Abstract
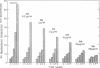
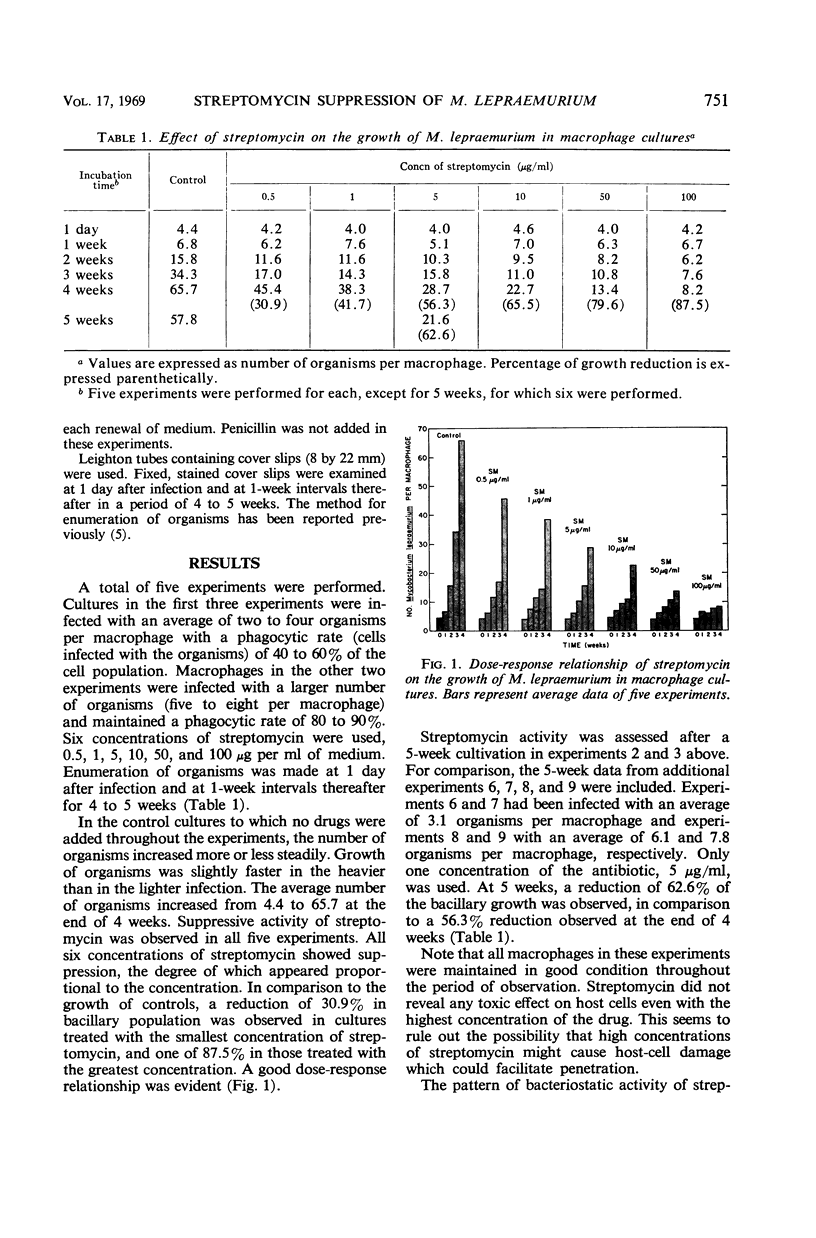
The effect of streptomycin on the growth of an obligate intracellular bacterium was studied in a new host-parasite cell system. The system consisted of Mycobacterium lepraemurium grown in cultures of mouse peritoneal macrophages. Since these organisms do not grow in bacteriological media, the influence of extracellular bacterial growth can be ruled out. The suppressive activity of streptomycin was observed in a total of five experiments. At the end of 4 weeks, the average number of organisms per macrophage for the controls was 65.7; for cultures with streptomycin at concentrations of 0.5, 1, 5, 10, 50, and 100 μg/ml of medium, it was 45.4, 38.3, 28.7, 22.7, 13.4, and 8.2, respectively. A good dose-response relationship was evident. M. lepraemurium which had been treated in macrophage cultures with various concentrations of the antibiotic for 6 to 8 weeks was used to infect fresh macrophages. These cultures were in turn treated with streptomycin. Resistance of the organisms to streptomycin did not occur.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- BAKER H. J. Effects of penicillin and streptomycin on staphylococci in cultures of mononuclear phagocytes. Ann N Y Acad Sci. 1954 Nov 17;58(7):1232–1245. doi: 10.1111/j.1749-6632.1954.tb45905.x. [DOI] [PubMed] [Google Scholar]
- BERTHRONG M., HAMILTON M. A. Tissue culture studies on resistance in tuberculosis. II. Monocytes from normal and immunized guinea pigs infected with virulent human tubercle bacilli. Am Rev Tuberc. 1959 Feb;79(2):221–231. doi: 10.1164/artpd.1959.79.2.221. [DOI] [PubMed] [Google Scholar]
- BRUMFITT W., GLYNN A. A., PERCIVAL A. FACTORS INFLUENCING THE PHAGOCYTOSIS OF ESCHERICHIA COLI. Br J Exp Pathol. 1965 Apr;46:215–226. [PMC free article] [PubMed] [Google Scholar]
- CHANG Y. T. LONG-TERM CULTIVATION OF MOUSE PERITONEAL MACROPHAGES. J Natl Cancer Inst. 1964 Jan;32:19–35. [PubMed] [Google Scholar]
- Change Y. T., Andersen R. N., Vaituzis Z. Growth of Mycobacterium lepraemurium in cultures of mouse peritoneal macrophages. J Bacteriol. 1967 Mar;93(3):1119–1131. doi: 10.1128/jb.93.3.1119-1131.1967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- FURNESS G. Interaction between Salmonella typhimurium and phagocytic cells in cell culture. J Infect Dis. 1958 Nov-Dec;103(3):272–277. doi: 10.1093/infdis/103.3.272. [DOI] [PubMed] [Google Scholar]
- GERBER D. F., WATKINS H. M. Growth of shigellae in monolayer tissue cultures. J Bacteriol. 1961 Dec;82:815–822. doi: 10.1128/jb.82.6.815-822.1961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HOLLAND J. J., PICKETT M. J. Intracellular behavior of Brucella variants in chick embryo cells in tissue culture. Proc Soc Exp Biol Med. 1956 Dec;93(3):476–479. doi: 10.3181/00379727-93-22792. [DOI] [PubMed] [Google Scholar]
