Abstract
Menthol, the cooling natural product of peppermint, is widely used in medicinal preparations for the relief of acute and inflammatory pain in sports injuries, arthritis and other painful conditions. Menthol induces the sensation of cooling by activating TRPM8, an ion channel in cold-sensitive peripheral sensory neurons. Recent studies identified additional targets of menthol, including the irritant receptor, TRPA1, voltage-gated ion channels and neurotransmitter receptors. It remains unclear which of these targets contribute to menthol-induced analgesia, or to the irritating side effects associated with menthol therapy.
Here, we use genetic and pharmacological approaches in mice to probe the role of TRPM8 in analgesia induced by L-menthol, the predominant analgesic menthol isomer in medicinal preparations. L-menthol effectively diminished pain behavior elicited by chemical stimuli (capsaicin, acrolein, acetic acid), noxious heat and inflammation (complete Freund's adjuvant). Genetic deletion of TRPM8 completely abolished analgesia by L-menthol in all these models, while other analgesics (acetaminophen) remained effective. Loss of L-menthol-induced analgesia was recapitulated in mice treated with a selective TRPM8 inhibitor, AMG2850. Selective activation of TRPM8 with WS-12, a menthol derivative we characterized as a specific TRPM8 agonist in cultured sensory neurons and in vivo, also induced TRPM8-dependent analgesia of acute and inflammatory pain. L-menthol and WS-12 induced analgesia was blocked by naloxone, suggesting activation of endogenous opioid-dependent analgesic pathways.
Our data show that TRPM8 is the principal mediator of menthol-induced analgesia of acute and inflammatory pain. In contrast to menthol, selective TRPM8 agonists may produce analgesia more effectively with diminished side effects.
INTRODUCTION
Menthol, the cooling natural product of peppermint, is widely used in preparations for pain relief in sports injuries, arthritis and other painful conditions [14; 25]. In animal models, topical, oral or systemic administration of menthol elicits analgesia of acute, inflammatory and neuropathic pain [17; 29; 40]. Menthol, and other cooling natural products such as eucalyptol, activate TRPM8, a Transient Receptor Potential (TRP) ion channel, an essential detector of cold stimuli in sensory neurons [2; 13; 37; 38; 41]. Menthol, through activation of TRPM8, may have effects similar to tissue cooling that reduces pain in some injury states. In TRPM8-deficient mice, cooling-induced analgesia was less effective towards formalin-induced and neuropathic pain [13; 31]. In rats, intrathecal injection of TRPM8 antisense DNA reduced the analgesic effects of menthol towards neuropathic pain [43; 51].
Studies using TRPM8 gene-deficient mice to probe the role of TRPM8 in menthol-induced analgesia have not been reported and some recent findings suggest that TRPM8-independent mechanisms are responsible for menthol analgesia. Menthol and eucalyptol have poor specificity for TRPM8 and interact with other TRP channels, including TRPA1 and TRPV3 [34; 60]. These ion channels may elicit pain and inflammatory symptoms in some patients treated with menthol preparations, but may also contribute to analgesia [21; 26; 61]. For example, eucalyptol was found to inhibit human TRPA1 channels, exerting a mild analgesic effect on pain elicited by TRPA1 agonists in human subjects [54]. Menthol has similar inhibitory effects on murine TRPA1 channels at higher concentrations, but not on human TRPA1 [28; 65]. Other studies suggest that menthol analgesia is mediated by TRP channel-independent mechanisms. Menthol was found to inhibit neuronal voltage-dependent Ca2+ channels [49; 52]. Menthol was shown to activate GABAA-receptors, which may induce central inhibition of nociception [11; 63; 67]. Menthol may also elicit analgesia by inactivating voltage-gated sodium channels mediating action potentials in sensory neurons [19]. Menthol was also found to inhibit nicotinic acetyl choline receptors, and serotonin-gated ion channels, known to contribute to pain signaling [22; 24].
In addition to the lack of selectivity, the contradictory outcomes of the above studies may have resulted from the use of different stereoisomers of menthol [27]. The predominant isomer (~98%) in natural mint oils is L-menthol, with small amounts of D-menthol and other isomers present [14]. L-menthol has a lower sensory detection threshold for cooling and more potent analgesic activity than D-menthol., and carries the “minty” smell associated with peppermint, whereas D-menthol has a phenolic smell [14; 16–18]. Most mentholated analgesic products exclusively contain L-menthol [9].
Here, we compare the analgesic effects of L-menthol in wild-type and TRPM8-deficient mice in models of acute cutaneous, visceral and inflammatory pain. L-menthol efficiently inhibited pain behavior induced by a diverse set of chemical and physical noxious stimuli. Menthol analgesia was completely abolished in Trpm8−/− mice, and in wild-type mice treated with a selective TRPM8 inhibitor. Moreover, a recently identified specific TRPM8 agonist recapitulated menthol's analgesic effects. These results suggest that TRPM8 is the principal mediator of menthol's analgesic effects in vivo.
MATERIALS AND METHODS
Animals
Experimental procedures were approved by the Institutional Animal Care and Use Committees of Yale University. Mice were housed at facilities accredited by the Association for Assessment and Accreditation of Laboratory Animal Care in standard environmental conditions (12 h light-dark cycle and 23 °C). Food and water were provided ad libitum. Trpa1−/− and Trpm8−/− mice were a gift from David Julius (UCSF) and backcrossed into C57BL/6 background using marker assisted backcrossing (Charles River Laboratories, Wilmington, MA). Trpv1−/− mice were purchased from Jackson Laboratories (Stock# 003770), Bar Harbor, ME, and crossed with Trpa1−/− mice to produce Trpv1−/−/Trpa1−/− mice. C57BL/6 wild-type mice were purchased from Charles River Laboratories. Male mice aged 8–12 weeks were used in all experiments
Chemicals
L-menthol and eucalyptol were purchased from Fisher Scientific (Pittsburgh, PA), WS-12 from Tocris Bioscience (Bristol, UK), AMG2850 was a gift from Amgen Inc. (Thousand Oaks, CA), CFA was from Rockland Immunochemicals (Gilbertsville, PA). Acetic acid, mustard oil, acetaminophen, capsaicin, acrolein, naloxone were from Sigma-Aldrich (St. Louis, MO).
Behavioral tests
Hot plate test
L-menthol at a dose of 10 mg/kg was prepared in DMSO, further diluted in PBS and was applied through oral gavage to wild type male C57BL/6 or Trpm8−/− mice (8–12 weeks old) in a volume of 50 ml/kg. WS-12 (10 mg/kg) was prepared in ethanol, further diluted in corn oil and injected (i.p.) to mice in a volume of 5 ml/kg. WS-12 showed poor solubility in aqueous media, and needed to be used at dosages equivalent to menthol in in vivo tests. After 30 min, mice were placed into a transparent chamber with a stainless steel hot plate set to a temperature of either 52°C or 55°C (IITC, Woodland Hills, CA). A baseline test of paw withdrawal latency was done one week before to make sure there were no differences among each group. Behavior was then videorecorded and the latency to exhibit nocifensive responses (such as lifting and licking of the hind paw or jumping) was calculated using a stop watch.
Acetic acid writhing test
Mice were first habituated in transparent observation chambers for 30 min before the test. Mice were given L-menthol (10 or 20 mg/kg), eucalyptol (200 mg/kg) or acetaminophen (50 mg/kg) by i.p. injection 30 min prior to testing, prepared in corn oil and administered i.p. to mice in a volume of 5 ml/kg. 30 min later, mice were injected (i.p.) with 0.6% acetic acid (10 ml/kg). Numbers of stretching (writhing) movements were recorded for 30 min by a camera located underneath the chamber and counted thereafter.
Tail flick test
Mice were restrained in a small restraining chamber allowing the free movement of the tails. Mice were trained in the chamber for 40 min daily for one week before the test. Tails of the mice were exposed to radiant heat, and the tail flick latency was automatically recorded by the testing device (IITC, Woodland Hills, CA). Tail flick latencies were averaged from 3 separate tests conducted within interval of 1 min. L-Menthol (10 or 20 mg/kg) is prepared in corn oil and then i.p. injected into mice at a volume of 2.5 ml/kg 30 min before testing.
Chemically induced nocifensive behavior
Mice were placed into transparent chambers and habituated for 30 min before testing. Compounds were injected into the hind paw of mice using a 1 ml syringe and 30G needle as follows: L-menthol (50 nmol) was co-injected with capsaicin (5 nmol) or acrolein (25 nmol) in a total volume of 50 μl dissolved in saline. WS-12 (6 nmol) was injected with capsaicin (2 nmol) or acrolein (30 nmol) in a total volume of 20 μl dissolved in corn oil. Nocifensive behavior (licking, flinching or biting of injected paw) was recorded for 10 min and quantified thereafter.
