Abstract
Purpose
Although modern cure rates for childhood acute lymphoblastic leukemia (ALL) exceed 80%, the outlook remains poor in patients with high risk disease and those who relapse, especially when allogeneic hematopoietic stem cell transplantation is not feasible. Strategies to improve outcome and prevent relapse are therefore required. Immunotherapy with antigen-specific T cells can have anti-leukemic activity without the toxicities seen with intensive chemotherapy and therefore represents an attractive strategy to improve the outcome of high risk patients with ALL. We explored the feasibility of generating tumor antigen-specific T cells ex vivo from the peripheral blood of 50 patients with ALL (26 NCI high risk and 24 standard risk) receiving maintenance therapy.
Experimental Design
Peripheral blood mononuclear cells were stimulated with autologous dendritic cells pulsed with complete peptide libraries of WT1, Survivin, MAGE-A3 and PRAME, antigens frequently expressed on ALL blasts.
Results
T-cell lines were successfully expanded from all patients, despite low lymphocyte counts and irrespective of NCI risk group. Antigen-specificity was observed in over 50% of patients after the initial stimulation and increased to over 90% after 3 stimulations as assessed in IFNγ-ELISpot and 51Cr-release assays. Moreover, tumor-specific responses were observed by reduction of autologous leukemia blasts in short- and long-term co-culture experiments.
Conclusion
This study supports the use of immunotherapy with adoptively transferred autologous tumor antigen-specific T cells to prevent relapse and improve the prognosis of patients with high risk ALL.
Keywords: immunotherapy, acute lymphoblastic leukemia, antigen-specific T cells
INTRODUCTION
The outcome of patients with acute lymphoblastic leukemia (ALL), the most common childhood leukemia, has significantly improved with the advent of intensive chemotherapy regimens that can achieve cure rates of over 90%. However, for patients classified as high-risk, according to NIH criteria, or those who relapse, the best chance of cure is allogeneic hematopoietic stem cell transplantation (HSCT) (1). Not all patients are eligible for HSCT and over a third of transplanted patients still relapse after HSCT for ALL (1, 2). Therefore, strategies to prevent relapse in patients with ALL, classified as high risk, are needed. Immunotherapy is an effective tool for the treatment of leukemia. First demonstrated in patients with chronic myeloid leukemia (CML) the infusion of unselected donor lymphocytes (DLI) has been shown to stably eradicate minimal residual disease (3, 4). In ALL, a high absolute lymphocyte count at the end of induction therapy is associated with improved survival, suggesting the importance of the host immune response in controlling disease (5). In addition, in the transplant setting there is strong evidence for a T-cell mediated graft-versus-leukemia effect (6). However, DLI rarely induces responses in patients relapsing after HSCT (7–9), suggesting that the anti-leukemic effects of T cells may require augmentation to prevent or treat relapsed ALL. Recently, T cells genetically modified with chimeric antigen receptors (CARs) targeting a CD19 epitope were shown to induce prolonged remissions in relapsed ALL patients (10, 11). However, relapse can occur by selection of a CD19 negative leukemia cell population (12). We have shown that T cells with native tumor-specificity can be activated and expanded using single epitopes of tumor antigens as stimulus in vitro for adoptive cell transfer or in vivo in response to vaccines (13, 14). Furthermore, we have demonstrated that, in healthy donors, it is possible to induce T cells specific for multiple tumor antigens which can target and kill acute myeloid leukemia cells (15).
We therefore chose to evaluate the feasibility of generating and expanding tumor antigen-specific T-cell lines from patients with ALL as a potential adoptive immunotherapeutic strategy to prevent relapse in high risk patients or patients not eligible for allogeneic HSCT. Acute lymphoblastic leukemia cells express a number of tumor-associated antigens (TAA). We selected WT1 (16), Survivin (17, 18), MAGE-A3 (19) and PRAME (6, 20) as target antigens for the generation of tumor antigen-specific T cells with the aim of broadening the applicability of T-cell therapy to the majority of patients with ALL, and reducing immune escape by the leukemia through emergence of clones deficient in TAA.
In this study we have developed a novel strategy to activate autologous T cells targeting multiple tumor antigens. We demonstrated that by utilizing both autologous dendritic cells and PHA-blasts as antigen-presenting cells we can successfully expand TAA-specific T-cell lines from 50 patients with ALL during maintenance therapy, irrespective of NCI risk status or lymphocyte count.
MATERIALS AND METHODS
Patient samples
Peripheral blood was obtained from 50 pediatric patients with ALL, receiving maintenance chemotherapy at the Texas Children's Cancer Center. All families had provided written informed consent on treatment protocols approved by the Baylor College of Medicine Institutional Review Board in accordance with the Declaration of Helsinki. Approximately 40 ml of blood was collected from 50 patients with ALL, 24 standard risk (SR) and 26 high risk (HR) patients, according to NCI Rome criteria (21) (Supplementary Table S1). Peripheral blood mononuclear cells (PBMC) were isolated by density gradient centrifugation and cryopreserved.
Generation of antigen-presenting cells
Monocyte-derived dendritic cells (DC) were generated by plate adherence of PBMC. PBMC were incubated for 2 hours in DC media (Cellgro DC media, Cellgenix, Freiburg, Germany) supplemented with 2mM Glutamax. Non-adherent cells were collected and washed. Adherent cells were cultured in DC media in the presence of IL4 (1000U/ml) and GM-CSF (800U/ml) (both R&D, Minneapolis, MN). On day 5, immature DC were matured in DC media with a cytokine cocktail consisting of IL4 (1000U/ml), GM-CSF (800U/ml), IL6 (10ng/ml), TNFα (10ng/ml), IL1β (10ng/ml) (all R&D) and PGE2 (1μg/ml) (Sigma-Aldrich), and were harvested after 48 hours of maturation for use as antigen presenting cells (APC).
For phytohemagglutinin (PHA)-blast generation, PBMC were stimulated with the mitogen phytohemagglutinin-P (PHA-P, 5μg/ml; Sigma-Aldrich, St. Louis, MO) in presence of IL2 to promote blast formation (PHA-blasts). PHA-blasts were cultured in RPMI 1640 supplemented with 5% human serum (Gem Cell, Gemini Bio-Products, West Sacramento, CA) and 2mM Glutamax (Invitrogen, Grand Island, NY) and Interleukin (IL) 2 (100U/ml) (Teceleukin, Chiron Therapeutics, Emeryville, CA).
Generation of TAA-specific T-cell lines
TAA-specific T-cell lines were generated from total PBMC. Matured DC were harvested and used as APC and peptide-pulsed with a mix of four peptide libraries (pepmixes, JPT Technologies, Berlin, Germany): WT1, Survivin, MAGE-A3 and PRAME. DC were used at a stimulator-to-effector ratio of 1:10. T cells were cultured in RPMI 1640 supplemented with 45% Clicks media (Irvine Scientific, Santa Ana, CA), 5% human AB serum and 2mM Glutamax. For initial stimulation, a cytokine mix containing IL7 (10ng/ml), IL12 (10ng/ml), IL15 (5ng/ml) and IL6 (100ng/ml) was added (15, 22). T cells were re-stimulated with peptide-pulsed autologous irradiated (30Gy) PHA-blasts at a ratio of 1:1 on day 10 to 12 and cultures were maintained in IL2 (50U/ml) supplemented media and re-stimulated every 7 days as described above for a minimum of 4 stimulation cycles, but could be kept in culture for up to 8 re-stimulations without loss of specificity. No further selection or enrichment of T cells was carried out at any point throughout the sensitization and expansion period.
IFNγ Enzyme-Linked Immunospot Assay (ELISpot)
Peptide recognition was tested in an Interferon-γ (IFNγ) Enzyme-linked Immunospot (ELISpot) assay (23). Recognition of the pooled TAAs as well as single antigens was tested as compared to no-peptide (media) control (labeled as control in all graphs) and irrelevant peptide (NY-Eso-1), that was not used for T-cell generation.
