Abstract
Fibroblast Growth Factors (FGFs) are expressed in many non-small cell lung cancer (NSCLC) primary tumors and derived cell lines, and mutations in FGF receptor 3 (FGFR3) have been identified in human lung adenocarcinoma. FGF9 has been implicated in the pathogenesis of NSCLC by synergizing with EGFR pathways or by providing an escape pathway mediating resistance to EGFR inhibition. To model pathogenic mechanisms mediated by FGF signals, we have established a mouse model in which FGF9 expression can be induced in adult lung epithelium. Here, we show that induced expression of FGF9 in adult lung leads to the rapid proliferation of distal airway epithelial cells that express the stem cell marker, Sca-1, and the proximal and distal epithelial markers, Sftpc and CC10, the rapid formation of Sftpc positive adenocarcinomas, and eventual metastasis in some mice. Furthermore, we have identified FGF receptor 3 (FGFR3) as the obligate receptor mediating the FGF9 oncogenic signal. These results identify an FGF9-FGFR3 signal as a primary oncogenic pathway for lung adenocarcinoma and suggest that this pathway could be exploited for customized therapeutic applications for both primary tumors and those that have acquired resistance to inhibition of other signaling pathways.
Keywords: Lung cancer, NSCLC, Fibroblast Growth Factor, FGF9, FGF receptor 3, FGFR3, metastasis, adenocarcinoma
Introduction
Non-small cell lung cancer (NSCLC) is a disease with considerable molecular heterogeneity (1-3), and thus, pathway-specific inhibitors have become attractive as candidates for customized therapy. Inhibitors of the Epidermal Growth Factor Receptor (EGFR) tyrosine kinase have proven effective in patients with activating mutations in the EGFR tyrosine kinase domain (4, 5). However, EGFR mutations account for only 10% of NSCLC patients in North America and 20-50% of NSCLC patients in Asia (6), and patients inevitably acquire resistance to EGFR inhibition (7). These observations highlight the need to identify additional signaling pathways that are activated in NSCLC as potential targets for new molecular therapies. One pathway of great interest in NSCLC is the Fibroblast Growth Factor (FGF) signaling pathway (8).
FGF signaling has been implicated in the etiology and pathogenesis of many cancers, including myeloma, bladder, prostate, cervical, ovarian endometrioid, endometrial, gastric, breast, melanoma, and lung (9). A subset of NSCLC tumor cells express FGF2 and its receptor, FGFR1 (3, 10). Several studies showed that FGF2 expression is associated with a poorer prognosis for patients with NSCLC (11, 12). FGF9 expression has also been identified in several tumor types, including breast, prostate, endometrioid, and lung cancer (13-16), suggesting an important role in tumorigenesis. Interestingly, three potentially activating missense mutations in FGFR3 were identified in a cohort of 183 lung adenocarcinomas recently subjected to whole genome sequence analysis, placing FGFR3 among the top 25 significantly mutated genes in lung adenocarcinoma (2). Another study showed increased expression of FGFR3 in primary human lung adenocarcinoma tissue, and in derived cell lines, micro RNA-mediated down regulation of FGFR3 correlated with decreased plating efficiency (17). Additionally, the FGFR1 gene is amplified in 22% of human squamous cell lung cancer and 3% of human lung adenocarcinoma (18, 19). Thus, the FGF signaling pathway may be a clinically relevant target for a subset of lung cancers.
Several NSCLC cell lines that acquired resistance to gefitinib (EGFR kinase inhibitor) were found to increase expression of FGF2, FGF9, FGFR1, FGFR2, and/or FGFR3 (7, 14, 20). Treatment of gefitinib-resistant cell lines that co-expressed either FGF2 or FGF9 and FGFR1 with a small molecule FGFR tyrosine kinase inhibitor, RO4383596, resulted in decreased expression of pERK and inhibition of cell growth. Cell lines that did not co-express both ligand and receptor did not show growth inhibition. These findings suggest that FGF mediated autocrine signaling may play an important role in the development and progression of NSCLC. In other studies, FGF9 expression was observed in 86 of 146 primary human NSCLC specimens, 77% of which were adenocarcinomas (21). FGF9 expression correlated with the expression of aryl hydrocarbon receptors and activation of aryl hydrocarbon receptors by benzopyrenes (known lung carcinogens) increased expression of FGF9 (21-23). FGF9 expression in an adenocarcinoma cell line increased its invasive properties in vitro and high FGF9 expression was associated with increased tumor stage and lymph node metastasis (23). Although, all these data point to the fact that alterations in FGF9 may play an important role in NSCLC, so far, no data exist supporting a role for FGF9 in cancer initiation.
Here, we show that induction of FGF9 in adult lung results in the rapid formation of epithelial tumors that resemble papillary adenocarcinomas. We further show that early targets of FGF9 include cells in the bronchioalveolar duct junction (BADJ) that co-express surfactant protein C (Sftpc), Clara cell antigen 10 (CC10, Scgb1a1), and Sca-1, suggesting that a cell with progenitor properties may be particularly sensitive to FGF9. Finally, we identify FGF receptor 3 (FGFR3) as an essential mediator of FGF9-induced tumorigenicity. This mouse model thus identifies FGF9-FGFR3 as an oncogenic signal that should be studied further in human lung cancer as a potential target for therapeutic intervention. Furthermore, this model should serve as a valuable tool to screen and evaluate pathway-specific pharmacological therapies.
Materials and Methods
Mice
All mouse strains, including Sftpc-rtTA, Tre-Fgf9-ires-eGfp, TetO-Cre, Fgfr3−/− and Rosa26 reporter (R26R), have been previously described (24-28). Sftpc-rtTA, Tre-Fgf9-ires-eGfp mice were maintained on the FVB genetic background. Other mice were maintained on a mixed FVB/129SV/J/C57B6/J background. Doxycycline diet was purchased from Bio-Serv Inc. (200mg/kg green pellets, S3888, USA). All mice were housed in a pathogen-free animal facility under the veterinary care of the Department of Comparative Medicine at Washington University School of Medicine, and used at the age of six-to-twelve weeks. All protocols were approved by the Washington University Animal Studies Committee and were performed in accordance with the Animal Welfare Act and the Guide for the Care and Use of Laboratory Animals.
Whole mount, histology analysis and immunohistochemistry
Mice were anesthetized with Avertin (2,2,2 tribromoethanol, 2-methyl-2-butanol) or KXA (31 mg/kg ketamine, 6 mg/kg xylazine, and 1 mg/kg acepromazine) and transcardially perfused with a vascular rinse of 0.9% NaCl followed by 10% buffered formalin phosphate (L-14449, Fisher Scientific, USA). Lung and other organs were dissected, whole mount eGFP fluorescence was viewed with a fluorescence dissecting microscope (SZX12-ILLD100, Olympus Optical CO. LTD). Tissues were postfixed in 10% phosphate buffered formalin overnight at 4°C. Frozen or paraffin sections were prepared using standard procedures. For histology, slides were stained with hematoxylin and eosin (H&E). For immunohistochemistry, sections were rehydrated and treated with 0.3% hydrogen peroxide in methanol for 15 min. to suppress the endogenous peroxidase activity. Antigen retrieval was achieved by boiling at 121°C for 15 min in 10 mM citrate buffer followed by gradual cooling to room temperature. Sections were incubated overnight with the primary antibodies at 4°C. Secondary antibodies were visualized using Broad Spectrum (AEC) Kit (95-9743, Zymed Laboratories Inc.) or Vectastain® Elite ABC (AEC) kit (PK-4005, Vector Laboratories). Sections were photographed on an Axioplan2 microscope (Zeiss). For immunofluorescence staining, 0.3% hydrogen peroxide treatment was omitted, and after incubation with the primary antibodies, appropriate Alexa Flour-coupled secondary antibodies (Molecular Probes) were applied at a 1:200 dilution. Sections were photographed on a ApoTome fluorescence microscope (Zeiss). The following primary antibodies were used for staining: FGF9 (AF-273-NA, R&D Systems, 1:100), Nkx2.1 (TTF-1, M3575, DakoCytomation, 1:100), CC10 (Scgb1a1, sc-9772, Santa Cruz Biotechnology Inc, 1:200); pro-SP-C (SftpC, AB3786, Millipore, 1:2,000); PCNA (sc-56, Santa Cruz Biotechnology Inc, 1:100); Sca-1 (557403, BD Pharmingen, 1:200); P-Erk (4370S, Cell Signaling, 1:200), E-Cadherin (610181, BD Pharmingen, 1:500), FGFR2 (sc-122, Santa Cruz Biotechnology Inc, 1:100), FGFR3 (rabbit polyclonal), GFP (NeuroMab N86/38, 1:500).
