Abstract
Glucagon-like peptide 1 (GLP-1) is best known as an incretin hormone, secreted from L cells in the intestine in response to nutrient ingestion to stimulate glucose-dependent insulin secretion. However, GLP-1 is also expressed in neurons, and plays a major role in regulation of homeostatic function within the central nervous system (CNS). This review summarizes our current state of knowledge on the role GLP-1 plays in neural coordination of the organismal stress response. In brain, the primary locus of GLP-1 production is in the caudal nucleus of the solitary tract (NTS) and the ventrolateral medulla of the hindbrain. GLP-1 immunoreactive fibers directly innervate hypophysiotrophic corticotropin-releasing hormone (CRH) neurons in the hypothalamic paraventricular nucleus (PVN), placing GLP-1 in prime position to integrate hypothalamo-pituitary-adrenocortical responses. Exogenous central GLP-1 activates HPA axis stress responses, and responses to a variety of stressors can be blocked by a GLP-1 receptor (GLP-1R) antagonist, confirming an excitatory role in glucocorticoid secretion. In addition, central infusion of GLP-1R agonist increases heart rate and blood pressure, and activates hypothalamic and brainstem neurons innervating sympathetic preganglionic neurons, suggesting a sympathoexcitatory role of GLP-1 in the CNS. Bioavailability of preproglucagon (PPG) mRNA and GLP-1 peptide is reduced by exogenous or endogenous glucocorticoid secretion, perhaps as a mechanism to reduce GLP-1-mediated stress excitation. Altogether, the data suggest that GLP-1 plays a key role in activation of stress responses, which may be connected with its role in central regulation of energy homeostasis.
Keywords: Anxiety, Chronic stress, HPA axis, Preproglucagon, Nucleus of the solitary tract, Paraventricular nucleus
1. Introduction
Successful adaptation to stress is required for the survival and well-being of all organisms. Stressors reflect real or perceived threats to homeostasis that trigger endocrine, autonomic and behavioral responses to promote physiological coping [1-4]. Prolonged or excessive exposure to stressful stimuli can impair adaptive mechanisms and increase the individual’s risk for developing numerous stress-associated diseases [5-11], hence requiring that stress be carefully managed by organism.
‘Stress responses’ are mediated by both autonomic (‘fight or flight’) responses and glucocorticoid hormone release by the hypothalamo-pituitary-adrenocortical (HPA) axis. The autonomic nervous system reacts rapidly to stressor exposure through activation of the sympathetic nervous system, which stimulates secretion of norepinephrine and epinephrine and thereby increases heart rate, blood pressure and blood glucose (among other effects). However, these responses are short lived, due in part to compensatory reactions driven by the parasympathetic nervous system [12]. Glucocorticoid effects are mediated by corticosteroid receptors, which act via genomic as well as non-genomic mechanisms to modulate intracellular signaling [12, 13, 14]. The HPA axis involves a neuroendocrine cascade, initiated by corticotrophin-releasing hormone (CRH) and arginine vasopressin (AVP) neurons of the hypothalamic paraventricular nucleus (PVN), which stimulate pituitary adrenocorticotrophic hormone (ACTH) release and subsequent adrenocortical synthesis and secretion of glucocorticoids. By virtue of the time delay in the neuroendocrine process and the intracellular/genomic actions of glucocorticoids, the HPA axis response occurs at longer latency than the sympathoadrenal response, and has more temporally extended cellular action. These systems act together to mobilize central and peripheral energetic resources over both immediate and protracted periods of time, allowing reaction to or anticipation of threat and recovery of energy homeostasis.
Stress responses are mediated by multiple overlapping circuits in forebrain limbic regions, hypothalamus and brainstem. In particular, the nucleus of the solitary tract (NTS) is a key brain region involved in the processing of autonomic and HPA axis stress responses [15, 16]. The NTS sends direct stress-excitatory signals to CRH neurons of the PVN [17, 18]. The NTS is also the primary central terminus for visceral afferent information from the vagus, glossopharyngeal, facial and trigeminal nerves [19]. In addition, NTS receives descending projections from forebrain limbic stress-excitatory regions, such as the prefrontal cortex and central nucleus of amygdala (CeA) [20-23], indicating possible involvement of the NTS in processing emotional and cognitive stress responses. Commensurate with a role in stress regulation, Fos is induced in NTS neurons following frank homeostatic challenges, including visceral illness [24], immune challenge [25, 26] and hypoxia [27]. Importantly, psychogenic stressors (e.g., restraint, forced swim, or immobilization) increase Fos activation in the NTS [28-30], indicating responsiveness to non-homeostatic stressors as well. The NTS is also activated under conditions of chronic unpredictable stress (as reflected in increased ΔFosB immunoreactivity), consistent with a role in long-term stress integration [31]. Stress excitatory signals from the NTS are conveyed via ascending catecholaminergic (noradrenergic and adrenergic) projections to the PVN [15, 17, 32], as lesions of these projection neurons reduce HPA axis responses to systemic stressors [33, 34]. In addition, the NTS houses numerous neuropeptides that are capable of modulating HPA axis stress responses, including glucagon-like peptide-1 (GLP-1), somatostatin, substance P, neuropeptide Y and enkephalin [15, 18, 35]. Of these peptides, GLP-1 appears to be in a unique position to influence stress responding [24, 36-39].
2. Anatomy of GLP-1 and GLP-1 receptor in the central nervous system
GLP-1 is an incretin hormone derived from post-translational processing of preproglucagon (PPG) [40]. It is produced both in the periphery (e.g., intestinal L cells) and in neurons in the central nervous system [40-42]. Peripheral GLP-1 secretion is dependent on a variety of factors, including ingested nutrients, taste receptor activation and influence of other neuronal and hormonal signals [43, 44]. GLP-1 secretion has a biphasic pattern depending on the amount of ingested nutrient with a rapid peak occurring at 15-30 min after nutrient ingestion, and a relatively later peak at 1-2 hour following a meal [45]. GLP-1 has a short half-life (<2 min), and it is degraded rapidly by the enzyme dipeptidyl peptidase-4 (DPP4) [46], leading to a very restricted time domain of biological activity. GLP-1 mediates its effect by binding to the G-protein coupled receptor GLP-1 receptor (GLP-1R), which is expressed abundantly in the periphery as well as in the CNS [40, 47, 48].
Although GLP-1 is best known for its stimulatory effect on insulin secretion, emerging evidence indicates an important role for the peptide in neural regulation of food intake and body weight [49-51], inhibition of water intake [52], maintenance of blood glucose level [53], regulation of heart rate and blood pressure [54-56], and modulation of HPA axis stress responses [24, 36-39]. Given that circulating GLP-1 is not thought to cross the blood brain barrier [57, 58], these effects are likely mediated by CNS GLP-1 neurons.