Millipore Multi Screen HTS filter plates (Millipore, Billerica, MA) were coated with IFNγ capture antibody (Mabtech, Nacka Strand, Sweden) at a concentration of 10μg/ml over night at 4°C. Plates were washed with PBS and blocked for 1 hour at 37°C to rule out non-specific protein binding. T cells were washed and re-suspended and stimulated with pepmix or single peptides at a concentration of 0.1mg/ml. The plates were incubated for 16–20 hours at 37°C. For development, plates were washed in PBS/0.05% Tween 20 and incubated with biotinylated IFNγ detection antibody (0.5μg/ml) (Mabtech) for 2 hours at 37°C, followed by incubation with streptavidin-coupled alkaline phosphatase complex (Vectastain, Vector Laboratories, Burlingame, CA) for 1 hour at room temperature and spots were developed by incubation with 3-Amino-9-ethylcarbazole substrate solution. Spot forming cells (SFC) were counted and evaluated by Zellnet Consulting (Fort Lee, NY) using an automated plate reader system (Karl Zeiss, Jena, Germany).
HLA-blocking experiments
HLA-restriction of antigen recognition was tested in IFNγ–ELISpot using autologous PHA-blasts pulsed with the relevant peptide (positive control) or without peptide (negative control) and blocking antibodies against HLA class I and class II (both BD Biosciences, San Jose, CA, USA). ELISpot plates were incubated and developed as described above.
51Chromium-release cytotoxicity assay
Cytolytic activity of T cells was assessed in a 51Chromium (51Cr)-release cytotoxicity assay. Autologous PHA-blasts were peptide-pulsed and labeled with 51Cr for one hour at 37°C, washed and re-suspended. Effector cells were used at effector-to-target ratios of 40:1 to 1.25:1 and tested against peptide-pulsed target cells (specific and irrelevant peptides (NY-Eso-1) not used for T cell generation) and unpulsed target cells and incubated for 4–6 hours. Spontaneous lysis was determined by measuring 51Cr -release into the supernatant on a gamma-counter. Spontaneous release was assessed by incubating target cells alone, maximum lysis by addition of 1% Triton X-100 (Sigma-Aldrich). Specific lysis was calculated as follows: Specific lysis [%] = 100 - ([experimental release - spontaneous release]/[maximum release - spontaneous release]).
Phenotyping of T-cell lines
T-cell lines were phenotyped by extracellular antibody staining with anti-CD3, CD4, CD8, CD45RA and RO, CCR7, CD62L, CD56, CD19 (all BD Biosciences) and analyzed on a BD FACS Calibur Flow Cytometer. Control samples labeled with appropriate isotype-matched antibodies were included in each experiment. Data were analyzed using FlowJo Flow Cytometry software (Treestar, Ashland, OR, USA).
Co-culture with autologous blast samples
To test recognition of autologous blasts, TAA-specific T-cell lines were co-cultured with each patient's autologous leukemic bone marrow sample, which had been cryopreserved at diagnosis. Co-culture experiments were carried out at ratios of 10:1 or 5:1 (T cells : leukemic blasts) for 3 days in presence of IL2 (50U/ml). Nonspecific autologous T cells were used as negative controls. Leukemia cells were quantified by co-staining with anti-CD10 and CD19 antibodies and samples were acquired on a BD FACS Calibur Flow Cytometer using Count Bright absolute counting beads (Molecular Probes, Eugene, OR) for quantification of absolute cell counts.
Enzyme-linked immunosorbent assays
Detection of cytokine release in the supernatants of co-cultured CTL was analyzed by an Enzyme-linked immunosorbent assay (ELISA) specific for IFNγ and IL4 (both R&D). For detection of IFNγ a 96-well microplate was coated with anti IFNγ capture antibody and incubated overnight. Cell culture supernatants were incubated and specific binding of IFNγ was detected by incubation with a biotinylated detection antibody, streptavidin-coupled horseradish-peroxidase and developed by incubation with substrate solution. Optical density of plates was read at 450nm with a wavelength correction at 570nm. For IL4, the same principle was applied and optical density determined at 490nm. Cytokine release was quantified in comparison to a specific standard; the assay was run in duplicates.
Colony forming unit assay
A colony forming unit (CFU) assay was carried out to show blast elimination in a long-term co-culture. T cells were co-cultured with autologous leukemic blasts at a 10:1 ratio in IMDM medium (Invitrogen) substituted with 10% FBS and 2mM Glutamax for 6 hours. After this pre-incubation, wells were collected and incubated in methocult methylcellulose media (Stemcell Technologies, Vancouver, Canada) in duplicates. Colonies were counted on day 14 and relative inhibition of colony formation calculated in comparison to nonspecific T cells as follows: Relative inhibition [%] = 100 – ([CFU specific T cells/CFU nonspecific T cells] *100).
Statistics
Data are summarized as mean ± standard deviation (SD) or mean ± standard error (SE), as noted in the text or figure legends. Student t-test was used to determine whether there was a statistically significant difference between samples, with two-tailed P values <0.05 indicating a significant difference.
RESULTS
TAA-specific T-cell lines can be reliably expanded from patients with ALL on maintenance therapy, irrespective of their NCI risk status or absolute lymphocyte count
TAA-specific T-cell lines were generated from peripheral blood obtained from 50 patients with ALL, 26 designated as high risk and as 24 standard risk according to NCI-Rome criteria, during the maintenance phase of chemotherapy. Blood samples, collected at least 3 months after the start of maintenance therapy to minimize effects of prior, more intensive chemotherapy, and were used for the generation of DC, PHA-blasts and T-cell lines. Stimulation of PBMC was carried out with antigen–pulsed DC using four complete peptide libraries spanning the entire amino acid sequence of the TAAs WT1, Survivin, MAGE-A3 and PRAME.
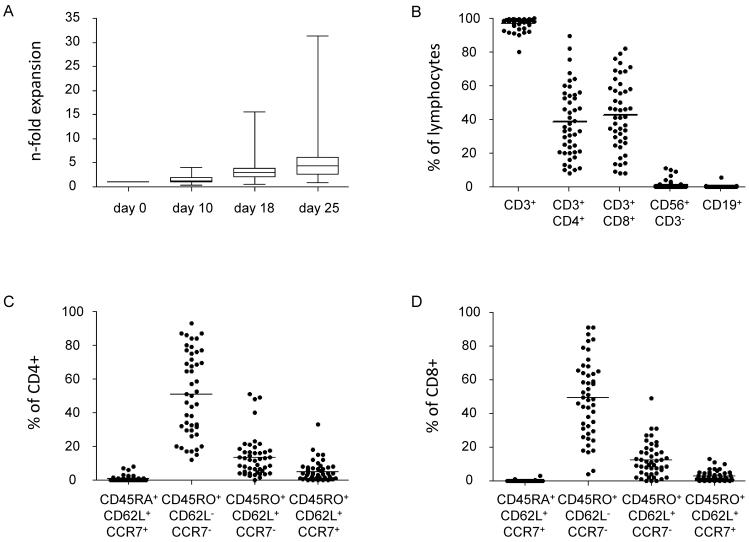
Generation of DCs as well as T-cell lines was possible from all samples obtained, despite 76% of patients having low ALC counts of <1000/μl (Supplementary Table S1). At least a minimal expansion of 1.2-fold could be achieved in all patients with a maximum 31.4-fold expansion (mean 6.4-fold) after 3 stimulations (Fig. 1A). Phenotyping of the ex vivo expanded T-cell lines showed a mean CD3+ content of 97.2% (range 80.3–99.9%) and varying distribution of CD4+ (mean 38.4% range 8.3–89.4%) and CD8+ (mean 42.6% range 7.9–82.1%) T cells, few NK cells (mean 1.3% range 0–10.9%) and rare residual B cells (mean 0.2% range 0–5.8%) (Fig. 1B). The majority of both CD4+ (mean 51.2%, range 12.0–93.0%) and CD8+ (mean 49.3%, range 4.0–91.0%) T cells were comprised of CD45RO+/CD62L−/CCR7− T-cells in accordance with an effector-memory phenotype. Very few naïve CD45RA+/CD62L+/CCR7+ cells (CD4+: mean 1.0%, range 0–8%; CD8+: mean 0.2%, range 0–3.0%) and central-memory CD45RO+/CD62L+/CCR7+ cells (CD4+: mean 5.2%, range 0.1–33.0%; CD8+: mean 2.9%, range 0–13.0%) were present after 3 re-stimulations (Figs. 1C, D).
