Abstract
Serotonin (5-HT), norepinephrine and orexins (ORX) are the three best established mediators of wake-related activation of hypoglossal (XII) motoneurons that innervate the muscles of the tongue. Since the tongue’s use is temporarily closely aligned with the rest-activity cycle, we tested whether expression of mRNA for relevant 5-HT, norepinephrine and ORX receptors varies in the XII nucleus with the rest-activity cycle. Adult rats (n=7–9/group) were decapitated at 8–9 am (near rest period onset) or at 6–7 pm (near active period onset). Tissue micropunches were extracted from medullary slices containing the XII motor and sensory external cuneate (ECN) nuclei. 5-HT2A, α1-adrenergic and ORX type 2 receptor mRNAs were quantified using RT-PCR. Only 5-HT2A receptor mRNA levels differed between the two time points and were higher at the active period onset; no differences were detected in the ECN. Consistent with the mRNA results, 5-HT2A protein levels were also higher in the XII nucleus at the active period onset than at rest onset. Thus, the endogenous serotonergic excitatory drive to XII motoneurons may be enhanced through circadian- or activity-dependent mechanisms that increase the availability of 5-HT2A receptors prior to the active period. Conversely, reduced levels of 5-HT2A receptors during the rest/sleep period may exacerbate the propensity for sleep-disordered breathing in subjects with anatomically compromised upper airway.
Keywords: circadian rhythm, norepinephrine, orexin, serotonin, sleep, tongue, obstructive sleep apnea, upper airway
1. INTRODUCTION
Hypoglossal (XII) motoneurons innervate the muscles of the tongue (genioglossus, hyoglossus, and geniohyoid). Under the normal, healthy conditions, motor functions of the tongue, such as food/fluid intake and phonation, are closely aligned with the rest-activity cycle. However, in subjects with anatomically compromised upper airway, activation of the tongue muscles is also required to maintain sufficient upper airway patency for breathing. In such subjects, upper airway muscle tone, including that of the tongue muscles, is elevated during wakefulness but declines during sleep, which leads to recurrent periods of flow limitation or complete upper airway obstructions, episodic hypoxemia and sleep disruption (Sauerland and Harper, 1976; Remmers et al., 1978; Suratt et al., 1988; Mezzanotte et al., 1992; Okabe et al., 1994; Katz and White, 2004; see Kubin and Davies, 2011; Horner, 2012; White and Younes, 2012 for reviews).
Similar to healthy humans (Chokroverty, 1980; Kuna et al., 1994; Katz and White, 2004; Brown et al., 2011), in rats, activity of the tongue muscles and XII motoneurons is high and variable during wakefulness, very low or absent during slow-wave sleep (SWS), and generally absent but punctuated by often large phasic twitches during rapid eye movement sleep (REMS) (Megirian et al., 1978; Lu et al., 2005; Lu and Kubin, 2009; Rukhadze et al., 2011). In obstructive sleep apnea (OSA) patients and rats with experimentally enhanced upper airway muscle tone through the means such as vagotomy, application of stimulants into the XII nucleus or chemical stimulation of breathing, XII motoneurons also exhibit inspiratory modulation (Hwang et al., 1983; Jelev et al., 2001; Morrison et al., 2003; Fenik et al., 2005) which in subjects with anatomically compromised upper airway plays an important role in protecting the airway against obstructions at the time when inspiratory negative pressure exerts a centripetal force on the airway walls. Thus, the accessory respiratory function of XII motoneurons in healthy subjects becomes extremely important in subjects with anatomical conditions that make the upper airway vulnerable to collapse, with both the tonic and inspiratory-modulated activity required to maintain adequate ventilation.
Several distinct neurochemical systems have been identified that collectively mediate the wakefulness-related excitatory effects onto XII motoneurons, thereby facilitating those functions of the tongue that are typically performed during wakefulness, as well as maintaining tongue activity in OSA patients. Of those, the best evidence is currently available for norepinephrine (NE) and serotonin (5-HT) which are released from brainstem cells and for orexins (ORX) which are produced by neurons located in the posterior, lateral hypothalamus (Aston-Jones and Bloom, 1981; Trulson and Trulson, 1982; Estabrooke et al., 2001; Fenik et al., 2002; Lee et al., 2005; Mileykovskiy et al., 2005; Takahash et al., 2008). NE, 5-HT and ORX cells have maximal activity during wakefulness, reduced activity during SWS and minimal or no activity during REMS. Terminals containing NE, 5-HT and ORX are present in the XII nucleus, and XII motoneurons express α1-adrenergic, 5-HT2 and ORX type 2 receptors that are all known to mediate excitatory effects (Kubin et al., 1992; Funk et al., 1994; Peyron et al., 1998; Fay and Kubin, 2000; Marcus et al., 2001; Volgin et al., 2001, 2002, 2003; Zhan et al., 2002; Fenik and Veasey, 2003). There is also pharmacological evidence that these modulators mediate major portions of the endogenous excitatory drives that maintain activity of XII motoneurons (Kubin et al., 1992; Fenik et al., 2005; Chan et al., 2006; Stettner and Kubin, 2013).
Under normal conditions, motor activity of the tongue is temporarily closely aligned with the rest-activity cycle. Mechanistically, this may be related to the fact that major excitatory drives to XII motoneurons originate in the premotor circuits that have sleep-wake dependent patterns of activity. However, it is also plausible that the sensitivity of XII motoneurons to these drives is regulated in a circadian manner with a pattern that reinforces the ability of the state-dependent systems to activate XII motoneurons at the time when it is most appropriate, i.e., during the active phase of the circadian cycle. Such a hypothetical mechanism could relay on a circadian- or use-dependent regulation of the availability of excitatory receptors in XII motoneurons. Indeed, 5-HT binding in different regions of the rat brain varies with the circadian time, suggesting that regulation of receptor availability is a potentially important mechanism by which effectiveness of neurotransmitter actions is aligned to the rest-activity cycle (Wesemann et al., 1983; Wesemann and Weiner, 1990; Weiner et al., 1992). Accordingly, our goal was to test whether such mechanisms operate for NE, 5-HT or ORX receptors located in the XII nucleus, as this would add a new dimension to both the normal regulation of tongue activity across the rest-activity cycle and the ability of central excitatory premotor pathways to maintain upper airway patency in OSA patients.