In the brain, GLP-1 is derived from posttranslational modification of PPG gene products [40]. Immunohistochemical and in situ hybridization studies indicate that PPG cell bodies are localized in the caudal (visceral) part of the NTS, the ventrolateral medulla, and in the glomerular layer of the olfactory bulb of the rat brain [18, 42, 59]. The hindbrain GLP-1 producing neurons are distinct from A2/C2 catecholaminergic neurons [60], suggesting that they comprise a parallel regulatory pathway. Recent studies have further documented PPG distribution using mice expressing yellow fluorescent protein under control of the PPG promoter (PPG-YFP mice). Anatomical analyses in PPG-YFP mice indicate caudal medullary localization corresponding to that seen in rat, and identify additional small groups of neurons in the raphe obscurus and in the medullary intermediate reticular nucleus [61]. Studies in non-human primates (macaca mulatta) show similar expression patterns of the PPG messenger RNA (mRNA) [62], indicating that the distribution of GLP-1 and PPG containing cell bodies in the CNS is highly conserved across species. Anatomical studies demonstrate that GLP-1 immunoreactive neurons project to multiple brain areas, including areas that are critical for regulation of HPA axis stress responses [18, 63]. Both retrograde tracing and electron microscopic studies indicate that NTS GLP-1 neurons send direct ascending projections to the PVN [18, 63], and make synaptic contacts with PVN CRH-containing neurons [64]. In addition, the GLP-1 neurons innervate other stress regulatory brain regions, such as the dorsomedial nucleus of hypothalamus and the supraoptic nucleus (SON) [63]. Most of the GLP-1 immunoreactive fibers form terminal-like appositions on oxytocin neurons in the magnocellular PVN and dorsal part of the SON, as well as CRH neurons in the dorsomedial parvocellular region of the PVN [63]. Importantly, retrograde tracing studies indicate that parvocellular PVN neurons projecting to the NTS region are apposed by GLP-1 immunoreactive terminals [63]. Brainstem PVN projections selectively target nuclei that control sympathetic and parasympathetic output (e.g., NTS, dorsal motor nucleus of the vagus) [65], suggesting that GLP-1 appositions are on preautonomic neuron populations. Together, these data suggest that central GLP-1 neurons innervate hypothalamic regions involved in multiple physiologic stress-regulatory pathways.
In rat, GLP-1 positive terminals and fibers are observed in extrahypothalamic stress-regulatory regions in forebrain, including the bed nucleus of the stria terminalis, the septal nuclei and the CeA [42], and are likely of NTS origin. Moreover, GLP-1 immunoreactive fibers are observed in hindbrain regions such as reticular formation, the dorsal motor nucleus of the vagus and in the intermediolateral (IML) cell column of the spinal cord (thoracic sympathetic level) [42, 62], regions involved in both HPA axis and autonomic regulation. The distribution of GLP-1 fibers is similar in PPG-YFP mice [61], indicating conservation across rodent species. Notably, PPG-YFP positive axon terminals are in close apposition to serotonergic neurons in the parapyramidal region of the ventral medulla, raphe pallidus and caudal raphe obscurus as well as in some brainstem cholinergic and catecholamineric neurons [66], indicating GLP-1 neurons project to various brainstem sites implicated in autonomic function. Importantly, GLP-1 neurons likely receive descending projections from the hypothalamus [67-69]. These afferent signals include oxytocin, melanin-concentrating hormone and orexin [67-69], all of which are known to modulate stress responses and autonomic function. Electron microcopy is needed to confirm whether these afferent signals make actual synaptic contacts at the ultrastructural level. Overall, the above studies suggest that NTS GLP-1 and PPG neurons project to numerous brain regions that are known to play important roles in integrating autonomic, endocrine and behavioral responses to stress. The functional significance of these afferent signals to GLP-1 neuronal activity remains to be determined.
The GLP-1R is distributed widely throughout the CNS. Expression of GLP-1R mRNA is observed in the olfactory bulb, the basal portion of the frontal cortex, nucleus accumbens, septal nuclei, amygdala, and posterior portion of the hippocampus [47]. It is highly expressed in various hypothalamic nuclei, such as SON, PVN, arcuate nucleus and preoptic area [47]. The GLP-1R is also highly expressed in the hindbrain, primarily in the dorsal vagal complex including the area postrema and NTS, as well as lateral reticular nucleus and raphe nuclei [47]. This distribution of GLP-1R mRNA is largely in agreement with previous studies using receptor binding assays (with the notable exception of thalamic signal: there is a high GLP-1R binding throughout the thalamus, where as GLP-1R mRNA is predominantly localized to the paraventricular and posterior thalamic nuclei) [70]. The GLP-1R is primarily expressed in neurons [71] (however, there is also evidence for GLP-1R mRNA in astrocytes and microglia in vivo [72]). Together, these studies suggest that GLP-1R is expressed abundantly in central regions that are critical for the regulation of metabolic, endocrine, behavioral and cardiovascular effects of stress. Overall, these studies provide evidence that central GLP-1 signaling is an important component of central stress neurocircuits.
3. Role of central GLP-1 neurons in neuroendocrine, behavioral and autonomic responses to stress
The brainstem, specifically the NTS, is critical for activation of the HPA axis [12]. Lesion and anatomical studies indicate the involvement of the NTS catecholaminergic neurons in PVN activation by systemic (for example, interleukin 1β injection, endotoxin exposure) [17, 33, 34] but not psychogenic stressors (for example, foot shock, restraint) [72-74], suggesting a stimulus-specific role in regulation of stress response. However, subsequent studies have also identified involvement of non-catecholaminergic NTS neurons in the stimulation of HPA axis responses to both systemic and psychogenic stressors [15]. Evidence of a role for central GLP-1 in the generation of stress responses was first demonstrated in 1997, whereby central infusion of GLP-1 significantly increased plasma corticosterone as early as 15-min following injection. In addition, exogenous GLP-1-induced c-Fos expression was observed in major stress regulatory hypothalamic nuclei, including the medial parvocellular PVN and magnocellular neurons of the PVN and SON. The stimulatory effect of GLP-1 on HPA activation is mediated by the central GLP-1R, as prior central administration of the GLP-1R antagonist exendin-9 (EX-9) blocks GLP-1-induced c-Fos expression [36]. In addition, systemic lithium chloride (LiCl) injection, which is a systemic stressor that produces visceral illness, can activate hindbrain PPG neurons [24], indicative of engagement of endogenous GLP-1 neurons during stressor exposure.
Later studies in our group noted that intracerebroventricular infusion of GLP-1 in rats significantly increased ACTH and corticosterone secretion, an effect that is accompanied by an increase in anxiety-like behavior in the elevated plus maze (EPM) [37]. By contrast, administration of GLP-1R antagonist inhibit HPA axis response to both psychogenic (EPM) as well as systemic (lithium chloride) stimuli [37], indicating that unlike ascending catecholaminergic neurons, GLP-1 neurons may be involved in generation of responses to multiple stressor modalities.
Anxiogenic and stress excitatory effects of central GLP-1 administration are region-specific. Local injection of GLP-1 into the PVN, but not amygdala increased corticosterone release, whereas amygdala, but not PVN, injection produces anxiogenic effects [37]. In addition, central infusion of GLP-1 also decreases number of drinking episodes in the punished drinking test, without affecting unpunished drinking, further supporting an anxiogenic effect of central GLP-1 [38]. Thus, these data suggest that central GLP-1 signaling mediates HPA axis activation via the PVN, and also modulates behavioral responses via interactions with other forebrain stress regulatory regions.
