Abstract
In multiple sclerosis (MS) and experimental autoimmune encephalomyelitis (EAE), relapses are markedly reduced during pregnancy. Exosomes are lipid-bound vesicles and are more abundant in the serum during pregnancy. We demonstrate that serum exosomes suppress T cell activation, promote the maturation of oligodendrocyte precursor cells (OPC), and pregnancy exosomes facilitate OPC migration into active CNS lesions. However, exosomes derived from both pregnant and non-pregnant mice reduced the severity of established EAE. Thus, during pregnancy, serum exosomes modulate the immune and central nervous systems and contribute to pregnancy-associated suppression of EAE.
Keywords: Pregnancy, Multiple Sclerosis, Experimental Autoimmune Encephalomyelitis, Exosome, Oligodendrocyte Precursor Cell
1. Introduction
Multiple sclerosis (MS) is a chronic, demyelinating disease of the central nervous system (CNS) characterized by a remitting-relapsing or chronic progressive disease course [1]. In MS, CD4+ Th1 cells, directed against myelin antigens, are thought to initiate neuroinflammation. Women develop MS nearly three times more often than men and typically present with a remitting-relapsing disease course [2]. Relapses are less frequent during pregnancy and the most dramatic reduction is observed during the third trimester. After delivery, disease activity rebounds, and relapse rates eventually return to pre-pregnancy levels [3].
We and others have shown that induction of experimental autoimmune encephalomyelitis (EAE) during pregnancy reduces the incidence and delays the onset of disease [4,5]. Mice immunized for EAE during the postpartum period exhibit increased disease severity [5]. Several mechanisms have been put forward to explain the dynamics between pregnancy and MS including a Th1/Th2 shift, suppression of the immune response by hormones such as estradiol and progesterone, and the pregnancy-specific estrogen, estriol. Langer-Gould et al. [4] implicated a pregnancy-specific serum factor as responsible for suppression of EAE, since serum from pregnant mice reduced proliferation and IL-2 production from myelin-specific T cells.
Exosomes are small (50–90 nm in diameter), lipid-bound vesicles of endosomal origin [6] and are abundant in the serum of pregnant women. Exosomes from the serum of pregnant women that deliver at term better suppress the activation of T cell signaling molecules than serum exosomes from women that deliver pre-term [7]. Further, exosomes from late pregnant mice are better able to suppress T cell proliferation than exosomes from virgin controls [8]. Together, these data suggest that serum exosomes may play a role in feto-maternal tolerance.
The pregnancy environment has been shown to afford a state of neuroprotection. The suppression of MS relapses associated with pregnancy has been correlated with a decrease in the number and area of white matter lesions [9]. A recent study reported a reduced risk of first clinical demyelinating event (by nearly 50%) for every birth [10]. Oligodendrocytes, the myelin-forming cell of the CNS, are continually generated from oligodendrocyte precursor cells (OPCs).. An increase in the generation of new oligodendrocytes and the number of myelinated axons has been observed during pregnancy [11]. Exosomes are taken up by several organ systems, including the brain, likely due to their small size and lipophilic nature. We therefore hypothesized that pregnancy-derived serum exosomes contribute to neuroprotection during EAE.
2. Materials and Methods
2.1. Mice
C57Bl/6 mice were purchased from The Jackson Laboratory (Bar Harbor, ME). Myelin oligodendrocyte glycoprotein (MOG) T cell receptor (TCR) transgenic mice on a C57Bl/6 background (2D2) were a kind gift from Dr. Vijay Kuchroo (Harvard Institute of Medicine, Boston, MA) [12]. Male mice were introduced to females for 3 d for the induction of pregnancy. Animals that were gestational day (GD) 16–18 were considered late pregnant. Mice were maintained on a 12-hour light/dark cycle and given food and water ad libitum.
2.2. EAE immunization
C57Bl/6 mice were immunized subcutaneously with 200 μg of MOG35–55 (Princeton Biomolecules, Langhorne, PA) and CFA containing 200 μg heat-killed Mycobacterium tuberculosis, Jamaica strain. Pertussis toxin (List Biological Laboratories, Campbell, CA) (250 ng) was injected i.p. on the day of immunization and 2 days later. Mice were monitored daily for clinical signs of disease and were scored as follows: 0, no observable signs; 1, limp tail; 2, limp tail and ataxia; 3, paralysis of one hind limb; 4, complete hind limb paralysis; 5, death.