We found that, of the three receptor systems investigated, 5-HT2A receptor mRNA and protein levels were higher in the XII nucleus at the active period onset than at the rest onset. This may result in a relatively enhanced endogenous serotonergic excitatory drive to XII motoneurons during the active period and a relatively reduced ability of 5-HT to activate XII motoneurons during the rest/sleep period. Preliminary data have been published (Kubin et al., 2010; Volgin et al., 2010).
2. MATHERIALS AND METHODS
Experiments were performed on 18 adult, male Sprague-Dawley rats (300–385g). All animal handling procedures followed the guidelines of the Guide for the Care and Use of Laboratory Animals of the National Institutes of Health and were approved by the Institutional Animal Care and Use Committee of the University of Pennsylvania.
2.1. Tissue extraction and mRNA quantification procedures
The animals were housed on a 12:12 light/dark schedule with lights on at 7 am and ad libitum access to food and water. They were decapitated under deep isoflurane anesthesia (4%) either between 8 and 9 am (just after the rest period onset) or between 6 and 7 pm (just prior to the active period onset). The brainstems ware rapidly removed and immersed in ice-cold artificial cerebrospinal fluid containing (in mM): 135 NaCl, 5 KCl, 1 CaCl2, 1 MgCl2, 10 HEPES, 10 glucose, and 20 mannitol; pH adjusted with NaOH to 7.4 and osmolarity set at 300±5 mOsm. The medulla was blocked, immersed in the same ice-cold medium, and transverse sections, 500–600 μm thick, were obtained using a tissue slicer (VSLM1; Lafayette, IN). Tissue micropunches, 500 μm in diameter, were extracted bilaterally from the XII nucleus and, for comparison, from the somatosensory external cuneate nucleus (ECN). From each pair of micropunch samples, one was used for total RNA extraction and the other was stored at −80° C for subsequent protein studies. The slices from which the punches were extracted were fixed in formalin, cut into 25 μm sections, mounted and stained with Neutral red to verify the proper placement of the punches (Fig. 1A).
Figure 1.
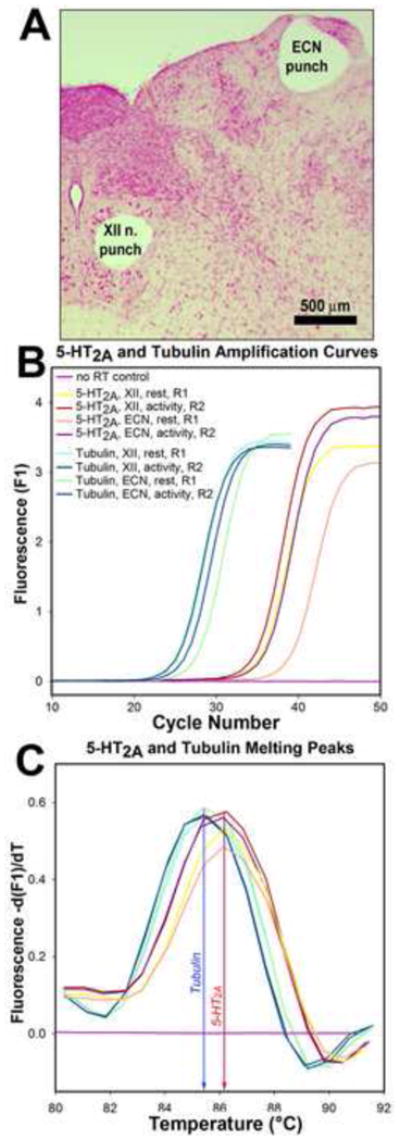
Example of tissue sampling and of the outputs from RT-PCR reactions. A: location of the tissue micropunches extracted from the XII nucleus and external cuneate nucleus (ECN) visualized in a Neutral red-stained section of a medullary slice. B: PCR amplification curves obtained with a set of cDNA samples from the XII nucleus and ECN from two rats, one at the rest period onset (R1) and the other at the active period onset (R2), and from one control mRNA sample that was not reverse-transcribed. C: melting curves for the set of reactions shown in B demonstrates that the PCR reactions yielded two distinct products that had melting peaks at the temperatures expected for the 5-HT2A receptor and tubulin cDNAs.
Total RNA was extracted from each micropunch using the RNeasy® Mini Kit (Qiagen, Valencia, CA), re-dissolved in 50 μl of RNase-free water and quantified by densitometry (BioPhotometer, Eppendorf, Germany). One half of the extract was treated with RNase-free DNase I (Roche Diagnostics, Mannheim, Germany) and reverse-transcribed using SuperScript® IIReverse Transcriptase (Life Technologies, Carlsbad, CA)in a total buffer volume of 50 μl. Subsequent PCR reactions were performed using LightCycler® system (Roche Diagnostics, Indianapolis, IN). Fixed aliquots of each cDNA sample (1 μl) were used for polymerase chain reactions (PCRs) with primer sets for the following genes: α1A- and α1B-adrenergic receptors (Volgin et al., 2001), 5-HT2A and 5-HT2C receptors (Volgin et al., 2003), ORX type 2 receptors (Volgin et al., 2002), and α1-tubulin (Volgin and Kubin, 2006) (see Table 1 for accession numbers). PCR amplification was performed in 20 μl of the reaction buffer containing 250 μM of dNTPs, 200 nM of the primers, 2.5 μl of SYBR Green I cDNA-sensitive dye (Sigma-Aldrich, Saint Louis, MO), 1 μl of cDNA sample, and 0.7 μl of Titanium© Taq DNA polymerase (Clontech, Palo Alto, CA). The PCRs comprised 30 s of initial denaturation at 95° C followed by repeated cycles of a 1 s spike at 95° C and 25 s of combined annealing-elongation at 68° C, and were completed with 30 s of final elongation (Fig. 1B). Subsequently, the products were subjected to linear heating (0.2° C/s) to 95° C to assess the quality of each reaction based on the position and shape of the peaks of the melting curves (Fig. 1C). After cooling, the PCR products were displayed on an ethidium bromide-stained 2% agarose gel to confirm that they were of the appropriate size. PCR reactions were calibrated using external cDNA standards produced in house for each cDNA target, as described previously (Volgin and Kubin, 2006). Ultimately, the target receptor mRNA levels were quantified as the number of cDNA copies per 1,000 copies of the housekeeping gene (tubulin) derived from the same micropunch sample.
Table 1.