For quantitation of cell number, multiple optical sections were scored manually to distinguish cell boundaries. Three different whole-lung longitudinal sections containing the main axial bronchi were scored for each mouse at each time point.
RNA isolation, cDNA synthesis, and RT–PCR analysis
Mouse lung total RNA was prepared, using the TRIzol LS Reagent (Life Technologies) following the manufacturer's instructions. One microgram of RNA was used for complementary DNA (cDNA) synthesis, employing M-MLV reverse transcriptase and oligo dTprimers (Promega). Fgf9 transcripts were amplified, using Klen Taq polymerase (Dept. of Biochemistry, Washington University). Amplification: 95°C for 3 min followed by 24-30 cycles of 95°C for 1 min, 55-65°C for 2 min and 72°C for 2 min, followed by a 5 min extension at 72°C. PCR primers were as follows: Fgf9, forward 5'-CAAGCTTGGATTGAAGAAAAGAACC-3', reverse, 5'-CAAGCTTGGATTGAAGAAAAGAACC-3′ (30 cycles); L19, forward 5′-CTGAAGGTGAAGGGGAATGTG-3′, reverse 5′-GGATAAAGTCTTGATGATCTC-3′ (24 cycles). The PCR products were electrophoresed on a 1% agarose-TAE gel and visualized by ethidium bromide staining.
Quantitative RT-PCR
Residual exonuclease–based fluorogenic PCR was performed using an ABI Prism 7700 Sequence Detection System (Applied Biosystems). TaqMan® Gene Expression Assay probes (Applied Biosystems) were used for mouse β2microglobulin (Mm00437764_m1) and mouse Fgf9 (Mm01319105_m1). The relative amounts of each gene mRNA in the samples were assessed by interpolation of their threshold cycles from a standard curve, which were then normalized against β2microglobulin mRNA.
In situ hybridization
RNA probes were transcribed and labeled with SP6 RNA polymerase (for antisense orientation) or T7 RNA polymerase (for sense orientation) using DIG-UTP labeling mix (Roche Applied Science).
Whole-mount β-Galactosidase staining
Lungs were dissected in ice-cold PBS and fixed with 0.5% glutaraldehyde in PBT (PBS, 0.1% Tween-20) overnight at 4°C. Tissues were washed in PBT twice for 10 min prior to incubation with β-Gal staining solution (2 mM MgCl2, 35 mM potassium ferrocyanide, 35 mM potassium ferricyanide, 1 mg/ml X-Gal in PBT) at room temperature in the dark. Following adequate color reaction, tissues were washed twice in PBT for, 10 min each, to stop the reaction. Paraffin sections were prepared with standard procedures and slides were counterstained with Fast Red. All staining patterns are representative of at least three mice.
Lung cell transplantation
Sftpc-rtTA, Tre-Fgf9-ires-eGfp mice were induced with Dox for 12 days. Lung cells were then isolated as described (29) with minor modifications. Mice were anesthetized and exsanguinated. The thoracic cavity was opened, and the lungs were exposed and perfused with 10 ml of PBS, 0.1 mM EDTA. The trachea was cannulated, and 3 ml of dispase (BD, Franklin, NJ) with 8U elastase solution (Worthington Biochemical, Lakewood, NJ) was instilled into the airways and incubated for 15 min at 37°C. Lung lobes were removed following dispase/elastase digestion, minced, and incubated with 7 ml of collagenase type II (3.6 U/ml) (Worthington Biochemical) plus 1 μl of DNase I (2 U/ml, Invitrogen, Carlsbad, CA) for 40 min at 37°C with rotation. Cells were passed through a 70 μm cell strainer (BD Biosciences, Bedford, MA), and red blood cells were lysed using RBC lysing buffer (eBioscience Inc., San Diego, CA).
The digested tissue was then dispersed with an 18-gauge needle, washed, and cells were resuspended in PBS. 5.5 × 106 cells / 100 μl were injected subcutaneously (n=4), or 2.25 × 106 cells / 100 μl were injected intravenously (n=2) through the tail vein of female wild type FVB mice.
Magnetic resonance imaging (MRI)
Respiratory-gated, spin-echo MR images were collected using a small-animal MR scanner built around an Oxford Instruments (Oxford, UK) 4.7 tesla, 40-cm bore magnet and interfaced to an Agilent/Varian (Santa Clara, CA) DirectDrive™ console. The magnet is equipped with Agilent/Magnex (Yarnton, UK) actively shielded, high-performance (21 cm ID; ~32 G/cm max gradient; ~400 μs rise-time) gradient coils and International Electric Company (Helsinki, Finland) high-performance (300 A; 200 V) gradient power supplies. All data were collected using a Stark Contrast (Erlangen, Germany) 2.5-cm, birdcage-style, quadrature rf coil. Prior to the imaging experiments, mice were anesthetized with isoflurane and were maintained on isoflurane/O2 (1% v/v) throughout data collection. Animal core-body temperature was maintained at 37±1 °C by circulation of warm air through the bore of the magnet. During the imaging experiments, the respiration rates for all mice were regular and ~2 sec−1. Synchronization of MR data collection with animal respiration was achieved with a respiratory-gating unit (30) and all images were collected during post-expiratory periods. Twenty-four to thirty contiguous coronal slices, ventral to dorsal, were collected for each mouse. Imaging parameters were TR ~3 s, TE = 30 ms, FOV 4.0 cm × 4.0 cm2, slice thickness = 0.5 mm, 128 × 128 data matrix, 4 averages.
Results
FGF9 overexpression in adult lung leads to adenocarcinoma
To investigate the immediate and long-term consequences of FGF9 expression in adult lung, we used a doxycycline-regulated inducible genetic system comprising the surfactant protein C–reverse tetracycline activator (Sftpc-rtTA) and tetracycline responsive, Tre-Fgf9-ires-eGfp transgenes (26, 31). In the absence of doxycycline, adult Sftpc-rtTA, Tre-Fgf9-ires-eGfp double transgenic mice were phenotypically normal and healthy, and their lung tissue did not express GFP and was histologically normal (Figure 1A-C,N). RT-PCR, eGFP fluorescence, in situ hybridization, and immunostaining of lung tissue in the absence of doxycycline showed very low or undetectable Fgf9 expression (Figure 2A-E, Supplementary Figure 1). However, in response to doxycycline (provided in chow), Fgf9 and eGFP expression were robustly induced (Figure 2A, F-H, Supplementary Figure 1).
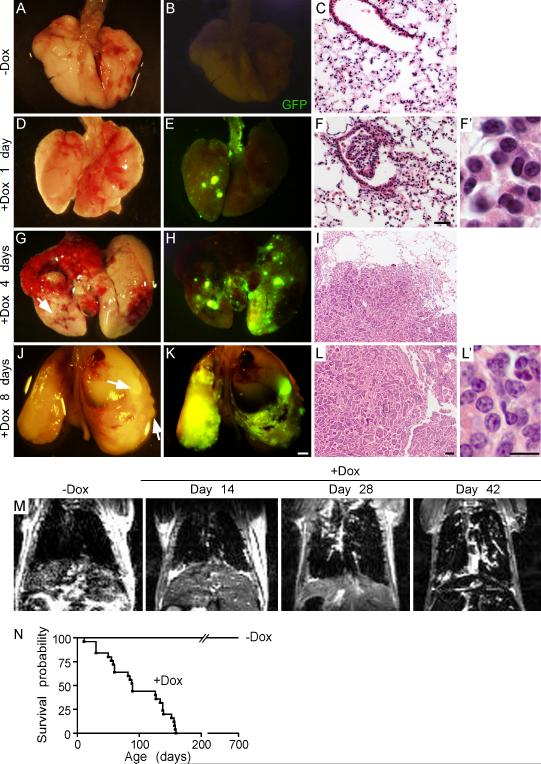
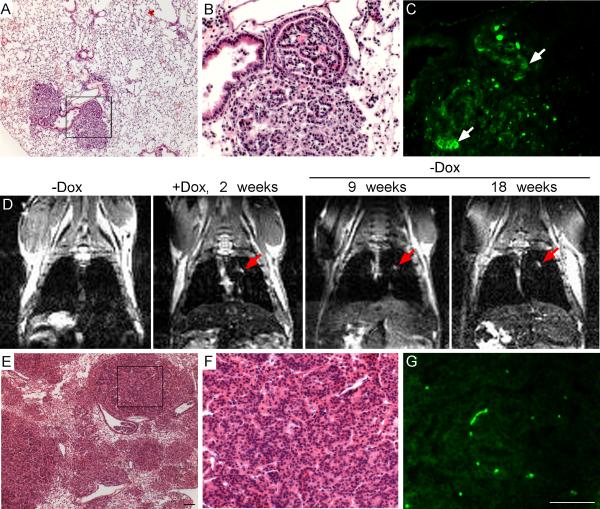
Figure 1.
