Summary
The gastrointestinal tract is lined by a series of epithelia that share functional requirements, but also have distinct, highly specialized roles. Distinct populations of somatic stem cells (SCs) regenerate these epithelia, yet the mechanisms that maintain regional identities of these SCs are not well understood. Here, we identify a role for the BMP-like Dpp signaling pathway in diversifying regenerative processes in the adult gastrointestinal tract of Drosophila. Dpp secreted from enterocytes at the boundary between the posterior midgut (PM) and the middle midgut (MM) sets up a morphogen gradient that selectively directs copper cell (CC) regeneration from gastric SCs in the MM and thus determines the size of the CC region. In vertebrates, deregulation of BMP signaling has been associated with Barrett’s metaplasia, where the squamous esophageal epithelium is replaced by a columnar epithelium, suggesting that the maintenance of regional SC identities by BMP is conserved.
Introduction
The intestinal epithelium of most animals undergoes rapid regeneration both in homeostatic conditions as well as in response to tissue damage. Mechanisms that ensure the functional diversity of newly formed intestinal cells have to be sustained throughout the lifespan of the organism. Such mechanisms are poorly understood, yet are likely to include short-range signaling interactions and cell autonomous cues that maintain diverse stem cell identities (such as the expression of region-specific homeotic factors), as well as long-range signals that impart positional information along the gastrointestinal tract, and are thus the basis for functional compartmentalization of this tissue (Barker et al., 2010).
Insults that perturb the maintenance of functional compartmentalization can have significant deleterious consequences for the animal. One example is Barrett’s metaplasia, in which the esophageal squamous epithelium acquires properties that are reminiscent of the gastric or intestinal epithelium. This transformation has been associated with acid reflux disease and is believed to be a cause of esophageal adenocarcinomas (Dvorak et al., 2011; Milano et al., 2007; Peters and Avisar, 2010). The histogenesis of Barrett’s metaplasia remains unclear, and has been proposed to include proximal migration of the gastric cardia, re-differentiation of the squamous epithelium, or colonization by cells from the esophageal gland ducts (Jankowski et al., 2000; Leedham et al., 2008; Maley et al., 2006; Sharma, 2009). Abnormal BMP signaling has been implicated in Barrett’s esophagus (Dvorak et al., 2011; Milano et al., 2007), yet the specific signaling mechanisms causing epithelial transformation are not well understood.
The adult Drosophila intestinal epithelium constitutes a productive, genetically accessible model system to study the maintenance of epithelial homeostasis, regenerative capacity, and stem cell function (Biteau et al., 2011). Based on morphological and functional characteristics, the midgut of flies can be subdivided into the anterior midgut (AM), the middle midgut (MM), which contains an acidic gastric or copper cell region (CCR (Dubreuil, 2004)), and the posterior midgut (PM). Stem cells (ISCs) can be found in each of these compartments (Biteau et al., 2011; Hou, 2010; Strand and Micchelli, 2011). ISCs in the PM express escargot (esg) and Delta (Dl), and divide asymmetrically to give rise to a precursor cell (the Dl−/esg+ Enteroblast, EB), which will further differentiate into either Pdm - expressing Enterocytes (ECs) or prospero (pros) - expressing Enteroendocrine cells (EEs) (Micchelli and Perrimon, 2006; Ohlstein and Spradling, 2006, 2007). In the CCR, esg+ gastric stem cells (GSSCs) respond to stress by inducing regeneration of three different cell types: Dve+/Labial+/Cut+ Copper cells (CCs, which secrete hydrochloric acid), Dve+/weak Labial+/Cut− interstitial cells and Pros+ endocrine cells (Strand and Micchelli, 2011).
The BMP signaling pathway has an evolutionarily conserved, recurrent function in development and homeostasis of gastrointestinal epithelia (Haramis et al., 2004; Que et al., 2006). In the mouse, intricate regulation of Bmp signaling activity is required for establishment of the esophageal epithelium (Jacobs et al., 2012). Bmp-4 activity has been proposed to influence cell identities in this tissue by regulating the expression of the homeotic gene Cdx (Que et al., 2006). In the mouse intestine, Bmp signaling is essential for differentiation of the secretory cell lineage (Auclair et al., 2007).
Similar to its role in vertebrates, the Bmp2/4 orthologue Dpp (Affolter and Basler, 2007) is involved in multiple aspects of gastrointestinal development in Drosophila (Nakagoshi, 2005). During embryogenesis, a morphogenetic gradient of Dpp signaling activity induces the high-threshold target labial (lab, encoding a homeobox transcription factor) and the low-threshold target dve, resulting in the specification of copper cells (Lab+) and interstitial cells (Dve+)(Nakagoshi, 2005). During larval development, in turn, Dpp is secreted by transient niche cells, preventing differentiation of adult midgut progenitors (Mathur et al., 2010), while in the adult posterior midgut, Dpp has been identified as a potential survival factor for enterocytes, secreted by tracheal cells that contact the intestine (Li et al., 2013). Whether Dpp signaling has additional functions in other regions of the adult gut has not been established. Here, we demonstrate that differential Dpp signaling activity segregates stem cell identities along the anterior-posterior axis of the adult gastrointestinal epithelium, and specifically regulates copper cell differentiation.