2.3. Exosome isolation and quantification
Exosomes were isolated from the serum of virgin (control) and pregnant female mice using differential centrifugation as previously described [13]. Briefly, peripheral blood was collected via retro orbital eye bleeds and serum was subjected to a series of centrifugations, the last for 1 h at 120,000 × g. The exosome pellet was washed and protein quantified, using a bicinchoninic acid assay (BCA) (Thermo Scientific, Pittsburgh, PA). Fresh exosomes were isolated for each experiment. Probing for actin and calreticulin via Western blot was done to verify the presence of isolated exosomes.
2.4. Intracellular cytokine staining
MOG TCR transgenic splenocytes were cultured with MOG35–55 (10 μg/ml) alone or with control- or pregnant-derived serum exosomes for 48 h including a 4-h incubation with BD Golgi Plug (brefeldin A). Cells were stained with PerCP- anti-CD4 mAb. After cells were permeabilized and fixed, intracellular IFN-γ was stained using allophycocyanin- anti-IFN-γ (BD Biosciences) mAbs. All samples were analyzed by flow cytometry (FACSCanto II; BD Biosciences).
2.5. Primary oligodendrocyte precursor cell (OPC) cultures
C57Bl/6 pups were sacrificed on postnatal day 2 by rapid decapitation and the cortices were removed. The tissue was triturated in 0.1% trypsin (Sigma) followed by DNase I (200 U/ml) (Sigma-Aldrich). Cells were resuspended and plated in poly-L-lysine-coated flasks for 8–10 d. Microglia were reduced in cultures by gentle shaking. More extended shaking resulted in detachment of OPCs which were plated onto poly-L-lysine-coated glass coverslips and cultured in, neurobasal (Invitrogen) medium containing PDGF (PeproTech, Rocky Hill, NJ) and B27 supplement (Invitrogen). After 48 h, control- or pregnant-derived exosomes were added for 72 h. Cells were then fixed with 4% paraformaldehyde.
2.6. Bromodeoxyuridine (BrdU) administration
BrdU solution was prepared freshly each day and mice with EAE were injected i.p. daily (50 mg/kg) from 15–21 dpi.
2.7. Immunohistochemistry of in vitro cultured OPCs
Following treatment with exosomes, coverslips containing OPC were washed and treated with 0.1% Triton X-100 (Sigma-Aldrich) and DAPI for 20 min at room temperature. Following washes, coverslips were mounted onto slides and the relative number of cells for each treatment (DAPI+ nuclei) was examined using MCID image analysis software (Interfocus Imaging, Cambridge, England). The MCID software controlled the motorized X-Y stage of an Axioplan 2 imaging microscope (Zeiss, Thornwood, NY) and 3% of each coverslip was counted.
2.8. Immunohistochemistry of spinal cord tissue
Mice were perfused with 4% paraformaldehyde, and spinal cords were removed and post-fixed for 2 h. Spinal cords were cryopreserved in 30% sucrose, frozen in O.C.T. Compound (Sakura Finetek, Torrance, CA), and sequential longitudinal sections (10 μm) were cut, slide mounted, and stored at −20°C. For BrdU staining, sections were rinsed and incubated in 2N HCL, then in 4% BSA/0.3%Triton X-100. Anti-NG2 (USBiological, Swampscott, MA) and anti-BrdU (AbD Serotec, Raleigh, NC) mAbs were applied for 20 h. Sections were rinsed, incubated with Alexa Fluor 488- and Alexa Fluor 546-conjugated secondary Abs (Molecular Probes), and washed.. Images were acquired using the Olympus FluoView FV1000 Spectral confocal microscope. A minimum of 5 lesions per animal were analyzed, lesion data for each animal were averaged, and analysis was performed on treatment means.
2.10. Statistical analysis
A Mann-Whitney U test was used to analyze mean clinical disease score. A two-tailed student’s t test was used for flow cytometric and immunohistochemical data. All data are graphed as mean ± SEM using GraphPad Prism software (GraphPad Software, San Diego, CA).
3. Results
3.1. Pregnancy suppresses ongoing, established EAE
To determine the effect of pregnancy on established EAE, C57Bl/6 mice were immunized with MOG35–55, and pregnancy was induced after disease onset (22–24 dpi). Mice that became pregnant exhibited a significantly less severe EAE disease course during pregnancy compared to unmated female controls (Fig. 1). Clinical disease did not progress after the initiation of pregnancy and resumed shortly before pups were delivered, suggesting that a factor present during the gestation period is responsible for inhibiting clinical EAE. The cumulative disease index of pregnant mice was significantly lower only during the gestation period compared to controls. Importantly, females that were exposed to males but did not become pregnant had a disease course similar to unmated control mice (Fig. 1). Taken together, these data suggest that pregnancy fosters a physiological environment that suppresses established EAE.
