Abstract
A single nucleotide polymorphism (SNP) in the α5 nicotinic acetylcholine receptor subunit gene, rs16969968, has been repeatedly associated with both smoking and respiratory health phenotypes. However, there remains considerable debate as to whether associations with lung cancer are mediated through effects on smoking behavior. Preclinical studies suggest that α5 receptor subunit expression and function may play a direct role in nicotine titration during self-administration. The present study investigated the association of CHRNA5 polymorphisms and smoking topography in 66 smokers asked to smoke 4 nicotine containing (nicotine yield = .60 mg) and 4 placebo (nicotine yield < .05 mg) cigarettes, during separate experimental sessions. Genotype at rs16969968 predicted nicotine titration, with homozygotes for the major allele (G:G) displaying significantly reduced puff volume in response to nicotine, while minor allele carriers (A:G or AA) produced equivalent puff volumes for placebo and nicotine cigarettes. The present results suggest that puff volume may be a more powerful objective phenotype of smoking behavior than self-reported cigarettes per day and nicotine dependence. Further, these results suggest that the association between rs16969968 and lung cancer may be mediated by the quantity of smoke inhaled.
Genome-wide association studies (GWAS) of tobacco smoking have consistently identified strong signals from polymorphisms in the long arm of chromosome 151. Most notably, polymorphisms in a cluster of genes coding for the α5, α3, and β4 nicotinic acetylcholine receptor (nAChR) subunits are associated with a variety of smoking-related phenotypes and health outcomes2. The first GWAS specific to nicotine dependence identified a strong association with a single nucleotide polymorphism (SNP) in the α5 receptor subunit gene at rs169699683. Homozygotes for the minor allele (i.e., A:A) were nearly twice as likely to be nicotine dependent as heterozygotes (A:G) or those without a minor allele (G:G). This SNP has since received considerable attention because of its biological relevance as a missense polymorphism; the minor allele produces an amino acid substitution in the α5 nAChR subunit protein (Asn398Asp) which reduces the Ca2+ permeability of certain nAChRs that incorporate the α5 subunit4, 5.
Subsequent studies have confirmed the association of rs16969968 with smoking status (e.g., smokers vs. non-smokers)4, 6-8, nicotine dependence3, 9-12, and cigarettes smoked per day (cpd)10, 13. In each case, the minor allele has been associated with increased risk for the smoking phenotype, with recessive3, 11, 13 or additive6, 7, 10 effects. Given the well-documented relationship between rs16969968 and smoking, it is not surprising that this SNP is also linked to respiratory health problems such as lung cancer2, 8, 13-17 and COPD8, 14. However, it has been argued that the association between this variant and lung cancer risk is not substantially mediated by changes in smoking intensity18. The majority of this work has used broad and subjective measures to define smoking behavior (e.g., cpd and pack years smoked) 2, 19. Measures that rely solely on self-report may not be as reliable, or sensitive to genetic effects, as objective measures of smoking. Additionally, such measures do not account for variation between individuals regarding nicotine and carcinogen exposure from each cigarette20, 21. Consequently, more objective measures of smoking behavior are needed to better estimate variation in health risk as a function of rs16969968 genotype2, 19.
Recent work has incorporated an examination of smokers' exposure to toxicants as objective measures of tobacco use. Following a single cigarette, higher levels of plasma nicotine and a tobacco-specific carcinogen are observed among carriers of a minor allele at rs16969968, relative to non-carriers22. In another study, higher levels of cotinine, a nicotine metabolite, were observed amongst rs16969968 minor allele carriers (or rs1051730; a proxy SNP for rs16969968 in Caucasian populations), even when controlling for cpd. As expected, this SNP was also more strongly associated with cotinine levels than with self-reported cpd19. These studies demonstrate that rs16969968 predicts aspects of smoking not accounted for by more global measures (e.g., cpd). Rather, more proximal and objective measures of smoking behavior (i.e., endophenotypes) may help to clarify the association of this SNP with lung cancer. Yet, additional work is needed to determine the mechanism that accounts for differences observed in toxicant exposure between genotypes.