Figure 1. Expansion and Phenotype of T-cell lines.
(A) N-fold (mean ± SD) expansion of antigen-specific T-cell lines (CTLs) generated from patients with ALL during maintenance therapy. Cell counts were assessed at the end of each re-stimulation on days 10, 18 and 25 of culture (n=50). (B) Phenotype of CTLs after the 3rd stimulation; gated on live lymphocytes. (C) T-cell subsets within the CD3+/CD4+ population. (D) T-cell subsets within the CD3+/CD8+ population.
Ex vivo expanded TAA-specific T-cells recognize multiple target antigens with broad epitope specificity
Antigen specificity of the ex vivo expanded T-cell lines was evaluated weekly in response to the mix of TAA and individual antigens in IFNγ-ELISpot assay. Specific reactivity against the pooled TAAs after the initial stimulation could be seen in 54% of SR and 65% of HR patients (data not shown). After 3 stimulations, 92% of SR and 96% of HR patients showed specific responses against at least one antigen. There was almost equal distribution of SR versus HR patients showing reactivity against one (21% SR; 19% HR), two (21% SR; 23% HR), three (21% SR; 23% HR), or all four antigens (29% SR; 19% HR) (Fig. 2A). The majority of the expanded TAA-specific T-cell lines elicited responses against PRAME (79% SR vs. 69% HR), followed by WT1 (71% SR vs. 54% HR) and MAGE-A3 (58% SR vs. 57% HR). In contrast, T-cell responses to survivin were seen in a minority of patients (42% SR vs. 27% HR) (Fig. 2B). Similarly, highest specificity as measured by spot counts in IFNγ-ELISpot assays, was seen in response to the pooled TAAs (SR: mean 312, range 31–970; HR: mean 258, range 17–777) and PRAME (SR: mean 230, range 0–851; HR: mean 173, range 2–681), followed by MAGE-A3 (SR: mean 168, range 0–1324; HR: mean 127, range 0–562), WT1 (SR: mean 121, range 2–534; HR: mean 145, range 2–642) and least against Survivin (SR: mean 109, range 0–541; HR: mean 84, range 2–498) (Fig. 2C).
Figure 2. Summary of antigen-specific responses in IFNγ-ELISpot and cytotoxicity assay.
(A) Number of antigens recognized in IFNγ-ELISpot by CTLs generated from patients classified as standard risk (SR, n=24, left panel) or high risk (HR, n=26, right panel). (B) Percent of patients recognizing each of the 4 antigens or the mix of all antigens (TAA) used for T-cell generation in IFNγ-ELISpot assay (SR white bars, HR black bars). (C) Number of spot forming cells (SFC)/105 cells (mean ± SE) in SR (n=24, white bars) and HR (n=26, black bars) patients for each of the antigens and TAA. Responses were assessed after the 3rd stimulation. (D) Cytolytic activity in a standard 51Cr-release assay against peptide-pulsed autologous target cells (PHA-blasts) at an effector-to-target (E:T) ratio of 20:1 (mean ± SE; SR n=24, white bars, HR n=26, black bars).
Cytolytic activity of T-cell lines was tested in a standard 51Cr-release assay against peptide-pulsed autologous PHA-blasts. Mean lysis of target cells pulsed with the pooled TAAs at an E:T ratio of 20:1 was 27% for CTL lines generated from the SR patients (range 0–77%) versus 31% for the HR patients (range 0–90%). Killing of targets pulsed with the individual antigens for the SR patients showed: mean 13% (range 0–66%) for WT1; mean 10%, (range 0–58%) for Survivin, mean 12%, (range 0–70%) for MAGE-A3 and mean 26%, (range 0–74%) for PRAME. No appreciable differences in cytolytic responses were seen in T-cell lines generated from HR patients: mean 15%, (range 0–100%) for WT1, mean 8%, (range 0–58%) for Survivin, 16%, (range 0–68%) for MAGE-A3; and mean 21% (range 0–55%) for PRAME (Fig. 2D). No killing (<6%) of unpulsed target cells was observed.
Polyclonal MHC class I and II-mediated T-cell responses can be elicited in vitro from patients on treatment for ALL
To compare the epitope-specific responses in T-cell lines generated from patients with ALL to those previously described in healthy donors (15, 24–28) we performed epitope mapping for WT1 in 11 T-cell lines. Reactivity against minipools of 15mer peptides overlapping by 11 amino acids spanning the whole sequence of WT1 were tested, as previously described (29). Subsequently, confirmation of single peptide recognition and HLA-restriction was performed (15).
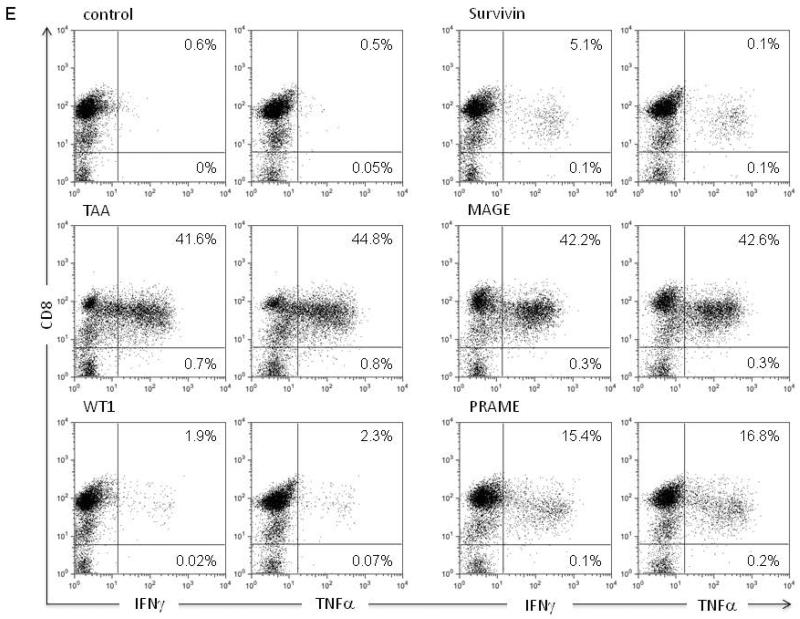
Results of the epitope mapping experiments of a representative TAA-specific T-cell line are shown in Fig. 3. As seen in Fig. 3A, IFNγ-ELISpot assays showed 70 SFC/105 cells in response to pooled TAAs after the initial stimulation, which increased to 200 SFC/105 cells after three in vitro stimulations. The expanded T-cell line recognized both WT1 and PRAME (Fig. 3B). Epitope mapping was then performed for WT1 which showed reactivity against two regions within WT1, peptides #9/10 (QWAPVLDFAPPGASAYGSL) and peptides #71/72 (LCGAQYRIHTHGVFRGIQD). Using HLA class I and class II blocking antibodies, the overlapping sequence of peptides #71/72 was confirmed to contain an HLA-class I restricted epitope previously published by Woelfl et. al (28) (Fig. 3C, Table 1). In contrast, recognition of peptides #9/10 was HLA class II-restricted, as confirmed by blocking experiments. This 15mer peptide is known to contain both HLA class I and II restricted epitopes, however with different HLA-restrictions from that seen in our patient (24) (Fig. 3D, Table 1). In all, analysis of the WT1-specific T-cell responses in 11 patient-derived T-cell lines identified a total of eleven 15mer class I and II-restricted peptides, as shown in Table 1. The majority of these peptides have previously been described and identified to be immunogenic in healthy volunteers (15, 24–28). Hence, these results demonstrate that we can successfully generate antigen-specific T cell-lines from patients with ALL, and the spectrum of the specificity in these lines is similar to that of healthy donors with a range of class I and II restricted epitopes (15, 24). Further, the polyfunctionality of the expanded tumor antigen-specific T cells is demonstrated in Fig. 3E where a T-cell line with specificity against all the targeted antigens released both IFNγ and TNFα in response to stimulation.
Figure 3. HLA-restriction of antigen recognition in two T-cell lines.
