Abstract
We used mouse models of pneumococcal colonization and disease combined with full genome sequencing to characterize three major drug resistant clones of S. pneumoniae that were recovered from the nasopharynx of PCV7-immunized children in Portugal. The three clones – serotype 6A (ST2191), serotype 15A (ST63) and serotype 19A (ST276) carried some of the same drug resistance determinants already identified in nasopharyngeal isolates from the pre-PCV7 era. The three clones were able to colonize efficiently the mouse nasopharyngeal mucosa where populations of these pneumococci were retained for as long as 21 days. During this period, the three clones were able to asymptomatically invade the olfactory bulbs, brain, lungs and the middle ear mucosa and established populations in these tissues. The virulence potential of the three clones was poor even at high inoculum (105 CFU per mouse) concentrations in the mouse septicemia model and was undetectable in the pneumonia model. Capsular type 3 transformants of clones 6A and 19A prepared in the laboratory produced lethal infection at low cell concentration (103 CFU per mouse) but the same transformants became impaired in their potential to colonize, indicating the importance of the capsular polysaccharide in both disease and colonization. The three clones were compared to the genomes of 56 S. pneumoniae strains for which sequence information was available in the public databank. Clone 15A (ST63) only differed from the serotype 19F clone G54 in a very few genes including serotype so that this clone may be considered the product of a capsular switch. While no strain with comparable degree of similarity to clone 19A (ST276) was found among the sequenced isolates, by MLST this clone is a single locust variant (SLV) of Denmark14-ST230 international clone. Clone 6A (ST2191) was most similar to the penicillin resistant Hungarian serotype 19A clone.
Introduction
The nasopharynx of preschool age children, particularly those attending day-care, is the main ecological niche for the gram-positive pathogen Streptococcus pneumoniae that asymptomatically colonizes these individuals at high rates [1]. Colonization is the first step for pneumococcal disease, which may range from non-invasive mucosal infection to severe invasive disease such as sepsis, bacteraemic pneumonia or meningitis [2]. Global estimates indicate that S. pneumoniae is responsible for 2 million deaths annually [3].
The development of pneumococcal resistance to penicillin and multiple other drugs is a global concern [4] as it can lead to disease treatment failures [5]. Surprisingly, although S. pneumoniae is a genetically diverse species capable of expressing over 90 different capsular types [6], only a limited number of these serotypes associated to a few pandemic clones have been responsible for the increase of drug resistant strains worldwide [4]. The “birthplace” of these drug resistant clones is believed to be the nasopharynx of young children, with a predisposition for upper-respiratory diseases and behavior traits that favor person-to-person contacts. These circumstances, combined with frequent antibiotic use, constitute ideal conditions for the selection, amplification and transmission of drug resistant clones [1].
The first anti-pneumococcal conjugate vaccine was introduced in the United States in 2000, when almost half of all invasive pneumococcal disease (IPD) was caused by pneumococci resistant to penicillin and/or macrolides [7]. The 7-valent pneumococcal conjugate vaccine (PCV7) targeted the most common serotypes causing IPD in children in the US, namely, serotypes 14, 6B, 19F, 18C, 23F, 4, and 9V [8], which also included the serotypes of highly successful drug resistant pandemic clones such as Spain23F-1, Spain6B-2 or Spain9V-3. In virtually all settings, and even with short vaccination schedules, the introduction of PCV7 has led to a significant decline in the frequency of PCV7 serotypes that were often involved with disease before the introduction of this vaccine [9-11].
A secondary but equally important expected outcome of immunization with this conjugate vaccine was to lower drug resistance levels by reducing the frequency of the PCV7 serotypes that were most often associated with antibiotic resistance prior to the introduction of the vaccine [12]. However, immunization with PCV7 has not only led to a serotype replacement but also to the concomitant expansion of drug resistant clones expressing non-PCV7 capsules [13]. The vaccine selected these clones by prevention of de novo acquisition of PCV7 serotypes and, at least in some cases, by unmasking of non-PCV7 serotypes [9] which – presumably – were already present in the nasopharynx as minority populations of pneumococci co-colonizing the chidren´s nasopharynx during the pre-vaccine era [9]. These non-PCV7 clones shared the same ecological niche with clones targeted by the vaccine and were consequently exposed to the same environmental insults, namely antibiotic pressure, which may explain their drug resistance patterns.
In 2001-2003, the impact of the PCV7 on colonization and antimicrobial resistance was first investigated in Portugal by sampling the nasopharynx of healthy day-care center attendees. This study documented replacement of PCV7 by non-PCV7 isolates which also carried resistance traits to several antimicrobial agents [14]. Among the drug resistant non-PCV7 pneumococci the dominant serotypes were 6A, 15A and 19A, which – in association with a few clonal lineages – accounted for 71% of the isolates [14].
S. pneumoniae expressing the same non-PCV7 serotypes and similar clonal types also expanded in countries other than Portugal where the PCV7 vaccine was introduced and these clones were shown to be able to cause human colonization as well as disease [14-19].
The present study focused on three drug resistant non-PCV7 clones isolated from the nasopharynx of healthy vaccinated children in Portugal [14]. Using several murine models, we examined the behavior of the 6A, 15A and 19A non-PCV7 clones in colonization and disease. Our data show that these three clones were highly competent in host colonization and were able to asymptomatically invade several tissues but remained poorly virulent in the mouse septicemia model and completely avirulent in the pneumonia model.
Materials and Methods
Ethics statement
All animal experiments were conducted with the approval of the Rockefeller University Institutional Animal Care and Use Committee (Permit Number: 09073).
Bacterial strains
The three drug resistant pneumococcal strains, expressing capsular types 6A, 15A and 19A, were recovered from the nasopharynx of healthy PCV7-immunized children in Portugal [14]. The strains were characterized for serotype, multi-locus sequence type (MLST) [20], susceptibility to antibiotics (penicillin, ceftriaxone, oxacillin, chloramphenicol, erythromycin, clindamycin, tetracycline, trimethoprim-sulfamethoxazole and levofloxacin) and pulsed-field gel electrophoresis (PFGE) type as described previously [14]. PFGE-based clonal types were defined as isolates with ≥80% relatedness using the Bionumerics software (Applied-Maths, Sint-Martens-Latem, Belgium). The three pneumococcal strains chosen for detailed study were selected on the basis of three criteria which included: i) serotype; ii) PFGE clonal type and iii) resistance to penicillin (Table 1 and Figure S1 in File S1).
Table 1. Major drug resistant non-PCV7 clones.
|
Representative strains
|
||||||
|---|---|---|---|---|---|---|
| Serotype | PFGE clonal type | Penicillin MIC associated | PFGE | Antibiotype | ST | |
| 6A | 6A-I | P(I) clone, MIC: 0.19 µg/mL | 6A-I | P(I) (MIC: 0.19 µg/mL), Tet | 2191 | |
| 6A - II | P(S) clone, MIC range: 0.023-0.064 µg/mL | |||||
| 15A | 15A-I | P(I) clone, MIC range: 0.125-0.19 µg/mL | 15A-I | P(I) (MIC: 0.19 µg/mL), Tet, Da, E | 63 | |
| 15A-II | P(S) clone, MIC: 0.016 µg/mL | |||||
| 19A | 19A-I | P(I) clone, MIC range: 0.5-0.75 µg/mL | 19A-I | P(I) (MIC: 0.75 µg/mL), SXT, Tet, Da, E | 276 | |
| 19A-II | P(I) clone, MIC: 0.19 µg/mL | |||||
| 19A-III | P(I) clone, MIC: 0.094 µg/mL | |||||
| 19A-IV | P(S) clone, MIC range: 0.016-0.023 µg/mL | |||||
P(S) – Penicillin Susceptible (P<0.094 µg/ml); P(I) – Penicillin Intermediate Resistance (0.094 µg/ml≤P<1.5 µg/ml) ; E – Erythromycin resistance (E≤15 µg/ml); Da – Clindamycin resistance (Da ≤15 µg/ml); Tet – Tetracycline resistance (Te ≤18 µg/ml); SXT - Trimethoprim-sulfamethoxazole resistance (SXT ≤15 µg/ml).
MIC – Minimal Inhibitory Concentration.
Transformants expressing the type 3 capsule were generated using 6A and 19A as the parental strains. The transforming DNA was from strain SV35T3, which was generated by transforming the SV35-T23 strain with a 22kB region from SV36T3 that contained genes for the type 3 capsule as well as a spectinomycin cassette to create a selectable marker [21]. Each of the two type 3 transformants was backcrossed three times to avoid recombination with unlinked noncapsular genes from the SV35T3 donor producing essentially isogenic strains. The D39 serotype 2 isolate was used as an arbitrary control strain in some animal experiments. Bacteria were grown without aeration at 37°C either in C+Y broth [22], Todd-Hewitt broth supplemented with 0.5% yeast extract (THY) or in Tryptic Soy agar (TSA) containing 5% defibrinated sheep blood supplemented with gentamicin (5 µg/mL) in a 5% CO2 atmosphere. Bacterial inocula were prepared by growing pneumococci in C+Y broth to an optical density of 0.8 (OD590). Cultures were then centrifuged and the pellets resuspended in a sterile saline solution to obtain the desired bacterial concentration.
Capsular switching methods
Competent cells were prepared by growing bacteria in THY broth to OD590=0.07-0.08. To carry out transformation, competent cells were diluted 1:20 in competence medium (TSB [pH 8.0], 0.16% bovine serum albumin, 0.01% CaCl2) containing whole genome transforming DNA (1,000 ng/mL) and the competence stimulating peptide (CSP, ~500 ng). The transforming DNA was purified from strain SV35T3 [21], and CSP1 and CSP2 were used to transform pneumococcal strains 6A and 19A, respectively. The transformation reaction (1 mL) was held 4 h at 37°C in a 1.5-mL Eppendorf tube and then challenged in TSA plates supplemented with 5% sheep blood and spectinomycin (125 µg/mL). Plates were incubated overnight at 37°C with 5% CO2. Transformants were confirmed by observation of the typical type 3 mucoid colony morphology, serotyping with specific sera [23] and detection of capsular type 3 by PCR amplification [24].