Given evidence that smokers can adjust the nicotine dose delivered from a cigarette by altering their smoking pattern (e.g., by puffing longer or deeper)23, it is plausible that smokers with a risk genotype inhale more toxicants by smoking each cigarette more intensively than non-carriers. This idea converges with pre-clinical work demonstrating α5 receptor involvement in nicotine self-administration24. For example, mice with a null mutation of the α5 receptor gene (Chrna5) self-administered more doses than wild type controls when nicotine was delivered in moderate to high concentrations, but not for low or placebo concentrations24. Unlike wild-type controls, knockouts failed to reduce rates of nicotine administration when nicotine dose concentration was increased beyond moderate levels. Thus, polymorphisms that interfere with the function of the α5 subunit in smokers may similarly alter the self-administration of nicotine delivered via cigarette smoking. A precise measure of nicotine self-administration in humans is smoking topography: puff number, volume, duration, and inter-puff-interval per cigarette. Compared to self-reported cpd, smokers' puff topography better predicts exposure to toxicants such as nicotine, carbon monoxide, and carcinogens25-27 and thus may serve as an endophenotype for smoking behavior and respiratory health.
Using data from our previously published work28, the present study sought to examine the influence of α5 receptor gene SNPs on smokers' puff topography. For this study, the topography outcome measure of interest was total puff volume per cigarette. It was hypothesized that minor allele carriers at rs16969968 would smoke nicotine-containing cigarettes more intensively (larger total puff volumes) than non-carriers, as is suggested by prior studies which have demonstrated the association of rs16969968 with nicotine and carcinogen exposure19, 22. Consistent with the nicotine self-administration data provided from pre-clinical genetic studies24, 29, 30, we expected no relationship between genotype and puff volume in response to placebo cigarettes. In addition, we explored the association of several other non-coding SNPs in CHRNA5 (rs11637635, rs17408276, rs3829787, rs4275821, rs588765, rs569207, & rs684513) with smokers' total puff volume per cigarette. Although the functional effects of these SNPs are not currently understood, each has been shown to predict smoking and/or risk of respiratory disease8, 9, 31-40.
Method
Participants
Eighty-three current cigarette smokers were recruited from the Tampa Bay area for a study investigating the effects of nicotine dose on neural indices of attention (the results of this primary study are not reported here). Eligible participants were required to be between the ages of 18-70 years and to have smoked 15 or more cpd for the past 2 years (biochemically verified by expired air carbon monoxide levels ≥ 10 ppm and urinary cotinine level ≥ 100 ng/mL). Participants were excluded from the study if they reported using nicotine containing products other than cigarettes within the past 3 months; were currently attempting to quit smoking (including use of smoking cessation medications); tested positive for psychoactive drug use or pregnancy; met criteria for a DSM-IV Axis I disorder (i.e., psychosis, major depressive episode, manic/hypomanic episode, panic disorder, current alcohol or substance abuse) as assessed by the Structured Clinical Interview for DSM disorders (SCID)41; reported any past head injury or loss of consciousness; reported any serious medical conditions such as cancer or cardiopulmonary disease; or were unable to read and understand the consent forms or questionnaires. This sample has been used previously to describe the influence of cigarette nicotine content on smoking topography28. Data was collected during a period from January, 2009 to May, 2012.
Procedure
An initial screening session was required to complete informed consent and establish eligibility status. During this session, participants provided demographic data and self-report measures related to smoking behavior, including the Fagerström Test for Nicotine Dependence (FTND)42. Participants were then scheduled to attend two 2.5 hour experimental sessions, each of which was preceded by overnight (i.e., 12 hours) abstinence from use of nicotine/tobacco (CO level ≤ 10 ppm or no greater than half of their CO level at the initial screening session) and alcohol (blood alcohol level <.001%). During each double-blind and counterbalanced session, participants were required to smoke either nicotine-containing (Quest 1, 8.9 mg) or placebo (Quest 3, 1.0 mg) cigarettes (Vector Tobacco Inc, Research Triangle Park, NC.). Four of the condition-assigned cigarettes were smoked ad libitum through a mouthpiece that was connected to a smoking topography device. Initiation of each cigarette was spaced approximately 40 minutes apart, and followed by the completion of the Modified Cigarette Evaluation Questionnaire (mCEQ)43. The participant was fitted with an electroencephalogram (EEG) cap as part of the primary study between smoking bouts 1 and 2 and was required to undergo tasks of attention and working-memory between smoking bouts 2 and 3 and bouts 3 and 4. This study was approved by the Moffitt Scientific Review Committee and the institutional review board of the University of South Florida. As such, it was conducted in accordance with the standards outlined in the 1964 Declaration of Helsinki.
