Abstract
Recent applications of culture-independent tools for microbiome profiling have revealed significant relationships between asthma and microbiota associated with the environment, gut or airways. Studies of the airway microbiome in particular represent a new frontier in pulmonary research. Although these studies are relatively new, current evidence suggests the possibility of new therapeutic strategies for the treatment or prevention of asthma. In this article, recent literature on microbiota and asthma are critically reviewed, with a particular focus on studies of the airway microbiome. Perspectives are presented on how growing knowledge of relationships between the microbiome and asthma is likely to translate into improved understanding of asthma pathogenesis, its heterogeneity, and opportunities for novel treatment approaches.
Keywords: Asthma, Microbiome, 16S ribosomal RNA, Microarray, Sequencing, Therapy, Gut microbiome, Respiratory microbiota, Treatment-resistant asthma
Introduction
The potential relationships between microbial exposures, infections, and asthma have long been of and continue to stimulate much scientific interest. Areas of investigation have spanned from the role of early life microbial exposures and gut microbial colonization in the development of allergy or asthma [1–6], to studies of airway infections in established asthma [7–12]. Historically, approaches for characterizing microbial exposures or infection patterns in samples of interest have included serologic studies, microbial cultures or microorganism-specific molecular tests.
In recent years, more detailed analysis of total microbial community composition has been made possible by the development of culture-independent techniques with greater sensitivity in detecting microbial species, in particular bacteria. Conventional culture methods have low sensitivity in bacterial detection for several reasons, including organisms that are fastidious to grow or are as yet non-culturable. However, a commonly applied culture-independent approach to detect bacteria takes advantage of features of broadly conserved bacterial genes to detect species in a sample using polymerase chain reaction amplification, without need for prior knowledge of the specific bacterial composition present. Coupled with high-throughput profiling platforms such as microarrays and next-generation sequencing, such culture-independent techniques have enabled much deeper characterization of microbiota associated with specific environments, including mammalian hosts. Such studies have generated unprecedented insight into the potential complexity and diversity of microbial communities that exist in the environment and within humans [13,14].
These developments have led to a new or re-direction of efforts to understand relationships between asthma and microbial exposures or infections. These include studies of microbiota in samples derived from different living environments [5,15]. Among human niches, the gut has been the most extensively studied, and relationships between gut microbiota and allergy or asthma have also been examined [2,6,16,17]. More recently, investigations of the airway microbiome in pulmonary health or disease have emerged (11–12, 18–22), advancing our knowledge about the diversity of airway microbiota associated with obstructive lung diseases. Ultimately the goal in human microbiome studies is to achieve an integrative understanding of how the “microbiome” – defined as the totality of microbes, their genetic elements and interactions in a given niche – contributes to the development or persistence of relevant diseases. Despite the nascency of microbiome-focused research in asthma, recently gathered insights are generating new hypotheses about the role of microbiota in the development, persistence or heterogeneity of asthma.
The overall purpose of this article is to discuss ways in which growing knowledge about relationships between specific microbiomes and asthma may lead to the development of new therapeutic strategies. A brief overview of technologies and related considerations in microbiome studies will first be discussed, followed by a summary of recent asthma-focused microbiome studies. Select studies on the environmental and gut microbiota in asthma are highlighted, as relevant reviews in these areas are available [15, 23–26]. Recent airway microbiome studies of asthma will be discussed in greater detail. Finally, perspectives will be offered on how current and anticipated future knowledge about microbiota associated with asthma could translate into improved and/or novel treatment strategies for asthma.
Technologies for microbiome studies
The vast majority of microbiome studies to date have focused on studies of bacteria. This is due in large part to well-developed techniques based on analysis of the 16S ribosomal RNA (rRNA) gene, a highly conserved locus of the bacterial genome. Properties of this gene that facilitate bacterial community analysis are the presence of broadly conserved sequences, which flank a number of hypervariable regions. Universal primers targeting conserved sequences can be used to amplify the 16S rRNA gene, and polymorphisms within the hypervariable regions allow for phylogenetic identification of species using existing large 16S rRNA gene sequence databases. A number of 16S rRNA-based molecular tools exist, with higher resolution bacterial community profiling achieved primarily with 16S rRNA-based phylogenetic microarray or next-generation sequencing approaches [27–33]. The common theme is that these tools enable interrogation of a sample without a priori expectation of the bacteria present, such that fastidious or difficult to culture species also can be detected. The diversity of bacterial species known to exist is great and reflected in current bacterial taxonomies (i.e. phylogenetic classification of organisms). As a starting reference point, taxonomic classification for some of the major bacterial groups comprising the human microbiome are shown in Table 1.
Table 1.
Examples of taxonomic classification for some of the major bacterial groups identified as members of the human microbiome, including the respiratory tract. Given the diversity of human microbiota, the table is not intended to be comprehensive. Listed bacterial phyla, classes, orders, families and genera include organisms that have been identified in lung microbiome studies.
| Phylum | ACTINOBACTERIA | BACTEROIDETES | FIRMICUTES | PROTEOBACTERIA |
|---|---|---|---|---|
| Classes | Actinobacteria | Bacteroidia Cytophagia Flavobacteria Sphingobacteria | Bacilli Clostridia Erysipelotrichia | Alphaproteobacteria Betaproteobacteria Deltaproteobacteria Epsilonproteobacteria Gammaproteobacteria |
| Orders | Acidimicrobiales Actinomycetales Bifidobacteriales Rubrobacterales | Bacteroidales Flavobacteriales Sphingobacteriales | Bacillales Clostridiales Lactobacillales | Burkholderiales Campylobacterales Enterobacteriales Moraxellaceae Neisseriales Pasteurellales Pseudomonadales Sphingomonadales |
| Families | Actinomycetaceae Bifidobacteriaceae Corynebacteriaceae Mycobacteriaceae Nocardiaceae Streptomycetaceae | Bacteroidaceae Flavobacteriaceae Porphyromonadaceae Prevotellaceae Sphingobacteriaceae | Bacillaceae Clostridiaceae Enterococcaceae Lachnospiraceae Lactobacillaceae Ruminococcaeae Streptoccocaceae Veillonellaceae | Bartonellaceae Burkholderiaceae Enterobacteriaceae Helicobacteraceae Neisseriaceae Pasteurellaceae Pseudomonadaceae Sphingomonadaceae |
| Genera | Actinomyces Bifidobacterium Corynebacterium Mycobacterium Nocardia Streptomyces | Bacteroides Capnocytophaga Porphyromonas Prevotella Sphingobacterium | Clostridium Faecalibacterium Lachnospira Ruminococcus Streptococcus Veillonella | Acinetobacter Escherichia Enterobacter Haemophilus Moraxella Pseudomonas Sphingomonas |
As with any biologic technique, available tools to study the microbiome each have respective advantages and disadvantages. Important considerations in interpreting results of microbiome studies include the depth of community characterization achieved. For example, in sequencing-based studies, the number of quality-filtered reads analyzed on a per sample basis can be important depending on the complexity of a sample type. Lower numbers of analyzed reads generally provide less community resolution, which can be tested statistically. For further insight various reviews have been published that discuss these considerations and also describe other approaches for bacterial microbiome studies [31–33].
