Abstract
Objective
Obese subjects exhibit decreased exercise capacity (VO2max). We have shown that vascular KATP channel mediates arteriolar dilation to muscle contraction. We hypothesize that exercise capacity is decreased in obesity due to impaired vascular KATP function.
Methods
VO2max was measured in LZR and OZR by treadmill running before and following treatment with the KATP blocker glibenclamide i.p. One week later the spinotrapezius muscle was prepared for in vivo microscopy. Arcade arteriolar diameters were measured following muscle contraction or application of the KATP opener cromakalim before and after glibenclamide application. In additional animals, LZR and OZR were treated with apocynin for 5 weeks. VO2max and arteriolar dilation experiments were repeated.
Results
OZR exhibited decreased VO2max, functional and cromakalim-induced vasodilation as compared to LZR. Glibenclamide had no effect on VO2max and functional vasodilation in OZR but significantly inhibited responses in LZR. Vascular superoxide levels and NADPH oxidase activity were increased in OZR but reduced in apocynin-treated OZR. Apocynin increased the VO2max, functional and cromakalim-induced vasodilation in OZR with no effect in LZR.
Conclusion
Exercise capacity is dependent on vascular KATP channel function. The reduced exercise capacity in OZR appears to be due in part to superoxide-mediated impairment in vascular KATP function.
Keywords: Obese, vascular, NADPH oxidase, superoxide, KATP channels
INTRODUCTION
Exercise is a valuable non-pharmacological treatment for obesity-associated metabolic disorders and cardiovascular dysfunction. However, a major consequence of obesity is reduced exercise capacity (VO2max), which might limit adequate exercise training in obese individuals. Previous studies have shown that the obesity-related metabolic and cardiovascular dysfunctions are associated with the impaired local functional hyperemia during muscle contraction (24, 29, 30). However, the majority of these studies were performed in anesthetized animals, leaving concerns as to how the local functional hyperemic response parallels with exercise capacity.
KATP channels in vascular smooth muscle cells (VSMCs) are important in mediating functional vasodilation in rats (16, 22). However, to our knowledge, the contribution of vascular KATP channel function to the maximal exercise capacity (VO2max) has not been investigated in conscious subjects or animals. In obese subjects and animals the elevated basal levels of vascular reactive oxygen species may impair KATP channel function (19). Our previous studies showed that, as compared with LZR, OZR exhibited higher levels of vascular superoxide and NADPH oxidase enzyme activity (28) which was associated with impaired functional vasodilation (28). The superoxide-mediated impairment of functional vasodilation is partially due to an abnormal TP-mediated vasoconstriction (3, 28). Our previous study suggests that the blunted functional arteriolar vasodilation in OZR is also due to an impaired VSMC KATP channel function (16). However, whether the impaired exercise capacity in conscious obese animals is due to ROS-induced vascular KATP channel dysfunction has not been determined. We hypothesized that the exercise capacity is partially mediated by vascular KATP channel function, which is impaired in OZR due to elevated vascular superoxide. Thus the current study determined the contribution of KATP channels to the maximal oxygen consumption (VO2max) during treadmill exercise in both LZR and OZR. We also determined whether a chronic inhibition of superoxide levels in OZR would improve KATP channel function, leading to increased exercise capability.
METHODS
Animals and antioxidant treatment
Male LZR and OZR (12–14 wks old) were acquired from Harlan Laboratories. Experimental protocols for this study wereapproved by the Institutional Animal Care and Use Committeeof the University of Mississippi Medical Center and were carriedout according to both the National Institutes of Health Guide for the Care and Use of Laboratory Animals and guidelines ofthe Animal Welfare Act. Rats were housed 1–3 rats per cage at 22°C (12:12-h light-dark cycle) with free accessto food and water.
Half of the LZR and OZR were control animals and half were treated with apocynin (2 mM in drinking water, approximately 46 mg/kg/d) (1). Rats were treated for 5 weeks and then VO2max measurement was performed. Apocynin treatment was temporarily stopped one day before starting each experiment to avoid any acute effects. After the exercise experiments, the same rats were treated with apocynin until the microcirculatory experiments.
Oral glucose tolerance test (OGTT) and blood pressure measurements
The blood pressure and OGTT were performed in other sets groups with or with apocynin treatment for 5 weeks. LZR and OZR were fasted 6 hrs before giving glucose solution via gavage (50% glucose solution, 3 ml/kg). Blood samples were taken immediately before glucose application and then at 15, 30, 60, 120, and 180 min. Blood samples were collected from a tail snip, and glucose levels were measured using a OnCall Plus blood glucose monitoring system (ACON Laboratories, Inc., CA).
In a separate experiment, all rats were catheterized in right carotid and blood pressure was measured under basal conditions. In brief, rats were anesthetized with isoflurane inhalation, and a catheter with 10% heparin was implanted in the right carotid artery. After recovery from anesthesia, the rats were allowed to equilibrate for 6 h, and the baseline blood pressures were measured (31)
VO2max during treadmill exercise
VO2max was measured as previously described (24). Briefly, a Columbus Instruments metabolic treadmill was used to measure oxygen consumption. Rats ran in the metabolic cage on a treadmill at 15° incline. The running was started at 10 meter/minutes for 5 minutes for a warm up. Then the speed was increased by 2 m/min every 2 minutes during the treadmill running and the VO2 was recorded. VO2max was determined when oxygen consumption reached a plateau and was not affected by increasing the workload. The rats were allowed to rest 24-hours and the same VO2max protocol was performed after i.p. glibenclamide administration (10 mg/kg ; 30 min prior exercise). We have shown that this treatment method inhibits KATP activation (31).
