Abstract
Researchers have identified environmental risks that predict subsequent psychological and medical problems. Based on these correlational findings, researchers have developed and tested complex developmental models and have examined biological moderating factors (e.g., gene–environment interactions).
In this context, we stress the critical need for researchers to use family-based, quasi-experimental designs when trying to integrate genetic and social science research involving environmental variables because these designs rigorously examine causal inferences by testing competing hypotheses.
We argue that sibling comparison, offspring of twins or siblings, in vitro fertilization designs, and other genetically informed approaches play a unique role in bridging gaps between basic biological and social science research. We use studies on maternal smoking during pregnancy to exemplify these principles.
RECENT RESEARCH HAS focused on early risks, such as prenatal factors, for later cognitive, behavioral, and health outcomes.1–7 This life course framework has been aided by recent advances in developmental theories and analyses of cascade models.8 These approaches have been complemented by a growing interest in understanding how biological factors moderate the influence of environmental risks by studies of gene–environment interaction (G×E).9,10 Researchers ultimately are trying to understand how genetic and biological influences, environmental risks, and behavior act and interact across development to result in psychological and physical health problems.11,12 In this context, the present article stresses the critical advantages of using family-based, quasi-experimental research designs when integrating genetic and social science research because these approaches allow for strong tests of causal inferences regarding hypothesized risks that are critical in developmental models and G×E studies. We use recent quasi-experimental studies on maternal smoking during pregnancy (SDP) as an exemplar.
MATERNAL SMOKING DURING PREGNANCY
SDP is associated with increased likelihood of various offspring problems, including preterm birth and low birth weight,13 infant mortality,14 cognitive deficits,15,16 obesity,17 and social and behavioral difficulties (e.g., childhood conduct problem, adolescent delinquency, and substance use problems).18,19 Several literature reviews have concluded that fetal exposure to maternal SDP causes these problems13,18,19 because the statistical associations are well-replicated, robust to the use of statistical controls for measured confounds, and consistent with basic research on SDP conducted in animals.20,21 Other researchers have hypothesized mechanisms through which maternal SDP influences offspring outcomes.22 For instance, researchers have postulated that maternal SDP alters the development of brain systems responsible for stress reactivity,23 reward sensitivity,19,24 and decision-making,25 which subsequently influence behavior and substance use problems.
However, all of the analytic models used to study mediating variables, including developmental cascade models, are based on strong causal assumptions.26 In particular, the models assume that there are no other causes of the associations among initial risks, the mediating variables, and the outcome of interest. It is clear, however, that maternal SDP is correlated with numerous environmental risks across multiple domains (e.g., maternal and paternal intellectual abilities, psychiatric problems, substance use problems, socioeconomic status, etc.) that also predict offspring problems.27 In addition, maternal SDP is influenced by genetic factors,28–30 with the genetic risk being correlated with both women’s liability for nicotine dependence problems29 and criminality,30 consistent with the finding that genetic factors often influence multiple behavioral traits.31,32 The fact that genetic factors influence SDP, therefore, means that genetic confounding factors could account for the association between SDP and offspring health and behavior problems.33,34 Given the inability of human research to adequately account for all environmental and genetic selection factors, numerous researchers have cautioned against drawing causal inferences regarding maternal SDP18,28,35–37 or, for that matter, all prenatal risks.38,39
Correctly identifying the processes through which genetic and environmental factors interact, therefore, requires that researchers appropriately rule out environmental and genetic confounds of the causal relation. The assumptions concerning causal inferences have profound implications for studies that explore G×E of SDP with measured genotypes,40,41 because all models assume that the putative environmental factor reflects purely environmental processes.9,42,43 Researchers must rule out genetic confounding of environmental variables when conducting G×E studies, because genetic selection factors caused by gene–environment correlations (rGE; a related but distinct set of mechanisms we describe in the following section) can confound the study of G×E.44,45 Correctly understanding how genetic factors interact with environmental risks, therefore, requires rigorously testing competing causal hypotheses about the environment. Improper conceptualization of environmental risks is a key limitation in current genomic studies of psychiatric problems.46
CONFOUNDING IN OBSERVATIONAL STUDIES
Typically, observational studies are composed of 1 person per family. This means that environmental risks can be correlated with—confounded by—all differences among families in genetic factors, environmental influences that make family members similar, and environments that make family members dissimilar. Confounds between genetic variants and environments that are pervasive47–49 can arise systematically through 2 basic types of rGE.33,50,51
Passive Gene–Environment Correlations
Correlations between genetic variants and environments can arise passively (i.e., the behavior and characteristics of the offspring do not cause the correlation). This occurs because parents provide both their children’s segregating genes and home environments. When an allele (version) of a genetic variant in parents is associated with their child rearing, the same allele in the offspring is passively correlated with their experienced child rearing.
Active and Evocative Gene–Environment Correlations
Genes and environments also become correlated when the genetically influenced behavior and characteristics of individuals actively select them into, or evoke changes in, their environments.
QUASI-EXPERIMENTAL DESIGNS
Numerous designs can be used to strengthen causal inferences regarding environmental risks by ruling out specific forms of confounding, including confounding because of rGE. Quasi-experiments are based on individuals who are not randomly assigned to conditions but use design features that rule out many plausible alternative explanations for an association.52 Family-based, quasi-experimental designs vary in their internal validity (ability to make strong causal inferences) and external validity (generalizability). These designs thus provide rigorous (but not probative) tests of causal inferences and help identify the mechanisms through which risks are associated with outcomes, which is critical for integrating genetic and social science research that relies on strong causal inferences.
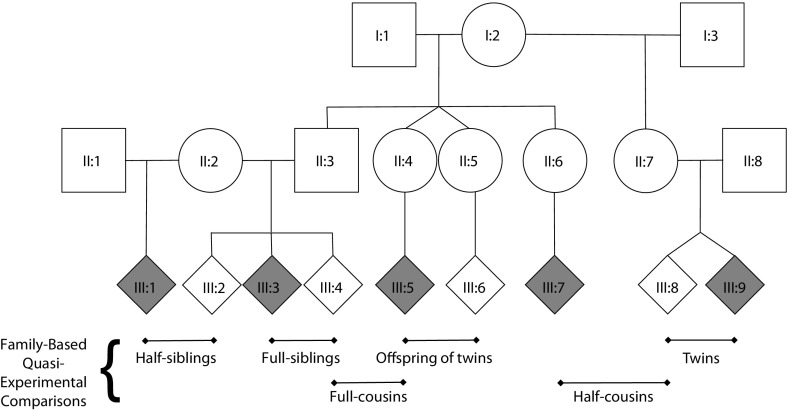
Family-based, quasi-experiments use “natural experiments” based on family relationships. When a study includes multiple family relationships that differ in their environmental exposure and genetic risk, quantitative genetic models can specifically test competing causal hypotheses.28,53–55 Although there are numerous family-based, quasi-experimental designs,34,56,57 we focus on the subset used to study SDP (e.g., we do not review adoption studies58). Figure 1 presents a family pedigree to illustrate several quasi-experimental designs.
FIGURE 1—
Schematic of extended family pedigree with examples of several family-based, quasi-experimental comparisons.
Note. I = first generation; II = second generation; III = third generation. Squares represent males. Circles represent females. Diamonds represent either males or females. Horizontal lines represent mating and sibling relationships. Vertical lines represent parent-child relationships. Inverted Vs represent twin pairs. Shaded diamonds represent individuals exposed to a risk factor (e.g., maternal smoking during pregnancy). Examples of differentially exposed siblings are presented, including half-siblings (III:1 and III:2, who share 25% of their genes); full-siblings (III:3 and III:4, who share 50% of their genes); and twins (III:8 and III:9, either fraternal twins, who share 50% of their genes, or identical twins, who share 100% of their genes). Differentially exposed cousins are also presented, including half-cousins (III:7 and III:8, who share 6.25% of their genetic factors); full-cousins (III:4 and III:5, who share 12.5% of their genetic factors); and offspring of twins (III:5 and III:6). If the twins (II:4 and II:5) are fraternal, their offspring (III:5 and III:6) would share 12.5% of their genetic factors. If the twins (II:4 and II:5) are identical, their offspring (III:5 and III:6) would share 25% of their genetic factors.