Finally, it is important to note that sample collection, processing and preparation methods also can affect the community composition characterized. Biases can be introduced at multiple steps including environmental or reagent sources of contamination, the variable efficiency of DNA extraction methods as some organisms are more difficult to lyse [34], and choice of 16S rRNA primers for PCR reactions. In particular, 16S rRNA sequencing protocols often utilize primers that target specific hypervariable region(s) of the gene. It is recognized that certain hypervariable regions do not capture well particular bacterial groups or species. Thus certain organisms may not be identified even if present in a sample [35–36]. The relative importance of these and other technical considerations will depend on the research questions, including the specific microbiome and types of samples to be studied, as well as potential bacterial groups of interest.
Environmental microbiota and asthma
A number of environmental factors have been associated with either risk for or protection against asthma or allergic sensitization (Figure 1). Microbial exposures can be invoked as plausible mechanisms for many of these associations. For example, subjects living in environments that afford greater exposure to animals (dogs, cats, farm animals) generally have lower risks for allergy or asthma [1, 3–5, 15, 37]. Raw milk consumption also has been associated with decreased atopy or asthma in childhood [38], while antibiotic use during pregnancy or perinatally is linked to increased risk for asthma-related outcomes [39]. However, meta-analyses of pooled studies have also suggested less strong or even absent associations of animal exposure and antibiotic use with asthma [40–42].
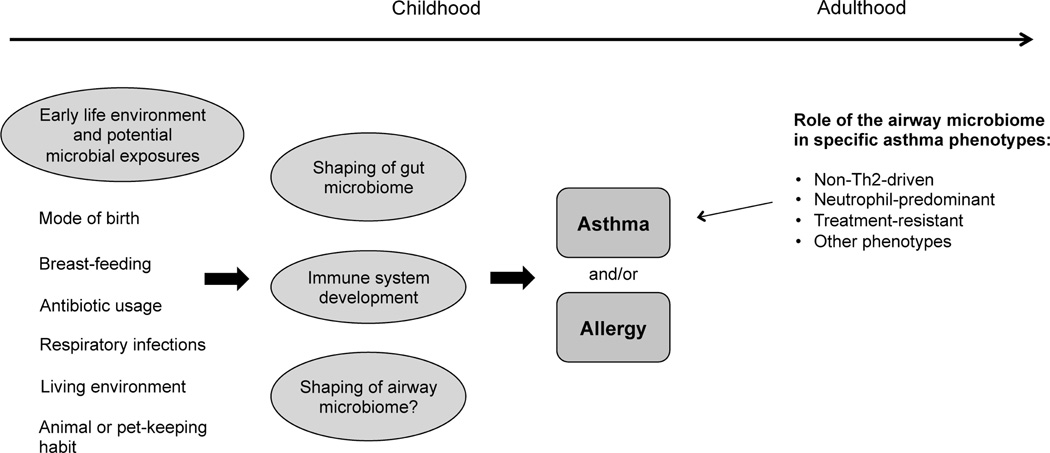
Figure 1.
Areas in which microbiota are likely to have an influential role in asthma. Microbial exposures associated with early life environmental factors contribute to risks for developing allergy, early onset asthma, or both. Among adults with asthma, the airway microbiome may be most influential in phenotypes associated with predominantly non-Th2 patterns of airway inflammation.
Studies in which indicators of microbial load or diversity have been measured, support the argument that environmental microbiota are likely an important mediator, if not a causal factor, in asthma development. Levels of endotoxin (produced by gram-negative bacteria) in mattress dust are inversely related to atopic asthma and sensitization in children [4]. Culture-independent surveys of bacteria and fungi in mattress and settled bedroom dust also suggest that microbial diversity is negatively associated with asthma risk [5]. Collectively these findings lead to speculation about how microbial exposures from one’s environment may modify risk for asthma in early life [15]. As one possibility, early evidence from the PASTURE study suggests microbial exposures may influence DNA methylation patterns over the first few years of life [43]. Thus stimuli from environmental microbiota could potentially shape expression of host genes and/or immune responses without necessarily effecting changes in host microbiota composition.
Gut microbiome and asthma
Gut microbiota provide antigenic stimulation to the immune system, educating its development in early life [23, 24]. Thus the composition of the gut microbiota can play an important role in shaping immune phenotype [25–26, 44]. Earlier studies of infant stool specimens have found that gut colonization patterns within the first three months of life differed between infants who did or did not develop allergic sensitization at 12 months of age [2]. Specifically more Clostridia and fewer Bifidobacteria species were identified from atopic children compared to non-atopic children. Different species within a specific bacterial family also may have different immune-stimulatory effects, as has been reported for Bifidobacteria as well as Lactobacilli [16, 45]. For example among breast-fed infants, Bifidobacterium bifidum was the main Bifidobacterium species found in fecal specimens from non-allergic infants, while B. adolescentis and B. longum were more prevalent in those who developed allergy [16].