Mouse models
Groups of 9-week-old female CD1 outbred mice obtained from the Charles River Laboratories (Wilmington, MA) were used in the colonization, pneumonia and septicemia models. When required, mice were anaesthetized by injecting 75 to 100-µL of a xylazine and ketamine mixture into the peritoneal cavity (0.2 ml Xylazine at 100 mg/mL and 1 ml of Ketamine at 100 mg/mL was mixed with 4.8 ml PBS buffer pH 7.4). The protocol for the animal experiments was approved by the Institutional Review Board of The Rockefeller University (Permit Number: 09073). Colony-forming units (CFU) inoculated in the mice were confirmed by counting of serial dilutions on TSA containing 5% blood supplemented with gentamicin. Mice were given food and water ad libitum, and monitored for survival on a daily basis during the experimental period. For sampling procedures mice were humanely euthanized by CO2 asphyxiation.
Colonization model
Each anesthetized mouse was inoculated intranasally with 10 µL of inoculum containing 108 CFU using a 20-µL micropipette. At defined time points after the challenge (2, 7, 14, and 21 days) groups of mice were euthanized and bacterial numbers assessed in the nasopharyngeal and middle ear mucosa, olfactory bulbs, brain, lungs and blood. The nasopharyngeal wash was performed by cannulation of the mouse trachea and posterior lavage with 500-µL of saline solution, which was collected through the nose. To sample the middle ear mucosa, we adapted the procedure used by Lai and colleagues [25]. In brief, we injected into each middle ear cavity 10 µL of sterile saline solution using a 10-µL micropipette. The saline solution was withdrawn and reintroduced three times (final volume recovered, ~13-µL per 2 ears). Blood collection (300 µL) was achieved by heart puncture. Using a tissue homogenizer (PYREX® Potter-Elvehjem), the lungs and brain or the olfactory bulbs were homogenized respectively in 1000 or 500 µL of sterile saline solution. Resulting solutions from all samples were diluted and plated for CFU counting. Mouse perfusion was performed before the lungs, olfactory bulbs and brain were harvested to avoid possible CFU contamination from the blood.
Pneumonia model
Each anesthetized mouse was inoculated intranasally with 50 µL of inoculum containing 105 or 107 CFU using a 100-µL micropipette and survival rates were followed for 7 days.
Septicemia model
Each mouse was injected intraperitoneally with 500-µL of inoculum containing 105 CFU and survival rates were followed for 7 days. At defined time points after the intraperitoneal injection (0.5, 3, 6, 12 and 24 h), 1-3 mice were euthanized and bacterial numbers assessed in the blood.
Statistics
Significant differences in CFU numbers were analyzed using the 2-tailed Mann-Whitney U test. Survival curves were analyzed using the log-rank (Mantel-Cox) test using Prism software from GraphPad Software Inc. In all analyses, a maximum error type I of 0.05 was considered for recognition of a significant difference.
Whole genome sequencing and assembly of the non-PCV7 strains
The genomes for strains 6A, 15A and 19A were sequenced using the 454 Life Science’s Titanium platform. The depth of coverage was as follows: type 6A: 36.3X coverage; type 15A: 33.9x coverage; type 19A: 37,5X coverage.
Fragment libraries and sequencing were performed following the manufactures’ guidelines outlines in GS FLX Titanium, General Library Preparation Method Manual (October 2008 Roche Molecular Systems, Nutley, NJ) and GS FLX Titanium emPCR and Sequencing Protocols (October 2008). The raw sequence reads were assembled into contigs using the Roche/454 Life Sciences GS de novo Newbler Assembler v2.3. The final assemblies for 15A, 6A and 19A contain 70, 87 and 49 contigs, respectively. The whole genome sequence for 15A, 6A, and 19A are currently being processed in GenBank and accession numbers will be available shortly.
Phylogenetic analysis
The three newly sequenced non-PCV7 strains were compared to a set of 56 fully sequenced S. pneumoniae strains listed in Table S1 in File S1.
Whole genome sequence alignment and phylogenetic tree
The whole genome sequence of the 59 strains was aligned using MAUVE under default parameters [26], and the core regions were extracted from the xmfa alignment file. The 1.5Mb core region of each of the 59 genomes was aligned using Mafft [27]. This alignment was used to compute a Maximum Likelihood phylogenetic tree using the GTRGAMMA model in RAxML [28], and branch support was computed with 1000 bootstrap replicates. The tree was visualized with FigTree software [29].
Results
PCV7 selects strains with non-PCV7 capsules and specific drug resistance traits
The PCV7 vaccine became available in Portugal in July 2001. In a three-year (2001-2003) surveillance study, a group of 238 PCV7-vaccinated children attending day-care in the Lisbon area was investigated regarding the impact of the PCV7 vaccine on colonization [14].
The PCV7 vaccine did not change the overall pneumococcal carriage rate (68.2% in the vaccines and 67.6% in the control) and importantly did not change the carriage rate of drug resistant strains (37.4% in the vaccines and 39.3% in the control). We focused on the latter finding and concluded that the PCV7 vaccine led to a replacement of drug resistant strains expressing PCV7 serotypes by drug resistant strains exhibiting non-PCV7 capsular types.
Among the non-PCV7 drug resistant strains the most prevalent serotypes were 15A (26 strains), 19A (22 strains) and 6A (6 strains), which together accounted for 71% (54/76) of the isolates [14]. Table 1 and Figure S1 in File S1 summarize the genetic diversity among these three major drug resistant non-PCV7 serotypes. Strains expressing serotypes 6A or 15A belonged to one of two PFGE clonal types, while strains that exhibited the 19A capsule belonged to one of four PFGE lineages. Furthermore, among the strains expressing the 6A serotype, the highest penicillin MIC (0.19 µg/mL) was associated with a single PFGE type named 6A-I. For serotype 15A, the highest penicillin MIC (0.19 µg/mL) was associated with the PFGE clone 15A-I, while for serotype 19A the PFGE clone presenting the highest penicillin MIC (0.75 µg/mL) was 19A-I. We next selected representative strains belonging to these specific PFGE types and characterized the isolates by MLST. The clone expressing the 6A serotype belonged to ST2191, the 15A serotype to ST63 and the 19A capsular type to ST276 (Table 1 and Figure S1 in File S1). These three clonal types became the most frequent drug resistant sequence types colonizing the nasopharynx of PCV7-immunized children in Portugal [14].
Whole genome sequence analysis of the three drug resistant non-PCV7 clones
To identify differences among the three non-PCV7 clones and place them in a species-wide perspective, we sequenced the full genomes and performed a phylogenetic analysis of the 6A, 15A and 19A clonal isolates and another 56 S. pneumoniae strains that capture much of the species diversity. The whole genome sequences of the 56 pneumococcal strains plus the three non-PCV7 clonal isolates were aligned generating a 1.5Mb region that is shared by all the isolates (referred to as the core WGS). Given that the average pneumococcal genome is 2.1Mb, this shows that ~71% of the genome of each individual strain is core [30]. This alignment-based method does not allow for gaps in any of the sequences, thus we selected the core set to maximize the number of informative positions. The core WGS from each genome was aligned using Mafft, and a Maximum Likelihood phylogenetic tree was computed using RAxML (Figure 1). The relative position of each non-PCV7 clone is supported by high bootstrap values in the inner branches. The 15A lineage is very closely related to G54, a drug resistant 19F clinical isolate, both presenting ST63 [31]. This high degree of similarity in the core region in combination with a difference in capsule type is consistent with one of these clones having evolved by serotype switching. In contrast, the 6A and 19A clones were not highly related to any of the remaining strains included in the currently available whole genome data set (Figure 1). The closest neighbor to the 6A clone is the Hungary-19A clinical isolate (ST268). The 19A clone is in the same branch as strains CDC3059_6 (ST199 clinical isolate), JJA (ST66 clinical isolated from Brazil), and the PMEN1 lineage (ST81). While it is possible that these strains have evolved by serotype switching, this data set did not capture any closely related strains. By MLST, the 15A clone is a representative of Sweden15A-ST63 international clone, the 19A is a SLV of Denmark14-ST230 international clone and for the 6A clone no obvious close relatives have been described but a double locus variant (DLV) has been identified in Greece.
Figure 1. Maximum likelihood phylogenetic tree based on the core genome polymorphisms of 59 Streptococcus pneumoniae.
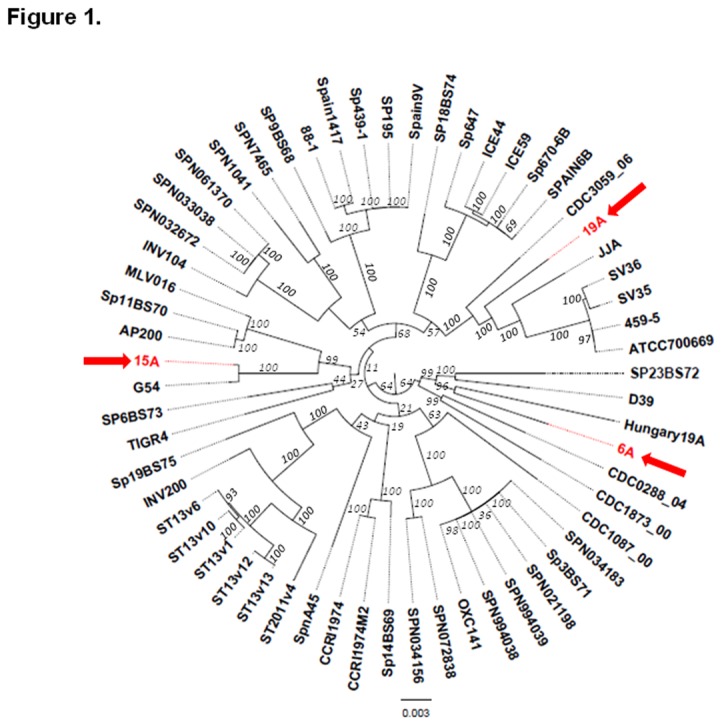
Branches are annotated with their bootstrap support (numbers in italics). Three non-PCV7 clones (6A, 15A and 19A) are highlighted in red. Strain 15A is highly similar to G54, strain 6A and 19A are not highly similar to any other strains in this set.
