Abstract
Purpose
To assess the efficacy of a single infusion of radiolabeled anti-prostate specific membrane antigen monoclonal antibody J591 (177Lu-J591) by PSA decline, measurable disease response, and survival.
Experimental Design
In this dual-center phase II study, 2 cohorts with progressive metastatic castration-resistant prostate cancer received one dose of 177Lu-J591 (15 patients at 65 mCi/m2, 17 at 70 mCi/m2) with radionuclide imaging. Expansion cohort (n=15) received 70 mCi/m2 to verify response rate and examine biomarkers.
Results
47 patients who progressed after hormonal therapies (55.3% also received prior chemotherapy) received 177Lu-J591. 10.6% experienced ≥ 50% decline in PSA, 36.2% experienced ≥ 30% decline, and 59.6% experienced any PSA decline following their single treatment. One of 12 with measurable disease experienced a partial radiographic response (8 with stable disease). Sites of prostate cancer metastases were targeted in 44 of 47 (93.6%) as determined by planar imaging. All experienced reversible hematologic toxicity with grade 4 thrombocytopenia occurring in 46.8% (29.8% received platelet transfusions) without significant hemorrhage. 25.5% experienced grade 4 neutropenia with 1 episode of febrile neutropenia. The phase I maximum tolerated dose (70 mCi/m2) resulted in more 30% PSA declines (46.9% vs 13.3%, p=0.048) and longer survival (21.8 vs 11.9 months, p=0.03), but also more grade 4 hematologic toxicity and platelet transfusions. No serious non-hematologic toxicity occurred. Those with poor PSMA imaging were less likely to respond.
Conclusion
A single dose of 177Lu-J591 was well-tolerated with reversible myelosuppression. Accurate tumor targeting and PSA responses were seen with evidence of dose-response. Imaging biomarkers appear promising.
Keywords: Prostate Cancer, Prostate-specific Membrane Antigen, Radioimmunotherapy, Monoclonal Antibody
INTRODUCTION
Prostate cancer is a radiosensitive disease and radiotherapy is an established form of definitive treatment for clinically localized prostate cancer and for palliation of painful bone metastases. Unsealed radiation sources (samarium-153, strontium-89, radium-223) targeting sites of increased bone metabolism/turnover as an indirect means to target bone metastases have demonstrated clinical benefit, including decreased pain, some PSA declines, and most importantly improvement in survival for Ra-223.(1-4) We have investigated the application of a tumor-targeted monoclonal antibody (mAb) as a means to deliver a cytotoxic payload directly and specifically to prostate cancer metastases not only in bone, but also soft tissue and visceral metastases. This approach combines the specificity of mAb targeting with the tumoricidal effects of beta radiation.
Prostate-specific membrane antigen (PSMA) is a non-secreted cell membrane protein with expression that is highly restricted to prostate epithelium and upregulated in prostate cancer.(5-10) Pathology studies indicate that PSMA is expressed by virtually all prostate cancers.(8, 11-14) PSMA was initially validated as an in vivo target for imaging utilizing radiolabeled mAb 7E11 (CYT-356, capromab), though therapeutic studies were disappointing.(15-18) Recognition that PSMA represented a prostate-cancer restricted target and that 7E11 targets an internal domain and is unable to bind to viable cells led to the development of mAbs to the exposed, extracellular domain of PSMA.(5,9,19-22) J591, a deimmunized mAb against the extracellular domain of PSMA is the lead clinical candidate.(22, 23)
Two independent phase I radioimmunotherapy (RIT) trials have been performed using Yttrium-90 (90Y) or Lutetium-177 (177Lu) linked via a DOTA chelate to J591 in patients with metastatic castration-resistant prostate cancer (CRPC).(24, 25) These trials defined the maximum tolerated dose (MTD), dosimetry, pharmacokinetics, and human anti-humanized antibody (HAHA) response, and demonstrated preliminary evidence of anti-tumor activity.
177Lu was chosen for further development based upon its physical properties, emitting both a short-range (0.2-0.3 mm) beta particle as well as gamma emission. As a result, it delivers a lower radiation dose to bone marrow relative to higher energy beta particles such as 90Y.(26) The gamma emission from 177Lu allows for ex vivo imaging in contrast to 90Y that, as a pure beta emitter, requires use of a surrogate isotope such as 111In for imaging. With RIT, tumor lesion geometry has been proposed to be an important factor and it has similarly been proposed that the emission characteristics of the isotope should probably be appropriately matched to the lesion size/volume to be treated to ideally focus energy within the tumor rather than in the tissue surrounding the lesion/s.(27)177Lu also has a longer physical half-life (6.7 days compared with 2.7 days for 90Y), resulting in longer tumor residence times. Because of these properties, higher activities can be delivered using 177Lu; in the phase I trials of radiolabeled J591, the MTD of 177Lu-J591 was 70 mCi/m2 compared with 17.5 mCi/m2 for 90Y-J591, with lower activity in bone marrow per amount of blood radioactivity.(24-26) Here we report safety and efficacy data for a phase II study of 177Lu-J591 in patients with metastatic CRPC.
PATIENTS AND METHODS
Adult subjects with progressive metastatic CRPC were eligible for enrollment. Histologic or cytologic confirmation of prostate cancer (primary or metastatic site) was required. Progressive CRPC was defined using modified Prostate Specific Antigen Working Group (PCWG1) criteria.(28) Continuous LHRH agonist therapy was required for subjects who had not undergone bilateral orchiectomy. Any number of previous regimens was allowed, provided the subject had not received anti-PSMA based therapy. Additional inclusion criteria included ECOG performance status 0 – 2, absolute neutrophil count ≥ 2000/mm3, platelet count ≥150,000/mm3, serum bilirubin ≤1.5x upper limit of normal (ULN), AST ≤ 2x ULN, PT/INR and aPTT ≤ 1.3x ULN (unless on anticoagulation) and serum creatinine ≤ 2.5 mg/dL.
Exclusion criteria included prior radiotherapy to > 25% of skeleton, prior 89Strontium or 153Samarium containing compounds, bone scan demonstrating confluent lesions involving both axial and appendicular skeleton (“superscan”), other active cancers, or clinically significant cardiac, renal, hepatic, pulmonary, thyroid, or psychiatric disease. Concurrent corticosteroids and/or adrenal hormone inhibitors, PC-SPES, finasteride, or dutasteride were not allowed. This registered study [clinicaltrials.gov NCT00195039] was approved by the institutional review boards of Weill Cornell Medical College and Memorial Sloan Kettering Cancer Center and all subjects provided written informed consent.
Treatment
Preparation and quality control of 177Lu-J591 was performed as previously described.(25) Subjects received a single dose of 177Lu-J591 consisting of J591 chelated at a specific activity of 12-15 mCi of 177Lu per mg of antibody plus sufficient non-radiolabeled, non-DOTA-conjugated (“naked”) J591 to achieve a total antibody dose of 20 mg. Although the MTD of the phase I dose escalation study was 70 mCi/m2,(25) based upon limited prior clinical experience with 177Lu-labeled mAbs as directed by the Food and Drug Administration, an initial cohort of 15 subjects received a dose of 177Lu of 65 mCi/m2 followed by 17 subjects at 70 mCi/m2. After analysis of the initial 32 subjects, an additional 15 were enrolled, underwent infusion of 111In-J591 with subsequent imaging to prospectively evaluate non-invasive assessment of PSMA expression as a predictive biomarker, then received a single dose of 177Lu-J591 at 70 mCi/m2. Each dose was administered without pre-medication by an IV infusion at a rate not to exceed 5 mg/min.