Recent studies have implicated other bacterial species or bacterial diversity in the gut with the development of asthma [6, 17]. In a prospective study of 117 children classified by the Asthma Predictive Index (API), the prevalence of Bacteroides fragilis and other anaerobic bacteria cultured from fecal samples taken at three weeks of age was higher in API-positive vs. API-negative subjects [17]. In a birth cohort study of 411 children at high-risk for asthma, stool samples collected at one and twelve months after birth were analyzed by 16S rRNA-based denaturing gradient gel electrophoresis (DGGE) and also by conventional cultures [6]. Reduced bacterial diversity, as estimated from DGGE band analysis, was inversely associated with allergic sensitization in the first six years of life, though not with the development of asthma. Collectively, evidence from studies of gut and environmental microbiota indicate that decreased exposure to a diversity of microbes, including specific microbial consortia, have negative implications for immune health that affect risks for allergy and asthma.
Insights from Animal Studies of the Microbiome
Studies described up to this point have been human investigations. Recent studies in animal models are noteworthy for their demonstration that gut microbiota are important drivers of immune responses that can modulate allergic inflammation and features of asthma in the airways [46–48]. For example, germ-free compared to specific pathogen-free mice exhibit increased accumulation of mucosal invariant natural killer T cells (iNKT) in the colonic lamina propria as well as in the lung [46]. These germ-free mice, in an ovalbumin-model of allergic asthma, also demonstrated increased airway resistance and eosinophils in lung lavage and tissue. Feeding of conventional microbiota to neonatal, but not adult, germ-free mice prevented iNKT cell accumulation in the lungs and mitigated any evidence of allergic inflammation and asthma. In another study, administration of vancomycin to neonatal mice led to reduced bacterial diversity and similarly enhanced susceptibility to allergic asthma in a murine model [48]. Finally, several mouse studies have demonstrated that oral administration of bacterial species with immunomodulatory properties can modulate features of allergic asthma in the lungs. These include oral supplementation with specific Bifidobacterium and Lactobacillus species, which have been shown to reduce Th2-cytokine production, eosinophilic inflammation and/or promote T-regulatory or Th17 immune responses [49–52]. Findings from these and other studies suggest that interventions delivered via the gut during a critical window of time may alter susceptibility to or attenuate allergic asthma.
Respiratory microbiota and asthma
Previous studies of respiratory bacterial infections and risk for asthma
Acute respiratory infections are known triggers of asthma exacerbation, but evidence of chronic airway colonization or infection by specific organisms also have been linked to asthma. Earlier studies, often based on serologic tests, suggested relationships between Mycoplasma pneumoniae and Chlamydophila pneumoniae infections and the onset of asthma [53–55]. Subsequent studies have applied PCR- based approaches to more directly evaluate the presence of these bacteria in respiratory specimens [7, 9]. In a study of 95 subjects with asthma and 58 healthy controls, C. pneumoniae was more frequently detected by PCR and/or immunoglobulin measurements in induced sputum from asthmatics, particularly among those with poorly controlled or non-atopic asthma [9]. Species-targeted PCR tests of endobronchial biopsies or bronchoalveolar lavage fluid found evidence of M. or C. pneumoniae in 56% of asthmatics, compared to only one healthy control [7]. However, despite many studies that have focused on M. pneumoniae or C. pneumoniae, their role in asthma remains inconclusive [56–58].
Recent studies have examined relationships between other airway-associated microbiota and asthma. In a study of 321 neonates, culture-based studies of hypopharyngeal samples collected at 1 month of age found that evidence of early upper airway colonization by Streptococcus pneumoniae, Haemophilus influenzae, and/or Moraxella catarrhalis, was significantly associated with persistent wheeze during the first 5 years of life, and with asthma at age 5 [10]. Blood eosinophils and total IgE at 4 years of age also were significantly higher in those with early colonization. These same species have been associated with acute wheezing episodes in children (overall odds ratio 2.9), independent of a similar association found with viral infections [59].
Recent studies of respiratory microbiota and asthma
Studies published within the past three years have begun to apply 16S rRNA-based sequencing and microarray techniques to profile more deeply airway microbiota and investigate their potential relationships to asthma. Results from these studies collectively indicate that the composition of bacterial microbiota detected from the lower respiratory tract differ in those with asthma compared to healthy subjects [11, 12, 60]. Whether a resident community of microbiota exists in the lower airways of healthy individuals, however, is unclear. A low level of bacterial burden is detectable, the composition of which tends to be similar to bacterial groups typically associated with the oropharyngeal niche [20]. Even with rigorous efforts to minimize oral contamination of lower airway specimens during their collection, it is challenging to discern whether detection of species traditionally viewed as oral flora represent carry-over contamination, transient microbial colonization from aspiration, or low-level permanent colonization. However, in the setting of obstructive airway diseases like asthma, the predominant bacterial communities associated with asthma have primarily represented bacterial groups that are not dominant members of the oral microbiome [12, 60].
One of the first studies to investigate the composition of bacterial airway microbiota among asthmatic patients utilized a traditional 16S rRNA clone ial sequencing approach to analyze respiratory samples from 24 adults (11 with asthma, 5 with COPD, 8 healthy controls) and 20 children (13 with difficult to control asthma, 7 healthy controls) [11]. Although this approach has much lower resolution in profiling bacterial communities compared to high-throughput microarray [61] or next-generation sequencing tools, members of the Proteobacteria phylum, in particular Haemophilus spp., were more commonly identified from bronchial brushings or lavage fluid from individuals with airway disease (asthma or COPD). In contrast, members of the Bacteroidetes phylum, such as Prevotella spp., were more frequently found in specimens from healthy subjects. All adult asthma and 60% of the COPD patients, however, were on inhaled corticosteroid therapies. Thus the impact of corticosteroid use on the findings is unclear. Potential differences in microbiota composition by airway geography also were explored in this study by comparing samples from the nasopharynx (NP), oropharynx and left upper lung lobe (LUL). In general NP bacterial communities were distinct from that found in the oropharynx or LUL. LUL communities also differed between patients with airway disease (asthma or COPD) versus healthy controls. Another limitation of this study was the grouping of COPD together with asthma patients in reported differences from healthy controls in airway bacterial community composition.