Neurotransmitter receptor mRNA levels quantified by RT-PCR near the time of the rest period onset (8–9 am) and near the active period onset (6–7 pm) in the XII nucleus and external cuneate nucleus (ECN).
| Transcript and its accession number | Transcript copy numbers* ±SE | |||||
|---|---|---|---|---|---|---|
| XII nucleus | ECN | |||||
| 8–9 am | 6–7 pm | p value | 8–9 am | 6–7 pm | p value | |
| 5-HT2A M30705 | 5.2±2.8 | 14.9±3.7 | 0.03 | 0.7±0.3 | 1.8±0.8 | 0.09 |
|
| ||||||
| 5-HT2C M21410 | 49.7±14.0 | 42.4±10.0 | 0.82 | 8.2±3.5 | 7.3±2.4 | 0.64 |
|
| ||||||
| α1AAR U07126 | 63.5±18.1 | 44.8±8.1 | 0.38 | 41.4±13.6 | 67.9±17.1 | 0.12 |
|
| ||||||
| α1BAR L08609 | 20.0±2.9 | 23.1±5.2 | 0.59 | 6.5±1.4 | 13.4±3.0 | 0.06 |
|
| ||||||
| ORX 2R AF041246 | 1291±476 | 711±175 | 0.77 | 807±255 | 1656±1038 | 0.85 |
|
| ||||||
| Tubulin, α1NM_022298 (absolute) | 9426±2191 | 11864±3277 | 0.54 | 3767±913 | 3496±1064 | 0.77 |
Receptor mRNA levels were quantified at each time point and anatomical location as the number of cDNA copies of the target cDNA per 1,000 copies of the housekeeping gene, tubulin, in the same sample. The bottom row shows the mean absolute cDNA copy numbers per sample for tubulin. p values describe for each transcript the statistical significance of the difference between the samples collected at the two different time points from the XII nucleus or the ECN (n=7–9 rats per group).
2.2. 5-HT2A receptor protein quantification
The amount of protein available from a single tissue micropunch like those collected in this study is, at best, sufficient for quantification of one selected target protein and one reference protein. Since our mRNA data pointed to 5-HT2A receptors as the ones whose levels were likely to vary with circadian time, we used the tissue punches that remained available after the mRNA study to quantify the levels of 5-HT2A receptor-like protein in the XII nucleus and ECN. Each micropunch sample was sonicated in 12 μl of the solubilizing buffer containing 7 M of urea, 2 M of thiourea, 0.25 mM Tris base, 4.0% 3-[(3-cholamidopropyl)dimethylammonio]-1-propanesulfonate( CHAPS)and 1.0% NP -40. Subsequently, proteins were separated by size on an SDS-PAGE gel and transferred onto nitrocellulose membranes (Mini-Protean-3 system, BioRad, Hercules, CA). Albumin-blocked membranes were incubated overnight with rabbit antibodies against 5-HT2Areceptors (1:250, catalog no. 24288, ImmunoStar, Hudson, WI) and β-actin (1:1,000, catalog no. 4970, Cell Signaling, Danvers, MA) at 4 C. Primary antibody binding sites were visualizedby incubation with donkey ECL anti -rabbit, horseradish peroxidase-conjugated IgG (1:10,000, catalog no. NA934V, GE Healthcare, Mickleton, NJ) and the SuperSignal West Dura chemiluminescent substrate (Pierce/Thermo Scientific, Hudson, NH). The chemiluminescent signal was detected using HyBlot CL autoradiography film (Denville Scientific, South Plainfield, NJ). Digital images of the lab eled bands corresponding to 5-HT2A receptor protein and β-actin were quantified using ImageJ software(National Institutes of Health; http://rsb.info.nih.gov/ij/index.html). After subtraction of background staining (Fig. 2B), the amounts of 5-HT2A and β-actin proteins were measured by integration of the intensity of staining within each band obtained from each micropunch sample and the amount of 5-HT2A protein was expressed relative to the density of the band for β-actin in the same tissue sample (Fig. 2C).
Figure 2.
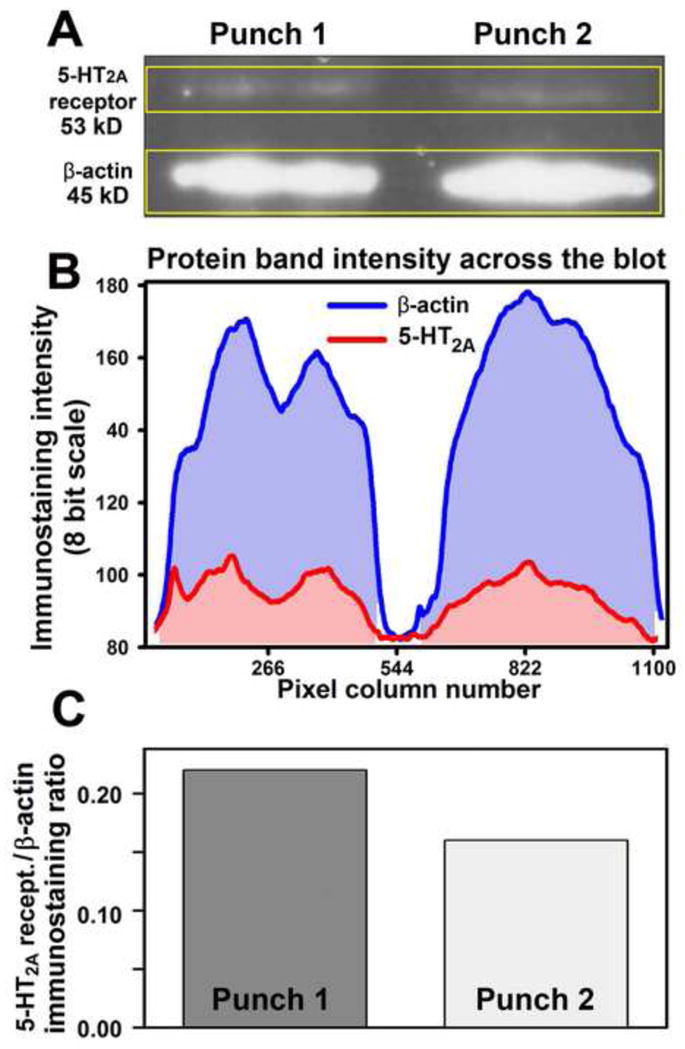
Quantification of 5-HT2A receptor protein in tissue micropunches by Western blotting. A: image of a blot double-labeled for 5-HT2A receptor protein and β-actin with two protein samples (lanes) obtained from tissue micropunches. The yellow frames enclose the areas scanned for densitometric measurements of protein amounts in each band. B: staining intensity for each of the two bands measured across the gel shown in A obtained after background subtraction. C: bars representing the ratios of 5-HT2A receptor to β-actin protein amounts for the data illustrated in A and B.