Whole genome sequencing allowed us to check for the presence of major genetic determinants known to play a role in several important processes such as, colonization, disease, competition, antibiotic resistance and natural transformation (Tables 2 and 3) [32-36]. Overall, in the three non-PCV7 clones the same set of genetic determinants was observed with the sole differences noted concerning capsular type and antibiotic resistance pattern. The rlrA pathogenicity island encoding pilus-like structures was missing from each of the three non-PCV7 clones.
Table 2. Streptococcus pneumoniae genetic determinants involved in colonization, competition, disease, antibiotic resistance and natural transformation.
|
Non-PCV7 clones
|
||||
|---|---|---|---|---|
| Pneumococcal genetic determinants | Main role | 6A | 15A | 19A |
| ST2191 | ST63 | ST276 | ||
| Colonization | ||||
| Capsule | Prevents entrapment in the nasal mucus, thereby allowing access to epithelial surfaces. Also inhibits effective opsonophagocytosis. | P (6A) | P (15A) | P (19A) |
| Choline binding protein (pspC) | Binds to human secretory component on a polymeric Ig receptor during the first stage of translocation across the epithelium. | P | P | P |
| Choline binding protein (pcpA) | Role in pneumococcal adhesion and biofilm formation. | P | P | P |
| nanA, bgaA and strH | Act sequentially to cleave terminal sugars from human glycoconjugates, which might reveal receptors for adherence. | P | P | P |
| hyl | Breaks down hyaluronan-containing extracellular matrix components. | P | P | P |
| pavA | Binds to fibronectin. | P | P | P |
| eno | Binds to plasminogen. | P | P | P |
| rlrA pathogenicity islet | The rlrA islet encodes pili-like structures contributing to adherence. | M | M | M |
| Competition in upper airway | ||||
| Bacteriocin Locus (blpC and βlpH) | Small antimicrobial peptide that targets members of the same species. | P | P | P |
| Disease | ||||
| ply | Cytolytic toxin that also activates complement. An important determinant of virulence in in vivo models of disease. Wide range of effects on host immune components at sub-lytic concentrations. | P | P | P |
| pspA | Prevents binding of C3 onto pneumococcal surface. Also binds lactoferrin. | P | P | P* |
| lytA | Digests the cell wall, which results in the release of ply. | P | P | P |
| psaA | Component of the ABC transport system, which is involved in resistance to oxidative stress. | P | P | P |
| piaA and piuA | Component of the ABC transport system. | P | P | P |
| nanA and nanB | Aid colonization by revealing receptors for adherence, modifying the surfaces of competing bacteria that are within the same niche and/or modifying the function of host clearance glycoproteins. | P | P | P |
| igA | Cleaves human IgA1. | P | P | P |
| srtA | Responsible for anchoring most LXPTG-containing proteins. | P | P | P |
Table 3. Streptococcus pneumoniae genetic determinants involved in colonization, competition, disease, antibiotic resistance and natural transformation (continued).
|
Non-PCV7 clones
|
||||
|---|---|---|---|---|
| Pneumococcal genetic determinants | Main role | 6A | 15A | 19A |
| ST2191 | ST63 | ST276 | ||
| Nature of the drug resistance genes | ||||
| Tetracycline (tetM) | Resistance to tetracycline. | P | P | P |
| Clindamycin (ermB) | Resistance to clindamycin. | M | P | P |
| Erythromycin (ermB) | Resistance to erythromycin. | M | P# | P |
| Penicillin-binding proteins (pbp2X,pbp1A, pbp1B, pbp2A, pbp2B) | Resistance to penicillin. | P | P | P |
| Natural transformation | ||||
| Competence operon (comABCDE) | Development of a competence state enabling DNA uptake for genetic transformation. | P | P | P |
The non-PCV7 clones are highly competent in colonizing and invading adjacent tissues asymptomatically
A murine colonization model was used to assess the capacity of the three major non-PCV7 clones to colonize the mouse nasal mucosa and invade adjacent tissues. To this end, mice were intranasally challenged with a 10-µL inoculum of each of the clones and were followed for a period of three weeks.
The 6A (ST2191) clone was observed in the nasopharynx at all time points over the 21-day experimental period, being also very often isolated from the nasopharyngeal adjacent tissues (lungs, olfactory bulbs, brain and middle ear), and was never present in the blood (Table 4). Out of 24 mice sacrificed, 79% showed the 6A clone colonizing the nasopharynx, while 71% presented it in the olfactory bulbs, 42% in the brain, 21% in the middle ear and 17% in the lungs (Table 4).
Table 4. Colonization and infection with the 6A clone.
| Colonization and infection1
|
|||||||
|---|---|---|---|---|---|---|---|
| Time (Days) | Mice | Nasopharynx | Olfactory bulbs | Brain | Lungs | Middle ear | Blood |
| 2 | 5 | 100% | 100% | 60% | 60% | 60% | 0% |
| 7 | 6 | 100% | 100% | 83% | 0% | 17% | 0% |
| 14 | 6 | 67% | 50% | 0% | 0% | 17% | 0% |
| 21 | 7 | 57% | 43% | 29% | 14% | 0% | 0% |
| Total | 24 | 79% | 71% | 42% | 17% | 21% | 0% |
| Total CFU recovered2 | |||||||
| Time (Days) | Mice | Nasopharynx | Olfactory bulbs | Brain | Lungs | Middle ear | Blood |
| 2 | 5 | 7.7E+04 | 1.8E+03 | 7.8E+01 | 5.8E+01 | 3.4E+01 | 0.0E+00 |
| 7 | 6 | 2.7E+04 | 4.3E+02 | 4.8E+02 | 0.0E+00 | 1.8E+00 | 0.0E+00 |
| 14 | 6 | 8.8E+02 | 1.2E+01 | 0.0E+00 | 0.0E+00 | 1.2E+00 | 0.0E+00 |
| 21 | 7 | 1.3E+03 | 5.0E+00 | 7.9E+00 | 3.7E+00 | 0.0E+00 | 0.0E+00 |
| Total | 24 | 2.7E+04 | 5.6E+02 | 1.4E+02 | 1.6E+01 | 9.4E+00 | 0.0E+00 |
At day 2, 7, 14, and 21 after intranasal inoculation (10µL) of the mice, several tissues (nasopharynx, olfactory bulbs, brain, lungs, middle ear and blood) were sampled to assess for the presence of live pneumococci.
1 Percentage of mice presenting pneumococci in the different tissues.
2 Average number of CFUs per mouse in the different tissues.
The 15A (ST63) clone was found in the nasopharynx, olfactory bulbs, brain, lungs and middle ear at all time points but was absent from the blood (Table 5). Out of 24 mice sacrificed, 96% had the 15A clone colonizing the nasopharynx, 75% presented it in the olfactory bulbs, 67% in the lungs, 54% in the brain and 46% in the middle ear (Table 5).
Table 5. Colonization and infection with the 15A clone.
| Colonization and infection1
|
|||||||
|---|---|---|---|---|---|---|---|
| Time (Days) | Mice | Nasopharynx | Olfactory bulbs | Brain | Lungs | Middle ear | Blood |
| 2 | 6 | 100% | 100% | 100% | 83% | 83% | 0% |
| 7 | 6 | 100% | 83% | 67% | 83% | 50% | 0% |
| 14 | 6 | 100% | 67% | 33% | 83% | 17% | 0% |
| 21 | 6 | 83% | 50% | 17% | 17% | 33% | 0% |
| Total | 24 | 96% | 75% | 54% | 67% | 46% | 0% |
| Total CFU recovered2 | |||||||
| Time (Days) | Mice | Nasopharynx | Olfactory bulbs | Brain | Lungs | Middle ear | Blood |
| 2 | 6 | 9.2E+04 | 1.9E+03 | 4.4E+02 | 1.2E+04 | 1.3E+01 | 0.0E+00 |
| 7 | 6 | 6.9E+04 | 5.1E+02 | 1.4E+02 | 4.8E+04 | 3.3E+00 | 0.0E+00 |
| 14 | 6 | 1.3E+04 | 6.6E+01 | 1.9E+01 | 8.2E+03 | 1.8E+00 | 0.0E+00 |
| 21 | 6 | 1.4E+04 | 4.2E+01 | 1.8E+01 | 3.4E+01 | 1.8E+00 | 0.0E+00 |
| Total | 24 | 4.7E+04 | 6.2E+02 | 1.5E+02 | 1.7E+04 | 4.9E+00 | 0.0E+00 |
At day 2, 7, 14, and 21 after intranasal inoculation (10µL) of the mice, several tissues (nasopharynx, olfactory bulbs, brain, lungs, middle ear and blood) were sampled to assess for the presence of live pneumococci.
1 Percentage of mice presenting pneumococci in the different tissues.
2 Average number of CFUs per mouse in the different tissues.
The 19A (ST276) clone was detected in the nasopharynx at all time points over the 21-day study. Concomitantly, the 19A clone was frequently isolated from the nasopharyngeal adjacent tissues (olfactory bulbs, brain, lungs and middle ear), while in the blood it was never found (Table 6). Out of 23 mice sacrificed, 78% showed the 19A clone colonizing the nasopharynx, 70% presented it in the olfactory bulbs, 48% in the middle ear and 35% in the brain and lungs (Table 6).