Central GLP-1 may also be required for the establishment and maintenance of chronic stress-induced HPA hyperactivity. Daily central infusion of GLP-1 enhances chronic stress-induced sensitization of corticosterone release in response to a novel stressor [39]. Conversely, HPA axis sensitization can be blocked by daily central administration of GLP-1 receptor antagonist, dHG-exendin [39], suggesting that endogenous GLP-1 may generate chronic stress HPA axis hyperactivity and by extension chronic stress-associated pathologies.
While excitatory effects of GLP-1 have been repeatedly demonstrated in pharmacological studies, experiments performed in GLP-1R knockout (KO) mice suggest a more complicated story. Mice with whole-body KO of the GLP-1R have increased startle responses (although only at high stimulus intensity) and an increase in risk-assessment behavior in the EPM (measured by increased center time) [76]. These KO mice also have increased corticosterone release following a mild stressor [76], suggesting a stress-inhibitory effect of central GLP-1R activation in mice. However, the adrenal glands of the GLP-1R KO mice are significantly smaller than those of controls, consistent with an overall decrease in pituitary-adrenal or autonomic drive. These discrepancies between the rodent pharmacology and mouse knock-out studies remains to be resolved.
GLP-1 may also be involved in the control of autonomic stress responses. In rats, both central and peripheral administration of GLP-1R agonist exendin-4 (EX-4) significantly increases heart rate and blood pressure in a dose-dependent manner [54]. In addition, EX-4 increases c-Fos expression in the medullary catecholaminergic neurons that project to sympathetic preganglionic neurons as well as the adrenal medulla, and was accompanied by increased transcription of tyrosine hydroxylase in the brainstem [54], suggesting that central GLP-1 system can activate cardiovascular responses by regulating sympathetic outflow. Additional studies indicate that catecholaminergic neurons in the area postrema can be activated by peripheral GLP-1, providing a potential anatomical substrate whereby peripheral GLP-1 can produce central drive to the autonomic nervous system [55]. Further studies in mice suggest a role for central GLP-1 signaling in the regulation of cardiovascular responses, as central infusion of GLP-1R agonist EX-4 decreases arterial blood flow whereas the GLP-1R antagonist exendin-9 (EX-9) has the opposite effect [56]. GLP-1 also modulates other autonomic processes such as colonic motility. Central infusion of GLP-1 significantly increased stress-induced fecal output [77]. Increase in fecal output is reversed by central administration of GLP-1R antagonist or CRH antagonist [77], indicating that an interaction between central GLP-1 signaling and CRH is necessary for stress-induced colonic motility. Collectively, these studies provide evidence that central GLP-1 signaling is an important component of stress regulation (Table 1).
TABLE 1.
Central GLP-1 signaling-mediated effects on neuroendocrine, behavioral and autonomic responses.
| Experimental Treatment |
Change | Comments |
|---|---|---|
| Central GLP-1 infusion in non- stressed rats |
↑ plasma ACTH | 15, 30 and 60 min after injection, [37]. |
| ↑ plasma corticosterone | 15, 30 and 60 min after injection, [36, 37]. | |
| ↑ plasma AVP | 15, 30 min after injection, [36]. | |
| ↓ plasma Glucose | Only at 20 min after injection, [36]. | |
| ↑ PVN Fos expression | 90 min after injection, [36]. | |
| ↑ arterial blood pressure | In dose-dependent manner, [54]. | |
| ↑ heart Rate | In dose-dependent manner, [54]. | |
| Central GLP-1 antagonism + acute psychogenic stress |
↓ plasma ACTH | 15, 30 and 60 min after stress, [37]. |
| ↓ plasma corticosterone | 15, 30 and 60 min after stress, [37]. | |
| ↓ anxiety-like behavior | Increased time spent in the open arms of EPM, [37]. | |
| Central GLP-1 + acute stress |
↑ Fecal output | 2 hour after restraint, [77]. |
| Central GLP-1 + chronic variable stress |
↑ plasma corticosterone in response to a novel stress |
No change in basal corticosterone response, [39]. |
| ↓ body weight | Compared to chronically stressed rats, [39]. |
All of these effects are significantly different from corresponding vehicle or saline treated rats (p<0.05). Numbers in the square brackets ([]) indicate respective reference.
4. Regulation of central GLP-1 signaling in response to stress: Functional clues?
The bioavailability of GLP-1 is critical for the regulation of central stress circuitry. Recent evidence suggests stress causes pronounced down-regulation of central GLP-1 protein and PPG mRNA expression [78]. Both acute restraint (psychogenic) and hypoxia (systemic) stress cause a rapid decrease in expression of PPG mRNA (as early as 30 min following stress onset). Down-regulation of PPG mRNA is accompanied by an increase in primary transcript (as measured by heteronuclear RNA expression), implying that loss of mRNA is not due to rapid decreases in transcription, and may in fact be due to mRNA degradation [78]. Decrements in PPG mRNA are accompanied by reduced GLP-1 immunoreactivity in the PVN [78], suggesting that acute stress results in a reduction of central GLP-1 bioavailability that may lead to less GLP-1 excitation and contribute to the recovery of the HPA axis after stress. Notably, stress-induced PPG mRNA decrements were blocked by adrenalectomy and could be mimicked by exogenous corticosterone, indicating that PPG down-regulation is mediated by glucocorticoids [78].
The reduction in GLP-1 bioavailability after acute stress led us to examine regulation following prolonged intermittent stress exposure (chronic variable stress). As was the case with acute stress, chronic stress also caused marked down-regulation of NTS PPG mRNA and PVN GLP-1 immunoreactivity. Again, stress-induced down-regulation was blocked by adrenalectomy with basal corticosterone replacement, and PPG mRNA decrements could be induced by exogenous steroid administration [79]. These data suggest that PPG down-regulation may be mediated by both short- and long-term ‘feedback’ effects of glucocorticoids, perhaps as an adaptive mechanism capable of limiting ‘overdrive’ of the HPA axis by stress.
Recent work from our group has compared regulation of the GLP-1 system in a chronic stress regimen that results in HPA axis habituation (repeated daily restraint) vs. one that does not (chronic variable stress). In this study male Sprague-Dawley rats (n=17/group) from Harlan (Indianapolis, IN, USA) were placed in a Plexiglas restraint tube for 30 min per day in the morning for 14 days or exposed to 14 days of chronic variable stress using our standard paradigm as reported previously [31]. On the morning after completion of the chronic stress regimen (day 15), rats were given a final 30-min restraint stress challenge. Tail blood was collected at 0, 30, 60, 90 and 120 min after the onset of restraint stress. Following restraint testing, rats were killed by decapitation. Plasma corticosterone levels were measured using radioimmunoassay as described previously [79]. NTS PPG mRNA expression was determined using quantitative real-time PCR (QRT-PCR) according to previously described methods [79]. Briefly, using the obex (bregma, 14.40 mm) as landmark, the NTS was dissected from the anterior margin (bregma, 11.04 mm; 3.36 mm from the obex to rostral margin) to the posterior margin (bregma, 15.72 mm; 1.32 mm from obex to caudal margin). Total RNA was isolated using Tri-reagent (Molecular Research Center) according to the manufacturer’s instructions, and treated by Turbo DNA-free (Ambion) to remove genomic DNA. cDNAs were synthesized using SuperScript TM III First-Strand Synthesis (Invitrogen) following manufacturer’s instructions. Primer sequences for PPG mRNA and the housekeeping gene L-32 were used as described before [79]. iCycler iQ Multi-Color Real Time PCR Detection System (Bio-Rad) was used to perform QRT-PCR, and cDNA amounts in each sample were detected using iQ SYBR Greeen Supermix (Bio-Rad). mRNA present in each sample was determined using the ΔΔCt method as described previously [80]. All experimental procedures were performed in accordance with the National Institutes of Health Guidelines for the Care and Use of Animals and approved by the University of Cincinnati Institutional Animal Care and Use Committee.