IFNγ-ELISpot responses during the course of 3 re-stimulation cycles against TAA after the initial stimulation (A) versus the 4 individual antigens after the 3rd stimulation (B). Epitope mapping for WT1 using a 15mer peptide library overlapping by 11 amino acids spanning the whole sequence of WT1 indicated recognition of two regions within the WT1 sequence: WT1 peptides #71 and #9. (C) IFNγ-secretion in response to peptides #71 can be inhibited by HLA-class I blocking antibody in ELISpot assay, which implies a CD8+-restricted recognition of this epitope. Autologous peptide-pulsed antigen presenting cells were used for stimulation or pre-incubated with blocking antibodies. (D) Peptide #9 recognition is reduced by HLA-class II blocking antibody indicating a CD4+-restricted recognition of this peptide. (Figures A–D: mean ± SD). (E) Intracellular detection of INFγ and TNFα after antigen stimulation in presence of co-stimulatory antibodies anti-CD28 and CD49d, Brefeldin and Monensin followed by intra- and extracellular antibody staining shows cytokine production at varying levels restricted to the CD8+ population. Plots shown are gated on live lymphocytes and CD3+ cells.
Table 1.
Immunogenic WT1 peptides identified by epitope mapping of WT1-specific T-cells derived from patients with acute lymphoblastic leukemia
| Patient ID | 15mer no. | AA# | Peptide sequence | HLA-restriction | HLA-A | HLA-B | HLA-DRB1 | HLA-DQB1 | Reference (AA#, HLA-restriction) |
|---|---|---|---|---|---|---|---|---|---|
| 948 | 9/10 | 33–47 | QWAPVLDFAP PGASAYGSL |
class II | 02/33 | 51/53 | 03/13 | 02/06 | AA#37–45 (HLA-A*0201)(27) AA#38–46 (HLA-A*0201)(24) AA#37–48 (HLA-DRB1*0402)(24) |
| 58 | 229–243 | MTSQLECMT WNQMNL |
class II | AA#235–243 (HLA-A24)(26) AA#235–243 (HLA-A*0201)(24) |
|||||
| 1024 | 9/10 | 33–47 | QWAPVLDFAP PGASAYGSL |
n.d. | 02/03 | 07/15 | 09/11 | 02/03 | AA#37–45 (HLA-A*0201)(27) AA#38–46 (HLA-A*0201)(24) AA#38–48 (HLA-DRB1*0402)(24) |
| 1106 | 32 | 125–139 | ARMFPNAPYL PSCLE |
class I | 24/68 | 08/35 | 11/13 | 03 | AA#126–134 (HLA-A*0201)(49) AA#124–138 (HLA-DR53)(25) |
| 1129 | 3 | 9–23 | NALLPAVPSL GGGGG |
n.d. | 02/33 | 65/35 | 04/13 | 03/06 | AA#6–15(HLA-A*0201, B*5701)(24) |
| 29 | 113–127 | GPPPPSQASS GQARM |
n.d. | ||||||
| 1137 | 12 | 55–69 | ASAYGSLGGP APPPA |
n.d. | 02/26 | 14/18 | 01/11 | 03/05 | AA#58–66 (HLA-A*0201)(24) |
| 59/60/61 | 233–255 | LECMTWNQM NLGATLKGVA AGSS |
n.d. | AA#235–243 (HLA-A24)(26) AA#235–242 (HLA-A*0201)(24) AA#238–246 (HLA-A*0101, A*0201, B*3508, C*1701)(24) AA#239–249 (HLA-*2402)(24) AA#235–249 (HLA-DRB1*1104)(24) AA#242–250 (HLA-A*0101, A*0201)(24) AA#243–252 (HLA-A*0203)(24) AA#246–253 (HLA-A*6901)(24) |
|||||
| 76/77 | 301–319 | RRVPGVAPTL VRSASETSE |
n.d. | ||||||
| 1221 | 71/72 | 271–289 | LCGAQYRIHT HGVFRGIQD |
n.d. | 03/24 | 07/48 | 04/16 | 03/05 | AA#286–293 (HLA-Cw7)(28) AA#269–278 (HLA-A*0101, B*3501)(24) |
| 1289 | 109/110 | 433–449 | RHHNMHQRN MTKLQLAL |
class I | 24 | 35/40 | 08/11 | 03/04 | AA#436–445 (HLA-A*0201, A*2402, B*4001)(24) |
| 1331 | 9/10 | 33–47 | QWAPVLDFAP PGASAYGSL |
class II | 02/31 | 07 | 15 | 06 | AA#37–45 (HLA-A*0201)(27) AA#38–46 (HLA-A*0201)(24) AA#38–48 (HLA-DRB1*0402)(24) |
| 71/72 | 271–289 | LCGAQYRIHT HGVFRGIQD |
class I | AA#286–293 (HLA-Cw7)(28) AA#269–278 (HLA-A*0101, B*3501)(24) |
|||||
| 1349 | 60 | 237–251 | TWNQMNLGA TLKGVA |
n.d. | 02/24 | 18/40 | 04/11 | 03/03 | AA#235–242 (HLA-A24)(26) AA#235–242 (HLA-A*0201)(24) AA#238–246 (HLA-A*0101, A*0201, B*3508, C*1701)(24) AA#239–249 (HLA-A*2402)(24) AA#235–249 (HLA-DRB1*1104)(24) AA#242–250 (HLA-A*0101, A*0201)(24) AA#243–252 (HLA-A*0203)(24) AA#246–253 (HLA-A*6901)(24) |
| 1485 | 9/10 | 33–47 | QWAPVLDFAP PGASAYGSL |
class II | 01/02 | 37/40 | 07/14 | 02/03 | AA#37–45 (HLA-A*0201)(27) AA#38–46 (HLA-A*0201)(24) AA#38–48 (HLA-DRB1*0402)(24) |
| 78 | 309–323 | TLVRSASETS EKRPF |
AA#317–327 (HLA-A1)(50) | ||||||
| 1516 | 71/72 | 271–289 | LCGAQYRIHT HGVFRGIQD |
02 | 49/51 | 11/14 | 03 | AA#286–293 (HLA-Cw7)(28) AA#269–278 (HLA-A*0101, B*3501)(24) |
n.d. not determined
Antigen recognition and anti-leukemic activity against autologous bone marrow blast samples
We next investigated whether TAA-specific T-cells generated from patients with ALL were able to recognize and kill autologous leukemia cells in vitro. Co-culture experiments were set up for 8 patient-derived TAA-specific T-cell lines using, as targets, autologous ALL blasts obtained from bone marrow aspirates that had been cryopreserved at diagnosis. Antigen-specificity as well as short- and long-term co-cultures were evaluated in IFNγ-ELISpot, flow-based and CFU assays. In addition, the co-culture supernatants were evaluated by IFNγ and IL4 ELISA assays. Nonspecific T-cells derived from the same patient were used as controls. In ELISpot assay at a 1:1 ratio of leukemia cells to T-cells, the TAA-specific T-cells showed increased IFNγ-release (mean 300 SFC/105 cells; range 8–1701) compared to control T cells (<10 SFC/105 cells) (Fig. 4A). No cytokine secretion by leukemia cells alone was observed. Co-culture of T cells at an E:T ratio of 10:1 in the presence of IL2 (50U/ml) showed a variable elimination of autologous leukemia blasts in vitro. After 3 days of co-culture with TAA-specific T cells there were 13.6% residual blasts remaining compared to 46.8% in the controls (calculated relative to the initial leukemia cell counts on day 0)(p=0.04) (Fig. 4B). Analysis of IFNγ and IL4-levels in the co-culture supernatants showed high concentrations of IFNγ by TAA-specific T cells after 3 days of co-culture with autologous blasts (mean 630 ± 364 pg/106 cells/ml) compared to control T cells (mean 0pg/106 cells/ml)(p=0.04). Lower levels of IL4 were secreted by TAA-specific T cells (mean 19.6 ± 3.3 pg/106 cells/ml; control T cells mean 7.6 ± 1.1 pg/106 cells/ml) after co-culture with autologous blasts (p=0.017)(Fig. 4C). Further, a specific inhibition of colony formation was observed in CFU assays after 14 days when leukemia blasts were co-cultured with TAA-specific T cells compared to control T cells. As shown in Fig. 4D, a mean relative inhibition of 42.3% of colony formation was observed (p=0.012).