Results and Discussion
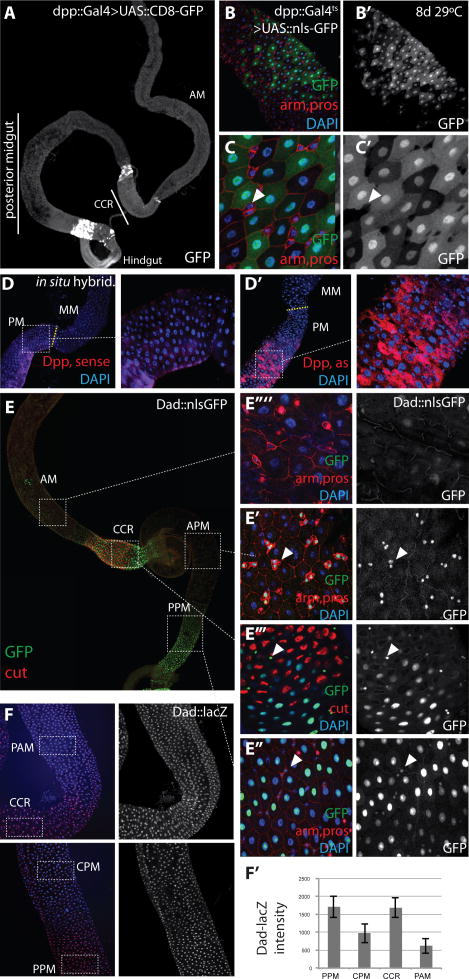
Using a Dpp::Gal4 promoter fusion that recapitulates Dpp expression during development (Staehling-Hampton et al., 1994), we observed Dpp expression in several regions of the adult gut, including in two groups of Enterocytes at the anterior and posterior ends of the posterior midgut (Fig. 1A). Since this expression pattern differs from the expression pattern of a Dpp::lacZ enhancer trap that has been used to describe expression of Dpp in tracheal cells (Li et al., 2013), we compared the expression of the two reporters in the gut. While the expression domains of both reporters overlap well in the third instar wing imaginal disc (Fig. S1A), Dpp::lacZ, but not Dpp::Gal4, was expressed in adult tracheal cells, and Dpp:Gal4, but not Dpp::lacZ was expressed in the adult intestinal epithelium (Fig. S1B, C). The Dpp::Gal4 promoter fusion and the Dpp::lacZ enhancer trap thus seem to recapitulate different aspects of Dpp gene regulation, which is known to be dynamic and complex (Schwyter et al., 1995). GFP expression in ECs in response to Dpp::Gal4 was not due to perdurance of GFP expressed during development, since expression of GFP in ECs could also be induced in the adult when developmental Gal4 activity was suppressed in a temperature-sensitive manner using Gal80ts (Fig. 1B, C, Fig. S1E; (McGuire et al., 2003)). To confirm that dpp RNA could be detected in the adult intestinal epithelium, we performed RNA in situ hybridization, and detected Dpp transcript in posterior midgut ECs close to the middle midgut (MM, containing the Copper Cell region, CCR), as well as close to the hindgut (Fig. 1D, Fig. S1F), recapitulating the expression profile observed with dpp::Gal4, UAS::GFP (Fig. S1E). ECs were identified based on their size, polyploid nuclei, and apical location within the epithelium. Interestingly, dpp expression was not uniform in ECs, but differed substantially between individual cells within the regions of high expression. This may reflect a very dynamic signal- or status- dependent regulation of dpp expression in these cells, the origin of which remains unresolved. We were unable to unambiguously confirm the expression of dpp in tracheal cells, as we observed sporadic signal using both sense and antisense probes against dpp in this tissue (Fig. S1D). However, we confirmed that the in situ signal in ECs indeed corresponds to dpp expression, by performing in situ hybridization on intestines of flies expressing dsRNA against dpp (DppRNAi) under the control of the pan-EC driver NP1::Gal4 (Fig. S1G). dpp transcript signal in the intestinal epithelium was significantly reduced in these flies.
Figure 1. Dpp activity gradient in the adult Drosophila intestine.
(A) Overview of dpp expression (white, dpp::Gal4, UAS::GFP) in the whole midgut (AM, anterior midgut; CCR, copper cell region). Note expression posterior to the CCR and at the posterior end of the posterior midgut.
(B-B′) Dpp expression (green in B, white in B′; dpp::Gal4, tubGal80ts; UAS::GFP) in ECs of the posterior midgut. To avoid developmental GFP expression from Dpp::Gal4, flies were reared at 18°C and then shifted as adults to 29°C for 8 days before dissection (green, GFP; red, armadillo and prospero; blue, DAPI).
(C-C′) Magnification of (B-B′). Dpp is expressed in ECs, characterized by big nuclei, but not in ISCs or EBs (arrowhead), characterized by high armadillo expression, small nuclei and absence of nuclear prospero.
(D-D′) RNA in situ hybridization of dpp. Dpp transcripts are detected by a dpp antisense (as, D′) probe (red) close to the boundary between the posterior midgut (MM) and the middle midgut (MM) (genotype is w1118; NP1::Gal4; tub::Gal80ts).
(E) Overview of dad expression (green, using dad::nlsGFP) in the gastrointestinal tract (APM, anterior posterior midgut; PPM, posterior posterior midgut).
(E′-E″″) Details of dad::nlsGFP expression in different regions. Note that dad is expressed in ISCs/EBs in the APM (E′, arrowheads), in ECs and weaker in ISCs/EBs in the PPM (E″), in ECs and GSSCs in the CCR (E‴), and rarely expressed in the AM (E″″).
(F and F′) Gradient expression of Dad::lacZ (detected by immunohistochemistry using B-gal antibody, Dad::lacZ expresses a nuclear bGalactosidase). Note that dad::lacZ expression is higher in the CCR and PPM, and lower in the Central Posterior Midgut (CPM) and the Posterior Anterior Midgut (PAM). (F′) Fluorescence intensity in individual nuclei was quantified using ImageJ.