2.3. Statistical analysis
All datasets with continuous variables were tested for normality and equal variance (Analyse-It, Leeds, UK). The significance of differences between mRNA or protein levels in tissue samples harvested from each of the two anatomic regions at the two different circadian times was examined using one-way ANOVA followed by post-hoc comparisons for each receptor subtype with Bonferroni’s correction for multiple comparisons. If normality criteria were not fulfilled, nonparametric analysis was performed using Kruskal-Wallis ANOVA. The variability of the means is characterized by the standard error (SE), and the differences were considered significant at p<0.05.
3. RESULTS
3.1. Receptor mRNA quantification in the XII nucleus and ECN
We compared mRNA levels for selected receptors that mediate wake-related activation of XII motoneurons between two time points separated by about 12 h and selected to represent a period just after the circadian rest phase onset and just before the active phase onset. When quantified relative to mRNA levels for tubulin, 5-HT2A receptor mRNA levels were significantly higher in the XII nucleus at the active period onset than at the rest period onset. For all other receptors studied (5-HT2C, α1A- and α1B-adrenergic, and ORX type 2), the differences between the two time points were not statistically significant (Table 1). In the ECN, the average mRNA levels of 5-HT2A, 5-HT2C and α1B adrenergic receptors were considerably lower than in the XII nucleus (p=0.002–0.09) and no significant differences were detected between the two time points (Table 1). It may be, however, of note that mRNA levels for 5-HT2A and α1B-adrenergic receptors exhibited relative prominent tendencies towards higher levels at the active period onset than at rest onset (p=0.09 and p=0.06, respectively).
3.2. 5-HT2A receptor protein levels in the XII nucleus and ECN
With the difference in 5-HT2A receptor mRNA levels in the XII nucleus between the rest onset and active period onset of a nearly 3-fold order and a moderate tendency in the same direction in the ECN, we then measured by means of Western blots whether the differences in 5-HT2A receptor protein levels followed the same pattern. We found a significantly higher 5-HT2A receptor to β-actin protein ratio in the XII nucleus samples collected at the active period onset when compared to the rest onset (2.0±0.3 vs. 1.0±0.2; p<0.02; Fig. 3, top panel). As with 5-HT2A receptor mRNA, the mean level of 5-HT2A receptor protein was considerably lower in the ECN than in the XII nucleus, and there was only a weak trend in the ECN for the relative level of the 5-HT2A receptor protein to be higher at the active period onset than at the rest onset (p=0.25; Fig. 3, bottom panel).
Figure 3.
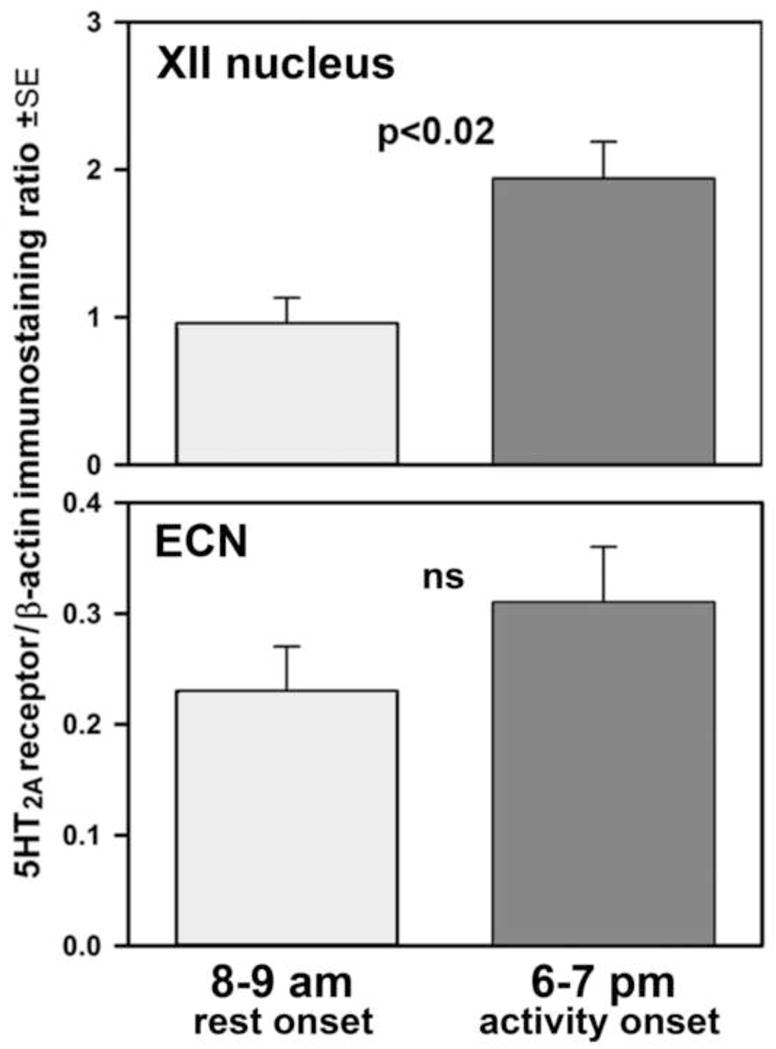
Mean 5-HT2A receptor protein levels in micropunches extracted from the XII nucleus (top panel) and external cuneate nucleus region (ECN; bottom panel) at rest onset (8–9 am) and active period onset (6–7 pm) normalized by the β-actin contents in the same protein samples (n=6–8 rats per group).