We briefly describe the various designs in the following, but Table 1 presents the major advantages and limitations or assumptions of each design, including the traditional comparison of unrelated individuals. In the following section, we also explain how combining multiple quasi-experimental designs enables researchers to further specify the mechanisms responsible for correlations between environmental risks and outcomes (e.g., SDP and offspring functioning).
TABLE 1—
Descriptions of Major Advantages and Limitations/Assumptions of Family Based Quasi-Experimental Designs for Examining Environmental Risks
| Research Design | Major Advantages | Major Limitations/Assumptions |
| 1. Traditional comparison of unrelated individuals | Economical; many samples exist that can be analyzed in this way. | Can only be used to assess correlations; cannot support any degree of causal inference. |
| Confound all genetic and environmental differences among individuals with the environmental factor. Statistical covariates can help control for these factors if they are measured and meet assumptions of statistical control. | ||
| Assume no carry-over effects. | ||
| 2. All sibling comparisons59–63 | Account for all selection factors that make the siblings similar (without having to include measured covariates of selection factors). These include genetic and environmental factors that are shared by siblings. | Do not rule out environmental selection factors that (1) vary among siblings, (2) are correlated with the environmental factor within families, and (3) are correlated with the outcome. Statistical covariates can be added to help control for such factors within the limits of statistical control. |
| Relatively easy to obtain large samples when all siblings, or a random selection of siblings, are assessed. | Do not account for factors correlated with age, birth order, and sex of siblings. Statistical covariates can be added to help control for such factors within the limits of statistical control. | |
| Are particularly sensitive to measurement unreliability. | ||
| Assume no carry-over effects from the environmental exposure of 1 sibling to the outcome of another. | ||
| Assume results generalize to other populations (e.g., singletons and families with no within-family variability). | ||
| Cannot examine influence of outcome of 1 sibling on outcome of another. | ||
| Cannot study risks that are shared by siblings. | ||
| Requires large samples for adequate statistical power. | ||
| 2A. Half-sibling comparisons (e.g., maternal half-siblings) | Account for all genetic selection factors passed down from 1 parent (e.g., mothers) that make siblings similar. Half-siblings share 25% of their genes. | Do not rule out genetic factors passed down from 1 parent (e.g., mothers) that make sibling dissimilar, which could influence exposure to the environmental risk. |
| Account for environmental selection factors that make siblings similar. | Do not rule out any genetic factors passed down from or the environmental factors associated with 1 parent (e.g., different fathers of maternal half-siblilngs). | |
| Assume results generalize to families with full-siblings. | ||
| 2B. Full-sibling comparisons | Account for all genetic selection factors passed down from both mothers and fathers that make siblings similar. Full-siblings share 50% of their genes. | Do not rule out genetic factors that make siblings dissimilar, which could influence exposure to the environmental risk. |
| Account for environmental selection factors that make siblings similar. | Assume results generalize to families with half-siblings. | |
| 2C. Fraternal twin comparisons | Account for all genetic selection factors passed down from both mothers and fathers that make siblings similar. Fraternal twins share 50% of their genes. | Do not rule out genetic factors make sibling dissimilar, which could influence exposure to the risk. |
| Account for environmental selection factors that make siblings similar. | Assume results generalize to other populations (e.g., nontwins). | |
| Account for all confounds associated with offspring age. | Cannot study prenatal and other risks that cotwins share. | |
| Account for shared prenatal factors that make twins similar. | Requires access to twin samples. | |
| 2D. Identical twins comparisons (co-twin control design)64 | Account for all genetic selection factors. Identical twins share 100% of their genes. | Assume results generalize to other populations (e.g., nontwins). |
| Account for environmental selection factors that make siblings similar. | Cannot study prenatal and other risks that co-twins share. | |
| Account for all confounds associated with offspring age. | Requires access to twin samples. | |
| Account for shared prenatal factors that make twins similar. | ||
| Account for factors correlated with sex. | ||
| 2E. Quantitative genetic analysis of multiple sibling comparison designs54,65,66 | Allow researchers to estimate the degree to which the association between an environmental risk and outcome is because of shared genetic liability and environmental factors that make siblings similar (both selection factors), as well as environmental factors that make siblings unique (consistent with a causal inference). | Requires assumptions about assortative mating and equal environmental influences across sibling types are met. |
| Can increase the statistical power of the estimate of environmental factors that make siblings unique (consistent with a causal inference). | Requires assumptions about equal variability in measures across sibling types are met. | |
| Requires access to large samples with multiple types of siblings. | ||
| 2F. Sibling comparisons in a bidirectional case-crossover design67 (i.e., comparison of subsets of sibling pairs with different patterns of exposure across birth order) | Can examine whether carry-over effects associated with timing of the risk account for a reduced sibling-comparison estimate. | Requires even larger samples of differentially exposed siblings (i.e., to compare families with exposure of the first-born offspring to families with exposure of the second-born offspring). |
| Cannot rule out all carry-over effects (e.g., those that influence all siblings in a family regardless of timing). | ||
| 3. All offspring of siblings/twins comparisons (e.g., maternal cousin comparisons)28,34,68–72 | Account for all factors that make the cousins similar. These include environmental factors and genetic factors that are shared by cousins. | Do not rule out selection factors that vary within an extended family. These include environmental factors unique to each nuclear family, such as the influence from spouses of the adult siblings (e.g., fathers). In addition, these include all genetic factors passed down from the spouses of the adult siblings (e.g., fathers). |
| Can explore environmental risks that are shared by siblings/twins. | Are particularly sensitive to measurement unreliability. | |
| Assume no carry-over effects. | ||
| Assume results generalize to other populations (e.g., extended families with no cousins). | ||
| Requires large samples for adequate statistical power. | ||
| 3A. Offspring of half-siblings comparisons (e.g., offspring of maternal half-siblings) | Account for part of the genetic selection factors passed down from 1 parent (e.g., mothers) that make cousins similar. These half-cousins share 6.25% of their genetic makeup. | Do not rule out genetic factors passed down from 1 parent (e.g., mothers) that make cousins dissimilar, which could influence exposure to the risk. |
| Account for environmental selection factors that make cousins similar. | Assume results generalize to families with offspring of full-siblings. | |
| Limited advantages because of minimal sharing of genetic factors. | ||
| 3B. Offspring of full-siblings comparisons (e.g., offspring of maternal full-siblings) | Account for part of the genetic selection factors passed down from 1 parent (e.g., mothers) that make cousins similar. These half-cousins share 12.5% of their genetic makeup. | Do not rule out genetic factors passed down from 1 parent (e.g., mothers) that make cousins dissimilar, which could influence exposure to the risk. |
| Account for environmental selection factors that make cousins similar. | Assume results generalize to families with offspring of half-siblings. | |
| Limited advantages because of minimal sharing of genetic factors. | ||
| 3C. Offspring of fraternal twins comparisons | Account for part of the genetic selection factors passed down from 1 parent (e.g., mothers) that make cousins similar. These half-cousins share 12.5% of their genetic makeup. | Do not rule out genetic factors passed down from 1 parent (e.g., mothers) that make cousins dissimilar, which could influence exposure to the risk. |
| Account for environmental selection factors that make cousins similar. | Assume results generalize to families with offspring of nontwins. | |
| Account for prenatal and age factors of the adult twins, which influence their offspring. | Requires access to twin samples. | |
| 3D. Offspring of identical twins comparisons73 | Account for part of the genetic selection factors passed down from 1 parent (e.g., mothers) that make cousins similar. These half-cousins share 25% of their genetic makeup. | Do not rule out genetic factors passed down from 1 parent (e.g., mothers) that make cousins dissimilar, which could influence exposure to the risk. |
| Account for environmental selection factors that make cousins similar. | Assume results generalize to families with offspring of nontwins. | |
| Account for prenatal and age factors of the adult twins, which influence their offspring. | Require access to twin samples. | |
| 3E. Quantitative genetic analysis of multiple offspring of siblings designs28,34,68–71 | Allow researchers to estimate the degree to which the association between a shared environmental risk and outcome is because of shared genetic liability and environmental factors that make cousins similar (both selection factors), as well as environmental factors that make cousins unique (consistent with a causal inference). | Requires assumptions about equal environmental influences across cousin types. |
| Can increase the statistical power of the estimate of environmental factors that make cousins unique (consistent with a causal inference). | Requires assumptions about equal variability in measures across cousin types. | |