Figure 4. Reactivity of tumor antigen-specific T-cell lines against autologous leukemia cells.
Summary of co-culture experiments of 8 patient-derived tumor antigen-specific T-cell lines (CTLs) against their autologous bone marrow leukemia blast samples cryopreserved at the time of diagnosis. Nonspecific T cells or unstimulated cells of the same patient were used as control in all experiments. (A) Summary of the IFNγ-ELISpot co-culture results of CTLs (n=8, black bar) vs. nonspecific T cells (n=8, white bar) (mean ± SE) at an E:T ratio of 1:1. (B) In a three-day co-culture at an E:T ratio of 10:1 leukemia cell counts were assessed by FACS analysis and compared to the absolute leukemia cell count obtained on day 0. Leukemia blasts were quantified by CD10 and CD19 co-staining; T cells were quantified by CD3 positivity. Absolute cell numbers were assessed by quantification with FACS counting beads and the percentage of remaining leukemia cells was calculated compared to cell numbers on day 0 (nonspecific T cells, control: white bars, CTLs: black bars) (mean ± SD). (C) IFNγ and IL4 ELISA of the co-culture supernatants after 24h (mean ± SE; control n=8, white bars, CTLs n=8, black bars). (D) Relative inhibition of colony formation of leukemic cells in CFU assays when co-cultured with CTLs (n=8, black bar) vs. nonspecific cells (n=8, white bar)(E:T ratio 10:1; mean ± SE).
Two representative examples for the leukemia-specific activity of the TAA-specific T cells are shown in Fig. 5 in a standard risk patient and supplementary Fig. S2 in a high risk patient, respectively. In patient ID#1032 (designated as SR), peptide reactivity against the pooled TAAs in ELISpot assay after initial (Fig. 5A) and three (Fig. 5B) stimulations showed recognition of WT1 and PRAME. Further, cytolytic activity against these antigens was demonstrated in a 51Cr-release assay using peptide-pulsed autologous PHA-blasts as targets (Fig. 5C). Co-culture of TAA-specific T-cells with autologous blasts showed reduction of autologous leukemia cells to 13% of the initial cell count after one day and to 4.8% after 3 days. In contrast, when nonspecific T cells derived from the same patient were incubated with autologous blasts, 23% of leukemia blasts remained on day 1 and 10% on day 3 of co-culture (Fig. 5D). Reduction of leukemia cell numbers were also apparent when applying absolute quantification with FACS counting beads. After 3 days, elimination of leukemia cells was observed when blasts were co-cultured with autologous TAA-specific T-cells but not control T cells (Fig. 5E). Activation of TAA-specific T cells by the autologous blasts was further demonstrated in IFNγ-ELISpot assays, where the T-cells showed markedly increased IFNγ-production (mean 252 SFC/105 cells) compared to the control (mean 128 SFC/105 cells) (Fig. 5F). To evaluate the expression of the targeted antigens on the ALL blasts, cytospins were stained by immunohistochemistry and showed weak positivity for MAGE-A3 and higher for Survivin (Fig. 5G). Similarly, in supplementary Figures S2 to S8 the results of the co-culture experiments with autologous blasts of the remaining patients are shown, including the 3-day co-culture and ELISpot assays as well as the immunohistochemistry of the leukemia blasts. There was no correlation between the generation of effective CTL and leukemia antigen expression in the blasts in the 8 patients where leukemia blasts and peripheral blood samples were available.
Figure 5. Antigen recognition and reactivity against autologous leukemia cells in a patient with standard risk acute lymphoblastic leukemia.
Antigen-specific CTLs of patient #1032 showed recognition of TAA after the initial stimulation (A), as well as recognition of PRAME and WT1 after the 3rd stimulation (B) in IFNγ-ELISpot (mean ± SD). (C) Cytolytic activity in a standard 51Cr-release assay against antigen-pulsed autologous target cells (PHA-blasts, PHAB) at E:T ratios from 40:1 to 1.2:1. (D) Percent of remaining leukemia cells in co-culture of CTLs with autologous leukemia blasts on day 1 and 3 in presence of IL2 (50U/ml) at an E:T ratio of 10:1. Leukemic cells were detected by co-staining with CD10 and CD19 antibodies, T cells by staining with CD3 antibody; nonspecific T cells (white bars) were used as control for unspecific lysis (CTLs: black bars). (E) Assessment of absolute leukemia cell counts in co-culture with CTLs (black line) and nonspecific T cells (dashed line) at an E:T ratio of 10:1 by FACS analysis using counting beads. (F) Co-culture at a 1:1 ratio of autologous leukemia cells to CTLs (black bar) or nonspecific T cells (white bar) in IFNγ-ELISpot assay (mean ± SD). (G) Immunohistochemistry of leukemia blasts.
DISCUSSION
Patients with ALL who have high risk disease or who relapse have a high rate of mortality and the best chance of cure is the allogeneic HSCT. There is a need for more effective treatment options for patients not eligible for allogeneic transplant and immunotherapy may be most effective for preventing relapse in high risk patients after chemotherapy. Therefore, we sought to develop a strategy to generate autologous T cells that target multiple TAAs and demonstrate specific anti-leukemic activity for use as adoptive T-cell immunotherapy to prevent relapse in pediatric ALL. Our rationale for obtaining samples during maintenance therapy was to demonstrate feasibility of collection at this time point for potential use as a front-line strategy, to augment therapy in high-risk patients in first remission. We show here that TAA-specific T cells can be generated from patients with ALL during maintenance therapy regardless of NCI risk group and in spite of low lymphocyte counts at the time of sample acquisition. Patients with high risk ALL might have more compromised immune systems, correlating with their poor outcome. Nevertheless, we observed no difference in the quality of TAA-specific T cells that could be generated from high and standard risk patients. None of the commonly known risk factors for relapse, including age and gender, affected our ability to ex vivo expand TAA-specific T cells from these patients and extensive in vitro testing showed anti-leukemic activity of the expanded products against autologous ALL blasts in long- and short-term co-culture experiments.
Antigen-specific responses were detectable within 7–10 days after just a single stimulation in more than half of the tested patients, indicating that the immunosuppression due to the chemotherapeutic drugs administered during maintenance therapy did not preclude the expansion of TAA-specific T cells in vitro. Moreover, upon subsequent re-stimulation, TAA-specific T cells were expanded from over 90% of patients, suggesting that the in vivo immune suppressive environment could be overcome in vitro. At least a minimal 1.2-fold expansion of the autologous T-cell lines was possible in all cases, using methods approved for good manufacturing practices. Nevertheless, for a clinical scale product the conditions need to be adjusted to achieve cell numbers necessary for infusion, e.g. through use of gas-permeable cell culture flasks (30).
After 3 weeks in culture, the T-cell lines expanded from patients with ALL displayed predominantly an effector-memory phenotype, although small populations of T-cells with central memory and naïve phenotypes were also detected. Effector-memory T-cells have been shown to have effective anti-tumor and anti-viral efficacy, but are short-lived in vivo, leading to tumor outgrowth after loss of the adoptively transferred cells (31). In contrast, it has been shown that naïve T cells or naïve-derived effector T cells exert the most effective anti-tumor effects as well as having better in vivo expansion and persistence than effector cells derived from memory subsets (31, 32) We and others previously showed that virus- and TAA-specific T cells can be expanded from naïve T cells derived from cord blood as well as from healthy donors (15, 23, 28, 33, 34). In contrast to virus-specific T cells where memory T cells are the main source of expanded T cells, naïve T cells appear to be an important source of TAA-specific T cells (15, 34). The lymphopenia following high dose chemotherapy may favor the in vitro generation of T cells from naïve cell populations. However, further experiments will be needed to define the origin of the TAA-specific T-cell lines. In addition to the ability to generate T-cell lines, the adoptive transfer of in vitro primed T cells into a lymphopenic milieu may favor the persistence and expansion of the transferred T cells, as has been shown by the infusion of predominantly effector-memory T-cells in the transplantation setting (35–37).
