Conspectus
During the three decades 1980–2010, magic angle spinning (MAS) NMR developed into the method of choice to examine many chemical, physical and biological problems. In particular, a variety of dipolar recoupling methods to measure distances and torsion angles can now constrain molecular structures to high resolution. However, applications are often limited by the low sensitivity of the experiments, due in large part to the necessity of observing spectra of low-γ nuclei such as the I = ½ species 13C or 15N. The difficulty is still greater when quadrupolar nuclei, like 17O or 27Al, are involved. This problem has stimulated efforts to increase the sensitivity of MAS experiments. A particularly powerful approach is dynamic nuclear polarization (DNP) which takes advantage of the higher equilibrium polarization of electrons (which conventionally manifests in the great sensitivity advantage of EPR over NMR). In DNP, the sample is doped with a stable paramagnetic polarizing agent and irradiated with microwaves to transfer the high polarization in the electron spin reservoir to the nuclei of interest. The idea was first explored by Overhauser and Slichter in 1953. However, these experiments were carried out on static samples, at magnetic fields that are low by current standards. To be implemented in contemporary MAS NMR experiments, DNP requires microwave sources operating in the subterahertz regime — roughly 150–660 GHz — and cryogenic MAS probes. In addition, improvements were required in the polarizing agents, because the high concentrations of conventional radicals that are required to produce significant enhancements compromise spectral resolution.
In the last two decades scientific and technical advances have addressed these problems and brought DNP to the point where it is achieving wide applicability. These advances include the development of high frequency gyrotron microwave sources operating in the subterahertz frequency range. In addition, low temperature MAS probes were developed that permit in-situ microwave irradiation of the samples. And, finally, biradical polarizing agents were developed that increased the efficiency of DNP experiments by factors of ~4 at considerably lower paramagnet concentrations. Collectively these developments have made it possible to apply DNP on a routine basis to a number of different scientific endeavors, most prominently in the biological and material sciences. This Account reviews these developments, including the primary mechanisms used to transfer polarization in high frequency DNP, and the current choice of microwave sources and biradical polarizing agents. In addition, we illustrate the utility of the technique with a description of applications to membrane and amyloid proteins that emphasizes the unique structural information that is available in these two cases.
Keywords: DNP, solid state NMR, magic angle spinning
Introduction
Magic angle spinning (MAS) nuclear magnetic resonance (NMR) has emerged as a powerful, nondestructive method that can be used to characterize the structure and dynamics of systems that are not accessible by either solution NMR or crystallography. In particular, the last three decades have witnessed the development of MAS techniques to probe various anisotropic interactions at the molecular and atomic scale via dipole recoupling techniques 1. As a consequence, it is possible to measure internuclear distances in amorphous and powder samples as well as in crystals. In principle, these measurements provide copious high resolution information about the structure and dynamics of a variety of biological systems such as peptides 2, membrane proteins 3–5, nanocrystals 6, amyloids 7–10, and materials science. Given this versatility, the recent rapid expansion of MAS NMR is expected to continue.
Despite the outstanding progress in this field, there remains an acute sensitivity problem since MAS NMR usually involves direct detection of 13C, 15N or another low-γ species. Cross polarization (CP) techniques and operation at higher magnetic fields have helped to address this issue. However, significantly higher sensitivity would help to bring MAS NMR into a regime where it is truly widely applicable. The subject of this Account is recent 102–103 fold improvements in MAS NMR sensitivity based on high frequency dynamic nuclear polarization (DNP). As we will see, high frequency DNP is significantly changing the landscape of what is possible with MAS NMR. This article illustrates this point with a discussion of polarization transfer mechanisms, polarizing agents, instrumentation, and recent applications of MAS DNP to complex heterogeneous systems.
DNP Mechanisms
DNP enhances NMR signals by transferring the large polarization of electrons to nearby nuclei via microwave (μw) irradiation of electron-nuclear transitions 11. Contemporary MAS DNP experiments on insulating solids are usually based either on the solid effect (SE), coupling an electron-nuclear spin pair, or the cross effect (CE), utilizing a pair of electrons in the form of a biradical and a nuclear spin. A third mechanism, thermal mixing (TM), involves multiple electrons and a homogeneously broadened EPR spectrum. However, at the high fields and low temperatures (80–110 K) currently used in MAS experiments, TM has thus far not provided an important polarization pathway. In all of these mechanisms it is necessary to add a stable paramagnetic polarizing agent to the sample and the most commonly used radicals are shown in Figure 1. Trityl and BDPA (or water soluble BDPA 12) support the SE, whereas the TEMPO based biradicals TOTAPOL 13 and bTbk 14 are used for the CE. The detailed polarization transfer schemes discussed below are closely linked to the shapes of the high field EPR spectra of these molecules.
Figure 1.
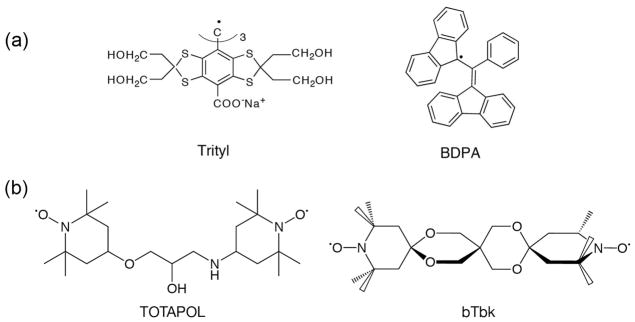
Polarizing agents commonly used for high field DNP experiments. (a) narrow line radicals trityl and BDPA used for the SE; (b) TEMPO based biradicals TOTAPOL and bis-TEMPO-bis-ketal (bTbk) used for the CE.
The Solid Effect
The SE can be understood using a two-spin model involving one electron and one nucleus, interacting via an electron-nuclear dipole coupling, and irradiation at nominally forbidden electron transitions at ωμw = ω0S ±ω0I illustrated in Figure 2. The Hamiltonian applicable to the two spin system is
Figure 2.
