Abstract
Eugenol and carvacrol, from the spices clove and oregano, respectively, are agonists of TRPV3 which is implicated in transduction of warmth and possibly heat pain. We presently investigated the temporal dynamics of lingual irritation elicited by these agents, and their effects on innocuous warmth and heat pain, using a half-tongue method in human subjects. The irritant sensation elicited by both eugenol and carvacrol decreased across repeated applications at a 1-min interstimulus interval (self-desensitization) which persisted for at least 10 min. Both agents also cross-desensitized capsaicin-evoked irritation. Eugenol and carvacrol significantly increased the magnitude of perceived innocuous warmth (44°C) for >10 min, and briefly (<5 min) enhanced heat pain elicited by a 49°C stimulus. Similar albeit weaker effects were observed when thermal stimuli were applied after the tongue had been desensitized by repeated application of eugenol or carvacrol, indicating that the effect is not due solely to summation of chemoirritant and thermal sensations. Neither chemical affected sensations of innocuous cool or cold pain. A separate group of subjects were asked to subdivide eugenol and carvacrol irritancy into subqualities, the most frequently-reported being numbing and warmth, with brief burning, stinging/pricking and tingle, confirming an earlier study. Eugenol, but not carvacrol, reduced detection of low-threshold mechanical stimuli. Eugenol and carvacrol enhancement of innocuous warmth may involve sensitization of thermal gating of TRPV3 expressed in peripheral warm fibers. The brief heat hyperalgesia following eugenol may involve a TRPV3-mediated enhancement of thermal gating of TRPV1 expressed in lingual polymodal nociceptors.
Introduction
The organic compounds eugenol and carvacrol are derived from the essential oils of cloves and oregano, respectively. Eugenol has been used for many years in dentistry as an analgesic or incorporated in temporary fillings due to its anesthetic and antimicrobial properties, [38] although its use may be limited by its irritant and cytotoxic properties. Carvacrol is used commercially mainly for its antibacterial and antifungal properties [47]. Eugenol and carvacrol are agonists of TRPV3, and are cyclic monoterpenes similar in chemical structure to camphor, propofol and thymol [53]. TRPV3 is a polymodal receptor that is activated by chemicals and warmth [44,52,55]. TRPV3 is expressed in many tissues including sensory neurons [56] and keratinocytes [10,44]. Mice lacking TRPV3 were reported to have deficits in sensitivity to both innocuous warmth and noxious heat [40], suggesting a role in heat pain, although this has recently been refuted [28].
Oral application of eugenol elicits a pungent sensation [13]. We presently investigated the oral irritant sensation of eugenol and the closely related chemical carvacrol. We first investigated temporal patterns of irritant sensation elicited by repeated applications using a halftongue method that employed a sensitive two-alternative forced-choice (2-AFC) paradigm coupled with bilateral magnitude ratings [14,15,32]. Given the proposed roles of TRPV3 in innocuous warmth and heat pain, we were particularly interested to determine if TRPV3 agonists affect the perceived sensations of warmth or heat pain, using a methodology that we previously employed to demonstrate heat hyperalgesia by TRPV1 and TRPA1 agonists, and cold hyperalgesia by the TRPM8 agonist menthol [1]. Given the use of eugenol as a local anesthetic, we additionally investigated if it or carvacrol affects mechanical sensitivity using a sensitive detection test. In a separate set of experiments we also reevaluated the sensory subqualities associated with irritation elicited by orally-applied eugenol and carvacrol. An abstract of portions of this work has appeared [31].
Materials and Methods
Subjects
The experimental protocol was approved by the University of California Davis Human Subjects Internal Review Board and all participants gave signed consent before data collection. A total of 524 subjects (153 male, 371 female, ages 18–53) were recruited from the University of California, Davis campus using the Psychology Research Participation System (Sona Systems Ltd.) web site. Subjects were told not to consume spicy food three days before, or to take pain medication the day of, or to eat/drink one hour before the start of the experiment. Twenty-five subjects participated in multiple experiments within this study, but no participant completed the same protocol more than once.
Chemical Stimuli
A working solution of 600 mM eugenol (Sigma-Aldrich, St. Louis, MO) in 4% ethanol and 1% Polysorbate-80 (Tween-80, Sigma) was made fresh daily. From a stock solution of 500 mM carvacrol (Sigma) in 40% ethanol and 10% Tween-80, a 50 mM working solution of carvacrol was made each day by dilution with deionized (DI) water. Each solution was vortexed/shaken into suspension just prior to application by pipette onto large (1.5 cm diameter, Whatman, GE Healthcare UK Ltd., Buckinghamshire, UK, 40 µL) or small (1 cm diameter, Whatman, 20 µL) filter papers. A stock solution of 0.1% capsaicin (3.3 mM) in 50% ethanol solution was diluted to 0.001% (0.033mM) in DI water. Capsaicin (0.033 mM) was pipetted unto large filter papers (1.5 cm diameter, 40 µL) and allowed to air-dry. Capsaicin-treated filter papers were reconstituted with DI water (40 µL) before application. The concentrations of eugenol and carvacrol were determined to approximately match the magnitude of irritation elicited by 0.033 mM capsaicin. This was done in pilot studies by applying one filter paper wetted with eugenol at various concentrations, and another wetted with 0.033 mM capsaicin, simultaneously on each side of the tongue and having subjects state on which side they experienced stronger irritation. A similar procedure was carried out with carvacrol. Subjects chose the side treated with capsaicin and either 600 mM eugenol or 50 mM carvacrol to be more intense in approximately the same numbers.
Stimulus application
We presently employed a split-tongue stimulus paradigm first reported by McBurney et al. [39]. This method allows simultaneous, side-by-side comparisons of sensations elicited by different stimuli on each side of the tongue. We have validated this method for detecting intensity differences elicited by differential bilateral irritant, gustatory and thermal stimulation of the tongue [1, 15, 16, 50]. For unilateral tongue application of chemicals, a large-sized filter paper soaked with the chemical of interest was held with sterile forceps and place onto one side of the anterior dorsal tongue surface. A filter paper soaked with vehicle was similarly placed onto the opposite side of the tongue. The side of chemical application was randomized across subjects. The subjects were asked to bring the tongue into the mouth and close the lips for the duration of the 30-sec stimulus period, after which the filter papers were removed. Subjects were then free to use a saliva ejector device (Sullivan Dental Products Inc, T&S Dental and Plastics Co., Myerstown, PA) to remove any excess saliva.
Thermal stimuli were delivered to the anterior dorsal tongue surface bilaterally using a square Peliter thermode (4.60 × 4.60 cm; NTE-2, Physitemp Instruments, Clifton, NJ). The thermode surface temperature was controlled via an electronic feedback circuit to within 0.2°C, and was preset to either 44°C (innocuous warmth), 49°C (noxious heat), 18°C (innocuous cold) or 4°C (noxious cold) using a specialized computer software program. The thermode surface was covered with Plastic wrap (Reynolds Wrap; Alcoa Consumer Products, Richmond, VA) as a sanitary barrier, and replaced after each subject. A thermocouple (IT-23, Physitemp) was placed at the center of the Peltier thermode, and connected to a digital thermometer (BAT-12, Physitemp) to continuously monitored the thermode-tongue interface temperature which was displayed using a Powerlab interface (ADInstruments, Colorado Springs, CO) running Chart software (ADInstruments). The interface temperature usually stabilized within 10 sec after contacting the subject’s tongue. The 44°C stimulus was perceived as innocuous warmth and resulted in a mean thermode-tongue interface temperature of 42.4°C +/− 0.64 (SD). This temperature was determined in pilot studies to be the lowest that reliably elicited a sensation of warmth, while temperatures below 44°C did not reliably elicit any sensation in some subjects. The 49°C stimulus was perceived as mildly-to-moderately painful and achieved a mean interface temperature of 47.1°C +/− 0.46. The 18°C stimulus was perceived as cool and achieved a mean interface temperature of 21.4°C +/− 0.56. This temperature was selected since higher temperatures did not reliably elicit sensations of innocuous cooling in pilot experiments. The 4°C stimulus was perceived as cold pain and achieved a mean interface temperature of 10.6°C +/− 1.55.
Low-threshold mechanical stimuli consisted of calibrated von Frey monofilaments having a bending force of 0.08 mN or 0.2 mN. Each filament was applied to the dorsal anterior tongue 10 times to the left and 10 times to the right side. The order of presentation of the two filaments, and side of stimulation, was randomized. In addition, 20 trials with no stimulation (blanks) were randomly interspersed with the stimulus trials, for a total of 60 trials per subject over a period of ~10 min. The subjects were asked to report if they detected a stimulus and if they were sure or not sure after each trial.