4. DISCUSSION
Our main finding is that, among the three distinct receptor systems that mediate wake-related activation of XII motoneurons, the levels of at least one receptor subtype, the 5-HT2A, exhibit a distinct and statistically significant difference in the XII nucleus between the time corresponding to the onset of the rest period and the time just preceding the active period when quantified at either the mRNA or protein levels. Importantly the direction of the difference is such that it should favor a stronger activation of XII motoneurons by 5-HT during the early part of the active period when compared to the beginning of the rest period. In contrast to 5-HT2A receptors, no differences between these two time points were suggested by measurements of mRNA levels for 5-HT2C receptor (another excitatory 5-HT receptor subtype present in the XII nucleus), nor for the α1-adrenergic or ORX type 2 receptors. Furthermore, no significant changes for any of these receptors were detected in ECN, a somatosensory nucleus located in the dorsal medulla relatively close to the XII motor nucleus. These results suggest that the mechanisms driven by the central circadian clock(s) or those secondary to the natural circadian pattern of tongue use can selectively influence the availability of one of several receptor systems that are known to mediate excitatory drive to XII motoneurons and are established mediators of their wake-related activation. As such, our findings unveil a potential mechanism by which the motor output to the tongue muscles is enhanced, or reinforced, during the time most appropriate for the use of the tongue (active period). Conversely, the same mechanism may also be seen as designed to reduce, or disfacilitate, activation of the tongue during the rest period when rats normally sleep and have a reduced need for complex and strong activation of the tongue muscles.
Our present measurements were limited to only two time points separated by approximately 12 h. We used this experimental design because it allows one to grossly assess circadian differences in receptor levels. With this limited approach, we were able to detect a large and statistically significant day-night difference for 5-HT2A receptors that consistently occurred at both the mRNA and protein levels. However, due to the limited temporal density of tissue sampling, our design might have been sub-optimal for capturing the full peak and trough of the process that determines 5-HT2A receptor levels in the XII nucleus, and it is possible that the other receptors for which we did not detect any circadian changes would exhibit circadian variations when tested more frequently or at different phases of the circadian cycle.
Nevertheless, our present positive findings with 5-HT2A receptors are important because they reveal a novel and little investigated mechanisms whose function might be to best align motoneuronal excitability with the active phase of the circadian cycle to achieve optimal motor performance. Additional studies with more frequent tissue sampling may reveal further intricacies of the processes that optimize the central control of the motor output relative to the circadian phases of rest and activity.
The rest-active period difference that we detected may be driven by the central circadian clock, the light-dark cycle, or the closely associated with these variables circadian changes in motoneuronal activity. In mammals, the main circadian clock is located in the suprachiasmatic nucleus (SCN) of the hypothalamus but multiple secondary molecular circadian pacemaker circuits are present in other brain regions, as well as in peripheral tissues and organs (Yamazaki et al., 2000; Reppert and Weaver, 2002; Yoo et al., 2004; Herichová et al., 2007; Kaneko et al., 2009; Hughes et al., 2012). Under most conditions, these additional clocks are synchronized by the neural and humoral outputs from the SCN but they are also capable of controlling the functions of different neural circuits and organs in a relatively autonomous manner. For example, in the hypertensive transgenic TGR(mRen-2)27 rats, normal circadian rhythms of blood pressure are inverted, with relatively higher blood pressure during the rest (light) phase than during the active (dark) phase, whereas circadian rhythms of heart rate and locomotor activity are normal (Lemmer et al., 1993; Witte and Lemmer, 1999). These rats have altered profiles of rhythmic expression of clock genes in the SCN and in the medullary regions that control arterial blood pressure (nucleus of the solitary tract and rostral ventrolateral medulla) (Herichová et al., 2007). In fruit flies (Drosophila), circadian rhythms of motor activity are associated with rhythmic circadian changes in the size of synaptic boutons on flight motoneurons that occur independently of synaptic activity or disruption of the rest-activity cycle and persist when motoneurons are disconnected from other major circadian clocks (Mehnert et al., 2007; Mehnert and Cantera, 2008). Thus, the neural circuits important for supporting the most essential functions of the organism appear to be equipped with their own mechanisms that optimize performance relative to the fundamental circadian rhythm of rest and activity both in mammals and evolutionarily lower species. The same is likely to be the case for mammalian motoneurons, and especially those that support alimentary functions. In rats, food intake is significantly higher during the dark (active) period than during the light (sleep) period (e.g., Madrid et al., 1993). Since the tongue plays a key role in alimentary behaviors, it is possible that the circadian change in 5-HT2A receptor expression that we detected is designed to support this function. Alternatively, it may have been driven by synaptic activity related to the circadian changes in the use of the tongue. Considering that the amounts of sleep, including REMS, vary between the dark and light periods, it is possible that certain cellular, humoral or neurochemical mechanisms specific for the generation of the distinct stages of sleep also contribute to the circadian variation of 5-HT2A receptor levels.
Expression of 5-HT receptors also can be regulated by ligand binding (see Sodhi and Sanders-Bush, 2004 for a review). Brain 5-HT levels exhibit circadian variation and, in rats, they are higher during the active phase than during the rest phase (Sanchez et al., 2008), a mechanism that can also contribute to the circadian variability of long-term facilitation of ventilation (c.f., the article by Mateika and Syed in this Special Issue). A strong stimulation of 5-HT2A receptors during the night may lead to receptor internalization resulting in a circadian variation whereby the initially high levels at the onset of the active period gradually decline with prolonged activation. This, in turn, may stimulate synthesis and transport of new receptors to the cell surface with a time constant of the entire process optimized to go along with the circadian period of rest and activity. Thus, there is a potential that the circadian variation of 5-HT2A receptor levels in the XII nucleus is driven by the circadian variation of 5-HT levels.
Studies conducted in different animal models suggested that the magnitude of endogenous activation of XII motoneurons by 5-HT is species-dependent. It is strong in cats (Kubin et al., 1992; Neuzeret et al., 2009) and dogs (Veasey et al., 1996) but relatively weaker than the effect of NE in rats (Fenik et al., 2005; Sood et al., 2005). However, it is important to note that the studies in rats were conducted during the rest period. Our present data suggest that the excitatory effects of 5-HT on XII motoneurons mediated by 5-HT2A receptors may be limited during the rest period by the receptor availability and that stronger effects could be detected during the active period.
Collectively, our findings suggest that the strength of serotonergic activation of XII motoneurons varies with the circadian cycle and that it is reinforced during the active period by an increased availability of 5-HT2A receptors. Under the normal conditions, this should properly support the main motor functions of the tongue. However, in patients with OSA, reduced levels of 5-HT2A receptors in XII motoneurons during the rest/sleep period would negatively impact the ability of the tongue muscles to protect the upper airway from collapse and to re-open the airway following an obstructive episode. The mechanisms that cause the circadian variation of 5-HT2A receptors in the XII nucleus need additional studies for their clear health relevance for sleep-disordered breathing and their potentially fundamental role in aligning the motor output with the natural periods of rest and activity.