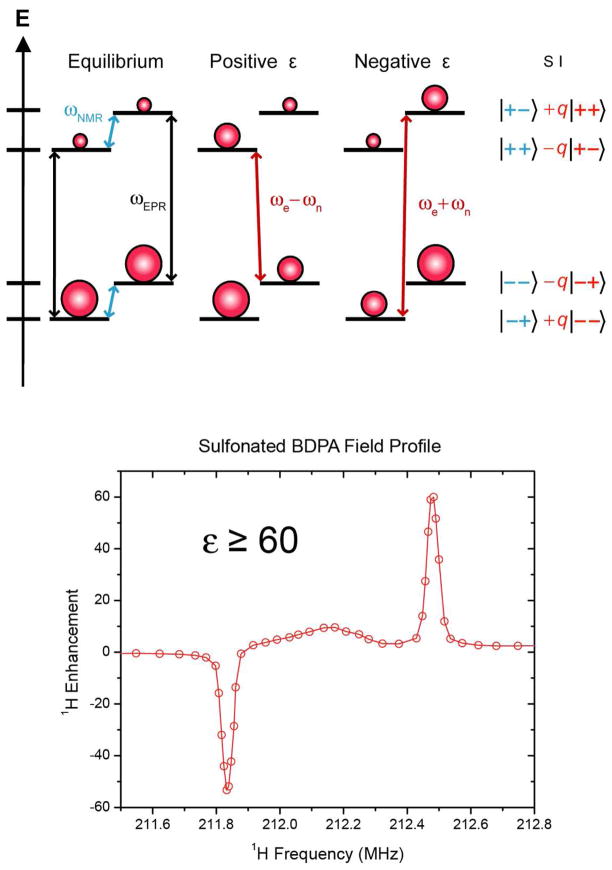
(top) Energy level diagram illustrating DNP via the solid effect (SE). At thermal equilibrium (left), populations of the same electron spin subspaces are governed by the Boltzmann distribution. Mixing of states in the nuclear and electron spin subspaces (right), leads to partially allowed double quantum (DQ) and zero quantum (ZQ) transitions, and positive and negative enhancements,
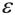 , respectively. The mixing of states is proportional to a constant q, which is inversely proportional to B0. Therefore, the enhancement in the Solid Effect DNP scales as
. (bottom) A plot of the enhancement from SA-BDPA12 as a function of magnetic field (1H frequency) showing the positive and negative enhancements. ωNMR and ωEPR are the NMR and EPR frequencies and ωe ±ωn are the sum and difference of the EPR and NMR frequencies.
, respectively. The mixing of states is proportional to a constant q, which is inversely proportional to B0. Therefore, the enhancement in the Solid Effect DNP scales as
. (bottom) A plot of the enhancement from SA-BDPA12 as a function of magnetic field (1H frequency) showing the positive and negative enhancements. ωNMR and ωEPR are the NMR and EPR frequencies and ωe ±ωn are the sum and difference of the EPR and NMR frequencies.
where ω0S and ω0I are the electron and nuclear Larmor frequencies, respectively, is the usual term in the electron-nuclear dipole Hamiltonian 15 and S and I are spin operators for electrons and nuclei, respectively. First order perturbation theory yields the mixed eigenstates shown in the figure where the mixing coefficient q = C/ω0I ≪ 1. The other terms in the electron-nuclear dipolar Hamiltonian (A, B, E and F in Van Vleck notation) also mix states, but the contributions are relatively small. Irradiation of the partially allowed transitions by μw’s gives rise to either positive (double quantum) or negative (single quantum) enhancement of the nuclear polarization as illustrated at the bottom of Figure 2.
The SE is the dominant DNP mechanism in systems where the polarizing agent exhibits a homogeneous EPR linewidth (δ) and an inhomogeneous spectral breadth (Δ) smaller than the nuclear Larmor frequency (δ, Δ< ω0I). This condition is satisfied by radicals with high molecular symmetry such as BDPA16, SA-BDPA12, and OX06317, where the g tensors are nearly isotropic and the hyperfine interaction is small. However, as the SE relies on the mixing of nuclear states by electron-nuclear coupling, the enhancement scales as . Therefore, the SE becomes less efficient at high magnetic fields (> 3 T). Nevertheless, recent research suggests that SE can be very efficient at high field provided that high microwave power and a large ω1S = γeB1S is available. Enhancements as high as ~144 have been achieved at 5 T. Finally, the SE can also be the dominant mechanism when transition metals complexes, e.g. with Gd3+, are used as polarizing agents. Since the broadening of the EPR line in these systems is mainly induced by the zero field splitting, the EPR line narrows at higher magnetic fields and metal-based polarizing agents may show improved performances at higher fields18.