2-AFC and magnitude ratings
After each chemical or thermal stimulus application, a two-alternative forced-choice (2-AFC) paradigm was employed by asking subjects to indicate by circling on a piece of paper on which side of the tongue they experienced a stronger irritant or thermal sensation. Immediately after the 2-AFC, subjects were asked to independently rate the magnitude of the sensation on each side, using the general labeled magnitude scale (gLMS; one for each side and time point). The subjects were given a sheet a paper with two gLMS scales for the 2 sides of the tongue and asked to independently rate each side for either irritancy or thermal ratings by marking a line on the vertical scale. The gLMS has verbal descriptors (no sensation, barely detectable, weak, moderate, strong, very strong, and strongest imaginable) spaced logarithmically along a vertical scale [22], and subjects marked the site on the scale corresponding to the perceived sensory magnitude. The scale used in this study was 100 mm long where: no sensation, 0; barely detectable, 1.4; weak, 6.1; moderate, 17.2; strong, 35.4; very strong, 53.3; and the strongest imaginable sensation of any kind, 100. For gLMS ratings, the distance of the mark from the end of the scale was measured in millimeters, a value of 1 was added to eliminate 0 scores (no sensation), and data were log transformed. [23]. The upper end of the gLMS is strongest sensation imaginable of any kind [4]. Subjects received individual instruction on how to use the gLMS at the beginning of the session.
Individual experimental procedures and data analysis
1. Sequential chemical application
Eugenol or carvacrol was applied sequentially 10 times to one side of the tongue at a 1-min interstimulus interval (ISI), with vehicle applied to the other side. The filter papers were removed after 30 sec and the subjects performed the 2-AFC and gLMS magnitude ratings. After a 10 min pause (minute 20), eugenol, carvacrol or capsaicin was applied for 30 seconds and subjects were asked to perform the same 2-AFC and gLMS ratings as before.
2-AFC data were analyzed_using the binomial test. gLMS ratings were measured from the end of the scale in millimeters, a value of one was added to avoid zero scores, and data were log transformed and subjected to repeated measures ANOVA for side of stimulation and time of application. Paired t-tests were used to compare chemical versus vehicle irritancy ratings at minute 20 for experiments using bilateral application of eugenol/carvacrol or capsaicin.
2. Effect of eugenol and carvacrol on thermal sensations
Eugenol or carvacrol was applied by large filter paper to one side of the tongue and removed after 30 sec. Subjects then immediately pressed the anterior dorsal tongue against the Peliter thermode that had been preheated to either 44°C, 49°C, 18°C or 4°C. The thermode temperatures for noxious heat (49°C) and noxious cold (4°C) are the same as used previously (Albin et al., 2007). Thermode temperatures for innocuous warmth (44°C) and cooling (18°C) were determined by pilot studies as the minimum temperature that could used to give reportable ratings using a gLMS. After the tongue-thermode interface temperature stabilized, or after 10 sec, subjects removed the tongue from the thermode and performed the 2-AFC (which side was warmer, colder or more painful) and bilateral gLMS ratings of the intensity of thermal sensation. This was repeated 1.5, 5 and 10 min after chemical application.
In a variant of this procedure, either eugenol or carvacrol was applied repeatedly 10 times at a 1- min interstimulus interval to desensitize one side of the tongue, followed immediately after the 10th application by pressing the tongue against the preheated or precooled Peltier thermode as described above. The aim was to isolate the thermal sensation by eliminating the chemical irritation. 2-AFC and rating data were analyzed as noted above.
3. Descriptive analysis of sensory qualities elicited by eugenol and carvacrol
At the beginning of the session, subjects were presented a list of sensory descriptors and their definitions (adapted from [21,25]). The sensations provided were burning (the sensation resulting from high temperatures, skin abrasions, or chemicals not necessarily accompanied by an actual increase in temperature, such as spicy food), stinging/pricking (small sharp sensations caused by a needle or insect bite, which can be constant or very brief), tingling (“pins-and-needles” sensation), numbing (a sensation produced by onset of an anesthetic, partial loss of sensation), cooling (decrease in temperature), heating (increase in temperature) and “other” if the subjects wanted to write down a sensation not provided on the list. “None” was also listed and it was indicated to the subjects that this option should be circled if there was an absence of any sensation (e.g. untreated tongue conditions). A large filter paper pre-soaked with eugenol or carvacrol was placed on one side of the tongue, and vehicle on the other. The side of chemical application was randomized across subjects. After removing the filter papers, subjects were asked to indicate as many sensations as they perceived on the tongue by circling the corresponding descriptor(s), once per min for 10 min, and again after a 10-min break (at minute 20). The percentage of subjects reporting each individual descriptor at each time point was calculated.
4. Effect of eugenol and carvacrol on tactile sensitivity
Eugenol or carvacrol was applied unilaterally for 30 sec with vehicle applied on the opposite side. Thirty sec after the filter papers were removed, the 0.08mN or 0.2mN von Frey filament, or no filament (blank), were applied as described above, with subjects reporting if they detected the stimulus or not and if they were sure or not sure. The responses were placed into a response matrix and an R-index was calculated for each side of the tongue [41]. The R-index measures the area under a receiver operation characteristics (ROC) curve based on signal detection theory; values range from 0.5–1 with higher numbers reflecting greater ability to discriminate between two stimulus intensities [41]. The comparison between treated side (eugenol or carvacrol) and the vehicle treated side was done by paired t-test similar to previously conducted studies [2, 49, 50]. Treatments were compared by paired t-tests.
Statistical analyses were made using SPSS software (Version 9.0) and error reported is the standard deviation (SD) for tongue-thermode interface measurements, or standard error of the mean (SEM) for all other measurements.
Results
Eugenol and carvacrol self-desensitization of oral irritation
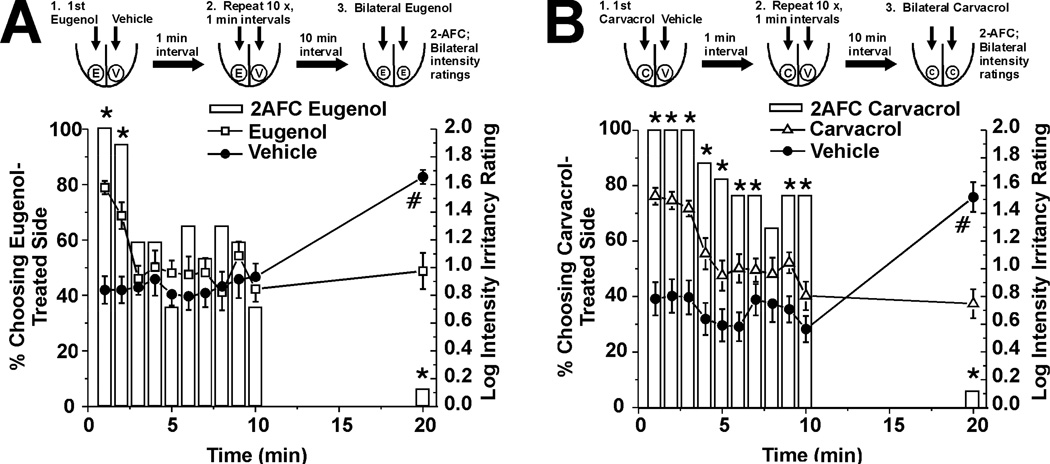
In these studies, subjects rated the composite irritant sensation elicited by lingual application of eugenol or carvacrol across repeated trials. The initial two applications of eugenol elicited strong irritation, as manifested by a significant proportion of subjects choosing the eugenol-treated side of the tongue as having a stronger sensation (Fig. 1A, bars, n=30), and assigning higher intensity ratings to that side (Fig. 1A, □). However, by the third application, subjects no longer reliably chose the treated side as stronger, and ratings declined to a low level corresponding to “barely detectable” on the gLMS and comparable to ratings on the vehicle-treated side (Fig. 1A, ●). This indicates desensitization of eugenol-evoked irritation after 3 applications. After the sequential stimuli and a 10-min rest period, eugenol was applied bilaterally. Desensitization of irritation was still strong, as manifested by a significant minority of subjects choosing the side previously receiving eugenol as having stronger irritation (Fig. 1A, right-hand bar), and by a significantly higher mean intensity rating on the side previously treated with vehicle (Fig. 1A, right-hand ●).
Figure 1.