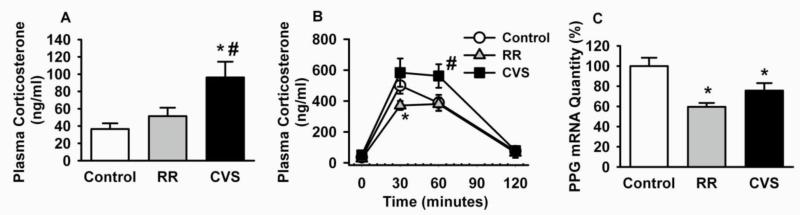
Figure. 1A illustrates that unlike chronic variable stress exposure, repeated restraint did not cause increased basal corticosterone release (determined 24 hours after the last stressor). Moreover, corticosterone responses to the final restraint stress were significantly dampened in the repeated restraint group relative to animals restrained for the first time, consistent with stress habituation (Figure. 1B). Importantly, NTS PPG mRNA was significantly decreased by both repeated restraint and chronic variable stress (Figure. 1C). We speculate that removal of GLP-1 drive may be an adaptive mechanism, reducing GLP-1 drive to the PVN. Continued HPA axis drive during chronic variable stress may be due to recruitment of other stress-excitatory pathways, e.g., increased activity of PVN-projecting catecholamine neurons or engagement of stress excitatory input from the hypothalamus [31, 79].
Figure. 1 A.
Basal plasma corticosterone response of repeated restraint (RR) compared to rats exposed to chronic variable stress (CVS) or unhandled control. Significant elevated basal AM plasma corticosterone levels were detected in animals exposed to CVS (*p<0.05 vs. control and #p<0.05 vs. RR, ANOVA). Basal corticosterone response is not increased in the RR group. B Time course analysis of the plasma corticosterone response to final 30-minute acute restraint stress on day 15. The corticosterone response was blunted in the RR group (*) due to habituation to the repeated stressor and adaptation over the period of repeated stress exposure. Corticosterone responses to novel restraint challenge were elevated in the CVS group (#) due to stress-induced facilitation. C NTS PPG mRNA expression was significantly decreased in CVS and RR groups (*p<0.05 vs. control), consistent with long-term down-regulation of GLP-1 even after exposure to a mild stressor (RR). These data suggest a role for GLP-1 removal in stress adaptation.
5. Perspective
This review highlights the importance of central GLP-1 signaling in controlling stress responses. The data indicate that hindbrain (largely NTS) GLP-1 neurons are involved in HPA axis drive and perhaps autonomic stress responses, and may even play a role in limbic processing of stressful stimuli (Figure. 2). In many ways, the stress-excitatory nature of central GLP-1 parallels its role in body weight regulation. A growing body of evidence indicates that GLP-1 has powerful anorectic effects [81]. Central injection of GLP-1 can be paired with tastants to support conditioned taste aversions, suggesting that stimulation of central GLP-1R may involved in illness responses [82, 83]. Importantly, the location of GLP-1 neurons in the NTS places GLP-1 at a critical point for regulating both central and peripheral GLP-1 signaling. The NTS receives neural relays from abdominal and thoracic viscera via the vagus nerve and can access blood-borne signals via the neighboring, blood-brain barrier deficient area postrema [19, 84, 85]. There is also evidence for local immune signaling at the level of the area postrema and perivascular cells, which may be responsible for relaying inflammatory stimuli to the PVN (and elsewhere) to promote glucocorticoid secretion [86]. Finally, the NTS receives descending information from the infralimbic cortex and central amygdaloid nuclei, which provide highly processed information that may be important in promoting stress responses in anticipation of a perceived threat [20, 21].
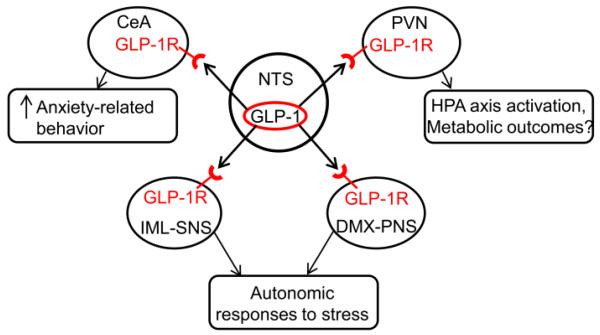
Figure. 2. Multiple actions of central GLP-1 signaling in regulating stress responses.
The paraventricular nucleus (PVN), central nucleus of amygdala (CeA), dorsal motor nucleus of the vagus nerve (DMX) and spinal cord contain GLP-1 receptor (GLP-1R) [47]. The central GLP-1 neurons in the NTS target the PVN CRH neurons and activate the HPA axis responses to stress [36, 37, 39]. The CeA mediates GLP-induced anxiety-related behavior [37]. GLP-1 positive fibers also innervate key autonomic regulatory regions such as DMX and IML, which may be involved in cardiovascular responses to stress [54-56, 61].
The GLP-1 system may also be a target for stress counter-regulatory feedback input from glucocorticoids. The rapid, glucocorticoid-dependent down-regulation of PPG production and depletion of PVN GLP-1 fibers is consistent with rapid removal of a potent stress-activating pathway. Subtraction of GLP-1 from a region such as the PVN may be consistent with the ‘refractory period’ seen 1-2 hours following stress cessation [87], serving the biological purpose of preventing HPA axis over-activation and its potentially deleterious consequences. Similarly long-term down-regulation may function to limit the impact of repeated activation of GLP-1 neurons, and may be an important adaptive mechanism to promote stress habituation and coping.
Finally, the potent stress-excitatory properties of NTS GLP-1 neurons underscores the high importance of the phylogenetically ‘primitive’ hindbrain in integrating reflexive, visceral and even emotional responses to stressors. The anatomical overlap of immune, cardiovascular, and abdominal inputs suggests that the NTS is a first-line monitor of homeostasis, detecting deviations from normal and driving appropriate behavioral, endocrine and neural responses to states such as infection, autonomic drive and satiety. The role of the NTS in emotional stress likely grows out of its vital homeostatic control functions, offering a means through which adjustments can be generated in accordance with anticipated outcomes rather than emergent physiological challenge.
Highlights.