The antigens we selected for T-cell generation are expressed in a wide variety of hematological disorders as well as solid tumors. Immunohistochemistry of the available patient samples showed only low levels of antigen expression in the leukemia cells, which might limit the anti-leukemic effects of the T cells in vivo, despite their high specificity. However, antigen expression in leukemic cells can be enhanced by epigenetically modifying drugs (38) and clinical trials in adults and pediatric patients with acute leukemia are currently under investigation. Furthermore, epigenetic modulation has been shown to render malignant cells more susceptible to T-cell recognition without impairing expansion or function of in vitro generated T cells (38–40). These observations suggest the feasibility of combining epigenetic modification and adoptive T-cell transfer.
Immunotherapeutic approaches for patients with acute leukemia have included vaccines, which generally target a single peptide epitope and clinical responses have been observed that appear to correlate with expansion of epitope-specific T cells in vivo. More recently, CD19-specific antibodies (41, 42) and CD19CAR gene-modified T-cells targeting the CD19-receptor on B cell malignancies have shown remarkable success for CD19+ malignancies including pediatric ALL (10–12). Our strategy offers a synergistic approach to these strategies. First, our technique covers multiple immunogenic epitopes. This offers the potential for a combined immunotherapeutic approach with vaccines to re-challenge the patients with the antigens used for T-cell generation and boost the adoptively transferred T cells in vivo. This may prolong their persistence and increase their potency. Second, our approach permits T-cell generation from all patients without limitation to certain HLA-types, unlike single peptide vaccine studies (14, 27, 43). Finally, the polyfunctionality and simultaneous targeting of multiple antigens may decrease the risk of tumor immune escape, such as the down-regulation of antigen expression, elimination of clones expressing the targeted epitope or outgrowth of antigen-negative populations, already observed with CD19-directed therapy (12). Therefore, to increase the breadth of the tumor-specific activity by T cells, TAA-specific T cells could be administered in combination with CD19 antibodies or could be genetically modified to express a CD19CAR.
Previous studies have shown the importance of CD4+ T-helper cells for the strength and persistence of in vivo immune responses against viral and tumor antigens (44, 45). In this study, tumor-specific responses were seen in both the CD4+ and CD8+ T-cell populations and immunogenic peptides restricted by MHC class I and II were detected by epitope mapping for WT1. The combination of CD4+ restricted epitopes with the commonly used CD8+ epitopes for T-cell stimulation can improve the survival of the generated T-cell lines and vaccination studies using a mix of MHC class I and II restricted peptides show a sustained activation and better survival of cytotoxic T cells (44–46). Furthermore, CD4+ T cells can also have a direct cytolytic effect on tumor cells (47, 48). Thus, the infusion of TAA-specific T cells containing antigen-specific CD4+ and CD8+ T cells may facilitate a better anti-leukemic effect in vivo.
While it is encouraging that our TAA-specific T cells reduced autologous leukemia blasts in co-culture experiments, it remains to be determined whether TAA-specific T cells have therapeutic efficacy in patients with ALL. It would be possible to generate a SCID/hu mouse model of ALL to further test the anti-leukemic effect of TAA-specific T cells. However, while such a model, using autologous T cells to prevent or treat ALL, might yield positive results, it would not guarantee success in man. Conversely, were the TAA-specific T cells ineffective in the murine model, we would not wish to abandon evaluating TAA-specific T cells in a carefully designed clinical trial, where we can track the fate and function of in vitro generated TAA and evaluate anti-leukemic efficacy by measuring residual disease. Given the safety of infused TAA-specific T cells generated in healthy donors,(13) and the lack of relevance of animal models for establishing safety, our next step will be to generate GMP grade TAA-specific T cells for a phase 1 clinical trial.
This study shows that the generation of TAA-specific T-cell lines from the peripheral blood of patients with ALL on maintenance therapy irrespective of NCI risk group is feasible, and that these T cells show anti-leukemic activity against autologous blasts in vitro. Therefore, adoptive immunotherapy with autologous TAA-specific T cells to boost anti-leukemic immune responses and prevent relapse appears to be a promising approach and may improve outcomes in high risk ALL both in the front-line setting, and as a new approach to treat relapsed patients not eligible for allogeneic stem cell transplant in combination with other therapeutic options.
Supplementary Material
TRANSLATIONAL RELEVANCE.
Although the majority of pediatric patients with acute lymphoblastic leukemia (ALL) have excellent outcomes, the prognosis remains poor in patients with high-risk disease and those who relapse, especially when allogeneic hematopoeietic stem cell transplantation is not feasible. Several new treatments are being investigated, including immune-based therapies. Immunotherapy approaches have included vaccines, which generally target a single HLA-restricted epitope. More recently, CD19-specific antibodies and CD19CAR gene-modified T-cells have shown remarkable successes for CD19+ malignancies, including pediatric ALL. Targeting single antigens can eliminate cells expressing the targeted epitope, but can lead to outgrowth of antigen-negative populations. This paper validates a novel approach expanding T-cells from pediatric patients with ALL, targeting multiple tumor-associated antigens. Simultaneous targeting of multiple antigens may decrease the risk of tumor immune escape when T cells are administered in vivo. Hence, this innovative immunotherapeutic strategy has the potential to increase the potency of vaccines and CD19-directed therapies to prevent relapse and improve the prognosis of patients with high-risk ALL.
Acknowledgments
Grant support This work was supported in parts by NIH grant 1P01CA148600-01 and CPRIT Grant RP100484. CMB was also supported by the career development award from the Leukemia Lymphoma Society and an award from the Gillson Longenbaugh Foundation. KRR was supported by the Kurt Groten Family Research Scholars' Program, the Gillson Longenbaugh Foundation, and the St. Baldrick's Pediatric Cancer Foundation.
Footnotes
Author contributions: GW designed, planned and performed experiments, analyzed the data, wrote the manuscript IC planned and performed experiments, analyzed data, contributed to the writing of the manuscript
RHR acquired patient samples, performed experiments, analyzed data
AJB reviewed data, contributed to the writing of the manuscript
AML reviewed data, contributed to the writing of the manuscript
KRR acquired patient samples, designed and planned the experiments, analyzed the data, reviewed the manuscript
CMB designed and planned experiments, analyzed the data, wrote the manuscript
Disclosure of potential conflicts of interest The authors declare no competing financial interests.