- HSU H. S., KAPRAL F. A. The suppressed multiplication of tubercle bacilli within macrophages derived from triiodothyronine-treated guinea pigs. Am Rev Respir Dis. 1960 Jun;81:881–887. doi: 10.1164/arrd.1960.81.6.881. [DOI] [PubMed] [Google Scholar]
- KESSEL R. W., BOUGHTON J., BRAUN W. Effect of meprobamate on the multiplication of Brucella abortus in monocytes. Science. 1961 Dec 8;134(3493):1882–1883. doi: 10.1126/science.134.3493.1882. [DOI] [PubMed] [Google Scholar]
- MACKANESS G. B. The phagocytosis and inactivation of staphylococci by macrophages of normal rabbits. J Exp Med. 1960 Jul 1;112:35–53. doi: 10.1084/jem.112.1.35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MAGOFFIN R. L., SPINK W. W. The protection of intracellular brucella against streptomycin alone and in combination with other antibiotics. J Lab Clin Med. 1951 Jun;37(6):924–930. [PubMed] [Google Scholar]
- POMALES-LEBRON A., STINEBRING W. R. Intracellular multiplication of Brucella abortus in normal and immune mononuclear phagocytes. Proc Soc Exp Biol Med. 1957 Jan;94(1):78–83. doi: 10.3181/00379727-94-22860. [DOI] [PubMed] [Google Scholar]
- RICHARDSON M., HOLT J. N. Synergistic action of streptomycin with other antibiotics of intracellular Brucella abortus in vitro. J Bacteriol. 1962 Oct;84:638–646. doi: 10.1128/jb.84.4.638-646.1962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SHAFFER J. M., KUCERA C. J., SPINK W. W. The protection of intracellular brucella against therapeutic agents and the bactericidal action of serum. J Exp Med. 1953 Jan;97(1):77–90. doi: 10.1084/jem.97.1.77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SHEPARD C. C. Nonacid-fast bacteria and HeLa cells: their uptake and subsequent intracellular growth. J Bacteriol. 1959 Jun;77(6):701–714. doi: 10.1128/jb.77.6.701-714.1959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SHEPARD C. C. Use of HeLa cells infected with tubercle bacilli for the study of antituberculous drugs. J Bacteriol. 1957 Apr;73(4):494–498. doi: 10.1128/jb.73.4.494-498.1957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SUTER E. The multiplication of tubercle bacilli within normal phagocytes in tissue culture. J Exp Med. 1952 Aug;96(2):137–150. doi: 10.1084/jem.96.2.137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stefanye D., Tresselt H. B., Spero L. OBSERVATIONS ON THE BEHAVIOR IN VITRO OF PASTEURELLA TULARENSIS AFTER PHAGOCYTOSIS. J Bacteriol. 1961 Mar;81(3):470–473. doi: 10.1128/jb.81.3.470-473.1961. [DOI] [PMC free article] [PubMed] [Google Scholar]