The expression profile of Dpp along the anterior-posterior axis of the gut suggested that Dpp signaling is active in the PM in a distal to central gradient and in the CCR in a posterior to anterior gradient. We tested this hypothesis using two reporters for Dpp signaling activity based on the Dpp signaling target gene Dad (Dad::lacZ, an enhancer trap line, and Dad::GFP, a promoter fusion that recapitulates the Dpp morphogen gradient in the developing wing disc, (Hamaratoglu et al., 2011)). In the wing imaginal disc, their expression domains overlap along a central to distal gradient in the anterior-posterior axis. Dad::lacZ, however, is expressed more broadly than Dad::GFP, suggesting that it responds to lower levels of Dpp signaling activity (Fig. S2A). This notion was confirmed in the adult intestine, as we found Dad::lacZ to be expressed more strongly than Dad::GFP in most ECs. Dad::GFP expression was high in the most posterior ECs of the PM, weaker in the central PM and the AM, and high in the MM, confirming the presence of gradients of Dpp signaling activity along the anterior-posterior axis of the adult intestinal epithelium. In stem cells, Dad::GFP expression was observed throughout the PM as well as in the MM (Fig. 1E, S2B, C). Similar to this pattern of Dad::GFP expression, the levels of Dad::lacZ expression vary in a distal-to-central gradient in the PM, are higher in the MM, and weaker in the AM (Fig. 1F, S2D). These results were further confirmed by staining against phosphorylated Mad, the transcription factor regulated by Dpp signaling, using the same antibody and staining protocol used in (Li et al., 2013) (Fig. S2E; note, however, that this antibody was raised against phosphorylated human pSmad3 and has significant background staining, as confirmed in clones deficient in Mad or the Dpp receptor Tkv, Fig. S6A, and see below).
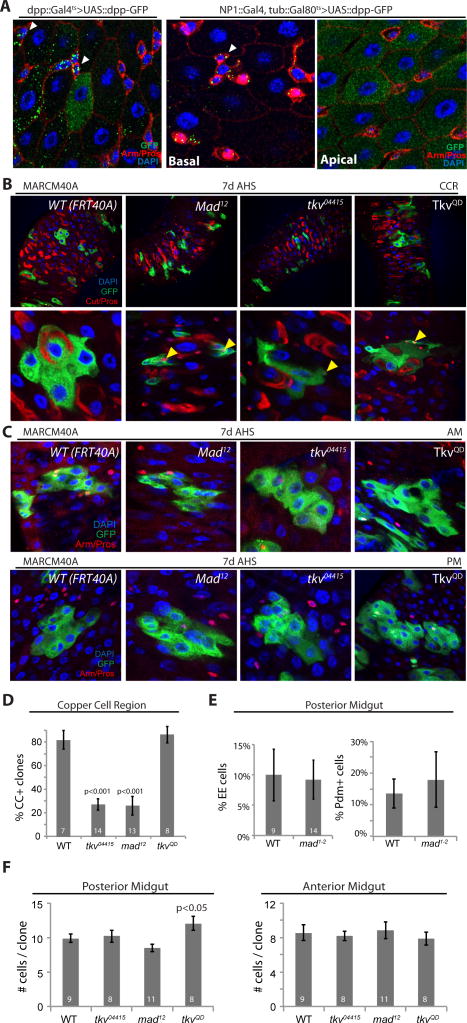
While Dpp signaling activity in the posterior PM has been shown to be critical for long-term survival of ECs (Li et al., 2013), the differential activation of Dpp signaling along the AP axis suggested an additional role for Dpp signaling in the intestine, potentially in the patterning of cells derived from ISCs and GSSCs during regeneration of the intestinal and/or gastric epithelium. To test whether EC-derived Dpp would signal to ISCs, we used a Dpp::GFP fusion expressed either by the Dpp::Gal4 driver itself, or the EC driver NP1::Gal4. Enrichment of extracellular Dpp::GFP was observed at the periphery of ISCs and GSSCs, indicating that EC-derived Dpp is able to act in a paracrine fashion to signal to these cells (Fig. 3A; Fig. S3A, B).
Figure 3. Dpp signaling is required for CC differentiation.
(A) EC-derived Dpp-GFP accumulates on ISCs/EBs. A Dpp-GFP fusion protein expressed in ECs either by Dpp::Gal4, tub::Gal80ts (left panel) or by NP1::Gal4, tub::Gal80ts (middle and right panels) can be detected on the surface of ISCs/EBs (arrowhead). Note that NP1::Gal4 induces Dpp::GFP in ECs apically (right panel), and extracellular Dpp::GFP accumulates on ISCs/EBs basally (middle panel).
(B) GFP-marked MARCM clones in the CCR from WT, Mad12, and tkv04415 mutant ISCs, as well as from TkvQD expressing ISCs at 7d after clone induction (AHS, after heat shock). (Green, GFP; red, Cut and Prospero; blue, DAPI; yellow arrowheads point to Pros+ enteroendocrine cells).
(C) GFP-marked MARCM clones in the AM and the PM (Green, GFP; red, cut and Prospero; blue, DAPI).
(D) Quantification of the ratio of Copper Cell (CC+) containing clones at 7d after heat shock in the CCR. The ratio of CC+ clones from tkv04415 or Mad12 is significantly decreased compared to WT. CCs were identified based on Cut staining. Note that Cut staining is extranuclear, while co-stained Prospero is detected within diploid EE nuclei. Average and SEM, p value from Student’s t test, and numbers of guts assessed are indicated.
(E) Quantification of percentage of EE and Pdm+ ECs in MARCM clones located in the PM at 7d after heat shock. There is no significant difference between WT and mad1–2 mutant clones. Average and SEM, number of guts are indicated.
(F) Quantification of clone sizes in the PM (left panel) and the AM (right panel) at 7d after heat shock. Average and SEM, p value from Student’s t test, and numbers of guts assessed are indicated.
To determine if the observed Dpp activity gradient in the midgut epithelium is important for anterior-posterior functional segregation of epithelial cell types, we disrupted the gradient using NP1::Gal4, which drives UAS-linked gene expression in all ECs, but not in ISCs or EEs, along the gut (Fig. S4A). Over-expression of Dpp using this driver activated the Dad::GFP reporter broadly in the PM and AM, and to a weaker degree in the CCR, while expression of DppRNAi was sufficient to reduce Dad::GFP expression in the PM and the CCR (Fig. S4B).