Self-desensitization of oral irritation elicited by eugenol and carvacrol. A. Eugenol (600 mM). Upper row shows schematic of experiment. Eugenol (E) was applied to one side and vehicle (V) to the other side of the tongue, 10 times in succession at 1-min intervals, with subjects stating which side had stronger irritation (2-AFC) and rating the intensity on each side after each stimulus application. After a 10-min hiatus, eugenol was applied bilaterally and 2-AFC and bilateral intensity ratings were obtained. The lower bar graph plots the percentage of subjects choosing the eugenol-treated side as having stronger irritation in 2-AFC (left-hand y-axis). *: p<0.05, binomial test; n=30). The line graphs plot mean ratings of irritation (log-transformed gLMS ratings; right-hand y-axis) vs. time for eugenol- (□) and vehicle-treated (●) sides. Error bars: SEM. There was a significant decrease in mean irritancy ratings over time (F(9,288) = 4.32, p<0.05 for min 0–10, repeated-measures ANOVA,). At min 20, eugenol was applied bilaterally. *: p<0.05, binomial test for 2-AFC. #: p<0.001, paired t-test for mean intensity ratings. B. As in A, for 50 mM carvacrol (n=17).
Similarly, carvacrol initially elicited strong irritation that exhibited desensitization across trials (Fig. 1B, n=17), albeit more slowly compared to eugenol. This was manifested by a significant decline after 4 trials in mean intensity ratings and after 8 trials in the 2-AFC (Fig. 1B). Ratings on the vehicle-treated side were consistently “barely detectable” in the gLMS (Fig. 1A, B; △). After a 10-min rest period, carvacrol was applied bilaterally. The side of the tongue previously receiving carvacrol was still desensitized, as indicated by a significant minority of subjects choosing that side as having stronger irritation in the 2-AFC (Fig. 1B, right-hand bar) and significantly lower intensity ratings on that side (Fig. 1B, △). Thus, eugenol and carvacrol exhibited a temporal pattern of desensitization across repeated applications, and this selfdesensization was still present after a 10-min rest period.
Eugenol and carvacrol cross-desensitization of capsaicin-evoked irritation
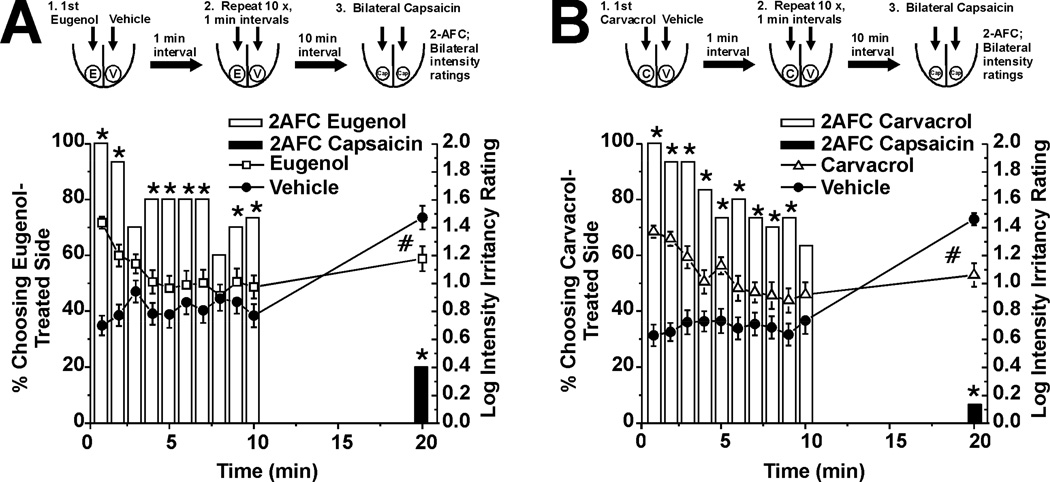
In this experiment we tested if eugenol or carvacrol cross-desensitize irritation elicited by capsaicin. We repeated the above experiment except that after the 10-min rest period, capsaicin was applied bilaterally. We confirmed that eugenol- and carvacrol-evoked irritation decreased over repeated applications (Fig 2A and 2B, respectively, n=30), as indicated by the decreasing number of subjects choosing the eugenol- or carvacrol-treated side as having stronger irritation in the 2-AFC (Fig 2A, B, open bars), and a decline in intensity ratings (Fig 2A, □, Fig. 2B, ●). After a 10-min rest period, capsaicin was applied bilaterally. Capsaicin-evoked irritation was significantly less on the side of the tongue previously receiving eugenol or carvacrol. In the 2-AFC, a significant minority of subjects chose the eugenol- or carvacrol-treated sides as having stronger irritation (Fig. 2A, B, black bars). Moreover, intensity ratings of capsaicin-evoked irritation were significantly greater on the vehicle-treated side (Fig. 2A, B, □, △ for eugenol and carvacrol, respectively). These data indicate that eugenol and carvacrol cross-desensitized the irritancy of capsaicin.
Fig. 2.
Eugenol and carvacrol self-desensitization and cross-desensitization of capsaicin-evoked irritation. A. Schematic and graph as in Fig. 1A, showing desensitization of eugenolevoked irritation. *: significant proportion of subjects choosing eugenol-treated side as having stronger irritation in the 2-AFC (bars; p<0.05, binomial test, n=30). Intensity ratings for the eugenol-treated side (□) decreased significantly over time (F(9,522) = 5.27, min 0–10, repeated-measures ANOVA, p<0.05). At 20 min, capsaicin was applied bilaterally. A significant minority of subjects chose the eugenol-treated side as having stronger irritation (black bar; *: p<0.05, binomial test, n=30). Also, the mean intensity rating of capsaicin-evoked irritation on the eugenol-treated side (□) was significantly less compared to the vehicle-treated side (●). #: p=0.02, paired t-test. B. Carvacrol (format as in A; n=30) also decreased significantly over time (F(9,522) = 4.94, min 0–10, repeated-measures ANOVA, p<0.05) and the mean intensity rating of capsaicin-evoked irritation on the carvacrol treated side (△) was significantly less compared to the vehicle treated side (●). #: p<0.001, paired t-test.
Eugenol and carvacrol enhancement of innocuous warmth
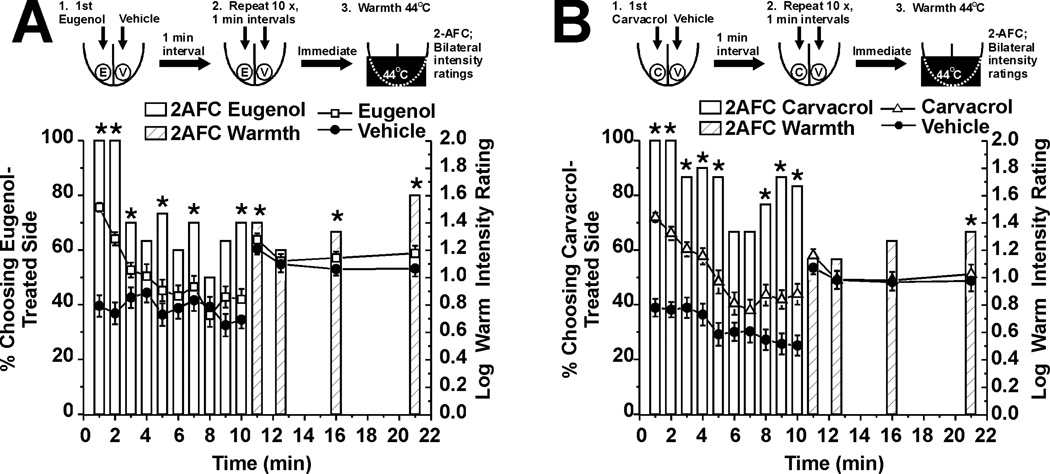
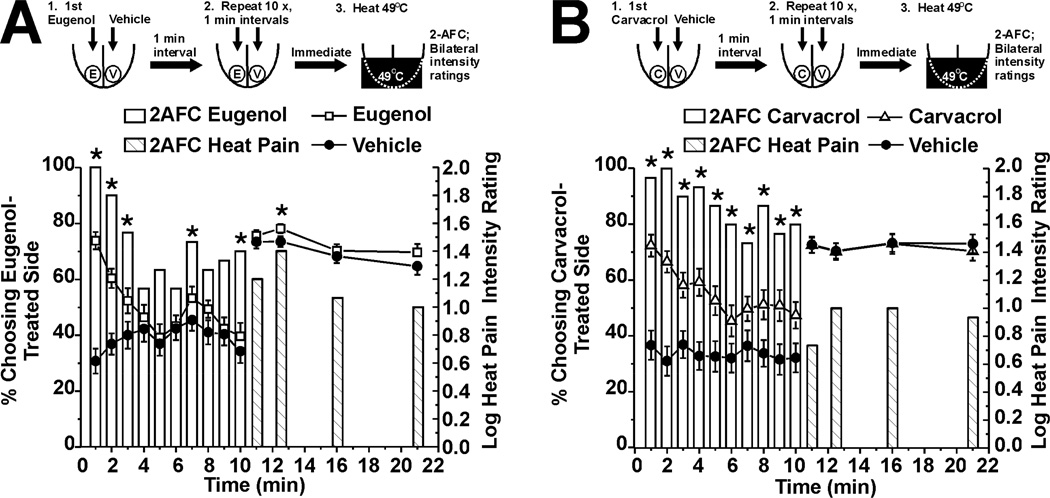
These experiments tested the hypothesis that eugenol and carvacrol enhance the sensation of innocuous warmth on the tongue. Immediately and 1.5 and 10 min after a single application of eugenol to one side of the tongue, a significant majority of subjects chose the eugenol-treated side to be warmer (Fig. 3A, bars, n=30). This was accompanied by significantly higher intensity ratings of warmth on the eugenol-treated side compared to the vehicle-treated side (Fig. 3A, □). A significant majority of subjects also chose the carvacrol-treated side as warmer immediately and 5 and 10 min after application (Fig. 3B, bars, n=30) and assigned significantly higher intensity ratings to that side (Fig. 3B, △). Both chemicals had an immediate enhancing effect that waned and subsequently returned, with eugenol showing a slower time course (Fig. 3).