| Requires access to large samples with multiple types of adult siblings. | ||
| 3F. Quantitative genetic analysis of the combination of sibling-comparisons and multiple offspring of siblings designs74–77 | Can help identify the background selection factors when sibling comparisons find no within-family estimate. The approach estimates the degree to which the association between an individual-level environmental risk and outcome is because of genetic liability (shared by parent and all offspring), environmental factors that make cousins similar, or environmental factors that make cousins unique (all selection factors). | Requires large sample of siblings nested within multiple types of adult siblings. |
| Requires advanced multilevel modeling to accurately distinguish intergenerational processes. | ||
| Require assumptions about equal environmental influences across cousin types. | ||
| Require assumptions about equal variability in measures across cousin types. | ||
| Are particularly sensitive to measurement unreliability. | ||
| 4. In vitro fertilization (IVF) comparisons78 | Use an adoption study “in utero” to separate genetic influences passed down from parents to offspring from environmental factors, including those in pregnancy. | Assume results generalize to other populations (e.g., families who didn’t go through fertility treatment). |
| Assume fertility treatments do not influence outcome of interest. | ||
| Requires large samples, especially when comparing subgroups of IVF. | ||
| Difficult to recruit large samples. | ||
| Potential restricted range in environmental risks. | ||
| Require access to fertility treatment samples. | ||
| 4A. Genetically related offspring comparisons (e.g., to mothers; homologous IVF or sperm donation). | Provide a group of mother-offspring pairs (similar to the comparison of unrelated individuals) who have gone through IVF treatment. | Have all of the limitations of the traditional comparison of unrelated individuals. |
| 4B. Genetically unrelated offspring comparisons (e.g., to mothers; embryo donation, egg donation, and gestational surrogacy). | Account for all genetic selection factors passed down from both mothers and fathers. | Do not rule out genetic factors that could influence exposure to the environmental risk. |
| Do not rule out environmental factors that correlate with the exposure. | ||
| 4C. Quantitative genetic analysis of multiple in vitro designs | Combination of genetically related and unrelated offspring allow researchers to estimate the degree to which the association between an environmental risk and outcome is because of shared genetic liability (a selection factor) or environmental factors correlated with the exposure (consistent with a causal inference). | Requires large samples to accurately estimate genetic and environmental estimates. |
Note. The table does not include every advantage and limitation/assumption. References to articles that more fully describe advantages and disadvantages of the methods are included in the text and in the table. The designs described in the table can be combined with other methods (e.g., longitudinal assessments and the use of statistical covariates) to help test and rule out competing explanations for the results.
Sibling-Comparison Designs
Comparisons of differentially exposed siblings can help account for certain types of confounding. Comparisons of siblings raised in the same family automatically exclude confounding of the exposure with all environmental factors that are shared in common by the siblings. Comparisons of siblings can also help rule out genetic confounding.59–61 The process of meiosis (the type of cell division that produces eggs and sperm) randomly distributes alleles from parents to each offspring. Although siblings differ in their genetic makeup, the random process of meiosis rules out systematic confounding between genetic variants and environmental exposures because of passive rGE. As such, the comparison of differentially exposed half-siblings, who share 25% of their genes on average (i.e., III:1 and III:2 in Figure 1), accounts for genetic factors passed down from 1 parent that make siblings similar. The comparison of full-siblings, who share 50% of their genes (i.e., III:3 and III:4), rules out the possibility that genetic factors passively passed down from either parent account for the association between the exposure and the outcome. If the probability of exposure cannot be influenced by the genetic characteristics of the offspring (e.g., random events or events that occur before conception), active and evocative rGE are also ruled out. To rule out genetic confounding arising from active or evocative rGE when studying other risks, the differentially exposed siblings have to be identical twins, who share 100% of their genetic sequences (i.e., III:8 and III:9).64 Because the twins must be discordant for the exposure, they cannot be used to study risks that are shared by twins, such as SDP.79,80
Like all quasi-experimental designs, sibling comparisons have a number of assumptions. For instance, sibling-comparison designs cannot rule out confounded environmental factors that vary within siblings that are highly correlated with both the exposure and the outcome. Comparisons of any type of siblings cannot always identify the selection factors responsible for associations between risks and outcomes.59,61 However, when combining different sibling-comparison designs (e.g., full- and half-siblings65 or fraternal and identical twins66), researchers can use quantitative genetic models to explore the degree to which an association is the result of genetic and shared environmental influences (selection factors) or environments that make siblings unique (consistent with a causal inference).54
There are also assumptions about the generalizability of the findings from the comparison of differentially exposed siblings.59,62 In addition, sibling comparisons are based on strict assumptions about carry-over effects (i.e., the possibility that exposure of 1 sibling influences the outcome of another).59–61,62,63 Fortunately, asking the same scientific question using different quasi-experimental approaches with different limitations in sequence allows researchers to robustly test causal hypotheses. For instance, with large enough samples, researchers can test the assumption of no carry-over effects by conducting bidirectional case-cross studies that explore differentially exposed sibling pairs who vary in their exposure across birth order (i.e., when the mother smoked more during the first or second pregnancy).67
Offspring of Siblings or Twins Designs
The comparison of differentially exposed cousins similarly helps to account for unmeasured genetic and environmental selection factors when examining a specific risk. Cousin comparisons account for all environmental factors shared by cousins that make them similar. Cousin comparisons also can help account for genetic selection, with the degree to which a cousin comparison accounts for genetic factors depending on their genetic relatedness. The offspring of half-siblings (i.e., III:7 and III:8, maternal half-cousins), for instance, share 6.25% of genetic variants, whereas the offspring of full-siblings (i.e., III:4 and III:5, maternal full-cousins) and fraternal twins (i.e., III:5 and III:6) share 12.5%. The offspring of identical twins design is a special case of a cousin comparison—the cousins share 25% of their genetic factors with each other (i.e., socially, the offspring are cousins, but genetically they are half-siblings).28,34,68–73
The advantage of the offspring of siblings or twins designs is that it can answer questions about risks that are almost always shared by siblings, like parental divorce. However, the design cannot account for all genetic and environmental confounds, especially the genetic and environmental influences passed down from the spouses of the twin parents.72 When studying individual-level risks, such as SDP, the design rules out fewer confounds than sibling comparisons (e.g., cousin comparisons do not account for as much genetic confounding as the comparison of full-siblings). However, the offspring of siblings or twins design has different assumptions and threats to its internal and external validity, making it a useful complement to sibling comparisons. For example, the comparison of differentially exposed first-born offspring of twins could be used to study a environmental risk free of the effects of birth order, which could confound sibling-comparison studies.59,61,63
When offspring of multiple types of adult siblings are included in a study (e.g., offspring of identical and fraternal twins), researchers can infer the extent to which environmental and genetic influences confound the statistical associations between an environmental risk and outcome.28,34,68–71 Again, one of the primary advantages of the offspring of siblings or twins designs is the ability to examine risks that are shared by siblings. However, researchers can combine siblings comparisons and offspring of siblings or twins designs to explore the degree to which genetic and environmental factors shared by siblings account for the associations between an individual-level risk factor and outcome.74–76 This is an important advantage, because sibling comparisons by themselves cannot identify the mechanisms responsible for a statistical association between a risk and an outcome when the comparison of differentially exposed siblings suggests no causal influence.59,61
In Vitro Fertilization Designs
The in vitro fertilization (IVF) design is a quasi-experimental approach that takes advantage of new reproductive technologies to conduct “cross fostering” studies in humans. The design includes both offspring who are genetically related to mothers (e.g., through homologous IVF) and not genetically related to their birth mothers (e.g., through embryo donation).78 The key test of a putative environmental risk factor, such as maternal SDP, is whether the risk is associated with an offspring outcome in families in which the women gave birth to genetically unrelated offspring. When IVF studies include both offspring who are genetically related to their mothers and offspring who are not genetically related, behavior genetic models can test whether genetic influences account for the statistical association between a risk factor and outcome.78 A limitation of this design is concern about the generalizability of findings from a sample of women who have had IVF.
