbs compared to axial symptoms including postural reflex disturbance.
Unexpectedly, age did not contribute significantly to AP. Spinal lesions were significantly associated with thoracolumbar AP, and this finding was consistent with a series of previous reports about camptocormia [3], [6], [34]. Although osteoporosis is a common complication of postmenopausal women [35], [36] and PD patients [37], the significant association with spinal lesions was revealed to be independent of sex, age and motor symptom severity in the present study. Severe spinal lesions seem to have some direct effect on vertebral alignment; however, another possible pathomechanism can be raised, namely, spinal lesions may trigger a peripheral-trauma-induced dystonia [38], [39] in paraspinal muscles. It has been reported that peripheral trauma induces movement disorders, and dystonia is the most frequent type (72%) [40]. PD may become a predisposing factor for trauma-induced dystonia because dystonia is one of the frequent complications in patients with PD. Additionally, subsequent secondary muscle or soft tissue degeneration might fix truncal AP in patients with advanced PD.
Although several antiparkinsonian drugs such as dopamine agonists[7], [8], [9], [10], [16], monoamine oxidase B inhibitor [14] (only selegiline is available in Japan to date), amantadine [12] or entacapone/levodopa/carbidopa [15] are nominated for causes of AP, they were not significant risk factors in our study.
Possible association of other factors such as contaminant myopathy or disturbance of body angle sensation could not be assessed in the present study, and these points are limitations of the study. Although this study had another limitation owing to its cross-sectional observational nature, the results suggest that body angles are shifted rightward in most patients with PD, and it is difficult to establish a clear cut-off point for body angles. TL AP is independent of NF AP and the significant risk factor for TL AP was comorbid orthopedic spinal lesions and motor disturbance of PD.
Supporting Information
Schematic representation of body angles. NF angle was defined as the angle between the two crossing lines: a line connecting the external acoustic foramen and the acromion, and another line connecting the acromion and the greater trochanter. Similarly, FB angle was the angle between a line connecting the acromion and the greater trochanter and a vertical line. On back view photographs, the angle between the line connecting the posterior process of the seventh cervical vertebra and that of the fifth lumbar vertebra and a vertical line was defined as LB angle.
(TIF)
Scattered plots of scale predictable variables. The relationship between possible predictable variables (age, onset age, duration, UPDRS-3, and daily dose of L-Dopa, dopamine agonists and dose of Dopa+agonists) was investigated in scattered diagram. There was a linear correlation between age and onset age, and therefore PD onset age was excluded from statistical analysis. There was multicollinearity between L-Dopa dose and L-Dopa+agonist dose, and therefore L-Dopa+agonist dose was eliminated from predictable variables.
(TIF)
(DOCX)
(DOCX)
Funding Statement
Drs. Hayashi, Tomita, and Kousaka report no disclosures. Drs. Umemura, Oeda and Sawada are funded by grants-in-aid from the National Hospital Organization. This does not alter the authors' adherence to all the neurology policies on sharing data and materials. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1. Finsterer J, Strobl W (2011) Causes of camptocormia. Disabil Rehabil 33: 1702–1703. [DOI] [PubMed] [Google Scholar]
- 2.Parkinson J (2002) An essay on the shaking palsy. 1817. J Neuropsychiatry Clin Neurosci 14: 223–236; discussion 222. [DOI] [PubMed]
- 3. Tiple D, Fabbrini G, Colosimo C, Ottaviani D, Camerota F, et al. (2009) Camptocormia in Parkinson disease: an epidemiological and clinical study. J Neurol Neurosurg Psychiatry 80: 145–148. [DOI] [PubMed] [Google Scholar]
- 4. Seki M, Takahashi K, Koto A, Mihara B, Morita Y, et al. (2011) Camptocormia in Japanese patients with Parkinson's disease: a multicenter study. Mov Disord 26: 2567–2571. [DOI] [PubMed] [Google Scholar]