Central GLP-1 plays an excitatory role in glucocorticoid secretion.
GLP-1 positive fibers innervate CRH neurons in the hypothalamic paraventricular nucleus.
Central infusions of GLP-1R agonists increase heart rate and blood pressure.
Bioavailability of central GLP-1 protein is reduced by exogenous or endogenous glucocorticoid secretion.
Acknowledgements
The authors thank Rong Zhang PhD and Ben Packard for their contributions to Figure 1. Preparation of this review was supported by US National Institute of Health Grant MH069860 (JPH).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- [1].Kathol RG, Anton R, Noyes R, Gehris T. Direct comparison of urinary free cortisol excretion in patients with depression and panic disorder. Biological psychiatry. 1989;25(7):873–878. doi: 10.1016/0006-3223(89)90267-9. [DOI] [PubMed] [Google Scholar]
- [2].Chrousos GP, Gold PW. The concepts of stress and stress system disorders. Overview of physical and behavioral homeostasis. JAMA. 1992;267(9):1244–1252. [PubMed] [Google Scholar]
- [3].Marti O, Gavalda A, Jolin T, Armario A. Effect of regularity of exposure to chronic immobilization stress on the circadian pattern of pituitary adrenal hormones, growth hormone, and thyroid stimulating hormone in the adult male rat. Psychoneuroendocrinology. 1993;18(1):67–77. doi: 10.1016/0306-4530(93)90056-q. [DOI] [PubMed] [Google Scholar]
- [4].Herman JP, Adams D, Prewitt C. Regulatory changes in neuroendocrine stress-integrative circuitry produced by a variable stress paradigm. Neuroendocrinology. 1995;61(2):180–190. doi: 10.1159/000126839. [DOI] [PubMed] [Google Scholar]
- [5].Charney DS, Deutch AY, Krystal JH, Southwick SM, Davis M. Psychobiologic mechanisms of posttraumatic stress disorder. Archives of general psychiatry. 1993;50(4):295–305. doi: 10.1001/archpsyc.1993.01820160064008. [DOI] [PubMed] [Google Scholar]
- [6].Coplan JD, Andrews MW, Rosenblum LA, Owens MJ, Friedman S, Gorman JM, Nemeroff CB. Persistent elevations of cerebrospinal fluid concentrations of corticotropin-releasing factor in adult nonhuman primates exposed to early-life stressors: implications for the pathophysiology of mood and anxiety disorders. Proceedings of the National Academy of Sciences of the United States of America. 1996;93(4):1619–1623. doi: 10.1073/pnas.93.4.1619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Grillon C, Southwick SM, Charney DS. The psychobiological basis of posttraumatic stress disorder. Molecular psychiatry. 1996;1(4):278–297. [PubMed] [Google Scholar]
- [8].Grippo AJ, Moffitt JA, Johnson AK. Cardiovascular alterations and autonomic imbalance in an experimental model of depression. American journal of physiology Regulatory, integrative and comparative physiology. 2002;282(5):R1333–1341. doi: 10.1152/ajpregu.00614.2001. [DOI] [PubMed] [Google Scholar]
- [9].Kim JJ, Diamond DM. The stressed hippocampus, synaptic plasticity and lost memories. Nature reviews Neuroscience. 2002;3(6):453–462. doi: 10.1038/nrn849. [DOI] [PubMed] [Google Scholar]
- [10].Brown ES, Varghese FP, McEwen BS. Association of depression with medical illness: does cortisol play a role? Biological psychiatry. 2004;55(1):1–9. doi: 10.1016/s0006-3223(03)00473-6. [DOI] [PubMed] [Google Scholar]
- [11].Nemeroff CB. Neurobiological consequences of childhood trauma. The Journal of clinical psychiatry. 2004;65(Suppl 1):18–28. [PubMed] [Google Scholar]
- [12].Ulrich-Lai YM, Herman JP. Neural regulation of endocrine and autonomic stress responses. Nature reviews Neuroscience. 2009;10(6):397–409. doi: 10.1038/nrn2647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].de Kloet ER, Joels M, Holsboer F. Stress and the brain: from adaptation to disease. Nature reviews Neuroscience. 2005;6(6):463–475. doi: 10.1038/nrn1683. [DOI] [PubMed] [Google Scholar]
- [14].Myers B, McKlveen JM, Herman JP. Neural Regulation of the Stress Response: The Many Faces of Feedback. Cell Mol Neurobiol. 2012 doi: 10.1007/s10571-012-9801-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Herman JP, Cullinan WE. Neurocircuitry of stress: central control of the hypothalamo-pituitary-adrenocortical axis. Trends Neurosci. 1997;20(2):78–84. doi: 10.1016/s0166-2236(96)10069-2. [DOI] [PubMed] [Google Scholar]
- [16].Herman JP, Figueiredo H, Mueller NK, Ulrich-Lai Y, Ostrander MM, Choi DC, Cullinan WE. Central mechanisms of stress integration: hierarchical circuitry controlling hypothalamo-pituitary-adrenocortical responsiveness. Front Neuroendocrinol. 2003;24(3):151–180. doi: 10.1016/j.yfrne.2003.07.001. [DOI] [PubMed] [Google Scholar]
- [17].Cunningham ET, Jr., Sawchenko PE. Anatomical specificity of noradrenergic inputs to the paraventricular and supraoptic nuclei of the rat hypothalamus. J Comp Neurol. 1988;274(1):60–76. doi: 10.1002/cne.902740107. [DOI] [PubMed] [Google Scholar]
- [18].Larsen PJ, Tang-Christensen M, Holst JJ, Orskov C. Distribution of glucagon-like peptide-1 and other preproglucagon-derived peptides in the rat hypothalamus and brainstem. Neuroscience. 1997;77(1):257–270. doi: 10.1016/s0306-4522(96)00434-4. [DOI] [PubMed] [Google Scholar]
- [19].Beckstead RM, Norgren R. An autoradiographic examination of the central distribution of the trigeminal, facial, glossopharyngeal, and vagal nerves in the monkey. J Comp Neurol. 1979;184(3):455–472. doi: 10.1002/cne.901840303. [DOI] [PubMed] [Google Scholar]
- [20].Schwaber JS, Kapp BS, Higgins GA, Rapp PR. Amygdaloid and basal forebrain direct connections with the nucleus of the solitary tract and the dorsal motor nucleus. J Neurosci. 1982;2(10):1424–1438. doi: 10.1523/JNEUROSCI.02-10-01424.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Van der Kooy D, Koda LY, McGinty JF, Gerfen CR, Bloom FE. The organization of projections from the cortex, amygdala, and hypothalamus to the nucleus of the solitary tract in rat. J Comp Neurol. 1984;224(1):1–24. doi: 10.1002/cne.902240102. [DOI] [PubMed] [Google Scholar]
- [22].Prewitt CM, Herman JP. Hypothalamo-Pituitary-Adrenocortical Regulation Following Lesions of the Central Nucleus of the Amygdala. Stress. 1997;1(4):263–280. doi: 10.3109/10253899709013746. [DOI] [PubMed] [Google Scholar]