Figure 1.
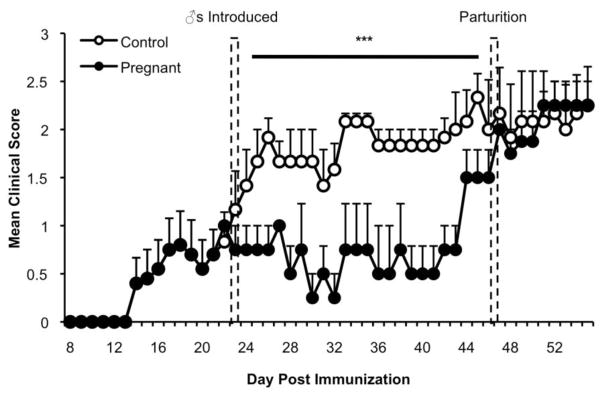
Pregnancy decreases the severity of established EAE. Mice were immunized with MOG35–55 and adjuvants. Female mice in the pregnant group were mated following the acute phase of disease for 3 d, 22–27 dpi, with immunized, age-, sex-, and strain-matched virgin mice as controls. All mice shown developed EAE. The gestation period is represented as the time between the hatched bars. Clinical signs of EAE were monitored (***P < 0.0001). The graph represents combined data from 4–6 mice per group.
3.2. Serum exosomes impact the activation of myelin-specific T cells
To determine if an alteration in the immune response mediated pregnancy-associated suppression of EAE, we analyzed immune cell phenotype and proliferative capacity during EAE and pregnancy. There were no differences in the populations of CD4+, CD8+, CD19+, CD11b+, CD11c+, and I-Ab+ cells, within the spleen or draining lymph nodes in mice with EAE during pregnancy (data not shown). Additionally, T cells from pregnant mice proliferated to the same extent and expressed similar levels of IFN-γ and T-bet as those from naïve mice following stimulation (data not shown). Finally, when T cells from immunized control and late pregnant mice were restimulated with antigen and transferred to naïve female mice for the induction of EAE, control- and late pregnancy-derived T cells transferred EAE with similar severity (data not shown). Together, these data suggest that there is not an intrinsic immune cell deficit during late pregnancy but rather, an environmental factor may be causing the immunomodulation.
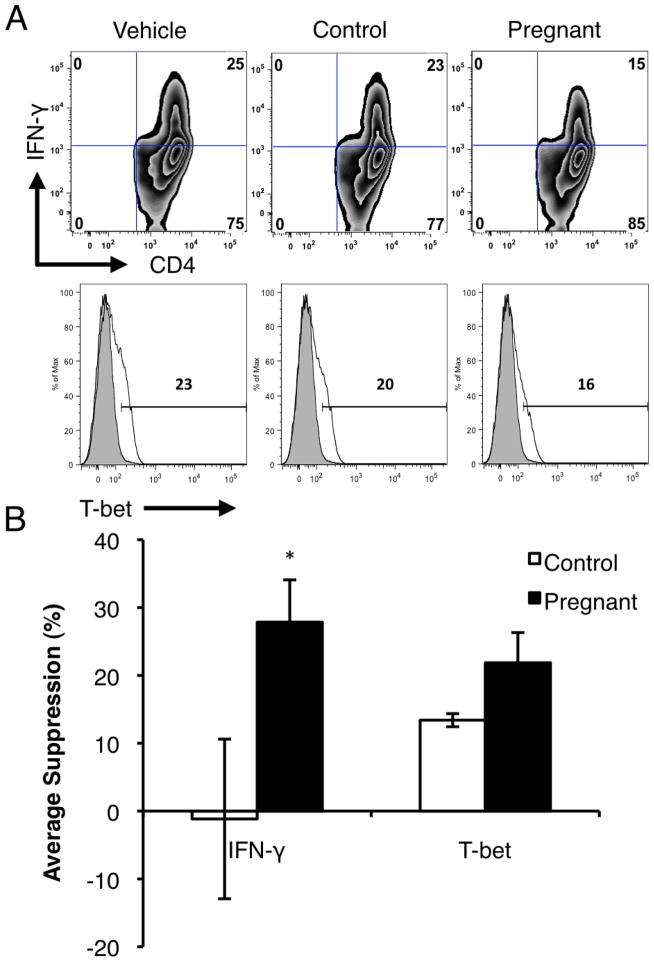
A serum factor, possibly exosomes, could be responsible for pregnancy-mediated suppression of EAE [4,8] To explore possible mechanisms, we first focused on the effects of exosomes on T cells. We measured intracellular IFN-γ and IL-17 in MOG-stimulated CD4+ T cells in the presence of serum exosomes. While exposure to control-derived exosomes did not alter IFN-γ expression in T cells, we observed a significant decrease in IFN-γ-expressing cells when activated T cells were exposed to pregnancy-derived exosomes (Fig. 2A). The decrease in IFN-γ expression correlated with a decrease in expression of the Th1 transcription factor, T-bet in CD4+ T cells (Fig. 2A). Additionally, this suppression of IFN-γ was significant when quantified over several experiments (Fig. 6B). The intracellular expression of IL-17 in CD4+ T cells did not differ with exosome treatment (data not shown). These data suggest that serum exosomes modulate immunity, dampening pathogenic Th1 cells, and point to a possible mediator for suppression of MS and EAE during pregnancy.