Functional and cromakalim-induced arteriolar dilation in spinotrapezius muscle
The right spinotrapezius muscle was used for in vivo experiments (24) one week after VO2max experiment was performed. In brief, rats were anesthetized with sodium pentobarbital (65 mg/kg, i.p.). Animals spontaneously breathed a gas mixture containing 30% oxygen and 70% nitrogen. The right spinotrapezius muscles were kept at in situ dimensions and continuously superfused with a physiological salt solution (in mM): 118.07 NaCl, 6.17 KCl, 2.55 CaCl2, and 25 NaHCO3, equilibrated with gases containing 5% CO2, 0% O2 and a balance of N2 (pH = 7.4, 35°C). Animals were allowed to stabilize for 30 minutes after surgery. A 3rd order arcade arteriole segment was selected for analysis.
Two silver-silver chloride electrodes (Harvard Instruments) were attached at both ends of the spinotrapezius and connected to a Grass S44 stimulator to induce muscle contraction. Arteriole diameters were obtained in the resting muscle and immediately following 2 minutes of electrical stimulations (4–5V, 1 Hz). After a 15-min recovery period, cromakalim (0.01, 0.1, and 1 μM) was added to the superfusion solution, and steady-state vasodilatory responses were measured. After the arteriole had returned to its resting diameter (~15–20 min), the tissue was treated with glibenclamide (1 μM) for 30 min via superfusion solution, and then the muscle stimulation and cromakalim treatment protocols were repeated. At the end of the experiment adenosine (10 μM) and SNP (10 μM) were added to the superfusate to determine maximal diameter and calculate basal vascular tone. When the experiment was complete, animals were euthanized by an overdose of sodium pentobarbital, followed by a pneumothorax.
Vascular superoxide levels and NADPH oxidase activity
Superoxide levels in aorta were measured using dihydroethidium (DHE) fluorescence as described previously (28). Aortic segments were rinsed in a physiological salt solution [PSS; containing (in mM) 119.0 NaCl, 4.7 KCl, 1.6 CaCl2, 1.18 NaH2PO4, 1.17 MgSO4, and 24.0 NaHCO3]. Aortic segments were incubated in light-protected PSS (37°C) containing 5 μM DHE for 30 min. Segments were rinsed in DHE-free PSS and split longitudinally and placed endothelium side down on a coverslip. A drop of Fluotrogel with tris buffer (EMS, PA) was applied to keep the tissue moist. The medial smooth muscle layer was visualized, and images were obtained using a laser scanning confocal microscope (Leica Microsystems).
NADPH oxidase activity was measured in femoral arteries using lucigenin chemiluminescence (28). Homogenates were prepared from femoral arteries. After homogenization tissues were centrifuged at 4°C at 12,000 g for 20 min. The homogenates were incubated with lucigenin (final concentration: 5 3M) for chemiluminescence detection using a Berthold luminometer. Background luminescence from buffer or tissue was determined and subtracted from all measurements. For measurement of NADPH oxidase activity, NADPH was added (100 μM final concentration), and chemiluminescence was measured. A luminescence reading was obtained for an overall measuring time of 5 min for each sample. Enzyme activity is expressed in relative light units (RLU) per minute and normalized by protein concentration from each sample.
Drugs and vasoactive agents
Apocynin, cromakalim, and glibenclamide were purchased from Sigma Chemical Company. Apocynin was fed by tap water. Glibenclamide and cromakalim were dissolved in 100% ethanol and applied topically. Glibenclamide was given i.p. by 2.5% of ethanol solution. We have previously shown that this ethanol concentration in the superfusion is approximately 1/1000 and does not have any effects on vascular reactivity (31).
Data analysis and statistical methods
Arteriolar diameter data were collected to a personal computer. The effects of glibenclamide on VO2max and vasodilatory responses were analyzed using two-way repeated-measures ANOVA. All of the other data were analyzed using two-way ANOVA. Where significant main effects occurred, individual groups were compared using the Holm-Sidak method. All data are presented as means ± SEM. Probability values of p < 0.05 were accepted as statistically significant for all comparisons.
