Abstract
Increased physical activity may protect against cognitive decline, the primary symptom of Alzheimer's disease (AD). In this study, we examined the relationship between physical activity and trajectories of cognitive functioning over serial assessments. Cognitively normal (Clinical Dementia Rating 0) middle aged and older adults (N=173, mean age 60.7 +/- 7.8 years) completed a self-report measure of physical activity and a battery of standard neuropsychological tests assessing processing speed, attention, executive functioning, and verbal memory. At baseline, individuals with higher physical activity levels performed better on tests of episodic memory and visuospatial functioning. Over subsequent follow-up visits, higher physical activity was associated with small performance gains on executive functioning and working memory tasks in participants with one or more copy of the apolipoprotein ε4 allele (APOE4). In APOE4 non-carriers, slopes of cognitive performance over time were not related to baseline physical activity. Our results suggest that cognitively normal older adults who report higher levels of physical activity may have slightly better cognitive performance, but the potential cognitive benefits of higher levels of physical activity over time may be most evident in individuals at genetic risk for AD.
Keywords: Alzheimer's disease, dementia, memory, physical activity, exercise, apolipoprotein E
Introduction
Physical activity has been identified as a possible protective factor against cognitive decline and subsequent development of symptomatic Alzheimer's disease (AD1,2). Exercise and physical fitness also may have potential benefits for other age-related health problems. The risk of diabetes, falls, and coronary heart disease may be reduced or ameliorated with increased physical fitness.3-5 Several clinical trials have been conducted in which sedentary older adults are randomly assigned to physical activity interventions (typically to an aerobic exercise condition) to determine if cognition improves as a result of introducing exercise as a lifestyle modification.6 The rationale for these studies is based on evidence that physically fit individuals have a lower risk of developing cognitive decline (for review see Denkinger and colleagues7), and that sedentary elderly persons will experience the same benefits by initiating increased physical activity. A meta-analysis of several intervention studies found that fitness training improved cognitive functioning in older adults by an average of 0.5 standard deviations, regardless of the study population (normal and clinical populations), training methods, or cognitive measures used.2 However, a recent meta-analytic review by Smith and colleagues8 that included several high quality randomized controlled trials9-11 found only modest effects of exercise on measures of attention and processing speed, executive function, and memory and no cumulative effect on working memory. Indeed, several studies in this meta-analysis showed little or no effect of exercise on cognition.
The presence of one or more copy of the apolipoprotein ε4 allele (APOE4) is a strong genetic risk factor for the development of AD and is associated with poorer cognitive performance in cognitively normal individuals.12-14 Some studies have reported that APOE4 carriers with higher levels of physical activity exhibit some cognitive benefits, in terms of improved performance and protection against cognitive decline. For example, Schuit and colleagues15 examined the risk for cognitive decline over 3-4 years in a large sample of elderly males. APOE4 carriers who reported higher levels of physical activity were less likely to decline than carriers who reported lower levels of physical activity.15 However, other studies have found no effects of APOE416,17, and others have shown that higher levels of physical activity were beneficial for cognitive performance only in APOE4 non-carriers.18
Proposed mechanisms through which physical activity may improve cognitive functioning, or delay onset of decline, include metabolic and vascular pathways that influence autophagy19, neurogenesis20, upregulation of brain growth factors21,22, and improvement of cerebral metabolic efficiency.20,23,24 Studies from our center have shown that cognitvely-normal older adults who report higher levels of exercise engagement have greater frontal lobe volume and less atrophy in the medial temporal lobe with age than those who are more sedentary.25 In addition, participants who reported less exercise engagement had decreased cerebrospinal fluid (CSF) amyloid-β (Aβ) levels, and increased levels of cortical fibrillar Aβ deposition, as indicated by Carbon-11 Pittsburgh Compound-B ([11C]PiB) binding, when compared with more active particpants, suggesting an association between exercise engagement and molecular biomarkers of AD.26,27 APOE4 carriers who reported more exercise engagement had reductions in amyloid burden, as evidenced by [11C]PiB binding, when compared with APOE4 carriers reporting less engagement.27
Although on balance, prior studies suggest that increased physical activity is beneficial for cognitive performance and brain health, there is little evidence to determine whether the reported cross-sectional associations are sustained longitudinally. In the present study, we examined the baseline association between self-reported levels of exercise and cognitive functioning, as measured by standard neuropsychological tests, and also for up to 6 years of follow-up in a sample of healthy middle-aged and older individuals participating in the Adult Children Study. We hypothesized that individuals who reported higher levels of physical activity at baseline would perform better on cognitive tests and show less cognitive decline over time compared with participants who reported lower physical activity levels.