Overexpression of FGF9 in the lungs leads to adenocarcinoma. (A, D, G, J) Images of whole lungs from Sftpc-rtTA, Tre-Fgf9-ires-eGfp mice induced with doxycycline for 0, 1, 4, and 8 days. (B, E, H, K) Corresponding images showing eGFP fluorescence. After one day of doxycycline induction, small eGFP positive nodules were detected on the lung surface (E). After 4 or 8 days of doxycycline induction, the lung surface showed increased size and number of eGFP positive nodules and some hemorrhagic lesions (G-K). (C, F, I, L) Representative histology (H&E stain) showing progression of epithelial tumors following exposure to doxycycline. (F) After one day of doxycycline induction, small lesions could be identified near BADJs. Nuclei were round or oval-shaped and of similar size (F’). Control double transgenic mice (C) were histologically normal. (I, L) After 4 or 8 days of doxycycline induction, progressively larger tumors contained cells with hyperchromatic nuclei containing prominent nucleoli (L'). (M) MRI showed the absence of lung nodules before doxycycline induction and progressive growth of lung nodules following doxycycline induction from 14-42 days. (N) Kaplan-Meier survival curve of Sftpc-rtTA, Tre-Fgf9-ires-eGfp mice not exposed to doxycycline (n=20) or induced with doxycycline (n=25). Median survival of doxycycline-induced mice was 89 days. Scale bars: C, F, 50 μm; I, L, 100 μm; F’, L’ 10 μm; K, 2.0 mm.
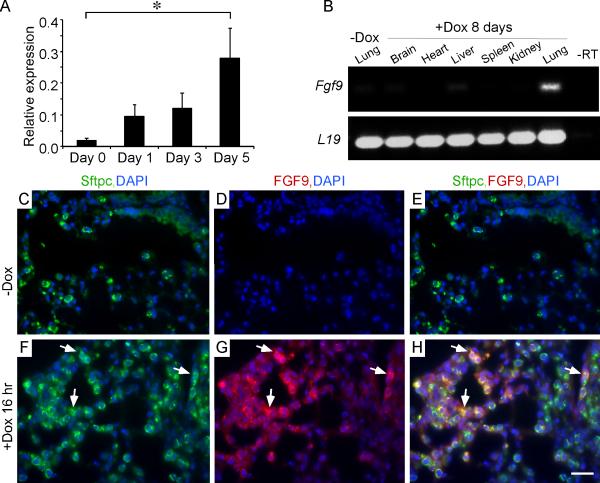
Figure 2.
FGF9 expression and localization following doxycycline induction of Sftpc-rtTA, Tre-Fgf9-ires-eGfp mice. (A) Quantitative RT-PCR analysis of Fgf9 expression following doxycycline induction for 0-5 days (data plotted as mean ± SEM, n=5 mice/time point). * p<0.05 compared to uninduced lung. (B) RT-PCR analysis of Fgf9 expression in different organs (brain, heart, liver, spleen, kidney, lung) following doxycycline induction of SftpC-rtTA, Tre-Fgf9-ires-eGfp mice showing specificity for lung tissue. (C-H) Expression of Sftpc and FGF9 in adult lung before (C-E) and 16 hr after doxycycline induction (F-H) showing FGF9 (red) co-localization with Sftpc (green) in distal bronchiolar epithelium (arrows). DAPI (blue). Scale bars: 20 μm.
Examination of whole lungs, one day following doxycycline (Dox) administration, revealed the rapid formation of small eGFP positive nodules visible on the lung surface (Figure 1D, E). Histological examination revealed small epithelial clusters, located in distal airways and BADJ regions with benign appearing nuclei (Figure 1F,F’). One day after induction, Fgf9 expression was readily detected by RT-PCR and four days after induction in situ histological analysis showed high Fgf9 and eGFP expression in tumor nodules (Figure 2A and Supplementary Figure 1). Consistent with the Sftpc-rtTA driver, induced FGF9 colocalized with Sftpc in lung tissue following 16 hr of doxycycline induction (Figure 2F-H). At 4 and 8 days of induction with doxycycline, the number and size of nodules continued to increase and the lung surface took on a cobblestone appearance with visible nodules corresponding to high eGFP fluorescence (Figure 1G,H,J,K). Histological examination after four days of doxycycline induction was consistent with papillary adenoma architecture (Figure 1I). After eight days of induction, the cells forming papillary structures became atypical with larger and hyperchromatic nuclei, consistent with the diagnosis of papillary adenocarcinoma (Figure 1L,L’). Other tissues examined (brain, heart, liver, spleen, kidney) did not express Fgf9 or eGFP and were histologically normal (Figure 2B and data not shown).
Magnetic resonance imaging (MRI) was used to assess tumor progression (Figure 1M). Due to low water content and the effects of magnetic susceptibility, healthy lung parenchyma was not visible on hydrogen MRI, and lungs from uninduced mice appeared clear of lesions. Consistent with the gross pathology, MRI showed progressive formation of multiple high-density nodules following doxycycline induction. Consistent with widespread lung tumorigenesis, the primary cause of death of doxycycline-induced mice appeared to be respiratory failure. The median survival of mice induced with doxycycline (n=25) was 89 days (Figure 1N). Mice not exposed to doxycycline remained disease free for over 700 days (n=20).
FGF9 targets the adult lung epithelium
During embryonic development, FGF9 primarily signals to mesenchyme, where it promotes proliferation and suppresses smooth muscle differentiation. Additionally, FGF9 directly and indirectly induces proliferation of developing lung epithelium, resulting in epithelial dilation (26, 32, 33). In contrast to the mesenchymal growth and epithelial dilation seen in response to FGF9 in embryos, FGF9 induction in adult lung resulted in epithelial proliferation with minimal stromal response. Immunostaining for Nkx2.1 (TTF-1) showed that the tumor nodules are derived from lung epithelium (Figure 3A,B). To characterize the type of lung epithelial cells, lung tissue was also stained for Sftpc and CC10. Interestingly, all tumor nodules were positive for Sftpc and negative for CC10 (Figure 3C-F), suggesting that the tumors arise from progenitor-like type II pneumocytes or progenitor cells that lose expression of CC10 as they progress towards malignancy.
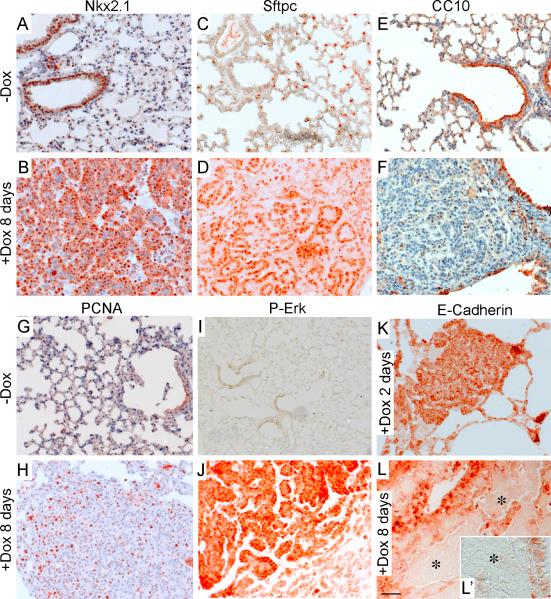
Figure 3.