Surprisingly, we did not observe a change in ISC proliferation rates (as measured by detecting the number of phospho-histone H3+ cells) when dpp was knocked down using either NP1::Gal, the regional dpp::Gal4, or the tracheal driver Btl::Gal4 (Fig. S3C). This result is inconsistent with the increase in ISC proliferation reported by Li et al. when DppRNAi was expressed in tracheal cells (we used the same DppRNAi construct used by Li et al., and reconfirmed our results using all other available DppRNAi constructs). One difference in the conditions used by us and Li et al. was the duration of DppRNAi expression, which in our case was induced by maintaining adult flies for 6 days at 29°C, while in Li et al was induced for 24 days. Since incubating flies at 29°C for 24 days induces age-related changes in the intestinal epithelium (Biteau et al., 2010), we refrained from using such a prolonged incubation.
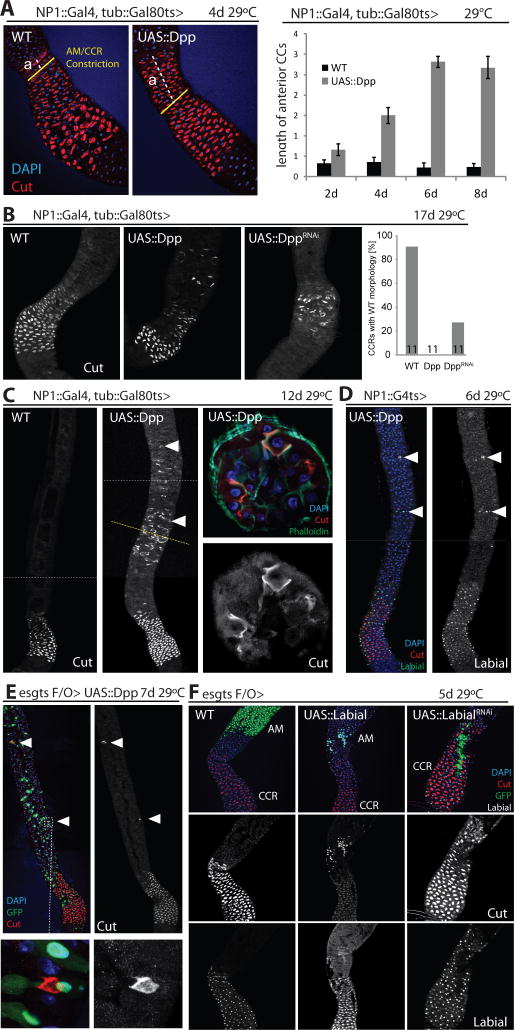
Broad activation of Dpp signaling in the intestinal epithelium caused a shortening of the PM relative to the CCR (the CCR was identified based on the presence of Cut+ cells in the MM; Fig. S4C), as well as a progressive expansion of the CCR into the AM, as measured by the number of Cut+ CCs present anterior to the constriction that delineates the boundary between the AM and the CCR (Fig. 2A, S4D). Accordingly, knockdown of Dpp resulted in a lengthening of the PM, while the CCR gradually disappeared (Fig. S4C, 2B). The lengthening and shortening of the PM in response to dpp knockdown or overexpression may represent a response to changes in the Dpp gradient that perturbs the balance between symmetric vs. asymmetric ISC divisions in the PM, influencing overall tissue growth. Similar plasticity of ISC divisions, regulated by insulin signaling, has been reported during fasting/refeeding cycles (O’Brien et al., 2011). Alternatively, changes in the balance between cell survival and regeneration may change overall tissue size. Further studies are needed to confirm this model.
Figure 2. Dpp is required for CCR integrity and sufficient to induce ectopic CCs in AM.
(A) Ectopic expression of Dpp (using NP1Gal4) induces progressive expansion of the CCR (red, anti-cut) into the AM. Left panel: representative images showing the distance (a, white lines) between the anterior border of Cut+ cells and the constriction delineating the anterior boundary of the CCR (yellow lines). Right panel: quantification of the length of ‘a’ at different timepoints after Dpp induction.
(B) Long-term (17 days) overexpression of Dpp using NP1::Gal4 causes dispersal of Cut+ (white) cells, while knockdown of Dpp results in loss of Cut+ cells in the CCR. The fraction of CCRs with wild-type morphology at this age (n=11) is quantified on the right.
(C) Appearance of Cut+ (white) cells in the AM (arrowheads) after long-term Dpp over-expression using NP1::Gal4. Yellow dotted line shows position of the cross section shown in the panels on the right. White dotted lines denote the border of individual confocal images used for reconstruction.
(D) The cut+ copper-like cells in the AM also express Labial (green, Labial; red, cut; blue, DAPI). Labial channel is shown separately on the right.
(E) Local expression of Dpp from cell clones induced using esgts-F/O is sufficient to induce Cut+ copper-like cells (arrowhead) in the AM, but not in the PM (data not shown). Lower panels show magnified example.
(F) Labial expression in ISC lineages is sufficient to promote Cut+ cell formation in the AM, and required for CC formation in the CCR. Clones of cells expressing Labial or dsRNA against Labial (LabialRNAi) were generated from ISCs or GSSCs using esgts-F/O.
Strikingly, prolonged expression of Dpp resulted in the appearance of ectopic Labial+/Cut+ copper-like cells in the AM, while Dpp knock-down caused a loss of CCs in the CCR (Fig. 2B–D, S5A). The CCR turns over more slowly than the AM or PM (Strand and Micchelli, 2011), and, accordingly, a strong phenotype emerged only when Dpp was knocked down for more than 14 days. These results suggested that sustained Dpp signaling in the adult CCR is required for long-term maintenance of normal CC numbers, while activation of Dpp signaling in the AM is sufficient to induce ectopic CC cell fates. Supporting this idea, local activation of Dpp signaling by expression of Dpp using esgtsF/O was sufficient to induce ectopic Cut+ CCs in the AM, but not in the PM (Fig. 2E; esgtsF/O allows lineage tracing of ISCs by heritably expressing Gal4 after a UAS::Flp-mediated recombination event; (Jiang et al., 2009)). The formation of Cut+ cells in response to Dpp expression using this strategy seems to be less efficient than in response to ubiquitous expression of Dpp using NP1::Gal4. This difference may be due to lower local levels of Dpp activity when Dpp is expressed in clones, or may reflect the slow formation and turnover of Cut+ cells in response to Dpp, resulting in progressive accumulation of these cells between 7 and 12 days after induction of Dpp expression regardless of the method.