Capsaicin-induced mechanical hyperalgesia
Mice were habituated for 1 h to wire mesh screen surface before testing. Paw withdrawal thresholds were determined using a series of von Frey filaments (0.008 to 6.00 g) pressed against the plantar surface of the hind paw in ascending order beginning with the finest fiber following standard procedures [10; 42]. The minimum force (g) that caused the mouse to withdraw its hind paw away from the filament was considered as the withdrawal threshold. For each paw, a von Frey hair was applied five times at 10-sec intervals. The threshold was determined when paw withdrawal was observed in more than three of five applications. A withdrawal response was considered valid only if the hind paw was removed completely from the platform. If the paw withdrawal response was ambiguous, the application was repeated. Thresholds were measured 0 min, 15 min, 30 min, 45 min and 60 min post-application of capsaicin (400 pmol) or capsaicin + L-menthol (20 nmol) in 20 μl volume.
CFA Test
CFA was injected into the plantar surface of the right hind paw of each mouse at a volume of 20 μl on day 0. For control mice, saline was injected instead. On days 1, 2 and 3, paw withdraw threshold (g) of the injected paw was evaluated by von Frey filaments as mentioned above.
Rotarod testing
Mice were placed on a rotating cylinder with the speed increasing from 5 to 40 rpm in 5 min (IITC, CA) on 4 consecutive days for habituation. Tests were repeated 3 times, with 5 min breaks. Fall latency times were determined by stopwatch and averaged for the 3 tests. All behavioral tests were performed by an experimenter blind to experimental conditions.
Cell culture and Ca2+ imaging
Adult mouse dorsal root ganglia were dissected and dissociated for 1 hour incubation in 0.28 WU/mL Liberase DH Research Grade high Dispase concentration (Roche, Germany), followed by washes with PBS, trituration, and straining of debris (70 μm cell strainer, Falcon, MA). Neurons were cultured in neurobasal-A medium (Invitrogen) with B-27 supplement, 0.5mM glutamine and 50 ng/mL NGF (Calbiochem) on 8-well chambered coverglass coated with polylysine (Sigma, MO) and laminin (Invitrogen). HEK-293T cells were cultured and transfected as described [26]. Ca2+ Imaging was performed 24 hours after DRG dissection or cell transfection. Medium was replaced by modified standard Ringer's bath solution (in mM): 140 NaCl, 5 KCl, 2 CaCl2, 2 MgCl2, 10 HEPES, pH 7.4. Cells were loaded with Fura 2-AM (10 μM, Calbiochem, CA) for 45 min and subsequently washed and imaged in standard bath solution. Ratiometric Ca2+-imaging was performed on an Olympus IX51 microscope with a Polychrome V monochromator (Till Photonics) and a PCO Cooke Sensicam QE CCD camera and Imaging Workbench 6 imaging software (Indec). Fura-2 emission images were obtained with exposures of 0.1 ms at 340 nm and 0.1 ms at 380 nm excitation wavelengths. The ratio of the fluorescence intensity obtained at 340 and 380 nm was used to determine the Ca2+ signal.
Statistical analysis
Statistical comparisons were made between groups using Student's t-test, or one-way analysis of variance (ANOVA), followed by Tukey post-hoc test with P < 0.05 considered significant. Data in bar graphs are as means and S.E.M.
RESULTS
TRPM8 is essential for L-menthol analgesia of acute heat- and capsaicin-induced pain in mice
Systemic or topical administration of L-menthol is known to increase pain thresholds in mice and rats in the hot plate test [17; 29; 57]. We compared the analgesic action of systemic L-menthol in wild-type C57BL6 and Trpm8−/− mice. L-menthol (10 mg/kg, p.o.) was administered 30 min before testing at plate temperatures of 52°C or 55°C. In wild-type mice, L-menthol approximately doubled paw withdrawal latencies at both temperatures (Fig. 1A). This analgesic effect was completely abolished in Trpm8−/− mice (Fig. 1A). Topical application of menthol to the mouse paws (30% in ethanol, 5 min before testing (52°C plate temperature) also produced a strong analgesic effect that was again abolished in Trpm8−/− mice (Fig. 1B, C). In control experiments, this concentration of L-menthol did not lead to acute nocifensive behavior when applied topically, compared to ethanol control animals (observed over 10min, n=7 animals each). We also observed that L-menthol has analgesic effects in the mouse tail flick test, another model of thermally induced pain. L-menthol (20 mg/kg, i.p., 30 min prior to testing) produced a significant increase in tail flick latencies in wild-type mice (Fig. 1D). This effect was again abolished in Trpm8−/− mice (Fig. 1D). The same dose of L-menthol (20 mg/kg, i.p.) did not change fall latencies in control rotarod experiments, suggesting a specific analgesic effect (Suppl. Fig. 1).
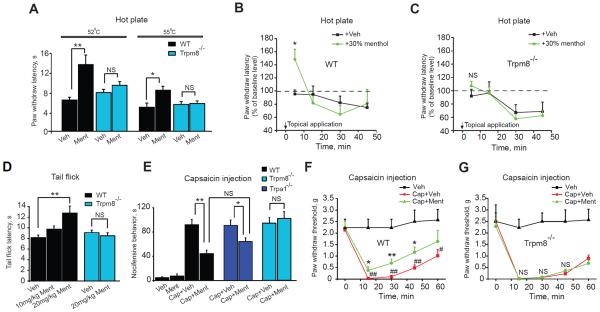
Fig.1.
Effects of L-menthol on thermal- and capsaicin-induced pain behavior in wild-type and Trpm8−/− mice.
(A) Paw withdrawal latencies in the hot plate test (52 or 55°C) in wild-type (black) and Trpm8−/−mice (blue) treated with L-menthol (Ment, 10 mg/kg) or vehicle (PBS, Veh) through oral gavage (p.o.) 30 min prior to testing. n=7–10 mice per group.
(B)Paw withdrawal latencies of wild-type mice in the hot plate test (52°C) following topical application of 30% L-menthol and ethanol (Veh). The two hind paws were immersed into vehicle or L-menthol solution for 5 seconds and allowed to dry for 1 min. n=7–9 mice per group.
(C) Paw withdrawal latencies of Trpm8−/− mice in the hot plate test following topical application of 30% L-menthol and ethanol. Experiment as in (B). n=8 mice per group.
(D) Tail flick latencies of wild-type and Trpm8−/− mice treated with vehicle or L-menthol (10 or 20 mg/kg, i.p.) 30 min before testing. n=6–7 mice per group.
(E) Effects of L-menthol on capsaicin-induced nocifensive behavior (flinching, lifting and biting) in wild-type mice, Trpm8−/− or Trpa1−/− mice. Vehicle (PBS), L-menthol (50 nmol), capsaicin (Cap, 5 nmol) or capsaicin in combination with L-menthol (5nmol+50 nmol) were injected (in 50 μl volume) into the plantar surface of the hind paw and nocifensive behavior was recorded for 10 min. n=6–12 mice per group.
(F) Effects of L-menthol on capsaicin-induced mechanical hyperalgesia in wild-type mice. Capsaicin (400 pmol) or capsaicin plus L-menthol (20 nmol) were injected into the plantar surface of the hind paw in 20 μl volume. Paw withdrawal thresholds were measured with von Frey hair filaments at time points indicated. n=6 mice per group.
(G) Effects of L-menthol on capsaicin-induced mechanical hyperalgesia in Trpm8−/− mice. Experiment performed as in (E). n=6 mice per group. **p < 0.01, *p < 0.05, NS: not significant (p > 0.05).
TRPV1, the capsaicin receptor, is a crucial mediator of heat-evoked pain in humans and rodents [8; 46; 47]. Since we observed that L-menthol inhibited heat-evoked pain, we asked whether L-menthol would also reduce nocifensive behavior caused by specific pharmacological activation of TRPV1 following injection of capsaicin into the plantar surface of the mouse hindpaw (5 nmol/50 μl) (Fig. 1E). Coinjection of L-menthol (intraplantar, 50 nmol/50 μl=1 mM) strongly diminished capsaicin-induced nocifensive responses in wild-type mice. In Trpm8−/− mice, capsaicin-induced nocifensive behavior was as robust as in wild-type mice (Fig. 1E). However, the analgesic effect of L-menthol was completely absent in Trpm8−/− mice (Fig. 1E). Since TRPA1 may functionally interact with TRPV1 in capsaicin-sensitive sensory neurons expressing both channels, and murine TRPA1 channels were found to be inhibited by high L-menthol concentration, we examined L-menthol's actions in Trpa1−/− mice. Trpa1−/− mice showed normal nocifensive responses to capsaicin, and we found that L-menthol's analgesic activity was not different from its activity in wild-type mice (Fig.1E, tested by ANOVA, followed by Tukey post-hoc test, p > 0.05]. Mechanical hyperalgesia caused by intraplantar injection of capsaicin (400 pmol/20 μl) was also inhibited by L-menthol when co-injected (20 nmol/20 μl=1 mM) in wild-type mice (Fig. 1F). Trpm8−/− mice showed normal mechanical hyperalgesia following injection of capsaicin, but L-menthol's analgesic effects were absent (Fig. 1G).