STUDIES OF MATERNAL SMOKING DURING PREGNANCY
Many studies of SDP using family-based, quasi-experimental designs suggest causal environmental effects on some outcomes, but not on others.81
Birth-Related and Perinatal Problems
Comparisons of siblings differentially exposed to maternal SDP have consistently found robust associations with birth-related complications, such as restricted fetal growth, preterm birth, and placental abruption, which support a causal inference.13,81 In particular, recent sibling-comparison studies with large representative samples have supported the hypothesis that that maternal SDP causes low infant birth weight79,82,83 and infant mortality.14 Offspring of twins29 and IVF studies84,85 have reported commensurate results regarding maternal SDP and offspring birth weight, which provides converging evidence for this causal hypothesis.
Psychosocial or Cognitive Outcomes
Sibling-comparison studies of SDP and childhood conduct and oppositional problems,76,82 attention or impulsivity problems,76,86 intellectual abilities,16,82 academic achievement or grades,15,77,82 psychological functioning during adolescence,75,87 suicidal behavior,88 substance use and substance problems,83 adolescent antisocial behavior,89 and adolescent and young adult criminal convictions87,89 have all reached the opposite conclusion—familial selection factors account for all of the associations. Although there is a robust correlation between maternal SDP and behavioral outcomes in between-family studies, differentially exposed siblings do not differ in the measured behaviors. Although there were concerns about the measurement of child functioning in some early sibling-comparison studies,60,90 all published sibling-comparison studies of behavioral outcomes to date, including studies predicting normative traits, have concluded that familial selection factors, not exposure to maternal SDP, account for the associations. This conclusion is reinforced by the fact that bidirectional case-cross studies67 of intellectual abilities16 suggest that some assumptions inherent in the sibling-comparison design (e.g., carry-over effects) were not violated and do not account for the findings.
Recent studies combined different quasi-experimental designs to test other assumptions in sibling comparisons and helped identify the confounded selection factors that actually account for the association between maternal SDP and offspring psychosocial or cognitive problems. In a study that included half- and full-siblings, as well as half- and full-cousins, the authors found that confounded genetic influences accounted for part of the association between SDP and offspring intellectual abilities and academic achievement.77 IVF studies likewise suggested that genetic factors passed down from women accounted for the statistical associations of SDP with offspring conduct problems84 and attention deficit or hyperactivity problems.85 Those results were also consistent with an offspring of twins study of SDP and offspring attention deficit or hyperactivity problems.91 Finally, a recent study that combined sibling comparisons with an offspring of siblings design found that genetic liability shared by parents and offspring accounted for the association between SDP and offspring stress-coping.75 The results of the quasi-experimental research was further supported by studies comparing unrelated individuals that included strong measured covariates in the analyses36,92 or propensity score matching.93 The converging evidence from studies using multiple designs, therefore, provides strong evidence that selection factors, including genetic confounds, account for the statistical associations between SDP and offspring psychosocial or cognitive problems.
SUTDIES OF MOLECULAR GENETIC RISKS
It is notable that similar types of quasi-experimental designs are performed to identify both environmental and genetic risk for outcomes. Transmission Disequlibrium Tests94 and similar within-family approaches95,96 have long been conducted in molecular genetic studies to account for several forms of confounding. The designs compare relatives (e.g., parent-offspring, siblings, etc.) to examine whether a proposed allele is segregating together with the disease more often than the other allele(s).
Price et al.97 recently used a similar family-based, quasi-experimental design to study women who SDP and who gave birth to fraternal twins who were discordant for specific genes. The study suggested that a variant in a gene coding enzymes that metabolize xenobiotic (foreign) substances in the fetus was significantly associated with fetal growth, even within fraternal twins (i.e., the twin with the susceptibility allele had less fetal growth). They also found that the magnitude of the association between SDP and offspring fetal growth was moderated by this genetic variant, suggesting a fetal genotype-by-maternal SDP interaction for fetal growth (an example of G×E).
INTEGRATING GENETICS AND SOCIAL SCIENCE RESEARCH
Family-based, quasi-experimental designs have profound implications for numerous research fields, although such studies are relatively new for many environmental risks. First, quasi-experimental studies have important ramifications for epidemiologic research. It is clear that observational studies that rely solely on including measured covariates in an analysis to control for selection factors do not adequately test alternative hypotheses, which has had severe consequences for the field.98 Rather, researchers must identify plausible alternative explanations for statistical associations ahead of time and use multiple designs to test competing causal hypotheses.34,52 This will often involve moving away from the standard practice of conducting observational designs using 1 person from each family. A number of such samples already exist. For example, nationally representative studies in the United States that included assessments of multiple siblings in families99–102 have enabled researchers to use several family-based, quasi-experimental designs. Far more family-based samples are needed in the future, however. Such research will require interdisciplinary efforts to combine quasi-experimental designs with more precise measurements of risks, confounding factors, mediating processes, and outcomes.
Second, quasi-experimental designs are of great value to prevention or intervention scientists. Conducting studies that support a credible degree of causal inference regarding environmental exposures can provide guidance and justification for randomized controlled trials that increase or decrease desirable or undesirable exposures.103–105 That is, quasi-experimental research can identify modifiable environmental risks that are associated with offspring development independent of genetic confounds and many environmental selection factors, consistent with a causal association. For example, quasi-experimental research strongly suggests that public health initiatives should aim to further reduce SDP because of its apparent causal effect on low birth weight, preterm birth, and infant mortality. Quasi-experimental research can also identify risks that are not causal, but instead are markers for other causal agents. As such, quasi-experimental research suggests that solely reducing maternal SDP is unlikely to reduce offspring psychosocial or behavior problems. Translating the findings from quasi-experimental studies into prevention and intervention efforts certainly will require more collaboration across traditionally disparate fields.106
Third, the use of family-based, quasi-experimental designs also can inform the burgeoning field of G×E.9,10 As discussed previously, research exploring the interaction between environments and genes is necessarily based on strong causal assumptions about the environmental influence.9,42,43 Given the quasi-experimental research to date, G×E studies with SDP are more justified when exploring pregnancy-related outcomes, such as low birth weight.97 By contrast, the growing body of quasi-experimental research suggests that researchers cannot assume that SDP has an environmentally mediated influence on psychosocial and cognitive problems. Recent G×E studies of maternal SDP,40,41 therefore, may have incorrectly interpreted the statistical interaction; the statistical interaction could represent gene-by-gene interactions instead of a G×E.
Finally, quasi-experimental research can help integrate genetic and social science research by informing clinical neuroscience research, an example of translational epidemiology.107–110 We certainly endorse integration of experimental methods in basic animal research with findings from human studies.111,112 There are limitations to the generalizability of animal studies,106 however, especially when studying pregnancy, because of the enormous biological differences across species.113 As such, the quasi-experimental studies of maternal SDP can help specify the mechanisms that clinical neuroscience research should explore further.114 Quasi-experimental studies can be seen as a critical bridge between basic neuroscience and epidemiology. Neuroscience research with animals has excellent internal validity because of the ability to conduct randomized controlled studies but somewhat limited external validity to human behavior. In contrast, traditional epidemiological studies have limited internal validity but stronger external validity.