Molecular identity of FGF9 induced tumors. (A-F) After eight days of doxycycline induction, tumor nodules prominently expressed the lung epithelial transcription factor, Nkx2.1 (A, B) and the type II pneumocyte marker, Sftpc (C, D), but were negative for expression of the Clara cell marker, CC10 (E, F). (G, H) Immunostaining for PCNA showing increased proliferation in FGF9-induced tumor nodules. (I, J) Immunostaining for p-Erk showing prominent expression in tumor nodules, but not in surrounding lung tissue or uninduced lung. (K, L) E-Cadherin is expressed in normal lung epithelium and small tumor nodules following doxycycline induction for two days (K). E-Cadherin expression is absent from larger tumors following doxycycline induction for eight days (asterisks in L, L’). Scale bars: L, 50 μm, L’ 25 μm.
We next assessed proliferation, differentiation, and FGFR signaling. Eight days after doxycycline induction, levels of Proliferating Cell Nuclear Antigen (PCNA) and phospho-Erk (P-Erk) were markedly elevated within tumor nodules. Additionally, E-Cadherin, an adhesion molecule expressed in differentiated lung epithelial cells was initially expressed in tumor nodules after two days of induction, but then lost from larger tumors after eight days of induction (Figure 3K,L,L’). Decreased E-Cadherin expression is associated with increased risk for metastasis of NSCLC (34). Consistent with the potential for FGF9-induced lung cancer to progress to malignancy, animals surviving four months of doxycycline induction developed ascites. Of five mice with ascites, two contained cells with malignant cytology that were positive for eGFP expression (Figure 4A,B). No evidence of metastatic seeding was identified in other tissues including brain and liver (data not shown).
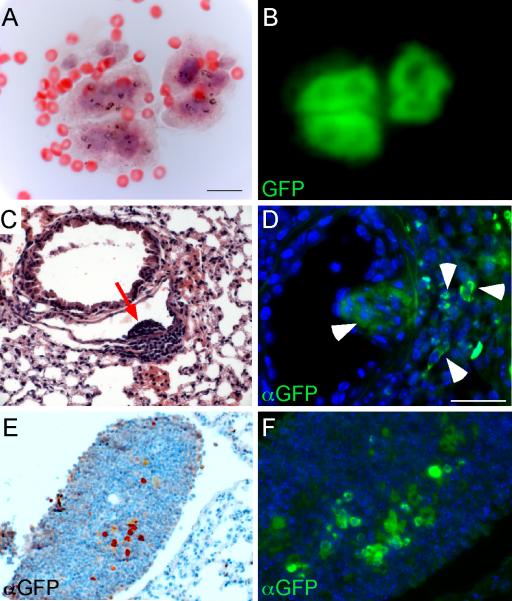
Figure 4.
Identification of eGFP-positive tumor cells in peritoneal fluid of tumor bearing mice, and lung and lymph node following transplantation. (A,B) Cells from ascites fluid showing malignant cytology (A) and expression of eGFP (B) after four months of doxycycline induction. (C, D) Lung histology from a wild type FVB mouse transplanted subcutaneously with 5.5 × 106 dissociated lung cells and then maintained on a doxycycline diet for five months showing a small clump of cells (C, red arrow) in a pulmonary blood vessel. Immunostaining for eGFP (D) identified GFP positive cells (arrowheads) in a similar vascular clump and in surrounding interstitial tissue. (E, F) eGFP-expressing cells (E, colorimetric detections; F, fluorescent detections) were also identified in a peribronchiolar lymph node in a wild type FVB mouse injected intravenously with 2.25 × 106 cells and maintained on doxycycline for 3 months. This mouse also had vascular and perivascular nodules in the lung. DAPI (blue). Scale bars: A, B, 20 μm; C-F, 25 μm.
To further assess malignant potential, syngeneic mice (FVB background), injected intravenously (n=4) or transplanted subcutaneously (n=2) with dissociated cells from FGF9-induced lung tissue and maintained on a doxycycline-containing diet for two to five months, developed small lung nodules that were often associated with blood vessels (Figure 4C). Immunostaining revealed that many of these nodules continued to express eGFP (Figure 4D). Additionally, in one intravenously injected mouse, a peribronchiolar lymph node was also found to contain eGFP positive cells (Figure 4E,F).
Persistence of lung tumors after withdrawal of FGF9 induction
To evaluate the reversibility of tumor formation, Sftpc-rtTA, Tre-Fgf9-ires-eGfp double transgenic mice were induced with doxycycline and then placed on a doxycycline-free diet. Two experimental designs were used. After 2-4 days induction, followed by 1 month doxycycline withdrawal (three mice), small tumor nodules persisted throughout the lungs. However, immunostaining for FGF9 showed that small clusters of cells within each nodule remained FGF9 positive after four weeks withdrawal (Figure 5A-C, arrow) while in surrounding cells, expression of FGF9 and eGFP (not shown) returned to baseline levels. Because some cells remained FGF9 positive, we induced two more mice (for two weeks) and then withdrew doxycycline for 18 weeks. In this second experiment, MRI was used to confirm that lungs were tumor-free before induction and to follow large tumor nodules during the withdrawal period (Figure 5D). Following doxycycline withdrawal, imaging at 9 and 18 weeks showed that large tumor nodules persisted with no apparent change in size (red arrow). Endpoint histological evaluation confirmed the persistence of tumors even after doxycycline withdrawal (Figure 5E,F). Immunostaining for FGF9 showed only background levels of FGF9 expression in most tumors (Figure 5G). These data indicate that once tumors are induced, they persist and become partially independent of FGF9. This is in contrast to lung tumors induced by expression of FGF3, FGF7 or FGF10 (targeted with Sftpc regulatory sequences), which showed rapid regression following doxycycline withdrawal (31, 35, 36).
Figure 5.
Persistence of lung tumors after withdrawal of FGF9 induction. (A-C) Example of lung histology from mice (n=3 mice) that were induced with doxycycline for 2-4 days followed by withdrawal of doxycycline for 1-2 months. Low magnification view of an H&E stained section (A) and high magnification view of the boxed region (B) showing focal tumor masses in otherwise normal lung tissue. (C) Adjacent section to A and B immunostained with an anti-FGF9 antibody showing small clusters of cells within the tumor that continued to express FGF9 (arrow). (D) Example of magnetic resonance imaging (n=2 mice) showing absence of lung masses before induction, clear lung masses following doxycycline induction for 2 weeks, and persistence of lung masses for 9 and 18 weeks after withdrawal of doxycycline (red arrows). (E-G) Endpoint histology from the mouse imaged in D. Low magnification (E) and high magnification (F) views of an H&E stained section. (G) FGF9 immunostaining of an adjacent section showing only background immunoreactivity (bright green spots are autofluorescent red blood cells). Scale bars: E, G, 100 μm.
FGF9 promotes proliferation of Sftpc, CC10 expressing cells in the bronchioalveolar duct junction
Initial studies suggest a very rapid response of adult lung tissues to FGF9, with small epithelial nodules visible within 24 hr after FGF9 induction. To determine which cells are potentially targeted for FGF9 expression, Sftpc-rtTA mice were crossed to TetO-Cre and ROSA26 reporter alleles. Following induction with doxycycline for 7 days, β-Galactosidase (β-Gal) activity was localized to type II pneumocytes (arrows in Supplementary Figure 2B) and a subset of bronchiolar epithelial cells (arrowheads in Supplementary Figure 2B). It should be noted that not only type II pneumocytes, but also distal airway epithelial cells, are potentially targeted for FGF9 induction with the Sftpc-rtTA allele.