In a larger study of 75 adults including subjects with mild-moderate asthma and healthy controls, between-group differences in lower airway bacterial community composition also were observed on analysis of bronchial epithelial brushings [12]. A significant correlation between the severity of airway hyperresponsiveness and bacterial diversity also was found. This extended to the identification of approximately 100 bacterial taxa that were strongly correlated with this pathophysiologic feature of asthma (a taxon being defined here as a group of bacterial species with > 97% homology in their 16S rRNA gene sequences). These included members of the Proteobacteria phylum, including families containing organisms with pathogenic potential such as Pseudomonadaceae, Enterobacteriaceae, Burkholderiaceae, and Neisseriaceae. Other highly correlated taxa included species with known functional repertoires that could be hypothesized to contribute to asthma-related disease mechanisms [12]. All asthmatics examined in this study had mild to moderate asthma, but were also taking a standardized moderate dose of inhaled fluticasone therapy. Thus, like the earlier discussed study [11], the potential influence of inhaled corticosteroids on the findings is unknown. However, the depth of bacterial community resolution achieved in this study was much greater, using a 16S rRNA-based microarray platform with the capacity to detect both very low and high abundance bacterial communities in a sample with equal efficiency [61]. This study also found that asthmatic subjects who improved after six weeks of clarithromycin therapy had higher airway bacterial diversity at baseline, compared to those that did not respond to therapy. In summary this study was one of the first to describe relationships between particular clinical features of asthma and the bacterial airway microbiome, suggesting that members of the bronchial microbiota may exert important functional effects.
Results of a recent small study indicate that differences in the composition of bacterial airway microbiota that have been associated with asthma are likely related to the disease of asthma itself [60], and not entirely attributable to inhaled corticosteroid use. 16S rRNA pyrosequencing analysis of induced sputum collected from 10 non-asthmatic and 10 mild asthma patients, none of whom were regularly taking inhaled corticosteroid therapy, found Proteobacteria present in significantly higher proportions in asthmatic vs. non-asthmatic patients. Specific bacterial families found in higher relative abundance among asthmatics included Enterobacteriaceae and Neisseriaceae. Although only 60% of the quality-filtered sequence reads could be confidently assigned to a bacterial family using a well-known 16S rRNA database, calculated bacterial diversity also was higher in the asthmatic group. Despite limitations of a small sample size and potential biases in bacterial community profiling due to the sample processing procedures used, the findings overall are consistent with that of previous studies [11, 12] and provide further evidence that airway microbiome features associated with asthma in studies to date are likely due to the disease itself.
Finally, it clearly would be of interest to investigate other types of airway microbiota and their potential role in asthma, such as fungi. While there have been some efforts to characterize fungal microbiota in the context of asthma [5, 62], it is important to note that best molecular approaches to profile fungal microbiota in a global manner are still active areas of research. Moreover, there is a need for better fungal sequence reference databases.
Avenues for new therapeutic approaches
Despite the relatively recent development of lung microbiome research, particularly with respect to asthma, it is not premature to begin to consider how growing knowledge in this area could transform our understanding of asthma and translate into novel or improved treatment approaches. Asthma is heterogeneous, however, and a variety of asthma phenotypes and classification schemes have been proposed [63]. Since this heterogeneity likely reflects different underlying disease mechanisms, contributions of the microbiome may be different in the etiopathogenesis of asthma compared to in established asthma and specific phenotypes, as suggested in Figure 1. Thus, determining where in the spectrum of asthma heterogeneity microbiome contributions are most important would shape the development of microbiome-based approaches to asthma treatment.
Current evidence points towards three areas in which the microbiome may play an important role in asthma: 1) the development of asthma in childhood that tends to be linked to allergic sensitization, 2) asthma in adults that is not associated with Th2 inflammation, such as neutrophil-predominant patterns, and 3) treatment-resistant asthma such as to corticosteroid therapy. Possible microbiome-driven therapeutic strategies for each of these scenarios are proposed and discussed further below.
Early-life microbiome composition and asthma development
Evidence from environmental and gut microbiome studies strongly suggests that microbial factors shape allergic sensitization and asthma development in susceptible individuals. Hence early life interventions that promote a ‘healthy’ human microbiome constitution may have benefits that include the possibility of asthma prevention. Specific environmental interventions to increase exposure to microbial diversity may be impractical due to individual circumstances. Alternative strategies might include oral supplementation with prebiotic nutrients, specific live microbial species (probiotics), or microbiota-derived products, to promote a gut microbiome composition that induces tolerogenic immune responses [25, 44]. Though these ideas are not new, hurdles remain from scientific and regulatory standpoints to investigate and develop probiotic-based therapies [64]. As these issues are being addressed in the probiotics field, approaches that succeed in achieving the same goal but do not require delivery of viable organisms may be more tenable.
Neutrophil variant forms of asthma
A large proportion of adult asthma patients do not exhibit hallmarks of Th2-driven airway inflammation. Thus it is particularly plausible that the airway microbiome may be important in the pathogenesis of certain non-Th2-driven phenotypes of asthma. For example, although neutrophilia is commonly seen in conjunction with inhaled corticosteroid use [63], sustained colonization of the airways by specific microbial species could also independently promote neutrophilic inflammation. Evidence in support of this is the consistent finding across airway microbiome studies of increased prevalence of Proteobacteria in asthmatic patients. This large phylum encompasses many known pathogenic species responsible for both acute respiratory and gastrointestinal illnesses, including H. influenzae, Pseudomonas, Neisseria, and Burkholderia species, as well as the Enterobacteriaceae family. Enterobacteriaceae comprise a large family of species typically associated with the GI tract (Klebsiella pneumoniae being one exception) but that also have been identified in microbiome studies of COPD as well as asthma [12, 21]. Neutrophilic inflammation promoted by Proteobacterial species in the context of asthma has been shown. For example, H. influenzae infection in an ovalbumin-murine model of allergic asthma caused induction of Th17 immune responses that promoted neutrophilic but suppressed eosinophilic inflammation [65]. Moreover, evidence of chronic H. influenzae infection was found one month after inoculation, and the airway inflammation seen in H. influenzae-infected allergic mice was resistant to steroid treatment in contrast to the steroid-responsive decrease in inflammation seen in uninfected allergic mice [66]. Human dendritic cells also produce greater pro-inflammatory cytokines (IL-23, IL-12p70) in response to H. influenzae and M. catarrhalis, compared to exposure to commensal species such as Veillonella [67].