Figure 2.
Pregnancy-derived serum exosomes suppress T cell activation. MOG TCR transgenic splenocytes were cultured with MOG35–55 (10 μg/ml) and exosomes (20 μg/ml) from serum of control or pregnant mice for 48 hours. (A) Intracellular IFN-γ and T-bet expression was measured by flow cytometry. Data from 3 independent experiments. (B) Suppression of intracellular IFN-γ and T-bet in CD4+ T cells by serum exosomes was compiled and analyzed over 3 independent experiments (*P < 0.05).
3.4. Serum exosomes enhance the function of OPCs in vitro and in vivo
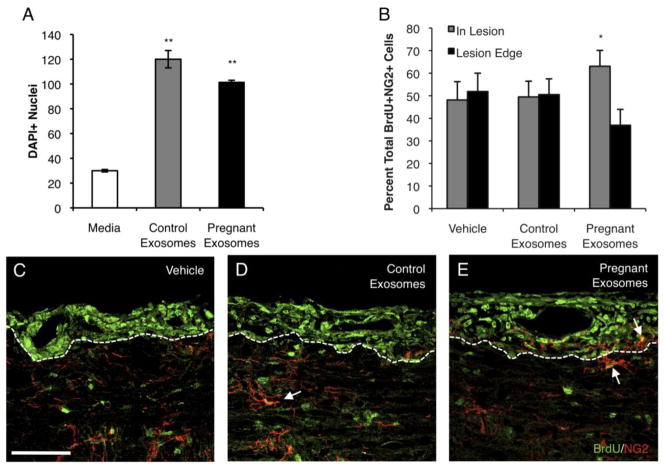
Due to the neuroprotective effects of pregnancy, the effect of serum exosomes on OPCs was examined. Following a 48-h equilibration period, OPCs were treated with medium alone, or medium supplemented with control- or pregnancy-derived serum exosomes for 72 h. Control and pregnant exosomes enhanced the expression of myelin basic protein (MBP) above that of medium-treated OPCs. Further, the complexity and branching of OPCs were increased when OPCs were treated with serum exosomes, regardless of origin (data not shown). In addition, quantification of OPC cell number revealed that more OPCs survived and/or proliferated in response to both control- and late pregnancy-derived serum exosomes in vitro (Fig. 3A). However, while control and pregnancy derived exosomes had similar effects on OPCs in vitro, only pregnancy-derived exosomes were able to modulate OPC function in vivo.
Figure 3.