SUMMARY
Researchers have identified environmental exposures that are correlated with psychological and medical problems. Based on these findings, researchers have developed complex developmental models and have tested biological moderating factors for these outcomes. We still know little about the true causes of these outcomes, however, because it remains unclear whether environmental risks are truly causal or whether part—or most—of the associations with these risks are attributable instead to familial confounding.115,116 It is important to stress that this is true of both social science research, which has typically focused on environmental risks, and genetic research with susceptibility genes or other biomarkers.96
In this article, we show examples of when genetic and environmental selection factors have been neglected, and causation may have been claimed erroneously. We also describe how family-based, quasi-experimental methods can help integrate genetics and the behavioral and social sciences to inform our understanding of the true causes of human health and development. We argue that rigorous translational epidemiological approaches—studies employing quasi-experimental methods—play a unique position in bridging gaps between basic biological research and social science research because the approaches rigorously test causal hypotheses.107–110 We used studies on maternal SDP to exemplify these principles.
Our advocacy for quasi-experimental designs in integrating genetic and social research parallels the calls to use these approaches by leading researchers in medicine,115,116 psychiatry,117,118 sociology,119 psychology,34,52 family studies,120 epidemiology,39,61 and economics.121 Ultimately, integrating genetic and social science research will require researchers to incorporate measures across multiple levels of analysis with strong quasi-experimental designs in a developmental context.120
Acknowledgments
Preparation of this article was supported by grants from the Eunice Kennedy Shriver National Institute of Child Health and Human Development (HD061817 and HD061384), the Swedish Research Council (Medicine), and the Swedish Prison and Probation Services.
References
- 1.Bale TL, Baram TZ, Brown AS et al. Early life programming and neurodevelopmental disorders. Biol Psychiatry. 2010;68(4):314–319. doi: 10.1016/j.biopsych.2010.05.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Huizink AC, Mulder JH, Buitelaar JK. Prenatal stress and risk for psychopathology: specific effects or induction of general susceptibility. Psychol Bull. 2004;130(1):115–142. doi: 10.1037/0033-2909.130.1.115. [DOI] [PubMed] [Google Scholar]
- 3.Beydoun H, Saftlas AF. Physical and mental health outcomes of prenatal maternal stress in human and animal studies: a review of recent evidence. Paediatr Perinat Epidemiol. 2008;22(5):438–466. doi: 10.1111/j.1365-3016.2008.00951.x. [DOI] [PubMed] [Google Scholar]
- 4.Glynn LM, Sandman CA. Prenatal origins of neurological development. Curr Direct Psychol Sci. 2011;20(6):384–389. [Google Scholar]
- 5.Barker DJP. Mothers, Babies and Health in Later Life. 2nd ed. Edinburgh, UK: Churchill Livingstone; 1998. [Google Scholar]
- 6.Gluckman PD, Hanson MA. Developmental plasticity and human disease: research directions. J Intern Med. 2007;261(5):461–471. doi: 10.1111/j.1365-2796.2007.01802.x. [DOI] [PubMed] [Google Scholar]
- 7.Gluckman PD, Hanson MA, Cooper C, Thornburg KL. Effect of in utero and early-life conditions on adult health and disease. N Engl J Med. 2008;359(1):61–73. doi: 10.1056/NEJMra0708473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Masten AS, Cicchetti D. Developmental cascades. Dev Psychopathol. 2010 doi: 10.1017/S0954579410000222. Part 1, 22(special issue 3):491–495;Part 2, 22(special issue 4):717–983. [DOI] [PubMed] [Google Scholar]
- 9.Dick DM. Gene-environment interaction in psychological traits and disorders. Annu Rev Clin Psychol. 2011;7(1):383–409. doi: 10.1146/annurev-clinpsy-032210-104518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Duncan LE, Keller MC. A critical review of the first 10 years of candidate gene-by-environment interaction research in psychiatry. Am J Psychiatry. 2011;168(10):1041–1049. doi: 10.1176/appi.ajp.2011.11020191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hernandez LM, Blazer DG. Genes, Behavior and the Social Environment: Moving Beyond the Nature/Nurture Debate. Washington, DC: National Academies Press; 2006. [PubMed] [Google Scholar]
- 12.Kendler K, Jaffee S, Romer D. The Dynamic Genome and Mental Health: The Role of Genes and Environments in Development. New York, NY: Oxford University; 2010. [Google Scholar]
- 13.Cnattingius S. The epidemiology of smoking during pregnancy: smoking prevalence, maternal characteristics, and pregnancy outcomes. Nicotine Tob Res. 2004;6(suppl 2):S125–S140. doi: 10.1080/14622200410001669187. [DOI] [PubMed] [Google Scholar]
- 14.Johansson ALV, Dickman PW, Kramer MS, Cnattingius S. Maternal smoking and infant mortality: does quitting smoking reduce the risk of infant death? Epidemiology. 2009;20(4):590–597. doi: 10.1097/EDE.0b013e31819dcc6a. [DOI] [PubMed] [Google Scholar]
- 15.Lambe M, Hultman C, Torrang A, MacCabe J, Cnattingius S. Maternal smoking during pregnancy and school performance at age 15. Epidemiology. 2006;17(5):524–530. doi: 10.1097/01.ede.0000231561.49208.be. [DOI] [PubMed] [Google Scholar]
- 16.Lundberg F, Cnattingius S, D’Onofrio B et al. Maternal smoking during pregnancy and intellectual performance in young adult Swedish male offspring. Paediatr Perinat Epidemiol. 2010;24(1):79–87. doi: 10.1111/j.1365-3016.2009.01073.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Iliadou AN, Koupil I, Villamor E et al. Familial factors confound the association between maternal smoking during pregnancy and young adult offspring overweight. Int J Epidemiol. 2010;39(5):1193–1202. doi: 10.1093/ije/dyq064. [DOI] [PubMed] [Google Scholar]
- 18.Wakschlag LS, Pickett KE, Cook E, Benowitz NL, Leventhal BL. Maternal smoking during pregnancy and severe antisocial behavior in offspring: a review. Am J Public Health. 2002;92(6):966–974. doi: 10.2105/ajph.92.6.966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Glantz MD, Chambers JC. Prenatal drug exposure effects on subsequent vulnerability to drug abuse. Dev Psychopathol. 2006;18(3):893–922. doi: 10.1017/s0954579406060445. [DOI] [PubMed] [Google Scholar]
- 20.Ernst M. Behavioral and neural consequences of prenatal exposure to nicotine. J Am Acad Child Adolesc Psychiatry. 2001;40(6):630–641. doi: 10.1097/00004583-200106000-00007. [DOI] [PubMed] [Google Scholar]
- 21.Shea AK, Steiner M. Cigarette smoking during pregnancy. Nicotine Tob Res. 2008;10(2):267–278. doi: 10.1080/14622200701825908. [DOI] [PubMed] [Google Scholar]
- 22.Cornelius MD, Ryan CM, Day NL, Godschmidt L, Willford JA. Prenatal tobacco effects on neuropsychological outcomes among preadolescents. J Dev Behav Pediatr. 2001;22(4):217–225. doi: 10.1097/00004703-200108000-00002. [DOI] [PubMed] [Google Scholar]
- 23.Koob GF. Stress, corticotropin releasing factor, and drug addiction. Ann NY Acad Sci. 1999;897:27–45. doi: 10.1111/j.1749-6632.1999.tb07876.x. [DOI] [PubMed] [Google Scholar]
- 24.Ferriero DM, Dempsey DA. Impact of addictive and harmful substances on fetal brain development. Curr Opin Neurol. 1999;12(2):161–166. doi: 10.1097/00019052-199904000-00006. [DOI] [PubMed] [Google Scholar]