Fig. 3.
Eugenol and carvacrol enhancement of innocuous warmth evoked by Peltier thermode set at 44°C. A. Eugenol. Upper row shows schematic of experiment. Eugenol and vehicle were applied, followed immediately by bilateral thermal stimulation of the tongue, after which 2-AFC and intensity ratings were obtained. Ratings were obtained again 1.5, 5 and 10 min later. Bar graph plots 2-AFC. *: p< 0.05, binomial test (n=30). Lines with symbols: warmth intensity ratings for eugenol- (□) and vehicle-treated (●) sides of tongue. &: F(3, 174) = 3.89, p<0.05, 2-way repeated measures ANOVA. B. Carvacrol (same format as A; n=30). &: F(3, 174) = 2.69, p<0.05, 2-way repeated measures ANOVA.
Because subjects may have summed the chemically- and thermally-evoked sensations (halo-dumping), we repeated the experiment following desensitization of irritation. Our aim was to determine if warmth sensation is enhanced by eugenol or carvacrol in the absence of chemically-evoked irritancy. Thus, either eugenol or carvacrol was applied 10 times at 1-min interstimulus intervals to the tongue, followed immediately by thermal stimulation with the Peltier thermode set at 44°C. Fig. 4A shows desensitization of eugenol-evoked irritation across trials as assessed by 2-AFC (open bars, n=30) and intensity ratings (□). Immediately and again 1.5, 5 and 10 min after the 10th application of eugenol, the thermal stimulus was applied to the tongue. A significant proportion of subjects chose the eugenol-treated side as warmer in the 2- AFC (hatched bars). Subjects also assigned numerically higher ratings of warmth to the eugenol-treated side (□) although the effect did not reach statistical significance. Enhancement of warmth following desensitization by carvacrol was even weaker and only apparent in the 2-AFC 10 min after the end of sequential stimulation (Fig. 4B, hatched bar to right), with no significant difference in intensity ratings of warmth (Fig. 4B, △, n=30). These results indicate that (a) warmth was enhanced by eugenol and carvacrol in the absence of chemical irritation, albeit more weakly compared to when both sensations are present simultaneously, (b) the 2-AFC is more sensitive than intensity ratings in detecting the warmth-enhancing effect, consistent with our prior experience using this methodology, and (c) halo-dumping may partly account for enhancement of warmth when the irritant sensations of eugenol and carvacrol are present.
Fig. 4.
Enhancement of perceived warmth after desensitization by eugenol and carvacrol. A. Eugenol. Upper row shows schematic of experiment in which eugenol and vehicle were applied 10 times, followed immediately by bilateral thermal stimulation of the tongue, and again 1.5, 5 and 10 min later. 2-AFC and intensity ratings obtained after each chemical and thermal stimulus. Lower bar graph plots 2-AFC results (open bars) for eugenol-evoked irritation. Hatched bars: 2-AFC for warmth (Peltier thermode set at 44°C). *: p<0.05, binomial test (n=30). Lines with symbols: warmth intensity ratings for eugenol- (□) and vehicle-treated (●) sides of tongue. B. Carvacrol (format as in A; n=30).
Eugenol and carvacrol enhancement of heat pain
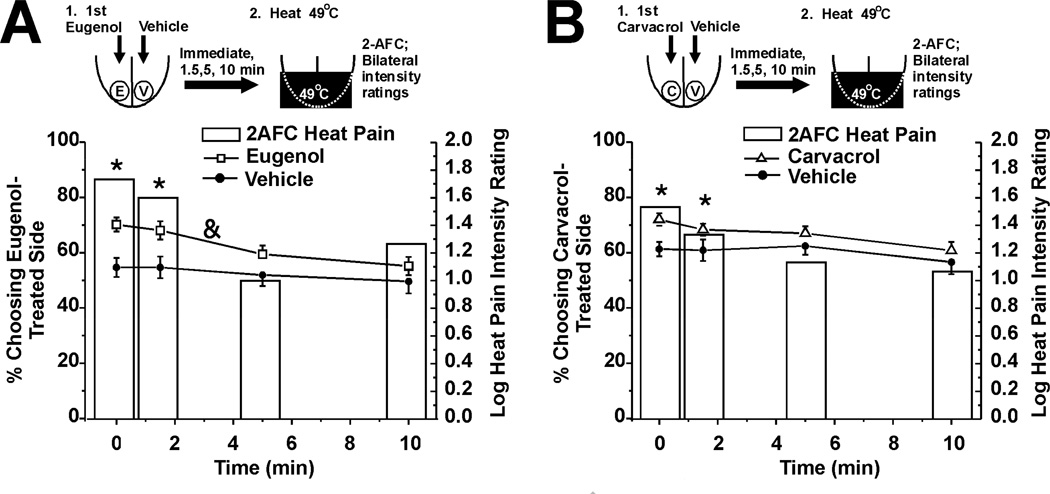
This experiment tested the hypothesis that eugenol and carvacrol enhance heat pain on the tongue. The same experiments as in the preceding section were repeated, except that the Peltier thermode was set at 49°C. Immediately and 1.5 min after a single unilateral application of eugenol, heat pain was enhanced as evidenced by a significant proportion of subjects choosing the eugenol-treated side as more painful in the 2-AFC (Fig. 5A, bars, n=30), and assigning significantly higher pain ratings to that side (Fig. 5A, □). Carvacrol also significantly enhanced heat pain in the 2-AFC, but not as assessed by intensity ratings (Fig. 5B, n=30).
Fig. 5.
Eugenol and carvacrol enhancement of heat pain evoked by Peltier thermode set at 49°C. A. Eugenol (format as in Fig. 3A). Bars: 2-AFC. *: p< 0.05, binomial test (n=30). Lines with symbols: warmth intensity ratings for eugenol- (□) and vehicle-treated (●) sides of tongue. &: F(3,174) = 3.09, p=0.028, 2-way repeated measures ANOVA. B. Carvacrol (same format as A; n=30).
To test for a halo-dumping effect, the experiments were repeated following desensitization of eugenol- and carvacrol-evoked irritation. One and one-half min after the end of sequential unilateral application of eugenol, heat pain was significantly enhanced in the 2-AFC (Fig. 6A, hatched bar, n=30). However, intensity ratings of heat pain did not differ significantly between the eugenol- and vehicle-treated sides (Fig. 6A, □, ●). Carvacrol had no effect on heat pain (Fig. 6B, n=30).
Fig. 6.
Enhancement of heat pain after desensitization by eugenol and carvacrol. A. Eugenol (format as in Fig. 4A). Open bars: 2-AFC for eugenol-evoked irritation. Hatched bars: 2-AFC for heat pain (Peltier thermode set at 49°C). *: p<0.05, binomial test (n=30). Lines with symbols: heat pain intensity ratings for eugenol- (□) and vehicle-treated (●) sides of tongue. B. Carvacrol (format as in A; n=30).