Evaluation During the Study
Subjects were monitored for at least 4 hours post mAb infusion. Complete blood counts (CBC) were performed at least weekly beginning 3 weeks after 177Lu-J591 infusion until 6 weeks or recovery and were repeated at least twice per week during periods of grade (Gr) 4 neutropenia and at least 3 times per week during periods of Gr 4 thrombocytopenia. Transfusions, filgrastim or pegfilgrastim (but not sargramostim), and red blood cell growth factors were permitted at the discretion of the treating physician. Chemistry panel including liver tests and PSA was performed at least every 4 weeks. Expansion cohort subjects had a baseline circulating tumor cell (CTC) count by CellSearch (Veridex) methodology at baseline and 4-6 weeks following 177Lu-J591 infusion.
A planar gamma camera image was obtained 5-7 days after 177Lu-J591 infusion (expansion cohort subjects also had pre-treatment imaging 3-4 days after 111In-J591 infusion) with SPECT images obtained in selected patients. Radiolabeled J591 images were compared to baseline clinical bone scintigraphy and cross sectional imaging. After planar gamma camera imaging, images were scored using 2 methods. A five point visual scale was performed by two independent radiologists and scored 0 (no uptake), 1 (weakly positive), 2 (definitely positive), 3 (equal intensity to liver), 4 (greater uptake than liver). Tumor Targeting Index (TuTI), a novel metric designed to semi-quantitatively score images was calculated for the most prominent lesions in each subject using the ratio of lesion count density (corrected for background) to whole body count density. TuTI = (lesion ROI count density – background count density)/(total body count density). Assessment of accurate uptake of radiolabeled mAb by known sites of disease was performed comparing visual scores and TuTI to areas of known metastatic disease on bone scan and CT/MRI. CT or MRI of abdomen/pelvis and bone scans were repeated 3 months after 177Lu-J591 infusion and every 3 months thereafter until progression. Radiographically measurable disease was defined as lymph nodes of at least 20 mm and non-osseous visceral disease of at least 10 mm in greatest diameter.
Statistical Plan
The primary endpoint of the study was response rate, evaluated by the measurable-disease response rate and post-treatment PSA decline rate, which was originally defined as the percent of patients who achieved a ≥50% decrease in PSA from baseline without requirement for confirmation. With an initial sample size of 32 patients, a two-sided 95% confidence interval (CI) for the response proportion was estimated to extend 0.10 from the observed proportion for an expected proportion of 10%. For an expected proportion of 15%, the CI was estimated to extend 0.12 from the observed proportion. The expansion cohort to bring the 70 mCi/m2 dose to 32 subjects allowed a two-sided 95% CI to be constructed to be within ± 11% of the expected ≥50% PSA decline response rate. A ≥30% response rate was added to the primary endpoint as an amendment based upon the survival association in chemotherapy trials published after this study began (29, 30) and a retrospective analysis of radiolabeled-J591 studies with a similar survival association; the 32 subjects allowed a 2-sided CI within ± 17% of the expected ≥30% PSA decline response rate. Kaplan-Meier survival analysis was used to estimate overall survival (OS), with median OS and 95% CI’s described. Descriptive statistics were performed to characterize the study sample.
Based upon observations made after study initiation, additional analyses were performed in post hoc fashion in the initial cohorts and prospectively in the expansion cohort. Fisher’s exact test was used to compare ≥30% PSA decline response proportions between the 65 mCi/m2 and 70 mCi/m2 dose cohorts and between quartiles of mean TuTI. The log-rank test was employed to compare OS between the two dose cohorts and between levels of PSA decline (≥30% vs <30% PSA decline). Median OS and 95% confidence intervals for median OS were stratified by dose cohort and level of PSA decline. All p-values are two-sided with statistical significance evaluated at the 0.05 alpha level. All analyses were performed in SAS Version 9.2 (SAS Institute, Inc., Cary, NC) and STATA Version 11.0 (StataCorp, College Station, TX).
RESULTS
In the initial portion of the trial, 32 subjects were treated between November, 2004 and February, 2008 at 2 centers; 15 additional subjects were treated in the expansion cohort between June 2009 and February 2012. Baseline demographics including prognostic variables are summarized in Table 1. All had progressed on multiple lines of hormonal therapy and the majority (55.3%) progressed on 1-4 lines of chemotherapy including docetaxel. There were no significant differences in any demographic or prognostic variables between the cohorts.
Table 1.
Baseline Characteristics (N=47)
| Age, years | |
| Median | 73.9 |
| Range | 49.7-90.4 |
| Gleason Sum | |
| 6 | 6 |
| 7 | 10 |
| 8 - 10 | 29 |
| unknown | 2 |
| PSA (ng/mL) | |
| Median | 74.4 |
| Range | 3.31-2184.6 |
| Sites of Metastases | |
| Bone | 46 (97.9%) |
| Lymph Node | 28 (59.6%) |
| Lung | 11 (23.4%) |
| Liver | 4 (8.4%) |
| Other | 3 (6.4%) |
| ECOG Performance Status | |
| 0 | 13 (27.7%) |
| 1 | 34 (72.3%) |
| 2 | 0 |
| LDH | |
| Median | 217.5 |
| Range | 134 - 647 |
| Hemoglobin | |
| Median | 11.9 |
| Range | 9.8 - 14.1 |
| Alkaline Phosphatase | |
| Median | 99 |
| Range | 23 - 1170 |
| CALGB Prognostic Score (60) | |
| Median | 149 |
| Range | 88 - 184 |
| # Previous Hormonal Therapies | |
| 1 | 6 (12.7%) |
| 2 | 21 (44.6%) |
| 3 | 14 (29.8%) |
| 4 | 6 (12.7%) |
| # Previous Chemotherapy Regimens | |
| 0 | 21 (44.6%) |
| 1 | 19 (40.4%) |
| ≥ 2 | 7 (14.9%) |
| Prior Radiation | |
| Prostate / Prostate bed | 21 (44.7%) |
| Palliative to bone metastasis | 3 (6.4%) |
| Other* | 1 (2.1%) |
Other: 1 subject received prior investigational radioimmunotherapy
Anti-tumor Effects and Survival
All subjects had progression by PSA prior to enrollment. Overall, five patients (10.6%; 95% CI=2.0-25.0%) experienced ≥ 50% decline in PSA from baseline, seventeen (36.2%) experienced ≥ 30% decline, and twenty eight (59.6%) experienced any PSA decline with median time to progression of 12 weeks (range 8-47 weeks) following their single treatment. Each subject’s best PSA response is depicted in Figure 1. Although the study was initially designed to have both cohorts analyzed together, a suggestion of dose-response was observed in favor of the 70 mCi/m2 cohort (the phase I MTD),(25) leading to the expansion cohort, confirming the dose-response relationship as depicted in Table 2, with 46.9% vs 13.3% with ≥30% PSA decline (p=0.048).(Individual PSA changes by dose received is depicted in Supplemental Figure 1A) Twelve of the 15 patients in cohort 3 had CTC counts measured at baseline and at 4-6 weeks following treatment (2 lab failures and 1 missed blood draw); 8 (66.7%) had ≥50% decline in CTC counts and 3 (25%) were unchanged at 0 or 1 per 7.5 mL blood (1 declined 27%).(Supplemental Figure 1B)
Figure 1.