The Cross Effect
When Δ >ω0I > δ, DNP is governed by the CE and scales with , leading to larger enhancements at higher magnetic fields. At highs, where the EPR spectrum is dominated by field g-anisotropy and inhomogeneously broadened, a three spin quantum mechanical treatment is possible19–21. The Hamiltonian for the nuclear spin and two electrons is
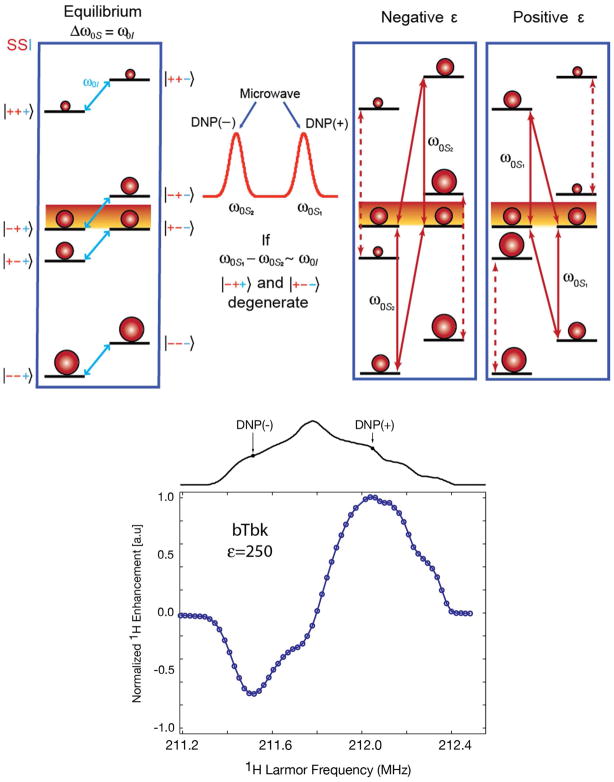
where the first three terms represent electron and nuclear Zeeman interactions, the fourth and fifth describe the electron-nuclear coupling (with A and B denoting the secular and psuedosecular hyperfine couplings22), the sixth represents the electron-electron dipolar coupling and the last describes exchange coupling. This leads to the energy level diagram for the CE is shown in Figure 3, and electron-electron-nuclear polarization transfer is maximized when the central energy levels are degenerate. This occurs when the matching condition |ωOS1 − ωOS2| = ωOI is fulfilled, where ωOS1 and ωOS2 are the Larmor frequencies of dipolar coupled electrons S1 and S2. The degeneracy leads to saturation of the four connected levels and enhanced nuclear polarization. A field profile obtained from bTbk is shown at the bottom of Figure 3, and roughly represents the negative 1st derivative of the EPR spectrum. Also shown are the positions in the EPR powder pattern that are irradiated for optimal positive and negative enhancements.
Figure 3.
(top) Energy diagram illustrating DNP via the CE. At equilibrium (left), under the matching condition, there is degeneracy and 1:1 population of the two shaded levels. The EPR spectrum of an ideal biradical for CE (middle) has two narrow lines separated by the nuclear Larmor frequency. Saturation of transitions near the first (second) EPR line gives rise to a positive (negative) DNP enhancement (right). (bottom) Field profile for bTbk with an enhancement ε= 230 14
Initially, high-field CE DNP experiments were performed with mono-radical species, such as TEMPO23,24. In this situation, the frequency matching condition is fulfilled only for the fraction of the radicals that adopt the correct relative orientation of their g-tensors. In order to improve CE DNP, we introduced biradicals such as bis-TEMPO-n-ethylene glycol (BTnE)19 and TOTAPOL 13, consisting of two tethered TEMPO moieties to obtain relatively short (~12 Å) electron-electron distances independent of concentration. With these polarizing agents, which have an e-e dipole coupling of 20–30 MHz, the enhancements were ~4-fold higher at an ~4-fold lower e concentration. Figure 4 shows recent results obtained using TOTAPOL from two standard samples, urea and proline. The observed ε= 181 and ε=134 are ~2-fold higher than we initially reported for TOTAPOL at this field 25 due to improvements in instrumentation – primarily gyrotron output power and lower temperatures (vide infra).
Figure 4.
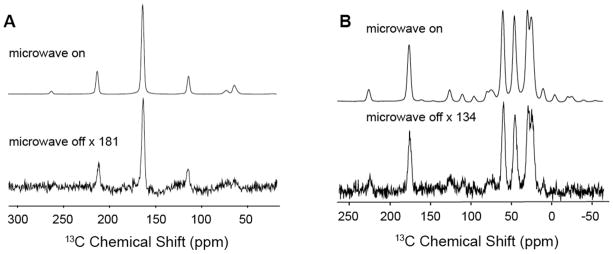
13C CP MAS NMR spectra of (A) 1M U-[13C-15N] urea and (B) 0.5 M U-[13C-15N] proline at 80 K with and without microwave irradiation. The DNP enhancements are ε=181 and ε=134, respectively. Both samples contained 10 mM TOTAPOL in a 60/30/10 ratio of d8-glycerol/D2O/H2O. Experimental parameters are: 4 scans, recycle delay 4 s, microwave power ~12.5 W, γB1(1H)=83 kHz, γB1(13C)=71 kHz, ωr/2π= 5 kHz.
Optimizing DNP Signal Enhancements
DNP enhancements are governed by a number of factors, including microwave power, concentration and design of the polarizing agent, temperature, solvent, and the relaxation times of the solvent and solute. We now review recent results aimed at optimizing the efficiency of DNP experiments with a focus on the influence of these parameters on the enhancements.
Microwave power
Gyrotrons 16,26,27 are capable of producing 10’s of watts of μw power with excellent frequency stability and low phase noise, making them the current microwave source of choice for DNP experiments. In particular, the low Q of the microwave circuit in the MAS NMR probe necessitates copious power to generate a sufficient B1 to excite DNP transitions. Furthermore, since the gyrotron is a fast wave device, it can operate at high powers for extended periods of time, as is required for multidimensional NMR experiments that involve signal averaging. Figure 5 (left) shows the enhancement as a function of μw power at 80 K obtained with a frequency tunable 250 GHz gyrotron28. The enhancement increases with power, does not saturate at our maximum available power of 12.5 W, and extrapolates to a limiting value εmax~240. Similar dependences of ε on μw power have been published elsewhere. 29,30 An alternative microwave source, that we explored sometime ago and currently in use in some labs31, is a low power (~10–100 mW) Gunn diode. However, the enhancements are lower: on one sample, ε ~25 with 10 mW from the Gunn diode versus ε=185 with 1 W from the gyrotron32. Thus, the data in Figure 5 suggest that with current technology the gyrotron is the microwave source of choice for DNP experiments, especially at microwave/ 1H NMR frequencies ≥ 400 MHz/263 GHz for 1H/e. Currently gyrotron-based DNP spectrometers are operating at microwave/ 1H NMR frequencies up to 460 GHz/700 MHz29 and are expected to go still higher. Nevertheless, microwave technology does improve with time, and it is possible that alternatives to the gyrotron and Gunn diode will be available in the future.