RESULTS
Apocynin treatment did not change the blood pressure and glucose tolerance in LZR and OZR
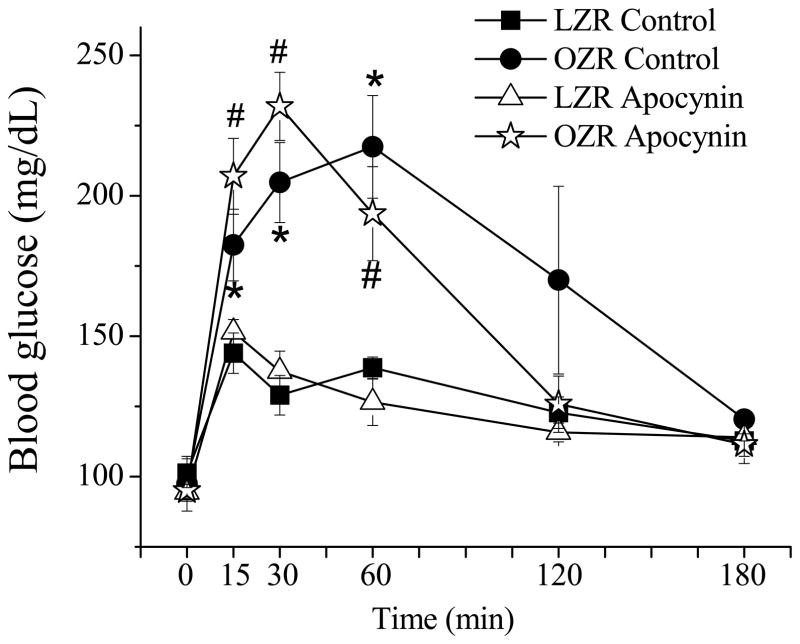
The blood pressures were not different between control conscious LZR (131 ± 2 mmHg) and OZR (125 ± 6 mmHg). Apocynin treatment had no effect on blood pressure (LZR: 127 ± 5 mmHg; OZR: 130 ± 6 mmHg). The fasting glucose levels were not different among groups (Fig. 1). With the OGTT blood glucose levels increased in all groups. OZR exhibited an elevated postprandial glucose level compared with the LZR (p < 0.05). Apocynin treatment has no effect on the postprandial hyperglycemia in the OZR.
Figure 1. Oral glucose tolerance test in LZR and OZR with and without apocynin treatment.
The fasting glucose levels were not significantly different between LZR and OZR both control and apocynin-treated groups. After gavage with a glucose solution, OZR exhibited significantly higher hyperglycemia compared with LZR in both control and apocynin-treated group (*, p < 0.05; #, p < 0.05). Apocynin treatment has no effect on insulin sensitivity in both LZR and OZR. (LZR control, n = 5; OZR control, n = 5; LZR apocynin, n = 4; OZR apocynin, n = 6).
VO2max is decreased in OZR associated with the superoxide-induced KATP dysfunction
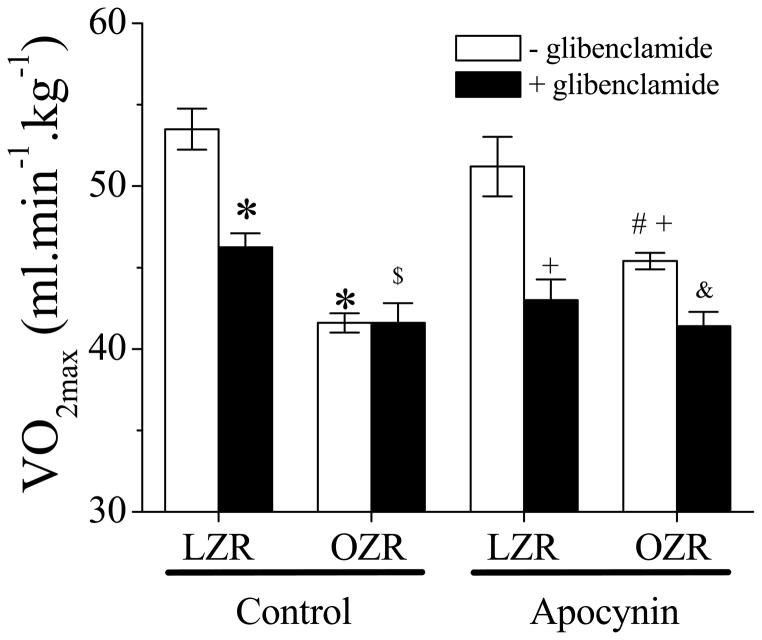
OZR exhibited an impaired VO2max as compared to the LZR group (Figure 2). Glibenclamide treatment (i.p.) significantly reduced VO2max in LZR but had no effect in OZR. After glibenclamide treatment, VO2max in OZR was still lower than in LZR. Apocynin treatment in the OZR partially restored the VO2max as compared to the OZR control or apocynin-treated LZR. In addition, glibenclamide exhibited an inhibitory effect on the VO2max in apocynin-treated OZR.
Figure 2. The effect of apocynin on VO2max in LZR and OZR before and following glibenclamide application (i.p.).
OZR exhibited an impaired VO2max (*, p<0.05 vs. LZR). Apocynin enhanced VO2max in OZR significantly (#, p<0.05 vs. OZR) but did not normalize it (+, p<0.05 vs. LZR with apocynin). Apocynin had no effect on LZR. Glibenclamide inhibited VO2max in all groups except in OZR control (&, p<0.05 vs. OZR with apocynin). OZR exhibited a less functional vasodilation after glibenclamide treatment in control group ($, p<0.05 vs. LZR+glibenclamide; n=5 for all groups).