PSA waterfall plot
Each individual subject’s best PSA response on study. Those subjects treated with 65 mCi/m2 of 177Lu-J591 (Cohort 1) are indicated in light gray while those that received 70 mCi/m2 of 177Lu-J591 (the phase I trial maximum tolerated dose) are indicated in blue (Cohort 2) or red (Cohort 3).
Table 2.
| DOSE (mCi/m2) COHORT N | 65 Cohort 1 n=15 | 70 Cohort 2 n=17 | 70 Cohort 3 n=15 | 70 Cohorts 2+3 n=32 | p value for dose comparison |
|---|---|---|---|---|---|
| Any PSA Decline | 46.7% | 70.6% | 60.0% | 65.6% | 0.35 |
| ≥30% PSA Decline* | 13.3% | 47.1% | 46.7% | 46.9% | 0.048 |
| ≥50% PSA Decline | 6.7% | 12.8% | 13.3% | 12.5% | 1.00 |
| Median Survival* | 11.9 mo | 19.8 mo | NR | 21.8 mo | 0.032 |
| Platelets Gr 3 | 40% | 13% | 6.7% | 9.4% | -- |
| Platelets Gr 4 | 27% | 53% | 53.3% | 56.3% | 0.069 |
| Platelet Transfusion* | 7% | 41% | 40.0% | 40.6% | 0.019 |
| Neutropenia Gr 3 | 53% | 13% | 46.7% | 28.1% | -- |
| Neutropenia Gr 4* | 0% | 48% | 26.7% | 37.5% | 0.005 |
p<0.05 for comparison between 65 and 70 mCi/m2
Only twelve (25.5%) patients had measurable disease; 1 experienced a partial response by RECIST(31) with confirmed 55% decrease in nodal metastases, 8 had stable disease, 2 with progressive disease, and 1 was lost to follow up prior to repeat image (with PSA increase of 10% from baseline at last evaluation).(Supplemental Figure 1C)
Median overall survival (OS) for all patients was 17.6 months (95% CI = 15.2, 20 months), with improved survival for the 70 mCi/m2 cohort as compared with the 65 mCi/m2 cohort (median OS = 21.8 months [95% CI = 16.3, 27.3 months] vs. 11.9 months [95% CI = 6.5, 17.3 months], respectively, P= 0.03) (Figure 2). As only a minority of patients had measurable disease, therapies with potential immune mechanisms may provide survival benefits independent of immediate response, and we had adequate follow up for survival analysis, we explored relationships between dose, PSA changes, and survival. In the overall study (all 3 cohorts), median OS for those with any PSA decline was 22.2 months [18.6, 25.7] compared to 11.4 months [8.4, 14.4] for those without PSA decline (P<0.01). The 17 patients with ≥ 30% PSA decline had a median OS of 22.2 months (95% CI = 18.4, 25.9 months) compared to 15.7 months (95% CI = 10.2, 21.3 months) among those with less than a 30% PSA decline (P=0.06).
Figure 2.
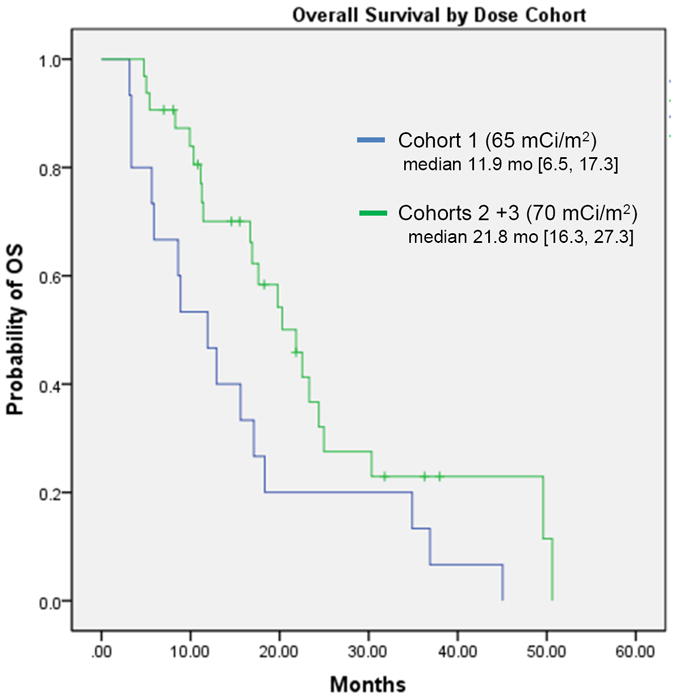
Overall survival
Probability of survival by dose received. [OS:overall survival; mo: months]
Imaging
Planar gamma camera imaging was performed on all patients. Forty four subjects (93.6%) had accurate targeting of known sites of disease when compared to baseline CT/MRI and bone scan images, though those with liver metastases were difficult to assess because of the antibody’s partial hepatic clearance (Figure 3). As our initial imaging data suggested significant variability of PSMA expression levels across the patient population, we therefore retrospectively explored the correlation between TuTI and PSA response in the initial cohorts. In the lowest quartile of mean TuTI’s (i.e. those with lowest PSMA expression by imaging), 12.5% experienced ≥ 30% PSA decline (0% with >50% decline), whereas in the 3 remaining quartiles 37.5% experienced ≥ 30% PSA decline (8.3% with >50% decline) (p=0.19). Prospective evaluation of this association using 111In-J591 imaging prior to 177Lu-J591 treatment in cohort 3 demonstrate the same trend (p=0.19). No association between imaging and toxicity was seen.
Figure 3.

Imaging
Left: 99mTc-MDP bone scan: Anterior (A) and posterior (B) images of pretreatment bony metastases. Right: 177Lu-J591 scan: Anterior (C) and posterior (D) total body images obtained via dual head gamma camera of sites of uptake 7 days after 177Lu-J591 administration. (Note: Radiolabeled antibody is partially cleared via the liver resulting in non-specific 177Lu localization).
Toxicity
Without pre-medication, 11 subjects (23.4%) experienced transient, reversible infusion reactions consisting of feelings of warmth (with or without temperature changes), cold (without episodes of hypothermia), flushing, rigors, or elevation of blood pressure. All completed drug infusion and four (8.5%) received pharmacologic intervention (diphenhydramine and/or acetaminophen; 2 received meperidine). Eight (17%) experienced transient grade (Gr) 1 transaminase elevation; 2 with Gr 2 (1 of whom had Gr 1 elevation at baseline). Treatment emergent adverse events are summarized in Table 3.
Table 3.