>L-Menthol requires TRPM8 for inhibition of TRPA1-induced acute and inflammatory pain
Acrolein, the major reactive irritant in cigarette smoke, stimulates sensory nerves through activation of TRPA1 [1; 4]. We recently reported that L-menthol inhibited acrolein-induced respiratory irritation responses in mice [64]. Acrolein is also generated endogenously as a product of lipid peroxidation, similar to other endogenous lipid peroxidation products thought to cause inflammatory pain through activation of TRPA1 following tissue injury [35; 39; 59]. To examine L-menthol's effects on TRPA1-induced pain behavior, we compared acute nocifensive responses of mice injected with acrolein (25 nmol/50 μl) into the plantar surface of the hindpaw, with or without coinjection of L-menthol (50 nmol/50 μl = 1mM). Wild-type mice showed obvious acrolein-induced TRPA1-dependent nocifensive behavior that was strongly inhibited by L-menthol (Fig. 2A, Suppl. Fig. 2A). Trpm8−/− mice showed equally strong responses to acrolein, but L-menthol's analgesic effects were completely absent (Fig. 2A).
Fig.2.
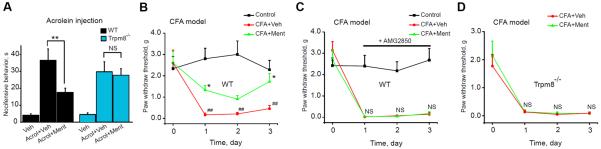
Effects of L-menthol on acute acrolein-induced pain and CFA-induced mechanical hyperalgesia in wild-type, Trpm8−/−, or TRPM8 antagonist-treated mice
(A) Nocifensive behavior in wild-type (black) or Trpm8−/− (blue) mice quantified over 10 min following hindpaw injection of vehicle (Veh, PBS), acrolein alone (Acrol, 25 nmol in 50 μl PBS) or acrolein plus L-menthol (50 nmol, co-injected). n=9–10 mice per group. **p < 0.01, NS: not significant (p > 0.05).
(B) Effects of L-menthol on CFA-induced mechanical hyperalgesia in wild-type mice, measured by von Frey hair analysis. Baseline thresholds were measured before CFA injection (day 0). On days 1–3, mice were injected into the plantar surface of the paw with L-menthol (Ment, 60 nmol in 20 μl, green) or vehicle (PBS, red) 30 min prior to testing. Non CFA-treated mice are shown for comparison (black). n=7–8 mice per group. *p < 0.05 vs. CFA+Veh, ##p < 0.01 vs. Control, NS: not significant from CFA+Veh.
(C) Effect of TRPM8 antagonists, AMG2850, on L-menthol inhibition of CFA-induced mechanical hyperalgesia in wild-type mice. Experiment performed as in (B). AMG2850 was administered (30 mg/kg, i.p.) 45 min before von Frey test. L-menthol was injected into hindpaw 15 min later. n=7–8 mice per group.
(D) Effects of L-menthol on CFA-induced mechanical hyperalgesia in Trpm8−/− mice. Experiment performed as in (B) except that Trpm8−/− mice were used. n=7–8 mice per group.
Systemic treatment of mice with a mixture of D- and L-menthol was recently reported to diminish mechanical pain hypersensitivity in the CFA (complete Freund's adjuvant) model of inflammatory pain [40]. Several pharmacological and gene knockout studies have implicated TRPA1 as a major mediator of hyperalgesia and allodynia in this model [12; 15; 32; 36]. We examined the effect of locally administered L-menthol (60 nmol/20 μl, or vehicle, intraplantar injection. 30 min before testing) by von Frey hair analysis of wild-type mice on the 3 days following CFA (20 μl) injection into the hindpaw (Fig. 2B). Mice injected with L-menthol showed significantly diminished mechanical hyperalgesia (Fig. 2B). The role of TRPM8 in the analgesic effect of L-menthol in the CFA model was probed in Trpm8−/− mice and in wild-type mice pre-treated with a recently developed specific TRPM8 inhibitor, AMG2850 [20]. In control experiments, AMG2850 (30 mg/kg i.p.) strongly inhibited behavioral responses of mice to acetone-induced evaporative cooling, a behavior known to be caused by TRPM8 activation (Suppl. Fig. 2B) [2; 13]. In the CFA mouse model, AMG2850 treatment (30 mg/kg i.p., 45 min before testing) completely abolished L-menthol induced analgesia (60 nmol/20 μl, i.p., 30 min before testing) (Fig. 2C). AMG2850 did not affect baseline mechanical sensitivity (Fig. 2C). Furthermore, L-menthol's analgesic effects were absent in Trpm8−/− mice in the CFA model (Fig. 2D). Taken together, these data suggest that the analgesia induced by L-menthol in TRPA1-mediated acute and inflammatory pain is mediated by activation of TRPM8.
Analgesia of visceral pain by L-menthol and eucalyptol requires TRPM8
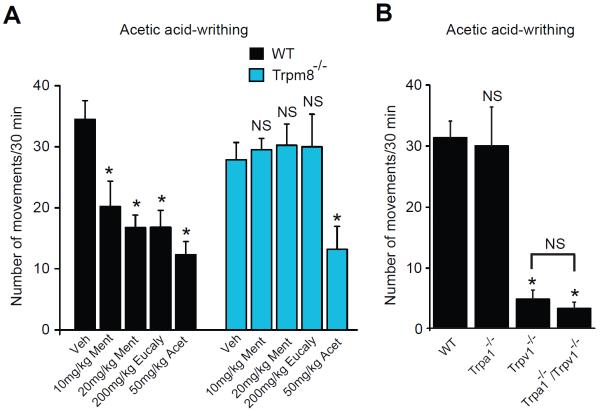
The menthol and eucalyptol receptors, TRPM8 and TRPA1, are expressed in sensory neurons innervating visceral organs, including the stomach, intestine, liver and bladder [23]. Many folk medical traditions recommend menthol- and eucalyptol (1,8-cineol)-containing essential oils for the treatment of visceral discomfort and pain. We examined the role of TRPM8 in L-menthol and eucalyptol-induced analgesia in the acetic acid model of visceral pain [17; 48]. In wild-type mice, writhing movements in response to acetic acid (0.6%, 10 ml/kg, i.p.) were strongly reduced by pre-treatment with either L-menthol (10 or 20 mg/kg, i.p.) or eucalyptol (200 mg/kg, i.p.) (Fig. 3A). Eucalyptol had no effects on locomotion in the rotarod assay at this dose (Suppl. Fig. 1). Trpm8−/− mice responded normally to acetic acid. However, the analgesic effects of L-menthol and eucalyptol were completely abolished in these mice (Fig. 3A). In control experiments, wild-type and Trpm8−/− mice were equally sensitive to analgesia by acetaminophen (50 mg/kg, i.p.). Acetic acid-induced writhing behavior was normal in Trpa1−/− mice (Fig. 3B). In contrast, this behavior was completely absent in Trpv1−/− mice, suggesting that TRPV1 is the major mediator of the pro-algesic effects of weak acids such as acetic acid (Fig. 3B).
Fig.3.
Effects of L-menthol and eucalyptol on mouse visceral pain behavior in the acetic acid writhing test.
(A) Quantification of writhing behavior in wild-type (black) or Trpm8−/− mice (blue) over 20 min following i.p. injection of 0.6% acetic acid (10 ml/kg). 30min prior to testing, mice were injected i.p. with L-menthol (10 mg/kg or 20 mg/kg, Ment) or eucalyptol (200 mg/kg, Eucaly) in 2.5 ml/kg volume in corn oil. Acetaminophen (Acet, 50 mg/kg) was used as a positive analgesic control.. n=5–8 mice per group.