- 5. Bloch F, Houeto JL, Tezenas du Montcel S, Bonneville F, Etchepare F, et al. (2006) Parkinson's disease with camptocormia. J Neurol Neurosurg Psychiatry 77: 1223–1228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Lepoutre AC, Devos D, Blanchard-Dauphin A, Pardessus V, Maurage CA, et al. (2006) A specific clinical pattern of camptocormia in Parkinson's disease. J Neurol Neurosurg Psychiatry 77: 1229–1234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Uzawa A, Mori M, Kojima S, Mitsuma S, Sekiguchi Y, et al. (2009) Dopamine agonist-induced antecollis in Parkinson's disease. Mov Disord 24: 2408–2411. [DOI] [PubMed] [Google Scholar]
- 8. Suzuki M, Hirai T, Ito Y, Sakamoto T, Oka H, et al. (2008) Pramipexole-induced antecollis in Parkinson's disease. J Neurol Sci 264: 195–197. [DOI] [PubMed] [Google Scholar]
- 9. Taguchi Y, Takashima S, Tanaka K (2008) Pramipexole-induced dropped head syndrome in Parkinson's disease. Intern Med 47: 2011–2012. [DOI] [PubMed] [Google Scholar]
- 10. Kashihara K, Ohno M, Tomita S (2006) Dropped head syndrome in Parkinson's disease. Mov Disord 21: 1213–1216. [DOI] [PubMed] [Google Scholar]
- 11. Fujimoto K (2006) Dropped head in Parkinson's disease. J Neurol 253 Suppl 7VII21–26. [DOI] [PubMed] [Google Scholar]
- 12. Kataoka H, Ueno S (2011) Dropped head associated with amantadine in Parkinson disease. Clin Neuropharmacol 34: 48–49. [DOI] [PubMed] [Google Scholar]
- 13. Cannas A, Solla P, Floris G, Tacconi P, Serra A, et al. (2009) Reversible Pisa syndrome in patients with Parkinson's disease on dopaminergic therapy. J Neurol 256: 390–395. [DOI] [PubMed] [Google Scholar]
- 14. Fasano A, Di Matteo A, Vitale C, Squintani G, Ferigo L, et al. (2011) Reversible Pisa syndrome in patients with Parkinson's disease on rasagiline therapy. Mov Disord 26: 2578–2580. [DOI] [PubMed] [Google Scholar]
- 15. Solla P, Cannas A, Congia S, Floris G, Aste R, et al. (2008) Levodopa/carbidopa/entacapone-induced acute Pisa syndrome in a Parkinson's disease patient. J Neurol Sci 275: 154–156. [DOI] [PubMed] [Google Scholar]
- 16. Cannas A, Solla P, Floris G, Borghero G, Tacconi P, et al. (2005) Reversible Pisa syndrome in Parkinson's disease during treatment with pergolide: a case report. Clin Neuropharmacol 28: 252. [DOI] [PubMed] [Google Scholar]
- 17. Broussolle E, Krack P, Thobois S, Xie-Brustolin J, Pollak P, et al. (2007) Contribution of Jules Froment to the study of parkinsonian rigidity. Mov Disord 22: 909–914. [DOI] [PubMed] [Google Scholar]
- 18. Wright WG, Gurfinkel VS, Nutt J, Horak FB, Cordo PJ (2007) Axial hypertonicity in Parkinson's disease: direct measurements of trunk and hip torque. Exp Neurol 208: 38–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Bonanni L, Thomas A, Varanese S, Scorrano V, Onofrj M (2007) Botulinum toxin treatment of lateral axial dystonia in Parkinsonism. Mov Disord 22: 2097–2103. [DOI] [PubMed] [Google Scholar]
- 20. Gerton BK, Theeler B, Samii A (2010) Backpack treatment for camptocormia. Mov Disord 25: 247–248. [DOI] [PubMed] [Google Scholar]
- 21. Margraf NG, Wrede A, Rohr A, Schulz-Schaeffer WJ, Raethjen J, et al. (2010) Camptocormia in idiopathic Parkinson's disease: a focal myopathy of the paravertebral muscles. Mov Disord 25: 542–551. [DOI] [PubMed] [Google Scholar]
- 22. Spuler S, Krug H, Klein C, Medialdea IC, Jakob W, et al. (2010) Myopathy causing camptocormia in idiopathic Parkinson's disease: a multidisciplinary approach. Mov Disord 25: 552–559. [DOI] [PubMed] [Google Scholar]
- 23. Charpentier P, Dauphin A, Stojkovic T, Cotten A, Hurtevent JF, et al. (2005) [Parkinson's disease, progressive lumbar kyphosis and focal paraspinal myositis]. Rev Neurol (Paris) 161: 459–463. [DOI] [PubMed] [Google Scholar]
- 24. Gibb WR, Lees AJ (1988) The relevance of the Lewy body to the pathogenesis of idiopathic Parkinson's disease. J Neurol Neurosurg Psychiatry 51: 745–752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Slawek J, Derejko M, Lass P, Dubaniewicz M (2006) Camptocormia or Pisa syndrome in multiple system atrophy. Clin Neurol Neurosurg 108: 699–704. [DOI] [PubMed] [Google Scholar]