- [23].Radley JJ, Arias CM, Sawchenko PE. Regional differentiation of the medial prefrontal cortex in regulating adaptive responses to acute emotional stress. J Neurosci. 2006;26(50):12967–12976. doi: 10.1523/JNEUROSCI.4297-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Rinaman L. Interoceptive stress activates glucagon-like peptide-1 neurons that project to the hypothalamus. The American journal of physiology. 1999;277(2 Pt 2):R582–590. doi: 10.1152/ajpregu.1999.277.2.R582. [DOI] [PubMed] [Google Scholar]
- [25].Ericsson A, Kovacs KJ, Sawchenko PE. A functional anatomical analysis of central pathways subserving the effects of interleukin-1 on stress-related neuroendocrine neurons. The Journal of neuroscience : the official journal of the Society for Neuroscience. 1994;14(2):897–913. doi: 10.1523/JNEUROSCI.14-02-00897.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Lacroix S, Rivest S. Functional circuitry in the brain of immune-challenged rats: partial involvement of prostaglandins. The Journal of comparative neurology. 1997;387(2):307–324. doi: 10.1002/(sici)1096-9861(19971020)387:2<307::aid-cne11>3.0.co;2-r. [DOI] [PubMed] [Google Scholar]
- [27].Teppema LJ, Veening JG, Kranenburg A, Dahan A, Berkenbosch A, Olievier C. Expression of c-fos in the rat brainstem after exposure to hypoxia and to normoxic and hyperoxic hypercapnia. The Journal of comparative neurology. 1997;388(2):169–190. doi: 10.1002/(sici)1096-9861(19971117)388:2<169::aid-cne1>3.0.co;2-#. [DOI] [PubMed] [Google Scholar]
- [28].Cullinan WE, Herman JP, Battaglia DF, Akil H, Watson SJ. Pattern and time course of immediate early gene expression in rat brain following acute stress. Neuroscience. 1995;64(2):477–505. doi: 10.1016/0306-4522(94)00355-9. [DOI] [PubMed] [Google Scholar]
- [29].Sawchenko PE, Li HY, Ericsson A. Circuits and mechanisms governing hypothalamic responses to stress: a tale of two paradigms. Progress in brain research. 2000;122:61–78. doi: 10.1016/s0079-6123(08)62131-7. [DOI] [PubMed] [Google Scholar]
- [30].Pezzone MA, Lee WS, Hoffman GE, Pezzone KM, Rabin BS. Activation of brainstem catecholaminergic neurons by conditioned and unconditioned aversive stimuli as revealed by c-Fos immunoreactivity. Brain research. 1993;608(2):310–318. doi: 10.1016/0006-8993(93)91472-5. [DOI] [PubMed] [Google Scholar]
- [31].Flak JN, Solomon MB, Jankord R, Krause EG, Herman JP. Identification of chronic stress-activated regions reveals a potential recruited circuit in rat brain. The European journal of neuroscience. 2012;36(4):2547–2555. doi: 10.1111/j.1460-9568.2012.08161.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32].Cunningham ET, Jr., Bohn MC, Sawchenko PE. Organization of adrenergic inputs to the paraventricular and supraoptic nuclei of the hypothalamus in the rat. The Journal of comparative neurology. 1990;292(4):651–667. doi: 10.1002/cne.902920413. [DOI] [PubMed] [Google Scholar]
- [33].Ritter S, Bugarith K, Dinh TT. Immunotoxic destruction of distinct catecholamine subgroups produces selective impairment of glucoregulatory responses and neuronal activation. J Comp Neurol. 2001;432(2):197–216. doi: 10.1002/cne.1097. [DOI] [PubMed] [Google Scholar]
- [34].Bienkowski MS, Rinaman L. Noradrenergic inputs to the paraventricular hypothalamus contribute to hypothalamic-pituitary-adrenal axis and central Fos activation in rats after acute systemic endotoxin exposure. Neuroscience. 2008;156(4):1093–1102. doi: 10.1016/j.neuroscience.2008.08.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [35].Sawchenko PE, Swanson LW, Grzanna R, Howe PR, Bloom SR, Polak JM. Colocalization of neuropeptide Y immunoreactivity in brainstem catecholaminergic neurons that project to the paraventricular nucleus of the hypothalamus. The Journal of comparative neurology. 1985;241(2):138–153. doi: 10.1002/cne.902410203. [DOI] [PubMed] [Google Scholar]
- [36].Larsen PJ, Tang-Christensen M, Jessop DS. Central administration of glucagon-like peptide-1 activates hypothalamic neuroendocrine neurons in the rat. Endocrinology. 1997;138(10):4445–4455. doi: 10.1210/endo.138.10.5270. [DOI] [PubMed] [Google Scholar]
- [37].Kinzig KP, D’Alessio DA, Herman JP, Sakai RR, Vahl TP, Figueiredo HF, Murphy EK, Seeley RJ, Figueredo HF. CNS glucagon-like peptide-1 receptors mediate endocrine and anxiety responses to interoceptive and psychogenic stressors. The Journal of neuroscience: the official journal of the Society for Neuroscience. 2003;23(15):6163–6170. doi: 10.1523/JNEUROSCI.23-15-06163.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [38].Moller C, Sommer W, Thorsell A, Rimondini R, Heilig M. Anxiogenic-like action of centrally administered glucagon-like peptide-1 in a punished drinking test. Progress in neuropsychopharmacology & biological psychiatry. 2002;26(1):119–122. doi: 10.1016/s0278-5846(01)00223-8. [DOI] [PubMed] [Google Scholar]
- [39].Tauchi M, Zhang R, D’Alessio DA, Seeley RJ, Herman JP. Role of central glucagon-like peptide-1 in hypothalamo-pituitary-adrenocortical facilitation following chronic stress. Experimental neurology. 2008;210(2):458–466. doi: 10.1016/j.expneurol.2007.11.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [40].Holst JJ. The physiology of glucagon-like peptide 1. Physiological reviews. 2007;87(4):1409–1439. doi: 10.1152/physrev.00034.2006. [DOI] [PubMed] [Google Scholar]
- [41].Han VK, Hynes MA, Jin C, Towle AC, Lauder JM, Lund PK. Cellular localization of proglucagon/glucagon-like peptide I messenger RNAs in rat brain. Journal of neuroscience research. 1986;16(1):97–107. doi: 10.1002/jnr.490160110. [DOI] [PubMed] [Google Scholar]
- [42].Jin SL, Han VK, Simmons JG, Towle AC, Lauder JM, Lund PK. Distribution of glucagonlike peptide I (GLP-I), glucagon, and glicentin in the rat brain: an immunocytochemical study. The Journal of comparative neurology. 1988;271(4):519–532. doi: 10.1002/cne.902710405. [DOI] [PubMed] [Google Scholar]
- [43].Rocca AS, Brubaker PL. Role of the vagus nerve in mediating proximal nutrient-induced glucagon-like peptide-1 secretion. Endocrinology. 1999;140(4):1687–1694. doi: 10.1210/endo.140.4.6643. [DOI] [PubMed] [Google Scholar]