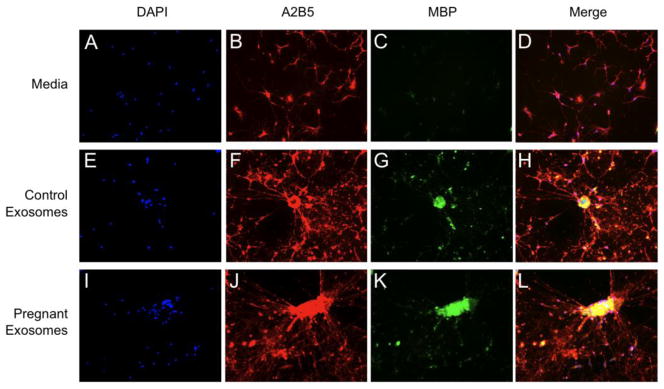
Pregnancy exosomes augment the function of OPCs. Mixed glia were plated and cultures enriched for OPCs. OPCs were cultured in medium alone or together with serum exosomes from control or pregnant mice for 72 h and labeled with DAPI (A) DAPI+ nuclei were counted on 3% of each coverslip (86 random fields) (**P < 0.01). Data are representative of 4 independent experiments. (B–E) Female mice were immunized with MOG35–55 and adjuvants, and treated i.v. with either vehicle, control- or pregnancy-derived serum exosomes 15 dpi. Following treatment, mice were injected with BrdU i.p. daily for 6 d. Mice were perfused and spinal cords removed and processed for immunohistochemistry. (B) The proportion of total BrdU+NG2+ cells was quantified within and outside of defined lesion areas (*P < 0.05). BrdU+NG2+ OPCs were normalized to the total number of BrdU+NG2+ cells counted per lesion. Representative lumbar spinal cord sections from (C) vehicle-, (D) control exosome-, (E) and pregnancy exosome-treated mice double-labeled for BrdU (green) and NG2 (red) by immunofluorescence, indicated by arrows (60x, scale bar, 50 μm). Data are representative of 4 mice per treatment, 5 lesions per animal.
EAE was induced and mice were treated with PBS (vehicle), or serum exosomes from control or late pregnant mice 15 dpi. Following treatment, mice were given BrdU i.p. daily for 6 d. To analyze proliferating OPCs, spinal cords from treated mice were stained for BrdU and NG2. Lesions were identified as areas of intense proliferation within the white matter of the spinal cord (Fig. 3C–E, outlined). The proportion of proliferating OPCs within lesions and at lesion edges was quantified. Interestingly, there was a significant increase in the number of actively proliferating OPCs migrating into lesion areas in mice with EAE that were treated with pregnancy-derived exosomes compared to OPCs found outside of lesions (Fig. 3B,E). These data suggest that pregnancy-derived exosomes facilitate OPC trafficking into lesion areas to initiate repair, as another mechanism to facilitate suppression of EAE during pregnancy.
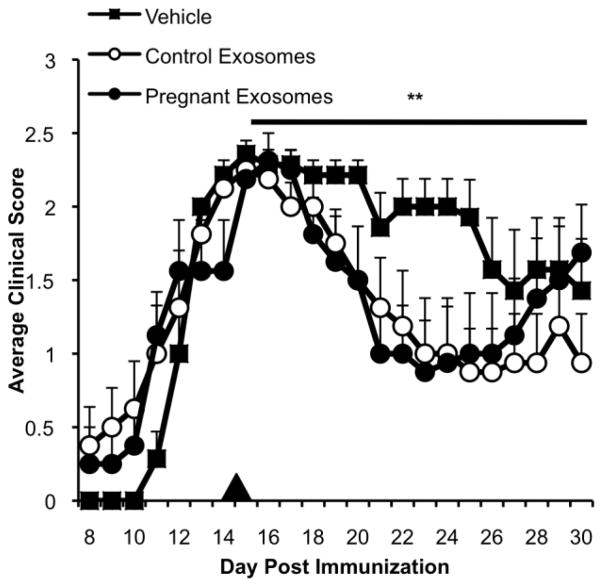
3.5. Control- and pregnancy-derived serum exosomes suppress established EAE
Since pregnancy-derived exosomes are able to inhibit Th1 cell activation, and enhance the survival, maturation, and migration of OPCs, we sought to determine if serum exosomes are able to suppress ongoing EAE. We administered a single dose (40 μg) of isolated control- or pregnancy-derived serum exosomes intravenously to mice with EAE 15 dpi, at the peak of disease. Following treatment, we observed a decrease in EAE severity in those mice receiving pregnancy as well as control exosomes, compared to mice treated with PBS (vehicle) (data not shown). It should be noted that the total protein concentration was normalized for the dose of exosomes. The overall impact of exosomes on EAE severity was significant; however, the effect of exosomes on disease course began to lessen 12 days after initiation of exosome treatment (27 dpi). Thus, serum exosomes, whether derived from pregnant or control mice, have suppressive capacity in the context of EAE and a single exosome administration has a time-limited action. Taken together, these data suggest that during pregnancy, serum exosomes contribute to immune modulation and neuroprotection; however, additional mechanisms are likely at work during pregnancy-associated suppression of EAE and MS.