- 25.Lotfipour S, Ferguson E, Leonard G et al. Orbitofrontal cortex and drug use during adolescence: role of prenatal exposure to maternal smoking and BDNF genotype. Arch Gen Psychiatry. 2009;66(11):1244–1252. doi: 10.1001/archgenpsychiatry.2009.124. [DOI] [PubMed] [Google Scholar]
- 26.Masten AS, Roisman GI, Long JD et al. Developmental cascades: linking academic achievement and externalizing and internalizing symptoms over 20 years. Dev Psychol. 2005;41(5):733–746. doi: 10.1037/0012-1649.41.5.733. [DOI] [PubMed] [Google Scholar]
- 27.Huizink AC, Mulder EJH. Maternal smoking, drinking or cannabis use during pregnancy and neurobehavioral and cognitive functioning in human offspring. Neurosci Biobehav Rev. 2006;30(1):24–41. doi: 10.1016/j.neubiorev.2005.04.005. [DOI] [PubMed] [Google Scholar]
- 28.D’Onofrio BM, Turkheimer E, Eaves LJ et al. The role of the children of twins design in elucidating causal relations between parent characteristics and child outcomes. J Child Psychol Psychiatry. 2003;44(8):1130–1144. doi: 10.1111/1469-7610.00196. [DOI] [PubMed] [Google Scholar]
- 29.Agrawal A, Knopik VS, Pergadia ML et al. Correlates of cigarette smoking during pregnancy and its genetic and environmental overlap with nicotine dependence. Nicotine Tob Res. 2008;10(4):567–578. doi: 10.1080/14622200801978672. [DOI] [PubMed] [Google Scholar]
- 30.Ellingson JM, Rickert ME, Lichtenstein P, Långström N, D’Onofrio BM. Disentangling the relationships between maternal smoking during pregnancy and co-occurring risk factors. Psychol Med. 2012;42(7):1547–1557. doi: 10.1017/S0033291711002534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Plomin R, DeFries JC, McClearn GE, McGuffin P. Behavioral Genetics. New York, NY: Worth Publishers; 2008. [Google Scholar]
- 32.Rutter M. Genes and Behavior: Nature-Nurture Interplay Explained. Malden, MA: Blackwell; 2006. [Google Scholar]
- 33.Scarr S, McCartney K. How people make their own environments: a theory of genotype greater than environment effects. Child Dev. 1983;54(2):424–435. doi: 10.1111/j.1467-8624.1983.tb03884.x. [DOI] [PubMed] [Google Scholar]
- 34.Rutter M, Pickles A, Murray R, Eaves LJ. Testing hypotheses on specific environmental causal effects on behavior. Psychol Bull. 2001;127(3):291–324. doi: 10.1037/0033-2909.127.3.291. [DOI] [PubMed] [Google Scholar]
- 35.Maughan B, Taylor A, Caspi A, Moffitt TE. Prenatal smoking and early childhood conduct problems. Arch Gen Psychiatry. 2004;61(8):836–843. doi: 10.1001/archpsyc.61.8.836. [DOI] [PubMed] [Google Scholar]
- 36.Silberg JL, Parr T, Neale MC, Rutter M, Angold A, Eaves LJ. Maternal smoking during pregnancy and risk to boys’ conduct disturbance: an examination of the causal hypothesis. Biol Psychiatry. 2003;53(2):130–135. doi: 10.1016/s0006-3223(02)01477-4. [DOI] [PubMed] [Google Scholar]
- 37.Fergusson DM. Prenatal smoking and antisocial behavior. Arch Gen Psychiatry. 1999;56(3):223–224. doi: 10.1001/archpsyc.56.3.223. [DOI] [PubMed] [Google Scholar]
- 38.Thapar A, Rutter M. Do prenatal risk factors cause psychiatric disorder? Be wary of causal claims. Br J Psychiatry. 2009;195(2):100–101. doi: 10.1192/bjp.bp.109.062828. [DOI] [PubMed] [Google Scholar]
- 39.Smith GD. Assessing intrauterine influences on offspring health outcomes: can epidemiological studies yield robust findings? Basic Clin Pharmacol Toxicol. 2008;102(2):245–256. doi: 10.1111/j.1742-7843.2007.00191.x. [DOI] [PubMed] [Google Scholar]
- 40.Wakschlag LS, Kistner EO, Pine DS et al. Interaction of prenatal exposure to cigarettes and MAOA genotype in pathways to youth antisocial behavior. Mol Psychiatry. 2010;15(9):928–937. doi: 10.1038/mp.2009.22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Becker K, El-Faddagh M, Schmidt MH, Esser G, Laucht M. Interaction of dopamine transporter genotype with prenatal smoke exposure on ADHD symptoms. J Pediatr. 2008;152(2):263–269. doi: 10.1016/j.jpeds.2007.07.004. [DOI] [PubMed] [Google Scholar]
- 42.Moffitt TE, Caspi A, Rutter M. Strategy for investigating interactions between measured genes and measured environments. Arch Gen Psychiatry. 2005;62(5):473–481. doi: 10.1001/archpsyc.62.5.473. [DOI] [PubMed] [Google Scholar]
- 43.Conley D, Rauscher E. Genetic Interactions with Prenatal Social Environment: Effects on Academic and Behavioral Outcomes. Cambridge, MA: National Bureau of Economic Research; 2010. [DOI] [PubMed] [Google Scholar]
- 44.Jaffee SR, Price TS. Gene-environment correlations: a review of the evidence and implications for prevention of mental illness. Mol Psychiatry. 2007;12(5):432–442. doi: 10.1038/sj.mp.4001950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Rutter M, Moffitt TE, Caspi A. Gene–environment interplay and psychopathology: multiple varieties but real effects. J Child Psychol Psychiatry. 2006;47(3-4):226–261. doi: 10.1111/j.1469-7610.2005.01557.x. [DOI] [PubMed] [Google Scholar]
- 46.Vrieze SI, Iacono WG, McGue M. Confluence of genes, environment, development, and behavior in a post Genome-Wide Association Study world. Dev Psychopathol. 2012;24(4):1195–1214. doi: 10.1017/S0954579412000648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Kendler KS, Baker JH. Genetic influences on measures of the environment: a systematic review. Psychol Med. 2007;37(5):615–626. doi: 10.1017/S0033291706009524. [DOI] [PubMed] [Google Scholar]
- 48.Plomin R, Bergeman CS. The nature of nurture: genetic influences on “environmental” measures. Behav Brain Sci. 1991;14(3):373–386. [Google Scholar]
- 49.Jaffee SR, Price TS. The implications of genotype–environment correlation for establishing causal processes in psychopathology. Dev Psychopathol. 2012;24(4):1253–1264. doi: 10.1017/S0954579412000685. [DOI] [PubMed] [Google Scholar]
- 50.Eaves LJ, Last K, Martin NG, Jinks JL. A progressive approach to non-additivity and genotype-environmental covariance in the analysis of human differences. Br J Math Stat Psychol. 1977;30(1):1–42. [Google Scholar]
- 51.Plomin R, DeFries JC, Loehlin JC. Genotype-environment interaction and correlation in the analysis of human behavior. Psychol Bull. 1977;84(2):309–322. [PubMed] [Google Scholar]
- 52.Shadish WR, Cook TD, Campbell DT. Experimental and Quasi-Experimental Designs for Generalized Causal Inference. New York, NY: Houghton Mifflin; 2002. [Google Scholar]
- 53.Schermerhorn AC, D’Onofrio BM, Turkheimer EN et al. A genetically informed study of associations between family functioning and child psychosocial adjustment. Dev Psychol. 2011;47(3):707–725. doi: 10.1037/a0021362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Turkheimer E, Harden KP. Behavior genetic research methods: testing quasi-causal hypotheses using multivariate twin data. In: Reis HT, Judd CM, editors. Handbook of Research Methods in Personality and Social Psychology. 2nd ed. Cambridge, UK: Cambridge University Press; In press. [Google Scholar]
- 55.Heath AC, Kessler RC, Neale MC, Hewitt JK, Eaves LJ, Kendler KS. Testing hypotheses about direction of causation using cross-sectional data. Behav Genet. 1993;23(1):29–50. doi: 10.1007/BF01067552. [DOI] [PubMed] [Google Scholar]
- 56.Lawlor DA, Mishra GD. Family Matters: Designing, Analysing, and Understanding Family-Based Studies in Life Course Epidemiology. Oxford, UK: Oxford University Press; 2009. [Google Scholar]
- 57.Eaves LJ, Last KA, Young PA, Martin NG. Model-fitting approaches to the analysis of human behaviour. Heredity. 1978;41(3):249–320. doi: 10.1038/hdy.1978.101. [DOI] [PubMed] [Google Scholar]