Treatment emergent adverse events
| CTCAE Toxicity | Grade 1-2 | Grade 3 | Grade 4 | Total |
|---|---|---|---|---|
| Non-Hematologic | ||||
| ALT (SGPT) | 6 (12.8%) | 6 (12.8%) | ||
| Anorexia | 11 (23.4%) | 11 (23.4%) | ||
| AST (SGOT) | 9 (19.2%) | 9 (19.2%) | ||
| Bruising (without thrombocytopenia) | 2 (4.3%) | 2 (4.3%) | ||
| Constipation | 5 (10.6%) | 5 (10.6%) | ||
| Creatinine | 5 (10.6%) | 5 (10.6%) | ||
| Diarrhea | 4 (8.5%) | 4 (8.5%) | ||
| Dizziness | 1 (2.1%) | 1 (2.1%) | ||
| Dyspepsia | 2 (4.3%) | 2 (4.3%) | ||
| Dyspnea | 3 (6.4%) | 3 (6.4%) | ||
| Edema: limb | 1 (2.1%) | 1 (2.1%) | ||
| Fatigue | 17 (36.2%) | 17 (36.2%) | ||
| Fever (without neutropenia) | 1 (2.1%) | 1 (2.1%) | ||
| Hemorrhage, GI: Oral cavity | 1 (2.1%) | 1 (2.1%) | ||
| Hypersensitivity (aka Infusion Reaction) | 11 (23.4%) | 11 (23.4%) | ||
| Nausea | 5 (10.6%) | 5 (10.6%) | ||
| Pain - Abdomen NOS | 2 (4.3%) | 2 (4.3%) | ||
| Pain - Joint | 1 (2.1%) | 1 (2.1%) | ||
| Petechiae/purpura | 1 (2.1%) | 1 (2.1%) | ||
| Rash/desquamation | 1 (2.1%) | 1 (2.1%) | ||
| Rigors/chills | 1 (2.1%) | 1 (2.1%) | ||
| Taste alteration (dysgeusia) | 1 (2.1%) | 1 (2.1%) | ||
| Weight Loss | 1 (2.1%) | 1 (2.1%) | ||
| Hematologic (see Table 2 for dose comparisons) | ||||
| Hemoglobin | 19 (40.1%) | 5 (10.6%) | 24 (51.1%) | |
| Leukocytes (total WBC) | 14 (29.8%) | 22 (46.8%) | 4 (8.5%) | 40 (85.1%) |
| Neutrophils (ANC) | 7 (14.9%) | 17 (36.2%) | 12 (25.5%) | 36 (76.6%) |
| Febrile neutropenia | 1 (2.1%) | 1 (2.1%) | ||
| Platelets | 4 (8.5%) | 10 (21.3%) | 22 (46.8%) | 36 (76.6%) |
All experienced hematologic toxicity, with nadir platelet and neutrophil counts occurring at a median of 4 weeks after 177Lu-J591 administration. Grade 4 thrombocytopenia occurred in 22 (46.8%) lasting a median of 7 (range 3-17) days; 14 received platelet transfusions (median 2, range 1-4 transfusions). None experienced significant hemorrhagic episodes. Three had Gr 1 ecchymosis at blood draw or other traumatic sites. Thirty nine (82.9%) experienced complete (i.e. at least 150,000/mcL) platelet recovery within a median of 25 days. Seven experienced recovery to Gr 1 (range 118-130,000/mcL peak platelet counts). One recovered to only grade 2 (59,000/mcL). Of those with incomplete recovery, all had concurrent progressive disease by PSA. Three who experienced partial platelet count recovery (i.e. increase from nadir) and subsequent decline had concurrent PSA rises and significant prostate cancer infiltration of bone marrow with otherwise normal hematopoietic elements on bone marrow biopsy. Twelve (25.5%) experienced Gr 4 neutropenia up to 17 days in duration (median 5, range 2-17 days); 1 had febrile neutropenia. Nine patients (19.1%) received filgrastim or pegfilgrastim. Hematologic toxicity was greater in the 70 mCi/m2 cohort (Table 2), with significantly more platelet transfusions and grade 4 neutropenia. No correlation between toxicity and sites of disease or number of bone metastases was observed, though there was a trend for more platelet transfusions in those who previously received radiotherapy (p=0.15 in univariate analysis, p=0.25 when correcting for 177Lu dose) and for those with lower baseline platelet counts (p=0.11). There was no difference in Gr 4 neutropenia with previous chemo- or radiotherapy.
DISCUSSION
Although RIT was first studied in solid tumors, the largest experience with RIT to date involves targeting the CD20 antigen (131I tositumomab or 90Y ibritumomab tiuxetan) in non-Hodgkin’s lymphoma. Radioimmunotherapy for solid tumors has lagged behind for several reasons, including a dearth of antigens of adequate tumor-specificity and concerns regarding tumor radio-resistance and antibody penetration. Other practical reasons have included difficulties in stably linking radionuclides to existing mAbs, shortfalls in existing (and readily available) radionuclides, and difficulty in clinical use (coordination between different specialties).(32) Prostate cancer is not subject to these limitations: (i) a highly tumor-restricted antigen, PSMA, has been identified; (ii) PC is radiosensitive; and (iii) metastatic PC can be identified at the stage of small volume lesions in bone marrow and lymph nodes that are well accessed by circulating antibody.
Radiation therapy may be delivered to primary and secondary sites of prostate cancer for curative or palliative intent via external beam or brachytherapy. Systemic radioisotope therapy targeting bone has also been successfully utilized. Samarium-153 and Strontium-89 are approved β-emitting agents for palliation of painful bony metastases.(1-4) Recently, an α-emitting agent has demonstrated a survival benefit in men with metastatic CRPC to bone.(4, 33) While bone-seeking radiopharmaceuticals may be seen as targeted agents with proven efficacy, they do not target tumor directly. Rather, their anti-tumor effect derives from radiopharmaceutical accumulation in proximity to malignant cells and/or stroma; these agents entirely ignore soft tissue and extra-osseous visceral metastases.
“Targeted” therapeutics offer a potential advantage in cancer therapy by sparing normal tissues. In prostate cancer, PSMA is an ideal target, as it is highly over-expressed by virtually all prostate cancers, and not significantly expressed by normal cells. The few sites that do express low levels of PSMA (e.g. renal proximal tubule lumen and brush border of small intestine) have minimal exposure to anti-PSMA mAb-based therapy, as these sites are not accessible to circulating intact mAb. In addition, recent therapeutic advances in targeting the AR-axis lead to increased PSMA expression.(34) We demonstrated safety and accurate tumor-targeting in previous studies using trace-labeled J591 in patients with advanced PC, but responses to the unarmed antibody in this patient population were limited.(22) These studies led to anti-PSMA-based RIT studies utilizing β- emitting radionuclides. Two phase I studies in patients with metastatic CRPC formed the basis for the current study.(24, 25) While a few efficacy studies have utilized mAbs against non-tumor-specific targets alone or in combination in solid tumor RIT,(35-40) this trial represents one of the few reported phase II studies of disease-specific single-agent RIT (i.e. targeted radiotherapy utilizing a disease-specific mAb) with mature follow up in solid tumor oncology.(41)
In this study, we successfully targeted known sites of metastatic disease in 93.6% of unselected metastatic CRPC subjects, confirming our previous results. More importantly, the initial evidence of anti-tumor efficacy observed in the phase I studies was supported,(24, 25) with the majority of subjects demonstrating PSA declines. Though PSA changes have never fully met criteria for surrogate endpoints, it is important to note that unlike other therapies including docetaxel,(42-44) J591 has no direct effect on PSA transcription, expression or secretion [NHB, unpublished data], PSA declines vs. increases following radiolabeled J591 therapy have been associated with radiographic response or progression,(24) and the data from this study as well as retrospective analysis of other radiolabeled J591 studies,(45) though preliminary, would suggest that patients with PSA declines lived significantly longer (P=0.01).