Lack of effect of eugenol or carvacrol in innocuous cold or cold pain
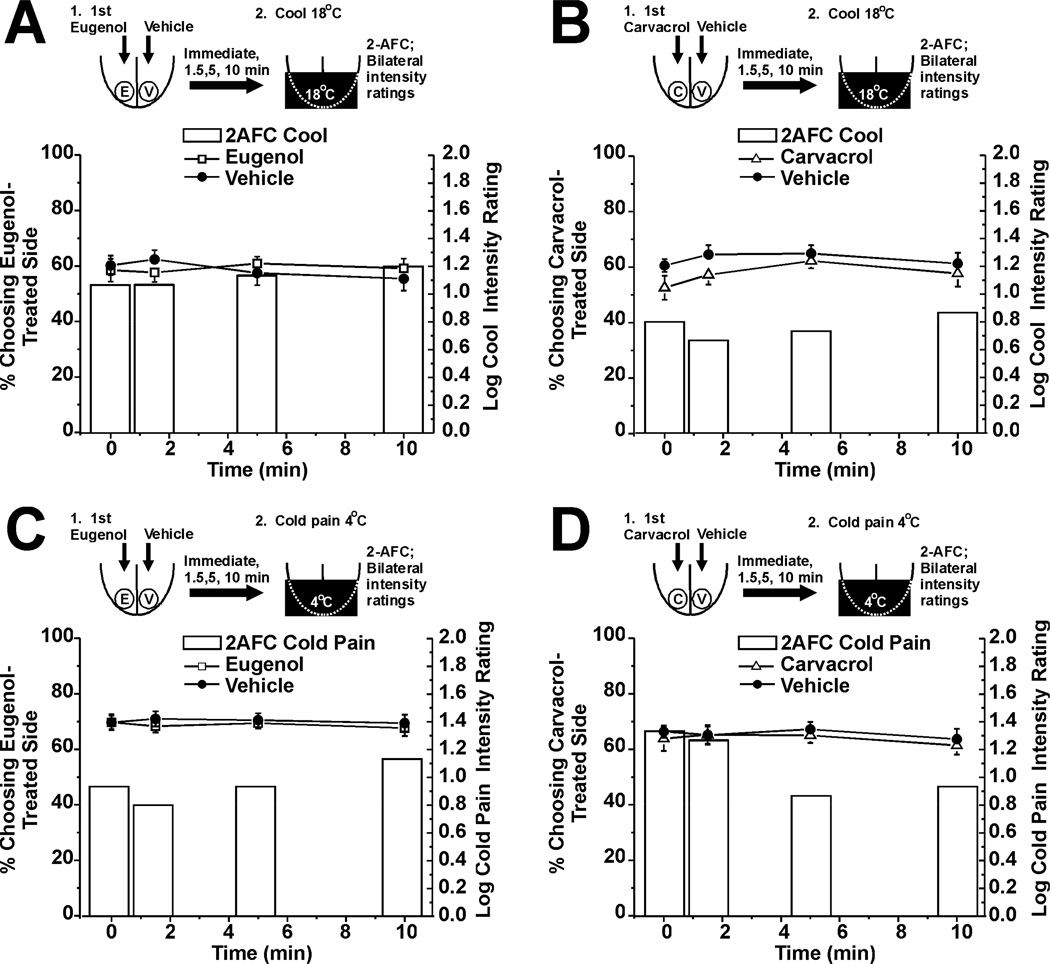
In these experiments we tested if eugenol or carvacrol affected sensations of innocuous cooling or cold pain on the tongue. Neither chemical had any effect, as assessed by 2-AFC and intensity ratings for innocuous cooling (Fig. 7A, B, n=30 for each) or cold pain (Fig. 7C, D, n=30 for each).
Fig. 7.
Lack of effect of eugenol and carvacrol on innocuous cold or cold pain. A. Eugenol had no effect on innocuous cold sensation elicited by 18°C stimulus (format as in Fig. 3A). Bars: 2-AFC (n=30). . Lines with symbols: cold intensity ratings. B: Carvacrol had no effect on cold sensation (format as in A; n=30). C. Eugenol had no effect on cold pain elicited by Peltier thermode set at 4°C (format as in A, n=30). D: Carvacrol had no effect on cold pain (format as in A, n=30).
Descriptive analysis of sensory qualities elicited by eugenol and carvacrol
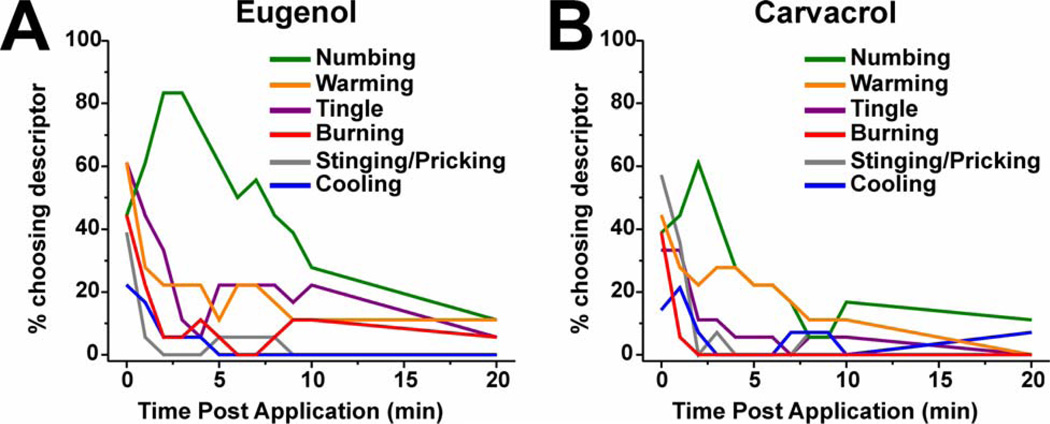
Irritation is a complex sensation that can be subdivided into a variety of contributing subqualities [6,7,11,13,25]. By having subjects choose freely from a list of descriptors, or select their own terms, we re-evaluated the subqualities of sensation elicited by lingual application of eugenol and carvacrol. For eugenol (n=18) and carvacrol (n=18), most subjects reported numbing, tingling, burning, stinging/pricking and/or warming immediately after application (Fig 8A, B). Following eugenol, numbing was reported most frequently (63.1%), followed by tingling and warming (27.2 and 23.7%, respectively, Fig. 8A). Burning and stinging/pricking were also reported immediately after eugenol but quickly decreased during the first few minutes (Fig. 8A). Following application of carvacrol, numbing was reported most frequently (27.8%) followed by warming (23.7%) and tingling (12.1%) (Fig.8B). Burning and stinging/pricking were also reported immediately after carvacrol application, but also declined very quickly. The descriptor “none” was the most frequently chosen descriptor following vehicle application (97.2% and 85.3% for sides opposite to eugenol and carvacrol application, respectively).
Fig. 8.
Sensory qualities elicited by lingual application of eugenol and carvacrol. A: Eugenol. Graph plots percentage of subjects reporting each perceived sensory quality immediately following 30 sec lingual application of eugenol, and each min thereafter for 10 min and again at 20 min post-application (n=18). B: Carvacrol (format as in A; n=18).
Eugenol reduces detection of weak tactile stimulation
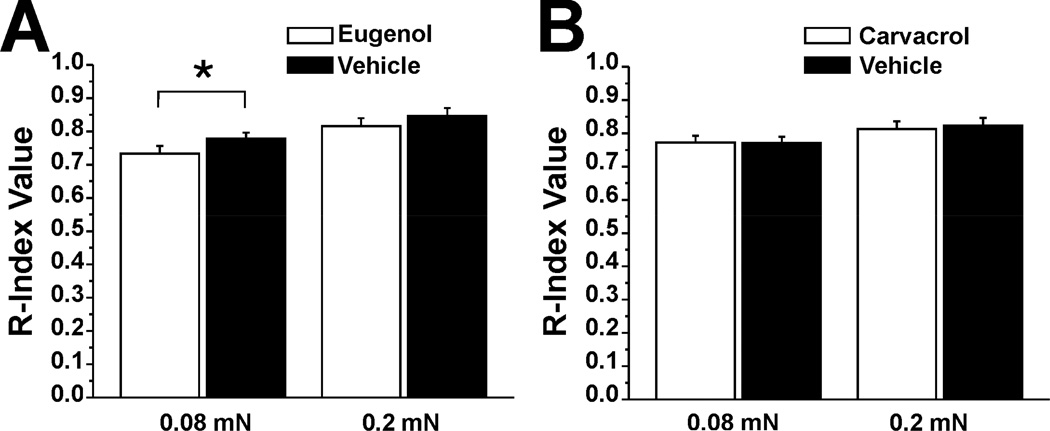
Because eugenol has been reported to act as a local anesthetic [38], we wished to test if it or carvacrol affected tactile sensitivity on the tongue. There was a significant decrease in the mean R-index for the 0.08 mN von Frey stimulus on the eugenol-treated compared to the vehicle treated side of the tongue (Fig 9A, n=30). Eugenol had no effect on detection of the stronger (0.2 mN) stimulus. Carvacrol had no effect on detection of either tactile stimulus (Fig 9B, n=29).
Fig. 9.
Eugenol but not carvacrol reduces detection of weak tactile stimulus. A. Eugenol. Significant difference in R-index measure of detection of weak (0.08 mN) von Frey stimulus between eugenol- vs. vehicle-treated side of tongue (*: p=0.45, paired t-test, n=30).Eugenol had no effect on detection of the stronger (0.2 mN) von Frey stimulus. B. Carvacrol had no effect on stimulus detection (format as A, n=29).