These studies indicate that airway infections by specific microbiota may induce, augment, or potentially even modify the predominant type of airway inflammation seen in asthma. Thus, a possible therapeutic strategy could be use of antimicrobials or vaccine therapies that target specific microbiota in asthmatics demonstrating evidence of such colonization. Conversely, microbiome-targeted treatment approaches could be construed from the perspective of how to promote a more functionally balanced airway microbiome, such that pathogenic microbiota are not able to exert dominant effects. This strategy has been studied and promulgated more in the gut microbiome literature [24–25, 68]. Studies of experimental respiratory infections also show that immune responses to pathogenic infections depend on the background microbiota present [69, 70]. Thus an understanding of microbiota that are negatively associated with features of asthma could be useful towards developing approaches to promote these microbiota, or specific functions they express that counteract detrimental inflammatory processes.
Treatment-Resistant Asthma
Treatment-resistant asthma is a particular challenge clinically often manifest in those with severe asthma. Novel therapeutic approaches are much needed. A number of factors may contribute to difficult to control asthma, including the possibility that prescribed therapies do not address underlying mechanisms of disease in patients. For example, inhaled corticosteroids are less effective in asthmatics without evidence of Th2-driven airway inflammation [71]. Another possible theory for treatment-resistant asthma is modulation of intended therapeutic effects by microbiota, whether at the host response level or of the therapy itself. Foundation for this proposed theory is based on hypothesized functions of microbiota we have noted in previous [12] and ongoing studies. Additional supportive evidence is extrapolated from the known capabilities of gut microbiota to transform therapeutic agents [72], and also from the field of bioremediation. Thus identification of microbiota that modulate responses to therapies may potentially lead to development of approaches that block these effects.
Conclusions
Studies of the lung microbiome using modern molecular tools are re-defining our perception and understanding of respiratory microbiota associated with asthma and other airway diseases. It is becoming increasingly clear that compared to healthy individuals, asthma is associated with a different lower airway microbiota composition. The asthmatic airway microbiome includes species with pathogenic potential as well as species with potential immunomodulatory or metabolic properties relevant to certain asthma-related disease mechanisms. As the field advances, it will be important to determine where microbiome contributions to asthma heterogeneity are significant. This would enable focused development of microbiome-driven therapies, such as manipulation of microbiota composition and/or their functional derivatives, as novel approaches to asthma treatment.
Acknowledgment
Yvonne J. Huang has received grant support from the National Institutes of Health.
Compliance with Ethics Guidelines
Conflict of Interest
Yvonne J. Huang has received grant support from Genentech.
Footnotes
Human and Animal Rights and Informed Consent
This article does not contain any studies with human or animal subjects performed by any of the authors.
References
Papers of particular interest, published recently, have been highlighted as:
• Of importance
- 1.Ownby DR, Johnson CC, Peterson EL. Exposure to dogs and cats in the first year of life and risk of allergic sensitization at 6 to 7 years of age. JAMA. 2002;288(8):963–972. doi: 10.1001/jama.288.8.963. [DOI] [PubMed] [Google Scholar]
- 2.Kalliomaki M, Kirjavainen P, Eerola E, et al. Distinct patterns of neonatal gut microflora in infants in whom atopy was and was not developing. J Allergy Clin Immunol. 2001;107:129–134. doi: 10.1067/mai.2001.111237. [DOI] [PubMed] [Google Scholar]
- 3.Havstad S, Wegienka G, Zoratti EM, et al. Effect of prenatal indoor pet exposure on the trajectory of total IgE levels in early childhood. J Allergy Clin Immunol. 2011;128:880–885. doi: 10.1016/j.jaci.2011.06.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Braun-Fahrländer C, Riedler J, Herz U, et al. Allergy and Endotoxin Study Team. Environmental exposure to endotoxin and its relation to asthma in school-age children. N Engl J Med. 2002;347(12):869–877. doi: 10.1056/NEJMoa020057. [DOI] [PubMed] [Google Scholar]
- 5.Ege MJ, Mayer M, Normand AC, et al. Exposure to environmental microorganisms and childhood asthma. N Engl J Med. 2011;364(8):701–709. doi: 10.1056/NEJMoa1007302. [DOI] [PubMed] [Google Scholar]
- 6.Bisgaard H, Li N, Bonnelykke K, et al. Reduced diversity of the intestinal microbiota during infancy is associated with increased risk of allergic disease at school age. J Allergy Clin Immunol. 2011;128(3):646–652. e1–e5. doi: 10.1016/j.jaci.2011.04.060. [DOI] [PubMed] [Google Scholar]
- 7.Martin RJ, Kraft M, Chu HW, et al. A link between chronic asthma and chronic infection. J Allergy Clin. Immunol. 2001;107(4):595–601. doi: 10.1067/mai.2001.113563. [DOI] [PubMed] [Google Scholar]
- 8.Kocabas A, Avsar M, Hanta I, et al. Chlamydophila pneumoniae infection in adult asthmatics patients. J. Asthma. 2008;45(1):39–43. doi: 10.1080/02770900701815735. [DOI] [PubMed] [Google Scholar]
- 9.Specjalski K, Jassem E. Chlamydophila pneumoniae, Mycoplasma pneumoniae infections, and asthma control. Allergy Asthma Proc. 2011;32(2):9–17. doi: 10.2500/aap.2011.32.3431. [DOI] [PubMed] [Google Scholar]
- 10.Bisgaard H, Hermansen MN, Buchvald F, et al. Childhood asthma after bacterial colonization of the airway in neonates. N Engl J Med. 2007;357(15):1487–1495. doi: 10.1056/NEJMoa052632. [DOI] [PubMed] [Google Scholar]
- 11.Hilty M, Burke C, Pedro H, et al. Disordered microbial communities in asthmatic airways. PLoS One. 2010;5(1):e8578. doi: 10.1371/journal.pone.0008578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Huang YJ, Nelson CE, Brodie EL, et al. Airway microbiota and bronchial hyperresponsiveness in patients with suboptimally controlled asthma. J Allergy Clin Immunol. 2011;127:372–381. e1–e3. doi: 10.1016/j.jaci.2010.10.048. This paper is the first to describe relationships between bacterial airway microbiota composition and clinical features of asthma, including airway hyperreactivity and response to prolonged clarithromycin therapy.