Methods
Participants
Participants were community residing volunteers (ages 45-74 at baseline) enrolled in the Adult Children Study (ACS) at the Knight Alzheimer Disease Research Center at Washington University School of Medicine in St Louis. The ACS is a longitudinal study to develop and validate biofluid markers, cognitive profiles, imaging features, and other indicators of the biological changes of AD that predate the onset of behavioral symptoms28,29 to aid in identifying individuals at high risk for developing symptomatic AD30 (see Xiong and colleagues31 for a detailed description of the ACS study). Individuals at genetic risk for AD because one of their biological parents had symptomatic AD with age at onset prior to 80 years are compared with a similarly aged control sample of individuals for whom neither parent had symptomatic AD (and lived at least to age 70 years). All ACS participants agree to complete the longitudinal ACS protocol, including clinical and neuropsychological assessments, seated blood pressure monitoring, height and weight for calculation of body mass index (BMI), blood collection for genotyping, lumbar puncture for CSF collection, structural and functional neuroimaging with magnetic resonance imaging (MRI), and positron emission tomography with the amyloid tracer, PET imaging with [C11]-PIB to examine Aβ in-vivo. Individuals with conditions that prevent completion of the protocol (e.g. metal implants that preclude MRI) are excluded. Younger participants (45-64 years at entry) are assessed every three years, while the older participants (65-74 years at entry) are evaluated annually with clinical and cognitive measures (CSF and imaging obtained every 3 years). At entry and each subsequent follow-up, each participant and their collateral source, a close friend or family member, are interviewed with standard instruments32 regarding cognitive and functional performance and that information is used by experienced clinicians to determine the Clinical Dementia Rating (CDR33). To be eligible for the ACS, participants must be CDR 0 (indicating cognitive normality) at baseline. Although the ACS has enrolled over 280 participants, the collection of data on physical activity was begun after the study had been launched. The sample for this study was the 173 participants who had complete baseline physical activity data and neuropsychological assessments. Ninety-one of the original 173 participants had returned for two or more neuropsychological assessments and comprise the sample for the longitudinal analyses. The Washington University Medical School Human Research Protection Office approved all procedures.
Measures
Physical Activity Assessment
Physical activity was assessed using items from the Nurses’ Health Study Exercise Questionnaire (NHSEQ), a self-report questionnaire designed for the Nurses Health Study II (For review see Colditz and colleagues34,35; refer to Appendix for item content). Participants completed the NHSEQ within approximately two months of their neuropsychological assessment (M = 51.4 days). The NHSEQ assesses total physical activity in minutes per week and has been demonstrated to be a reliable and valid measure for assessing physical activity.36 Similar forms of this questionnaire have been widely used in epidemiological studies, which have shown correlations between physical activity and health factors such as diabetes, blood pressure, and body mass index.5,37 Participants are asked to describe the amount of time they engage in both leisure (e.g., jogging) and home (e.g., mowing the lawn) physical activity during an average week over the previous year. For each item, participants reported the amount of time spent in that activity from 0 minutes up to 11+ hours. Total physical activity was calculated using two methods: 1) a metabolic equivalent measurement (MET) was derived by multiplying the sum of minutes in a given activity by their MET values. MET values represent the kilocalories utilized during one hour of activity, as compared to the kilocalories needed for an hour of rest.38 For example, if an individual spent 3 hours (180 minutes) per week jogging, it would be equivalent to 1250 METs of physical activity. 2) The second calculation used an ordinal scoring method to rate physical activity from 0 (none) to 9 (most) time spent per week in each of 10 categories, forming a total physical activity score ranging from 0 to 90. The response fields for estimated minutes of physical activity in the NHSEQ (see Appendix) have unequal ranges and contain some overlap, resulting in highly skewed data. In addition, the amount of effort expended (i.e., light, moderate or vigorous) for each activity is left unspecified, making it difficult to estimate an accurate MET value. Although both scoring methods were considered in initial analyses, we focused results on the ordinal scoring method as the preferred method.
Appendix.
1. DURING THE PAST YEAR, what was your average time per WEEK spent at each of the following recreational activities? (Mark an “X” in the box that reflects your activity)
| Zero | 1-4 Min. |
5-19 Min. |
20-59 Min. |
One Hour |
1-1.5 Hrs. |
2-3 Hrs. |
4-6 Hrs. |
7-10 Hrs. |
11+ Hrs. |
|
|---|---|---|---|---|---|---|---|---|---|---|
| Walking for exercise or walking to work | ||||||||||
| Jogging (slower than 10 min/mile) | ||||||||||
| Running (10 min/mile or faster) | ||||||||||
| Bicycling (include stationary machine) | ||||||||||
| Tennis, squash, racquetball | ||||||||||
| Lap swimming | ||||||||||
| Other aerobic exercise (aerobic dance, ski or stair machine, etc.) | ||||||||||
| Lower intensity exercise (yoga, stretching, toning) | ||||||||||
| Other vigorous activities (e.g. lawn mowing) | ||||||||||
| Weight Training (include free weights, or machines such as Nautilus) |
(Adapted from Harvard Nurses Health Study34
Neuropsychological Assessment
All participants completed a standard battery of cognitive tests within approximately two weeks of their clinical assessment yielding the CDR. Tests administered included the Free and Cued Selective Reminding Test for verbal memory (FCSR39); the Trailmaking Test Part A for speeded visuospatial function (TMTA) and Part B for working memory/executive function (TMTB40); category fluency for working memory/executive function (Animal Naming41); and the Letter-Number Sequencing task for working memory/executive function (LNS42). Tests were selected and domains were created based on a previous factor analysis from our center.43 Participants also completed the Geriatric Depression Scale-Short Form (GDS44).
APOE genotyping
TaqMan assays (Applied Biosystems, Foster City, USA) for both rs429358 (ABI#C_3084793_20) and rs7412 (ABI#C_904973_10) were used for APOE genotyping. Allele calling was performed using the allelic discrimination analysis module of ABI Sequence Detection Software. Positive controls for each of six possible APOE genotypes were included on the genotyping plate. Individuals were then classified as ε4+ (44, 34, 24) or ε4- (33, 23, 22).
Statistical Analyses
Spearman correlations were used to assess bivariate relationships between independent variables and outcome measures for model building. Based on our initial analyses and well-established associations with cognitive performance, age at baseline, gender, education, GDS score at baseline, parental history of AD (coded as yes/no based on having one or more affected parent), and presence or absence of one or more copy of the APOE ε4 allele (indicating a genetic risk for AD) were modeled as covariates in all initial analyses.