Discussion
The TRPV3 agonists, eugenol and carvacrol, elicited oral irritation that declined across repeated applications of both chemicals and persisted at least 10 min (self-desensitization). Both chemicals enhanced sensations of innocuous warmth and heat pain, but had no effect on innocuous cool or cold pain sensations. Eugenol also reduced detection of a weak tactile stimulus. Possible mechanisms of action are discussed below.
Desensitization
Eugenol and carvacrol exhibited self-desensitization, with the time course being faster for eugenol (Fig. 1). Desensitization has also been reported for the TRPM8 agonist menthol [16], and the TRPA1 agonists cinnamaldehyde [45], nicotine [15] and mustard oil [51]. The mechanism may involve desensitization of TRPV3. Prolonged exposure to monoterpenoids desensitized TRPV3 currents recorded in transfected HEK293 and human epithelial-derived cell lines [48].
Both eugenol and carvacrol cross-desensitized capsaicin-evoked oral irritation. (Fig. 2), consistent with cross-desensitization among other TRP channel agonists [16,24,32,49]. TRPV3 and TRPV1 are co-expressed in primary afferent neurons [19,52], supporting a peripheral site of interaction between TRPV3 and TRPV1 agonists. Eugenol activates TRPV1 [57] and TRPA1 [56] and induced desensitization, possibly via a calcium-dependent mechanism [54]. Carvacrol also activated and rapidly desensitized TRPA1 currents in transfected HEK293 cells [56]. Unlike the TRPV3 agonists, repeated application of capsaicin elicited a progressive rise in oral irritation (sensitization) [14,20,45,51] characterized by a burning quality. Thus, we speculate that the cross-desensitizing effect of eugenol and carvacrol on capsaicin-evoked irritation is mediated indirectly via activation of TRPV3, rather than via a direct effect of the TRPV3 agonists at TRPA1 or TRPV1.
Enhancement of warmth and heat pain
Eugenol and carvacrol enhanced the perception of innocuous warmth elicited by the 44°C (42.4 °C surface temperature) stimulus. We believe that this temperature was insufficient to excite thermal nociceptors innervating the tongue, since human lingual heat pain thresholds are >45°C [1,26,30]. The enhancement of warmth was still present, albeit weaker, following desensitization of the tongue to eugenol and carvacrol irritation (Fig. 4). This implies that to some extent, subjects may have summed the chemical irritant and thermal sensations when reporting their overall perception of warmth, a phenomenon referred to as halo-dumping [12]. Nevertheless, following desensitization of the tongue, enhancement of warmth was still detected using the 2-AFC. We speculate that TRPV3 agonists weakly sensitized responses of TRPV3-expressing warm fibers to innocuous thermal stimuli, while simultaneously desensitizing the chemically-evoked responses. However, we cannot rule out the possibility that the TRPV3 agonists act indirectly, for example by inducing the release of prostaglandin E2 [27] or other inflammatory agents [56] from epithelial cells that might increase the excitability of trigeminal nerve endings to warming.
Eugenol and carvacrol also enhanced heat pain on the tongue elicited by the 49°C stimulus. Eugenol had a stronger effect that was detected in both the 2-AFC and intensity ratings. Following desensitization of the tongue with eugenol, heat pain was still enhanced in the 2-AFC although intensity ratings were numerically but not significantly larger (Fig. 6A). This effect might be due to TRPV3-mediated enhancement of thermal gating by TRPV1 co-expressed in the same lingual nociceptive nerve endings (see above). Using the same psychophysical approach, we previously reported that capsaicin and mustard oil briefly enhanced heat pain [1]. Capsaicin enhancement of heat pain was still strong in the capsaicin-desensitized tongue, arguing against a halo-dumping effect and in favor of sensitization of the heat-sensing region on TRPV1. In the present study, enhancement of heat pain was lost following desensitization of the tongue by carvacrol (Fig. 6B). This suggests that the weak enhancement of heat pain by carvacrol in the naïve tongue (Fig. 5B) may have been due largely to summation of chemically- and thermally-evoked sensations, such that the effect was no longer detectable in the absence of chemicallyevoked irritation.
Neither eugenol nor carvacrol had any significant effect on innocuous cold or cold pain sensations (Fig.7). This corroborates a role for TRPV3 in sensing innocuous warmth [29] but not cold [40]. We previously reported that the TRPM8 agonist, menthol, significantly enhanced cold but not heat pain; TRPA1 agonists cinnamaldehyde and mustard oil also weakly enhanced cold pain while the TRPV1 agonist capsaicin did not [1]. Thus, the ability of TRP channel agonists to modulate temperature sensitivity appears to be specific to the range of thermal sensitivity of the particular TRP channel.
Sensory qualities
Following application of eugenol or carvacrol to the tongue, most subjects selected more than one sensory quality as being present, which is similar to reports using other chemical irritants [6,7,11,13,25]. The most frequently reported qualities were numbing followed by tingling and warming (Fig. 8), consistent with an earlier study reporting a dominant and prolonged numbing effect of eugenol [13]. Other irritants including ibuprofen [6,7], carbonated water [21, 49] and alkylamides such as hydroxyl-alpha sanshools and their derivatives [2,9] elicit numbing and tingling sensations. The mechanisms underlying these paraesthetic sensory qualities may involve inhibition of potassium channels [5] and/or activation of TRPV1 and TRPA1 in trigeminal sensory nerve endings (see [33] for further discussion).Eugenol inhibition of voltage-gated sodium channels [42], could possibly relate to an anesthetic effect associated with numbing and tingling.
The warming quality elicited by eugenol and carvacrol may be attributable to activation of TRPV3 expressed in lingual epithelial cells and/or trigeminal sensory nerve endings in the tongue. We recently presented preliminary data that ~25% of rat trigeminal ganglion (TG) cells responded to application of eugenol or carvacrol, with ~10% of these being unresponsive to algogens [34]; these might represent innocuous warm fibers. However, the vast majority of eugenol- or carvacrol-sensitive TG cells additionally responded to capsaicin, mustard oil and menthol, suggesting that TRPV3 is coexpresssed with TRPV1, TRPA1 and/or TRPM8 in trigeminal nociceptive nerve endings. Carvacrol activates and desensitizes TRPA1, relevant to its pungent quality [3]. Lingual application of eugenol and carvacrol excited a majority of noxious heat-sensitive neurons in rat trigeminal subnucleus caudalis [34], consistent with the idea that TRPV3 agonists activate trigeminal pain pathways to account for their burning and stinging/pricking qualities.
Tactile sensitivity
Because of the reported anesthetic action of eugenol [38], we tested if it and carvacrol affect lingual touch sensitivity. Eugenol reduced detection of a weak mechanical stimulus on the tongue (Fig. 9A). Eugenol was previously reported to reduce nerve compound action potentials [8,35] and to inhibit voltage-gated sodium [42] and potassium channels [36], P2X3 [37], and hyperpolarization-activated cyclic nucleotide-gated channels [58]. Importantly, eugenol enhanced perceived warmth and heat pain but did not affect cold sensitivity, arguing against a local anesthetic action. We speculate that multiple mechanisms of action account for the different effects of eugenol. The self- and cross-desensitizing actions of TRPV3 agonists, and their ability to weakly enhance sensitivity to increasing but not decreasing temperatures, are attractive features with implications for the use of these agents in oral hygiene products, analgesic balms, and other everyday cosmetic applications.
Eugenol and carvacrol are derived from the oils of clove and oregano, respectively, and impart warming, numbing and irritant sensations in the oral cavity by interacting with the warm-sensitive ion channel TRPV3. Here we show that these agents induce oral irritation in a manner that exhibits self-desensitization and cross-desensitization of capsaicin-evoked irritancy, and enhancement of warmth and heat pain, but not cold sensation.
Acknowledgements
This study was supported by grants from the National Institutes of Health (DE013685, AR057194).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflict of Interest
The authors do not declare any conflict of interest.