Functional and KATP-mediated arteriolar vasodilation is impaired in OZR associated with elevated NADPH oxidase activity
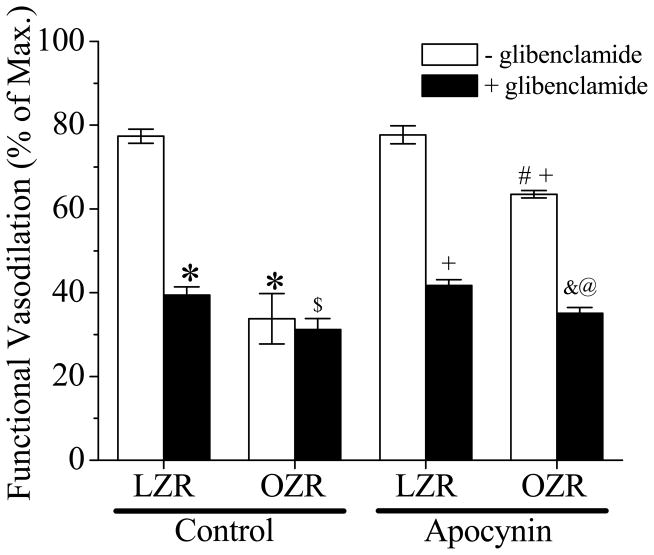
As shown in Figure 3, the arteriolar functional vasodilation in the spinotrapezius was significantly impaired in OZR compared with LZR. Glibenclamide significantly inhibited functional vasodilation in LZR but had no effect in the OZR. After glibenclamide treatment, functional vasodilation in OZR was significantly decreased as compared to LZR. Apocynin treatment partially restored the functional vasodilation in OZR (p < 0.05 vs. OZR control and apocynin-treated LZR) but had no effect in LZR. In addition, glibenclamide inhibited functional vasodilation in apocynin-treated OZR.
Figure 3. The effect of apocynin on functional vasodilation in spinotrapezius muscle in LZR and OZR with or without glibenclamide application.
OZR exhibited blunt functional vasodilation (*, p<0.05 vs. LZR). Apocynin enhanced functional vasodilation in OZR significantly (#, p<0.05 vs. OZR) but did not normalize it (+, p<0.05 vs. LZR with apocynin). Apocynin had no effect in LZR. Glibenclamide pretreatment (30 min) inhibited functinal vasodilation in all groups except in OZR control (&, p<0.05 vs. OZR with apocynin). OZR exhibited a less functional vasodilation after glibenclamide pretreatment in both control and apocynin groups ($, p<0.05 vs. LZR+glibenclamide; @, p<0.05 vs. LZR with apocynin+glibenclamide, n=7 for LZR, n=5 for OZR, n=6 for both LZR and OZR with apocynin).
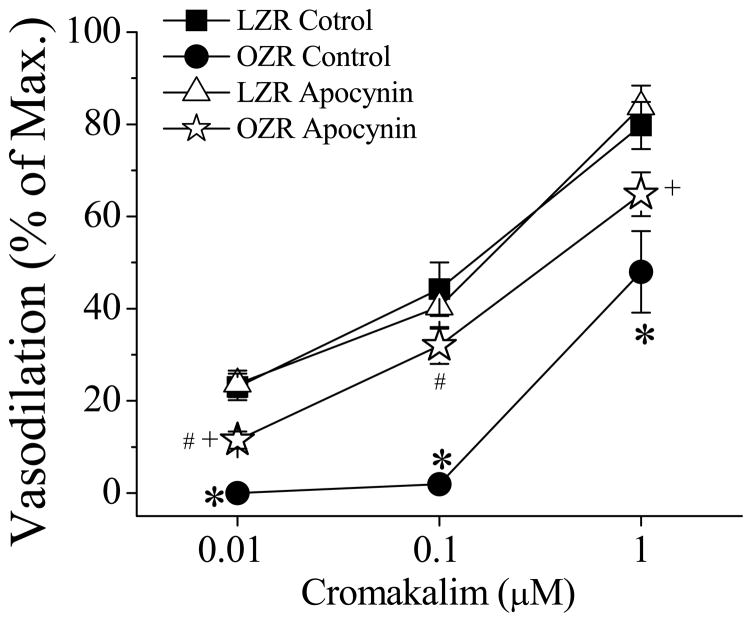
In another set of experiments we compared the spinotrapezius arteriolar responses to cromakalim between LZR and OZR before and after glibenclamide treatment (Figure 4). Cromakalim induced arteriolar vasodilation in a concentration-dependent manner. Cromakalim-induced vasodilation was impaired in OZR compared to LZR. Apocynin treatment increased cromakalim-induced vasodilation in OZR with no effect in LZR. Glibenclamide (1 μM) abolished cromakalim-induced vasodilation in all groups (data not shown). Apocynin had no effect on vascular diameter when applied topically. In these two sets of experiments, the arteriolar diameters before and after glibenclamide treatment along with the maximal arteriolar diameters in the spinotrapezius of LZR and OZR with or without apocynin treatment were not significantly different (Table 1).
Figure 4. Cromakalim induced vasodilation in spinotrapezius muscle in LZR and OZR with or without apocynin.
Cromakalim (0.01, 0.1, and 1 μM) induced vasodilation in concentration-dependent manner. OZR exhibited an impaired vasodilation response to cromakalim (*, p<0.05 vs. LZR). Apocynin significantly enhanced cromakalim-induced vasodilation in OZR (#, p<0.05 vs. OZR) but did not normalize it (+, p<0.05 vs. LZR with apocynin; n=7 for LZR, n=5 for OZR, n=6 for both LZR and OZR with apocynin)
Table 1.
Basal (before and after glibenclamide) and maximal arteriol diameters
| (μm) | LZR | OZR | LZR-A | OZR-A |
|---|---|---|---|---|
| Basal diameter | 18±1 | 19±1 | 20±1 | 20±1 |
| After glibenclamide | 19±1 | 19±1 | 19±1 | 20±1 |
| Max. diameter | 44±4 | 44±5 | 47±4 | 42±3 |
Values are means±SEM; n=7 for LZR, n=5 for OZR, n=6 for apocynin-treated LZR (LZR-A), and n=6 for apocynin-treated OZR (OZR-A). There is no significant difference in basal or maximal arteriolar diameters among all 4 groups.