Numerous publications evaluating PSMA expression have indicated that 84-100% of prostate cancers are PSMA-positive.(8, 11-14) Therefore, patient selection based on PSMA expression was not performed in this study. Even though receptor sites are not saturated, it has been shown that the amount of radiolabeled mAb uptake is proportionate to the level of antigen expression;(46) it is logical that the level of PSMA expression might correlate with response to PSMA-targeted therapy and provide a predictive parameter to identify those less likely to respond (i.e. those with no or low PSMA expression). Post-hoc analysis of the initial cohorts suggested imaging-based scoring of PSMA expression may correlate with subsequent response. Since using 177Lu-J591 as the imaging agent carries the toxicity associated with beta-emission, we performed a pre-treatment scan utilizing 111In-J591 in the prospective cohort, demonstrating the same trend for a lower likelihood of response for poor-imagers. However, planar or even SPECT imaging, is qualitative by nature which may limit clinical utility. Use of quantitative imaging, such as anti-PSMA-based positron emission tomography (PET),(34,47) may be more effective in selecting the best candidates (or more practically ruling out poor candidates given general expression levels) for a PSMA-targeted therapeutic.
As described in the methods section, initial plans were for a single-arm phase II study at the phase I MTD / recommended phase II dose (70 mCi/m2). Based upon limited prior experience with 177Lu and discussions with the FDA, a cohort treated at a slightly lower dose (65 mCi/m2) was used with the expectation that neither efficacy nor toxicity would be significantly different. In the initial cohorts, we observed preliminary evidence suggestive of a dose-response relationship which led to an expansion cohort which validated the increased PSA response rates seen with a single infusion of 70 mCi/m2 of 177Lu-J591; this group also experienced improved survival.
In RIT clinical trials, factors such as antibody internalization and the physical properties of the radionuclide, including the type of particle(s) emitted, half-life, and path-length are important in designing the appropriate clinical strategy.(27, 48) Whereas PSMA/J591 is an excellent antigen/antibody pair in PC, the physical properties of 177Lu theoretically make it most optimal for patients with micro-metastatic disease. Consequently, the patients treated in this phase II trial may be a less suitable cohort in which to demonstrate durable responses. The observed anti-tumor activity together with the additional safety data suggest that 177Lu-J591 targeted radiotherapy may be safe and effective in PC patients with micro-metastatic disease. A multi-institutional trial has begun to test this hypothesis (clinicaltrials.gov NCT00859781) and pre-clinical work is ongoing on J591-alpha particle emitters.
One concern related to RIT is the possibility that treatment may result in damaged bone marrow that might prevent patients from receiving subsequent therapy. The dose-limiting toxicity of RIT in general is transient myelosuppression, which typically occurs in a delayed fashion compared to cytotoxic chemotherapy.(49) Myelodysplastic syndrome and acute leukemia have been reported with anti-CD20 based RIT for non-Hodgkin’s lymphoma,(50) though larger studies have not substantiated this effect.(51, 52) In this study all subjects were treated at or near the MTD (i.e. at or near a dose leading to significant myelosuppression). While all subjects recovered normal neutrophil counts, 7 did not fully recover a normal platelet count. This effect cannot be attributed solely to the radioisotope however, because all of these subjects had clinically progressive prostate cancer and the 3 who underwent bone marrow biopsy revealed infiltrative metastases, so it is plausible that their lack of complete recovery was secondary to progression of their prostate cancer. As with patients receiving chemotherapy, not all have full recovery of blood counts as evidenced by the patients treated on recent post-chemotherapy studies with baseline and ongoing thrombocytopenia post-docetaxel.(53, 54) In preliminary review of our overall anti-PSMA-based RIT experience through 2009 (109 patients), excluding re-treated patients, 98% and 87% had full recovery of neutrophils and platelets respectively.(55) Of the remaining, all but 4 recovered to Gr 1 neutropenia and/or thrombocytopenia. The most common reason for lack of complete hematologic recovery was CRPC progression (PSA and or scan progression with confirmatory bone marrow biopsy revealing significant prostate cancer metastases). No cases of post-RIT myelodysplasia and/or leukemia have been observed.(55)
In summary, a single dose of 177Lu-J591 was well-tolerated with reversible myelosuppression. PSA responses were seen with evidence of a 177Lu dose-response relationship. This study further validates PSMA as an excellent PC-restricted target as well as the performance of the J591 antibody in vivo. The anti-tumor activity seen suggests clinical potential of targeting other types of cytotoxic agents to PSMA. Future directions in progress with anti-PSMA RIT include i) studies to improve patient selection utilizing imaging and CTC and immunohistochemical PSMA-expression analysis, ii) improving therapeutic margin with dose-fractionation, (23, 56) iii) utilizing taxane radiosensitization and tumor debulking (combination studies),(23, 57) and iv) “targeted salvage radiotherapy” exploring 177Lu-J591 in the biochemically recurrent population, a setting in which the physical properties of 177Lu should be more optimally suited.(23,58,59) In addition, a randomized phase III registration trial in men with metastatic CRPC is planned.
Supplementary Material
STATEMENT OF TRANSLATIONAL RELEVANCE.
Targeted therapies are of relevance to many fields of medicine. Prostate specific membrane antigen (PSMA) represents a highly restricted, over-expressed prostatecancer cell-surface protein. J591, a de-immunized monoclonal antibody targeting the external domain of PSMA has been successfully radiolabeled with β-emitting radionuclides. Here, we report a phase II trial of a single dose of 177Lu-J591 that successfully targets known sites of disease in men with progressive metastatic, castration-resistant prostate cancer. Declines in prostate specific antigen were demonstrated, with a dose-response relationship seen. Circulating tumor cell count control occurred in the majority of patients tested. Non-invasive assessment of PSMA expression via imaging may prove to be a predictive biomarker. Based upon this and other clinical trials plus the physical properties of 177Lu (short path length), a randomized study is ongoing targeting a theoretically more optimal micro-metastatic disease opulation (i.e. castration-resistant prostate cancer without metastases) and a phase III registration trial is planned.
Acknowledgments
The authors would like to thank Madhu Mazumdar, Kristen Petrillo, Jodi Selzer, Sandra Flynn, Alicia Lewis, John P. Leonard
Sources of Support: Prostate Cancer Foundation, National Institutes of Health (ULI RR024996, 1-KL2-RR024997-01, K23 CA102544-05, PTBF5405), Department of Defense (W81XWH-04-1-0267), David H. Koch Foundation, Peter M. Sacerdote Foundation, Robert Dow Foundation, Robert H. McCooey Memorial Cancer Research Fund
Footnotes
Conflict of interest: NHB is an inventor on patents that are assigned to Cornell Research Foundation (“CRF”) for the J591 antibody described in this article. Dr. Bander is a paid consultant to and owns stock in BZL Biologics, the company to which the patents were licensed by CRF for further research and development.