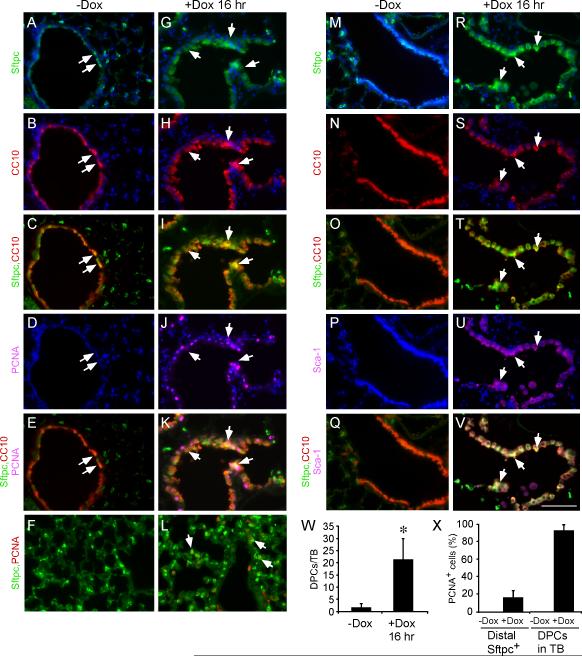
To identify which cells initially proliferate in response to FGF9, lung sections were stained for PCNA after 16 hr doxycycline induction. Interestingly, the majority of PCNA positive cells were localized to distal bronchiolar epithelium (Supplementary Figure 2C,D). A small population of cells in the distal bronchiolar epithelium, referred to as bronchioalveolar stem cells (BASCs), coexpress Sftpc and CC10 and have been reported to have progenitor-like properties. Although BASCs can contribute to alveolar and bronchiolar lineages in vitro, it is controversial as to whether they can contribute to all lung epithelial lineages in vivo (37-40). To determine whether these proliferating cells expressed markers of putative progenitor cells, lung tissue was immunostained for Sftpc and CC10 (Figure 6). In uninduced lung, 2 ± 1.7 Sftpc, CC10 positive cells (double-positive cells) could be identified within 25 cells of the BADJ (defined as the terminal bud, TB) (Figure 6W) (40). Of these, none were positive for PCNA (Figure 6A-F, X). Following induction of FGF9, 22 ± 8.3 cells within 25 cells of the BADJ were Sftpc, CC10 positive, and 94 ± 0.1% of these were also positive for PCNA (Figure 6G-K, W, X). In contrast, only 17 ± 0.1% of peripheral Sftpc positive cells were also positive for PCNA (Figure 6F, L, X).
Figure 6.
FGF9 induces Sftpc and Sca-1 expression in CC10 positive cells and promotes proliferation of bronchiolar epithelial cells. (A-L) Immunofluorescence showing Sftpc, CC10 and PCNA expression in lungs from Sftpc-rtTA, Tre-Fgf9-ires-eGfp control (A-F, -Dox) and 16 hr induced (G-L, +Dox) mice. A-E and G-K show distal bronchiolar epithelial regions and F, L show alveolar regions. Arrows indicate cells expressing multiple markers as indicated. (M-V) Immunofluorescence showing Sftpc, CC10 and Sca-1 expression in lungs from Sftpc-rtTA, Tre-Fgf9-ires-eGfp control (M-Q, -Dox) and 16 hr induced (R-V, +Dox) mice. All of the Sftpc, CC10 double-positive cells in induced mice are also Sca-1 positive. Arrows indicate examples of Sftpc, CC10 double-positive cells. (W) Quantification of Sftpc, CC10 double-positive cells in the terminal bud (TB) region (defined as within 25 cells of the BADJ) after doxycycline induction for 16 hr. (X) Quantification of the ratio of PCNA positive cells to Sftpc, CC10 double-positive cells compared with the ratio of PCNA positive cells to distal Sftpc positive cells showing that Sftpc, CC10 double-positive cells have a much higher proliferation rate compared to distal Sftpc positive cells. Data plotted as mean ± SD, * p<0.001. DAPI (blue). Scale bar: 50 μm.
Sca-1 is a marker of a broad population of bronchiolar progenitor cells, including BASCs (29, 37). Lungs induced to express FGF9 showed a high level of Sca-1 immunoreactivity in cells that were also positive for Sftpc and CC10 (Figure 6R-V) compared to very low Sca-1 expression in uninduced lung tissue (Figure 6M-Q). Together, these data identify a putative population of epithelial cells located in distal bronchiolar epithelium as the primary cell type that proliferates in response to FGF9. However, within the time frame of 16 hr, it is unlikely that the small population of pre-existing BASCs could proliferate in response to FGF9 to an extent that would displace pre-existing CC10 positive cells in the distal bronchiolar epithelium.
FGFR3 is essential for FGF9-induced lung adenocarcinoma
Epithelial FGFR2 is required for lung branching morphogenesis (41). FGFR3 and FGFR4, together, regulate alveolar septation in the neonatal lung (42). In adult lung, immunostaining showed that FGFR2 and FGFR3 are expressed in lung epithelium and in FGF9-induced adenocarcinoma (Figure 7A-D). Furthermore, following induction of FGF9, FGFR3-expressing cells in the BADJ also co-express Sca-1 (Supplementary Figure 3). Since over 90% of human NSCLC cell lines were found to express FGFR3b (43) and FGFR3b is a unique target for the FGF9 sub-family (44), we hypothesized that FGFR3 could mediate the in vivo epithelial response to FGF9.
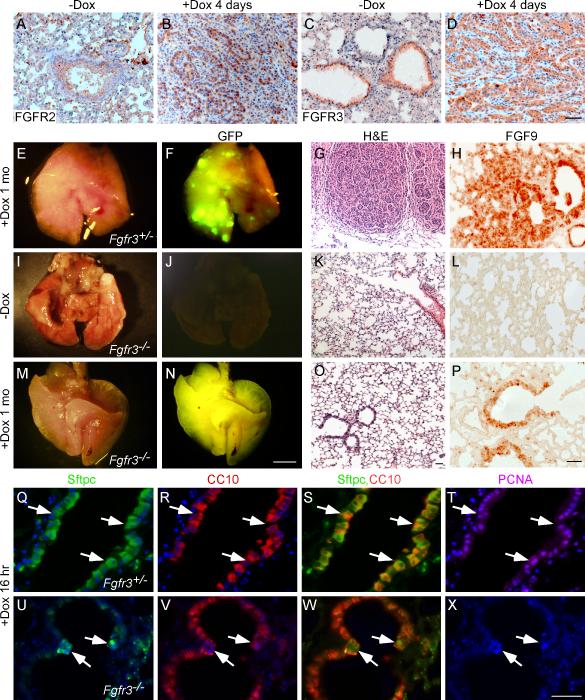
Figure 7.
FGFR3 is essential for FGF9-induced lung adenocarcinoma. (A-D) immunohistochemistry staining for FGFR2 (A, B) and FGFR3 (C, D) showing that both FGFRs are expressed in lung epithelial cells and tumors from Sftpc-rtTA, Tre-Fgf9-ires-eGfp control mice and mice induced with doxycycline for four days, respectively. (E, I, M) Images of whole lungs from Sftpc-rtTA, Tre-Fgf9-ires-eGfp mice lacking either one allele (Fgfr3+/−) or both alleles (Fgfr3−/−) of Fgfr3, as indicated. Mice were either not induced (I) or induced with doxycycline for one month (E, M). (F, J, N) Corresponding fluorescent images showing eGFP expression. Lungs that are heterozygous for Fgfr3 show surface nodules, while lungs homozygous for the Fgfr3 null allele have a smooth surface appearance. (G, K, O) Representative histology (H&E) of the lungs shown in E, I, M. Note that SftpC-rtTA, Tre-Fgf9-ires-eGfp, Fgfr3−/− mice, induced with doxycycline for one month show normal histology while mice heterozygous for Fgfr3 develop adenocarcinoma. (H, L, P) Immunohistochemistry of the lungs in E, I, M showing FGF9 expression in tumor nodules (H) and in normal lung epithelium (P). (Q-X) Sftpc, CC10 and PCNA imunostaining of SftpC-rtTA, Tre-Fgf9-ires-eGfp, Fgfr3+/− and Fgfr3−/− lungs induced with doxycycline for one month. Lungs heterozygous for Fgfr3 showed a large increase in Sftpc, CC10 double-positive cells (arrows) (Q-S) and most of the double-positive cells are also positive for PCNA (T). In contrast, lungs that lacked Fgfr3 showed few Sftpc, CC10 double-positive cells (arrows) (U-W) and were negative for PCNA. DAPI (blue). Scale bars: D, O, P, X, 50 μm; N, 2.0 mm.