Table 6. Colonization and infection with the 19A clone.
| Colonization and infection1
|
|||||||
|---|---|---|---|---|---|---|---|
| Time (Days) | Mice | Nasopharynx | Olfactory bulbs | Brain | Lungs | Middle ear | Blood |
| 2 | 5 | 100% | 100% | 100% | 80% | 100% | 0% |
| 7 | 6 | 100% | 100% | 50% | 50% | 67% | 0% |
| 14 | 6 | 83% | 67% | 0% | 17% | 33% | 0% |
| 21 | 6 | 33% | 17% | 0% | 0% | 0% | 0% |
| Total | 23 | 78% | 70% | 35% | 35% | 48% | 0% |
| Total CFU recovered2 | |||||||
| Time (Days) | Mice | Nasopharynx | Olfactory bulbs | Brain | Lungs | Middle ear | Blood |
| 2 | 5 | 7.9E+04 | 3.2E+03 | 1.2E+03 | 1.6E+02 | 1.5E+02 | 0.0E+00 |
| 7 | 6 | 4.9E+04 | 4.3E+02 | 1.2E+02 | 5.1E+01 | 1.4E+01 | 0.0E+00 |
| 14 | 6 | 3.6E+03 | 7.5E+00 | 0.0E+00 | 1.8E+01 | 1.8E+00 | 0.0E+00 |
| 21 | 6 | 1.0E+03 | 3.3E+00 | 0.0E+00 | 0.0E+00 | 0.0E+00 | 0.0E+00 |
| Total | 23 | 3.3E+04 | 9.0E+02 | 3.4E+02 | 5.7E+01 | 4.2E+01 | 0.0E+00 |
At day 2, 7, 14, and 21 after intranasal inoculation (10µL) of the mice, several tissues (nasopharynx, olfactory bulbs, brain, lungs, middle ear and blood) were sampled to assess for the presence of live pneumococci.
1 Percentage of mice presenting pneumococci in the different tissues.
2 Average number of CFUs per mouse in the different tissues.
The capsular type 2 strain D39 was used as an arbitrary control in all these experiments. The D39 strain was found in the nasopharynx and the adjacent tissues at low CFU numbers, with the exception of the lungs, and was never detected in the blood (Table 7). Out of 10 mice sacrificed, 70% presented the D39 strain in the lungs, 50% presented it in the nasopharynx, 30% in the brain and 10% in the middle ear or the olfactory bulbs (Table 7).
Table 7. Colonization and infection with the control D39 strain.
| Colonization and infection1
|
|||||||
|---|---|---|---|---|---|---|---|
| Time (Days) | Mice | Nasopharynx | Olfactory bulbs | Brain | Lungs | Middle ear | Blood |
| 2 | 3 | 100% | 33% | 33% | 67% | 33% | 0% |
| 7 | 2 | 50% | 0% | 50% | 100% | 0% | 0% |
| 14 | 3 | 33% | 0% | 33% | 100% | 0% | 0% |
| 21 | 2 | 0% | 0% | 0% | 0% | 0% | 0% |
| Total | 10 | 50% | 10% | 30% | 70% | 10% | 0% |
| Total CFU recovered2 | |||||||
| Time (Days) | Mice | Nasopharynx | Olfactory bulbs | Brain | Lungs | Middle ear | Blood |
| 2 | 3 | 4.7E+03 | 1.1E+01 | 4.0E+00 | 3.3E+02 | 8.3E+00 | 0.0E+00 |
| 7 | 2 | 6.5E+02 | 0.0E+00 | 5.5E+00 | 1.5E+02 | 0.0E+00 | 0.0E+00 |
| 14 | 3 | 4.0E+00 | 0.0E+00 | 4.0E+00 | 9.3E+03 | 0.0E+00 | 0.0E+00 |
| 21 | 2 | 0.0E+00 | 0.0E+00 | 0.0E+00 | 0.0E+00 | 0.0E+00 | 0.0E+00 |
| Total | 10 | 1.3E+03 | 2.7E+00 | 3.4E+00 | 2.4E+03 | 2.1E+00 | 0.0E+00 |
At day 2, 7, 14, and 21 after intranasal inoculation (10µL) of the mice, several tissues (nasopharynx, olfactory bulbs, brain, lungs, middle ear and blood) were sampled to assess for the presence of live pneumococci.
1 Percentage of mice presenting pneumococci in the different tissues.
2 Average number of CFUs per mouse in the different tissues.
For all clones and in virtually all cases, when mice presented pneumococci in the brain, higher or similar CFU numbers were also found in the nasopharynx and olfactory bulbs of the experimental animals (data not shown). Although all the non-PCV7 clonal lineages tested in this colonization model were able to invade and persist in tissues adjacent to the nasopharynx, no mice presented any signs of disease (e.g. piloerection, eyelid closure or hunched posture) during the 21 days of the experiment (Tables 4-6).
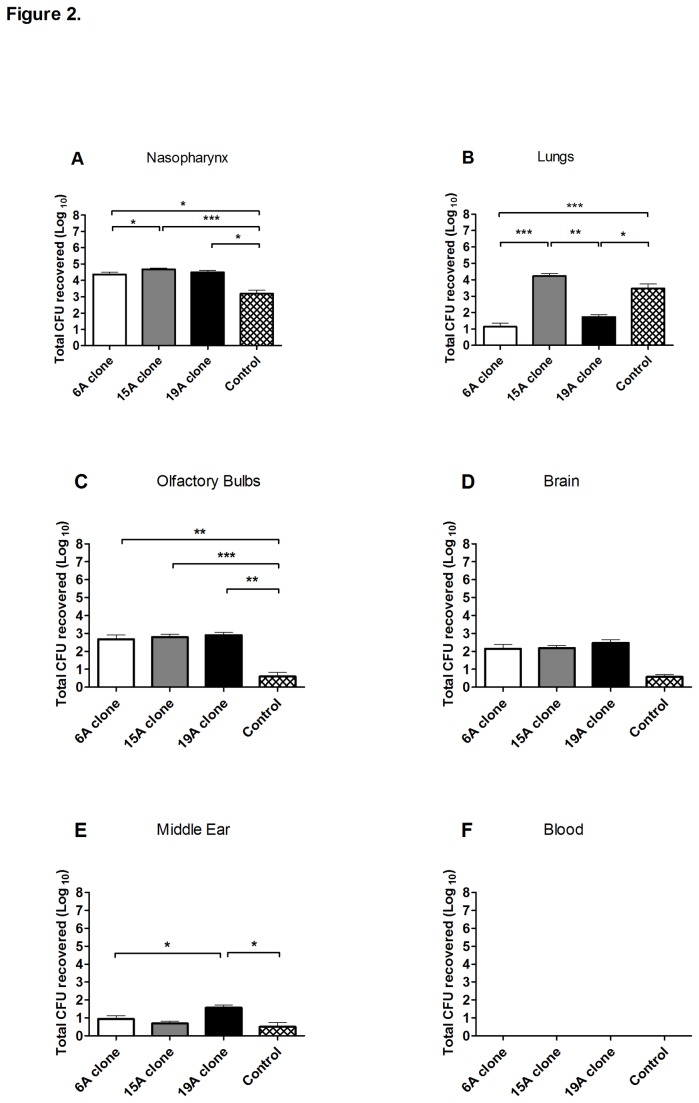
In general, the three non-PCV7 clones exhibited similar potential to asymptomatically colonize, invade and persist within the nasopharynx’s adjacent tissues (Figure 2). Along the 21-day experiment the non-PCV7 clones presented a mean of ~104 CFUs recovered from the nasopharynx, while the control D39 strain presented significantly lower CFU counts (p<0.05). In the lungs, the 15A and D39 strains presented significantly higher CFU counts than the 6A and 19A strains (p<0.05). Regarding the olfactory bulbs, the three non-PCV7 clones presented significantly higher CFU numbers than D39 strain, while in the brain no significant differences were found. Lastly, in the middle ear, 19A presented the highest mean CFU numbers (p<0.05) (Figure 2).
Figure 2. Murine colonization model: persistence of three antibiotic resistant S. pneumoniae clones in mouse tissues.
Mice were intranasally inoculated with 108 CFU of the non-PCV7 clones (6A, 15A or 19A) or the control strain (D39). At days 2, 7, 14, and 21 after inoculation, CFU counting was performed in the nasopharynx, olfactory bulbs, brain, lungs, and middle ear. Each bar represents the mean of the total CFU recovered (Log10) throughout the 21-day experiment for each strain in 10-24 mice ± SEM. (A) nasopharynx, (B) lungs, (C) olfactory bulbs, (D) brain, (E) middle ear and (F) blood. The 0 value on the Y-axis represents the absence of detectable CFUs. The asterisks indicates significant differences, * p<0.05, ** p<0.01 and *** p<0.001 according to 2-tailed Mann-Whitney U test.
Taken together, the above results indicate that the three non-PCV7 clones specialized in colonizing the host nasopharyngeal surface accompanied by asymptomatic invasion and persistence in neighbouring tissues.
The non-PCV7 clones show none or poor virulence, in the pneumonia and septicemia models respectively
To investigate the potential of the three non-PCV7 clonal types to cause lung infection, we used a murine pneumonia model. Mice were challenged intranasally with a 50-µL inoculum of either the non-PCV7 clones or the control D39 strain and survival was assessed over a 7-day period. Using 105 or 107 CFU inocula, the non-PCV7 clones appeared to be harmless, causing no death or any significant sign of disease (e.g. piloerection, eyelid closure or hunched posture) in the mice challenged. In contrast, using the highly virulent D39 strain in the same range of CFUs caused lethality in all mice (data not shown).