References
- 1.Porter AT, McEwan AJ, Powe JE, Reid R, McGowan DG, Lukka H, et al. Results of a randomized phase-III trial to evaluate the efficacy of strontium-89 adjuvant to local field external beam irradiation in the management of endocrine resistant metastatic prostate cancer. Int J Radiat Oncol Biol Phys. 1993;25:805–13. doi: 10.1016/0360-3016(93)90309-j. [DOI] [PubMed] [Google Scholar]
- 2.Quilty PM, Kirk D, Bolger JJ, Dearnaley DP, Lewington VJ, Mason MD, et al. A comparison of the palliative effects of strontium-89 and external beam radiotherapy in metastatic prostate cancer. Radiother Oncol. 1994;31:33–40. doi: 10.1016/0167-8140(94)90411-1. [DOI] [PubMed] [Google Scholar]
- 3.Sartor O, Reid R, Hoskin P, Quick D, Ell P, Coleman R, et al. Samarium-153-lexidronam complex for treatment of painful bone metastases in hormone-refractory prostate cancer. Urology. 2004;63:940–5. doi: 10.1016/j.urology.2004.01.034. [DOI] [PubMed] [Google Scholar]
- 4.Nilsson S, Franzen L, Parker C, Tyrrell C, Blom R, Tennvall J, et al. Bone-targeted radium-223 in symptomatic, hormone-refractory prostate cancer: A randomised, multicentre, placebo-controlled phase II study. Lancet Oncol. 2007;8:587–94. doi: 10.1016/S1470-2045(07)70147-X. [DOI] [PubMed] [Google Scholar]
- 5.Horoszewicz JS, Kawinski E, Murphy GP. Monoclonal antibodies to a new antigenic marker in epithelial prostatic cells and serum of prostatic cancer patients. Anticancer Res. 1987;7:927–35. [PubMed] [Google Scholar]
- 6.Israeli RS, Powell CT, Fair WR, Heston WD. Molecular cloning of a complementary DNA encoding a prostate-specific membrane antigen. Cancer Res. 1993;53:227–30. [PubMed] [Google Scholar]
- 7.Israeli RS, Powell CT, Corr JG, Fair WR, Heston WD. Expression of the prostate-specific membrane antigen. Cancer Res. 1994;54:1807–11. [PubMed] [Google Scholar]
- 8.Wright GL, Haley C, Beckett ML, Schellhammer PF. Expression of prostate-specific membrane antigen in normal, benign, and malignant prostate tissues. Urologic Oncology: Seminars and Original Investigations. 1995;1:18–28. doi: 10.1016/1078-1439(95)00002-y. [DOI] [PubMed] [Google Scholar]
- 9.Troyer JK, Beckett ML, Wright GL., Jr Detection and characterization of the prostate-specific membrane antigen (PSMA) in tissue extracts and body fluids. Int J Cancer. 1995;62:552–8. doi: 10.1002/ijc.2910620511. [DOI] [PubMed] [Google Scholar]
- 10.Sokoloff RL, Norton KC, Gasior CL, Marker KM, Grauer LS. A dual-monoclonal sandwich assay for prostate-specific membrane antigen: Levels in tissues, seminal fluid and urine. Prostate. 2000;43:150–7. doi: 10.1002/(sici)1097-0045(20000501)43:2<150::aid-pros10>3.0.co;2-b. [DOI] [PubMed] [Google Scholar]
- 11.Bostwick DG, Pacelli A, Blute M, Roche P, Murphy GP. Prostate specific membrane antigen expression in prostatic intraepithelial neoplasia and adenocarcinoma: A study of 184 cases. Cancer. 1998;82:2256–61. doi: 10.1002/(sici)1097-0142(19980601)82:11<2256::aid-cncr22>3.0.co;2-s. [DOI] [PubMed] [Google Scholar]
- 12.Kusumi T, Koie T, Tanaka M, Matsumoto K, Sato F, Kusumi A, et al. Immunohistochemical detection of carcinoma in radical prostatectomy specimens following hormone therapy. Pathol Int. 2008;58:687–94. doi: 10.1111/j.1440-1827.2008.02294.x. [DOI] [PubMed] [Google Scholar]
- 13.Mannweiler S, Amersdorfer P, Trajanoski S, Terrett JA, King D, Mehes G. Heterogeneity of prostate-specific membrane antigen (PSMA) expression in prostate carcinoma with distant metastasis. Pathol Oncol Res. 2009;15:167–72. doi: 10.1007/s12253-008-9104-2. [DOI] [PubMed] [Google Scholar]
- 14.Ananias HJ, van den Heuvel MC, Helfrich W, de Jong IJ. Expression of the gastrin-releasing peptide receptor, the prostate stem cell antigen and the prostate-specific membrane antigen in lymph node and bone metastases of prostate cancer. Prostate. 2009;69:1101–8. doi: 10.1002/pros.20957. [DOI] [PubMed] [Google Scholar]
- 15.Kahn D, Williams RD, Haseman MK, Reed NL, Miller SJ, Gerstbrein J. Radioimmunoscintigraphy with In-111-labeled capromab pendetide predicts prostate cancer response to salvage radiotherapy after failed radical prostatectomy. J Clin Oncol. 1998;16:284–9. doi: 10.1200/JCO.1998.16.1.284. [DOI] [PubMed] [Google Scholar]
- 16.Kahn D, Williams RD, Manyak MJ, Haseman MK, Seldin DW, Libertino JA, et al. 111Indium-capromab pendetide in the evaluation of patients with residual or recurrent prostate cancer after radical prostatectomy. the ProstaScint study group. J Urol. 1998;159:2041–6. doi: 10.1016/S0022-5347(01)63239-7. [DOI] [PubMed] [Google Scholar]
- 17.Deb N, Goris M, Trisler K, Fowler S, Saal J, Ning S, et al. Treatment of hormone-refractory prostate cancer with 90Y-CYT-356 monoclonal antibody. Clin Cancer Res. 1996;2:1289–97. [PubMed] [Google Scholar]
- 18.Kahn D, Austin JC, Maguire RT, Miller SJ, Gerstbrein J, Williams RD. A phase II study of [90Y] yttrium-capromab pendetide in the treatment of men with prostate cancer recurrence following radical prostatectomy. Cancer Biother Radiopharm. 1999;14:99–111. doi: 10.1089/cbr.1999.14.99. [DOI] [PubMed] [Google Scholar]
- 19.Troyer JK, Feng Q, Beckett ML, Wright GL. Biochemical characterization and mapping of the 7E11-C5.3 epitope of the prostate-specific membrane antigen. Urologic Oncology: Seminars and Original Investigations. 1995;1:29–37. doi: 10.1016/1078-1439(95)00004-2. [DOI] [PubMed] [Google Scholar]
- 20.Liu H, Moy P, Kim S, Xia Y, Rajasekaran A, Navarro V, et al. Monoclonal antibodies to the extracellular domain of prostate-specific membrane antigen also react with tumor vascular endothelium. Cancer Res. 1997;57:3629–34. [PubMed] [Google Scholar]
- 21.Liu H, Rajasekaran AK, Moy P, Xia Y, Kim S, Navarro V, et al. Constitutive and antibody-induced internalization of prostate-specific membrane antigen. Cancer Res. 1998;58:4055–60. [PubMed] [Google Scholar]