Measures
Genetics
Buccal cells were collected for genotyping. Participants were required to rinse their mouths with water, use a tongue depressor to gently scrape the inside of their cheeks and tongue, and then rinse their mouth with saline solution.
Smoking topography
Cigarettes were smoked through a mouthpiece connected to a pressure transducer, via the Clinical Research Support System (Borgwaldt, KC, Richmond VA). Inhalation-induced pressure changes were amplified, digitized, and sampled at a rate of 1000 Hz, and software converted signals to air flow (ml/sec) for data integration. This device is effective for quantifying smoke exposure and has negligible effects on smoking behavior25, 44.
Data Analyses
Genotyping
Genomic DNA was extracted from buccal cells using the Gentra Puregene tissue kit (Qiagen, Valencia, CA) according to the manufacturer's protocol. DNA samples were genotyped using the Illumina GoldenGate™ assay (Illumina, San Diego, CA) and were called using the BeadStudio algorithm at the Moffitt Cancer Center's Molecular Genomic Core.
Statistical analysis
The primary analysis investigated the effects of minor allele carrier status at rs16969968 and cigarette nicotine content on total puff volume. Secondary analyses tested this same effect for polymorphisms at the following non-coding SNPs: rs11637635, rs17408276, rs3829787, rs4275821, rs588765, rs569207, and rs684513. Because our sample contained relatively few individuals homozygous for the minor allele with regard to several of our SNPs (i.e., 4.50% for rs16969968), genotype was dichotomized to increase statistical power. That is, minor allele carriers (i.e., heterozygotes and minor homozygotes) were compared with non-carriers (i.e., major homozygous).
To examine SNP effects and potential interactions with cigarette nicotine content, we used mixed-model repeated measures analyses with a scaled identity covariance structure. Specifically, models included fixed effects for genotype, nicotine content (nicotine vs. placebo), and the interaction of these two factors, with cigarette trial as a covariate and random effect. Bonferroni-corrected planned comparisons were then conducted to further characterize interactive effects that included genotype (i.e., genotype or genotype X nicotine content). All models were also reexamined while controlling for other significant predictors of puff topography (e.g., FTND, race, and ethnicity), and are reported below.
Results
Sample Characteristics
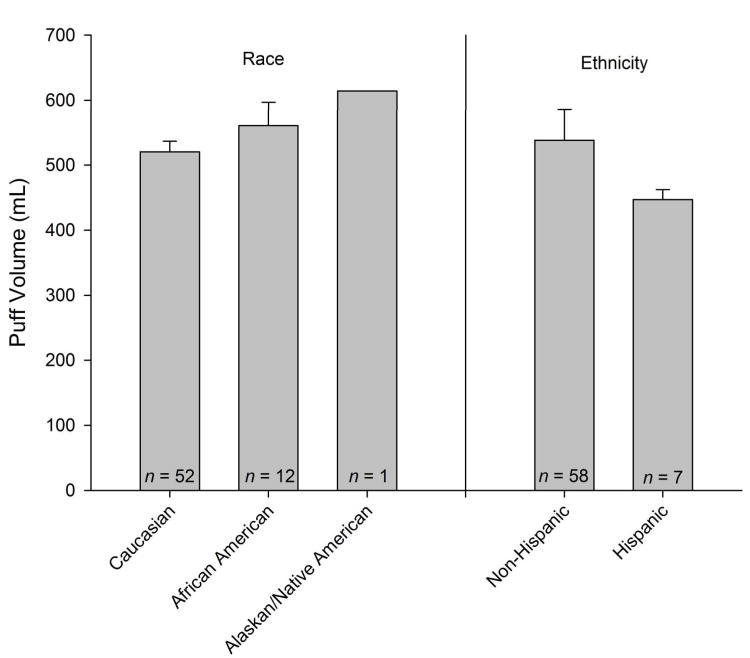
Seventeen participants were excluded from the analysis due to either procedural errors in the smoking topography equipment (n = 16) or missing genotype data (n = 1). The remaining 66 participants (50 males),self-identified their race as Caucasian (n = 52), African American (n = 12), or American Indian or Alaskan Native (n = 1). One participant did not identify a racial background. Seven participants self-identified their ethnicity as Hispanic, while the remainder identified as non-Hispanic (n = 58), or did not report (n = 1). Participants had an average age of 39.6 (SD = 12.1) years, smoked 22.5 (SD = 6.9) cpd, and had a moderate nicotine dependence score of 5.77 (SD = 1.87) on the FTND. Table 1 presents the frequencies of carrier status across all SNPs. Generally, there were no carrier status differences in self-reported smoking measures. However, minor allele carriers at rs11637635 [t(60) = 2.06, p = .04] and rs17408276 [t(60) = 2.57, p = .01] showed lower levels of nicotine dependence as assessed by the FTND. Minor carriers at rs11637635 [t(64) = 2.08, p = .04], rs17408276 [t(64) = 2.41, p = .02] and rs588765 [t (64) = 2.11, p = .04] also reported smoking fewer cpd. Additionally, Caucasians were more likely to carry a minor allele at rs17408276 [ χ2 (1, N = 65) = 11.29, p = .004], rs3829787 [ χ2 (1, N = 65) = 16.25, p < .001], and rs4275821 [ χ2 (1, N = 65) = 12.57, p = .002]. Minor allele frequencies (MAF) for each SNP are presented in Table 2.
Table 1.
Results for total puff volume across all SNPs with nicotine content and genotype effects, controlling for cigarette trial and nicotine dependence (FTND). Carriers are defined as individuals with at least one copy of the minor allele. M = mean, SE = standard error. Gene, and Gene × Nicotine effects which met traditional significance (p < .05) are presented in bold.
| Cigarette Type | ||||||
|---|---|---|---|---|---|---|
|
| ||||||
| Nicotine | Placebo | |||||
|
| ||||||
| SNP | Minor Allele | Frequency (%) | M | (SE) | M | (SE) |
| rs16969968 | Non Carrier | 59.4 | 467.86 | 21.03 | 531.75 | 21.10 |
| Carrier | 40.6 | 536.36 | 25.38 | 537.42 | 25.71 | |
| rs11637635 | Non Carrier | 28.1 | 507.07 | 25.33 | 559.13 | 26.06 |
| Carrier | 71.9 | 477.41 | 22.82 | 510.42 | 22.75 | |
| rs17408276 | Non Carrier | 31.2 | 498.44 | 24.52 | 546.52 | 25.13 |
| Carrier | 68.8 | 482.38 | 24.16 | 516.04 | 24.08 | |
| rs3829787 | Non Carrier | 29.7 | 513.51 | 24.25 | 562.63 | 24.88 |
| Carrier | 70.3 | 464.26 | 24.03 | 498.03 | 23.97 | |
| rs4275821 | Non Carrier | 28.1 | 520.99 | 24.37 | 580.53 | 25.07 |
| Carrier | 71.9 | 458.48 | 22.99 | 488.87 | 22.93 | |
| rs588765 | Non Carrier | 23.4 | 515.30 | 26.79 | 552.55 | 27.72 |
| Carrier | 76.6 | 475.90 | 22.62 | 514.45 | 22.56 | |
| rs637137 | Non Carrier | 65.6 | 497.00 | 22.05 | 525.22 | 22.41 |
| Carrier | 34.4 | 481.42 | 25.08 | 538.46 | 24.66 | |
| rs684513 | Non Carrier | 73.4 | 493.85 | 21.45 | 524.59 | 21.75 |
| Carrier | 26.6 | 484.25 | 27.472 | 542.88 | 26.798 | |
| Overall | Nicotine | Gene | Gene × Nicotine | ||||
|---|---|---|---|---|---|---|---|
|
| |||||||
| M | (SE) | F | p | F | p | F | p |
| 499.80 | 19.68 | 7.63 | 0.006 | 3.05 | 0.083 | 7.14 | 0.008 |
| 536.89 | 23.89 | ||||||
| 533.10 | 23.17 | 10.61 | 0.001 | 2.51 | 0.115 | 0.53 | 0.466 |
| 493.92 | 21.73 | ||||||
| 522.48 | 22.50 | 10.47 | 0.001 | 0.82 | 0.366 | 0.33 | 0.568 |
| 499.21 | 23.08 | ||||||
| 538.07 | 22.07 | 10.44 | 0.001 | 4.99 | 0.027 | 0.36 | 0.550 |
| 481.14 | 22.97 | ||||||
| 550.76 | 22.09 | 11.89 | 0.001 | 9.82 | 0.002 | 1.25 | 0.264 |
| 473.67 | 21.91 | ||||||
| 533.93 | 24.34 | 7.38 | 0.007 | 2.18 | 0.141 | 0.00 | 0.963 |
| 495.17 | 21.60 | ||||||
| 511.11 | 21.05 | 12.01 | 0.001 | 0.00 | 0.958 | 1.37 | 0.242 |
| 509.94 | 22.77 | ||||||
| 509.22 | 20.52 | 11.22 | 0.001 | 0.03 | 0.855 | 1.09 | 0.296 |
| 513.56 | 24.58 | ||||||
Table 2.