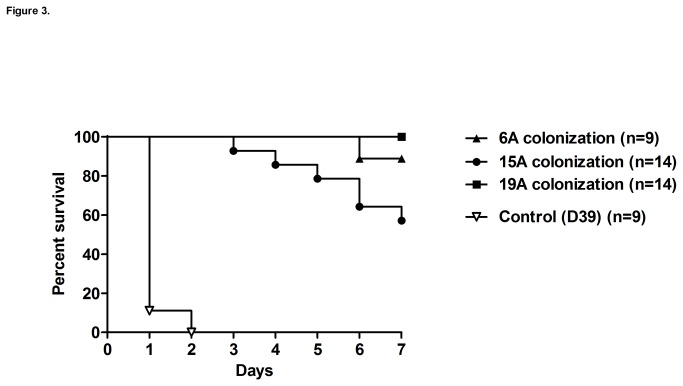
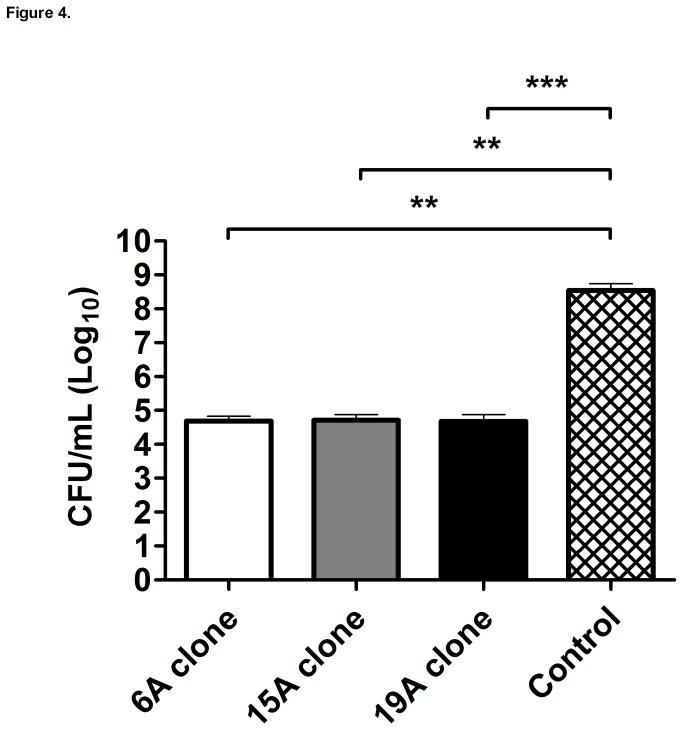
We used an intraperitoneal model to evaluate the potential of non-PCV7 clones to cause septicemia. Mice were challenged with the non-PCV7 clones or the control D39 strain and survival was assessed over a 7-day period. Using a 105 CFU inoculum the non-PCV7 clones were poorly virulent as compared to strain D39 which killed all mice within 2 days of the challenge (Figure 3). Consistent with the results from this survival experiment, we observed that during the first 24 h after the intraperitoneal challenge the D39 control strain already displayed mean titers of 3.5 x108 CFUs per mL of blood, a significantly higher load than that presented by the three non-PCV7 clones (~104 CFU/mL), which were virtually avirulent in this intraperitoneal septicemia model (Figure 4).
Figure 3. Intraperitoneal murine septicemia model: survival curves.
Mice were intraperitoneally challenged with 105 CFU of the non-PCV7 clones, 6A, 15A or 19A and the control strain (D39). Survival was followed to assess the virulence potential of the strains. Survival curves for the non-PCV7 clones were significantly different from the D39 control strain (Mantel-Cox test, p<0.05).
Figure 4. Intraperitoneal murine septicemia model: strain growth.
Mice were intraperitoneally challenged with 105 CFU of the non-PCV7 clones (6A, 15A or 19A) or the control strain (D39). At 0.5, 3, 6, 12 and 24 hours after intraperitoneal injection, CFU counting was performed in the blood. Each bar represents the log10 of the mean CFU/mL throughout the 24-h experiment for each strain in 13-14 mice ± SEM. The 0 value on the Y-axis represents the absence of detectable CFUs. The asterisks indicates significant differences, ** p<0.01 and *** p<0.001 according to 2-tailed Mann-Whitney U test.
Contribution of capsular type to colonization and disease
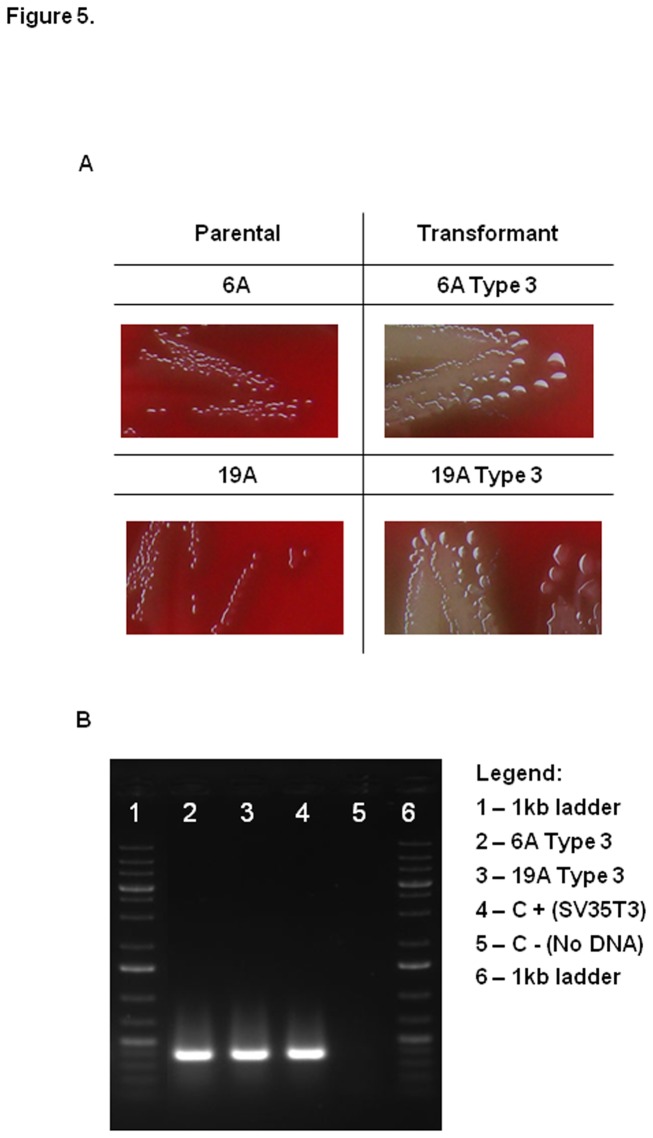
To investigate whether the capsule or the genetic background is the preponderant factor driving the potential of the non-PCV7 clones to colonize or cause disease (septicemia), we generated isogenic strains expressing different capsular types. Two non-PCV7 clones, 6A and 19A, were transformed in the laboratory to express the capsular type 3. Figure 5 shows the morphology of the parental and type 3 transformants (mucoid), as well as PCR amplification confirming the presence of the type 3 operon in the transformants’ genome.
Figure 5. Colony morphology and PCR for detection of capsular type 3.
(A) Colony morphology of parental and Type 3 transformant strains. (B) PCR amplification for detection of capsular type 3.
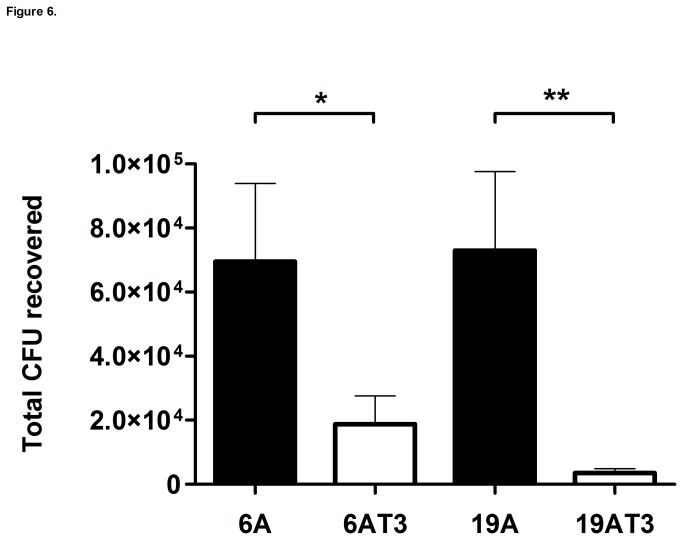
The nasopharynx of mice was colonized with the 6A or 19A clones and with the corresponding type 3 transformants (6A Type 3 and 19A Type 3). At day 2 after the challenge, the CFUs in the nasopharynx of the mice were quantified for comparison (Figure 6). The 6A clone presented on average 7x104 CFU, and the corresponding type 3 a significantly reduced bacterial load (1x104 CFUs) in the nasopharynx (p<0.05). The 19A clonal isolate also colonized each mouse with an average of 7x104 CFU, and the 19A type 3 transformant also showed a significantly lower (p<0.05) average number of CFUs recovered per mouse (3x103) as compared to the wild-type clone (Figure 6).
Figure 6. Impact of capsular polysaccharide in colonization.
Mice were intranasally challenged with S. pneumoniae strains (108 CFU), wild-type (black columns) or the type 3 transformants (white columns) in a 10-µL volume. At day 2, after inoculation, nasal lavage (500 µL) was performed to assess for the presence of live pneumococci. Values represent the mean of the total CFU ± SEM recovered from 5 to 7 mice per strain. The 0 value on the Y-axis represents the absence of detectable CFU. The asterisks indicates significant differences, * p<0.05 and ** p<0.01, according 2-tailed Mann-Whitney U test.