Figure 5.
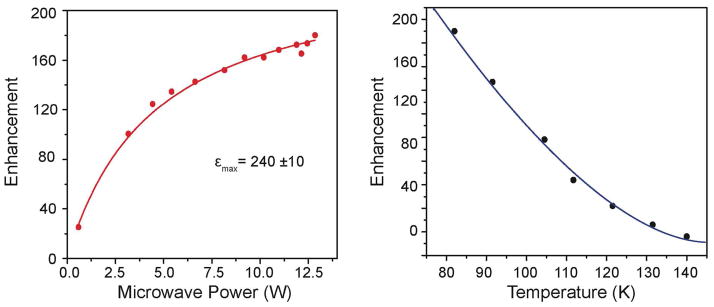
13C CP DNP enhancements of U-[13C ,15N] urea with 10 mM TOTAPOL plotted as a function of μw power at 80 K (left) and as a function of temperature at 12.5 W μw (right). ωr/2π= 7 kHz.
Temperature and polarizing agents and
Both the sample temperature and the nature of the polarizing agent profoundly influence the DNP enhancements. Lower temperatures improve both the SE and CE enhancements, most likely due to longer electron and proton relaxation times. Figure 5 (right) plots recent data showing the temperature dependence of the 1H DNP signal enhancement in the range 80–140 K at 250 GHz/380 MHz with the TOTAPOL/urea sample described above. Note that the DNP enhancement increases as the temperature approaches 80 K, by a factor of 3.6 in the range 110 to 80 K.
It is well known that high concentrations of paramagnets dramatically broaden NMR linewidths and attenuate integrated NMR signal intensities. It is for this reason that we developed biradical polarizing agents with a ~20–30 MHz intramolecular e-e dipole coupling which yield ~4-fold larger enhancements at ~4-fold lower electron concentration than monoradicals such as TEMPO19. Our experiments performed on urea, proline, bacteriorhodopsin (bR), and PI3-SH3 fibrils 33,34 suggest that optimal radical concentration of TOTAPOL is 10–20 mM.
Finally, we note that biradicals such as bTbk14 (Figure 1) and bTbk-py35 have the TEMPO moieties locked at ~90° with respect to one another, and therefore yield a relative orientation of the two g-tensors that better satisfies the CE matching condition. These polarizing agents have produced enhancements as large as 250 (Figure 1)14.
Some time ago, some of us reported that 2H labeled solvents improve 1H DNP enhancements, and that even with 90–95% 2H labeling we can still efficiently CP to low abundance nuclei in the target molecule19,36. Thus, while 1H dilution attenuates relaxation processes, even dilute protons mediate 1H-1H spin diffusion, with the overall result of a higher enhancement. For example, Akbey et al. 37 reported that perdeuteration of the α-spectrin-SH3 domain led to three to five times higher DNP enhancement (ε~148) than obtained with protonated SH3. In a more recent example we prepared 98% perdeuterated U-[2H,13C,15N] bR and with 15 mM TOTAPOL and observed ε= 72, whereas for U-[1H,13C,15N]-bR we obtained ε~35–4333,38. Thus, perdeuterated proteins will likely be important for biological applications of DNP.
Applications of DNP MAS NMR
To date one of the most interesting examples of the application of DNP is to the light-driven ion pump bR, which is a 26.6 kDa trans-membrane protein containing a retinal chromophore. bR has been studied intensively since its discovery in the 1970’s, but the mechanism by which it enforces vectorial action is still not understood and MAS NMR studies can potentially elucidate the relevant structural details of the intermediates in its photocycle (Figure 6 (left)). However, many of the intermediates can only be cryo-trapped at low (~5%) concentrations, so that high signal-to-noise, and therefore DNP, is required to observe their MAS NMR spectra 33,38.
Figure 6.
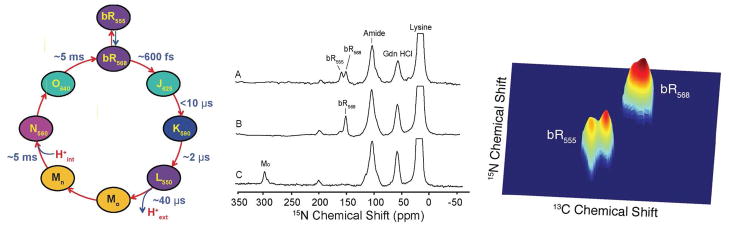
(left) The ion-motive photocycle of bR. The subscript for each intermediate represents the wavelength (in nm) of maximum visible absorption. (middle) 15N CP DNP spectra [ζ-15N-Lys] bR prepared with 15 mM TOTAPOL in 60/30/10 ratio of d8-glycerol/D2O/H2O in 0.3 M guanidinium hydrochloride at pH 10. (A) the dark adapted (DA) state comprises a thermal equilibrium mixture of bR555 and bR568 (B) LA (bR568) accumulated by 532 nm irradiation of the rotating sample for 4 hours 273 K (C) the Mo intermediate created by 532 nm irradiation of rotating LA at 230 K. The spectra of all three intermediates were obtained in roughly 2 hours with a spinning frequency of 7 kHz. (right) 2D spectrum obtained from DA bR illustrating the splittings observed at low temperature due to inequivalent sites.
The retinal cofactor is covalently bonded to Lys216 via a protonated Schiff base linkage, and the sensitivity of the unique 15N chemical shift of the Schiff base to its local environment provides an excellent marker and probe of each photocycle intermediate. In the dark-adapted (DA) state, bR exhibits two conformations: bR555 and bR568 in a ratio of 60:40 (Figure 6 (middle, A). After irradiation at 532 nm, DA is converted to light adapted (LA) state in which only bR568 remains (Figure 6 (middle, B)). Upon the absorption of a photon, the retinal isomerizes and cycles the protein through several intermediates that can be cryo-trapped for observation in situ. Figure 6 (middle, C) shows the 1D spectrum of Mo. With low temperature DNP it is possible to perform 2D spectroscopy and a 13C-15N spectrum of DA bR is shown in Figure 6 (right) showing that at 90 K there are actually four forms of bR present – two each of the bR555 and bR568.