Labial is a target gene for Dpp signaling during endoderm induction in the Drosophila embryo (Panganiban et al., 1990), and, accordingly, Dpp over-expression in the adult AM (but not the PM) is sufficient to induce labial gene expression (Fig. S5B). Labial expression was observed in both small (diploid) and polyploid cells, while Cut was expressed only in polyploid cells, suggesting that labial expression is induced early during differentiation of ISC daughter cells, while Cut is only expressed once the cells mature (Fig. S5A). Supporting this view, when Dpp is expressed from ECs, Dl+ ISCs in the AM induce dad::GFP and become Labial+ (in wild-type conditions these cells are dad::GFP and Labial negative; Fig. S5C). To test if Dpp-induced CC differentiation is mediated by Labial, we assessed whether ectopic expression or knock-down of Labial in Flp-out clones would be sufficient to perturb CCR regeneration. Indeed, over-expression of Labial was sufficient to induce Cut+ cell clones in the AM, while CCR-resident clones in which Labial was knocked down lacked Cut+ CCs (Fig. 2F, S5D). Furthermore, Dpp over-expression is only sufficient to induce ectopic Cut+ cells in the AM, where it also induces labial transcription, but not in the PM, where it fails to induce labial expression (Fig. S5B). Taken together, these results indicate that Dpp-mediated induction of labial is sufficient and required for CC cell generation in the AM and MM.
To confirm the role of Dpp signaling in CC specification, we performed lineage tracing using mosaic analysis with a repressible cell marker (MARCM, (Lee and Luo, 2001)). We generated ISCs homozygous mutant for Dpp signaling components (the receptor Thickveins, Tkv, and the transcription factor Mothers against Dpp, Mad) by MARCM using the loss of function alleles tkv04415 (Perrimon et al., 1996) and mad12 (Sekelsky et al., 1995) (both alleles resulted in decreased p-Smad3 staining in intestinal cells, Fig. S6A). For these mutants, we determined ISC proliferation rates in the AM and PM (clonal growth, Fig. 3C, S6B) and cell differentiation in the PM (we quantified both the percentage of Pros+ EEs and the percentage of Pdm+ ECs in mutant clones, Fig. 3E), and did not observe significant differences compared to wild-type cell clones (results were confirmed using the loss of function alleles tkva12 and mad1–2, Fig. S6C). When a constitutively active Tkv construct (TkvQD, (Nellen et al., 1996)) was over-expressed in PM ISCs, clone growth was moderately, but significantly increased (Fig. 3C, F, S6B). Clone formation in the CCR is significantly more infrequent than in the PM or AM, due to the intrinsic quiescence of gastric stem cells (Strand and Micchelli, 2011). However, we obtained MARCM clones in the CCR in about 50% of all examined guts. Compared to wild-type clones, loss of tkv or mad resulted in a significant reduction in the fraction of clones containing Cut+ CCs, while clone growth was not significantly affected (Fig. 3B, D, S6D, E), supporting a role for Dpp signaling in CC differentiation.
Taken together, our results indicate that high Dpp signaling activity in gastric stem cells of the CCR promotes the differentiation of CCs by regulating labial expression. The Dpp signaling gradient in the MM, generated by continuous Dpp expression at the MM/PM boundary, is thus critical for functional maintenance of the CCR in the adult. While ectopic Dpp activity is sufficient to induce labial expression and promote the formation of CCs from ISCs in the AM, a similar phenotype was not observed in the PM, suggesting that ISCs and/or their daughter cells differ between AM and PM in their response to Dpp signaling.