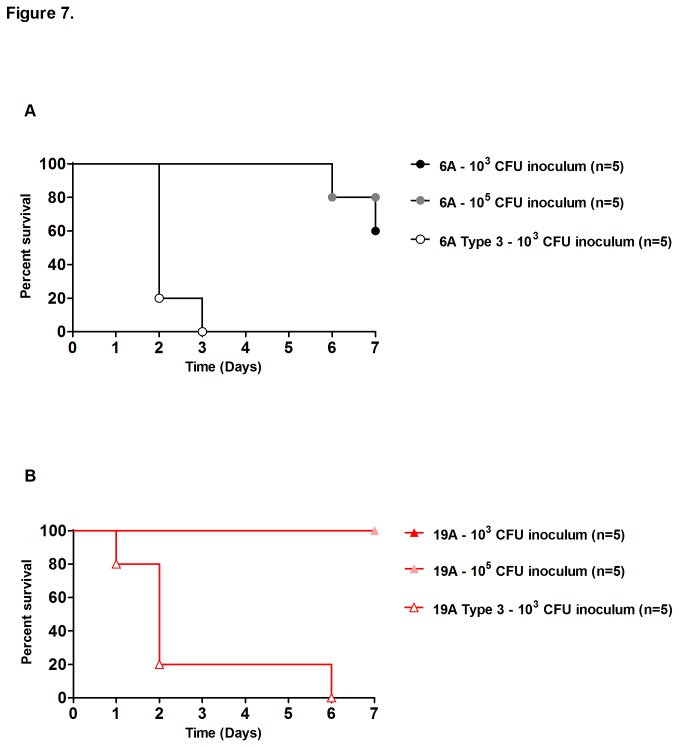
Next, mice were infected intraperitoneally with the 6A or 19A clones and the corresponding type 3 transformant strains. Following infection, mice survival was assessed over a 7-day period (Figure 7). When the infection inoculum of 6A or 19A was 103 or 105 CFU per mouse, the vast majority of the animals survived the challenge without any signs of disease (e.g. piloerection, eyelid closure or hunched posture). However, when challenged with as few as 103 CFU of the type 3 transformant strains, all animals died before day 7, presenting signs of disease as early as one day after the intraperitoneal challenge (Figure 7). Analysis of the survival curves indicated statistically significant differences (p<0.01) when comparing the parental strain with the corresponding type 3 transformant (Figure 7).
Figure 7. Impact of capsular polysaccharide on survival in a murine model of septicemia.
(A) and (B), isogenic strains expressing either capsular types 6A or 19A and the corresponding capsular type 3 transformants were injected into the peritoneal cavity of CD1 mice. Survival was followed to assess the virulence potential of the capsular types. Five mice per serotype/inoculum were tested. Survival curves for parental strains (6A and 19A clones) were significantly different from the corresponding type 3 transformants (Mantel-Cox test, p<0.01).
These results indicate that the capsular type can have a major and opposing impact on colonization and disease.
Discussion
The commercial introduction of the 7-valent anti-pneumococcal conjugate vaccine (PCV7) in Portugal, in 2001, caused an extensive change in the nature of S. pneumoniae strains colonizing the nasopharynx of pre-school aged children, which represents the major natural reservoir of this bacterial pathogen [1,37,38]. In a study conducted in Portugal, among PCV7-vaccinated children, drug resistant strains expressing capsular polysaccharides included in the vaccine were replaced by strains that were also drug resistant but produced capsules of different chemical composition [14]. The overwhelming majority (71%) of the “new” drug resistant colonizing strains expressed three serotypes, 6A, 15A or 19A – polysaccharides that are not included in the vaccine. Interestingly, these non-PCV7 serotypes were represented by “novel” PFGE types [14] and molecular typing described in the present communication further identified the major non-PCV7 strains as serotype 6A with sequence type (ST) ST2191, serotype 19A as ST276 and the capsular type 15A as ST63.
As to the possible origin of these non-PCV7 clones we compared them to the genomes of 56 fully sequenced S. pneumoniae strains (Figure 1).
The capsular type 15A (ST63) strain was found to only differ from the fully sequenced 19F clinical isolate G54 in the chemical composition of the capsular polysaccharide indicating that this lineage has the capacity to undergo in vivo capsular switch. A capsular switch may produce “vaccine escape” recombinants [39] that can avoid the vaccine-induced immune pressure. The importance of such genetic events in the in vivo evolution of S. pneumoniae has been documented [21]. In Portugal and elsewhere, both 15A and 19F variants of ST63, the Sweden15A-ST63 PMEN international clone [14], had been detected before introduction of PCV7 [14].
As to the 6A (ST2191) and the 19A (ST276) clones, the genomes of the currently available fully sequenced S. pneumoniae showed no isolates with comparable sequence identity. However, by MLST, ST276 is a SLV of Denmark14-ST230 clone [14], and this lineage has been in circulation in Portugal since the pre-PCV7 era [14].
The approximate “position” of these three non-PCV7 clones on a phylogenetic tree is shown in Figure 1.
The major purpose of the studies described in this communication was to use the adult mouse models of pneumococcal colonization and disease to evaluate the disease potential of the three major clonal types of non-PCV7 isolates that were selected in vivo among vaccine-treated Portuguese children. The main conclusion that emerged from these tests was that the three clones were highly efficient in causing colonization and long-term carriage, but exhibited only poor invasive potential.
Because of the discrepancy between high rate of colonization and poor performance in terms of virulence, it was important to test if the three major non-PCV7 clones were equipped with genetic determinants of known virulence factors. Tables 2 and 3 show that the genetic determinants of each of the major known virulence factors of S. pneumoniae were carried by the three non-PCV7 clones. The exception was the rlrA pathogenicity islet or pilus islet-1 (PI-1), mostly prevalent in the pre-PCV7 era among vaccine type strains [40], but absent from the three non-PCV7 clones studied here.
The results of experiments presented in Figure 7 clearly demonstrate that the primary factor determining the degree of virulence in these strains – as measured in the mouse intraperitoneal model – was the chemistry of the capsular polysaccharide. We replaced the 6A and the 19A capsules with the capsular type 3, by genetic transformation in the laboratory and produced type 3 derivatives of the 6A (ST2191) and 19A (ST276) that were able to induce rapid lethal infection in the mouse at low bacterial inocula in the range of 103 CFU per animal – suggesting that the bacterial structure primarily responsible for the lethality of infection was the capsular polysaccharide and the genetic background of the two non-PCV7 clones did not prevent the expression of high degrees of virulence in these laboratory produced capsular type 3 derivatives of the non-PCV7 clones.
Most interestingly, the in vitro capsular switch that caused the massive increase in virulence had the opposite effect on the colonizing potential of the same type 3 transformants, which had significantly reduced capacity to colonize the experimental animals (Figure 6). These findings parallel experience with pneumococcal infectious disease in which the most invasive capsular types (capsular type 1, 4, 5, 7F, etc) have rarely been identified in the human colonizing flora [41,42].
Several recent reports [43,44] suggest that the frequency of pneumococcal disease by non-PCV7 isolates is reduced in the post PCV7 era. These findings find a parallel with a low degree of virulence of the three non-PCV7 clones in the mouse models. The serotype 19A (ST276) and 15A (ST 63) clones have been identified as S. pneumoniae clonal types most frequently recovered from pneumococcal infections worldwide – in countries that introduced the PCV7 vaccine [15,16,45,46]. For reasons unknown, representatives of the third major colonizing clone with serotype 6A (ST2191) have not been recovered from pneumococcal disease. In contrast, colonization by each of the three major non-PCV7 clonal lineages have been widely reported [14,17,19].
No data are currently available concerning the severity of disease caused by drug resistant non-PCV7 strains. Limited experience with another multi-drug resistant clone – PMEN1 – documented that this clone had the capacity to cause the entire spectrum of pneumococcal diseases and thus appeared to pay “no price” for the drug resistance in terms of a diminished virulence [47].
The major clonal types selected for characterization in this study showed resistance to several antibacterial agents. In a previous study the frequency of resistant strains among colonizing isolates recovered from vaccinated and non-vaccinated children were shown to be comparable. For instance the frequency of penicillin resistance was ~24% among the colonizing pneumococci recovered from vaccinated and non-vaccinated attendees in day-care centers in Portugal [14]. The major clonal types selected for characterization showed resistance to penicillin in the range of 0.19 to 0.75 µg/mL suggesting the presence of mutant pbp genes [48]. Whether or not the murM determinant previously implied in the mechanism of resistance to penicillin carries mutations remains to be determined in the non-PCV7 strains [49].
The newly emerged non-PCV7 strains carry the same antibiotic resistance mechanisms against tetracycline (tetM), clindamycin and erythromycin (ermB) that were already identified in the vaccine type strains.
The newly emerged clones also carry the competence operon (Table 3) and acquisition of antibiotic resistance determinants from the majority vaccine type strains through genetic transformation seems to have occurred during the pre-PCV7 era [49]. Clones 6A (ST2191), 19A (ST276) and 15A (ST63) must have pre-existed as minority components of the complex nasopharyngeal flora in the era prior to the introduction of the PCV7 vaccine [9,50] and must have “witnessed” the same selective pressure of antibiotic use that has led to the emergence of drug resistance lineages in the pre-PCV7 era. This notion is consistent with the surprisingly high frequency of drug resistance strains detected among the newly emerged non-PCV7 strains which is similar (about 40%) to the frequency of antibiotic resistance among colonizing strains characterized during the pre-PCV7 era [14,51,52].
We observed in the mouse model of colonization that the three major non-PCV7 clones have the capacity to colonize the nasopharyngeal mucosa for prolonged times and to invade and prevail within neighboring tissue (olfactory bulbs, brain, lungs or the middle ear) without causing lethal infections or any signs of disease in the experimental animals (Figure 2). This latter finding strongly suggests that stable pneumococcal populations can be established within tissues, protecting pneumococci against mucus flow or competition with the microflora present on the nasopharynx’s surface. Pneumococci from within the nasopharynx mucosal tissue [53] or deeper tissues could then constitute a reservoir able to “seed” the nasopharyngeal surface, ensuring recurrent colonization of this mucosa.
Interestingly, and in accordance with previously reported data [54,55], the colonized mice with brain infection did not present bacteria in the blood, clearly showing that pneumococci can enter the brain directly from the nasopharynx bypassing the blood [55].