(B) Effects of acetic acid i.p. injection in Trpa1−/−, Trpv1−/− and Trpa1−/−/Trpv1−/− -mice. Treatment and analysis are as in (A). n=6–9 mice/ group. *p < 0.05, NS: not significant (p > 0.05)
WS-12 is a specific agonist of TRPM8 channels in sensory neurons
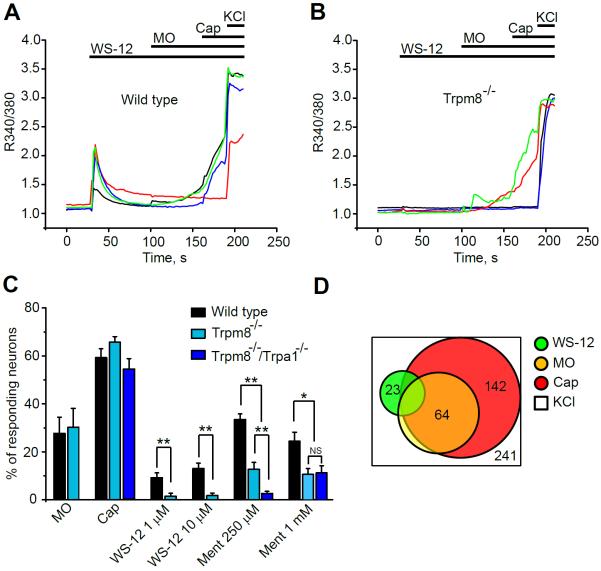
TRPM8 agonists such as L-menthol, eucalyptol or icillin were recently found to interact with other TRP ion channels, including TRPA1 and TRPV3, and other neuronal targets, including sodium- and calcium channels, and GABA receptors [28; 34; 40; 50; 53; 60; 63]. These interactions may contribute to analgesia but may also underlie the irritating and pro-inflammatory side effects observed in many patients treated with these agents. The development of specific TRPM8 agonists would allow more focused studies of the analgesic effects of this ion channel, and may provide improved analgesics with diminished side effects. In tests on heterologously expressed TRP ion channels the menthol derivative, WS-12, was identified as a potentially selective agonists of TRPM8 [5; 33; 62]. In control Ca2+-imaging experiments in HEK293t cells, WS-12 (10 μM) activated mouse TRPM8 (mTRPM8), but neither activated nor inhibited mTRPA1 (pre-activated by mustard oil (MO)), and minimally inhibited human TRPA1 (Suppl. Fig. 3). In contrast, menthol and eucalyptol both strongly inhibited mTRPA1 and hTRPA1 channels pre-activated by MO (Suppl. Fig. 3).
The specificity of WS-12 for TRPM8 has not been systematically corroborated in cultured primary neurons, or in vivo. In Ca2+-imaging experiments we challenged cultured mouse DRG neurons with a TRPM8-saturating dose of WS-12 (1 μM, or 10 μM), and subsequently with MO (70 μM), capsaicin (Cap, 1 μM) and KCl (40 mM). Approximately 10–14% of cultured wild-type neurons were responsive to WS-12 (Fig. 4A, C). These responses were almost completely abolished in DRG neurons cultured from Trpm8−/− mice (Fig. 4B, C). Absence of TRPM8 did not affect responsiveness to capsaicin or mustard oil (Fig. 4B, C). L-menthol (250 μM) activated a larger population of DRG neurons (approximately 34%) than WS-12. In neurons dissociated from Trpm8−/− mice, a significant number of neurons retained sensitivity to L-menthol (~13%). Recent studies reported that TRPA1 is activated by menthol, a result we confirmed in the present study (Suppl. Fig. 3). To examine the contribution of TRPA1 to the residual TRPM8-independent neuronal responses to L-menthol, we analyzed the effect of L-menthol in neurons cultured from Trpm8−/−/Trpa1−/− double-deficient mice. L-menthol responsiveness was almost completely abolished in these neurons, suggesting that TRPA1 mediates the residual L-menthol-induced Ca2+-influx (Fig. 4 C). At a very high concentrations (1 mM) L-menthol's effects were diminished, likely due to inhibition of murine TRPA1 channels, with L-menthol activating some neurons in the absence of TRPA1 and TRPM8 (Fig. 4 C). Population analysis revealed that a subset of WS-12 responsive neurons (13 of 23) also responded to capsaicin, suggesting that TRPM8 and TRPV1 are co-expressed in a subpopulation of DRG neurons. Taken together, the above data suggest that WS-12 is a highly potent and specific TRPM8 channel agonist in DRG neurons.
Fig. 4.
Effects of cooling agent WS-12 on mouse DRG neurons, measured by ratiometric Ca2+-imaging.
(A) Representative Ca2+-signals in wild-type DRG neurons elicited by WS-12 (1 μM), followed by mustard oil (MO, 70 μM), capsaicin (Cap, 1 μM) and KCl (40 mM).
(B) Representative Ca2+-signals in Trpm8−/− DRG neurons. Experiments performed as in (A).
(C) Percentage of wild-type, Trpm8−/− and Trpa1−/−/Trpm8−/−DRG neurons responding to Cap, MO, WS-12 (1 or 10 μM) and L-menthol (Ment, 250 μM or 1 mM). Each column shows average percentages from 5 to 8 separate tests and each test contains 40–60 neurons. Neurons were defined as responsive when the increase in Fura-2 emission ratio (340 nm/380 nm) in a given neuron exceeded 10% of the KCl response. **p <0.01, *p < 0.05, NS: no significance (p > 0.05).
(D) Population analysis of wild-type DRG neurons responding to WS-12, MO and Cap. Agonists were applied as in (A). (n = 241 neurons, 6 fields).
Analgesia of capsaicin-, acrolein- and heat-induced pain in mice by selective TRPM8 activation with WS-12
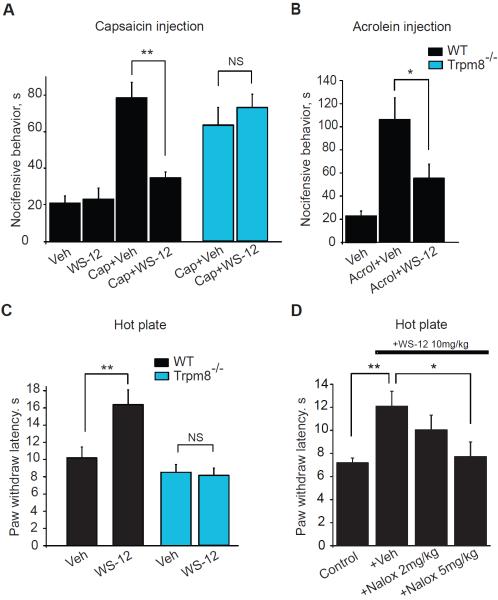
While WS-12 has been characterized as a cooling agent in humans, its effects on nociception have not been examined in vivo [62]. We asked whether WS-12, through specific activation of TRPM8, produces analgesic effects similar to L-menthol. We first examined the effects of WS-12 on capsaicin induced nocifensive behavior. Similar to L-menthol, the co-injection of WS-12 (6 nmol/20 μl) with capsaicin (2 nmol/20 μl) into the mouse hindpaw significantly reduced capsaicin induced nocifensive behavior (Fig. 5A). The analgesic effect of WS-12 was completely abolished in Trpm8−/− mice (Fig. 5A). WS-12 (6 nmol/20 μl) also diminished acrolein (30 nmol/20 μl)-induced nocifensive behavior when coinjected into the hindpaw (Fig. 5B). In the hot plate test (52°C), systemic treatment with WS-12 (10 mg/kg, i.p.) produced an obvious analgesic effect similar to L-menthol, and this effect was again abolished in Trpm8−/− mice (Fig. 5C). WS-12 did not change falling latencies in mice in the rotarod test at this dose (10 mg/kg, i.p.) (Suppl. Fig. 1).
Fig. 5.
Effects of WS-12 on acute thermal-, capsaicin- and acrolein-induced nocifensive behavior.
(A) Nocifensive behavior of wild-type (black) or Trpm8−/− (blue) mice following local injection of vehicle (corn oil), WS-12 (6 nmol), capsaicin (2 nmol), and capsaicin (2 nmol) in combination with WS-12 (6 nmol), all administered in 20 μl injection volume. **p < 0.01, NS: not significant (p > 0.05). n=6–12 mice per group.
(B) Nocifensive behavior in response to paw injections of vehicle (corn oil), acrolein (30 nmol/20 μl), and acrolein and WS-12 (6 nmol/20 μl) together, 20 μl injection volume each recorded as in (A). n=6–7 mice per group. *p < 0.05.
(C) Paw withdrawal latencies in the hot plate test (52°C) in wild-type (black) or Trpm8−/− mice, injected i.p. with vehicle (corn oil) or WS-12 (10 mg/kg) in a volume of 5 ml/kg, 30 min prior to testing. n =6–8 mice per group.
(D) Effects of naloxone on WS-12 induced analgesia in the hot plate test (52°C). WS-12 (10 mg/kg) was administered as in (C) 30 min before testing. 15 min later, naloxone (Nalox, 2 or 5 mg/kg, i.p.) or vehicle (Veh, PBS) in a volume of 5 ml/kg was injected. n=8–12 mice per group.
Pharmacological studies using opioid receptor antagonists indicate that menthol's analgesic effects depend on endogenous opioid pathways [17; 57]. We used naloxone, a non-selective opioid receptor antagonist, to examine whether WS-12 induced analgesia in the hot plate test would also depend on opioid receptor function. Inhibiting opioid receptors (naloxone, 5 mg/kg, i.p.) significantly diminished the analgesic effect of WS-12 (52°C plate temperature), suggesting that both menthol and WS-12 activate similar opioid mechanisms downstream of TRPM8 (Fig. 5D).