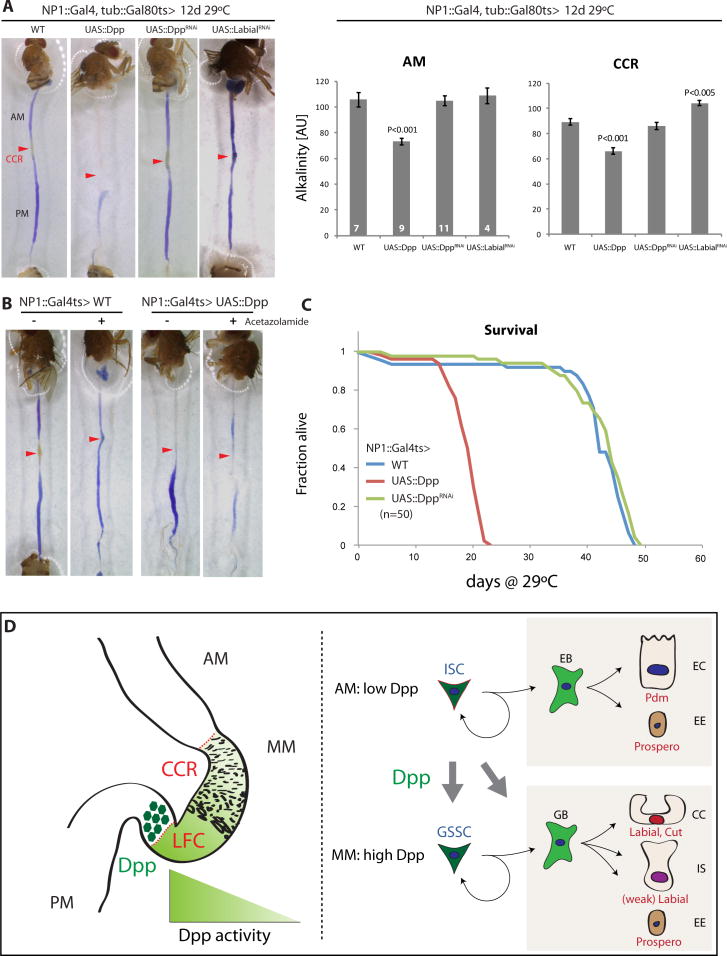
To assess how disrupting the compartmentalization of cellular identities along the gastrointestinal tract influences intestinal physiology, we first tested whether intestinal pH homeostasis was influenced by perturbation of the Dpp activity gradient in the gut epithelium (Fig. 4A). Since CCs are acid secreting cells, the intestinal pH is very low in the CCR, a fact that can be visualized colorimetrically by feeding animals Bromophenolblue (Shanbhag and Tripathi, 2009). In wildtype animals, the AM and PM show a deep blue coloration, while the CCR is yellow, reflecting its low pH. When the dpp gradient was perturbed by Dpp over-expression, however, the AM showed similar acidification, supporting the notion that the newly formed Cut+ CCs in this region are functional acid-secreting cells (Fig. 4A, S7A, B). This acidification could be rescued using the carbonic anhydrase inhibitor Acetazolamide, which can interfere with acidification of the insect CCR (Shanbhag and Tripathi, 2009), confirming the disruption of pH homeostasis in the AM by ectopic Dpp activation (Fig. 4B). Loss of Dpp (DppRNAi) did not significantly perturb the pH balance of the gut, indicating that the loss of CCs is not yet severe enough at the assessed timepoint (12 days after induction) to impair acidification of the CCR. However, knockdown of labial (using LabialRNAi) was sufficient to impair maintenance of the acidic region (Fig. 4A), confirming that labial expression in the MM is required to maintain a functional CCR.
Figure 4. Disrupting Dpp signaling impairs pH homeostasis in the Drosophila midgut.
(A) Dpp overexpression results in acidification of the AM, while knockdown of Labial reduces acidity of the MM, as determined by the pH indicator dye Bromophenol Blue. Quantification of alkalinity in the AM and the CCR was performed using the method described in Fig S7B.
(B) Supplementation with the Carbonic Anhydrase inhibitor Acetazolamide (100 μM) prevents the acidification of the AM in Dpp gain-of-function conditions.
(C) Lifespan of females flies of the indicated genotypes at 29°C. Cohorts of 50 individuals were compared.
(D) Model for the function of Dpp signaling in the maintenance of CC regeneration. A gradient of Dpp signaling activity is set up in the MM by EC-derived Dpp from the PM/MM boundary (left panel). Dpp is required for the formation of CCs from GSSCs, and can induce GSSC-like lineages in the AM (right panel; EB: Enteroblast, GB: Gastroblast).
We also assessed the lifespan of flies with Dpp gain and loss of function conditions in the gut. While feeding was not affected (as determined using the CAFÉ assay; (Ja et al., 2007); Fig. S7C), we observed a significant shortening of lifespan when Dpp was over-expressed, suggesting that perturbation of the pH balance in the intestine has deleterious physiological consequences for the animal (Fig. 4C).
Our results identify Dpp signaling as a critical factor maintaining the diversity of regenerative processes in the adult intestinal epithelium of flies (Fig. 4D). In addition to the survival function of Dpp in ECs (Li et al., 2013), Dpp thus also acts as a morphogen to influence cell differentiation in the regenerating intestinal epithelium. Interestingly, however, when lineage-tracing mutant SCs, we only observed strong loss of function phenotypes for Dpp pathway components in the CCR and not in the PM or AM (when analyzing differentiation capacity and ISC proliferation). While Dpp signaling is thus sufficient and required for the formation of CCs from gastric stem cells in the middle midgut, the exact function of the central-distal Dpp gradient in the posterior midgut remains unclear.
It is likely that additional determinants of regional identity influence the response of stem cells to the Dpp signal. Such integration would explain the different responses of PM ISCs and AM ISCs to increased Dpp signaling activity, which results in ectopic CC formation in the AM, but not in the PM, and will be of interest for further study.
The generation of gastric cells from AM ISCs, as well as the acidification of the AM in Dpp gain of function conditions, has similarities with changes in the esophagus of patients with Barrett’s metaplasia, indicating that perturbation of the Bmp/Dpp signaling gradient has evolutionarily conserved consequences for gut homeostasis. Our data suggest that the Drosophila intestine may serve as a model to explore the cellular and molecular mechanisms causing such metaplasias.
Experimental procedures
Fly Lines and Husbandry
See supplemental information for origin of fly lines and specific handling information.
qRT-PCR analysis
Total RNA from ten dissected guts was extracted using Trizol (Invitrogen). cDNA was synthesized using an oligo-dT primer. Real-time PCR was performed on a Bio-Rad CFX96 detection system. Relative expression of Dpp and labial was normalized to Actin5C. See supplemental information for primer sequences.
Immunostaining and Microscopy
Immunostaining and in situ hybridization was performed using standard protocols. See supplemental information for details.
Primary antibodies and dilution: rabbit anti-pSMad3 (Epitomics), 1:300; rabbit anti-β-galactosidase (Cappel), 1:5000; rabbit anti-GFP (Invitrogen), 1:500; anti-phospho-Histone H3 Ser10 (Upstate), 1:1000; and mouse anti-cut, anti-Prospero, anti-Armadillo, anti-β-galactosidase, and anti-Delta (Developmental Studies Hybridoma Bank), 1:300, 1:250, 1:100, 1:500, and 1:100, respectively; rabbit anti-labial (gift from T. Kaufman), 1:200; rat anti-pdm2 (gift from C.Q.Doe), 1:10. Fluorescent secondary antibodies were from Jackson Immunoresearch. DAPI was used to stain DNA. All images were taken on a Zeiss LSM 700 confocal microscope and processed using Adobe Photoshop, Illustrator and Image J.