We have also published the first DNP MAS NMR spectra of the K and L intermediates 33,38. While the K state showed just one Schiff base signal, it relaxed to several L states, of which all but one are dead ends (relaxing back to bR). The data for the functional L state (the one that relaxes to M) suggest that its Schiff base has a strong counterion. One of the possible explanations would support the hypothesis that bR is an inward OH− pump, rather than an outward H+ pump.
With the sensitivity available from DNP, it is also possible to record 3D correlation spectra for the individual resonances in the DA state which has an effective molecular weight of ~ 85 kDa. Figure 7 shows cross-peaks between the Schiff base 15N and 13C-12,13,14,15,20 of the retinal and 13C-ε of Lys216 in the dark adapted state of bR. The experiment has been conducted by using a Gaussian pulse to select the signals arising from the 15N of Lys216 in both bR568 and bR555 followed by a 15N-13C and then 13C-13C diffusion via RFDR mixing. Again the spectrum of bR would not be accessible sans DNP.
Figure 7.
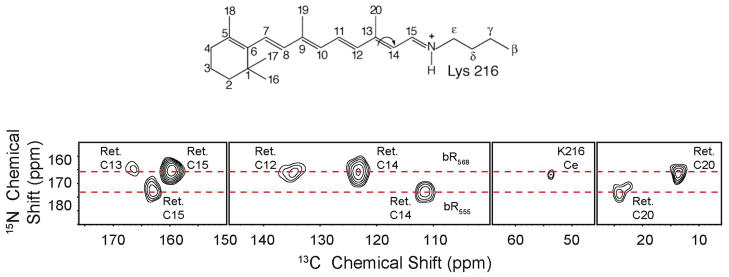
15N-13C spectrum obtained from dark adapted U-[13C,15N]-bR after selective excitation of the 15N Schiff base, CP to the 13C-15 of the retinal and 13Cε of Lys216, followed by RFDR mixing. The spectrum shows cross-peaks between the Schiff base 15N and 13C-12,13,14,15,20 on the retinal chromophore and 13Cε Lys216 27,33. The arrow indicates the trans-cis isomerization of the C13=C14 bond that occurs during the photocycle.
Amyloid fibrils
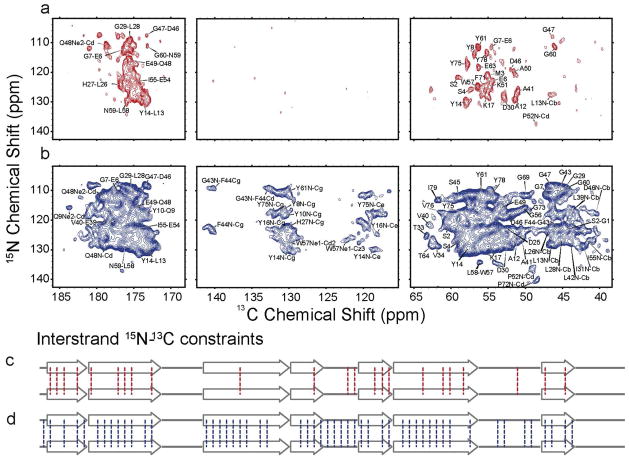
MAS NMR is also essential for studies of the structure of amyloid fibrils. Intermolecular 13C-13C or 15N-13C distances derived from MAS DNP experiments provide otherwise unavailable structural constraints. The most straightforward approach is to measure long-range 13C-15N distances with a ZF-TEDOR experiment 40. However, for distances ≥5 Å, the efficiency is low (< 5%), which vastly extends the acquisition time and severely limits the number of constraints that can be observed. The application of DNP to overcome this situation has been demonstrated on mixed samples of [15N, 12C] PI3-SH3 /[14N,13C] PI3-SH3 (50:50 molar ratio). Figure 8 compares the 15N-13C intermolecular correlation spectra obtained with ZF-TEDOR recoupling (τmix = 16 ms) at 750 MHz without DNP and at 400 MHz with DNP, collected in 16 days and 32 hours respectively. The number of intermolecular 15N-13C constraints detected was more than doubled due to the DNP with ε ~ 30 on 13C. The additional constraints obtained from DNP permitted us to establish that the PI3-SH3 protein strands are aligned in a parallel and in-register β-sheet arrangement 34.
Figure 8.
Comparison between room temperature and DNP enhanced, low temperature correlation spectra of PI3-SH3. The spectra were obtained with ZF-TEDOR recoupling (τmix = 16 ms) from sample prepared from partially labeled fibrils [15N, 12C] PI3-SH3 /[14N,13C] PI3-SH3 (50:50 molar ratio). (a) 15N-13C intermolecular correlations in PI3-SH3 fibrils at 300 K obtained at 750 MHz in 16 days of acquisition time. (b). Same sample and identical spectral regions were recorded at 100 K and 400 MHz with DNP enhancement in 32 h. (c). Illustration of the 23 interstrand contacts established from 13C -15N peaks in the 750 MHz spectra acquired at 300 K in a. (d) the 52 interstrand contacts established from the 400 MHz DNP enhanced spectra recorded at 100 K shown in (d). 34
In additon, it is clear that the approaches described here are widely applicable to other areas of science, in particular materials problems – polymers, zeolites, surfaces, semiconductors, etc. These experiments will likely include spectroscopy of quadrupolar species such as 17O41 and 27Al42 as well as I=1/2 species. We refer the interested reader to other articles in this issue for a complete discussion of these very interesting applications.
Finally we note that, while most of the results described here were obtained at 250 GHz/380 MHz or 263 GHz/400 MHz, DNP experiments have recently been performed at 460 GHz/700 MHz29 and at 395 GHz/600 MHz and 527 GHz/800 MHz [http://www.bruker.com/products/mr/nmr/dnp-nmr/overview.html]. Thus, DNP is rapidly moving to higher frequency where the chemical shift resolution will improve and additional systems will become accessible.