In the experimental model we used adult outbred mouse. However, pneumococcal disease, particularly otitis media, is a most frequent affliction in children and a more appropriate model to evaluate the disease potential of the three non-PCV7 clones would be to also test their virulence potential in the infant mouse model, ideally in combination with a simultaneous viral infection, which represents the most frequent scenario of human pediatric disease [56]. Experiments evaluating the disease potential of the three non-PCV7 clones in such an infant mouse model are currently in progress.
Supporting Information
Figure S1, PFGE clonal types associated to serotypes 6A, 15A and 19A. Table S1, List of the 59 sequenced Streptococcus pneumoniae strains used in the phylogenetic analysis.
(PDF)
Acknowledgments
We thank Florian Gehre (the Rockefeller University, NY, USA), Susana Lima and Léa Zinck (Champalimaud Neuroscience Programme, Oeiras, Portugal) and Dinora Lopes, Vitor Santos and Rosário Tito (Instituto de Higiene e Medicina Tropical – IHMT) for assistance with the experimental animal model procedures.
Funding Statement
This work was supported by a grant (Bridges to Better Medicine Technology Innovation Fund) from the Rockefeller University to AT and the PNEUMOPATH project (contract HEALTH-F3-2009-222983) from the European Commission to HL. NF was supported by a Postdoctoral fellowship from Fundação para a Ciência e a Tecnologia (SFRH/BPD/73229/2010). NLH was supported by the National Institute on Deafness And Other Communication Disorders of the National Institutes of Health (NIH) Award Number R00DC011322. GDH, EP, JE and AA were supported by Allegheny General Hospital, Allegheny-Singer Research Institute and NIH grants DC002148 (GDE), DC02148 – 16S1 (GDE), AI080935 (GDE). The funders had no role in study design, data collection and analysis, decisions to publish, or preparation of the manuscript.
References
- 1. de Lencastre H, Tomasz A (2002) From ecological reservoir to disease: the nasopharynx, day-care centres and drug-resistant clones of Streptococcus pneumoniae . J Antimicrob Chemother 50 Suppl S2: 75-81. PubMed: 12556436. [DOI] [PubMed] [Google Scholar]
- 2. Bogaert D, De Groot R, Hermans PW (2004) Streptococcus pneumoniae colonisation: the key to pneumococcal disease. Lancet Infect Dis 4: 144-154. doi:10.1016/S1473-3099(04)00938-7. PubMed: 14998500. [DOI] [PubMed] [Google Scholar]
- 3. O’Brien KL, Wolfson LJ, Watt JP, Henkle E, Deloria-Knoll M et al. (2009) Burden of disease caused by Streptococcus pneumoniae in children younger than 5 years: global estimates. Lancet 374: 893-902. doi:10.1016/S0140-6736(09)61204-6. PubMed: 19748398. [DOI] [PubMed] [Google Scholar]
- 4. McGee L, McDougal L, Zhou J, Spratt BG, Tenover FC et al. (2001) Nomenclature of major antimicrobial-resistant clones of Streptococcus pneumoniae defined by the pneumococcal molecular epidemiology network. J Clin Microbiol 39: 2565-2571. doi:10.1128/JCM.39.7.2565-2571.2001. PubMed: 11427569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Tleyjeh IM, Tlaygeh HM, Hejal R, Montori VM, Baddour LM (2006) The impact of penicillin resistance on short-term mortality in hospitalized adults with pneumococcal pneumonia: a systematic review and meta-analysis. Clin Infect Dis 42: 788-797. doi:10.1086/500140. PubMed: 16477555. [DOI] [PubMed] [Google Scholar]
- 6. Calix JJ, Porambo RJ, Brady AM, Larson TR, Yother J et al. (2012) Biochemical, genetic, and serological characterization of two capsule subtypes among Streptococcus pneumoniae Serotype 20 strains: discovery of a new pneumococcal serotype. J Biol Chem 287: 27885-27894. doi:10.1074/jbc.M112.380451. PubMed: 22736767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Whitney CG, Farley MM, Hadler J, Harrison LH, Lexau C et al. (2000) Increasing prevalence of multidrug-resistant Streptococcus pneumoniae in the United States. N Engl J Med 343: 1917-1924. doi:10.1056/NEJM200012283432603. PubMed: 11136262. [DOI] [PubMed] [Google Scholar]
- 8. CDC (2000) Preventing pneumococcal disease among infants and young children: recommendations from the Advisory Committee on Immunizations Practices. MMWR Morb Mortal Wkly Rep 49: 1-38. PubMed: 10993565.10993565 [Google Scholar]
- 9. Frazão N, Sá-Leão R, de Lencastre H (2010) Impact of a single dose of the 7-valent pneumococcal conjugate vaccine on colonization. Vaccine 28: 3445-3452. doi:10.1016/j.vaccine.2010.02.070. PubMed: 20197144. [DOI] [PubMed] [Google Scholar]
- 10. van Gils EJ, Veenhoven RH, Hak E, Rodenburg GD, Bogaert D et al. (2009) Effect of reduced-dose schedules with 7-valent pneumococcal conjugate vaccine on nasopharyngeal pneumococcal carriage in children: a randomized controlled trial. JAMA 302: 159-167. doi:10.1001/jama.2009.975. PubMed: 19584345. [DOI] [PubMed] [Google Scholar]
- 11. WHO (2007) Pneumococcal conjugate vaccine for childhood immunization - WHO position paper. Wkly Epidemiol Rec 82: 93-104. PubMed: 17380597. [PubMed] [Google Scholar]
- 12. Dagan R, Klugman KP (2008) Impact of conjugate pneumococcal vaccines on antibiotic resistance. Lancet Infect Dis 8: 785-795. doi:10.1016/S1473-3099(08)70281-0. PubMed: 19022193. [DOI] [PubMed] [Google Scholar]
- 13. Hanage WP, Huang SS, Lipsitch M, Bishop CJ, Godoy D et al. (2007) Diversity and antibiotic resistance among nonvaccine serotypes of Streptococcus pneumoniae carriage isolates in the post-heptavalent conjugate vaccine era. J Infect Dis 195: 347-352. doi:10.1086/510249. PubMed: 17205472. [DOI] [PubMed] [Google Scholar]
- 14. Frazão N, Brito-Avô A, Simas C, Saldanha J, Mato R et al. (2005) Effect of the seven-valent conjugate pneumococcal vaccine on carriage and drug resistance of Streptococcus pneumoniae in healthy children attending day-care centers in Lisbon. Pediatr Infect Dis J 24: 243-252. doi:10.1097/01.inf.0000154326.77617.3e. PubMed: 15750461. [DOI] [PubMed] [Google Scholar]
- 15. Aguiar SI, Pinto FR, Nunes S, Serrano I, Melo-Cristino J et al. (2009) Denmark14-230 clone as an increasing cause of pneumococcal infection in Portugal within a background of diverse serotype 19A lineages. J Clin Microbiol 48: 101-108. PubMed: 19864476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Gertz RE Jr., Li Z, Pimenta FC, Jackson D, Juni BA et al. (2010) Increased penicillin nonsusceptibility of nonvaccine-serotype invasive pneumococci other than serotypes 19A and 6A in post-7-valent conjugate vaccine era. J Infect Dis 201: 770-775. doi:10.1086/650496. PubMed: 20178139. [DOI] [PubMed] [Google Scholar]
- 17. Hanage WP, Bishop CJ, Huang SS, Stevenson AE, Pelton SI et al. (2011) Carried pneumococci in Massachusetts children: the contribution of clonal expansion and serotype switching. Pediatr Infect Dis J 30: 302-308. doi:10.1097/INF.0b013e318201a154. PubMed: 21085049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Muñoz-Almagro C, Esteva C, de Sevilla MF, Selva L, Gene A et al. (2009) Emergence of invasive pneumococcal disease caused by multidrug-resistant serotype 19A among children in Barcelona. J Infect 59: 75-82. doi:10.1016/j.jinf.2009.05.012. PubMed: 19576637. [DOI] [PubMed] [Google Scholar]
- 19. Vestrheim DF, Høiby EA, Aaberge IS, Caugant DA (2010) Impact of a pneumococcal conjugate vaccination program on carriage among children in Norway. Clin Vaccine Immunol 17: 325-334. doi:10.1128/CVI.00435-09. PubMed: 20107006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Enright MC, Spratt BG (1998) A multilocus sequence typing scheme for Streptococcus pneumoniae: identification of clones associated with serious invasive disease. Microbiology 144(11): 3049-3060. doi:10.1099/00221287-144-11-3049. [DOI] [PubMed] [Google Scholar]
- 21. Hu FZ, Eutsey R, Ahmed A, Frazao N, Powell E et al. (2012) In vivo capsular switch in Streptococcus pneumoniae - analysis by whole genome sequencing. PLOS ONE 7: e47983. doi:10.1371/journal.pone.0047983. PubMed: 23144841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Kharat AS, Tomasz A (2006) Drastic reduction in the virulence of Streptococcus pneumoniae expressing type 2 capsular polysaccharide but lacking choline residues in the cell wall. Mol Microbiol 60: 93-107. doi:10.1111/j.1365-2958.2006.05082.x. PubMed: 16556223. [DOI] [PubMed] [Google Scholar]
- 23. Sørensen UB (1993) Typing of pneumococci by using 12 pooled antisera. J Clin Microbiol 31: 2097-2100. PubMed: 8370735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Pai R, Gertz RE, Beall B (2006) Sequential multiplex PCR approach for determining capsular serotypes of Streptococcus pneumoniae isolates. J Clin Microbiol 44: 124-131. doi:10.1128/JCM.44.1.124-131.2006. PubMed: 16390959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Lai WC, Pakes SP, Stefanu C, Lu YS (1986) Comparison of Chalquest and Hayflick media, with and without ammonium reineckate, for isolating Mycoplasma pulmonis from rats. J Clin Microbiol 23: 817-821. PubMed: 3711268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Darling AC, Mau B, Blattner FR, Perna NT (2004) Mauve: multiple alignment of conserved genomic sequence with rearrangements. Genome Res 14: 1394-1403. doi:10.1101/gr.2289704. PubMed: 15231754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Katoh K, Toh H (2008) Recent developments in the MAFFT multiple sequence alignment program. Brief Bioinform 9: 286-298. doi:10.1093/bib/bbn013. PubMed: 18372315. [DOI] [PubMed] [Google Scholar]