References
- 1.Albin KC, Carstens MI, Carstens E. Modulation of oral heat and cold pain by irritant chemicals. Chem Senses. 2008;33(1):3–15. doi: 10.1093/chemse/bjm056. [DOI] [PubMed] [Google Scholar]
- 2.Albin KC, Simons CT. Psychophysical evaluation of a sanshool derivative (alkylamide) and the elucidation of mechanisms subserving tingle. PLoS One. 2010;5(3):e9520. doi: 10.1371/journal.pone.0009520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bandell M, Story GM, Hwang SW, Viswanath V, Eid SR, Petrus MJ, Earely TJ, Patapoutian A. Noxious cold ion channel TRPA1 is activated by pungent compounds and bradykinin. Neuron. 2004;41(6):849–857. doi: 10.1016/s0896-6273(04)00150-3. [DOI] [PubMed] [Google Scholar]
- 4.Bartoshuk LM, Duffy VB, Green BG, Hoffman HJ, Ko CW, Lucchina LA, Marks LE, Snyder DJ, Weiffenbach JM. Valid across-group comparisons with labeled scales: the gLMS versus magnitude matching. Physiol Behav. 2004;82:109–114. doi: 10.1016/j.physbeh.2004.02.033. [DOI] [PubMed] [Google Scholar]
- 5.Bautista DM, Sigal YM, Milstein AD, Garrison JL, Zorn JA, Tsuruda PR, Nicoll RA, Julius D. Pungent agents from Szechuan peppers excite sensory neurons by inhibiting two-pore potassium channels. Nat Neurosci. 2008;11:772–779. doi: 10.1038/nn.2143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bennett SM, Hayes JE. Differences in the chemesthetic subqualities of capsaicin, ibuprofen, and olive oil. Chem Senses. 2012;37(5):471–478. doi: 10.1093/chemse/bjr129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Breslin PAS, Gingrich TN, Green BG. Ibuprofen as a chemesthetic stimulus evidence of a novel mechanism of throat irritation. Chem Senses. 2001;26(1):55–65. doi: 10.1093/chemse/26.1.55. [DOI] [PubMed] [Google Scholar]
- 8.Brodin P, Røed A. Effects of eugenol on rat phrenic nerve and phrenic nerve-diaphragm preparations. Archs Oral Biol. 1984;29(8):611–615. doi: 10.1016/0003-9969(84)90130-4. [DOI] [PubMed] [Google Scholar]
- 9.Bryant BP, Mezine I. Alkylamides that produce tingling paresthesia activate tactile and thermal trigeminal neurons. Brain Res. 1999;842:452–460. doi: 10.1016/s0006-8993(99)01878-8. [DOI] [PubMed] [Google Scholar]
- 10.Chung MK, Lee H, Mizuno A, Suzuki M, Caterina MJ. TRPV3 and TRPV4 mediate warmth-evoked currents in primary mouse keratinocytes. J Biol Chem. 2004;279:21569–21575. doi: 10.1074/jbc.M401872200. [DOI] [PubMed] [Google Scholar]
- 11.Cicerale S, Breslin PAS, Beauchamp GK, Keast RSJ. Sensory characterization of the irritant properties of oleocanthal a natural anti-inflammatory agent in extra virgin olive oils. Chem Senses. 2009;34(4):333–339. doi: 10.1093/chemse/bjp006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Clark CC, Lawless HT. Limiting response alternatives in time-intensity scaling: an examination of the halo-dumping effect. Chem Senses. 1994;19(6):583–594. doi: 10.1093/chemse/19.6.583. [DOI] [PubMed] [Google Scholar]
- 13.Cliff M, Heymann H. Descriptive analysis of oral pungency. Journal of Sensory Studies. 1992;7(4):279–290. [Google Scholar]
- 14.Dessirier JM, O'Mahony M, Carstens E. Oral irritant effects of nicotine: psychophysical evidence for decreased sensation following repeated application and lack of cross-desensitization to capsaicin. Chem Senses. 1997;22(5):483–492. doi: 10.1093/chemse/22.5.483. [DOI] [PubMed] [Google Scholar]
- 15.Dessirier J-M, Nguyen N, Sieffermann J-M, Carstens E, O’Mahony M. Oral irritant properties of piperine and nicotine: Psychophysical evidence for asymmetrical desensitization effects. Chem Senses. 1999;24(4):405–413. doi: 10.1093/chemse/24.4.405. [DOI] [PubMed] [Google Scholar]
- 16.Dessirier J-M, O’Mahony M, Carstens E. Oral irritant properties of menthol: sensitizing and desensitizing effects of repeated application and cross-desensitization to nicotine. Physiol Behav. 2001;73(1–2):25–36. doi: 10.1016/s0031-9384(01)00431-0. [DOI] [PubMed] [Google Scholar]
- 17.Dessirier JM, O'Mahony M, Sieffermann JM, Carstens E. Mecamylamine inhibits nicotine but not capsaicin irritation on the tongue: psychophysical evidence that nicotine and capsaicin activate separate molecular receptors. Neurosci Lett. 1998;240(2):65–68. doi: 10.1016/s0304-3940(97)00930-0. [DOI] [PubMed] [Google Scholar]
- 18.Dessirier JM, Simons CT, O'Mahony M, Carstens E. The oral sensation of carbonated water: cross-desensitization by capsaicin and potentiation by amiloride. Chem Senses. 2001;26(6):639–643. doi: 10.1093/chemse/26.6.639. [DOI] [PubMed] [Google Scholar]
- 19.Facer P, Casula MA, Smith GD, Benham CD, Chessell IP, Bountra C, Sinisi M, Birch R, Anand P. Differential expression of the capsaicin receptor TRPV1 and related novel receptors TRPV3, TRPV4 and TRPM8 in normal human tissues and changes in traumatic and diabetic neuropathy. BMC Neurol. 2007;7:11. doi: 10.1186/1471-2377-7-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Green BG. Capsaicin sensitization and desensitization on the tongue produced by brief exposures to a low concentration. Neurosci Lett. 1989;107(1–3):173–178. doi: 10.1016/0304-3940(89)90812-4. [DOI] [PubMed] [Google Scholar]
- 21.Green BG. The effects of temperature and concentration on the perceived intensity and quality of carbonation. Chem Senses. 1992;17(4):435–450. [Google Scholar]
- 22.Green BG, Shaffer GS, Gilmore MM. Derivation evaluation of a semantic scale of oral sensation magnitude with apparent ratio properties. Chem. Senses. 1993;18:683–702. [Google Scholar]
- 23.Green BG, Dalton P, Cowart B, Shaffer G, Rankin K, Higgins J. Evaluating the 'Labeled Magnitude Scale' for measuring sensations of taste and smell. Chem Senses. 1996;21:323–334. doi: 10.1093/chemse/21.3.323. [DOI] [PubMed] [Google Scholar]
- 24.Green BG, McAuliffe BL. Menthol desensitization of capsaicin irritation Evidence of a short-term anti-nociceptive effect. Physiol Behav. 2000;68(5):631–639. doi: 10.1016/s0031-9384(99)00221-8. [DOI] [PubMed] [Google Scholar]
- 25.Green BG. Psychophysical Measurement of Oral Chemesthesis. In: Simon SA, Nicolelis MAL, editors. Methods in Chemosensory Reseach. Methods and New Frontiers in Neuroscience Series. Boca Raton FL: CRC Press; 2002. pp. 3–19. [Google Scholar]
- 26.Grushka M, Sessle BJ, Howley TP. Psychophysical assessment of tactile, pain and thermal sensory functions in burning mouth syndrome. Pain. 1987;28(2):169–184. doi: 10.1016/0304-3959(87)90114-X. [DOI] [PubMed] [Google Scholar]
- 27.Huang SM, Lee H, Chung M-K, Park U, Yu YY, Bradshaw HB, Coulombe PA, Walker JM, Caterina MJ. Overexpressed TRPV3 ion channels in skin keratinocytes modulate pain sensitivity via prostaglandin E2 . J Neurosci. 2011a;28(51):13727–13737. doi: 10.1523/JNEUROSCI.5741-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Huang SM, Li X, Yu Y, Wang J, Caterina MJ. TRPV3 and TRPV4 are not major contributors to mouse heat sensation. Mol Pain. 2011b;7:37. doi: 10.1186/1744-8069-7-37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Joshi NK, Mahara N, Thomas A. The TRPV3 receptor as a pain target: A therapeutic promise or just some more new biology? Open Drug Discov J. 2010;2:89–97. [Google Scholar]
- 30.Kaplan I, Levin T, Papoiu AD, Patel N, Patel T, Calderon S, Littner M, McGlone F, Yosipovitch G. Thermal sensory and pain thresholds in the tongue and chin change with age, but are not altered in burning mouth syndrome. Skin Res Technol. 2011;17(2):196–200. doi: 10.1111/j.1600-0846.2010.00483.x. [DOI] [PubMed] [Google Scholar]