Conclusions
There are currently two important mechanisms that mediate high field DNP processes, namely the SE and the CE. In addition, there are a number of important experimental factors that influence the magnitudes of the enhancements, including microwave power, temperature, and the nature of the polarizing agent. With currently available technology — gyrotron microwave sources, MAS at 80 K, biradical polarizing agents, and partially deuterated proteins — it is possible to obtain enhancements of ≥100 on many samples. This enhancement, together with the improved Boltzmann factor of 300 K/80 K=3.75 due to the lower temperature, yields sensitivity gains of ≥375 and time savings of >105. Historically, increases in sensitivity of NMR experiments by factors of 102–103 have dramatically changed the landscape of what is possible with NMR, and we are beginning to witness the next step in this movement due to high frequency DNP. We have illustrated this point with applications of MAS DNP experiments to membrane proteins and fibrils which are typical of the biological materials that will be studied in the future. These results clearly illustrate that many experiments that are not possible sans DNP, become feasible avec DNP. Thus, it is clear that the increased availability of commercial instruments to perform DNP experiments will open many new avenues of scientific and technical endeavor.
Acknowledgments
We thank Jeffrey Bryrant, Ajay Thakkar, David J. Ruben for their extensive technical assistance, and Drs. Christopher Turner, Bjorn Corzilius, Yongchao Su and Marvin J. Bayro for their insightful discussions. This work was supported by National Institute of Health Grants EB002804, EB001960, EB003151, EB001035, GM095843 and EB002026.
Biographies
Qing Zhe Ni received the B.S. degree in chemistry, with minors in physics and mathematics, summa cum laude from Florida State University in 2010. She is currently pursuing the Ph.D. degree in the Department of Chemistry at MIT.
Eugenio Daviso received his Laurea degree in Industrial Chemistry from the Università degli Studi di Torino in 2001, and his Ph.D. in Chemistry with Prof. Huub de Groot and Dr. Joerg Matysik in the Netherlands in 2008. He is currently a postdoc at the Francis Bitter Magnet Laboratory and Department of Chemistry, MIT.
Thach V. Can studied physics at Vietnam National University, Hanoi, and then received his M.S. from the Florida State University. Currently he is a Ph.D. student in the Department of Chemistry at MIT.
Evgeny Markhasin obtained his M.S. degree from the University of Nizhniy Novogorod in Russia and is currently a Ph.D. student in the Chemistry Department at MIT.
Sudheer K. Jawla received his M.Sc. degree in physics from the IIT-Delhi, New Delhi, India, in 2004 and Ph.D. degree in physics from the EPFL, Lausanne, Switzerland in 2010. He is currently a postdoc at Plasma Science and Fusion Center, MIT.
Judy Herzfeld received her B.A. degree in Chemistry from the Barnard College in 1967, her Ph.D. in chemical physics from MIT in 1972, and an M.P.P from the Kennedy School of Government at Harvard University in 1973. She has served on the faculties of Amherst College, Harvard Medical School and, since 1985, the Department of Chemistry at Brandeis University.
Robert G. Griffin received his B.S. degree in 1964 from the University of Arkansas, and his Ph.D. from Washington University, St. Louis, MO, in 1969. He has been at the Francis Bitter Magnet Laboratory since 1972 and a faculty member in the Department of Chemistry at MIT since 1998.
Timothy M. Swager received his B.S. from Montana State University and Ph.D. from CalTech. He is currently Professor of Chemistry at MIT and one of his many interests is the synthesis of new polarizing agents for DNP.
Richard J. Temkin received his B.A. degree in physics from Harvard College, and his M.A. and Ph.D. degrees in physics from MIT. Since 1974, he has been with MIT, first at the Francis Bitter National Magnet Laboratory and later at the Plasma Science and Fusion Center (PSFC) and the Department of Physics.
Footnotes
Author Contributions:
The manuscript was written through contributions of all authors. All authors have given approval to the final version of the manuscript.
References
- 1.Griffin RG. Dipolar recoupling in MAS spectra of biological solids. Nature Structural Biology. 1998;5:508–512. doi: 10.1038/749. [DOI] [PubMed] [Google Scholar]
- 2.Rienstra CM, Hohwy M, Mueller LJ, Jaroniec CP, Reif B, Griffin RG. Determination of multiple torsion-angle constraints in U-13C,15N-labeled peptides: 3D 1H-15N-13C-1H dipolar chemical shift spectroscopy in rotating solids. J Am Chem Soc. 2002;124:11908–11922. doi: 10.1021/ja020802p. [DOI] [PubMed] [Google Scholar]
- 3.Thompson LK, McDermott AE, Raap J, van der Wielen CM, Lugtenburg J, Herzfeld J, Griffin RG. Rotational Resonance NMR Study of the Active Site Structure in Bacteriorhodopsin: COnformation of the Schiff Base Linkage. Biochemistry. 1992;31:7931. doi: 10.1021/bi00149a026. [DOI] [PubMed] [Google Scholar]