- 26. Solla P, Cannas A, Costantino E, Orofino G, Lavra L, et al. (2012) Pisa syndrome in a patient with progressive supranuclear palsy. J Clin Neurosci 19: 922–923. [DOI] [PubMed] [Google Scholar]
- 27. Gilman S, Wenning GK, Low PA, Brooks DJ, Mathias CJ, et al. (2008) Second consensus statement on the diagnosis of multiple system atrophy. Neurology 71: 670–676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Litvan I, Agid Y, Jankovic J, Goetz C, Brandel JP, et al. (1996) Accuracy of clinical criteria for the diagnosis of progressive supranuclear palsy (Steele-Richardson-Olszewski syndrome). Neurology 46: 922–930. [DOI] [PubMed] [Google Scholar]
- 29. Sako W, Nishio M, Maruo T, Shimazu H, Matsuzaki K, et al. (2009) Subthalamic nucleus deep brain stimulation for camptocormia associated with Parkinson's disease. Mov Disord 24: 1076–1079. [DOI] [PubMed] [Google Scholar]
- 30. Umemura A, Oka Y, Ohkita K, Yamawaki T, Yamada K (2010) Effect of subthalamic deep brain stimulation on postural abnormality in Parkinson disease. J Neurosurg 112: 1283–1288. [DOI] [PubMed] [Google Scholar]
- 31. Konczak J, Krawczewski K, Tuite P, Maschke M (2007) The perception of passive motion in Parkinson's disease. J Neurol 254: 655–663. [DOI] [PubMed] [Google Scholar]
- 32. Jobst EE, Melnick ME, Byl NN, Dowling GA, Aminoff MJ (1997) Sensory perception in Parkinson disease. Arch Neurol 54: 450–454. [DOI] [PubMed] [Google Scholar]
- 33. Vaugoyeau M, Azulay JP (2010) Role of sensory information in the control of postural orientation in Parkinson's disease. J Neurol Sci 289: 66–68. [DOI] [PubMed] [Google Scholar]
- 34. Djaldetti R, Mosberg-Galili R, Sroka H, Merims D, Melamed E (1999) Camptocormia (bent spine) in patients with Parkinson's disease–characterization and possible pathogenesis of an unusual phenomenon. Mov Disord 14: 443–447. [DOI] [PubMed] [Google Scholar]
- 35. Sipila S, Poutamo J (2003) Muscle performance, sex hormones and training in peri-menopausal and post-menopausal women. Scand J Med Sci Sports 13: 19–25. [DOI] [PubMed] [Google Scholar]
- 36. Christenson ES, Jiang X, Kagan R, Schnatz P (2012) Osteoporosis management in post-menopausal women. Minerva Ginecol 64: 181–194. [PubMed] [Google Scholar]
- 37. Abou-Raya S, Helmii M, Abou-Raya A (2009) Bone and mineral metabolism in older adults with Parkinson's disease. Age Ageing 38: 675–680. [DOI] [PubMed] [Google Scholar]
- 38. Jankovic J, Van der Linden C (1988) Dystonia and tremor induced by peripheral trauma: predisposing factors. J Neurol Neurosurg Psychiatry 51: 1512–1519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Jankovic J (2009) Peripherally induced movement disorders. Neurol Clin 27: 821–832, vii. [DOI] [PubMed]
- 40. van Rooijen DE, Geraedts EJ, Marinus J, Jankovic J, van Hilten JJ (2011) Peripheral trauma and movement disorders: a systematic review of reported cases. J Neurol Neurosurg Psychiatry 82: 892–898. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Schematic representation of body angles. NF angle was defined as the angle between the two crossing lines: a line connecting the external acoustic foramen and the acromion, and another line connecting the acromion and the greater trochanter. Similarly, FB angle was the angle between a line connecting the acromion and the greater trochanter and a vertical line. On back view photographs, the angle between the line connecting the posterior process of the seventh cervical vertebra and that of the fifth lumbar vertebra and a vertical line was defined as LB angle.
(TIF)
Scattered plots of scale predictable variables. The relationship between possible predictable variables (age, onset age, duration, UPDRS-3, and daily dose of L-Dopa, dopamine agonists and dose of Dopa+agonists) was investigated in scattered diagram. There was a linear correlation between age and onset age, and therefore PD onset age was excluded from statistical analysis. There was multicollinearity between L-Dopa dose and L-Dopa+agonist dose, and therefore L-Dopa+agonist dose was eliminated from predictable variables.
(TIF)
(DOCX)
(DOCX)