The increased basal levels of vascular superoxide in OZR are due in part to elevated NADPH oxidase activity
Figure 5 shows that OZR exhibited elevated vascular NADPH oxidase activity (Figure 5) along with increased superoxide levels (Figure 6) as compared with OZR. Chronic treatment with apocynin significantly inhibited NADPH oxidase activity and superoxide levels in OZR with a minimal effect in LZR. In addition, although the enzyme activity was similar between treated LZR and OZR, the superoxide levels were still higher in the treated OZR than in the treated LZR (Figure 6).
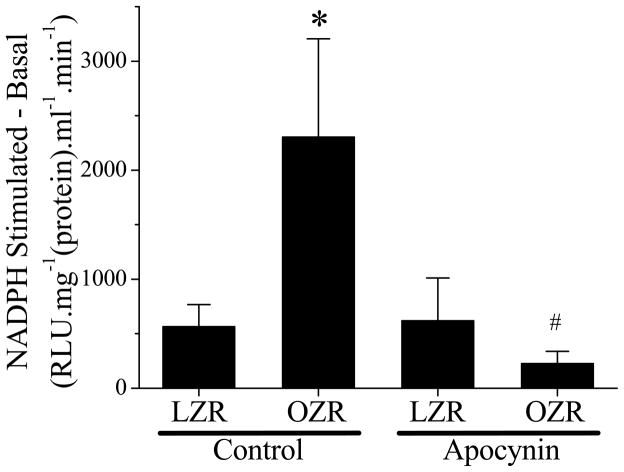
Figure 5. Femoral vascular NADPH oxidase activity in LZR and OZR with or without apocynin treatment.
Basal superoxide levels were subtracted from the NADPH-stimulated superoxide levels and normalized by protein concentration. OZR exhibited significant high NADPH oxidase activity (*, p<0.05 vs. LZR). Apocynin treatment reduced NADPH oxidase activity in OZR significantly and had no effect on LZR (#, p<0.05 vs. OZR; n=6 for LZR, n=5 for OZR, n=6 for both LZR and OZR with apocynin treatment).
Figure 6. Superoxide levels in LZR and OZR with or without apocynin treatment.
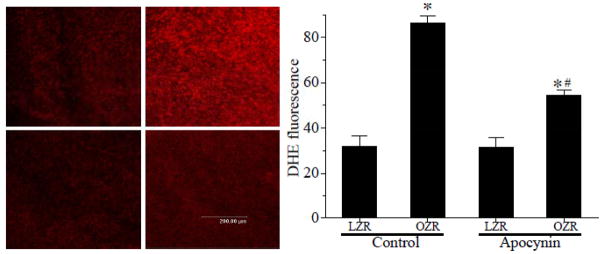
Confocal images obtained using a laser scanning confocal microscopy from DHE-treated aortic segments (split longitudinally). Superoxide levels between control and apocynin-treated LZR and OZR were compared. OZR had increased fluorescence compared with LZR (*, p<0.05 vs. LZR control). Apocynin treatment significantly reduced the fluorescence in OZR (#, p<0.05 vs. OZR control) but failed to normalize the superoxide level ((*, p<0.05 vs. LZR apocynin-treated; n=7 for LZR, n=5 for OZR, n=6 for both LZR and OZR with apocynin).)
DISCUSSION
The major findings of this study were: 1) Exercise capacity (VO2max) is dependent on vascular KATP channel function; 2) In OZR, the elevated superoxide impairs the vascular KATP channel function, leading to blunted exercise capacity and functional vasodilation.
KATP channels have been found to be physiologically important in numerous tissues, including VSMC (22). The activation of vascular KATP channels is reported to mediate the functional vasodilation in skeletal muscles (2, 23). In the vasculature of skeletal muscles, VSMC KATP channels were shown to mediate functional vasodilation of a single pre-capillary arteriole (16). However the role of KATP channels on maximal exercise capacity (VO2max) has not, to our knowledge, been determined. Since exercise capacity (VO2max) is tightly linked to the maximal increase in blood flow to exercising muscles (27), the current study was designed to determine the contribution of KATP channel activation to VO2max. We found that KATP channel blocker glibenclamide inhibits the VO2max (Figure 2) as well as the functional vasodilation in LZR (Figure 3).
However there are other potential roles for KATP channels in affecting oxygen consumption. Glibenclamide may inhibit mitochondrial KATP channels and subsequently reduce VO2. However, there is evidence that the decrease in oxygen consumption following inhibition of KATP channels is mainly due to a decreased tissue blood flow instead of inhibited mitochondria respiration (5). Activation of coronary VSMC KATP channels can increase coronary blood flow and subsequently affect cardiac output (7). However, during exercise, the coronary hyperemia is not affected by inhibition of KATP channels (7, 8, 26). Thus the current data along with published studies suggest that skeletal muscle vascular KATP activation plays an important role in exercise capacity.