- 13.Sogin ML, Morrison HG, Huber JA, et al. Microbial diversity in the deep sea and the underexplored "rare biosphere". Proc Natl Acad Sci USA. 2006;103(32):12115–12120. doi: 10.1073/pnas.0605127103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Human Microbiome Jumpstart Reference Strains Consortium. Nelson KE, Weinstock GM, Highlander SK, et al. A catalog of reference genomes from the human microbiome. Science. 2010;328(5981):994–999. doi: 10.1126/science.1183605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Heederik Dick, Erika von Mutius. Does diversity of environmental microbial exposure matter for the occurrence of allergy and asthma? J Allergy Clin Immunol. 2012;130(1):44–50. doi: 10.1016/j.jaci.2012.01.067. This review provides an excellent summary of recent environmental microbiome studies in asthma.
- 16.Ouwehand AC, Isolauri E, He F, et al. Differences in Bifidobacterium flora composition in allergic and healthy infants. J Allergy Clin Immunol. 2001;108:144–145. doi: 10.1067/mai.2001.115754. [DOI] [PubMed] [Google Scholar]
- 17.Vael C, Nelen V, Verhulst SL, et al. Early intestinal Bacteroides fragilis colonisation and development of asthma. BMC Pulm Med. 2008;8:19. doi: 10.1186/1471-2466-8-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Rogers GB, Carroll MP, Serisier DJ, et al. Characterization of bacterial community diversity in cystic fibrosis lung infections by use of 16s ribosomal DNA terminal restriction fragment length polymorphism profiling. J Clin Microbiol. 2004;42(11):5176–5183. doi: 10.1128/JCM.42.11.5176-5183.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Harris JK, De Groote MA, Sagel SD, et al. Molecular identification of bacteria in bronchoalveolar lavage fluid from children with cystic fibrosis. Proc Natl Acad Sci USA. 2007;104(51):20529–20533. doi: 10.1073/pnas.0709804104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Charlson ES, Bittinger K, Haas AR, et al. Topographical continuity of bacterial populations in the healthy human respiratory tract. Am J Respir Crit Care Med. 2011;184:957.e63. doi: 10.1164/rccm.201104-0655OC. This study of lung microbiota in healthy subjects using detailed sampling procedures addresses issues of oral and environmental sources of contamination in lung microbiome studies.
- 21.Huang YJ, Kim E, Cox MJ, et al. A persistent and diverse airway microbiota present during chronic obstructive pulmonary disease exacerbations. OMICS. 2010;14(1):9–59. doi: 10.1089/omi.2009.0100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Erb-Downward JR, Thompson DL, Han MK, et al. Analysis of the lung microbiome in the ‘healthy’ smoker and in COPD. PLoS One. 2011;6(2):e16384. doi: 10.1371/journal.pone.0016384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Abt MC, Artis D. The intestinal microbiota in health and disease: the influence of microbial products on immune cell homeostasis. Curr Opin Gastroenterol. 2009;25(6):496–502. doi: 10.1097/MOG.0b013e328331b6b4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Fujimura KE, Slusher NA, Cabana MD, et al. Role of the gut microbiota in defining human health. Expert Rev Anti Infect Ther. 2010;8(4):435–454. doi: 10.1586/eri.10.14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. McLoughlin RM, Mills KH. Influence of gastrointestinal commensal bacteria on the immune responses that mediate allergy and asthma. J Allergy Clin Immunol. 2011;127:1097–1107. doi: 10.1016/j.jaci.2011.02.012. This article provides an excellent summary of immunomodulatory effects of gut microbiota and their potential role in the development of allergy and asthma.
- 26.Kozyrskyj AL, Bahreinian S, Azad MB. Early life exposures: impact on asthma and allergic disease. Curr Opin Allergy Clin Immunol. 2011 Oct;11(5):400–406. doi: 10.1097/ACI.0b013e328349b166. [DOI] [PubMed] [Google Scholar]
- 27.Brodie EL, Desantis TZ, Joyner DC, et al. Application of a high-density oligonucleotide microarray approach to study bacterial population dynamics during uranium reduction and reoxidation. Appl Environ Microbiol. 2006;72:6288e98. doi: 10.1128/AEM.00246-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.DeSantis TZ, Brodie EL, Moberg JP, et al. High-density universal 16S rRNA microarray analysis reveals broader diversity than typical clone library when sampling the environment. Microb Ecol. 2007;53:371e83. doi: 10.1007/s00248-006-9134-9. [DOI] [PubMed] [Google Scholar]
- 29.Rajilić-Stojanović M, Heilig HG, Molenaar D, et al. Development and application of the human intestinal tract chip, a phylogenetic microarray: analysis of universally conserved phylotypes in the abundant microbiota of young and elderly adults. Environ Microbiol. 2009;11(7):1736–1751. doi: 10.1111/j.1462-2920.2009.01900.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Hazen TC, Dubinsky EA, DeSantis TZ, et al. Deep-sea oil plume enriches indigenous oil-degrading bacteria. Science. 2010;330(6001):204–208. doi: 10.1126/science.1195979. This article includes detailed description of a new generation 16S rRNA-based microarray platform and its deep profiling capabilities for bacterial microbiome studies.