Dye- and drug-feeding experiments
Bromophenol blue sodium salt (B5525, Sigma, dissolved in food at 0.2%) was used as pH indicator, showing yellow at pH 2.35, blue at pH 4, and a variable green/yellow color in between. Dissections of dye-containing intestines were performed in PBS, leaving the head and posterior cuticle intact to prevent dye leakage. Images were taken immediately after each gut was dissected to avoid color changes caused by incubation in PBS.
After boiling, the food was allowed to cool down to 60°C–65°C before it was supplemented with 0.2% BPB and/or 100 uM acetazolamide (A6011, sigma), dissolved in DMSO.
Supplementary Material
Highlights.
AP gradients of Dpp signaling activity are maintained in the adult Drosophila gut
Dpp signaling is required for copper cell regeneration
Activation of Dpp signaling induces ectopic copper cells in the anterior midgut
Dpp gradients maintain gut compartmentalization into basic and acidic regions
Acknowledgments
This work was supported by the National Institute on General Medical Sciences (NIH RO1 GM100196), and the Ellison Medical Foundation (AG-SS-2224-08) to H.J.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Affolter M, Basler K. The Decapentaplegic morphogen gradient: from pattern formation to growth regulation. Nat Rev Genet. 2007;8:663–674. doi: 10.1038/nrg2166. [DOI] [PubMed] [Google Scholar]
- Auclair BA, Benoit YD, Rivard N, Mishina Y, Perreault N. Bone morphogenetic protein signaling is essential for terminal differentiation of the intestinal secretory cell lineage. Gastroenterology. 2007;133:887–896. doi: 10.1053/j.gastro.2007.06.066. [DOI] [PubMed] [Google Scholar]
- Barker N, Bartfeld S, Clevers H. Tissue-resident adult stem cell populations of rapidly self-renewing organs. Cell Stem Cell. 2010;7:656–670. doi: 10.1016/j.stem.2010.11.016. [DOI] [PubMed] [Google Scholar]
- Biteau B, Hochmuth CE, Jasper H. Maintaining tissue homeostasis: dynamic control of somatic stem cell activity. Cell Stem Cell. 2011;9:402–411. doi: 10.1016/j.stem.2011.10.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Biteau B, Karpac J, Supoyo S, DeGennaro M, Lehmann R, Jasper H. Lifespan extension by preserving proliferative homeostasis in Drosophila. PLoS Genet. 2010;6:e1001159. doi: 10.1371/journal.pgen.1001159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dubreuil RR. Copper cells and stomach acid secretion in the Drosophila midgut. Int J Biochem Cell Biol. 2004;36:745–752. doi: 10.1016/j.biocel.2003.07.004. [DOI] [PubMed] [Google Scholar]
- Dvorak K, Goldman A, Kong J, Lynch JP, Hutchinson L, Houghton JM, Chen H, Chen X, Krishnadath KK, Westra WM. Molecular mechanisms of Barrett’s esophagus and adenocarcinoma. Ann N Y Acad Sci. 2011;1232:381–391. doi: 10.1111/j.1749-6632.2011.06062.x. [DOI] [PubMed] [Google Scholar]
- Hamaratoglu F, de Lachapelle AM, Pyrowolakis G, Bergmann S, Affolter M. Dpp signaling activity requires Pentagone to scale with tissue size in the growing Drosophila wing imaginal disc. PLoS Biol. 2011;9:e1001182. doi: 10.1371/journal.pbio.1001182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haramis AP, Begthel H, van den Born M, van Es J, Jonkheer S, Offerhaus GJ, Clevers H. De novo crypt formation and juvenile polyposis on BMP inhibition in mouse intestine. Science. 2004;303:1684–1686. doi: 10.1126/science.1093587. [DOI] [PubMed] [Google Scholar]
- Hou SX. Intestinal stem cell asymmetric division in the Drosophila posterior midgut. J Cell Physiol. 2010;224:581–584. doi: 10.1002/jcp.22194. [DOI] [PubMed] [Google Scholar]
- Ja WW, Carvalho GB, Mak EM, de la Rosa NN, Fang AY, Liong JC, Brummel T, Benzer S. Prandiology of Drosophila and the CAFE assay. Proc Natl Acad Sci U S A. 2007;104:8253–8256. doi: 10.1073/pnas.0702726104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacobs IJ, Ku WY, Que J. Genetic and cellular mechanisms regulating anterior foregut and esophageal development. Dev Biol. 2012;369:54–64. doi: 10.1016/j.ydbio.2012.06.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jankowski JA, Harrison RF, Perry I, Balkwill F, Tselepis C. Barrett’s metaplasia. Lancet. 2000;356:2079–2085. doi: 10.1016/S0140-6736(00)03411-5. [DOI] [PubMed] [Google Scholar]
- Jiang H, Patel PH, Kohlmaier A, Grenley MO, McEwen DG, Edgar BA. Cytokine/Jak/Stat signaling mediates regeneration and homeostasis in the Drosophila midgut. Cell. 2009;137:1343–1355. doi: 10.1016/j.cell.2009.05.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee T, Luo L. Mosaic analysis with a repressible cell marker (MARCM) for Drosophila neural development. Trends Neurosci. 2001;24:251–254. doi: 10.1016/s0166-2236(00)01791-4. [DOI] [PubMed] [Google Scholar]
- Leedham SJ, Preston SL, McDonald SA, Elia G, Bhandari P, Poller D, Harrison R, Novelli MR, Jankowski JA, Wright NA. Individual crypt genetic heterogeneity and the origin of metaplastic glandular epithelium in human Barrett’s oesophagus. Gut. 2008;57:1041–1048. doi: 10.1136/gut.2007.143339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Z, Zhang Y, Han L, Shi L, Lin X. Trachea-derived dpp controls adult midgut homeostasis in Drosophila. Dev Cell. 2013;24:133–143. doi: 10.1016/j.devcel.2012.12.010. [DOI] [PubMed] [Google Scholar]