Similar with human studies (10, 21), we found that OZR exhibited impaired functional vasodilation along with a decreased VO2max as compared with LZR. However, there are limitations comparing VO2max between LZR and OZR due to the differences in skeletal muscle-to-fat mass ratio. Since apocynin treatment did not change the body weight in either LZR or OZR, we assume that the muscle-fat mass ratio was not altered with the apocynin treatment. The impaired VO2max and hyperemia in obesity may involve an impaired functional vasodilation (24) and decreased vascular density in skeletal muscle (18). Frisbee et al. showed a decreased microvascular density in the gastrocnemius muscle of OZR, with antioxidant treatment improving the vascular density (11). Therefore, the improved VO2max in OZR following apocynin treatment may be due to a combination of both an increased functional vasodilation and microvascular density. Our previous study showed that cromakalim-induced KATP vasodilation in isolated small arteries is impaired in OZR, even though the KATP channel protein expression is similar between LZR and OZR (16). We confirmed an impaired cromakalim-induced KATP arteriolar dilation in vivo (Figure 4). In addition, we found that inhibiting KATP channels significantly decreased functional vasodilation and VO2max in the LZR (Figures 2 and 3). Such inhibitory effects were minimized in OZR, suggesting that the blunted functional vasodilation and exercise capability in OZR is, at least partly, due to an impaired KATP channel function.
Vascular NADH/NADPH oxidase has been shown to be the major source of superoxide. (13). Apocynin inhibits superoxide release by blocking migration of p47phox, a critical subunit of NADPH oxidase, to the cell membrane, which is critical in initiating assembly of the functional NADPH oxidase complex (25). In addition, apocynin has a direct anti-oxidant effect by decreasing peroxynitrite (ONOO-) (20) and superoxide levels (14, 15). Similar to our previous findings (28), we found increased vascular superoxide levels in OZR. Chronic apocynin treatment normalized the vascular NADPH oxidase activity in OZR with no effect in LZR. These results suggest that the decreased enzyme activity in OZR is due to a chronic effect from the apocynin treatment. The mechanism for the “selective” inhibition of NADPH oxidase activity in OZR is not clear. A possible mechanism is that ROS-dependent cascades may amplify the signals that promote NADPH oxidase upregulation. In obesity the increased cellular stress secondary to elevated ROS may further activate NADPH oxidase (17), thus inhibition of superoxide may eventually prevent the upregulation of NADPH oxidase. Additionally, the chronic apocynin treatment may also interfere with the circulating superoxide levels, especially since there is evidence in Type II diabetes that circulating superoxide dismutase is impaired (12). To address whether there was any improvement in the metabolic disorders following apocynin treatment, we performed an oral glucose tolerance test and found that the impaired glucose homeostasis was not affected by apocynin treatment. This confirms our previous study that the impaired glucose homeostasis in OZR impairs functional vasodilation through an increased ROS (28).
In the current study, we showed that the impaired functional and cromakalim-induced KATP-dependent vasodilations were improved following chronic apocynin treatment, suggesting an inhibitory effect of ROS on vascular KATP activation. The effects of ROS on KATP channel function are controversial. Mawatari et al. found that ROS inhibits KATP activation in VSMCs in vitro (19). However, Chai et al. reported that ROS can directly activate KATP channels in human embryonic kidney (HEK) 293 cells and rabbit ventricular cardiomyocytes (4). These results suggest that the acute and chronic effects of ROS on KATP function are different and may depend on the methodology and tissues chosen for study. Chronic apocynin treatment normalized the vascular NADPH oxidase activity but only partially restored the superoxide levels in OZR (Figures 5 and 6). These results raise the possibility for an increased superoxide production independent of the NADPH oxidase pathway in OZR. For example, an elevated xanthine oxidase activity is found in both obese human and animal models (6, 9). With the partially restored superoxide levels in OZR after apocynin treatment, the KATP channel-mediated vasodilations and the VO2max in the OZR were improved but still blunted as compared with LZR. Although the mechanisms are unclear, these results provide indirect evidence that exercise capacity is partially dependent on vascular KATP channel function.
Conclusions
Exercise capacity (VO2max) is dependent on KATP channel function in Zucker rats. Impaired KATP channel function in OZR is responsible for a portion of the impaired exercise capacity. Reduction of ROS by chronic inhibition of NADPH oxidase activity improves functional vasodilation and exercise capacity via enhancing KATP channel function in OZR. The impaired exercise capacity in obesity is partially due to increased NADPH oxidase-dependent ROS production and resultant impaired KATP channel dysfunction. These results explain the beneficial effects of reductions in ROS on vascular function and exercise capacity in obesity.
PERSPECTIVE
Obesity exhibits impaired exercise capacity and functional vasodilation. The current study suggests that impaired vascular KATP channel function contributes to the impairment in exercise capacity in OZR. Reduction of ROS and/or NADPH oxidase activity by apocynin treatment improves vascular KATP channel function. This results in increased exercise capacity in OZR, which may have implications for the mechanisms and future treatments of impaired exercise capability in obesity.
Acknowledgments
The study was supported by NIH HL-89581, HL-51971, AHA postdoctoral fellowship, and T32 HL-105324.
We wish to thank Mohamad Sebai and Lynn Lee for their technical help with these experiments.