DISCUSSION
In the present study we have dissected the role of TRPM8 in the analgesic effects of L-menthol, the predominant menthol isomer in widely used analgesic treatments. L-menthol effectively attenuated pain behavior in all models of acute (capsaicin, heat, acrolein, acetic acid) and inflammatory pain (CFA) examined in this study. These analgesic effects were completely abolished in TRPM8-deficient mice, supporting a crucial role of TRPM8 in menthol-induced analgesia vs. other proposed targets.
TRPM8 was essential for L-menthol to inhibit nocifensive responses triggered by both TRPV1- and TRPA1-activated pain pathways. L-menthol attenuated nocifensive behavior elicited by injection of capsaicin or by noxious heat in the hot plate and tail flick tests, responses associated with activation of TRPV1. TRPM8 was also crucial for L-menthol-induced analgesia in the acetic acid model of visceral pain. Acetic acid-induced pain was absent in TRPV1-deficient mice, suggesting that activation of acid-sensitive TRPV1 channels is the key nociceptive mechanism causing writhing behavior. This observation is in line with prior studies demonstrating analgesic effects of selective TRPV1 antagonists in this model and suggests that the roles of other proposed targets such as acid-sensitive ion channels (ASICs) or TRPA1 in nociception caused by weak acids needs to be reexamined [44; 56].
TRPM8 was also necessary for L-menthol to inhibit nocifensive behavior elicited by direct chemical stimulation of TRPA1 with acrolein. Acrolein, a reactive electrophile, is the major noxious irritant in tobacco smoke, and we recently reported that L-menthol effectively blocked acrolein-induced respiratory irritation responses mediated by airway-innervating chemosensory neurons in mice [1; 64]. The physiological effects of menthol in cigarettes are currently the focus of controversial discussions and regulatory efforts since menthol may facilitate smoke inhalation and smoking induced diseases [64]. In that previous study we demonstrated that a TRPM8 inhibitor, AMTB, partially abolished L-menthol's counterirritant effects towards acrolein and other respiratory irritants in tobacco smoke [64]. The specificity of AMTB remains poorly characterized and its potency and pharmacokinetics in vivo require further study. Our present data derived from Trpm8−/− mice show complete absence of L-menthol's analgesic effects towards acrolein-induced pain. This result supports a role of TRPM8 in L-menthol induced counterirritation in the respiratory system that is innervated by sensory neurons showing TRP channel expression patterns and connectivity very similar to nociceptors [3]. Acrolein is also a lipid peroxidation product and resembles other endogenous reactive products generated during inflammation and activating TRPA1 [35; 39; 59]. We demonstrated that L-menthol diminished inflammatory mechanical hyperalgesia in the CFA model in which TRPA1 has been implicated as a major pro-algesic target. This effect was absent in Trpm8−/− mice and mice treated with AMG2850, a more potent and selective TRPM8 inhibitor [20]. The analgesic effects of menthol towards mechanical stimulation were generally weaker than its effects on chemically or thermally induced nociception, confirming earlier studies demonstrating only small effects of menthol on mechanical sensing in rodents [29; 30]. In all pain models examined in this study, Trpm8−/− mice showed pain behaviors equivalent to wild-type mice. Acetaminophen worked effectively as an analgesic in Trpm8−/− mice, demonstrating that these mice have no general defects preventing analgesia.
While not as comprehensive as for menthol, our examination of eucalyptol's analgesic effects points to a crucial role of TRPM8 as an analgesic target of this widely used natural product. In the acetic acid model, eucalyptol (1,8-cineol) strongly reduced writhing behavior, with an efficacy very similar to L-menthol. This effect was abolished in TRPM8-deficient mice, supporting the idea that activation of TRPM8 by eucalyptol activates analgesic pathways. This finding differs from the outcome of a recent study observing that eucalyptol is acting as an analgesic through inhibition of TRPA1 [54]. As explained above, our data suggest that acetic acid-induced pain behavior is triggered by activation of TRPV1, demonstrating that eucalyptol can inhibit pain responses triggered by pathways that do not involve TRPA1. Eucalyptol's analgesic effects may depend on its pharmacological actions on one, or both, ion channels, depending on the pain-inducing stimulus.
With WS-12 we identified a specific pharmacological tool enabling identification and characterization of TRPM8-expressing cultured sensory neurons. WS-12, previously characterized as a selective agonist of TRPM8 in heterologous expression systems, exhibited high specificity for TRPM8 in cultured DRG neurons [33]. WS-12 induced Ca2+-influx in DRG neurons of wild-type mice, but not in neurons cultured from TRPM8-deficient mice. In DRG cultures, L-menthol activated a larger population of sensory neurons than WS-12 and a significant number of Trpm8−/− neurons retained sensitivity to L-menthol, indicating that L-menthol activated additional TRPM8-independent Ca2+-influx pathways. This residual response was absent in DRG neurons from Trpm8−/−/Trpa1−/− double deficient mice, suggesting that TRPM8 and TRPA1 are the major excitatory L-menthol receptors in sensory neurons [28]. WS-12-responsive cells fell within two categories. One cell population was responsive to WS-12 and also to mustard oil and capsaicin, the agonists of TRPA1 and TRPV1, respectively. This population may represent a nociceptor population [55; 58]. The other neuronal population was responsive to WS-12, but not to capsaicin or mustard oil. It is likely that this population initiates the sensation of cool temperatures when TRPM8 is activated [55; 58].
WS-12 also showed high specificity for TRPM8 in vivo. WS-12 induced analgesia of TRPV1 (heat, capsaicin)- and TRPA1(acrolein)-activated nociception. Similar to L-menthol induced analgesia, WS-12-induced analgesia was completely absent in TRPM8-deficient mice. Both analgesia by L-menthol and WS-12 was diminished when opioid pathways were blocked by naloxone treatment, suggesting that both agents activate similar TRPM8-dependent analgesic pathways, and that TRPM8-induced analgesia relies on activation of endogenous opioid receptors. L-menthol analgesia may involve signaling by central κ-opioid receptors, since central administration of a κ-opioid was shown to diminish L-menthol-induced analgesia [17; 57].
L-menthol produced TRPM8-dependent analgesia of acute and inflammatory pain over a wide range of concentrations and administration routes (oral, intraperitoneal, intraplantar or topical), from estimated systemic levels of 60μM (at 10mg/kg, assuming equal distribution in a 25g mouse without metabolization) or 120μM (20mg/kg), to a topical concentration in the molar range (at 30% in ethanol). In addition to activating TRPM8 channels, these concentrations are sufficient or by far exceed the concentrations needed to exert pharmacological effects on other targets implicated as mediators of menthol-induced analgesia, including voltage-dependent Ca2+ channels, GABAA-receptors, sodium channels, peripheral nicotinic acetyl choline receptors or serotonin-gated ion channels [11; 19; 22; 24; 49; 52; 63; 67]. The role of these alternative targets in L-menthol-induced analgesia should have been unmasked in Trpm8−/− mice. However, since we did not observe any residual analgesic effects of L-menthol in Trpm8−/− mice, the interaction of these targets with menthol may not affect nociception, or these alternative targets may play a role in menthol analgesia only in specific situations. Some analgesic effects mediated by other targets may be specific for other menthol isoforms such as D-menthol that was shown to induce analgesia by inhibiting neuronal sodium channels when these were activated by a specific peptide toxin [19]. However, D-menthol was shown to have no analgesic effects in the acetic acid induced model and other pain models [17]. Since L-menthol is by far the predominant menthol isomer in analgesic preparations, analgesia induced by other menthol isomers may not be relevant in the context of current treatment regimens.
The L-menthol concentrations used in our in vivo studies is sufficient to affect the activity of TRPA1 channels in sensory neurons. Both mouse and human TRPA1 are activated by menthol at lower concentrations, and mouse (but not human) TRPA1 is inhibited by menthol at high concentrations in vitro, findings we confirmed in our imaging analysis of heterologously expressed TRPA1 channels [28; 34]. However, acrolein-induced nocifensive behavior, clearly TRPA1-dependent, was unaffected by co-injection of 1 mM L-menthol, a concentration shown to inhibit mTRPA1 channels in vitro [28]. This apparent lack of inhibition may be due to TRPM8- and TRPA1-independent pro-algesic effects of menthol at high concentrations, as reported in earlier studies [6; 28; 45]. Indeed, we observed that L-menthol at 1mM activated Ca2+-influx into DRG-neurons from Trpm8−/−/Trpa1−/− double-deficient mice. Metabolic mechanisms may also play a role in the in vivo effects of L-menthol, as shown by us in a previous study demonstrating that L-menthol-induced respiratory irritation depends on the activity of cytochrome P450 enzymes that may convert menthol into reactive intermediates activating TRPA1 in airway-innervating sensory fibers [64].