To evaluate the contribution of FGFR3 to FGF9-induced tumorigenesis, the Sftpc-rtTA, Tre-Fgf9-ires-eGfp alleles were mated with mice harboring a null allele of Fgfr3 (Fgfr3−) to generate double transgenic mice that are heterozygous or null for Fgfr3. Adult mice were then induced with doxycycline for variable lengths of time (16 hr to 6 months) and their lungs were examined for eGFP expression and for any histopathology. Induction of FGF9 expression in Fgfr3−/+ mice (n=20) resulted in the robust formation of tumor nodules after one week (not shown) or one month (Figure 7E-H) on doxycycline. In contrast, induction of FGF9 expression in Fgfr3−/− mice (total n=26) did not result in the formation of lung tumors, and lung histology remained normal after induction for one (Figure 7M-P, n=4) to six months (data not shown, n=4). Expression of the Tre-Fgf9-ires-eGfp transgene was monitored by GFP fluorescence and immunostaining for FGF9. After one month induction, Sftpc-rtTA, Tre-Fgf9-ires-eGfp, Fgfr3−/− lungs were positive for eGFP fluorescence and expressed immunoreactive FGF9 in airway epithelium even though they were histologically normal (Figure 7J,L,N,P).
We next evaluated the early response of distal bronchiolar epithelial cells to FGF9 expression. After 16 hr induction, lungs were examined for expression of Sftpc and CC10 and for any increased proliferation. Fgfr3−/+ mice responded to FGF9 induction with increased PCNA labeling and increased Sftpc, CC10 colabeling of cells in the distal bronchiolar epithelium (Figure 7Q-T). In contrast, Fgfr3−/− mice showed no increased cell proliferation and few double-positive cells in the distal bronchiolar epithelium (Figure 7U-X). These data demonstrate that FGFR3 is required for FGF9-induced activation of pre-existing CC10 positive cells in the distal bronchiolar epithelium and ensuing oncogenesis in adult lungs.
Discussion
Studies on primary human lung adenocarcinomas and derived cell lines revealed an association between FGF9 expression and tumorigenesis; however, little is known about the ability of FGF9 to directly induce lung tumors in vivo. To evaluate the oncogenic potential of FGF9 in adult lung, Fgf9 mRNA was selectively and conditionally expressed in distal lung epithelial cells and type II pneumocytes. Forced induction of Fgf9 rapidly caused multifocal papillary adenomas, which progressed to adenocarcinoma with the ability to metastasize. Although the cell of origin of these tumors cannot be unequivocally identified, we show that cells in the distal bronchiolar epithelium express FGFR3, rapidly enter the cell cycle in response to FGF9, and express markers (Sftpc, CC10, Sca-1) that are associated with progenitor-like cells. We also show that FGFR3 is absolutely essential for the response of distal bronchiolar epithelial cells to FGF9 and the formation of lung tumors. Importantly, after induction of FGF9 is stopped, the tumors persist, suggesting that they rapidly become independent of the initiating FGF9 stimulus. These data demonstrate that unlike other FGF ligands, FGF9 may uniquely target the expansion of lung epithelial progenitor-like cells and induce the formation of papillary adenomas that progress to adenocarcinoma.
Identification of the cell of origin of FGF9-induced lung lesions remains an important goal. Tumors may derive from alveolar type II pneumocytes, BASCs, or Clara cells. Examination of cell proliferation at early time points following FGF9 induction showed that the highest density of proliferating cells are located near the BADJ and 94% of these also express Sftpc and CC10. Nevertheless, 17% of type II pneumocytes were also PCNA positive. Between one and two days following FGF9 induction, papillary adenomas were associated with the BADJ and often occupied the distal bronchiole, again suggesting that some expanding epithelial tumors arise from distal bronchiolar epithelial cells. Supporting this model, clusters of proliferating type II pneumocytes peripheral to the BADJ were rarely observed. Careful sequential examination at early time points suggests the conversion of “facultative” Clara cells into Sftpc, CC10 double-positive cells following induction of FGF9. Ensuing papillary adenoma formation appeared to be contiguous with expansion of columnar distal bronchiolar epithelial cells that subsequently lose expression of CC10.
Similar to what is observed in this FGF9 induction model, increased numbers of Sftpc, CC10 double-positive cells have often been observed in mice with reduced expression of proteins that normally function to suppress cell proliferation, such as PTEN (45), MapK14 (i.e., p38α) (46), p27CK (47) and GATA-6 (48). Increased numbers of proliferating Sftpc, CC10 double-positive cells have been observed in response to K-ras activation (37, 40). However, Xu et al., concluded that Sftpc, CC10 double-positive cells only underwent hyperplasia and that tumors were most likely to arise from alveolar type II cells. Another possibility is that Sftpc, CC10 double-positive cells could progressively lose CC10 expression. Loss of CC10 expression has been associated with increased tumorigenicity in human NSCLC (49) and in mouse models of lung cancer (50).
Several mouse models for the role of FGFs in lung tumorigenesis have been described in which epithelial hyperplasia and/or adenoma were observed (31, 35, 36). In these models, expression of members of the Fgf7 subfamily (Fgf3, Fgf7, Fgf10) was induced in mouse lung using the Sftpc-rtTA regulatory system. However, in all these models, invasive cancer was not observed and the adenomatous hyperplasia was reversible upon withdrawal of doxycycline. In contrast, tumors arising from FGF9 induction do not appear to regress upon withdrawal of doxycycline. FGFs 3, 7, and 10 activate epithelial splice variants of FGFR1 and FGFR2, but do not have known activity towards FGFR3 (44). In contrast, FGF9 has the unique ability to activate both b and c splice variants of FGFR3 (44). We showed that FGFR3 is predominantly expressed in bronchiolar epithelial cells, which is consistent with published data showing high-level expression of Fgfr3 in adult rat lung (51). Collectively, these data suggest that FGF9 is activating an FGFR3-expressing lung epithelial progenitor-like cell that can then progress through oncogenic stages in the absence of the inducing ligand. This is in sharp contrast to FGFs 3, 7, and 10, which activate FGFR2-expressing lung epithelial cells that only undergo hyperplasia. Differences in FGF responsiveness may be due to the unique expression of FGFR3 in progenitor-like cells or differences in the signaling properties of FGFR2 and FGFR3 in progenitor-like cells or Clara cells. In support of a potential pathogenic role for FGFR3 in lung cancer, whole genome sequencing identified FGFR3 among 25 significantly mutated genes in human lung adenocarcinoma (2).
Induction of FGF9 expression in embryonic lung in vivo or addition of FGF9 to lung explant cultures in vitro results in mesenchymal proliferation and epithelial dilation, but no epithelial tumors (26, 33). However, these embryonic epithelial and mesenchymal responses to FGF9 are not mediated by FGFR3 because both control and Fgfr3−/− lung explant cultures showed a similar response to FGF9 in vitro (data not shown).
In summary, lung tumors robustly arise in mice with lung epithelial-specific induction of FGF9. The morphologic features in this mouse model are similar to human adenocarcinoma, and also closely reflect the rapid progression in a subset of human lung adenocarcinomas, providing a model that can be experimentally manipulated to resolve questions concerning the role of FGF9/FGFR3 in lung adenocarcinoma development. Furthermore, the FGFR-TK pathway has become increasingly recognized as an attractive target for cancer therapy (52), and the ability to induce FGF9 in a mouse model thus provides a powerful tool for assessing the short- and long-term efficacy of anti-FGF pathway molecules for cancer treatment and perhaps prevention (53).
Supplementary Material
Acknowledgement
We thank C. Smith and C. Ward for animal husbandry and genotyping and J. Engelbach for imaging. This work was funded by grants from the March of Dimes Foundation, NIH R01 HL111190, ImClone Systems, the Digestive Diseases Research Core Center Grant P30 DK052574 (transgenic mouse production), funds from the Department of Developmental Biology, the Alvin J. Siteman Cancer Center, the Mallinckrodt Institute of Radiology and a generous gift from Edward and Linda Ornitz.
Footnotes
Conflict of interest:
The authors do not have any actual, potential, or perceived conflict of interest with regard to this manuscript.