Baseline Analyses
Hierarchical linear regression models were fitted to simultaneously examine the effects of age, gender, education, APOE status, GDS score, parental history of AD, and ordinal-scale physical activity on cognitive performance on each test using the R statistical software package, version 2.13.2 (www.r-project.org). APOE status, parental history status, GDS score, and their interaction terms with physical activity explained no unique variance (all p's > .20) and were therefore excluded from the final models. Thus the final model included the following terms, testing the null hypothesis βi=0: Y = β0 + β1*(age) + β2*(gender) + β3*(education) + β4*(physical activity) + ε, where Y is the standardized score for each cognitive test. The statistical test for each regression coefficient thus reflects the unique effect of each variable on cognitive performance after accounting for all the other variables.
Longitudinal Analyses
Linear mixed effects modeling was used to examine the interactive effects of physical activity and time on cognitive performance using the lme4 (Bates & Maechler, 2010) and languageR (Baayen, 2009) packages in R. Models included terms for physical activity (ordinal NHSEQ scale), age (centered at 64.88 years at first assessment), gender, baseline cognitive performance for each test, APOE4 status, parental history of AD status, GDS score at baseline, education (centered at 15.71 years at first assessment), time (entered as both a fixed and random effect), and the interaction with time with each covariate. The term for time refers to the period between completing the physical activity assessment and the psychometric assessment at each follow-up. These within-subject analyses provided estimates of change in cognitive performance relative to physical activity level at baseline. Model validity was assessed with likelihood ratio tests comparing the models with fixed effect to the null models with only random effects. Results were rejected in which the model including fixed effects did not differ significantly from the null model. P-values for mixed-effects models were estimated with Markov-chain Monte Carlo sampling in languageR.
To further explore significant interactive effects, we created variables for “high” physical activity and “low” physical activity based on MET equivalent values as established by the American Heart Association's recommendation of 150 minutes/week of moderate exercise46 (using an estimated average of 6.67 METs for moderate exercise activities). Finally, longitudinal slopes of BMI were contrasted between high and low physical activity groups.
Results
Baseline Analyses
Demographic characteristics are shown in Table 1. The baseline analyses included 173 cognitively normal individuals ages 45-75 years (M = 60.7 years; 64.7% female). There were no differences in age, gender, education, parental history of AD, or APOE4 status between those reporting high levels of physical activity and low levels of physical activity. Individuals reporting low levels of physical activity scored slightly higher on the GDS (p = .040), but the mean score was well below the level of clinical significance and was influenced strongly by restricted range and floor effects. There were no significant differences in age, gender, education, or GDS score between APOE4 carriers and non-carriers. The results from hierarchical multiple regression models are presented in Table 2.
Table 1.
Participant Characteristics
| Baseline (N = 173) | Longitudinal (N = 91) | |
|---|---|---|
| Age | ||
| Mean (SD) | 60.7 years (±7.81) | 64.9 years (± 7.36) |
| Number of follow-up assessments | ||
| Mean (SD) | -- | 3.39 (1.20) |
| Mean Years of Education (SD) | 15.91 years (±2.55) | 15.71 years (± 2.58) |
| APOE Carriers, n (%)* | N = 172* | |
| ε2,2 | 2 (1.2) | 2 (2.2) |
| ε2,3 | 15 (8.7) | 7 (7.7) |
| ε2,4 | 6 (3.5) | 3 (3.3) |
| ε3,3 | 94 (54.3) | 52 (57.1) |
| ε3,4 | 44 (25.4) | 21 (23.0) |
| ε4,4 | 11 (6.4) | 4 (4.4) |
| Gender, n (%) | ||
| Males | 61 (35.3) | 34 (37.4) |
| Females | 112 (64.7) | 57 (62.6) |
| Race, n (%) | ||
| White | 151 (87.3) | 85 (93.4) |
| African American | 32 (12.1) | 6 (6.6) |
| Body Mass Index (Mean, SD) | 27.67 (5.2) | 27.99 (5.8) |
| Geriatric Depression Scale-Short Form | ||
| Mean (SD) | 0.88 (0.9) | 0.68 (0.71) |
| Parental History of AD, n (%) | 91 (52.6) | 40 (44.0) |
| NHSEQ Physical Activity (Mean, SD) | ||
| Weekly total physical activity (ordinal) | 13.81 (8.7) | 14.02 (7.91) |
| MET minutes/week | 1748.3 (1786.6) | 1716.84 (1716.86) |
One participant did not have genotype data.
Table 2.
Summary of Regression Analyses Predicting Cognitive Scores from Physical Activity Levels at Baseline (N=173).
| Cognitive Task | B for physical activity | Δ R2 for physical activity | Total R2 (all IVs) |
|---|---|---|---|
| Trails A | .179 | .035* | .196*** |
| Trails B | .119 | .004 | .224*** |
| Free & Cued Selective Reminding Task | .182 | .031* | .171*** |
| Letter-Number Sequencing | .090 | .001 | .120** |
| Animals | .138 | .027a | .120** |
Note: All models include age, gender, and education.