- 4.Griffiths JM, Lakshmi KV, Bennett AE, Raap J, Vanderwielen CM, Lugtenburg J, Herzfeld J, Griffin RG. Dipolar Correlation NMR-Spectroscopy of a Membrane-Protein. J Am Chem Soc. 1994;116:10178–10181. [Google Scholar]
- 5.Cady SD, Schmidt-Rohr K, Wang J, Soto C, DeGrado W, Hong M. Structure of the amantadine binding site of influenza M2 proton channels in lipid bilayers. Nature. 2010;463:689–692. doi: 10.1038/nature08722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Castellani F, van Rossum B, Diehl A, Schubert M, Rehbein K, Oschkinat H. Structure of a protein determined by solid-state magic-angle-spinning NMR spectroscopy. Nature. 2002;420:98–102. doi: 10.1038/nature01070. [DOI] [PubMed] [Google Scholar]
- 7.Jaroniec CP, MacPhee CE, Bajaj VS, McMahon MT, Dobson CM, Griffin RG. High Resolution Molecular Structure of a Peptide in an Amyloid Fibril Determined by MAS NMR Spectroscopy. Proc Nat’l Acad Sci. 2004;101:711–716. doi: 10.1073/pnas.0304849101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Tycko R. Molecular structure of amyloid fibrils: insights from solid-state NMR. Quarterly Reviews of Biophysics. 2006;39:1–55. doi: 10.1017/S0033583506004173. [DOI] [PubMed] [Google Scholar]
- 9.Wasmer C, Lange A, Van Melckebeke H, Siemer AB, Riek R, Meier BH. Amyloid fibrils of the HET-s(218–289) prion form a beta solenoid with a triangular hydrophobic core. Science. 2008;319:1523–1526. doi: 10.1126/science.1151839. [DOI] [PubMed] [Google Scholar]
- 10.Bayro MJ, Maly T, Birkett N, MacPhee C, Dobson CM, Griffin RG. High-resolution MAS NMR analysis of PI3-SH3 amyloid fibrils: Backbone conformation and implications for protofilament assembly and structure. Biochemistry. 2010;49:7474–7488. doi: 10.1021/bi100864t. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Maly T, Debelouchina GT, Bajaj VS, Hu K-N, Joo C-G, Mak-Jurkauskas ML, Sirigiri JR, Wel PCAvd, Herzfeld J, Temkin RJ, Griffin RG. Dynamic Nuclear Polarization at High Magnetic Fields. J Chem Physics. 2008;128:052211. doi: 10.1063/1.2833582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Haze O, Corzilius B, Smith AA, Griffin RG, Swager TM. Water-Soluble Narrow-Line Radicals for Dynamic Nuclear Polarization. J Am Chem Soc. 2012;134:14287–14290. doi: 10.1021/ja304918g. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Song C, Hu KN, Joo CG, Swager TM, Griffin RG. TOTAPOL – A Biradical Polarizing Agent for Dynamic Nuclear Polarization Experiments in Aqueous Media. J Am Chem Soc. 2006;128:11385–90. doi: 10.1021/ja061284b. [DOI] [PubMed] [Google Scholar]
- 14.Matsuki Y, Maly T, Ouari O, Lyubenova S, Herzfeld J, Prisner T, Tordo P, Griffin RG. Dynamic Nuclear Polarization using a Rigid Biradical. Angewandte Chemie. 2009;48:4996–5000. doi: 10.1002/anie.200805940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Goldman M. Spin Temperature and Nuclear Magnetic Resonance in Solids. Oxford University Press; London: 1970. [Google Scholar]
- 16.Becerra LR, Gerfen GJ, Temkin RJ, Singel DJ, Griffin RG. Dynamic Nuclear Polarization with a Cyclotron Resonance Maser at 5 T. Phys Rev Lett. 1993;71:3561–3564. doi: 10.1103/PhysRevLett.71.3561. [DOI] [PubMed] [Google Scholar]
- 17.Hu KN, Bajaj VS, Rosay MM, Griffin RG. High Frequency Dynamic Nuclear Polarization Using Mixtures of TEMPO and Trityl Radicals. J Chem Phys. 2007;126:044512. doi: 10.1063/1.2429658. [DOI] [PubMed] [Google Scholar]
- 18.Corzilius B, Smith AA, Barnes AB, Luchinat C, Bertini I, Griffin RG. High Field Dynamic Nuclear Polarization with High-Spin Transition Metal Ions. Jour Amer Chem Soc. 2011;133:5648–5651. doi: 10.1021/ja1109002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hu KN, Yu HH, Swager TM, Griffin RG. Dynamic nuclear polarization with biradicals. J Am Chem Soc. 2004;126:10844–10845. doi: 10.1021/ja039749a. [DOI] [PubMed] [Google Scholar]
- 20.Hu KN, Song C, Yu H-h, Swager TM, Griffin RG. High-Frequency Dynamic Nuclear Polarization Using Biradicals: A Multifrequency EPR Lineshape Analysis. J Chem Phys. 2008;128:052321. doi: 10.1063/1.2816783. [DOI] [PubMed] [Google Scholar]
- 21.Hu KN, Debelouchina GT, Smith AA, Griffin RG. Quantum mechanical theory of dynamic nuclear polarization in solid dielectrics. J Chem Physics. 2011;134:125105. doi: 10.1063/1.3564920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Schweiger A, Jeschke G. Principles of pulsed electron paramagnetic resonance. Oxford University Press; 2001. [Google Scholar]
- 23.Bajaj VS, Farrar CT, Hornstein MK, Mastovsky I, Vieregg J, Bryant J, Elena B, Kreischer KE, Temkin RJ, Griffin RG. Dynamic nuclear polarization at 9T using a novel 250 GHz gyrotron microwave source. J Mag Res. 2003;160:85–90. doi: 10.1016/j.jmr.2011.09.010. [DOI] [PubMed] [Google Scholar]
- 24.Rosay M, Lansing JC, Haddad KC, Bachovchin WW, Herzfeld J, Temkin RJ, Griffin RG. High Frequency Dynamic Nuclear Polarization in MAS Spectra of Membrane and Soluble Proteins. J Am Chem Soc. 2003;125:13626–27. doi: 10.1021/ja036898k. [DOI] [PubMed] [Google Scholar]