- 28. Stamatakis A (2006) RAxML-VI-HPC: maximum likelihood-based phylogenetic analyses with thousands of taxa and mixed models. Bioinformatics 22: 2688-2690. doi:10.1093/bioinformatics/btl446. PubMed: 16928733. [DOI] [PubMed] [Google Scholar]
- 29. Rambaut A (2012)igTree 1: 4 https://tree.bio.ed.ac.uk/software/figtree/ Available: 2012 [Google Scholar]
- 30. Donati C, Hiller NL, Tettelin H, Muzzi A, Croucher NJ et al. (2010) Structure and dynamics of the pan-genome of Streptococcus pneumoniae and closely related species. Genome Biol 11: R107. doi:10.1186/gb-2010-11-2-107. PubMed: 21034474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Dopazo J, Mendoza A, Herrero J, Caldara F, Humbert Y et al. (2001) Annotated draft genomic sequence from a Streptococcus pneumoniae type 19F clinical isolate. Microb Drug Resist 7: 99-125. doi:10.1089/10766290152044995. PubMed: 11442348. [DOI] [PubMed] [Google Scholar]
- 32. Glover DT, Hollingshead SK, Briles DE (2008) Streptococcus pneumoniae surface protein PcpA elicits protection against lung infection and fatal sepsis. Infect Immun 76: 2767-2776. doi:10.1128/IAI.01126-07. PubMed: 18391008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Hiller NL, Eutsey RA, Powell E, Earl JP, Janto B et al. (2011) Differences in genotype and virulence among four multidrug-resistant Streptococcus pneumoniae isolates belonging to the PMEN1 clone. PLOS ONE 6: e28850. doi:10.1371/journal.pone.0028850. PubMed: 22205975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Kadioglu A, Weiser JN, Paton JC, Andrew PW (2008) The role of Streptococcus pneumoniae virulence factors in host respiratory colonization and disease. Nat Rev Microbiol 6: 288-301. doi:10.1038/nrmicro1871. PubMed: 18340341. [DOI] [PubMed] [Google Scholar]
- 35. Sánchez-Beato AR, López R, García JL (1998) Molecular characterization of PcpA: a novel choline-binding protein of Streptococcus pneumoniae . FEMS Microbiol Lett 164: 207-214. doi:10.1016/S0378-1097(98)00206-7. PubMed: 9675866. [DOI] [PubMed] [Google Scholar]
- 36. Tettelin H, Nelson KE, Paulsen IT, Eisen JA, Read TD et al. (2001) Complete genome sequence of a virulent isolate of Streptococcus pneumoniae . Science 293: 498-506. doi:10.1126/science.1061217. PubMed: 11463916. [DOI] [PubMed] [Google Scholar]
- 37. Sá-Leão R, Tomasz A, de Lencastre H (2001) Multilocus sequence typing of Streptococcus pneumoniae clones with unusual drug resistance patterns: genetic backgrounds and relatedness to other epidemic clones. J Infect Dis 184: 1206-1210. doi:10.1086/323663. PubMed: 11598847. [DOI] [PubMed] [Google Scholar]
- 38. Simões AS, Pereira L, Nunes S, Brito-Avô A, de Lencastre H et al. (2011) Clonal evolution leading to maintenance of antibiotic resistance rates among colonizing Pneumococci in the PCV7 era in Portugal. J Clin Microbiol 49: 2810-2817. doi:10.1128/JCM.00517-11. PubMed: 21632898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Brueggemann AB, Pai R, Crook DW, Beall B (2007) Vaccine escape recombinants emerge after pneumococcal vaccination in the United States. PLOS Pathog 3: e168. doi:10.1371/journal.ppat.0030168. PubMed: 18020702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Basset A, Trzcinski K, Hermos C, O’Brien KL, Reid R et al. (2007) Association of the pneumococcal pilus with certain capsular serotypes but not with increased virulence. J Clin Microbiol 45: 1684-1689. doi:10.1128/JCM.00265-07. PubMed: 17392439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Kronenberg A, Zucs P, Droz S, Mühlemann K (2006) Distribution and invasiveness of Streptococcus pneumoniae serotypes in Switzerland, a country with low antibiotic selection pressure, from 2001 to 2004. J Clin Microbiol 44: 2032-2038. doi:10.1128/JCM.00275-06. PubMed: 16757594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Sá-Leão R, Pinto F, Aguiar S, Nunes S, Carriço JA et al. (2011) Analysis of invasiveness of pneumococcal serotypes and clones circulating in Portugal before widespread use of conjugate vaccines reveals heterogeneous behavior of clones expressing the same serotype. J Clin Microbiol 49: 1369-1375. doi:10.1128/JCM.01763-10. PubMed: 21270219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Yildirim I, Hanage WP, Lipsitch M, Shea KM, Stevenson A et al. (2010) Serotype specific invasive capacity and persistent reduction in invasive pneumococcal disease. Vaccine 29: 283-288. doi:10.1016/j.vaccine.2010.10.032. PubMed: 21029807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Yildirim I, Stevenson A, Hsu KK, Pelton SI (2012) Evolving picture of invasive pneumococcal disease in massachusetts children: a comparison of disease in 2007-2009 with earlier periods. Pediatr Infect Dis J 31: 1016-1021. PubMed: 22673142. [DOI] [PubMed] [Google Scholar]
- 45. Ardanuy C, Tubau F, Pallares R, Calatayud L, Domínguez MA et al. (2009) Epidemiology of invasive pneumococcal disease among adult patients in barcelona before and after pediatric 7-valent pneumococcal conjugate vaccine introduction, 1997-2007. Clin Infect Dis 48: 57-64. doi:10.1086/597108. PubMed: 19035779. [DOI] [PubMed] [Google Scholar]
- 46. Mahjoub-Messai F, Doit C, Koeck JL, Billard T, Evrard B et al. (2009) Population snapshot of Streptococcus pneumoniae serotype 19A isolates before and after introduction of seven-valent pneumococcal Vaccination for French children. J Clin Microbiol 47: 837-840. doi:10.1128/JCM.01547-08. PubMed: 19109482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Roberts RB, Tomasz A, Corso A, Hargrave J, Severina E (2001) Penicillin-resistant Streptococcus pneumoniae in metropolitan New York hospitals: case control study and molecular typing of resistant isolates. Microb Drug Resist 7: 137-152. doi:10.1089/10766290152045011. PubMed: 11442340. [DOI] [PubMed] [Google Scholar]
- 48. Hakenbeck R, Grebe T, Zähner D, Stock JB (1999) beta-lactam resistance in Streptococcus pneumoniae: penicillin-binding proteins and non-penicillin-binding proteins. Mol Microbiol 33: 673-678. doi:10.1046/j.1365-2958.1999.01521.x. PubMed: 10447877. [DOI] [PubMed] [Google Scholar]
- 49. Filipe SR, Tomasz A (2000) Inhibition of the expression of penicillin resistance in Streptococcus pneumoniae by inactivation of cell wall muropeptide branching genes. Proc Natl Acad Sci U S A 97: 4891-4896. doi:10.1073/pnas.080067697. PubMed: 10759563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Croucher NJ, Finkelstein JA, Pelton SI, Mitchell PK, Lee GM et al. (2013) Population genomics of post-vaccine changes in pneumococcal epidemiology. Nat Genet, 45: 656–63. PubMed: 23644493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Mato R, Sanches IS, Simas C, Nunes S, Carriço JA et al. (2005) Natural history of drug-resistant clones of Streptococcus pneumoniae colonizing healthy children in Portugal. Microb Drug Resist 11: 309-322. doi:10.1089/mdr.2005.11.309. PubMed: 16359190. [DOI] [PubMed] [Google Scholar]
- 52. Sá-Leão R, Nunes S, Brito-Avô A, Frazão N, Simões AS et al. (2009) Changes in pneumococcal serotypes and antibiotypes carried by vaccinated and unvaccinated day-care centre attendees in Portugal, a country with widespread use of the seven-valent pneumococcal conjugate vaccine. Clin Microbiol Infect 15: 1002-1007. doi:10.1111/j.1469-0691.2009.02775.x. PubMed: 19392883. [DOI] [PubMed] [Google Scholar]
- 53. Briles DE, Novak L, Hotomi M, van Ginkel FW, King J (2005) Nasal colonization with Streptococcus pneumoniae includes subpopulations of surface and invasive pneumococci. Infect Immun 73: 6945-6951. doi:10.1128/IAI.73.10.6945-6951.2005. PubMed: 16177374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Marra A, Brigham D (2001) Streptococcus pneumoniae causes experimental meningitis following intranasal and otitis media infections via a nonhematogenous route. Infect Immun 69: 7318-7325. doi:10.1128/IAI.69.12.7318-7325.2001. PubMed: 11705903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. van Ginkel FW, McGhee JR, Watt JM, Campos-Torres A, Parish LA et al. (2003) Pneumococcal carriage results in ganglioside-mediated olfactory tissue infection. Proc Natl Acad Sci U S A 100: 14363-14367. doi:10.1073/pnas.2235844100. PubMed: 14610280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Heikkinen T, Thint M, Chonmaitree T (1999) Prevalence of various respiratory viruses in the middle ear during acute otitis media. N Engl J Med 340: 260-264. doi:10.1056/NEJM199901283400402. PubMed: 9920949. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Figure S1, PFGE clonal types associated to serotypes 6A, 15A and 19A. Table S1, List of the 59 sequenced Streptococcus pneumoniae strains used in the phylogenetic analysis.
(PDF)