p < .05
p < .01
p<.001
trend at p<.10
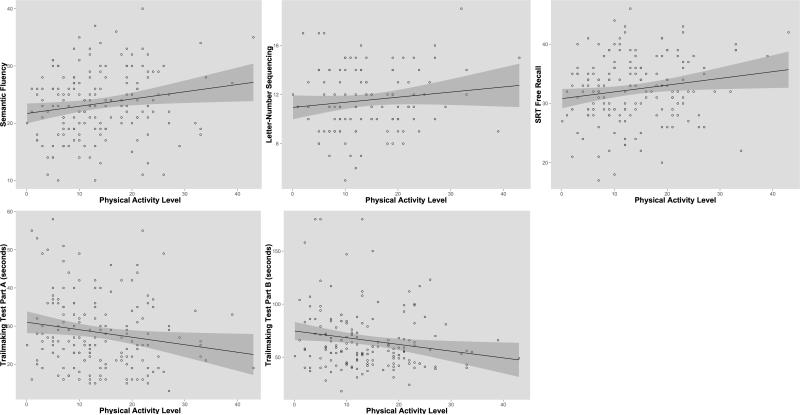
The linear combination of age, gender, education, and ordinal physical activity values together accounted for 20% of the variability in TMTA performance, whereas the unique variance accounted for by physical activity alone was 3.5%. Thus, a 1-SD increase in physical activity was associated with a 0.18-SD increase in TMTA performance. On the FCSR, all variables together accounted for 17% of the variability, and physical activity accounted for 3.1% of the variability. A 1-SD increase in physical activity was associated with a 0.18-SD increase in performance on FCSR. There was a trend-level association with Animal Naming, where physical activity explained 2.7% of the variance. LNS and TMTB performance were not related to physical activity levels at baseline (p's > .40). Scatter plots for raw scores and ordinal physical activity values are presented in Figure 1.
Figure 1.
Scatterplots of raw cognitive scores and physical activity levels at baseline. A positive correlation (Trailmaking Tests A & B are reversed scored) was evident across all tests administered. Significant effects of physical activity on cognitive functioning were seen on Trail Making Test Part A (p<.05) and Selective Reminding Test Free Recall (p<.05).
Longitudinal Analyses
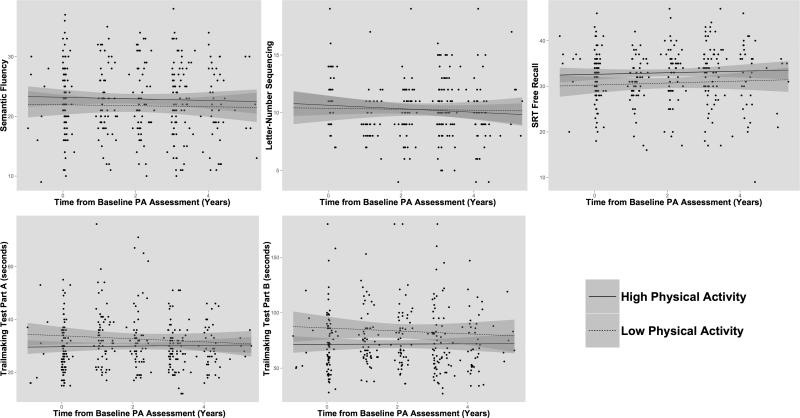
From the baseline sample, 91 participants were selected who had complete neuropsychological data for two or more follow-up assessments. The mean age of longitudinal participants was slightly older at baseline (M = 64.9 years) than the cross-sectional sample and 62.6% were female. Results from linear mixed models, which included terms for age at baseline physical activity assessment, gender, education, baseline cognitive performance on each test, APOE4 status, GDS score, and parental history of AD found no significant relationships between ordinal physical activity and slopes of cognitive performance on any individual test (all p's > .30; Figure 2). Analyses were repeated using the MET equivalent and results remained not significant (all p's > .40).
Figure 2.
Cognitive performance over follow-up assessments. Raw cognitive scores are shown for each participant from baseline through longitudinal follow-up. The trend lines represent “high” and “low” physical activity (PA) groups defined by AHA guidelines (above/below 1000 METs × minutes). No significant effects were seen for the interaction between physical activity and time from baseline physical activity assessment.
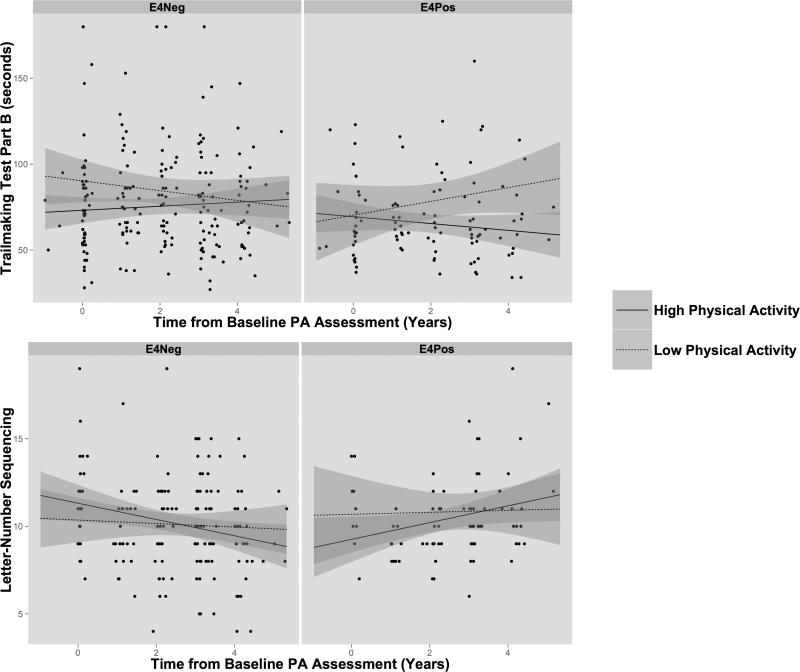
Addition of an interaction term for time, physical activity and APOE4 status revealed significant associations on the Trail Making Test Part B (p=.004) and the Letter-Number Sequencing test (p=.042), measures of working memory/executive function. Follow-up analyses revealed that APOE4 carriers who reported higher levels of physical activity showed improved performance over time on the TMTB (p=.038), whereas no such effect was seen in non-carriers (Figure 3). On LNS, APOE4 carriers in the high physical activity group again showed improvement over time (p=.047) while the non-carriers showed no change. Of note was a nonsignificant downward trend in LNS performance in the high physical activity non-carriers. However, results from LNS must be interpreted with caution due to the reduced number of participants with complete Letter-Number Sequencing data at follow-up visits. There were no differences in longitudinal slopes of BMI between participants reporting high and low levels of physical activity, using AHA MET values (all p's >.40).