References
- 1.Ding L, Getz G, Wheeler DA, Mardis ER, McLellan MD, Cibulskis K, et al. Somatic mutations affect key pathways in lung adenocarcinoma. Nature. 2008;455:1069–75. doi: 10.1038/nature07423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Imielinski M, Berger AH, Hammerman PS, Hernandez B, Pugh TJ, Hodis E, et al. Mapping the hallmarks of lung adenocarcinoma with massively parallel sequencing. Cell. 2012;150:1107–20. doi: 10.1016/j.cell.2012.08.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Govindan R, Ding L, Griffith M, Subramanian J, Dees ND, Kanchi KL, et al. Genomic landscape of non-small cell lung cancer in smokers and never-smokers. Cell. 2012;150:1121–34. doi: 10.1016/j.cell.2012.08.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Lynch TJ, Bell DW, Sordella R, Gurubhagavatula S, Okimoto RA, Brannigan BW, et al. Activating mutations in the epidermal growth factor receptor underlying responsiveness of non-small-cell lung cancer to gefitinib. N Engl J Med. 2004;350:2129–39. doi: 10.1056/NEJMoa040938. [DOI] [PubMed] [Google Scholar]
- 5.Herbst RS, Heymach JV, Lippman SM. Lung cancer. N Engl J Med. 2008;359:1367–80. doi: 10.1056/NEJMra0802714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Sequist LV, Lynch TJ. EGFR tyrosine kinase inhibitors in lung cancer: an evolving story. Annu Rev Med. 2008;59:429–42. doi: 10.1146/annurev.med.59.090506.202405. [DOI] [PubMed] [Google Scholar]
- 7.Oxnard GR, Arcila ME, Chmielecki J, Ladanyi M, Miller VA, Pao W. New strategies in overcoming acquired resistance to epidermal growth factor receptor tyrosine kinase inhibitors in lung cancer. Clin Cancer Res. 2011;17:5530–7. doi: 10.1158/1078-0432.CCR-10-2571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Semrad TJ, Mack PC. Fibroblast growth factor signaling in non-small-cell lung cancer. Clin Lung Cancer. 2012;13:90–5. doi: 10.1016/j.cllc.2011.08.001. [DOI] [PubMed] [Google Scholar]
- 9.Turner N, Grose R. Fibroblast growth factor signalling: from development to cancer. Nat Rev Cancer. 2010;10:116–29. doi: 10.1038/nrc2780. [DOI] [PubMed] [Google Scholar]
- 10.Kuhn H, Kopff C, Konrad J, Riedel A, Gessner C, Wirtz H. Influence of basic fibroblast growth factor on the proliferation of non-small cell lung cancer cell lines. Lung Cancer. 2004;44:167–74. doi: 10.1016/j.lungcan.2003.11.005. [DOI] [PubMed] [Google Scholar]
- 11.Donnem T, Al-Shibli K, Al-Saad S, Busund LT, Bremnes RM. Prognostic Impact of Fibroblast Growth Factor 2 in Non-small Cell Lung Cancer: Coexpression with VEGFR-3 and PDGF-B Predicts Poor Survival. J Thorac Oncol. 2009;4:578–85. doi: 10.1097/JTO.0b013e31819f2e38. [DOI] [PubMed] [Google Scholar]
- 12.Iwasaki A, Kuwahara M, Yoshinaga Y, Shirakusa T. Basic fibroblast growth factor (bFGF) and vascular endothelial growth factor (VEGF) levels, as prognostic indicators in NSCLC. Eur J Cardiothorac Surg. 2004;25:443–8. doi: 10.1016/j.ejcts.2003.11.031. [DOI] [PubMed] [Google Scholar]
- 13.Li ZG, Mathew P, Yang J, Starbuck MW, Zurita AJ, Liu J, et al. Androgen receptor- negative human prostate cancer cells induce osteogenesis in mice through FGF9-mediated mechanisms. J Clin Invest. 2008;118:2697–710. doi: 10.1172/JCI33093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Marek L, Ware KE, Fritzsche A, Hercule P, Helton WR, Smith JE, et al. Fibroblast growth factor (FGF) and FGF receptor-mediated autocrine signaling in non-small-cell lung cancer cells. Mol Pharmacol. 2009;75:196–207. doi: 10.1124/mol.108.049544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Abdel-Rahman WM, Kalinina J, Shoman S, Eissa S, Ollikainen M, Elomaa O, et al. Somatic FGF9 mutations in colorectal and endometrial carcinomas associated with membranous beta-catenin. Hum Mutat. 2008;29:390–7. doi: 10.1002/humu.20653. [DOI] [PubMed] [Google Scholar]
- 16.Hendrix ND, Wu R, Kuick R, Schwartz DR, Fearon ER, Cho KR. Fibroblast growth factor 9 has oncogenic activity and is a downstream target of Wnt signaling in ovarian endometrioid adenocarcinomas. Cancer Res. 2006;66:1354–62. doi: 10.1158/0008-5472.CAN-05-3694. [DOI] [PubMed] [Google Scholar]
- 17.Kang J, Lee SY, Kim YJ, Park JY, Kwon SJ, Na MJ, et al. microRNA-99b acts as a tumor suppressor in non-small cell lung cancer by directly targeting fibroblast growth factor receptor 3. Exp Ther Med. 2012;3:149–53. doi: 10.3892/etm.2011.366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Dutt A, Ramos AH, Hammerman PS, Mermel C, Cho J, Sharifnia T, et al. Inhibitor-sensitive FGFR1 amplification in human non-small cell lung cancer. PLoS One. 2011;6:e20351. doi: 10.1371/journal.pone.0020351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Weiss J, Sos ML, Seidel D, Peifer M, Zander T, Heuckmann JM, et al. Frequent and Focal FGFR1 Amplification Associates with Therapeutically Tractable FGFR1 Dependency in Squamous Cell Lung Cancer. Sci Transl Med. 2010;2:62ra93. doi: 10.1126/scitranslmed.3001451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ware KE, Marshall ME, Heasley LR, Marek L, Hinz TK, Hercule P, et al. Rapidly Acquired Resistance to EGFR Tyrosine Kinase Inhibitors in NSCLC Cell Lines through De-Repression of FGFR2 and FGFR3 Expression. PLoS One. 2010;5:e14117. doi: 10.1371/journal.pone.0014117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Wang CK, Chang H, Chen PH, Chang JT, Kuo YC, Ko JL, et al. Aryl hydrocarbon receptor activation and overexpression upregulated fibroblast growth factor-9 in human lung adenocarcinomas. Int J Cancer. 2009;125:807–15. doi: 10.1002/ijc.24348. [DOI] [PubMed] [Google Scholar]
- 22.Ueng TH, Hung CC, Kuo ML, Chan PK, Hu SH, Yang PC, et al. Induction of Fibroblast Growth Factor-9 and Interleukin-1{alpha} Gene Expression by Motorcycle Exhaust Particulate Extracts and Benzo(a)pyrene in Human Lung Adenocarcinoma Cells. Toxicol Sci. 2005;87:483–96. doi: 10.1093/toxsci/kfi251. [DOI] [PubMed] [Google Scholar]
- 23.Ueng TH, Chang YL, Tsai YY, Su JL, Chan PK, Shih JY, et al. Potential roles of fibroblast growth factor-9 in the benzo(a)pyrene-induced invasion in vitro and the metastasis of human lung adenocarcinoma. Arch Toxicol. 2010 doi: 10.1007/s00204-010-0547-3. [DOI] [PubMed] [Google Scholar]
- 24.Perl AK, Wert SE, Nagy A, Lobe CG, Whitsett JA. Early restriction of peripheral and proximal cell lineages during formation of the lung. Proc Natl Acad Sci U S A. 2002;99:10482–7. doi: 10.1073/pnas.152238499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Soriano P. Generalized lacZ expression with the ROSA26 Cre reporter strain. Nat Genet. 1999;21:70–1. doi: 10.1038/5007. [DOI] [PubMed] [Google Scholar]
- 26.White AC, Xu J, Yin Y, Smith C, Schmid G, Ornitz DM. FGF9 and SHH signaling coordinate lung growth and development through regulation of distinct mesenchymal domains. Development. 2006;133:1507–17. doi: 10.1242/dev.02313. [DOI] [PubMed] [Google Scholar]
- 27.Perl AK, Tichelaar JW, Whitsett JA. Conditional gene expression in the respiratory epithelium of the mouse. Transgenic Res. 2002;11:21–9. doi: 10.1023/a:1013986627504. [DOI] [PubMed] [Google Scholar]