- Maley CC, Galipeau PC, Finley JC, Wongsurawat VJ, Li X, Sanchez CA, Paulson TG, Blount PL, Risques RA, Rabinovitch PS, et al. Genetic clonal diversity predicts progression to esophageal adenocarcinoma. Nat Genet. 2006;38:468–473. doi: 10.1038/ng1768. [DOI] [PubMed] [Google Scholar]
- Mathur D, Bost A, Driver I, Ohlstein B. A transient niche regulates the specification of Drosophila intestinal stem cells. Science. 2010;327:210–213. doi: 10.1126/science.1181958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGuire SE, Le PT, Osborn AJ, Matsumoto K, Davis RL. Spatiotemporal rescue of memory dysfunction in Drosophila. Science. 2003;302:1765–1768. doi: 10.1126/science.1089035. [DOI] [PubMed] [Google Scholar]
- Micchelli CA, Perrimon N. Evidence that stem cells reside in the adult Drosophila midgut epithelium. Nature. 2006;439:475–479. doi: 10.1038/nature04371. [DOI] [PubMed] [Google Scholar]
- Milano F, van Baal JW, Buttar NS, Rygiel AM, de Kort F, DeMars CJ, Rosmolen WD, Bergman JJ, JVAM, Wang KK, et al. Bone morphogenetic protein 4 expressed in esophagitis induces a columnar phenotype in esophageal squamous cells. Gastroenterology. 2007;132:2412–2421. doi: 10.1053/j.gastro.2007.03.026. [DOI] [PubMed] [Google Scholar]
- Nakagoshi H. Functional specification in the Drosophila endoderm. Dev Growth Differ. 2005;47:383–392. doi: 10.1111/j.1440-169X.2005.00811.x. [DOI] [PubMed] [Google Scholar]
- Nellen D, Burke R, Struhl G, Basler K. Direct and long-range action of a DPP morphogen gradient. Cell. 1996;85:357–368. doi: 10.1016/s0092-8674(00)81114-9. [DOI] [PubMed] [Google Scholar]
- O’Brien LE, Soliman SS, Li X, Bilder D. Altered modes of stem cell division drive adaptive intestinal growth. Cell. 2011;147:603–614. doi: 10.1016/j.cell.2011.08.048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohlstein B, Spradling A. The adult Drosophila posterior midgut is maintained by pluripotent stem cells. Nature. 2006;439:470–474. doi: 10.1038/nature04333. [DOI] [PubMed] [Google Scholar]
- Ohlstein B, Spradling A. Multipotent Drosophila intestinal stem cells specify daughter cell fates by differential notch signaling. Science. 2007;315:988–992. doi: 10.1126/science.1136606. [DOI] [PubMed] [Google Scholar]
- Panganiban GE, Reuter R, Scott MP, Hoffmann FM. A Drosophila growth factor homolog, decapentaplegic, regulates homeotic gene expression within and across germ layers during midgut morphogenesis. Development. 1990;110:1041–1050. doi: 10.1242/dev.110.4.1041. [DOI] [PubMed] [Google Scholar]
- Perrimon N, Lanjuin A, Arnold C, Noll E. Zygotic lethal mutations with maternal effect phenotypes in Drosophila melanogaster. II. Loci on the second and third chromosomes identified by P-element-induced mutations. Genetics. 1996;144:1681–1692. doi: 10.1093/genetics/144.4.1681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peters JH, Avisar N. The molecular pathogenesis of Barrett’s esophagus: common signaling pathways in embryogenesis metaplasia and neoplasia. Journal of gastrointestinal surgery : official journal of the Society for Surgery of the Alimentary Tract. 2010;14(Suppl 1):S81–87. doi: 10.1007/s11605-009-1011-7. [DOI] [PubMed] [Google Scholar]
- Que J, Choi M, Ziel JW, Klingensmith J, Hogan BL. Morphogenesis of the trachea and esophagus: current players and new roles for noggin and Bmps. Differentiation. 2006;74:422–437. doi: 10.1111/j.1432-0436.2006.00096.x. [DOI] [PubMed] [Google Scholar]
- Schwyter DH, Huang JD, Dubnicoff T, Courey AJ. The decapentaplegic core promoter region plays an integral role in the spatial control of transcription. Mol Cell Biol. 1995;15:3960–3968. doi: 10.1128/mcb.15.7.3960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sekelsky JJ, Newfeld SJ, Raftery LA, Chartoff EH, Gelbart WM. Genetic characterization and cloning of mothers against dpp, a gene required for decapentaplegic function in Drosophila melanogaster. Genetics. 1995;139:1347–1358. doi: 10.1093/genetics/139.3.1347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shanbhag S, Tripathi S. Epithelial ultrastructure and cellular mechanisms of acid and base transport in the Drosophila midgut. J Exp Biol. 2009;212:1731–1744. doi: 10.1242/jeb.029306. [DOI] [PubMed] [Google Scholar]
- Sharma P. Clinical practice. Barrett’s esophagus. N Engl J Med. 2009;361:2548–2556. doi: 10.1056/NEJMcp0902173. [DOI] [PubMed] [Google Scholar]
- Staehling-Hampton K, Jackson PD, Clark MJ, Brand AH, Hoffmann FM. Specificity of bone morphogenetic protein-related factors: cell fate and gene expression changes in Drosophila embryos induced by decapentaplegic but not 60A. Cell Growth Differ. 1994;5:585–593. [PubMed] [Google Scholar]
- Strand M, Micchelli CA. Quiescent gastric stem cells maintain the adult Drosophila stomach. Proc Natl Acad Sci U S A. 2011;108:17696–17701. doi: 10.1073/pnas.1109794108. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.