- 22.Bander NH, Nanus DM, Milowsky MI, Kostakoglu L, Vallabahajosula S, Goldsmith SJ. Targeted systemic therapy of prostate cancer with a monoclonal antibody to prostate-specific membrane antigen. Semin Oncol. 2003;30:667–76. doi: 10.1016/s0093-7754(03)00358-0. [DOI] [PubMed] [Google Scholar]
- 23.Tagawa ST, Beltran H, Vallabhajosula S, Goldsmith SJ, Osborne J, Matulich D, et al. Anti-prostate-specific membrane antigen-based radioimmunotherapy for prostate cancer. Cancer. 2010;116:1075–83. doi: 10.1002/cncr.24795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Milowsky MI, Nanus DM, Kostakoglu L, Vallabhajosula S, Goldsmith SJ, Bander NH. Phase I trial of yttrium-90-labeled anti-prostate-specific membrane antigen monoclonal antibody J591 for androgen-independent prostate cancer. J Clin Oncol. 2004;22:2522–31. doi: 10.1200/JCO.2004.09.154. [DOI] [PubMed] [Google Scholar]
- 25.Bander NH, Milowsky MI, Nanus DM, Kostakoglu L, Vallabhajosula S, Goldsmith SJ. Phase I trial of 177lutetium-labeled J591, a monoclonal antibody to prostate-specific membrane antigen, in patients with androgen-independent prostate cancer. J Clin Oncol. 2005;23:4591–601. doi: 10.1200/JCO.2005.05.160. [DOI] [PubMed] [Google Scholar]
- 26.Vallabhajosula S, Kuji I, Hamacher KA, Konishi S, Kostakoglu L, Kothari PA, et al. Pharmacokinetics and biodistribution of 111In- and 177Lu-labeled J591 antibody specific for prostate-specific membrane antigen: prediction of 90Y-J591 radiation dosimetry based on 111In or 177Lu? J Nucl Med. 2005;46:634–41. [PubMed] [Google Scholar]
- 27.O’Donoghue JA, Bardies M, Wheldon TE. Relationships between tumor size and curability for uniformly targeted therapy with beta-emitting radionuclides. J Nucl Med. 1995;36:1902–9. [PubMed] [Google Scholar]
- 28.Bubley GJ, Carducci M, Dahut W, Dawson N, Daliani D, Eisenberger M, et al. Eligibility and response guidelines for phase II clinical trials in androgen-independent prostate cancer: Recommendations from the prostate-specific antigen working group. J Clin Oncol. 1999;17:3461–7. doi: 10.1200/JCO.1999.17.11.3461. [DOI] [PubMed] [Google Scholar]
- 29.Petrylak DP, Ankerst DP, Jiang CS, Tangen CM, Hussain MH, Lara PN, Jr, et al. Evaluation of prostate-specific antigen declines for surrogacy in patients treated on SWOG 99-16. J Natl Cancer Inst. 2006;98:516–521. doi: 10.1093/jnci/djj129. [DOI] [PubMed] [Google Scholar]
- 30.Armstrong AJ, Garrett-Mayer E, OU Yang YC, Carducci MA, Tannock I, de Wit R, et al. Prostate-specific antigen and pain surrogacy analysis in metastatic hormone-refractory prostate cancer. J Clin Oncol. 2007;25:3965–70. doi: 10.1200/JCO.2007.11.4769. [DOI] [PubMed] [Google Scholar]
- 31.Therasse P, Arbuck SG, Eisenhauer EA, Wanders J, Kaplan RS, Rubinstein L, et al. New guidelines to evaluate the response to treatment in solid tumors. european organization for research and treatment of cancer, national cancer institute of the united states, national cancer institute of canada. J Natl Cancer Inst. 2000;92:205–16. doi: 10.1093/jnci/92.3.205. [DOI] [PubMed] [Google Scholar]
- 32.Divgi C. Editorial: What ails solid tumor radioimmunotherapy? Cancer Biother Radiopharm. 2006;21:81–4. doi: 10.1089/cbr.2006.21.81. [DOI] [PubMed] [Google Scholar]
- 33.Parker C, Heinrich D, O’Sullivan JM, Fossa S, Chodacki A, Demkow T, et al. Overall survival benefit of radium-223 chloride (alpharadin) in the treatment of patients with symptomatic bone metastases in castration-resistant prostate cancer: A phase III randomized trial (ALSYMPCA) ECCO-ESMO European Multidisciplinary Cancer Congress. 2011 Abstract 1LBA. [Google Scholar]
- 34.Evans M, Smith-Jones PM, Wongvipat J, Navarro V, Kim S, Bander NH, et al. Noninvasive measurement of androgen receptor signaling with a positron-emitting radiopharmaceutical that targets prostate-specific membrane antigen. PNAS. 2011;108:9578–82. doi: 10.1073/pnas.1106383108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Behr TM, Liersch T, Greiner-Bechert L, Griesinger F, Behe M, Markus PM, et al. Radioimmunotherapy of small-volume disease of metastatic colorectal cancer. Cancer. 2002;94:1373–81. doi: 10.1002/cncr.10308. [DOI] [PubMed] [Google Scholar]
- 36.Reardon DA, Akabani G, Coleman RE, Friedman AH, Friedman HS, Herndon JE, 2nd, et al. Salvage radioimmunotherapy with murine iodine-131-labeled antitenascin monoclonal antibody 81C6 for patients with recurrent primary and metastatic malignant brain tumors: Phase II study results. J Clin Oncol. 2006;24:115–22. doi: 10.1200/JCO.2005.03.4082. [DOI] [PubMed] [Google Scholar]
- 37.Yu L, Ju DW, Chen W, Li T, Xu Z, Jiang C, et al. 131I-chTNT radioimmunotherapy of 43 patients with advanced lung cancer. Cancer Biother Radiopharm. 2006;21:5–14. doi: 10.1089/cbr.2006.21.5. [DOI] [PubMed] [Google Scholar]
- 38.Chen ZN, Mi L, Xu J, Song F, Zhang Q, Zhang Z, et al. Targeting radioimmunotherapy of hepatocellular carcinoma with iodine (131I) metuximab injection: Clinical phase I/II trials. Int J Radiat Oncol Biol Phys. 2006;65:435–44. doi: 10.1016/j.ijrobp.2005.12.034. [DOI] [PubMed] [Google Scholar]
- 39.Li L, Quang TS, Gracely EJ, Kim JH, Emrich JG, Yaeger TE, et al. A phase II study of anti-epidermal growth factor receptor radioimmunotherapy in the treatment of glioblastoma multiforme. J Neurosurg. 2010;113:192–8. doi: 10.3171/2010.2.JNS091211. [DOI] [PubMed] [Google Scholar]
- 40.Salaun PY, Campion L, Bournaud C, Faivre-Chauvet A, Vuillez JP, Taieb D, et al. Phase II trial of anticarcinoembryonic antigen pretargeted radioimmunotherapy in progressive metastatic medullary thyroid carcinoma: biomarker response and survival improvement. J Nucl Med. 2012;53:1185–92. doi: 10.2967/jnumed.111.101865. [DOI] [PubMed] [Google Scholar]
- 41.Brouwers AH, Mulders PF, de Mulder PH, van den Broek WJ, Buijs WC, Mala C, et al. Lack of efficacy of two consecutive treatments of radioimmunotherapy with 131I-cG250 in patients with metastasized clear cell renal cell carcinoma. J Clin Oncol. 2005;23:6540–48. doi: 10.1200/JCO.2005.07.732. [DOI] [PubMed] [Google Scholar]