- 28.Colvin JS, Bohne BA, Harding GW, McEwen DG, Ornitz DM. Skeletal overgrowth and deafness in mice lacking fibroblast growth factor receptor 3. Nat Genet. 1996;12:390–7. doi: 10.1038/ng0496-390. [DOI] [PubMed] [Google Scholar]
- 29.Teisanu RM, Lagasse E, Whitesides JF, Stripp BR. Prospective isolation of bronchiolar stem cells based upon immunophenotypic and autofluorescence characteristics. Stem Cells. 2009;27:612–22. doi: 10.1634/stemcells.2008-0838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Garbow JR, Dugas JP, Song SK, Conradi MS. A Simple, Robust Hardware Device for Passive or Active Respiratory Gating in MRI and MRS Experiments. Concepts Magn Reson. 2004;21B:40–8. [Google Scholar]
- 31.Tichelaar JW, Lu W, Whitsett JK. Conditional expression of fibroblast growth factor-7 in the developing and mature lung. J Biol Chem. 2000;275:11858–64. doi: 10.1074/jbc.275.16.11858. [DOI] [PubMed] [Google Scholar]
- 32.del Moral PM, De Langhe SP, Sala FG, Veltmaat JM, Tefft D, Wang K, et al. Differential role of FGF9 on epithelium and mesenchyme in mouse embryonic lung. Dev Biol. 2006;293:77–89. doi: 10.1016/j.ydbio.2006.01.020. [DOI] [PubMed] [Google Scholar]
- 33.Yin Y, White AC, Huh SH, Hilton MJ, Kanazawa H, Long F, et al. An FGF-WNT gene regulatory network controls lung mesenchyme development. Dev Biol. 2008;319:426–36. doi: 10.1016/j.ydbio.2008.04.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Yoo JY, Yang SH, Lee JE, Cho DG, Kim HK, Kim SH, et al. E-cadherin as a predictive marker of brain metastasis in non-small-cell lung cancer, and its regulation by pioglitazone in a preclinical model. J Neurooncol. 2012 doi: 10.1007/s11060-012-0890-8. [DOI] [PubMed] [Google Scholar]
- 35.Clark JC, Tichelaar JW, Wert SE, Itoh N, Perl AK, Stahlman MT, et al. FGF-10 disrupts lung morphogenesis and causes pulmonary adenomas in vivo. Am J Physiol Lung Cell Mol Physiol. 2001;280:L705–15. doi: 10.1152/ajplung.2001.280.4.L705. [DOI] [PubMed] [Google Scholar]
- 36.Zhao B, Chua SS, Burcin MM, Reynolds SD, Stripp BR, Edwards RA, et al. Phenotypic consequences of lung-specific inducible expression of FGF-3. Proc Natl Acad Sci U S A. 2001;98:5898–903. doi: 10.1073/pnas.101116598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Kim CF, Jackson EL, Woolfenden AE, Lawrence S, Babar I, Vogel S, et al. Identification of bronchioalveolar stem cells in normal lung and lung cancer. Cell. 2005;121:823–35. doi: 10.1016/j.cell.2005.03.032. [DOI] [PubMed] [Google Scholar]
- 38.Rawlins EL, Okubo T, Xue Y, Brass DM, Auten RL, Hasegawa H, et al. The role of Scgb1a1+ Clara cells in the long-term maintenance and repair of lung airway, but not alveolar, epithelium. Cell Stem Cell. 2009;4:525–34. doi: 10.1016/j.stem.2009.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.McQualter JL, Yuen K, Williams B, Bertoncello I. Evidence of an epithelial stem/progenitor cell hierarchy in the adult mouse lung. Proc Natl Acad Sci U S A. 2010;107:1414–9. doi: 10.1073/pnas.0909207107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Xu X, Rock JR, Lu Y, Futtner C, Schwab B, Guinney J, et al. Evidence for type II cells as cells of origin of K-Ras-induced distal lung adenocarcinoma. Proc Natl Acad Sci U S A. 2012;109:4910–5. doi: 10.1073/pnas.1112499109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Abler LL, Mansour SL, Sun X. Conditional gene inactivation reveals roles for Fgf10 and Fgfr2 in establishing a normal pattern of epithelial branching in the mouse lung. Dev Dyn. 2009;238:1999–2013. doi: 10.1002/dvdy.22032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Weinstein M, Xu X, Ohyama K, Deng CX. FGFR-3 and FGFR-4 function cooperatively to direct alveogenesis in the murine lung. Development. 1998;125:3615–23. doi: 10.1242/dev.125.18.3615. [DOI] [PubMed] [Google Scholar]
- 43.Fischer H, Taylor N, Allerstorfer S, Grusch M, Sonvilla G, Holzmann K, et al. Fibroblast growth factor receptor-mediated signals contribute to the malignant phenotype of non-small cell lung cancer cells: therapeutic implications and synergism with epidermal growth factor receptor inhibition. Mol Cancer Ther. 2008;7:3408–19. doi: 10.1158/1535-7163.MCT-08-0444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Zhang X, Ibrahimi OA, Olsen SK, Umemori H, Mohammadi M, Ornitz DM. Receptor specificity of the fibroblast growth factor family. The complete mammalian FGF family. J Biol Chem. 2006;281:15694–700. doi: 10.1074/jbc.M601252200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Yanagi S, Kishimoto H, Kawahara K, Sasaki T, Sasaki M, Nishio M, et al. Pten controls lung morphogenesis, bronchioalveolar stem cells, and onset of lung adenocarcinomas in mice. J Clin Invest. 2007;117:2929–40. doi: 10.1172/JCI31854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Ventura JJ, Tenbaum S, Perdiguero E, Huth M, Guerra C, Barbacid M, et al. p38alpha MAP kinase is essential in lung stem and progenitor cell proliferation and differentiation. Nat Genet. 2007;39:750–8. doi: 10.1038/ng2037. [DOI] [PubMed] [Google Scholar]
- 47.Besson A, Hwang HC, Cicero S, Donovan SL, Gurian-West M, Johnson D, et al. Discovery of an oncogenic activity in p27Kip1 that causes stem cell expansion and a multiple tumor phenotype. Genes Dev. 2007;21:1731–46. doi: 10.1101/gad.1556607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Zhang Y, Goss AM, Cohen ED, Kadzik R, Lepore JJ, Muthukumaraswamy K, et al. A Gata6-Wnt pathway required for epithelial stem cell development and airway regeneration. Nat Genet. 2008;40:862–70. doi: 10.1038/ng.157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Linnoila RI, Szabo E, DeMayo F, Witschi H, Sabourin C, Malkinson A. The role of CC10 in pulmonary carcinogenesis: from a marker to tumor suppression. Ann N Y Acad Sci. 2000;923:249–67. doi: 10.1111/j.1749-6632.2000.tb05534.x. [DOI] [PubMed] [Google Scholar]
- 50.Hicks SM, Vassallo JD, Dieter MZ, Lewis CL, Whiteley LO, Fix AS, et al. Immunohistochemical analysis of Clara cell secretory protein expression in a transgenic model of mouse lung carcinogenesis. Toxicology. 2003;187:217–28. doi: 10.1016/s0300-483x(03)00060-x. [DOI] [PubMed] [Google Scholar]
- 51.Claus P, Grothe C. Molecular cloning and developmental expression of rat fibroblast growth factor receptor 3. Histochem Cell Biol. 2001;115:147–55. doi: 10.1007/s004180000215. [DOI] [PubMed] [Google Scholar]
- 52.Lamont FR, Tomlinson DC, Cooper PA, Shnyder SD, Chester JD, Knowles MA. Small molecule FGF receptor inhibitors block FGFR-dependent urothelial carcinoma growth in vitro and in vivo. Br J Cancer. 2011;104:75–82. doi: 10.1038/sj.bjc.6606016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Olive KP, Tuveson DA. The use of targeted mouse models for preclinical testing of novel cancer therapeutics. Clin Cancer Res. 2006;12:5277–87. doi: 10.1158/1078-0432.CCR-06-0436. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.