Figure 3.
Trail Making Test Park B and Letter-Number Sequencing performance over follow-up assessments in APOE4 carriers and non-carriers. Raw cognitive scores are shown for each participant from baseline through longitudinal follow-up. The trend lines represent “high” and “low” physical activity (PA) groups defined with AHA guidelines (above/below 1000 METs × minutes). Significant effects for APOE4 status were seen for APOE4 carriers in the high physical activity group.
Discussion
This study had two major aims. The first was to determine if higher levels of physical activity were related to better cognitive performance at baseline. The second was to determine if baseline physical activity levels would exert a protective influence on cognitive trajectories over subsequent assessments. Analyses at baseline confirmed that participants who reported higher levels of physical activity performed slightly better on tests of speeded visuospatial functioning and verbal episodic memory than individuals who reported less physical activity. The positive association between physical activity levels and cognitive performance over time was limited to small performance gains in participants with at least one copy of the apolipoprotein ε4 allele, and was in different cognitive domains than the group differences at baseline. APOE4 carriers who reported higher levels of physical activity showed small improvements over time on tests of executive functioning and working memory. APOE4 non-carriers showed no effects of physical activity on cognitive performance over time.
Prior studies of exercise and cognition have yielded mixed results, but in general there is a small beneficial effect when sedentary individuals incorporate increases in exercise as an intervention.8 What remains difficult to ascertain is whether individuals who have sustained higher levels of physical activity exhibit any long-term cognitive benefits. These benefits may manifest as a “slowing” of normal age-related cognitive changes—indicated by a more horizontal slope of cognitive performance across the lifespan. Another manifestation may be a delay in the cognitive declines experienced at the onset of a neurodegenerative disease like AD. Perhaps individuals who are more physically fit will be more cognitively resilient to brain pathology, akin to the concept of cognitive reserve.47,48 The literature on cognitive reserve suggests that multiple factors, including education, prosocial behaviors, and cognitively enriching activity may all serve to delay the onset of cognitive decline associated with neurodegenerative disease.49,50 As a preliminary examination of physical activity and cognitive functioning, our data indicate a small positive association between baseline physical activity ratings and cognitive trajectories only in carriers of the APOE4 allele. This finding is novel in that we are unaware of any longitudinal studies that have examined the interactive effects of physical activity and APOE4 status on cogntive performance in cognitively normal individuals using sensitive neuropsychological tests (i.e., not brief dementia screening instruments). APOE4 carriers are at increased risk of developing AD,tend to develop symptomatic AD at youger ages, and show evidence of early cognitive changes prior to the appearance of symptoms of AD.13,51 Additionally, APOE4 carriers show loss of brain volume and cortical thickness52,53, increased amyloid burden54, and functional brain alterations55,56 well before the clinical diagnosis of AD. It may be that APOE4 carriers are more vulnerable to cognitive changes at younger ages and are therefore more likely to benefit from factors associated with cognitive reserve, including physical activity. Our data thus may reflect a threshold effect of cognitive reserve, wherein APOE4 carriers at midlife and at older ages have reached a threshold for vulnerability at which physical activity may exert a protective influence14, perhaps by delaying or preventing amyloid deposition and structural brain changes. Our preliminary results are consistent with prior studies that demonstrate that APOE4 carriers who report higher levels of exercise engagement exhibit lower levels of [11C]PiB binding than more sedentary individuals.27 As the ACS study progresses, we will assess whether these findings extend to individuals who become demented at later stages, possibly providing further insight about the relationship between physical activity and cognitive reserve.
There are several important limitations of our study. Our assessment of physical activity was provided through a self-report questionnaire used in the Nurses Health Study.34 While this measure has shown adequate convergent validity with other assessments of physical activity36, it may be influenced by recall bias and demand characteristics, among other limitations.57,58 Physical activity was assessed only at baseline, thus it is possible that some participants may have changed their physical activity levels during the course of the study. However, there is some evidence that exercise regimens and physical activity levels established in young adulthood remain relatively stable across the lifespan.59 In addition, there were no differences in slopes of BMI between the high and low physical activity groups, suggesting that on average, the physical activity regimens of study participants remained stable across follow-up assessments. Due to the structure of the ACS study, the number of assessments and length of time between cognitive assessments was inconsistent depending on the age at entry of the participants. Participants who entered before age 65 returned for follow-up every 3 years until age 65, when all participants are assessed annually. To account for this, time was entered as both a random and fixed effect in analyses, but the variability in the length of time between assessments may make our assessment of longitudinal trends less robust in our younger participants. As the ACS continues enrollment, we plan to increase the sample size and number of follow-up assessments to improve the strength of these conclusions. Future longitudinal studies should endeavor to include objective indices of physical fitness at each timepoint, including heart rate, maximal oxygen consumption (VO2 Max), and pulse wave velocity. Further in-depth investigations might consider how certain types of exercise (e.g., aerobic vs. stretching) might differentially impact cognitive domains.7
Overall, our results suggest that among healthy middle-aged and older adults, those who self-report higher levels of physical activity may have slightly better cognitive performance at any given timepoint. Over time, physical activity may be differentially beneficial for cognitively normal adults at midlife or later, depending upon their genetic risk for AD. However, it remains unclear whether the adoption of higher levels of physical activity at midlife will alter cognitive trajectories for APOE4 carriers. If future studies support a causal link between increased physical activity and improved (or maintained) levels of cognitive functioning in APOE4 carriers, it is possible that exercise interventions may delay or prevent the onset of symptomatic AD.