- 31.Hamady M, Knight R. Microbial community profiling for human microbiome projects: tools, techniques, and challenges. Genome Res. 2009;19:1141e52. doi: 10.1101/gr.085464.108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Han MK, Huang YJ, Lipuma JJ, et al. Significance of the microbiome in obstructive lung disease. Thorax. 2012;67(5):456–463. doi: 10.1136/thoraxjnl-2011-201183. This review summarizes recent lung microbiome studies of different diseases including an overview of molecular techniques for microbiome profiling.
- 33. Loman NJ, Constantinidou C, Chan JZ, et al. High-throughput bacterial genome sequencing: an embarrassment of choice, a world of opportunity. Nat Rev Microbiol. 2012;10(9):599–606. doi: 10.1038/nrmicro2850. An excellent summary of next-generation sequencing technologies for microbial genome sequencing with discussion of related considerations and challenges.
- 34. Yuan S, Cohen DB, Ravel J, et al. Evaluation of Methods for the Extraction and Purification of DNA from the Human Microbiome. PLoS ONE. 2012;7(3):e33865. doi: 10.1371/journal.pone.0033865. This study highlights the effects of different sample extraction methods on bacterial community characterization by next-generation sequencing.
- 35.Soergel DA, Dey N, Knight R, et al. Selection of primers for optimal taxonomic classification of environmental 16S rRNA gene sequences. ISME J. 2012;6(7):1440–1444. doi: 10.1038/ismej.2011.208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Kumar PS, Brooker MR, Dowd SE, et al. Target Region Selection Is a Critical Determinant of Community Fingerprints Generated by 16S Pyrosequencing. PLoS ONE. 2011;6(6):e20956. doi: 10.1371/journal.pone.0020956. This study discusses differences in bacterial community profiles generated by pyrosequencing using primers targeting different hypervariable regions of the 16S rRNA.
- 37.Ege MJ, Mayer M, Schwaiger K, et al. Environmental bacteria and childhood asthma. Allergy. 2012 Dec;67(12):1565–1571. doi: 10.1111/all.12028. [DOI] [PubMed] [Google Scholar]
- 38.Loss G, Apprich S, Waser M, et al. The protective effect of farm milk consumption on childhood asthma and atopy: the GABRIELA study. J Allergy Clin Immunol. 2011;128:766–773e4. doi: 10.1016/j.jaci.2011.07.048. [DOI] [PubMed] [Google Scholar]
- 39.Stensballe LG, Simonsen J, Jensen SM, et al. Use of Antibiotics during Pregnancy Increases the Risk of Asthma in Early Childhood. J Pediatr. 2013;162(4):832.e3–838.e3. doi: 10.1016/j.jpeds.2012.09.049. [DOI] [PubMed] [Google Scholar]
- 40.Lodge CJ, Allen KJ, Lowe AJ, et al. Perinatal cat and dog exposure and the risk of asthma and allergy in the urban environment: a systematic review of longitudinal studies. Clin Dev Immunol. 2012;2012:176484. doi: 10.1155/2012/176484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Lødrup Carlsen KC, Roll S, et al. Does Pet Ownership in Infancy Lead to Asthma or Allergy at School Age? Pooled Analysis of Individual Participant Data from 11 European Birth Cohorts. PLoS ONE. 2012;7(8):e43214. doi: 10.1371/journal.pone.0043214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Penders J, Kummeling I, Thijs C. Infant antibiotic use and wheeze and asthma risk: a systematic review and meta-analysis. Eur Respir Jrnl. 2011;38(2):295–302. doi: 10.1183/09031936.00105010. [DOI] [PubMed] [Google Scholar]
- 43.Michel S, Busato F, Genuneit J, et al. Farm exposure and time trends in early childhood may influence DNA methylation in genes related to asthma and allergy. Allergy. 2013;68(3):355–364. doi: 10.1111/all.12097. [DOI] [PubMed] [Google Scholar]
- 44. Round JL, O'Connell RM, Mazmanian SK. Coordination of tolerogenic immune responses by the commensal microbiota. J Autoimmun. 2010;34(3):J220–J225. doi: 10.1016/j.jaut.2009.11.007. This review provides an excellent summary of immune tolerance mechanisms induced by microbiota.
- 45.Mileti E, Matteoli G, Iliev ID, et al. Comparison of the immunomodulatory properties of three probiotic strains of Lactobacilli using complex culture systems: prediction for in vivo efficacy. PLoS One. 2009;4(9):e7056. doi: 10.1371/journal.pone.0007056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Olszak T, An D, Zeissig S, et al. Microbial exposure during early life has persistent effects on natural killer T cell function. Science. 2012;336(6080):489–493. doi: 10.1126/science.1219328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Herbst T, Sichelstiel A, Schär C, Yadava K, Bürki K, Cahenzli J, McCoy K, Marsland BJ, Harris NL. Dysregulation of allergic airway inflammation in the absence of microbial colonization. Am J Respir Crit Care Med. 2011;184(2):198–205. doi: 10.1164/rccm.201010-1574OC. [DOI] [PubMed] [Google Scholar]
- 48.Russell SL, Gold MJ, Hartmann M, et al. Early life antibiotic-driven changes in microbiota enhance susceptibility to allergic asthma. EMBO Rep. 2012 May 1;13(5):440–447. doi: 10.1038/embor.2012.32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Karimi K, Inman MD, Bienenstock J, et al. Lactobacillus reuteri-induced regulatory T cells protect against an allergic airway response in mice. Am J Respir Crit Care Med. 2009;179:186–193. doi: 10.1164/rccm.200806-951OC. [DOI] [PubMed] [Google Scholar]
- 50.MacSharry J, O'Mahony C, Shalaby KH, et al. Immunomodulatory effects of feeding with Bifidobacterium longum on allergen-induced lung inflammation in the mouse. Pulm Pharmacol Ther. 2012;25(4):325–334. doi: 10.1016/j.pupt.2012.05.011. [DOI] [PubMed] [Google Scholar]