- 25.Barnes AB, Corzilius B, Mak-Jurkauskas ML, Andreas LB, Bajaj VS, Matsuki Y, Belenky ML, Lugtenburg J, Sirigiri JR, Temkin RJ, Herzfeld J, Griffin RG. Resolution and polarization distribution in cryogenic DNP/MAS experiments. Phys Chem Chem Phys. 2010:12. doi: 10.1039/c003763j. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Becerra LR, Gerfen GJ, Bellew BF, Bryant JA, Hall DA, Inati SJ, Weber RT, Un S, Prisner TF, McDermott AE, Fishbein KW, Kreischer KE, Temkin RJ, Singel DJ, Griffin RG. A Spectrometer for Dynamic Nuclear-Polarization and Electron- Paramagnetic-Resonance at High-Frequencies. Journal of Magnetic Resonance Series A. 1995;117:28–40. [Google Scholar]
- 27.Bajaj VS, Hornstein MK, Kreischer KE, Sirigiri JR, Woskov PP, Mak-Jurkauskas ML, Herzfeld J, Temkin RJ, Griffin RG. 250 GHz CW gyrotron oscillator for dynamic nuclear polarization in biological solid state NMR. J Magn Reson. 2007;189:251–279. doi: 10.1016/j.jmr.2007.09.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Barnes AB, Nanni EA, Griffin RG, Temkin RJ. A 250 GHz gyrotron with a 3 GHz tuning bandwidth for dynamic nuclear polarization. J Magn Reson. 2012;221:147–153. doi: 10.1016/j.jmr.2012.03.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Barnes AB, Markhasin E, Daviso E, Michaelis VK, Nanni EA, Jawla S, Mena E, DeRocher R, Thakkar A, Woskow P, Herzfeld JRJT, Griffin RG. Dynamic Nuclear Polarization at 700 MHz/460 GHz. J Magn Reson. 2012;221:1–7. doi: 10.1016/j.jmr.2012.08.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Barnes AB, Mak-Jurkauskas ML, Matsuki Y, Bajaj VS, Wel PCAvd, DeRocher R, Bryant J, Sirigiri JR, Temkin RJ, Lugtenburg J, Herzfeld J, Griffin RG. Cryogenic sample exchange NMR probe for magic angle spinning dynamic nuclear polarization. Jour Magnetic Resonance. 2009;198:261–270. doi: 10.1016/j.jmr.2009.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Thurber KR, Yau WM, Tycko R. Low-temperature dynamic nuclear polarization at 9.4 T with a 30 mW microwave source. Jour Magn Resonance. 2010;204:303–313. doi: 10.1016/j.jmr.2010.03.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Gerfen GJ, Becerra LR, Hall DA, Griffin RG, Temkin RJ, Singel DJ. High-Frequency (140 GHz) Dynamic Nuclear-Polarization - Polarization Transfer to a Solute in Frozen Aqueous-Solution. J Chem Phys. 1995;102:9494–9497. [Google Scholar]
- 33.Bajaj VS, Mak-Jurkauskas ML, Belenky M, Herzfeld J, Griffin RG. Functional and shunt states of bacteriorhodopsin resolved by 250-GHz dynamic nuclear polarization-enhanced solid-state NMR. Proc Nat’l Acad Sci. 2009;106:9244–49. doi: 10.1073/pnas.0900908106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Bayro MJ, Debelouchina GT, Eddy MT, Birkett NR, MacPhee CE, Rosay M, Maas WE, Dobson CM, Griffin RG. Intermolecular structure determination of amyloid fibrils with magic-angle spinning, dynamic nuclear polarization NMR. J Am Chem Soc. 2011;133:13967–13974. doi: 10.1021/ja203756x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kiesewetter M, Corzilius B, Smith AA, Griffin RG, Swager TM. Dynamic Nuclear Polarization with a Water-soluble Rigid Biradical. J Am Chem Soc. 2012;134:4537–4540. doi: 10.1021/ja212054e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Rosay M, Weis V, Kreischer KE, Temkin RJ, Griffin RG. Two-dimensional 13C-13C correlation spectroscopy with magic angle spinning and dynamic nuclear polarization. J Am Chem Soc. 2002;124:3214–3215. doi: 10.1021/ja0176752. [DOI] [PubMed] [Google Scholar]
- 37.Akbey Ü, Franks WT, Linden A, Lange S, Griffin RG, Rossum B-Jv, Oschkinat H. Dynamic Nuclear Polarization of Deuterated Proteins. Angewandte Chemie International Edition. 2010;49:7803–7806. doi: 10.1002/anie.201002044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Harbison GS, Herzfeld J, Griffin RG. Solid-State 15N Nuclear Magnetic-Resonance Study of the Schiff-Base in Bacteriorhodopsin. Biochemistry. 1983;22:1–5. doi: 10.1021/bi00270a600. [DOI] [PubMed] [Google Scholar]
- 39.Harbison GS, Smith SO, Pardoen JA, Courtin JML, Lugtenburg J, Herzfeld J, Mathies RA, Griffin RG. Solid-State C-13 Nmr Detection of a Perturbed 6-S-Trans Chromophore in Bacteriorhodopsin. Biochemistry. 1985;24:6955–6962. doi: 10.1021/bi00345a031. [DOI] [PubMed] [Google Scholar]
- 40.Jaroniec CP, Filip C, Griffin RG. 3D TEDOR NMR experiments for the simultaneous measurement of multiple carbon-nitrogen distances in uniformly C-13, N-15- labeled solids. J Am Chem Soc. 2002;124:10728–10742. doi: 10.1021/ja026385y. [DOI] [PubMed] [Google Scholar]
- 41.Michaelis VK, Markhasin E, Daviso E, Herzfeld J, Griffin RG. Dynamic Nuclear Polarization of Oxygen-17. J Phys Chem Lett. 2012;3:2030–2034. doi: 10.1021/jz300742w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Vitzthum V, Mieville P, Carnevale D, Caporini MA, Gajan D, Cope C, Lelli M, Zagdoun A, Rossini AJ, Lesage A, Emsley L, Bodenhausen G. Dynamic nuclear polarization of quadrupolar nuclei using cross polarization from protons: surface-enhanced aluminium-27 NMR. Chem Commun. 2012;48:1988–1990. doi: 10.1039/c2cc15905h. [DOI] [PubMed] [Google Scholar]