- 31.Klein AH, Iodi Carstens M, Carstens E. TRPV3 agonists induce a temporally desensitizing pattern of oral irritation and affect lingual temperature sensitivity. Chem. Senses. 2011a;31:A40. [Google Scholar]
- 32.Klein AH, Iodi Carstens M, Zanotto KL, Sawyer CM, Ivanov M, Cheung S, Carstens E. Self-and cross-desensitization of oral irritation by menthol and cinnamaldehyde (CA) via peripheral interactions at trigeminal sensory neurons. Chem Senses. 2011b;36(2):199–208. doi: 10.1093/chemse/bjq115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Klein AH, Sawyer CM, Zanotto KL, Ivanov MA, Cheung S, Carstens MI, Furrer S, Simons CT, Slack JP, Carstens E. A tingling sanshool derivative excites primary sensory neurons and elicits nocifensive behavior in rats. J Neurophysiol. 2011c;105(4):1701–1710. doi: 10.1152/jn.00922.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Klein AH, Joe CL, Davoodi A, Takechi K, Iodi Carstens M, Carstens E. Program No. 784.14/NN18. 2012 Neuroscience Meeting Planner. New Orleans, LA: Society for Neuroscience; 2012. Eugenol and carvacrol increased lingual heat responses in trigeminal subnucleus caudalis (Vc) neurons and activate capaicin-sensitive trigeminal ganglion neurons in rats (abstract) Online. [Google Scholar]
- 35.Kozam G. The effect of eugenol on nerve transmission. Oral Surg Oral Med Oral Pathol. 1977;44:799–805. doi: 10.1016/0030-4220(77)90390-5. [DOI] [PubMed] [Google Scholar]
- 36.Li HY, Park CK, Jung SJ, Choi S-Y, Lee SJ, Park K, Kim JS, Oh SB. Eugenol inhibits K+ currents in trigeminal ganglion neurons. J Dent Res. 2007;86(9):898–902. doi: 10.1177/154405910708600918. [DOI] [PubMed] [Google Scholar]
- 37.Li HY, Lee BY, Kim JS, Jung SJ, Oh SB. Eugenol inhibits ATP-induced P2X currents in trigeminal ganglion neurons. Korean J Physiol Pharmacol. 2008;12(6):315–321. doi: 10.4196/kjpp.2008.12.6.315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Markowitz K, Moynihan M, Liu M, Kim S. Biological properties of eugenol and zinc-oxide eugenol. Oral Surg Oral Med Oral Pathol. 1992;73:729–737. doi: 10.1016/0030-4220(92)90020-q. [DOI] [PubMed] [Google Scholar]
- 39.McBurney DH, Kasschau RA, Bogart LM. The effect of adaptation on taste jnds. Percept Psychophys. 1967;2:175–178. [Google Scholar]
- 40.Moqrich A, Hwang SW, Earley TJ, Petrus MJ, Murray AN, Spencer KSR, Andahazy M, Story GM, Patapoutian A. Impaired thermosensation in mice lacking TRPV3, a heat and camphor sensor in the skin. Science. 2005;307(5714):1468–1472. doi: 10.1126/science.1108609. [DOI] [PubMed] [Google Scholar]
- 41.O’Mahony M. Understanding discrimination tests: A user-friendly treatment of response bias rating and ranking R-index tests and their relationship to signal detection. J Sens Studies. 1992;7(1):1–47. [Google Scholar]
- 42.Park CK, Li HY, Yeon K-Y, Jung SJ, Choi S-Y, Lee SJ, Lee S, Park K, Kim JS, Oh SB. Eugenol inhibits sodium currents in dental afferent neurons. J Dent Res. 2006;85(10):900–904. doi: 10.1177/154405910608501005. [DOI] [PubMed] [Google Scholar]
- 43.Park CK, Kim K, Jung SJ, Kim MJ, Ahn DK, Hong SD, Kim JS, Oh SB. Molecular mechanism for local anesthetic action of eugenol in the rat trigeminal system. Pain. 2009;144(1–2):84–94. doi: 10.1016/j.pain.2009.03.016. [DOI] [PubMed] [Google Scholar]
- 44.Peier AM, Reeve AJ, Andersson DA, Moqrich A, Earley TJ, Hergarden AC, Story GM, Colley S, Hogenesch JB, McIntyre P, Bevan S, Patapoutian A. A heat sensitive TRP channel expressed in keratinocytes. Science. 2002;296:2046–2049. doi: 10.1126/science.1073140. [DOI] [PubMed] [Google Scholar]
- 45.Prescott J, Swain-Campbell N. Responses to repeated oral irritation by capsacin, cinnamaldehyde and ethanol in PROP tasters and non-tasters. Chem Senses. 2000;25(3):239–246. doi: 10.1093/chemse/25.3.239. [DOI] [PubMed] [Google Scholar]
- 46.Ruparel NB, Patwardhan AM, Akopian AN, Hargreaves KM. Homologous and heterologous desensitization of capsaicin and mustard oil responses utilize different cellular pathways in nociceptors. Pain. 2008;135(3):271–279. doi: 10.1016/j.pain.2007.06.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Shabab A, Haghighati F, Baeeri M, Jamalifar H, Abdollahi M. A clinical, microbiological and immunological comparison between subgingival irrigation with Dental and chlorhexidine in advanced periodontitis. Arch Med Sci. 2011;7(1):154–160. doi: 10.5114/aoms.2011.20622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Sherkheli MA, Benecke H, Doerner JF, Kletke O, Vogt-Eisele AK, Gisselmann G, Hatt H. Monoterpenoids induced agonist-specific desensitization of transient receptor potential vanilloid-3 ion channels. J Pharm Pharmaceut Sci. 2009;12(1):116–128. doi: 10.18433/j37c7k. [DOI] [PubMed] [Google Scholar]
- 49.Simons CT, Dessirier JM, Carstens MI, O'Mahony M, Carstens E. Neurobiological and psychophysical mechanisms underlying the oral sensation produced by carbonated water. J Neurosci. 1999;19(18):8134–44. doi: 10.1523/JNEUROSCI.19-18-08134.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Simons CT, O'Mahony M, Carstens E. Taste suppression following lingual capsaicin pre-treatment in humans. Chem Senses. 2002;27(4):353–365. doi: 10.1093/chemse/27.4.353. [DOI] [PubMed] [Google Scholar]
- 51.Simons CT, Iodi Carstens M, Carstens E. Oral irritation by mustard oil: self-desensitization and cross-desensitization with capsaicin. Chem Senses. 2003;28(6):459–465. doi: 10.1093/chemse/28.6.459. [DOI] [PubMed] [Google Scholar]
- 52.Smith GD, Gunthorpe MJ, Kelsell RE, Hayes PD, Reilly P, Facer P, Wright JE, Jerman JC, Walhin JP, Ooi L, Egerton J, Charles KJ, Smart D, Randall AD, Anand P, Davis JB. TRPV3 is a temperature-sensitive vanilloid receptor-like protein. Nature. 2002;418(6894):186–190. doi: 10.1038/nature00894. [DOI] [PubMed] [Google Scholar]
- 53.Voet-Eisele AK, Weber K, Sherkheli MA, Vielhaber G, Panten J, Gisselmann G, Hatt H. Monoterpenoid agonists of TRPV3. Br J Pharmacol. 2007;151(4):530–540. doi: 10.1038/sj.bjp.0707245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Vyklický L, Nováková-Tousová K, Benedikt J, Samad A, Touska F, Vlachová V. Calcium-dependent desensitization of vanilloid receptor TRPV1: a mechanism possibly involved in analgesia induced by topical application of capsaicin. Physiol Res. 2008;57(S3):S59–S68. doi: 10.33549/physiolres.931478. [DOI] [PubMed] [Google Scholar]
- 55.Xu H, Ramsey IS, Kotecha SA, Moran MM, Chong JA, Lawson D, Ge P, Lilly J, Silos-Santiago, Xie Y, Distefano PS, Curtis R, Clapham DE. TRPV3 is a calcium-permeable temperature-sensitive ion channel. Nature. 2002;418(6894):181–186. doi: 10.1038/nature00882. [DOI] [PubMed] [Google Scholar]
- 56.Xu H, Delling M, Jun JC, Clapham D. Oregano, thyme and clove-derived flavors and skin sensitizers activate specific TRP channels. Nat Neurosci. 2006;9:628–635. doi: 10.1038/nn1692. [DOI] [PubMed] [Google Scholar]
- 57.Yang BH, Piao ZG, Kim YB, Lee CH, Lee JK, Park K, Kim JS, Oh SB. Activation of vanilloid receptor 1 (VR1) by eugenol. J Dent Res. 2003;82:781–785. doi: 10.1177/154405910308201004. [DOI] [PubMed] [Google Scholar]
- 58.Yeon KY, Chung G, Kim YH, Hwang JH, Davies AJ, Park MK, Ahn DK, Kim JS, Jung SJ, Oh SB. Eugenol reverses mechanical allodynia after peripheral nerve injury by inhibiting hyperpolarization-activated cyclic nucleotide-gated (HCN) channels. Pain. 2011;152(9):2108–2016. doi: 10.1016/j.pain.2011.05.018. [DOI] [PubMed] [Google Scholar]