Acknowledgements
The authors acknowledge the Clinical Cores of the Adult Children Study and the Knight Alzheimer's Disease Research Center (P50 AG05681; JC Morris, PI) for the clinical assessments, Martha Storandt, PhD and Denise Maue Dreyfus, MA for psychometric assessments, and Alison Goate, DPhil. and the Knight ADRC Genetics Core for genotype data. We thank all participants for volunteering for this research.
Sources of Support: Funding for this study was provided by the National Institute on Aging (P01-AG26276, P50-AG005681, PO1-AG003991; JC Morris, PI), the generous support of F. Simmons and O. Mohan, The Farrell Family Research Foundation, and The Charles F. and Joanne Knight Alzheimer's Initiative.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Geda YE, Roberts RO, Knopman DS, et al. Physical exercise, aging, and mild cognitive impairment: a population-based study. Archives of Neurology. 2010;67(1):80–86. doi: 10.1001/archneurol.2009.297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Colcombe S, Kramer AF. Fitness effects on the cognitive function of older adults: a meta-analytic study. Psychol Sci. 2003;14(2):125–130. doi: 10.1111/1467-9280.t01-1-01430. [DOI] [PubMed] [Google Scholar]
- 3.Gregg EW, Pereira MA, Caspersen CJ. Physical activity, falls, and fractures among older adults: a review of the epidemiologic evidence. J Am Geriatr Soc. 2000;48(8):883–893–893. doi: 10.1111/j.1532-5415.2000.tb06884.x. [DOI] [PubMed] [Google Scholar]
- 4.Manson JE, Nathan DM, Krolewski AS, Stampfer MJ, Willett WC, Hennekens CH. A Prospective Study of Exercise and Incidence of Diabetes Among US Male Physicians. JAMA. 1992;268(1):63–67. [PubMed] [Google Scholar]
- 5.Manson JE, Hu FB, Rich-Edwards JW, et al. A Prospective Study of Walking as Compared with Vigorous Exercise in the Prevention of Coronary Heart Disease in Women. N. Engl. J. Med. 1999;341(9):650–658. doi: 10.1056/NEJM199908263410904. [DOI] [PubMed] [Google Scholar]
- 6.Jak AJ. The impact of physical and mental activity on cognitive aging. Curr Top Behav Neurosci. 2012;10:273–291. doi: 10.1007/7854_2011_141. [DOI] [PubMed] [Google Scholar]
- 7.Denkinger M, Nikolaus T, Denkinger C, Lukas A. Physical activity for the prevention of cognitive decline. 2012;45(1):11–16. doi: 10.1007/s00391-011-0262-6. [DOI] [PubMed] [Google Scholar]
- 8.Smith PJ, Blumenthal JA, Hoffman BM, et al. Aerobic Exercise and Neurocognitive Performance: A Meta-Analytic Review of Randomized Controlled Trials. Psychosomatic Medicine. 2010;72(3):239–252. doi: 10.1097/PSY.0b013e3181d14633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lautenschlager NT, Cox KL, Flicker L. Effect of physical activity on cognitive function in older adults at risk for Alzheimer disease. 2008 doi: 10.1001/jama.300.9.1027. [DOI] [PubMed] [Google Scholar]
- 10.Williamson JD, Espeland M. Changes in cognitive function in a randomized trial of physical activity: results of the lifestyle interventions and independence for elders pilot study. 2009 doi: 10.1093/gerona/glp014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hoffman BM, Blumenthal JA, Babyak MA, et al. Exercise fails to improve neurocognition in depressed middle-aged and older adults. 2008;40(7):1344. doi: 10.1249/MSS.0b013e31816b877c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Saunders AM, Strittmatter WJ, Schmechel D, et al. Association of apolipoprotein E allele epsilon 4 with late-onset familial and sporadic Alzheimer's disease. Neurology. 1993;43(8):1467–1472. doi: 10.1212/wnl.43.8.1467. [DOI] [PubMed] [Google Scholar]
- 13.Bondi MW, Salmon DP, Monsch AU, et al. Episodic memory changes are associated with the APOE-epsilon 4 allele in nondemented older adults. Neurology. 1995;45(12):2203–2206. doi: 10.1212/wnl.45.12.2203. [DOI] [PubMed] [Google Scholar]
- 14.Etnier JL, Caselli RJ, Reiman EM, et al. Cognitive performance in older women relative to ApoE-epsilon4 genotype and aerobic fitness. Medicine & Science in Sports & Exercise. 2007;39(1):199–207. doi: 10.1249/01.mss.0000239399.85955.5e. [DOI] [PubMed] [Google Scholar]
- 15.Schuit AJ, M Feskens EJ, Launer LJ, Kromhout D. Physical activity and cognitive decline, the role of the apolipoprotein e4 allele. Medicine & Science in Sports & Exercise. 2001:772–777. doi: 10.1097/00005768-200105000-00015. [DOI] [PubMed] [Google Scholar]
- 16.Lindsay J. Risk Factors for Alzheimer's Disease: A Prospective Analysis from the Canadian Study of Health and Aging. American journal of epidemiology. 2002;156(5):445–453. doi: 10.1093/aje/kwf074. [DOI] [PubMed] [Google Scholar]
- 17.Taaffe DR, Irie F, Masaki KH, et al. Physical activity, physical function, and incident dementia in elderly men: the Honolulu-Asia Aging Study. J Gerontol A Biol Sci Med Sci. 2008;63(5):529–535. doi: 10.1093/gerona/63.5.529. [DOI] [PubMed] [Google Scholar]