While TRPM8 accounts for likely all of the analgesic activity of L-menthol, interaction of L-menthol with TRPA1 and other targets may have pro-algesic and inflammatory effects. Indeed, topical menthol treatment frequently is accompanied by skin irritation, and menthol inhalation can exacerbate asthma in some patients, both conditions in which TRPA1 has a documented role [1; 7; 66]. The replacement of L-menthol in analgesic and counterirritant treatments by TRPM8-specific agents such as WS-12 may prevent these pro-inflammatory effects and allow a more focused analgesic therapy.
Supplementary Material
Suppl. Fig. 1 Locomotor activity in mice injected i.p. with L-menthol (20 mg/kg, Ment), eucalyptol (200 mg/kg, Eucaly) and WS-12 (10 mg/kg) (vehicle1: corn oil+3% DMSO, vehicle2: corn oil+14% ethanol), 30 min prior to testing by rotarod assay. n=5–7 mice/group. NS: not significant (p > 0.05).
Suppl. Fig. 2 (A) Nocifensive behavior in wild-type (black) or Trpa1−/− (red) mice quantified over 10 min following hindpaw injection of acrolein (25 nmol in 50 μl PBS). Mice in vehicle group (grey) were injected with just 50 μl PBS (veh.). n=8–12 mice per group. ##p < 0.01 vs. Veh group, **p < 0.01 vs. WT + Acrolein group.
(B) Effects of selective TRPM8 antagonist, AMG2850, on behavior elicited by acetone-induced evaporative cooling. Licking and flinching responses were measured in mice for 1 min following application of acetone (50 μl) to the hind paw. AMG2850 (30 mg/kg i.p.) was administered 45 min before the test. **p < 0.01 vs. WT control group. n=5–7 mice per group.
Suppl. Fig. 3 Comparison of the effects of L-menthol, eucalyptol, WS-12 and icilin on human or mouse TRPM8 and TRPA1 channels, measured by ratiometric Ca2+ imaging in HEK293T cells.
(A) Effects of L-menthol (Ment), eucalyptol (Euca), WS-12 (WS) and icilin on mouse TRPA1 channels. Ionomycin (Iono, 1.5 μM) was used as control.
(B) Effects of L-menthol, eucalyptol, WS-12 and icilin on mouse TRPA1 channels. Cells were also superfused with mustard oil (MO, 100 μM) and ionomycin.
(C) Effects of L-menthol, eucalyptol, WS-12 and icilin on mouse TRPA1 channels pre-activated with mustard oil. TRPA1 currents were inhibited with ruthenium red (RR) at the end of the recordings (10 μM).
(D) Effects of L-menthol, eucalyptol, WS-12 and icilin on human TRPA1 channels pre-activated with mustard oil. Responses of n=30–40 cells were averaged in each experiment. The scale bar shows the time and ratio of 340nm/380nm.
ACKNOWLEDGEMENTS
This work was supported by grants R01ES015056 (National Institute for Environmental Health Sciences to S.E.J.), R01HL105635 and R01HL105635-02S1 (National Heart, Lung and Blood Institute to S.E.J. and J.B.M.). We thank Diana Bautista (UC Berkeley) for Trpa1−/−/Trpm8−/− mice and Narender Gavva (Amgen, Thousand Oaks, CA) for the gift of AMG2850.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
The authors declare no conflicts of interest.
REFERENCES
- [1].Bautista DM, Jordt SE, Nikai T, Tsuruda PR, Read AJ, Poblete J, Yamoah EN, Basbaum AI, Julius D. TRPA1 mediates the inflammatory actions of environmental irritants and proalgesic agents. Cell. 2006;124:1269–1282. doi: 10.1016/j.cell.2006.02.023. [DOI] [PubMed] [Google Scholar]
- [2].Bautista DM, Siemens J, Glazer JM, Tsuruda PR, Basbaum AI, Stucky CL, Jordt SE, Julius D. The menthol receptor TRPM8 is the principal detector of environmental cold. Nature. 2007;448:204–208. doi: 10.1038/nature05910. [DOI] [PubMed] [Google Scholar]
- [3].Bessac BF, Jordt SE. Breathtaking TRP channels: TRPA1 and TRPV1 in airway chemosensation and reflex control. Physiology (Bethesda) 2008;23:360–370. doi: 10.1152/physiol.00026.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Birrell MA, Belvisi MG, Grace M, Sadofsky L, Faruqi S, Hele DJ, Maher SA, Freund-Michel V, Morice AH. TRPA1 agonists evoke coughing in guinea pig and human volunteers. Am J Respir Crit Care Med. 2009;180:1042–1047. doi: 10.1164/rccm.200905-0665OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Bodding M, Wissenbach U, Flockerzi V. Characterisation of TRPM8 as a pharmacophore receptor. Cell Calcium. 2007;42:618–628. doi: 10.1016/j.ceca.2007.03.005. [DOI] [PubMed] [Google Scholar]
- [6].Braz JM, Basbaum AI. Differential ATF3 expression in dorsal root ganglion neurons reveals the profile of primary afferents engaged by diverse noxious chemical stimuli. Pain. 2010;150:290–301. doi: 10.1016/j.pain.2010.05.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Caceres AI, Brackmann M, Elia MD, Bessac BF, del Camino D, D'Amours M, Witek JS, Fanger CM, Chong JA, Hayward NJ, Homer RJ, Cohn L, Huang X, Moran MM, Jordt SE. A sensory neuronal ion channel essential for airway inflammation and hyperreactivity in asthma. Proc Natl Acad Sci U S A. 2009;106:9099–9104. doi: 10.1073/pnas.0900591106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Caterina MJ, Leffler A, Malmberg AB, Martin WJ, Trafton J, Petersen-Zeitz KR, Koltzenburg M, Basbaum AI, Julius D. Impaired nociception and pain sensation in mice lacking the capsaicin receptor. Science. 2000;288:306–313. doi: 10.1126/science.288.5464.306. [DOI] [PubMed] [Google Scholar]
- [9].Chen C, Luo W, Isabelle LM, Gareau KD, Pankow JF. The stereoisomers of menthol in selected tobacco products. A brief report. Nicotine Tob Res. 2011;13:741–745. doi: 10.1093/ntr/ntr031. [DOI] [PubMed] [Google Scholar]
- [10].Chen CC, Zimmer A, Sun WH, Hall J, Brownstein MJ. A role for ASIC3 in the modulation of high-intensity pain stimuli. Proc Natl Acad Sci U S A. 2002;99:8992–8997. doi: 10.1073/pnas.122245999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Corvalan NA, Zygadlo JA, Garcia DA. Stereo-selective activity of menthol on GABA(A) receptor. Chirality. 2009;21:525–530. doi: 10.1002/chir.20631. [DOI] [PubMed] [Google Scholar]
- [12].da Costa DS, Meotti FC, Andrade EL, Leal PC, Motta EM, Calixto JB. The involvement of the transient receptor potential A1 (TRPA1) in the maintenance of mechanical and cold hyperalgesia in persistent inflammation. Pain. 2010;148:431–437. doi: 10.1016/j.pain.2009.12.002. [DOI] [PubMed] [Google Scholar]
- [13].Dhaka A, Murray AN, Mathur J, Earley TJ, Petrus MJ, Patapoutian A. TRPM8 is required for cold sensation in mice. Neuron. 2007;54:371–378. doi: 10.1016/j.neuron.2007.02.024. [DOI] [PubMed] [Google Scholar]
- [14].Eccles R. Menthol and related cooling compounds. J Pharm Pharmacol. 1994;46:618–630. doi: 10.1111/j.2042-7158.1994.tb03871.x. [DOI] [PubMed] [Google Scholar]
- [15].Eid SR, Crown ED, Moore EL, Liang HA, Choong KC, Dima S, Henze DA, Kane SA, Urban MO. HC-030031, a TRPA1 selective antagonist, attenuates inflammatory- and neuropathy-induced mechanical hypersensitivity. Mol Pain. 2008;4:48. doi: 10.1186/1744-8069-4-48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Emberger R HREH. Synthesis and sensory characteristics on menthol enantiomers and their derivatives for the use of nature-identical peppermint oils. Topics in Flavor Research. 1988:201–218. [Google Scholar]
- [17].Galeotti N, Di Cesare Mannelli L, Mazzanti G, Bartolini A, Ghelardini C. Menthol: a natural analgesic compound. Neurosci Lett. 2002;322:145–148. doi: 10.1016/s0304-3940(01)02527-7. [DOI] [PubMed] [Google Scholar]
- [18].Galeotti N, Ghelardini C, Mannelli L, Mazzanti G, Baghiroli L, Bartolini A. Local anaesthetic activity of (+)- and (−)-menthol. Planta Med. 2001;67:174–176. doi: 10.1055/s-2001-11515. [DOI] [PubMed] [Google Scholar]