HIGHLIGHTS.
Activity of hypoglossal (XII) motoneurons helps maintain airflow in the upper airway.
Serotonin (5-HT) mediates wake-related activation of XII motoneurons.
5-HT2A receptor levels are higher in XII nucleus at 6–7 pm than at 8–9 am.
Receptor changes in motoneurons help align motor performance with circadian rhythm.
Acknowledgments
This study was supported by the National Institutes of Health grants HL-47600 and HL-116508, and a research fellowship from the Deutsche Forschungsgemeinschaft (DFG Ste1899/1-1) to GMS.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Aston-Jones G, Bloom FE. Activity of norepinephrine-containing locus coeruleus neurons in behaving rats anticipates fluctuations in the sleep-waking cycle. J Neurosci. 1981;1:876–886. doi: 10.1523/JNEUROSCI.01-08-00876.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown EC, Hudson AL, Butler JE, McKenzie DK, Bilston LE, Gandevia SC. Single motor unit recordings in human geniohyoid reveal minimal respiratory activity during quiet breathing. J Appl Physiol. 2011;110:1054–1059. doi: 10.1152/japplphysiol.00454.2010. [DOI] [PubMed] [Google Scholar]
- Chan E, Steenland HW, Liu H, Horner RL. Endogenous excitatory drive modulating respiratory muscle activity across sleep-wake states. Am J Respir Crit Care Med. 2006;174:1264–1273. doi: 10.1164/rccm.200605-597OC. [DOI] [PubMed] [Google Scholar]
- Chokroverty S. Phasic tongue movements in human rapid eye-movement sleep. Neurology. 1980;30:665–668. doi: 10.1212/wnl.30.6.665. [DOI] [PubMed] [Google Scholar]
- Estabrooke IV, McCarthy MT, Ko E, Chou TC, Chemelli RM, Yanagisawa M, Saper CB, Scammell TE. Fos expression in orexin neurons varies with behavioral state. J Neurosci. 2001;21:1656–1662. doi: 10.1523/JNEUROSCI.21-05-01656.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fay R, Kubin L. Pontomedullary distribution of 5-HT2A receptor-like protein in the rat. J Comp Neurol. 2000;418:323–345. [PubMed] [Google Scholar]
- Fenik P, Veasey SC. Pharmacological characterization of serotonergic receptor activity in the hypoglossal nucleus. Am J Respir Crit Care Med. 2003;167:563–569. doi: 10.1164/rccm.200202-107OC. [DOI] [PubMed] [Google Scholar]
- Fenik VB, Davies RO, Kubin L. REM sleep-like atonia of hypoglossal (XII) motoneurons is caused by loss of noradrenergic and serotonergic inputs. Am J Respir Crit Care Med. 2005;172:1322–1330. doi: 10.1164/rccm.200412-1750OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fenik VB, Marchenko V, Janssen P, Davies RO, Kubin L. A5 cells are silenced when REM sleep-like signs are elicited by pontine carbachol. J Appl Physiol. 2002;93:1448–1456. doi: 10.1152/japplphysiol.00225.2002. [DOI] [PubMed] [Google Scholar]
- Funk GD, Smith JC, Feldman JL. Development of thyrotropin-releasing hormone and norepinephrine potentiation of inspiratory-related hypoglossal motoneuron discharge in neonatal and juvenile mice in vitro. J Neurophysiol. 1994;72:2538–2541. doi: 10.1152/jn.1994.72.5.2538. [DOI] [PubMed] [Google Scholar]
- Herichová I, Mravec B, Stebelová K, Krizanová O, Jurkovicová D, Kvetnanský R, Zeman M. Rhythmic clock gene expression in heart, kidney and some brain nuclei involved in blood pressure control in hypertensive TGR(mREN-2)27 rats. Mol Cell Biochem. 2007;296:25–34. doi: 10.1007/s11010-006-9294-4. [DOI] [PubMed] [Google Scholar]
- Horner RL. Neural control of the upper airway: Integrative physiological mechanisms and relevance for sleep disordered breathing. Compr Physiol. 2012;2:479–535. doi: 10.1002/cphy.c110023. [DOI] [PubMed] [Google Scholar]
- Hughes ME, Hong HK, Chong JL, Indacochea AA, Lee SS, Han M, Takahashi JS, Hogenesch JB. Brain-specific rescue of Clock reveals system-driven transcriptional rhythms in peripheral tissue. PLoS Gen. 2012;8:e1002835. doi: 10.1371/journal.pgen.1002835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hwang JC, Bartlett D, Jr, St John WM. Characterization of respiratory-modulated activities of hypoglossal motoneurons. J Appl Physiol. 1983;55:793–798. doi: 10.1152/jappl.1983.55.3.793. [DOI] [PubMed] [Google Scholar]
- Jelev A, Sood S, Liu H, Nolan P, Horner RL. Microdialysis perfusion of 5-HT into hypoglossal motor nucleus differentially modulates genioglossus activity across natural sleep-wake states in rats. J Physiol (Lond) 2001;532:467–481. doi: 10.1111/j.1469-7793.2001.0467f.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaneko K, Yamada T, Tsukita S, Takahashi K, Ishigaki Y, Oka Y, Katagiri H. Obesity alters circadian expressions of molecular clock genes in the brainstem. Brain Res. 2009;1263:58–68. doi: 10.1016/j.brainres.2008.12.071. [DOI] [PubMed] [Google Scholar]
- Katz ES, White DP. Genioglossus activity during sleep in normal control subjects and children with obstructive sleep apnea. Am J Respir Crit Care Med. 2004;170:553–560. doi: 10.1164/rccm.200403-262OC. [DOI] [PubMed] [Google Scholar]
- Kubin L, Davies RO. Mechanisms of upper airway hypotonia. In: Pack AI, editor. Sleep Apnea. Pathogenesis, Diagnosis, and Treatment. 2. Informa Healthcare; St. Helier, Jersey, UK: 2011. pp. 82–127. [Google Scholar]