List of Abbreviations
- DHE
Dihydroethidium
- LZR
Lean Zucker rats
- NADPH oxidase
Nicotinamide adenine dinucleotide phosphate-oxidase
- OGTT
Oral glucose tolerance test
- OZR
Obese Zucker rats
- ROS
Reactive oxygen species
- VO2max
Maximal oxygen consumption
- VSMC
Vascular smooth muscle cells
Reference List
- 1.Bäumer AT, Krüger CA, Falkenberg J, Freyhaus HT, Rösen R, Fink K, Rosenkranz S. The NAD(P)H oxidase inhibitor apocynin improves endothelial NO/superoxide balance and lowers effectively blood pressure in spontaneously hypertensive rats: comparison to calcium channel blockade. Clin Exp Hypertens. 2007;29(5):287–99. doi: 10.1080/10641960701500398. [DOI] [PubMed] [Google Scholar]
- 2.Bank AJ, Sih R, Mullen K, Osayamwen M, Lee PC. Vascular ATP-dependent potassium channels, nitric oxide, and human forearm reactive hyperemia. Cardiovasc Drugs Ther. 2000;14(1):23–9. doi: 10.1023/a:1007835003493. [DOI] [PubMed] [Google Scholar]
- 3.Butcher JT, Goodwill AG, Stanley SC, Frisbee JC. Blunted temporal activity of microvascular perfusion heterogeneity in metabolic syndrome: a new attractor for peripheral vascular disease? Am J Physiol Heart Circ Physiol. 2012 doi: 10.1152/ajpheart.00805.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Chai Y, Zhang DM, Lin YF. Activation of cGMP-dependent protein kinase stimulates cardiac ATP-sensitive potassium channels via a ROS/calmodulin/CaMKII signaling cascade. PLoS One. 2011;6(3):e18191. doi: 10.1371/journal.pone.0018191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Chen Y, Traverse JH, Zhang J, Bache RJ. Selective blockade of mitochondrial KATP channels does not impair myocardial oxygen consumption. Am J Physiol Heart Circ Physiol. 2001;281(2 50-2):H738–H744. doi: 10.1152/ajpheart.2001.281.2.H738. [DOI] [PubMed] [Google Scholar]
- 6.Chiney MS, Schwarzenberg SJ, Johnson LA. Altered xanthine oxidase and N-acetyltransferase activity in obese children. Br J Clin Pharmacol. 2011;72(1):109–15. doi: 10.1111/j.1365-2125.2011.03959.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Dick GM, Tune JD. Role of potassium channels in coronary vasodilation. Exp Biol Med (Maywood) 2010;235(1):10–22. doi: 10.1258/ebm.2009.009201. [DOI] [PubMed] [Google Scholar]
- 8.Duncker DJ, Van Zon NS, Altman JD, Pavek TJ, Bache RJ. Role of K-ATP channels in coronary vasodilation during exercise. Circulation. 1993;88:1245–1253. doi: 10.1161/01.cir.88.3.1245. [DOI] [PubMed] [Google Scholar]
- 9.Erdei N, Tóth A, Pásztor ET, Papp Z, Edes I, Koller A, Bagi Z. High-fat diet-induced reduction in nitric oxide-dependent arteriolar dilation in rats: role of xanthine oxidase-derived superoxide anion. Am J Physiol Heart Circ Physiol. 2006 Nov;291(5):H2107–15. doi: 10.1152/ajpheart.00389.2006. Epub 2006. [DOI] [PubMed] [Google Scholar]
- 10.Fossum E, Hoieggen A, Moan A, Rostrup M, Nordby G, Kjeldsen SE. Relationship between insulin sensitivity and maximal forearm blood flow in young men. Hypertension. 1998;32:838–843. doi: 10.1161/01.hyp.32.5.838. [DOI] [PubMed] [Google Scholar]
- 11.Frisbee JC. Reduced nitric oxide bioavailability contributes to skeletal muscle microvessel rarefaction in the metabolic syndrome. Am J Physiol Regul Integr Comp Physiol. 2005;289(2):R307–R316. doi: 10.1152/ajpregu.00114.2005. [DOI] [PubMed] [Google Scholar]
- 12.Fujita H, Sakamoto T, Komatsu K, Fujishima H, Morii T, Narita T, Takahashi T, Yamada Y. Reduction of circulating superoxide dismutase activity in type 2 diabetic patients with microalbuminuria and its modulation by telmisartan therapy. Hypertens Res. 2011;34(12):1302–8. doi: 10.1038/hr.2011.127. [DOI] [PubMed] [Google Scholar]
- 13.Griendling KK, Ushio-Fukai M. NADH/NADPH Oxidase and Vascular Function. Trends Cardiovasc Med. 1997;7(8):301–7. doi: 10.1016/S1050-1738(97)00088-1. [DOI] [PubMed] [Google Scholar]
- 14.Hayashi T, Juliet PA, Kano-Hayashi H, Tsunekawa T, Dingqunfang D, Sumi D, Matsui-Hirai H, Fukatsu A, Iguchi A. NADPH oxidase inhibitor, apocynin, restores the impaired endothelial-dependent and -independent responses and scavenges superoxide anion in rats with type 2 diabetes complicated by NO dysfunction. Diabetes Obes Metab. 2005;7(4):334–43. doi: 10.1111/j.1463-1326.2004.00393.x. [DOI] [PubMed] [Google Scholar]
- 15.Heumüller S, Wind S, Barbosa-Sicard E, Schmidt HH, Busse R, Schröder K, Brandes RP. Apocynin is not an inhibitor of vascular NADPH oxidases but an antioxidant. Hypertension. 2008;51(2):211–7. doi: 10.1161/HYPERTENSIONAHA.107.100214. [DOI] [PubMed] [Google Scholar]