- [19].Gaudioso C, Hao J, Martin-Eauclaire MF, Gabriac M, Delmas P. Menthol pain relief through cumulative inactivation of voltage-gated sodium channels. Pain. 2012;153:473–484. doi: 10.1016/j.pain.2011.11.014. [DOI] [PubMed] [Google Scholar]
- [20].Gavva NR, Davis C, Lehto SG, Rao S, Wang W, Zhu DX. Transient receptor potential melastatin 8 (TRPM8) channels are involved in body temperature regulation. Mol Pain. 8:36. doi: 10.1186/1744-8069-8-36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Green BG. The sensory effects of l-menthol on human skin. Somatosens Mot Res. 1992;9:235–244. doi: 10.3109/08990229209144774. [DOI] [PubMed] [Google Scholar]
- [22].Hans M, Wilhelm M, Swandulla D. Menthol suppresses nicotinic acetylcholine receptor functioning in sensory neurons via allosteric modulation. Chem Senses. 2012;37:463–469. doi: 10.1093/chemse/bjr128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Harrington AM, Hughes PA, Martin CM, Yang J, Castro J, Isaacs NJ, Blackshaw LA, Brierley SM. A novel role for TRPM8 in visceral afferent function. Pain. 2011;152:1459–1468. doi: 10.1016/j.pain.2011.01.027. [DOI] [PubMed] [Google Scholar]
- [24].Heimes K, Hauk F, Verspohl EJ. Mode of action of peppermint oil and (−)-menthol with respect to 5-HT3 receptor subtypes: binding studies, cation uptake by receptor channels and contraction of isolated rat ileum. Phytother Res. 2011;25:702–708. doi: 10.1002/ptr.3316. [DOI] [PubMed] [Google Scholar]
- [25].Johar P, Grover V, Topp R, Behm DG. A comparison of topical menthol to ice on pain, evoked tetanic and voluntary force during delayed onset muscle soreness. Int J Sports Phys Ther. 2012;7:314–322. [PMC free article] [PubMed] [Google Scholar]
- [26].Jordt SE, Bautista DM, Chuang HH, McKemy DD, Zygmunt PM, Hogestatt ED, Meng ID, Julius D. Mustard oils and cannabinoids excite sensory nerve fibres through the TRP channel ANKTM1. Nature. 2004;427:260–265. doi: 10.1038/nature02282. [DOI] [PubMed] [Google Scholar]
- [27].Journigan VB, Zaveri NT. TRPM8 ion channel ligands for new therapeutic applications and as probes to study menthol pharmacology. Life Sci. 2012 doi: 10.1016/j.lfs.2012.10.032. [DOI] [PubMed] [Google Scholar]
- [28].Karashima Y, Damann N, Prenen J, Talavera K, Segal A, Voets T, Nilius B. Bimodal action of menthol on the transient receptor potential channel TRPA1. J Neurosci. 2007;27:9874–9884. doi: 10.1523/JNEUROSCI.2221-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Klein AH, Sawyer CM, Carstens MI, Tsagareli MG, Tsiklauri N, Carstens E. Topical application of L-menthol induces heat analgesia, mechanical allodynia, and a biphasic effect on cold sensitivity in rats. Behav Brain Res. 2010;212:179–186. doi: 10.1016/j.bbr.2010.04.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Klein AH, Sawyer CM, Takechi K, Davoodi A, Ivanov MA, Carstens MI, Carstens E. Topical hindpaw application of L-menthol decreases responsiveness to heat with biphasic effects on cold sensitivity of rat lumbar dorsal horn neurons. Neuroscience. 2012;219:234–242. doi: 10.1016/j.neuroscience.2012.05.061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].Knowlton WM, Palkar R, Lippoldt EK, McCoy DD, Baluch F, Chen J, McKemy DD. A sensory-labeled line for cold: TRPM8-expressing sensory neurons define the cellular basis for cold, cold pain, and cooling-mediated analgesia. J Neurosci. 2013;33:2837–2848. doi: 10.1523/JNEUROSCI.1943-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32].Lennertz RC, Kossyreva EA, Smith AK, Stucky CL. TRPA1 mediates mechanical sensitization in nociceptors during inflammation. PloS One. 2012;7:e43597. doi: 10.1371/journal.pone.0043597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [33].Ma S, G G, Ak VE, Jf D, H H Menthol derivative WS-12 selectively activates transient receptor potential melastatin-8 (TRPM8) ion channels. Pak J Pharm Sci. 2008;21:370–378. [PubMed] [Google Scholar]
- [34].Macpherson LJ, Hwang SW, Miyamoto T, Dubin AE, Patapoutian A, Story GM. More than cool: promiscuous relationships of menthol and other sensory compounds. Mol Cell Neurosci. 2006;32:335–343. doi: 10.1016/j.mcn.2006.05.005. [DOI] [PubMed] [Google Scholar]
- [35].Macpherson LJ, Xiao B, Kwan KY, Petrus MJ, Dubin AE, Hwang S, Cravatt B, Corey DP, Patapoutian A. An ion channel essential for sensing chemical damage. J Neurosci. 2007;27:11412–11415. doi: 10.1523/JNEUROSCI.3600-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [36].McGaraughty S, Chu KL, Perner RJ, Didomenico S, Kort ME, Kym PR. TRPA1 modulation of spontaneous and mechanically evoked firing of spinal neurons in uninjured, osteoarthritic, and inflamed rats. Mol Pain. 2010;6:14. doi: 10.1186/1744-8069-6-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [37].McKemy DD. The molecular and cellular basis of cold sensation. ACS Chem Neurosci. 2013;4:238–247. doi: 10.1021/cn300193h. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [38].McKemy DD, Neuhausser WM, Julius D. Identification of a cold receptor reveals a general role for TRP channels in thermosensation. Nature. 2002;416:52–58. doi: 10.1038/nature719. [DOI] [PubMed] [Google Scholar]
- [39].Onyango AN. Small reactive carbonyl compounds as tissue lipid oxidation products; and the mechanisms of their formation thereby. Chem Phys Lipids. 2012;165:777–786. doi: 10.1016/j.chemphyslip.2012.09.004. [DOI] [PubMed] [Google Scholar]
- [40].Pan R, Tian Y, Gao R, Li H, Zhao X, Barrett JE, Hu H. Central mechanisms of menthol-induced analgesia. J Pharmacol Exp Ther. 2012;343:661–672. doi: 10.1124/jpet.112.196717. [DOI] [PubMed] [Google Scholar]
- [41].Peier AM, Moqrich A, Hergarden AC, Reeve AJ, Andersson DA, Story GM, Earley TJ, Dragoni I, McIntyre P, Bevan S, Patapoutian A. A TRP channel that senses cold stimuli and menthol. Cell. 2002;108:705–715. doi: 10.1016/s0092-8674(02)00652-9. [DOI] [PubMed] [Google Scholar]
- [42].Pitcher GM, Ritchie J, Henry JL. Paw withdrawal threshold in the von Frey hair test is influenced by the surface on which the rat stands. J Neurosci Methods. 1999;87:185–193. doi: 10.1016/s0165-0270(99)00004-7. [DOI] [PubMed] [Google Scholar]
- [43].Proudfoot CJ, Garry EM, Cottrell DF, Rosie R, Anderson H, Robertson DC, Fleetwood-Walker SM, Mitchell R. Analgesia mediated by the TRPM8 cold receptor in chronic neuropathic pain. Curr Biol. 2006;16:1591–1605. doi: 10.1016/j.cub.2006.07.061. [DOI] [PubMed] [Google Scholar]
- [44].Rigoni M, Trevisani M, Gazzieri D, Nadaletto R, Tognetto M, Creminon C, Davis JB, Campi B, Amadesi S, Geppetti P, Harrison S. Neurogenic responses mediated by vanilloid receptor-1 (TRPV1) are blocked by the high affinity antagonist, iodo-resiniferatoxin. Br J Pharmacol. 2003;138:977–985. doi: 10.1038/sj.bjp.0705110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [45].Robbins A, Kurose M, Winterson BJ, Meng ID. Menthol activation of corneal cool cells induces TRPM8-mediated lacrimation but not nociceptive responses in rodents. Invest Ophthalmol Vis Sci. 2012;53:7034–7042. doi: 10.1167/iovs.12-10025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [46].Round P, Priestley A, Robinson J. An investigation of the safety and pharmacokinetics of the novel TRPV1 antagonist XEN-D0501 in healthy subjects. Br J Clin Pharmacol. 2011;72:921–931. doi: 10.1111/j.1365-2125.2011.04040.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [47].Rowbotham MC, Nothaft W, Duan WR, Wang Y, Faltynek C, McGaraughty S, Chu KL, Svensson P. Oral and cutaneous thermosensory profile of selective TRPV1 inhibition by ABT-102 in a randomized healthy volunteer trial. Pain. 2011;152:1192–1200. doi: 10.1016/j.pain.2011.01.051. [DOI] [PubMed] [Google Scholar]