- Kubin L, Tojima H, Davies RO, Pack AI. Serotonergic excitatory drive to hypoglossal motoneurons in the decerebrate cat. Neurosci Lett. 1992;139:243–248. doi: 10.1016/0304-3940(92)90563-m. [DOI] [PubMed] [Google Scholar]
- Kuna ST, Smickley JS, Vanoye CR, McMillan TH. Cricothyroid muscle activity during sleep in normal adult humans. J Appl Physiol. 1994;76:2326–2332. doi: 10.1152/jappl.1994.76.6.2326. [DOI] [PubMed] [Google Scholar]
- Kubin L, Stettner GM, Volgin DV. Neurosci Meeting Planner Online. Society for Neuroscience; San Diego, CA: 2010. Serotonin (5-HT) type 2A receptor mRNA and protein levels are higher in the rat hypoglossal (XII) motor nucleus at activity onset than at rest onset; p. Abstr 589.6. (Abstract) [Google Scholar]
- Lee MG, Hassani OK, Jones BE. Discharge of identified orexin/hypocretin neurons across the sleep-waking cycle. J Neurosci. 2005;25:6716–6720. doi: 10.1523/JNEUROSCI.1887-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lemmer B, Mattes A, Böhm M, Ganten D. Circadian blood pressure variation in transgenic hypertensive rats. Hypertension. 1993;22:97–101. doi: 10.1161/01.hyp.22.1.97. [DOI] [PubMed] [Google Scholar]
- Lu J, Kubin L. Electromyographic activity at the base and tip of the tongue across sleep-wake states in rats. Respir Physiol Neurobiol. 2009;167:307–315. doi: 10.1016/j.resp.2009.06.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu J, Mann GL, Ross RJ, Morrison AR, Kubin L. Differential effect of sleep-wake states on lingual and dorsal neck muscle activity in rats. Respir Physiol Neurobiol. 2005;147:191–203. doi: 10.1016/j.resp.2005.05.007. [DOI] [PubMed] [Google Scholar]
- Madrid JA, Lopez-Bote C, Martín E. Effect of neonatal androgenization on the circadian rhythm of feeding behavior in rats. Physiol Behav. 1993;53:329–335. doi: 10.1016/0031-9384(93)90213-y. [DOI] [PubMed] [Google Scholar]
- Marcus JN, Aschkenasi CJ, Lee CE, Chemelli RM, Saper CB, Yanagisawa M, Elmquist JK. Differential expression of orexin receptors 1 and 2 in the rat brain. J Comp Neurol. 2001;435:6–25. doi: 10.1002/cne.1190. [DOI] [PubMed] [Google Scholar]
- Megirian D, Cespuglio R, Jouvet M. Rhythmical activity of the rat’s tongue in sleep and wakefulness. EEG Clin Neurophysiol. 1978;44:8–13. doi: 10.1016/0013-4694(78)90100-1. [DOI] [PubMed] [Google Scholar]
- Mehnert KL, Beramendi A, Elghazali F, Negro P, Kyriacou CP, Cantera R. Circadian changes in Drosophila motor terminals. Dev Neurobiol. 2007;67:415–421. doi: 10.1002/dneu.20332. [DOI] [PubMed] [Google Scholar]
- Mehnert KL, Cantera R. A peripheral pacemaker drives the circadian rhythm of synaptic boutons in Drosophila independently of synaptic activity. Cell Tis sRes. 2008;334:103–109. doi: 10.1007/s00441-008-0670-0. [DOI] [PubMed] [Google Scholar]
- Mezzanotte WS, Tangel DJ, White DP. Waking genioglossal electromyogram in sleep apnea patients versus normal controls (a neuromuscular compensatory mechanism) J Clin Invest. 1992;89:1571–1579. doi: 10.1172/JCI115751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mileykovskiy BY, Kiyashchenko LI, Siegel JM. Behavioral correlates of activity in identified hypocretin/orexin neurons. Neuron. 2005;46:787–798. doi: 10.1016/j.neuron.2005.04.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morrison JL, Sood S, Liu H, Park E, Liu X, Nolan P, Horner RL. Role of inhibitory amino acids in control of hypoglossal motor outflow to genioglossus muscle in naturally sleeping rats. J Physiol. 2003;552:975–991. doi: 10.1113/jphysiol.2003.052357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neuzeret PC, Sakai K, Gormand F, Petitjean T, Buda C, Sastre JP, Parrot S, Guidon G, Lin JS. Application of histamine or serotonin to the hypoglossal nucleus increases genioglossus muscle activity across the wake-sleep cycle. J Sleep Res. 2009;18:113–121. doi: 10.1111/j.1365-2869.2008.00708.x. [DOI] [PubMed] [Google Scholar]
- Okabe S, Hida W, Kikuchi Y, Taguchi O, Takishima T, Shirato K. Upper airway muscle activity during REM and non-REM sleep of patients with obstructive apnea. Chest. 1994;106:767–773. doi: 10.1378/chest.106.3.767. [DOI] [PubMed] [Google Scholar]
- Peyron C, Tighe DK, van den Pol AN, de Lecea L, Heller HC, Sutcliffe JG, Kilduff TS. Neurons containing hypocretin (orexin) project to multiple neuronal systems. J Neurosci. 1998;18:9996–10015. doi: 10.1523/JNEUROSCI.18-23-09996.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Remmers JA, deGroot WJ, Sauerland EK, Anch AM. Pathogenesis of upper airway occlusion during sleep. J Appl Physiol. 1978;44:931–938. doi: 10.1152/jappl.1978.44.6.931. [DOI] [PubMed] [Google Scholar]
- Reppert SM, Weaver DR. Coordination of circadian timing in mammals. Nature. 2002;418:935–941. doi: 10.1038/nature00965. [DOI] [PubMed] [Google Scholar]
- Rukhadze I, Kamani H, Kubin L. Quantitative differences among EMG activities of muscles innervated by subpopulations of hypoglossal and upper spinal motoneurons during non-REM sleep - REM sleep transitions: a window on neural processes in the sleeping brain. Arch Ital Biol. 2011;149:499–515. doi: 10.4449/aib.v149i4.1385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanchez S, Sanchez C, Paredes SD, Cubero J, Rodriguez AB, Barriga C. Circadian variations of serotonin in plasma and different brain regions of rats. Mol Cell Biochem. 2008;317:105–111. doi: 10.1007/s11010-008-9836-z. [DOI] [PubMed] [Google Scholar]