- 16.Hodnett BL, Xiang L, Dearman JA, Carter CB, Hester RL. K(ATP)-mediated vasodilation is impaired in obese Zucker rats. Microcirculation. 2008;15.6:485–94. doi: 10.1080/10739680801942240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Jiang F, Zhang Y, Dusting GJ. NADPH oxidase-mediated redox signaling: roles in cellular stress response, stress tolerance, and tissue repair. Pharmacol Rev. 2011;63(1):218–42. doi: 10.1124/pr.110.002980. [DOI] [PubMed] [Google Scholar]
- 18.Lillioja S, Young AA, Culter CL, Ivy JL, Abbott WG, Zawadzki JK, Yki-Järvinen H, Christin L, Secomb TW, Bogardus C. Skeletal muscle capillary density and fiber type are possible determinants of in vivo insulin resistance in man. J Clin Invest. 1987;80(2):415–24. doi: 10.1172/JCI113088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Mawatari K, Yasui S, Morizumi R, Hamamoto A, Furukawa H, Koyama K, Hattori A, Yoshioka E, Yoshida M, Nakano M, Teshigawara K, Harada N, Hosaka T, Takahashi A, Nakaya Y. Reactive oxygen species induced by diamide inhibit insulin-induced ATP-sensitive potassium channel activation in cultured vascular smooth muscle cells. Asia Pac J Clin Nutr. 2008;17 (Suppl 1):162–6. [PubMed] [Google Scholar]
- 20.Muijsers RB, van Den Worm E, Folkerts G, Beukelman CJ, Koster AS, Postma DS, Nijkamp FP. Apocynin inhibits peroxynitrite formation by murine macrophages. Br J Pharmacol. 2000;130(4):932–6. doi: 10.1038/sj.bjp.0703401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Nyholm B, Nielsen MF, Kristensen K, Nielsen S, Østergård T, Pedersen SB, Christiansen T, Richelsen B, Jensen MD, Schmitz O. Evidence of increased visceral obesity and reduced physical fitness in healthy insulin-resistant first-degree relatives of type 2 diabetic patients. Eur J Endocrinol. 2004;150(2):207–14. doi: 10.1530/eje.0.1500207. [DOI] [PubMed] [Google Scholar]
- 22.Quayle JM, Standen NB. K(ATP) channels in vascular smooth muscle. Cardiovasc Res. 1994;28(6):797–804. doi: 10.1093/cvr/28.6.797. [DOI] [PubMed] [Google Scholar]
- 23.Saito Y, Mckay M, Eraslan A, Hester RL. Functional hyperemia in striated muscle is reduced following blockade of ATP-sensitive potassium channels. Am J Physiol. 1996;270:H1649–H1654. doi: 10.1152/ajpheart.1996.270.5.H1649. [DOI] [PubMed] [Google Scholar]
- 24.Sebai M, Lu S, Xiang L, Hester RL. Improved functional vasodilation in obese Zucker rats following exercise training. Am J Physiol Heart Circ Physiol. 2011;301(3):H1090–6. doi: 10.1152/ajpheart.00233.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Stolk J, Hiltermann TJ, Dijkman JH, Verhoeven AJ. Characteristics of the inhibition of NADPH oxidase activation in neutrophils by apocynin, a methoxy-substituted catechol. Am J Respir Cell Mol Biol. 1994;11(1):95–102. doi: 10.1165/ajrcmb.11.1.8018341. [DOI] [PubMed] [Google Scholar]
- 26.Tune JD, Richmond KN, Gorman MW, Feigl EO. KATP channels, nitric oxide, and adenosine are not required for local metabolic coronary vasodilation. Am J Physiol Heart Circ Physiol. 2001;280:H868–75. doi: 10.1152/ajpheart.2001.280.2.H868. [DOI] [PubMed] [Google Scholar]
- 27.Wilmore JH, Costill DL. Physiology of Sport and Exercise. 3. Champaign, IL: Human Kinetics; 2005. [Google Scholar]
- 28.Xiang L, Dearman J, Abram SR, Carter C, Hester RL. Insulin resistance and impaired functional vasodilation in obese Zucker rats. Am J Physiol Heart Circ Physiol. 2008;294.4:H1658–H1666. doi: 10.1152/ajpheart.01206.2007. [DOI] [PubMed] [Google Scholar]
- 29.Xiang L, Naik J, Hester RL. Exercise-induced increase in skeletal muscle vasodilatory responses in obese Zucker rats. Am J Physiol Regul Integr Comp Physiol. 2005;288.4:R987–R991. doi: 10.1152/ajpregu.00702.2004. [DOI] [PubMed] [Google Scholar]
- 30.Xiang L, Naik JS, Hodnett BL, Hester RL. Altered arachidonic acid metabolism impairs functional vasodilation in metabolic syndrome. Am J Physiol Regul Integr Comp Physiol. 2006;290.1:R134–R138. doi: 10.1152/ajpregu.00295.2005. [DOI] [PubMed] [Google Scholar]
- 31.Xiang L, Lu S, Fuller W, Aneja A, Russell GV, Jones LB, Hester R. Impaired blood pressure recovery to hemorrhage in obese Zucker rats with orthopedic trauma. Am J Physiol Heart Circ Physiol. 2012;302(1):H340–8. doi: 10.1152/ajpheart.00439.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]