REFERENCE LIST
- (1).Kennedy-Nasser AA, Bollard CM, Myers GD, Leung KS, Gottschalk S, Zhang Y, et al. Comparable outcome of alternative donor and matched sibling donor hematopoietic stem cell transplant for children with acute lymphoblastic leukemia in first or second remission using alemtuzumab in a myeloablative conditioning regimen. Biol Blood Marrow Transplant. 2008 Nov;14(11):1245–52. doi: 10.1016/j.bbmt.2008.08.010. [DOI] [PubMed] [Google Scholar]
- (2).Eapen M, Zhang MJ, Devidas M, Raetz E, Barredo JC, Ritchey AK, et al. Outcomes after HLA-matched sibling transplantation or chemotherapy in children with acute lymphoblastic leukemia in a second remission after an isolated central nervous system relapse: a collaborative study of the Children's Oncology Group and the Center for International Blood and Marrow Transplant Research. Leukemia. 2008 Feb;22(2):281–6. doi: 10.1038/sj.leu.2405037. [DOI] [PubMed] [Google Scholar]
- (3).Kolb HJ, Schattenberg A, Goldman JM, Hertenstein B, Jacobsen N, Arcese W, et al. Graft-versus-leukemia effect of donor lymphocyte transfusions in marrow grafted patients. Blood. 1995 Sep 1;86(5):2041–50. [PubMed] [Google Scholar]
- (4).Porter DL, Orloff GJ, Antin JH. Donor mononuclear cell infusions as therapy for B-cell lymphoproliferative disorder following allogeneic bone marrow transplant. Transplant Sci. 1994 Sep;4(1):12–4. [PubMed] [Google Scholar]
- (5).Rabin KR, Gramatges MM, Borowitz MJ, Palla SL, Shi X, Margolin JF, et al. Absolute lymphocyte counts refine minimal residual disease-based risk stratification in childhood acute lymphoblastic leukemia. Pediatr Blood Cancer. 2012 Sep;59(3):468–74. doi: 10.1002/pbc.23395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (6).Rezvani K, Yong AS, Tawab A, Jafarpour B, Eniafe R, Mielke S, et al. Ex vivo characterization of polyclonal memory CD8+ T-cell responses to PRAME-specific peptides in patients with acute lymphoblastic leukemia and acute and chronic myeloid leukemia. Blood. 2009 Mar 5;113(10):2245–55. doi: 10.1182/blood-2008-03-144071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (7).Choi SJ, Lee JH, Lee JH, Kim S, Lee YS, Seol M, et al. Treatment of relapsed acute lymphoblastic leukemia after allogeneic bone marrow transplantation with chemotherapy followed by G-CSF-primed donor leukocyte infusion: a prospective study. Bone Marrow Transplant. 2005 Jul;36(2):163–9. doi: 10.1038/sj.bmt.1705024. [DOI] [PubMed] [Google Scholar]
- (8).Levine JE, Barrett AJ, Zhang MJ, Arora M, Pulsipher MA, Bunin N, et al. Donor leukocyte infusions to treat hematologic malignancy relapse following allo-SCT in a pediatric population. Bone Marrow Transplant. 2008 Aug;42(3):201–5. doi: 10.1038/bmt.2008.135. [DOI] [PubMed] [Google Scholar]
- (9).Collins RH, Jr., Goldstein S, Giralt S, Levine J, Porter D, Drobyski W, et al. Donor leukocyte infusions in acute lymphocytic leukemia. Bone Marrow Transplant. 2000 Sep;26(5):511–6. doi: 10.1038/sj.bmt.1702555. [DOI] [PubMed] [Google Scholar]
- (10).Kochenderfer JN, Dudley ME, Feldman SA, Wilson WH, Spaner DE, Maric I, et al. B-cell depletion and remissions of malignancy along with cytokine-associated toxicity in a clinical trial of anti-CD19 chimeric-antigen-receptor-transduced T cells. Blood. 2012 Mar 22;119(12):2709–20. doi: 10.1182/blood-2011-10-384388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (11).Savoldo B, Ramos CA, Liu E, Mims MP, Keating MJ, Carrum G, et al. CD28 costimulation improves expansion and persistence of chimeric antigen receptor-modified T cells in lymphoma patients. J Clin Invest. 2011 May;121(5):1822–6. doi: 10.1172/JCI46110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (12).Grupp SA, Kalos M, Barrett D, Aplenc R, Porter DL, Rheingold SR, et al. Chimeric Antigen Receptor-Modified T Cells for Acute Lymphoid Leukemia. N Engl J Med. 2013 Mar 25; doi: 10.1056/NEJMoa1215134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (13).Rezvani K, Price DA, Brenchley JM, Kilical Y, Gostick E, Sconocchia G, et al. Transfer of PR1-specific T-cell clones from donor to recipient by stem cell transplantation and association with GvL activity. Cytotherapy. 2007;9(3):245–51. doi: 10.1080/14653240701218524. [DOI] [PubMed] [Google Scholar]
- (14).Rezvani K, Yong AS, Mielke S, Savani BN, Musse L, Superata J, et al. Leukemia-associated antigen-specific T-cell responses following combined PR1 and WT1 peptide vaccination in patients with myeloid malignancies. Blood. 2008 Jan 1;111(1):236–42. doi: 10.1182/blood-2007-08-108241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (15).Weber G, Gerdemann U, Caruana I, Savoldo B, Hensel NF, Rabin KR, et al. Generation of multi-leukemia antigen-specific T cells to enhance the graft-versus-leukemia effect after allogeneic stem cell transplant. Leukemia. 2013 Mar 1; doi: 10.1038/leu.2013.66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (16).Boublikova L, Kalinova M, Ryan J, Quinn F, O'Marcaigh A, Smith O, et al. Wilms' tumor gene 1 (WT1) expression in childhood acute lymphoblastic leukemia: a wide range of WT1 expression levels, its impact on prognosis and minimal residual disease monitoring. Leukemia. 2006 Feb;20(2):254–63. doi: 10.1038/sj.leu.2404047. [DOI] [PubMed] [Google Scholar]
- (17).Ambrosini G, Adida C, Altieri DC. A novel anti-apoptosis gene, survivin, expressed in cancer and lymphoma. Nat Med. 1997 Aug;3(8):917–21. doi: 10.1038/nm0897-917. [DOI] [PubMed] [Google Scholar]
- (18).Esh AM, Atfy M, Azizi NA, El Naggar MM, Khalil EE, Sherief L. Prognostic significance of survivin in pediatric acute lymphoblastic leukemia. Indian J Hematol Blood Transfus. 2011 Mar;27(1):18–25. doi: 10.1007/s12288-010-0053-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (19).Martinez A, Olarte I, Mergold MA, Gutierrez M, Rozen E, Collazo J, et al. mRNA expression of MAGE-A3 gene in leukemia cells. Leuk Res. 2007 Jan;31(1):33–7. doi: 10.1016/j.leukres.2006.05.009. [DOI] [PubMed] [Google Scholar]
- (20).Steinbach D, Viehmann S, Zintl F, Gruhn B. PRAME gene expression in childhood acute lymphoblastic leukemia. Cancer Genet Cytogenet. 2002 Oct 1;138(1):89–91. doi: 10.1016/s0165-4608(02)00582-4. [DOI] [PubMed] [Google Scholar]
- (21).Smith M, Arthur D, Camitta B, Carroll AJ, Crist W, Gaynon P, et al. Uniform approach to risk classification and treatment assignment for children with acute lymphoblastic leukemia. J Clin Oncol. 1996 Jan;14(1):18–24. doi: 10.1200/JCO.1996.14.1.18. [DOI] [PubMed] [Google Scholar]
- (22).Gerdemann U, Katari U, Christin AS, Cruz CR, Tripic T, Rousseau A, et al. Cytotoxic T lymphocytes simultaneously targeting multiple tumor-associated antigens to treat EBV negative lymphoma. Mol Ther. 2011 Dec;19(12):2258–68. doi: 10.1038/mt.2011.167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (23).Weber G, Karbach J, Kuci S, Kreyenberg H, Willasch A, Koscielniak E, et al. WT1 peptide-specific T cells generated from peripheral blood of healthy donors: possible implications for adoptive immunotherapy after allogeneic stem cell transplantation. Leukemia. 2009 Sep;23(9):1634–42. doi: 10.1038/leu.2009.70. [DOI] [PubMed] [Google Scholar]
- (24).Doubrovina E, Carpenter T, Pankov D, Selvakumar A, Hasan A, O'Reilly RJ. Mapping of novel peptides of WT-1 and presenting HLA alleles that induce epitope-specific HLA-restricted T cells with cytotoxic activity against WT-1(+) leukemias. Blood. 2012 Aug 23;120(8):1633–46. doi: 10.1182/blood-2011-11-394619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (25).Kobayashi H, Nagato T, Aoki N, Sato K, Kimura S, Tateno M, et al. Defining MHC class II T helper epitopes for WT1 tumor antigen. Cancer Immunol Immunother. 2006 Jul;55(7):850–60. doi: 10.1007/s00262-005-0071-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (26).Ohminami H, Yasukawa M, Fujita S. HLA class I-restricted lysis of leukemia cells by a CD8(+) cytotoxic T-lymphocyte clone specific for WT1 peptide. Blood. 2000 Jan 1;95(1):286–93. [PubMed] [Google Scholar]