- Sauerland EK, Harper RM. The human tongue during sleep: electromyographic activity of the genioglossus muscle. Exp Neurol. 1976;51:160–170. doi: 10.1016/0014-4886(76)90061-3. [DOI] [PubMed] [Google Scholar]
- Sodhi MS, Sanders-Bush E. Serotonin and brain development. Int Rev Neurobiol. 2004;59:111–174. doi: 10.1016/S0074-7742(04)59006-2. [DOI] [PubMed] [Google Scholar]
- Sood S, Morrison JL, Liu H, Horner RL. Role of endogenous serotonin in modulating genioglossus muscle activity in awake and sleeping rats. Am J Respir Crit Care Med. 2005;172:1338–1347. doi: 10.1164/rccm.200502-258OC. [DOI] [PubMed] [Google Scholar]
- Stettner GM, Kubin L. Antagonism of orexin receptors in the posterior hypothalamus reduces hypoglossal and cardiorespiratory excitation from the perifornical hypothalamus. J Appl Physiol. 2013;114:119–130. doi: 10.1152/japplphysiol.00965.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suratt PM, McTier RF, Wilhoit SC. Upper airway muscle activation is augmented in patients with obstructive sleep apnea compared with that in normal subjects. Am Rev Respir Dis. 1988;137:889–894. doi: 10.1164/ajrccm/137.4.889. [DOI] [PubMed] [Google Scholar]
- Takahash K, Lin JS, Sakai K. Neuronal activity of orexin and non-orexin waking-active neurons during wake-sleep states in the mouse. Neuroscience. 2008;153:860–870. doi: 10.1016/j.neuroscience.2008.02.058. [DOI] [PubMed] [Google Scholar]
- Trulson ME, Trulson VM. Activity of nucleus raphe pallidus neurons across the sleep-waking cycle in freely moving cats. Brain Res. 1982;237:232–237. doi: 10.1016/0006-8993(82)90572-8. [DOI] [PubMed] [Google Scholar]
- Veasey SC, Panckeri KA, Hoffman EA, Pack AI, Hendricks JC. The effects of serotonin antagonists in an animal model of sleep-disordered breathing. Am J Respir Crit Care Med. 1996;153:776–786. doi: 10.1164/ajrccm.153.2.8564132. [DOI] [PubMed] [Google Scholar]
- Volgin DV, Mackiewicz M, Kubin L. α1B receptors are the main postsynaptic mediators of adrenergic excitation in brainstem motoneurons, a single-cell RT-PCR study. J Chem Neuroanat. 2001;22:157–166. doi: 10.1016/s0891-0618(01)00124-7. [DOI] [PubMed] [Google Scholar]
- Volgin DV, Saghir M, Kubin L. Developmental changes in the orexin 2 receptor mRNA in hypoglossal motoneurons. NeuroReport. 2002;13:433–436. doi: 10.1097/00001756-200203250-00014. [DOI] [PubMed] [Google Scholar]
- Volgin DV, Fay R, Kubin L. Postnatal development of serotonin 1B, 2A and 2C receptors in brainstem motoneurons. Eur J Neurosci. 2003;17:1179–1188. doi: 10.1046/j.1460-9568.2003.02545.x. [DOI] [PubMed] [Google Scholar]
- Volgin DV, Kubin L. Chronic intermittent hypoxia alters hypothalamic transcription of genes involved in metabolic regulation. Auton NeurosciBas Clin. 2006;126:93–99. doi: 10.1016/j.autneu.2006.03.013. [DOI] [PubMed] [Google Scholar]
- Volgin DV, Stettner GM, Kubin L. Serotonin 2A receptor mRNA levels, but not mRNAs for other excitatory receptors that mediate wake-related activation of hypoglossal (XII) motoneurons, are higher in the XII nucleus at wake onset than at sleep onset. Sleep. 2010;33(Suppl):A20, Abstr act 0047. [Google Scholar]
- Weiner N, Clement HW, Gemsa D, Wesemann W. Circadian and seasonal rhythms of 5-HT receptor subtypes, membrane anisotropy and 5-HT release in hippocampus and cortex of the rat. Neurochem Int. 1992;21:7–14. doi: 10.1016/0197-0186(92)90062-v. [DOI] [PubMed] [Google Scholar]
- Wesemann W, Weiner N. Circadian rhythm of serotonin binding in rat brain. Prog Neurobiol. 1990;35:405–428. doi: 10.1016/0301-0082(90)90029-g. [DOI] [PubMed] [Google Scholar]
- Wesemann W, Weiner N, Rotsch M, Schulz E. Serotonin binding in rat brain: circadian rhythm and effect of sleep deprivation. J Neur Transm. 1983;18(Suppl):287–294. [PubMed] [Google Scholar]
- White DP, Younes MK. Obstructive sleep apnea. Compr Physiol. 2012;2:2541–2594. doi: 10.1002/cphy.c110064. [DOI] [PubMed] [Google Scholar]
- Witte K, Lemmer B. Development of inverse circadian blood pressure pattern in transgenic hypertensive TGR(mREN2)27 rats. Chronobiol Int. 1999;16:293–303. doi: 10.3109/07420529909116859. [DOI] [PubMed] [Google Scholar]
- Yamazaki S, Numano R, Abe M, Hida A, Takahashi R, Ueda M, Block GD, Sakaki Y, Menaker M, Tei H. Resetting central and peripheral circadian oscillators in transgenic rats. Science. 2000;288:682–685. doi: 10.1126/science.288.5466.682. [DOI] [PubMed] [Google Scholar]
- Yoo SH, Yamazaki S, Lowrey PL, Shimomura K, Ko CH, Buhr ED, Siepka SM, Hong HK, Oh WJ, Yoo OJ, Menaker M, Takahashi JS. PERIOD2::LUCIFERASE real-time reporting of circadian dynamics reveals persistent circadian oscillations in mouse peripheral tissues. Proc Natl Acad Sci USA. 2004;101:5339–5346. doi: 10.1073/pnas.0308709101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhan G, Shaheen F, Mackiewicz M, Fenik P, Veasey SC. Single cell laser dissection with molecular beacon polymerase chain reaction identifies 2A as the predominant serotonin receptor subtype in hypoglossal motoneurons. Neuroscience. 2002;113:145–154. doi: 10.1016/s0306-4522(02)00137-9. [DOI] [PubMed] [Google Scholar]