4. Discussion
MS patients that become pregnant have fewer disease relapses, especially during the third trimester. Relapses typically rebound after parturition, taking nearly a year to return to pre-pregnancy levels [3]. Serum exosomes are thought to contribute to disease suppression due to their ability to suppress T cell function and their hypothesized importance in feto-maternal tolerance [7,8,14]. Harnessing the unique mechanism by which serum exosomes mediate pregnancy-associated suppression of MS may lead to a novel therapeutic modality.
In this study, we sought to identify a serum factor responsible for pregnancy-associated suppression of EAE and MS. Serum exosomes from pregnant mice were isolated and characterized. The suppressive effects of serum exosomes in vitro led us to examine their effects on T cell encephalitogenicity and OPC function. Serum exosomes from pregnant animals were able to dampen Th1 responses and enhance OPC maturation and trafficking to lesion areas during EAE, positioning them at a therapeutic juncture between immunomodulation and neuroprotection during pregnancy and disease
Regulation of immune cells by exosomes has been demonstrated in several animal models. Exosomes derived from dendritic cells made to over-express IL-4, FasL or IL-10 were able to suppress inflammation in mice with delayed-type hypersensitivity or collagen-induced arthritis (CIA) [13,15,16]. In fact, administration of exosomes derived from IL-10 transduced dendritic cells was able to delay the onset and reduce the severity of CIA to the same extent as injection of parental dendritic cells [13]. Additionally, the placenta has been reported to contribute to local immune suppression by exosomes. First trimester trophoblast cells lack surface FasL, but are able to induce Fas-mediated apoptosis of activated T cells by compartmentalizing FasL within exosomes [17].. We found that pregnancy-derived serum exosomes suppress IFN-γ production and T-bet expression in activated T cells.. The suppression of IFN-γ by pregnancy-derived exosomes not only slows the inflammatory response, but also may contribute to oligodendrocyte sparing during neuroinflammation, as IFN-γ is able to directly induce oligodendrocyte death. Previously, we have reported that IL-17 production from lymph node cells of immunized SJL mice is reduced during pregnancy [8]. Surprisingly, IL-17 expression was not altered in T cells exposed to pregnancy-derived exosomes.
Surprisingly, control- and pregnancy-derived exosomes were both able to suppress EAE with similar proficiency. The concentration of exosomes administered to mice with EAE was normalized for protein content, and based on preliminary 2D gel analysis, the proteomic profile of pregnancy exosomes differs from that of control exosomes. Pregnancy-derived exosomes were enriched in leukemia inhibitory factor receptor (LIFR), ceruloplasmin, serine protease inhibitors (corticosteroid-binding globulin and alpha-1-antitrypsin 1–5), complement components (C4-B and C5), and signaling molecules (fibrinogen-like protein 1 and fibrinogen gamma chain) while transporter protein (apolipoprotein A-I and A-IV), and peroxidase/esterase protein (serum paraoxonase/arylesterase 1 and liver carboxylesterase N) family members were down-regulated in pregnancy exosomes. Exosomes are also known to contain nucleic acids including RNAs and microRNAs. Together, this suggests that other macromolecular components of exosomes may be important in determining disease modulation during pregnancy.
The study of neuroprotective properties of pregnancy-derived exosomes focused on the oligodendrocyte. Exosomes represent a unique treatment modality as they have been shown to cross the blood brain barrier and fuse with their target, incorporating exosomal surface receptors into the plasma membrane of the recipient cell [18]. Interestingly, LIFR is enriched on the surface of pregnancy-derived exosomes and is likely incorporated into the plasma membrane of exosome-treated OPCs, causing OPCs to be more receptive to LIF-mediated signaling. LIF is a neurotropic factor in the IL-6 family of cytokines that has been shown to completely rescue oligodendrocyte loss during EAE. In addition, LIF also functions as a critical mediator of remyelination and repair [19]. Ceruloplasmin is another protein up-regulated in pregnancy-derived serum exosomes that may contribute to OPC maturation as it is a ferroxidase, able to catalyze the oxidation of ferrous iron [20]. The conversion from ferrous to ferric iron is essential for iron uptake by oligodendrocytes and efficient downstream myelin production [21]. Together, LIFR and ceruloplasmin may contribute to exosome-induced survival and maturation of OPCs during pregnancy.