- [44].Hansen L, Holst JJ. The effects of duodenal peptides on glucagon-like peptide-1 secretion from the ileum. A duodeno--ileal loop? Regulatory peptides. 2002;110(1):39–45. doi: 10.1016/s0167-0115(02)00157-x. [DOI] [PubMed] [Google Scholar]
- [45].Herrmann C, Goke R, Richter G, Fehmann HC, Arnold R, Goke B. Glucagon-like peptide-1 and glucose-dependent insulin-releasing polypeptide plasma levels in response to nutrients. Digestion. 1995;56(2):117–126. doi: 10.1159/000201231. [DOI] [PubMed] [Google Scholar]
- [46].Demuth H-U, McIntosh CHS, Pederson RA. Type 2 diabetes--therapy with dipeptidyl peptidase IV inhibitors. Biochimica et biophysica acta. 2005;1751(1):33–44. doi: 10.1016/j.bbapap.2005.05.010. [DOI] [PubMed] [Google Scholar]
- [47].Merchenthaler I, Lane M, Shughrue P. Distribution of pre-pro-glucagon and glucagon-like peptide-1 receptor messenger RNAs in the rat central nervous system. The Journal of comparative neurology. 1999;403(2):261–280. doi: 10.1002/(sici)1096-9861(19990111)403:2<261::aid-cne8>3.0.co;2-5. [DOI] [PubMed] [Google Scholar]
- [48].Willard FS, Sloop KW. Physiology and emerging biochemistry of the glucagon-like peptide-1 receptor. Experimental diabetes research. 2012;2012:470851. doi: 10.1155/2012/470851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [49].Barrera JG, Sandoval DA, D’Alessio DA, Seeley RJ. GLP-1 and energy balance: an integrated model of short-term and long-term control. Nat Rev Endocrinol. 2011;7(9):507–516. doi: 10.1038/nrendo.2011.77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [50].Hayes MR, Bradley L, Grill HJ. Endogenous hindbrain glucagon-like peptide-1 receptor activation contributes to the control of food intake by mediating gastric satiation signaling. Endocrinology. 2009;150(6):2654–2659. doi: 10.1210/en.2008-1479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [51].Zhao S, Kanoski SE, Yan J, Grill HJ, Hayes MR. Hindbrain leptin and glucagon-like-peptide-1 receptor signaling interact to suppress food intake in an additive manner. International journal of obesity. 2012;36(12):1522–1528. doi: 10.1038/ijo.2011.265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [52].Tang-Christensen M, Larsen PJ, Goke R, Fink-Jensen A, Jessop DS, Moller M, Sheikh SP. Central administration of GLP-1-(7-36) amide inhibits food and water intake in rats. The American journal of physiology. 1996;271(4 Pt 2):R848–856. doi: 10.1152/ajpregu.1996.271.4.R848. [DOI] [PubMed] [Google Scholar]
- [53].Sandoval D. CNS GLP-1 regulation of peripheral glucose homeostasis. Physiology & behavior. 2008;94(5):670–674. doi: 10.1016/j.physbeh.2008.04.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [54].Yamamoto H, Lee CE, Marcus JN, Williams TD, Overton JM, Lopez ME, Hollenberg AN, Baggio L, Saper CB, Drucker DJ, Elmquist JK. Glucagon-like peptide-1 receptor stimulation increases blood pressure and heart rate and activates autonomic regulatory neurons. The Journal of clinical investigation. 2002;110(1):43–52. doi: 10.1172/JCI15595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [55].Yamamoto H, Kishi T, Lee CE, Choi BJ, Fang H, Hollenberg AN, Drucker DJ, Elmquist JK. Glucagon-like peptide-1-responsive catecholamine neurons in the area postrema link peripheral glucagon-like peptide-1 with central autonomic control sites. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2003;23(7):2939–2946. doi: 10.1523/JNEUROSCI.23-07-02939.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [56].Cabou C, Campistron G, Marsollier N, Leloup C, Cruciani-Guglielmacci C, Penicaud L, Drucker DJ, Magnan C, Burcelin R. Brain glucagon-like peptide-1 regulates arterial blood flow, heart rate, and insulin sensitivity. Diabetes. 2008;57(10):2577–2587. doi: 10.2337/db08-0121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [57].Orskov C, Poulsen SS, Moller M, Holst JJ. Glucagon-like peptide I receptors in the subfornical organ and the area postrema are accessible to circulating glucagon-like peptide I. Diabetes. 1996;45(6):832–835. doi: 10.2337/diab.45.6.832. [DOI] [PubMed] [Google Scholar]
- [58].Kastin AJ, Akerstrom V, Pan W. Interactions of glucagon-like peptide-1 (GLP-1) with the blood-brain barrier. Journal of molecular neuroscience: MN. 2002;18(1-2):7–14. doi: 10.1385/JMN:18:1-2:07. [DOI] [PubMed] [Google Scholar]
- [59].Vrang N, Hansen M, Larsen PJ, Tang-Christensen M. Characterization of brainstem preproglucagon projections to the paraventricular and dorsomedial hypothalamic nuclei. Brain research. 2007;1149:118–126. doi: 10.1016/j.brainres.2007.02.043. [DOI] [PubMed] [Google Scholar]
- [60].Rinaman L. Hindbrain noradrenergic A2 neurons: diverse roles in autonomic, endocrine, cognitive, and behavioral functions. American journal of physiology Regulatory, integrative and comparative physiology. 2011;300(2):R222–235. doi: 10.1152/ajpregu.00556.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [61].Llewellyn-Smith IJ, Reimann F, Gribble FM, Trapp S. Preproglucagon neurons project widely to autonomic control areas in the mouse brain. Neuroscience. 2011;180:111–121. doi: 10.1016/j.neuroscience.2011.02.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [62].Vrang N, Larsen PJ. Preproglucagon derived peptides GLP-1, GLP-2 and oxyntomodulin in the CNS: role of peripherally secreted and centrally produced peptides. Prog Neurobiol. 2010;92(3):442–462. doi: 10.1016/j.pneurobio.2010.07.003. [DOI] [PubMed] [Google Scholar]
- [63].Tauchi M, Zhang R, D’Alessio DA, Stern JE, Herman JP. Distribution of glucagon-like peptide-1 immunoreactivity in the hypothalamic paraventricular and supraoptic nuclei. Journal of chemical neuroanatomy. 2008;36(3-4):144–149. doi: 10.1016/j.jchemneu.2008.07.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [64].Sarkar S, Fekete C, Legradi G, Lechan RM. Glucagon like peptide-1 (7-36) amide (GLP-1) nerve terminals densely innervate corticotropin-releasing hormone neurons in the hypothalamic paraventricular nucleus. Brain Res. 2003;985(2):163–168. doi: 10.1016/s0006-8993(03)03117-2. [DOI] [PubMed] [Google Scholar]
- [65].Swanson LW, Kuypers HG. The paraventricular nucleus of the hypothalamus: cytoarchitectonic subdivisions and organization of projections to the pituitary, dorsal vagal complex, and spinal cord as demonstrated by retrograde fluorescence double-labeling methods. J Comp Neurol. 1980;194(3):555–570. doi: 10.1002/cne.901940306. [DOI] [PubMed] [Google Scholar]