- 58.Leve LD, Neiderhiser JM, Scaramella LV, Reiss D. The early growth and development study: using the prospective adoption design to examine genotype-environment interplay. Behav Genet. 2010;40(3):306–314. doi: 10.1007/s10519-010-9353-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Lahey BB, D’Onofrio BM. All in the family: comparing siblings to test causal hypotheses regarding environmental influences on behavior. Curr Dir Psychol Sci. 2010;19(5):319–323. doi: 10.1177/0963721410383977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Rutter M. Proceeding from observed correlation to causal inference: the use of natural experiments. Perspect Psychol Sci. 2007;2(4):377–395. doi: 10.1111/j.1745-6916.2007.00050.x. [DOI] [PubMed] [Google Scholar]
- 61.Donovan SJ, Susser E. Commentary: advent of sibling designs. Int J Epidemiol. 2011;40(2):345–349. doi: 10.1093/ije/dyr057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Frisell T, Oberg S, Kuja-Halkola R, Sjolander A. Sibling comparison designs: bias from non-shared confounders and measurement error. Epidemiology. 2012;23(5):713–720. doi: 10.1097/EDE.0b013e31825fa230. [DOI] [PubMed] [Google Scholar]
- 63.Susser E, Eide MG, Begg M. Invited commentary: the use of sibship studies to detect familial confounding. Am J Epidemiol. 2010;172(5):537–539. doi: 10.1093/aje/kwq196. [DOI] [PubMed] [Google Scholar]
- 64.McGue M, Osler M, Christensen K. Causal inference and observational research: the utility of twins. Perspect Psychol Sci. 2010;5(5):546–556. doi: 10.1177/1745691610383511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Tierney C, Merikangas KR, Risch N. Feasibility of half-sibling designs for detecting a genetic component to a disease. Genet Epidemiol. 1994;11(6):523–538. doi: 10.1002/gepi.1370110608. [DOI] [PubMed] [Google Scholar]
- 66.Neale MC, Cardon LRD. Methodology for Genetic Studies of Twins and Families. Dordrecht, The Netherlands: Kluwer Academic Press; 1992. [Google Scholar]
- 67.Meyer KA, Williams P, Hernandez-Diaz S, Cnattingius S. Smoking and the risk of oral clefts: exploring the impact of study designs. Epidemiology. 2004;15(6):671–678. doi: 10.1097/01.ede.0000142148.51230.60. [DOI] [PubMed] [Google Scholar]
- 68.Heath AC, Kendler KS, Eaves LJ, Markell D. The resolution of cultural and biological inheritance: informativeness of different relationships. Behav Genet. 1985;15(5):439–465. doi: 10.1007/BF01066238. [DOI] [PubMed] [Google Scholar]
- 69.Silberg JL, Eaves LJ. Analyzing the contribution of genes and parent-child interaction to childhood behavioral and emotional problems: a model for the children of twins. Psychol Med. 2004;34(2):347–356. doi: 10.1017/s0033291703008948. [DOI] [PubMed] [Google Scholar]
- 70.D’Onofrio BM, Turkheimer EN, Emery RE et al. A genetically informed study of marital instability and its association with offspring psychopathology. J Abnorm Psychol. 2005;114(4):570–586. doi: 10.1037/0021-843X.114.4.570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Gottesman, Bertelsen A. Confirming unexpressed genotypes for schizophrenia. Arch Gen Psychiatry. 1989;46(10):867–872. doi: 10.1001/archpsyc.1989.01810100009002. [DOI] [PubMed] [Google Scholar]
- 72.Eaves LJ, Silberg JL, Maes HH. Revisiting the children of twins: can they be used to resolve the environmental effects of dyadic parental treatment on child behavior? Twin Res Hum Genet. 2005;8(4):283–290. doi: 10.1375/1832427054936736. [DOI] [PubMed] [Google Scholar]
- 73.Nance WE, Corey LA. Genetic models for the analysis of data from the families of identical twins. Genetics. 1976;83(4):811–826. doi: 10.1093/genetics/83.4.811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Harden KP, Lynch SK, Turkheimer E et al. A behavior genetic investigation of adolescent motherhood and offspring mental health problems. J Abnorm Psychol. 2007;116(4):667–683. doi: 10.1037/0021-843X.116.4.667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Kuja-Halkola R, D’Onofrio BM, Illiadou A, Pawitan Y, Langstrom N, Lichtenstein P. Prenatal smoking exposure and stress coping in late adolescence: no causal link. Int J Epidemiol. 2010;39(6):1531–1540. doi: 10.1093/ije/dyq133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.D’Onofrio BM, Van Hulle CA, Waldman ID et al. Smoking during pregnancy and offspring externalizing problems: an exploration of genetic and environmental confounds. Dev Psychopathol. 2008;20(1):139–164. doi: 10.1017/S0954579408000072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.D’Onofrio BM, Singh AL, Iliadou A et al. A quasi-experimental study of maternal smoking during pregnancy and offspring academic achievement. Child Dev. 2010;81(1):80–100. doi: 10.1111/j.1467-8624.2009.01382.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Thapar A, Harold G, Rice F et al. Do intrauterine or genetic influences explain the foetal origins of chronic disease? A novel experimental method for disentangling effects. BMC Med Res Methodol. 2007;7(6):25. doi: 10.1186/1471-2288-7-25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Turkheimer E, D’Onofrio BM, Maes HH, Eaves LJ. Analysis and interpretation of twin studies with measured environments. Child Dev. 2005;76(6):1217–1233. doi: 10.1111/j.1467-8624.2005.00846.x. [DOI] [PubMed] [Google Scholar]
- 80.Purcell S, Koenen KC. Environmental mediation and the twin design. Behav Genet. 2005;35(4):491–498. doi: 10.1007/s10519-004-1484-9. [DOI] [PubMed] [Google Scholar]
- 81.Knopik VS. Maternal smoking during pregnancy and child outcomes: real or spurious effect? Dev Neuropsychol. 2009;34(1):1–36. doi: 10.1080/87565640802564366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Gilman SE, Gardener H, Buka SL. Maternal smoking during pregnancy and children’s cognitive and physical development: a causal risk factor? Am J Epidemiol. 2008;168(5):522–531. doi: 10.1093/aje/kwn175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.D’Onofrio BM, Rickert ME, Långström N et al. Familial confounding of the associations between maternal smoking during pregnancy and offspring substance use problems: converging evidence across samples and measures. Arch Gen Psychiatry. 2012;69(11):1140–1150. doi: 10.1001/archgenpsychiatry.2011.2107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Rice F, Harold GT, Boivin J, Hay DF, Van den Bree M, Thapar A. Disentangling prenatal and inherited influences in humans with an experimental design. Proc Natl Acad Sci U S A. 2009;106(7):2464–2467. doi: 10.1073/pnas.0808798106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Thapar A, Rice F, Hay D et al. Prenatal smoking might not cause attention-deficit/hyperactivity disorder. Evidence from a novel design. Biol Psychiatry. 2009;66(8):722–727. doi: 10.1016/j.biopsych.2009.05.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Obel C, Olsen J, Henriksen TB et al. Is maternal smoking during pregnancy a risk factor for hyperkinetic disorder?—findings from a sibling design. Int J Epidemiol. 2011;40(2):338–345. doi: 10.1093/ije/dyq185. [DOI] [PubMed] [Google Scholar]
- 87.D’Onofrio BM, Singh AL, Iliadou A et al. Familial confounding of the association between maternal smoking during pregnancy and offspring criminality: a population-based study in Sweden. Arch Gen Psychiatry. 2010;67(5):529–538. doi: 10.1001/archgenpsychiatry.2010.33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Cnattingius S, Svensson T, Granath F, Iliadou A. Maternal smoking during pregnancy and risks of suicidal acts in young offspring. Eur J Epidemiol. 2011;26(6):485–492. doi: 10.1007/s10654-011-9556-7. [DOI] [PubMed] [Google Scholar]