- [48].Santos FA, Rao VS. Antiinflammatory and antinociceptive effects of 1,8-cineole a terpenoid oxide present in many plant essential oils. Phytother Res. 2000;14:240–244. doi: 10.1002/1099-1573(200006)14:4<240::aid-ptr573>3.0.co;2-x. [DOI] [PubMed] [Google Scholar]
- [49].Sidell N, Verity MA, Nord EP. Menthol blocks dihydropyridine-insensitive Ca2+ channels and induces neurite outgrowth in human neuroblastoma cells. J Cell Physiol. 1990;142:410–419. doi: 10.1002/jcp.1041420226. [DOI] [PubMed] [Google Scholar]
- [50].Story GM, Peier AM, Reeve AJ, Eid SR, Mosbacher J, Hricik TR, Earley TJ, Hergarden AC, Andersson DA, Hwang SW, McIntyre P, Jegla T, Bevan S, Patapoutian A. ANKTM1, a TRP-like channel expressed in nociceptive neurons, is activated by cold temperatures. Cell. 2003;112:819–829. doi: 10.1016/s0092-8674(03)00158-2. [DOI] [PubMed] [Google Scholar]
- [51].Su L, Wang C, Yu YH, Ren YY, Xie KL, Wang GL. Role of TRPM8 in dorsal root ganglion in nerve injury-induced chronic pain. BMC Neurosci. 2011;12:120. doi: 10.1186/1471-2202-12-120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [52].Swandulla D, Carbone E, Schafer K, Lux HD. Effect of menthol on two types of Ca currents in cultured sensory neurons of vertebrates. Pflugers Arch. 1987;409:52–59. doi: 10.1007/BF00584749. [DOI] [PubMed] [Google Scholar]
- [53].Swandulla D, Schafer K, Lux HD. Calcium channel current inactivation is selectively modulated by menthol. Neurosci Lett. 1986;68:23–28. doi: 10.1016/0304-3940(86)90223-5. [DOI] [PubMed] [Google Scholar]
- [54].Takaishi M, Fujita F, Uchida K, Yamamoto S, Shimizu MS, Uotsu CH, Shimizu M, Tominaga M. 1,8-cineole, a TRPM8 agonist, is a novel natural antagonist of human TRPA1. Mol Pain. 2012;8:86. doi: 10.1186/1744-8069-8-86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [55].Takashima Y, Ma L, McKemy DD. The development of peripheral cold neural circuits based on TRPM8 expression. Neuroscience. 2010;169:828–842. doi: 10.1016/j.neuroscience.2010.05.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [56].Tang L, Chen Y, Chen Z, Blumberg PM, Kozikowski AP, Wang ZJ. Antinociceptive pharmacology of N-(4-chlorobenzyl)-N'-(4-hydroxy-3-iodo-5-methoxybenzyl) thiourea, a high-affinity competitive antagonist of the transient receptor potential vanilloid 1 receptor. J Pharmacol Exp Ther. 2007;321:791–798. doi: 10.1124/jpet.106.117572. [DOI] [PubMed] [Google Scholar]
- [57].Taniguchi Y DY, Saita M, Noda K. Antinociceptive effects of counterirritants. Nihon yakurigaku zasshi. 1994;104:433–446. doi: 10.1254/fpj.104.433. [DOI] [PubMed] [Google Scholar]
- [58].Teichert RW, Raghuraman S, Memon T, Cox JL, Foulkes T, Rivier JE, Olivera BM. Characterization of two neuronal subclasses through constellation pharmacology. Proc Natl Acad Sci U S A. 2012;109:12758–12763. doi: 10.1073/pnas.1209759109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [59].Trevisani M, Siemens J, Materazzi S, Bautista DM, Nassini R, Campi B, Imamachi N, Andre E, Patacchini R, Cottrell GS, Gatti R, Basbaum AI, Bunnett NW, Julius D, Geppetti P. 4-Hydroxynonenal, an endogenous aldehyde, causes pain and neurogenic inflammation through activation of the irritant receptor TRPA1. Proc Natl Acad Sci U S A. 2007;104:13519–13524. doi: 10.1073/pnas.0705923104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [60].Vogt-Eisele AK, Weber K, Sherkheli MA, Vielhaber G, Panten J, Gisselmann G, Hatt H. Monoterpenoid agonists of TRPV3. Br J Pharmacol. 2007;151:530–540. doi: 10.1038/sj.bjp.0707245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [61].Wasner G, Schattschneider J, Binder A, Baron R. Topical menthol--a human model for cold pain by activation and sensitization of C nociceptors. Brain. 2004;127:1159–1171. doi: 10.1093/brain/awh134. [DOI] [PubMed] [Google Scholar]
- [62].Watson HR HR, Rowsell DG, Spring DJ. New compounds with menthol cooling effect. J Soc Cosmet Chem. 1978;29:185–200. [Google Scholar]
- [63].Watt EE, Betts BA, Kotey FO, Humbert DJ, Griffith TN, Kelly EW, Veneskey KC, Gill N, Rowan KC, Jenkins A, Hall AC. Menthol shares general anesthetic activity and sites of action on the GABA(A) receptor with the intravenous agent, propofol. Eur J Pharmacol. 2008;590:120–126. doi: 10.1016/j.ejphar.2008.06.003. [DOI] [PubMed] [Google Scholar]
- [64].Willis DN, Liu B, Ha MA, Jordt SE, Morris JB. Menthol attenuates respiratory irritation responses to multiple cigarette smoke irritants. FASEB J. 2011;25:4434–4444. doi: 10.1096/fj.11-188383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [65].Xiao B, Dubin AE, Bursulaya B, Viswanath V, Jegla TJ, Patapoutian A. Identification of transmembrane domain 5 as a critical molecular determinant of menthol sensitivity in mammalian TRPA1 channels. J Neurosci. 2008;28:9640–9651. doi: 10.1523/JNEUROSCI.2772-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [66].Yosipovitch G, Szolar C, Hui XY, Maibach H. Effect of topically applied menthol on thermal, pain and itch sensations and biophysical properties of the skin. Arch Dermatol Res. 1996;288:245–248. doi: 10.1007/BF02530092. [DOI] [PubMed] [Google Scholar]
- [67].Zhang XB, Jiang P, Gong N, Hu XL, Fei D, Xiong ZQ, Xu L, Xu TL. A-type GABA receptor as a central target of TRPM8 agonist menthol. PloS One. 2008;3:e3386. doi: 10.1371/journal.pone.0003386. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Suppl. Fig. 1 Locomotor activity in mice injected i.p. with L-menthol (20 mg/kg, Ment), eucalyptol (200 mg/kg, Eucaly) and WS-12 (10 mg/kg) (vehicle1: corn oil+3% DMSO, vehicle2: corn oil+14% ethanol), 30 min prior to testing by rotarod assay. n=5–7 mice/group. NS: not significant (p > 0.05).
Suppl. Fig. 2 (A) Nocifensive behavior in wild-type (black) or Trpa1−/− (red) mice quantified over 10 min following hindpaw injection of acrolein (25 nmol in 50 μl PBS). Mice in vehicle group (grey) were injected with just 50 μl PBS (veh.). n=8–12 mice per group. ##p < 0.01 vs. Veh group, **p < 0.01 vs. WT + Acrolein group.
(B) Effects of selective TRPM8 antagonist, AMG2850, on behavior elicited by acetone-induced evaporative cooling. Licking and flinching responses were measured in mice for 1 min following application of acetone (50 μl) to the hind paw. AMG2850 (30 mg/kg i.p.) was administered 45 min before the test. **p < 0.01 vs. WT control group. n=5–7 mice per group.
Suppl. Fig. 3 Comparison of the effects of L-menthol, eucalyptol, WS-12 and icilin on human or mouse TRPM8 and TRPA1 channels, measured by ratiometric Ca2+ imaging in HEK293T cells.
(A) Effects of L-menthol (Ment), eucalyptol (Euca), WS-12 (WS) and icilin on mouse TRPA1 channels. Ionomycin (Iono, 1.5 μM) was used as control.
(B) Effects of L-menthol, eucalyptol, WS-12 and icilin on mouse TRPA1 channels. Cells were also superfused with mustard oil (MO, 100 μM) and ionomycin.
(C) Effects of L-menthol, eucalyptol, WS-12 and icilin on mouse TRPA1 channels pre-activated with mustard oil. TRPA1 currents were inhibited with ruthenium red (RR) at the end of the recordings (10 μM).
(D) Effects of L-menthol, eucalyptol, WS-12 and icilin on human TRPA1 channels pre-activated with mustard oil. Responses of n=30–40 cells were averaged in each experiment. The scale bar shows the time and ratio of 340nm/380nm.