Currently, there are no approved therapies for the induction of remyelination in MS; therefore, there is a need for further understanding of mechanisms underlying myelin re-formation in CNS lesions. When mice with EAE were systemically treated with pregnancy-derived exosomes, there was a significant increase in the proportion of OPCs within lumbar spinal cord lesions. Several factors are thought to mediate OPC recruitment including growth factors, cytokines, and chemokines. Pregnancy-derived exosomes contain tropic factors that support the survival of CNS cell types and may act either on OPCs themselves or on proximal supporting cells, altering the CNS microenvironment during inflammation to facilitate OPC trafficking. The recruitment of OPCs to lesion areas is critical for remyelination to occur [22]. Therefore, the promotion of proliferating OPC trafficking to active spinal cord lesions by serum exosomes may provide a novel mechanism by which pregnancy affords a state of neuroprotection.
In conclusion, serum exosomes are a prominent mediator both of immune modulation and neuroprotection during pregnancy. While pregnancy-derived exosomes are able to affect both immune and CNS compartments, it appears an overall change in disease course is difficult to establish when exosomes alone are used therapeutically. Serum exosomes or exosomal components may serve as a useful adjunctive therapy alongside other currently approved drugs to enhance the therapeutic effect. Harnessing the mechanism by which this specialized serum factor is able to achieve neuroprotection and mediate immune modulation while maintaining overall immune integrity can be an invaluable step in formulating a novel therapy for MS.
Figure 4.
Figure 5.
Highlights.
Pregnancy results in suppression of established EAE
Serum exosomes contribute to the pregnancy-induced suppression of EAE
Pregnancy exosomes facilitate OPC migration into CNS lesions
Acknowledgments
We thank Dr. Vijay K. Kuchroo for the gift of MOG TCR transgenic mice, and Drs. Amy E. Lovett-Racke and Michael Racke for their insightful scientific discussion. Thanks to Ingrid Gienapp, Todd Shawler, Ping Wei, and Todd Lash for their outstanding technical support, and The Ohio State University Campus Chemical Instrument Center Mass Spectrometry and Proteomics Facility for DIGE processing and analysis.
Footnotes
Disclosures
The authors have no financial conflicts of interest.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Compston A, Coles A. Multiple sclerosis. Lancet. 2008;372:1502–1517. doi: 10.1016/S0140-6736(08)61620-7. [DOI] [PubMed] [Google Scholar]
- 2.Whitacre CC. Sex differences in autoimmune disease. Nat Immunol. 2001;2:777–780. doi: 10.1038/ni0901-777. [DOI] [PubMed] [Google Scholar]
- 3.Confavreux C, Hutchinson M, Hours MM, Cortinovis-Tourniaire P, Moreau T. Rate of pregnancy-related relapse in multiple sclerosis. Pregnancy in Multiple Sclerosis Group. N Engl J Med. 1998;339:285–291. doi: 10.1056/NEJM199807303390501. [DOI] [PubMed] [Google Scholar]
- 4.Langer-Gould A, Garren H, Slansky A, Ruiz PJ, Steinman L. Late pregnancy suppresses relapses in experimental autoimmune encephalomyelitis: evidence for a suppressive pregnancy-related serum factor. J Immunol. 2002;169:1084–1091. doi: 10.4049/jimmunol.169.2.1084. [DOI] [PubMed] [Google Scholar]
- 5.McClain MA, Gatson NN, Powell ND, Papenfuss TL, Gienapp IE, Song F, Shawler TM, Kithcart A, Whitacre CC. Pregnancy suppresses experimental autoimmune encephalomyelitis through immunoregulatory cytokine production. J Immunol. 2007;179:8146–8152. doi: 10.4049/jimmunol.179.12.8146. [DOI] [PubMed] [Google Scholar]
- 6.Thery C, Zitvogel L, Amigorena S. Exosomes: composition, biogenesis and function. Nat Rev Immunol. 2002;2:569–579. doi: 10.1038/nri855. [DOI] [PubMed] [Google Scholar]
- 7.Taylor DD, Akyol S, Gercel-Taylor C. Pregnancy-associated exosomes and their modulation of T cell signaling. J Immunol. 2006;176:1534–1542. doi: 10.4049/jimmunol.176.3.1534. [DOI] [PubMed] [Google Scholar]