- [66].Llewellyn-Smith IJ, Gnanamanickam GJ, Reimann F, Gribble FM, Trapp S. Preproglucagon (PPG) neurons innervate neurochemically identified autonomic neurons in the mouse brainstem. Neuroscience. 2013;229:130–143. doi: 10.1016/j.neuroscience.2012.09.071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [67].Rinaman L, Rothe EE. GLP-1 receptor signaling contributes to anorexigenic effect of centrally administered oxytocin in rats. Am J Physiol Regul Integr Comp Physiol. 2002;283(1):R99–106. doi: 10.1152/ajpregu.00008.2002. [DOI] [PubMed] [Google Scholar]
- [68].Zheng H, Patterson LM, Berthoud HR. Orexin-A projections to the caudal medulla and orexin-induced c-Fos expression, food intake, and autonomic function. J Comp Neurol. 2005;485(2):127–142. doi: 10.1002/cne.20515. [DOI] [PubMed] [Google Scholar]
- [69].Zheng H, Patterson LM, Morrison C, Banfield BW, Randall JA, Browning KN, Travagli RA, Berthoud HR. Melanin concentrating hormone innervation of caudal brainstem areas involved in gastrointestinal functions and energy balance. Neuroscience. 2005;135(2):611–625. doi: 10.1016/j.neuroscience.2005.06.055. [DOI] [PubMed] [Google Scholar]
- [70].Goke R, Larsen PJ, Mikkelsen JD, Sheikh SP. Distribution of GLP-1 binding sites in the rat brain: evidence that exendin-4 is a ligand of brain GLP-1 binding sites. Eur J Neurosci. 1995;7(11):2294–2300. doi: 10.1111/j.1460-9568.1995.tb00650.x. [DOI] [PubMed] [Google Scholar]
- [71].Hamilton A, Holscher C. Receptors for the incretin glucagon-like peptide-1 are expressed on neurons in the central nervous system. Neuroreport. 2009;20(13):1161–1166. doi: 10.1097/WNR.0b013e32832fbf14. [DOI] [PubMed] [Google Scholar]
- [72].Iwai T, Ito S, Tanimitsu K, Udagawa S, Oka J. Glucagon-like peptide-1 inhibits LPS-induced IL-1beta production in cultured rat astrocytes. Neurosci Res. 2006;55(4):352–360. doi: 10.1016/j.neures.2006.04.008. [DOI] [PubMed] [Google Scholar]
- [73].Li HY, Ericsson A, Sawchenko PE. Distinct mechanisms underlie activation of hypothalamic neurosecretory neurons and their medullary catecholaminergic afferents in categorically different stress paradigms. Proc Natl Acad Sci U S A. 1996;93(6):2359–2364. doi: 10.1073/pnas.93.6.2359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [74].Li HY, Sawchenko PE. Hypothalamic effector neurons and extended circuitries activated in “neurogenic” stress: a comparison of footshock effects exerted acutely, chronically, and in animals with controlled glucocorticoid levels. J Comp Neurol. 1998;393(2):244–266. [PubMed] [Google Scholar]
- [75].Ritter S, Watts AG, Dinh TT, Sanchez-Watts G, Pedrow C. Immunotoxin lesion of hypothalamically projecting norepinephrine and epinephrine neurons differentially affects circadian and stressor-stimulated corticosterone secretion. Endocrinology. 2003;144(4):1357–1367. doi: 10.1210/en.2002-221076. [DOI] [PubMed] [Google Scholar]
- [76].MacLusky NJ, Cook S, Scrocchi L, Shin J, Kim J, Vaccarino F, Asa SL, Drucker DJ. Neuroendocrine function and response to stress in mice with complete disruption of glucagon-like peptide-1 receptor signaling. Endocrinology. 2000;141(2):752–762. doi: 10.1210/endo.141.2.7326. [DOI] [PubMed] [Google Scholar]
- [77].Gulpinar MA, Bozkurt A, Coskun T, Ulusoy NB, Yegen BC. Glucagon-like peptide (GLP-1) is involved in the central modulation of fecal output in rats. Am J Physiol Gastrointest Liver Physiol. 2000;278(6):G924–929. doi: 10.1152/ajpgi.2000.278.6.G924. [DOI] [PubMed] [Google Scholar]
- [78].Zhang R, Jankord R, Flak JN, Solomon MB, D’Alessio DA, Herman JP. Role of glucocorticoids in tuning hindbrain stress integration. J Neurosci. 2010;30(44):14907–14914. doi: 10.1523/JNEUROSCI.0522-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [79].Zhang R, Packard BA, Tauchi M, D’Alessio DA, Herman JP. Glucocorticoid regulation of preproglucagon transcription and RNA stability during stress. Proc Natl Acad Sci U S A. 2009;106(14):5913–5918. doi: 10.1073/pnas.0808716106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [80].Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) Method. Methods. 2001;25(4):402–408. doi: 10.1006/meth.2001.1262. [DOI] [PubMed] [Google Scholar]
- [81].Barrera JG, Jones KR, Herman JP, D’Alessio DA, Woods SC, Seeley RJ. Hyperphagia and increased fat accumulation in two models of chronic CNS glucagon-like peptide-1 loss of function. J Neurosci. 2011;31(10):3904–3913. doi: 10.1523/JNEUROSCI.2212-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [82].Rinaman L. A functional role for central glucagon-like peptide-1 receptors in lithium chloride-induced anorexia. Am J Physiol. 1999;277(5 Pt 2):R1537–1540. doi: 10.1152/ajpregu.1999.277.5.R1537. [DOI] [PubMed] [Google Scholar]
- [83].Lachey JL, D’Alessio DA, Rinaman L, Elmquist JK, Drucker DJ, Seeley RJ. The role of central glucagon-like peptide-1 in mediating the effects of visceral illness: differential effects in rats and mice. Endocrinology. 2005;146(1):458–462. doi: 10.1210/en.2004-0419. [DOI] [PubMed] [Google Scholar]
- [84].Kalia M, Sullivan JM. Brainstem projections of sensory and motor components of the vagus nerve in the rat. J Comp Neurol. 1982;211(3):248–265. doi: 10.1002/cne.902110304. [DOI] [PubMed] [Google Scholar]
- [85].Cunningham ET, Jr., Miselis RR, Sawchenko PE. The relationship of efferent projections from the area postrema to vagal motor and brain stem catecholamine-containing cell groups: an axonal transport and immunohistochemical study in the rat. Neuroscience. 1994;58(3):635–648. doi: 10.1016/0306-4522(94)90087-6. [DOI] [PubMed] [Google Scholar]
- [86].Ericsson A, Arias C, Sawchenko PE. Evidence for an intramedullary prostaglandin-dependent mechanism in the activation of stress-related neuroendocrine circuitry by intravenous interleukin-1. J Neurosci. 1997;17(18):7166–7179. doi: 10.1523/JNEUROSCI.17-18-07166.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [87].Keller-Wood ME, Dallman MF. Corticosteroid inhibition of ACTH secretion. Endocr Rev. 1984;5(1):1–24. doi: 10.1210/edrv-5-1-1. [DOI] [PubMed] [Google Scholar]