- (27).Rezvani K, Brenchley JM, Price DA, Kilical Y, Gostick E, Sewell AK, et al. T-cell responses directed against multiple HLA-A*0201-restricted epitopes derived from Wilms' tumor 1 protein in patients with leukemia and healthy donors: identification, quantification, and characterization. Clin Cancer Res. 2005 Dec 15;11(24 Pt 1):8799–807. doi: 10.1158/1078-0432.CCR-05-1314. [DOI] [PubMed] [Google Scholar]
- (28).Wolfl M, Kuball J, Ho WY, Nguyen H, Manley TJ, Bleakley M, et al. Activation-induced expression of CD137 permits detection, isolation, and expansion of the full repertoire of CD8+ T cells responding to antigen without requiring knowledge of epitope specificities. Blood. 2007 Jul 1;110(1):201–10. doi: 10.1182/blood-2006-11-056168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (29).Kern F, Surel IP, Faulhaber N, Frommel C, Schneider-Mergener J, Schonemann C, et al. Target structures of the CD8(+)-T-cell response to human cytomegalovirus: the 72-kilodalton major immediate-early protein revisited. J Virol. 1999 Oct;73(10):8179–84. doi: 10.1128/jvi.73.10.8179-8184.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (30).Vera JF, Brenner LJ, Gerdemann U, Ngo MC, Sili U, Liu H, et al. Accelerated production of antigen-specific T cells for preclinical and clinical applications using gas-permeable rapid expansion cultureware (G-Rex) J Immunother. 2010 Apr;33(3):305–15. doi: 10.1097/CJI.0b013e3181c0c3cb. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (31).Hinrichs CS, Borman ZA, Cassard L, Gattinoni L, Spolski R, Yu Z, et al. Adoptively transferred effector cells derived from naive rather than central memory CD8+ T cells mediate superior antitumor immunity. Proc Natl Acad Sci U S A. 2009 Oct 13;106(41):17469–74. doi: 10.1073/pnas.0907448106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (32).Hinrichs CS, Borman ZA, Gattinoni L, Yu Z, Burns WR, Huang J, et al. Human effector CD8+ T cells derived from naive rather than memory subsets possess superior traits for adoptive immunotherapy. Blood. 2011 Jan 20;117(3):808–14. doi: 10.1182/blood-2010-05-286286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (33).Hanley PJ, Cruz CR, Savoldo B, Leen AM, Stanojevic M, Khalil M, et al. Functionally active virus-specific T cells that target CMV, adenovirus, and EBV can be expanded from naive T-cell populations in cord blood and will target a range of viral epitopes. Blood. 2009 Aug 27;114(9):1958–67. doi: 10.1182/blood-2009-03-213256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (34).Quintarelli C, Dotti G, De AB, Hoyos V, Mims M, Luciano L, et al. Cytotoxic T lymphocytes directed to the preferentially expressed antigen of melanoma (PRAME) target chronic myeloid leukemia. Blood. 2008 Sep 1;112(5):1876–85. doi: 10.1182/blood-2008-04-150045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (35).Rezvani K, Yong AS, Mielke S, Savani BN, Jafarpour B, Eniafe R, et al. Lymphodepletion is permissive to the development of spontaneous T-cell responses to the self-antigen PR1 early after allogeneic stem cell transplantation and in patients with acute myeloid leukemia undergoing WT1 peptide vaccination following chemotherapy. Cancer Immunol Immunother. 2012 Jul;61(7):1125–36. doi: 10.1007/s00262-011-1187-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (36).Heslop HE, Slobod KS, Pule MA, Hale GA, Rousseau A, Smith CA, et al. Long-term outcome of EBV-specific T-cell infusions to prevent or treat EBV-related lymphoproliferative disease in transplant recipients. Blood. 2010 Feb 4;115(5):925–35. doi: 10.1182/blood-2009-08-239186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (37).Leen AM, Myers GD, Sili U, Huls MH, Weiss H, Leung KS, et al. Monoculture-derived T lymphocytes specific for multiple viruses expand and produce clinically relevant effects in immunocompromised individuals. Nat Med. 2006 Oct;12(10):1160–6. doi: 10.1038/nm1475. [DOI] [PubMed] [Google Scholar]
- (38).Goodyear O, Agathanggelou A, Novitzky-Basso I, Siddique S, McSkeane T, Ryan G, et al. Induction of a CD8+ T-cell response to the MAGE cancer testis antigen by combined treatment with azacitidine and sodium valproate in patients with acute myeloid leukemia and myelodysplasia. Blood. 2010 Sep 16;116(11):1908–18. doi: 10.1182/blood-2009-11-249474. [DOI] [PubMed] [Google Scholar]
- (39).Bhatla T, Wang J, Morrison DJ, Raetz EA, Burke MJ, Brown P, et al. Epigenetic reprogramming reverses the relapse-specific gene expression signature and restores chemosensitivity in childhood B-lymphoblastic leukemia. Blood. 2012 May 31;119(22):5201–10. doi: 10.1182/blood-2012-01-401687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (40).Cruz CR, Gerdemann U, Leen AM, Shafer JA, Ku S, Tzou B, et al. Improving T-cell therapy for relapsed EBV-negative Hodgkin lymphoma by targeting upregulated MAGE A4. Clin Cancer Res. 2011 Nov 15;17(22):7058–66. doi: 10.1158/1078-0432.CCR-11-1873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (41).Handgretinger R, Zugmaier G, Henze G, Kreyenberg H, Lang P, von SA. Complete remission after blinatumomab-induced donor T-cell activation in three pediatric patients with post-transplant relapsed acute lymphoblastic leukemia. Leukemia. 2011 Jan;25(1):181–4. doi: 10.1038/leu.2010.239. [DOI] [PubMed] [Google Scholar]
- (42).Topp MS, Kufer P, Gokbuget N, Goebeler M, Klinger M, Neumann S, et al. Targeted therapy with the T-cell-engaging antibody blinatumomab of chemotherapy-refractory minimal residual disease in B-lineage acute lymphoblastic leukemia patients results in high response rate and prolonged leukemia-free survival. J Clin Oncol. 2011 Jun 20;29(18):2493–8. doi: 10.1200/JCO.2010.32.7270. [DOI] [PubMed] [Google Scholar]
- (43).Keilholz U, Letsch A, Busse A, Asemissen AM, Bauer S, Blau IW, et al. A clinical and immunologic phase 2 trial of Wilms tumor gene product 1 (WT1) peptide vaccination in patients with AML and MDS. Blood. 2009 Jun 25;113(26):6541–8. doi: 10.1182/blood-2009-02-202598. [DOI] [PubMed] [Google Scholar]
- (44).Hung K, Hayashi R, Lafond-Walker A, Lowenstein C, Pardoll D, Levitsky H. The central role of CD4(+) T cells in the antitumor immune response. J Exp Med. 1998 Dec 21;188(12):2357–68. doi: 10.1084/jem.188.12.2357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (45).Marzo AL, Kinnear BF, Lake RA, Frelinger JJ, Collins EJ, Robinson BW, et al. Tumor-specific CD4+ T cells have a major “post-licensing” role in CTL mediated anti-tumor immunity. J Immunol. 2000 Dec 1;165(11):6047–55. doi: 10.4049/jimmunol.165.11.6047. [DOI] [PubMed] [Google Scholar]
- (46).Maslak PG, Dao T, Krug LM, Chanel S, Korontsvit T, Zakhaleva V, et al. Vaccination with synthetic analog peptides derived from WT1 oncoprotein induces T-cell responses in patients with complete remission from acute myeloid leukemia. Blood. 2010 Jul 15;116(2):171–9. doi: 10.1182/blood-2009-10-250993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (47).Guo Y, Niiya H, Azuma T, Uchida N, Yakushijin Y, Sakai I, et al. Direct recognition and lysis of leukemia cells by WT1-specific CD4+ T lymphocytes in an HLA class II-restricted manner. Blood. 2005 Aug 15;106(4):1415–8. doi: 10.1182/blood-2005-01-0413. [DOI] [PubMed] [Google Scholar]
- (48).Knutson KL, Disis ML. Tumor antigen-specific T helper cells in cancer immunity and immunotherapy. Cancer Immunol Immunother. 2005 Aug;54(8):721–8. doi: 10.1007/s00262-004-0653-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (49).Gao L, Bellantuono I, Elsasser A, Marley SB, Gordon MY, Goldman JM, et al. Selective elimination of leukemic CD34(+) progenitor cells by cytotoxic T lymphocytes specific for WT1. Blood. 2000 Apr 1;95(7):2198–203. [PubMed] [Google Scholar]
- (50).Asemissen AM, Keilholz U, Tenzer S, Muller M, Walter S, Stevanovic S, et al. Identification of a highly immunogenic HLA-A*01-binding T cell epitope of WT1. Clin Cancer Res. 2006 Dec 15;12(24):7476–82. doi: 10.1158/1078-0432.CCR-06-1337. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.