- 8.Gatson NN, Williams JL, Powell ND, McClain MA, Hennon TR, Robbins PD, Whitacre CC. Induction of pregnancy during established EAE halts progression of CNS autoimmune injury via pregnancy-specific serum factors. J Neuroimmunol. 2011;230:105–113. doi: 10.1016/j.jneuroim.2010.09.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.van Walderveen MA, Tas MW, Barkhof F, Polman CH, Frequin ST, Hommes OR, Valk J. Magnetic resonance evaluation of disease activity during pregnancy in multiple sclerosis. Neurology. 1994;44:327–329. doi: 10.1212/wnl.44.2.327. [DOI] [PubMed] [Google Scholar]
- 10.Ponsonby AL, Lucas RM, van der Mei IA, Dear K, Valery PC, Pender MP, Taylor BV, Kilpatrick TJ, Coulthard A, Chapman C, Williams D, McMichael AJ, Dwyer T. Offspring number, pregnancy, and risk of a first clinical demyelinating event: the AusImmune Study. Neurology. 2012;78:867–874. doi: 10.1212/WNL.0b013e31824c4648. [DOI] [PubMed] [Google Scholar]
- 11.Gregg C, Shikar V, Larsen P, Mak G, Chojnacki A, Yong VW, Weiss S. White matter plasticity and enhanced remyelination in the maternal CNS. J Neurosci. 2007;27:1812–1823. doi: 10.1523/JNEUROSCI.4441-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bettelli E, Pagany M, Weiner HL, Linington C, Sobel RA, Kuchroo VK. Myelin oligodendrocyte glycoprotein-specific T cell receptor transgenic mice develop spontaneous autoimmune optic neuritis. J Exp Med. 2003;197:1073–1081. doi: 10.1084/jem.20021603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kim SH, Lechman ER, Bianco N, Menon R, Keravala A, Nash J, Mi Z, Watkins SC, Gambotto A, Robbins PD. Exosomes derived from IL-10-treated dendritic cells can suppress inflammation and collagen-induced arthritis. J Immunol. 2005;174:6440–6448. doi: 10.4049/jimmunol.174.10.6440. [DOI] [PubMed] [Google Scholar]
- 14.Sabapatha A, Gercel-Taylor C, Taylor DD. Specific isolation of placenta-derived exosomes from the circulation of pregnant women and their immunoregulatory consequences. Am J Reprod Immunol. 2006;56:345–355. doi: 10.1111/j.1600-0897.2006.00435.x. [DOI] [PubMed] [Google Scholar]
- 15.Kim SH, Bianco NR, Shufesky WJ, Morelli AE, Robbins PD. Effective treatment of inflammatory disease models with exosomes derived from dendritic cells genetically modified to express IL-4. J Immunol. 2007;179:2242–2249. doi: 10.4049/jimmunol.179.4.2242. [DOI] [PubMed] [Google Scholar]
- 16.Kim SH, Bianco N, Menon R, Lechman ER, Shufesky WJ, Morelli AE, Robbins PD. Exosomes derived from genetically modified DC expressing FasL are anti-inflammatory and immunosuppressive. Mol Ther. 2006;13:289–300. doi: 10.1016/j.ymthe.2005.09.015. [DOI] [PubMed] [Google Scholar]
- 17.Abrahams VM, Straszewski-Chavez SL, Guller S, Mor G. First trimester trophoblast cells secrete Fas ligand which induces immune cell apoptosis. Mol Hum Reprod. 2004;10:55–63. doi: 10.1093/molehr/gah006. [DOI] [PubMed] [Google Scholar]
- 18.Mathivanan S, Ji H, Simpson RJ. Exosomes: extracellular organelles important in intercellular communication. J Proteomics. 2010;73:1907–1920. doi: 10.1016/j.jprot.2010.06.006. [DOI] [PubMed] [Google Scholar]
- 19.Marriott MP, Emery B, Cate HS, Binder MD, Kemper D, Wu Q, Kolbe S, Gordon IR, Wang H, Egan G, Murray S, Butzkueven H, Kilpatrick TJ. Leukemia inhibitory factor signaling modulates both central nervous system demyelination and myelin repair. Glia. 2008;56:686–698. doi: 10.1002/glia.20646. [DOI] [PubMed] [Google Scholar]
- 20.Roeser HP, Lee GR, Nacht S, Cartwright GE. The role of ceruloplasmin in iron metabolism. J Clin Invest. 1970;49:2408–2417. doi: 10.1172/JCI106460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Todorich B, Pasquini JM, Garcia CI, Paez PM, Connor JR. Oligodendrocytes and myelination: the role of iron. Glia. 2009;57:467–478. doi: 10.1002/glia.20784. [DOI] [PubMed] [Google Scholar]
- 22.Zhang SC, Ge B, Duncan ID. Adult brain retains the potential to generate oligodendroglial progenitors with extensive myelination capacity. Proc Natl Acad Sci U S A. 1999;96:4089–S4094. doi: 10.1073/pnas.96.7.4089. [DOI] [PMC free article] [PubMed] [Google Scholar]