- 89.D’Onofrio BM, Van Hulle CA, Goodnight JA, Rathouz PJ, Lahey BB. Is maternal smoking during pregnancy a causal environmental risk factor for adolescent antisocial behavior? Testing etiological theories and assumptions. Psychol Med. 2012;42(7):1535–1545. doi: 10.1017/S0033291711002443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Talati A, Weissman MM. In utero smoking exposure warrants further investigation. Arch Gen Psychiatry. 2010;67(10):1094. doi: 10.1001/archgenpsychiatry.2010.119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Knopik VS, Sparrow EP, Madden PAF et al. Contributions of parental alcoholism, prenatal substance exposure, and genetic transmission to child ADHD risk: a female twin study. Psychol Med. 2005;35(5):625–635. doi: 10.1017/s0033291704004155. [DOI] [PubMed] [Google Scholar]
- 92.Batty GD, Der G, Deary IJ. Effect of maternal smoking during pregnancy on offspring’s cognitive ability: empirical evidence for complete confounding in the US National Longitudinal Survey of Youth. Pediatrics. 2006;118(3):943–950. doi: 10.1542/peds.2006-0168. [DOI] [PubMed] [Google Scholar]
- 93.Boutwell BB, Beaver KM. Maternal cigarette smoking during pregnancy and offspring externalizing behavioral problems: a propensity score matching analysis. Int J Environ Res Public Health. 2010;7(1):146–163. doi: 10.3390/ijerph7010146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Spielman RS, McGinnis RE, Ewens WJ. Transmission test for linkage disequilibrium: the insulin gene region and insulin-dependent diabetes mellitus (IDDM) Am J Hum Genet. 1993;52(3):506–516. [PMC free article] [PubMed] [Google Scholar]
- 95.Fulker DW, Cherny SS, Sham PC, Hewitt JK. Combined linkage and association sib-pair analysis for quantitative traits. Am J Hum Genet. 1999;64(1):259–267. doi: 10.1086/302193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Turkheimer E. Genome wide association studies of behavior are social science. In: Plaisance KS, Redydon TAC, editors. Philosophy of Behavioral Biology. New York, NY: Springer; 2012. pp. 43–64. [Google Scholar]
- 97.Price TS, Grosser T, Plomin R, Jaffee SR. Fetal genotype for the xenobiotic metabolizing enzyme NQO1 influences intrauterine growth among infants whose mothers smoked during pregnancy. Child Dev. 2010;81(1):101–114. doi: 10.1111/j.1467-8624.2009.01383.x. [DOI] [PubMed] [Google Scholar]
- 98.Taubes G. Epidemiology faces its limits. Science. 1995;269(5221):164–169. doi: 10.1126/science.7618077. [DOI] [PubMed] [Google Scholar]
- 99.Baker P, Mott FL. NLSY Child Handbook. Columbus, OH: Center for Human Resource Research; 1989. [Google Scholar]
- 100.Chase-Lansdale PL, Mott FL, Brooks-Gunn J, Phillips DA. Children of the National Longitudinal Survey of Youth: a unique research opportunity. Dev Psychol. 1991;27(6):918–931. [Google Scholar]
- 101.Harris KM, Halpern CT, Whitsel E et al. The National Longitudinal Study of Adolescent Health: research design. Add Health. 2009 Available at: http://www.cpc.unc.edu/projects/addhealth/design. Accessed April 25, 2013. [Google Scholar]
- 102.Reiss D, Neiderhiser JM, Hetherington EM, Plomin R. The Relationship Code: Deciphering Genetic and Social Patterns in Adolescent Development. Cambridge, MA: Harvard University Press; 2000. [Google Scholar]
- 103.Cicchetti D. Developmental psychopathology: reactions reflections projections. Dev Rev. 1993;13(4):471–502. [Google Scholar]
- 104.Coie JD, Watt NF, West SG et al. The science of prevention: a conceptual-framework and some directions for a national research-program. Am Psychol. 1993;48(10):1013–1022. doi: 10.1037//0003-066x.48.10.1013. [DOI] [PubMed] [Google Scholar]
- 105.National Research Council & Institute of Medicine. Preventing Mental, Emotional, and Behavioral Disorders among Young People: Progress and Possibilities. Washington, DC: National Academies Press; 2009. [PubMed] [Google Scholar]
- 106.Zerhouni EA. Translational and clinical science — time for a new vision. N Engl J Med. 2005;353(15):1621–1623. doi: 10.1056/NEJMsb053723. [DOI] [PubMed] [Google Scholar]
- 107.Gaziano JM. The evolution of population science. JAMA. 2010;304(20):2288–2289. doi: 10.1001/jama.2010.1691. [DOI] [PubMed] [Google Scholar]
- 108.Weissman MM, Brown AS, Talati A. Translational epidemiology in psychiatry: linking population to clinical and basic sciences. Arch Gen Psychiatry. 2011;68(6):600–608. doi: 10.1001/archgenpsychiatry.2011.47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Hiatt RA. Invited commentary: the epicenter of translational science. Am J Epidemiol. 2010;172(5):525–527. doi: 10.1093/aje/kwq212. [DOI] [PubMed] [Google Scholar]
- 110.Khoury MJ, Gwinn M, Ioannidis JPA. The emergence of translational epidemiology: from scientific discovery to population health impact. Am J Epidemiol. 2010;172(5):517–524. doi: 10.1093/aje/kwq211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Caspi A, Moffitt TE. Gene-environment interactions in psychiatry: joining forces with neuroscience. Nat Rev Neurosci. 2006;7(7):583–590. doi: 10.1038/nrn1925. [DOI] [PubMed] [Google Scholar]
- 112.Wahlsten D. The hunt for gene effects pertinent to behavioral traits and psychiatric disorders: from mouse to human. Dev Psychobiol. 2012;54(5):475–492. doi: 10.1002/dev.21043. [DOI] [PubMed] [Google Scholar]
- 113.Mitchell BF, Taggert MJ. Are animal models relevant to key aspects of human parturition? Am J Physiol Regul Integr Comp Physiol. 2009;297(3):R525–R545. doi: 10.1152/ajpregu.00153.2009. [DOI] [PubMed] [Google Scholar]
- 114.D’Onofrio BM, Rathouz PJ, Lahey BB. The importance of understanding gene-environment correlations in the development of antisocial behavior. In: Kendler KS, Jaffee SR, Romer D, editors. The Dynamic Genome and Mental Health: The Role of Genes and Environments in Youth Development. New York, NY: Oxford University Press; 2011. [Google Scholar]
- 115.Academy of Medical Sciences Working Group. Identifying the Environmental Causes of Disease: How Should We Decide What to Believe and When to Take Action? London, UK: Academy of Medical Sciences; 2007. [Google Scholar]
- 116.British Academy of Science Working Group. Social Science and Family Policy. London, UK: British Academy Policy Center; 2010. [Google Scholar]
- 117.Kendler KS. Psychiatric genetics: a methodological critique. Am J Psychiatry. 2005;162(1):3–11. doi: 10.1176/appi.ajp.162.1.3. [DOI] [PubMed] [Google Scholar]
- 118.Lahey BB, D’Onofrio BM, Waldman ID. Using epidemiologic methods to test hypotheses regarding causal influences on child and adolescent mental disorders. J Child Psychol Psychiatry. 2009;50(1-2):53–62. doi: 10.1111/j.1469-7610.2008.01980.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Freese J. Genetics and the social science explanation of individual outcomes. Am J Sociol. 2008;114(suppl):S1–S35. doi: 10.1086/592208. [DOI] [PubMed] [Google Scholar]
- 120.D’Onofrio BM, Lahey BB. Biosocial influences on the family: a decade review. J Marriage Fam. 2010;72(3):762–782. doi: 10.1111/j.1741-3737.2010.00729.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Duncan GJ. Give us this day our daily breadth. Child Dev. 2012;83(1):6–15. doi: 10.1111/j.1467-8624.2011.01679.x. [DOI] [PubMed] [Google Scholar]