- 51.Harb H, van Tol EA, Heine H, et al. Neonatal supplementation of processed supernatant from Lactobacillus rhamnosus GG improves allergic airway inflammation in mice later in life. Clin Exp Allergy. 2013;43(3):353–364. doi: 10.1111/cea.12047. [DOI] [PubMed] [Google Scholar]
- 52.Zhang B, An J, Shimada T, Liu S, et al. Oral administration of Enterococcus faecalis FK-23 suppresses Th17 cell development and attenuates allergic airway responses in mice. Int J Mol Med. 2012 Aug;30(2):248–254. doi: 10.3892/ijmm.2012.1010. [DOI] [PubMed] [Google Scholar]
- 53.Hahn DL, Dodge RW, Golubjatnikov R. Association of Chlamydia pneumoniae (strain TWAR) infection with wheezing, asthmatic bronchitis, and adult-onset asthma. JAMA. 1991;266(2):225–230. [PubMed] [Google Scholar]
- 54.Yano T, Ichikawa Y, Komatu S, Arai S, Oizumi K. Association of Mycoplasma pneumoniae antigen with initial onset of bronchial asthma. Am J Respir Crit Care Med. 1994;149(5):1348–1353. doi: 10.1164/ajrccm.149.5.8173777. [DOI] [PubMed] [Google Scholar]
- 55.Ten Brinke A, Van Dissel JT, Sterk PJ, et al. Persistent airflow limitation in adult-onset nonatopic asthma is associated with serologic evidence of Chlamydia pneumoniae infection. J Allergy Clin Immunol. 2001;107(3):449–454. doi: 10.1067/mai.2001.113047. [DOI] [PubMed] [Google Scholar]
- 56.Johnston SL, Martin RJ. Chlamydophila pneumoniae and Mycoplasma pneumoniae: a role in asthma pathogenesis? Am J Respir Crit Care Med. 2005;172(9):1078–1089. doi: 10.1164/rccm.200412-1743PP. [DOI] [PubMed] [Google Scholar]
- 57.Metz G, Kraft M. Effects of atypical infections with Mycoplasma and Chlamydia on asthma. Immunol Allergy Clin North Am. 2010;30(4):575–585. vii–viii. doi: 10.1016/j.iac.2010.08.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Hong SJ. The role of Mycoplasma pneumoniae infection in asthma. Allergy Asthma Immunol Res. 2012;4(2):59–61. doi: 10.4168/aair.2012.4.2.59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Bisgaard H, Hermansen MN, Bonnelykke K, et al. Association of bacteria and viruses with wheezy episodes in young children: prospective birth cohort study. BMJ. 2010;341:c4978. doi: 10.1136/bmj.c4978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Marri PR, Stern DA, Wright AL, et al. Asthma-associated differences in microbial composition of induced sputum. J Allergy Clin Immunol. 2013;131(2):346.e3–352.e3. doi: 10.1016/j.jaci.2012.11.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.DeSantis TZ, Brodie EL, Moberg JP, et al. High-density universal 16S rRNA microarray analysis reveals broader diversity than typical clone library when sampling the environment. Microbial Ecology. 2007;53(3):371–383. doi: 10.1007/s00248-006-9134-9. [DOI] [PubMed] [Google Scholar]
- 62.van Woerden HC, Gregory C, Brown R, et al. Differences in fungi present in induced sputum samples from asthma patients and non-atopic controls: a community based case control study. BMC Infect Dis. 2013;13:69. doi: 10.1186/1471-2334-13-69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Wenzel SE. Asthma phenotypes: the evolution from clinical to molecular approaches. Nat Med. 2012 May 4;18(5):716–725. doi: 10.1038/nm.2678. This review discusses insights into asthma heterogeneity and phenotypes using combined clinical and molecular approaches to patient characterization.
- 64.Rauch M, Lynch SV. The potential for probiotic manipulation of the gastrointestinal microbiome. Curr Opin Biotechnol. 2012 Apr;23(2):192–201. doi: 10.1016/j.copbio.2011.11.004. [DOI] [PubMed] [Google Scholar]
- 65. Essilfie AT, Simpson JL, Horvat JC, et al. Haemophilus influenzae infection drives IL-17-mediated neutrophilic allergic airways disease. PLoS Pathog. 2011;7(10):e1002244. doi: 10.1371/journal.ppat.1002244. Interesting murine study showing modulation of eosinophilic allergic airway inflammation and asthma responses by acute infection with Haemophilus influenzae.
- 66. Essilfie AT, Simpson JL, Dunkley ML, et al. Combined Haemophilus influenzae respiratory infection and allergic airways disease drives chronic infection and features of neutrophilic asthma. Thorax. 2012;67:588–599. doi: 10.1136/thoraxjnl-2011-200160. Followup to previous study showing corticosteroid-resistant nature of inflammation resulting from H. influenzae-induced modulation of allergic airway disease.
- 67.Larsen JM, Steen-Jensen DB, Laursen JM, et al. Divergent pro-inflammatory profile of human dendritic cells in response to commensal and pathogenic bacteria associated with the airway microbiota. PLoS One. 2012;7(2):e31976. doi: 10.1371/journal.pone.0031976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Kinross JM, Darzi AW, Nicholson JK. Gut microbiome-host interactions in health and disease. Genome Med. 2011;3(3):14. doi: 10.1186/gm228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Henriksson G, Helgeland L, Midtvedt T, et al. Immune response to I in nasal mucosa is modulated by the normal microbiota. Am J Respir Cell Mol Biol. 2004;31(6):657–662. doi: 10.1165/rcmb.2004-0207OC. [DOI] [PubMed] [Google Scholar]
- 70.Ichinohe T, Pang IK, Kumamoto Y, et al. Microbiota regulates immune defense against respiratory tract influenza A virus infection. Proc Natl Acad Sci USA. 2011;108(13):5354–5359. doi: 10.1073/pnas.1019378108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Woodruff PG, Modrek B, Choy DF, et al. T-helper type 2-driven inflammation defines major subphenotypes of asthma. Am J Respir Crit Care Med. 2009;180(5):388–395. doi: 10.1164/rccm.200903-0392OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Wilson ID, Nicholson JK. The role of gut microbiota in drug response. Curr Pharm Des. 2009;15(13):1519–1523. doi: 10.2174/138161209788168173. [DOI] [PubMed] [Google Scholar]