- 18.Podewils LJ, Guallar E, Kuller LH, et al. Physical activity, APOE genotype, and dementia risk: findings from the Cardiovascular Health Cognition Study. American journal of epidemiology. 2005;161(7):639–651. doi: 10.1093/aje/kwi092. [DOI] [PubMed] [Google Scholar]
- 19.He C, Bassik MC, Moresi V, et al. Exercise-induced BCL2-regulated autophagy is required for muscle glucose homeostasis. Nature. 2012;481(7382):511–515. doi: 10.1038/nature10758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hillman CH, Erickson KI, Kramer AF. Be smart, exercise your heart: exercise effects on brain and cognition. Nature Reviews Neuroscience. 2008;9(1):58–65. doi: 10.1038/nrn2298. [DOI] [PubMed] [Google Scholar]
- 21.Vaynman S, Ying Z, Gomez-Pinilla F. Exercise induces BDNF and synapsin I to specific hippocampal subfields. 2004;76(3):356–362. doi: 10.1002/jnr.20077. [DOI] [PubMed] [Google Scholar]
- 22.Gold SM, Schulz K-H, Hartmann S, et al. Basal serum levels and reactivity of nerve growth factor and brain-derived neurotrophic factor to standardized acute exercise in multiple sclerosis and controls. J. Neuroimmunol. 2003;138(1-2):99–105. doi: 10.1016/s0165-5728(03)00121-8. [DOI] [PubMed] [Google Scholar]
- 23.Convit A. Links between cognitive impairment in insulin resistance: an explanatory model. Neurobiology of aging. 2005;26(Suppl 1):31–35. doi: 10.1016/j.neurobiolaging.2005.09.018. [DOI] [PubMed] [Google Scholar]
- 24.Pereira AC, Huddleston DE, Brickman AM, et al. An in vivo correlate of exercise-induced neurogenesis in the adult dentate gyrus. Proc Natl Acad Sci USA. 2007;104(13):5638–5643. doi: 10.1073/pnas.0611721104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Bugg JM, Head D. Exercise moderates age-related atrophy of the medial temporal lobe. Neurobiology of aging. 2011;32(3):506–514. doi: 10.1016/j.neurobiolaging.2009.03.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Liang KY, Mintun MA, Fagan AM, et al. Exercise and Alzheimer's disease biomarkers in cognitively normal older adults. Annals of neurology. 2010;68(3):311–318. doi: 10.1002/ana.22096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Head D, Bugg JM, Goate AM, et al. Exercise Engagement as a Moderator of the Effects of APOE Genotype on Amyloid Deposition. Archives of Neurology. 2012 doi: 10.1001/archneurol.2011.845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Price JL, Morris JC. Tangles and plaques in nondemented aging and “preclinical” Alzheimer's disease. Annals of neurology. 1999;45(3):358–368. doi: 10.1002/1531-8249(199903)45:3<358::aid-ana12>3.0.co;2-x. [DOI] [PubMed] [Google Scholar]
- 29.Goldman WP, Price JL, Storandt M, et al. Absence of cognitive impairment or decline in preclinical Alzheimer's disease. 2001;56(3):361–367. doi: 10.1212/wnl.56.3.361. [DOI] [PubMed] [Google Scholar]
- 30.Coats M. Antecedent Biomarkers of Alzheimer's Disease: The Adult Children Study. Journal of Geriatric Psychiatry and Neurology. 2005;18(4):242–244. doi: 10.1177/0891988705281881. [DOI] [PubMed] [Google Scholar]
- 31.Xiong C, Roe CM, Buckles V, et al. Role of Family History for Alzheimer Biomarker Abnormalities in the Adult Children Study. Archives of Neurology. 2011;68(10):1313. doi: 10.1001/archneurol.2011.208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Morris JC, Weintraub S, Chui HC, et al. The Uniform Data Set (UDS): clinical and cognitive variables and descriptive data from Alzheimer Disease Centers. Alzheimer Dis Assoc Disord. 2006;20(4):210–216. doi: 10.1097/01.wad.0000213865.09806.92. [DOI] [PubMed] [Google Scholar]
- 33.Morris JC. The Clinical Dementia Rating (CDR): current version and scoring rules. Neurology. 1993;43(11):2412–2414. doi: 10.1212/wnl.43.11.2412-a. [DOI] [PubMed] [Google Scholar]
- 34.Colditz GA, Manson JE, Hankinson SE. The Nurses’ Health Study: 20-year contribution to the understanding of health among women. Journal of Women's Health. 1997;6(1):49–62. doi: 10.1089/jwh.1997.6.49. [DOI] [PubMed] [Google Scholar]
- 35.Colditz GA, Hankinson SE. The Nurses’ Health Study: lifestyle and health among women. Nature Reviews Cancer. 2005;5(5):388–396. doi: 10.1038/nrc1608. [DOI] [PubMed] [Google Scholar]
- 36.Erickson JB. Reproducibility and validity of a self-administered lifetime physical activity questionnaire in women. 2000 [Google Scholar]
- 37.Yaffe K, Barnes D, Nevitt M, Lui LY, Covinsky K. A prospective study of physical activity and cognitive decline in elderly women: women who walk. Archives of internal medicine. 2001;161(14):1703–1708. doi: 10.1001/archinte.161.14.1703. [DOI] [PubMed] [Google Scholar]
- 38.Ainsworth BE, Haskell WL, Leon AS, et al. Compendium of physical activities: classification of energy costs of human physical activities. Medicine & Science in Sports & Exercise. 1993;25:71–71. doi: 10.1249/00005768-199301000-00011. [DOI] [PubMed] [Google Scholar]