- 42.Kuroda K, Liu H, Kim S, Guo M, Navarro V, Bander NH. Docetaxel down-regulates the expression of androgen receptor and prostate-specific antigen but not prostate-specific membrane antigen in prostate cancer cell lines: Implications for PSA surrogacy. Prostate. 2009;69:1579–85. doi: 10.1002/pros.21004. [DOI] [PubMed] [Google Scholar]
- 43.Zhu ML, Horbinski CM, Garzotto M, Qian DZ, Beer TM, Kyprianou N. Tubulin-targeting chemotherapy impairs androgen receptor activity in prostate cancer. Cancer Res. 2010;70:7992–8002. doi: 10.1158/0008-5472.CAN-10-0585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Darshan MS, Loftus MS, Thadani-Mulero M, Levy BP, Escuin D, Zhou XK, et al. Taxane-induced blockade to nuclear accumulation of the androgen receptor predicts clinical responses in metastatic prostate cancer. Cancer Res. 2011;71:6019–29. doi: 10.1158/0008-5472.CAN-11-1417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Tagawa ST, Akhtar NH, Osborne JR, Vallabhajosula S, Beltran H, Saran A. Anti-prostate specific membrane antigen (PSMA)-based radioimmunotherapy for metastatic castration-resistant prostate cancer (CRPC): A decade of experience with radiolabeled (RL)-J591. J Urol. 2012;187:e387–e388. [Google Scholar]
- 46.O’Donoghue JA, Smith-Jones PM, Humm JL, Ruan S, Pryma DA, Jungbluth AA, et al. 124I-huA33 antibody uptake is driven by A33 antigen concentration in tissues from colorectal cancer patients imaged by immuno-PET. J Nucl Med. 2011;52:1878–85. doi: 10.2967/jnumed.111.095596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Holland JP, Divilov V, Bander NH, Smith-Jones PM, Larson SM, Lewis JS. 89Zr-DFO-J591 for immunoPET of prostate-specific membrane antigen expression in vivo. J Nucl Med. 2010;51:1293–300. doi: 10.2967/jnumed.110.076174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Bouchelouche K, Tagawa ST, Goldsmith SJ, Turkbey B, Capala J, Choyke P. PET/CT imaging and radioimmunotherapy of prostate cancer. Semin Nucl Med. 2011;41:29–44. doi: 10.1053/j.semnuclmed.2010.08.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Cheson BD, Leonard JP. Monoclonal antibody therapy for B-cell non-hodgkin’s lymphoma. N Engl J Med. 2008;359:613–26. doi: 10.1056/NEJMra0708875. [DOI] [PubMed] [Google Scholar]
- 50.Roboz GJ, Bennett JM, Coleman M, Ritchie EK, Furman RR, Rossi A, et al. Therapy-related myelodysplastic syndrome and acute myeloid leukemia following initial treatment with chemotherapy plus radioimmunotherapy for indolent non-hodgkin lymphoma. Leuk Res. 2007;31:1141–4. doi: 10.1016/j.leukres.2006.11.011. [DOI] [PubMed] [Google Scholar]
- 51.Bennett JM, Kaminski MS, Leonard JP, Vose JM, Zelenetz AD, Knox SJ, et al. Assessment of treatment-related myelodysplastic syndromes and acute myeloid leukemia in patients with non-hodgkin lymphoma treated with tositumomab and iodine I131 tositumomab. Blood. 2005;105:4576–82. doi: 10.1182/blood-2004-12-4690. [DOI] [PubMed] [Google Scholar]
- 52.Czuczman MS, Emmanouilides C, Darif M, Witzig TE, Gordon LI, Revell S, et al. Treatment-related myelodysplastic syndrome and acute myelogenous leukemia in patients treated with ibritumomab tiuxetan radioimmunotherapy. J Clin Oncol. 2007;25:4285–92. doi: 10.1200/JCO.2006.09.2882. [DOI] [PubMed] [Google Scholar]
- 53.de Bono JS, Logothetis CJ, Molina A, Fizazi K, North S, Chu L, et al. Abiraterone and increased survival in metastatic prostate cancer. N Engl J Med. 2011;364:1995–2005. doi: 10.1056/NEJMoa1014618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Scher HI, Fizazi K, Saad F, Taplin ME, Sternberg CN, Miller K, et al. Increased survival with enzalutamide in prostate cancer after chemotherapy. N Engl J Med. 2012;367:1187–97. doi: 10.1056/NEJMoa1207506. [DOI] [PubMed] [Google Scholar]
- 55.Tagawa ST, Parmar S, Pena J, Petrillo K, Matulich D, Selzer J, et al. Bone marrow recovery and subsequent chemotherapy following radiolabeled anti-prostate-specific membrane antigen (PSMA) monoclonal antibody J591 in patients (pts) with metastatic castrate-resistant prostate cancer (metCRPC) J Clin Oncol. 2009;27(Suppl) abstr e16004. [Google Scholar]
- 56.Tagawa ST, Vallabahajosula S, Osborne J, Goldsmith SJ, Petrillo K, Tyrell L, et al. Phase I trial of fractionated-dose 177lutetium radiolabeled anti-prostate-specific membrane antigen (PSMA) monoclonal antibody J591 (177Lu-J591) in patients (pts) with metastatic castration-resistant prostate cancer (metCRPC) J Clin Oncol. 2010;28(Suppl) Abst 4667. [Google Scholar]
- 57.Beltran H, Vallabhajosula S, Kelly WK, Whang Y, Osborne J, Petrillo K, et al. Phase I dose escalation study of Docetaxel/Predisone and fractionated 177Lu -J591 for metastatic castrate resistant prostate cancer (metCRPC) J Clin Oncol. 2010;28(Suppl) Abst TPS 247. [Google Scholar]
- 58.Tagawa ST, Osborne J, Christos PJ, Vallabhajosula S, Petrillo K, Nadeau K, et al. A randomized phase II trial of 177lu radiolabeled monoclonal antibody J591 (177Lu-J591) and ketoconazole in patients (pts) with high-risk castrate biochemically relapsed prostate cancer (PC) after local therapy. J Clin Oncol. 2010;28(Suppl) Abst TPS248. [Google Scholar]
- 59.Kosuri S, Akhtar NH, Smith M, Osborne JR, Tagawa ST. Review of salvage therapy for biochemically recurrent prostate cancer: the role of imaging and rationale for systemic salvage targeted anti-prostate-specific membrane antigen radioimmunotherapy. Adv Urol. 2012:921674. doi: 10.1155/2012/921674. Epub 2012 May 28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Halabi S, Small EJ, Kantoff PW, Kattan MW, Kaplan EB, Dawson NA, et al. Prognostic model for predicting survival in men with hormone-refractory metastatic prostate cancer. J Clin Oncol. 2003;21:1232–7. doi: 10.1200/JCO.2003.06.100. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