- 39.Grober E, Buschke H, Crystal H, Bang S, Dresner R. Screening for dementia by memory testing. Neurology. 1988;38(6):900–900. doi: 10.1212/wnl.38.6.900. [DOI] [PubMed] [Google Scholar]
- 40.Armitage SG. An analysis of certain psychological tests used for the evaluation of brain injury. Psychological Monographs. 1946:i–48. [Google Scholar]
- 41.Goodglass H, Kaplan E. The assessment of aphasia and related disorders. 1983 [Google Scholar]
- 42.Wechsler D. Wechsler Adult Intelligence Scale® – Third Edition (WAIS®–III) Harcourt Assessments; 1997. [Google Scholar]
- 43.Johnson DK, Storandt M, Morris JC, Langford ZD, Galvin JE. Cognitive profiles in dementia: Alzheimer disease vs healthy brain aging. Neurology. 2008;71(22):1783–1789. doi: 10.1212/01.wnl.0000335972.35970.70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Yesavage JA, Brink TL, Rose TL, et al. Development and validation of a geriatric depression screening scale: a preliminary report. J Psychiatr Res. 1982;17(1):37–49. doi: 10.1016/0022-3956(82)90033-4. [DOI] [PubMed] [Google Scholar]
- 45.Binder EF, Storandt M, Birge SJ. The relation between psychometric test performance and physical performance in older adults. J Gerontol A Biol Sci Med Sci. 1999;54(8):M428–32. doi: 10.1093/gerona/54.8.m428. [DOI] [PubMed] [Google Scholar]
- 46.Association AH. American Heart Association Complete Guide to Women's Heart Health. Clarkson Potter; 2011. [Google Scholar]
- 47.Stern Y. What is cognitive reserve? Theory and research application of the reserve concept. J Int Neuropsychol Soc. 2002;8(3):448–460. [PubMed] [Google Scholar]
- 48.Mortimer J, Graves AB. Education and Other Socioeconomic Determinants of Dementia and Alzheimers-Disease. Neurology. 1993;43(8):S39–S44. [Google Scholar]
- 49.Roe CM, Mintun MA, D'Angelo G, Xiong C, Grant EA, Morris JC. Alzheimer disease and cognitive reserve: variation of education effect with carbon 11-labeled Pittsburgh Compound B uptake. Archives of Neurology. 2008;65(11):1467. doi: 10.1001/archneur.65.11.1467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Scarmeas N, Zarahn E, Anderson KE, et al. Association of life activities with cerebral blood flow in Alzheimer disease: implications for the cognitive reserve hypothesis. Archives of Neurology. 2003;60(3):359. doi: 10.1001/archneur.60.3.359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Caselli RJ, Dueck AC, Osborne D, et al. Longitudinal modeling of age-related memory decline and the APOE epsilon4 effect. N. Engl. J. Med. 2009;361(3):255–263. doi: 10.1056/NEJMoa0809437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Donix M, Burggren AC, Suthana NA, et al. Longitudinal changes in medial temporal cortical thickness in normal subjects with the APOE-4 polymorphism. NeuroImage. 2010;53(1):37–43. doi: 10.1016/j.neuroimage.2010.06.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Schuff N, Woerner N, Boreta L, et al. MRI of hippocampal volume loss in early Alzheimer's disease in relation to ApoE genotype and biomarkers. Brain. 2008;132(4):1067–1077. doi: 10.1093/brain/awp007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Morris JC, Roe CM, Xiong C, et al. APOE predicts amyloid-beta but not tau Alzheimer pathology in cognitively normal aging - Morris - 2009 - Annals of Neurology - Wiley Online Library. Annals of neurology. 2010;67(1):122–131. doi: 10.1002/ana.21843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Scarmeas N. Altered PET Functional Brain Responses in Cognitively Intact Elderly Persons at Risk for Alzheimer Disease (Carriers of the 4 Allele). American Journal of Geriatric Psychiatry. 2004;12(6):596–605. doi: 10.1176/appi.ajgp.12.6.596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Sheline YI, Morris JC, Snyder AZ, et al. APOE4 allele disrupts resting state fMRI connectivity in the absence of amyloid plaques or decreased CSF Aβ42. The Journal of Neuroscience. 2010;30(50):17035–17040. doi: 10.1523/JNEUROSCI.3987-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Shephard RJ. Limits to the measurement of habitual physical activity by questionnaires * Commentary. British Journal of Sports Medicine. 2003;37(>3):197–206. doi: 10.1136/bjsm.37.3.197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Miller DI, Taler V, Davidson PSR, Messier C. Measuring the impact of exercise on cognitive aging: methodological issues. Neurobiology of aging. 2012;33(3):622.e29–43. doi: 10.1016/j.neurobiolaging.2011.02.020. [DOI] [PubMed] [Google Scholar]
- 59.Malina RM. Physical activity and fitness: pathways from childhood to adulthood. Am. J. Hum. Biol. 2001;13(2):162–172. doi: 10.1002/1520-6300(200102/03)13:2<162::AID-AJHB1025>3.0.CO;2-T. [DOI] [PubMed] [Google Scholar]