Minor allele frequency, puff volume and demographic characteristics by racial and ethnic group. Means are presented for puff volumes and demographic values with standard deviation expressed in parentheses. CPD = cigarettes per day.
| Race | |||
|---|---|---|---|
|
| |||
| Minor Allele | Caucasian | African American | |
|
| |||
| N (% of sample) | 52 (78.79) | 12 (18.18) | |
| MAF | |||
| rs16969968 | A (A/G) | 0.25 | 0.08 |
| rs11637635 | A (A/G) | 0.48 | 0.25 |
| rs17408276 | C (C/T) | 0.48 | 0.13 |
| rs3829787 | A (A/G) | 0.48 | 0.08 |
| rs4275821 | C (C/T) | 0.48 | 0.17 |
| rs569207 | A (A/G) | 0.20 | 0.25 |
| rs588765 | T (T/C) | 0.54 | 0.29 |
| rs637137 | A (A/T) | 0.20 | 0.25 |
| rs684513 | G (G/C) | 0.16 | 0.13 |
| Demographic | |||
| Age | 39.40 (12.15) | 42.42 (12.34) | |
| CPD | 22.17 (6.35) | 24.42 (8.53) | |
| FTND | 5.48 (1.80) | 6.92 (1.62) | |
| Puff Volume | |||
| Nicotine | 500.69 (167.46) | 522.61 (106.37) | |
| Placebo | 530.881 (118.70) | 639.96 (124.60) | |
| All | 520.36 (116.66) | 560.59 (126.44) | |
|
| |||
| Ethnicity | |||
|
| |||
| American Indian or Alaskan Native | Non-Hispanic | Hispanic | |
|
| |||
| 1 (1.52) | 58 (87.88) | 7 (10.61) | |
|
| |||
| 0.50 | 0.22 | 0.21 | |
| 0.50 | 0.45 | 0.43 | |
| 0.50 | 0.42 | 0.43 | |
| 0.50 | 0.42 | 0.43 | |
| 0.50 | 0.44 | 0.43 | |
| 0.00 | 0.19 | 0.29 | |
| 0.50 | 0.51 | 0.43 | |
| 0.00 | 0.19 | 0.29 | |
| 0.00 | 0.14 | 0.29 | |
| 32.00 | 39.67 (11.90) | 38.29 (15.11) | |
| 25.00 | 22.48 (6.67) | 22.71 (8.56) | |
| 9.00 | 5.83 (1.83) | 5.29 (2.43) | |
| 638.14 | 516.53 (160.09) | 413.02 (106.19) | |
| 590.31 | 555.83 (119.87) | 483.11 (149.06) | |
| 614.22 | 538.20 (115.17) | 447.17 (126.57) | |
Predictors of total puff volume
Ethnicity, race, FTND, cpd, number of quit attempts over the past year predicted total puff volume (ps < .05). On average, total puff volumes were lower amongst Caucasians when compared with participants identifying with a different racial background (12 African Americans and 1 Native American/Alaskan). Hispanic ethnicity was associated with reduced puff volumes, and FTND was positively associated with total puff volume. Puff volumes for racial and ethnic subgroups are illustrated in Figure 1. Puff volume was not predicted by age, gender, age of 1st cigarette, age of regular smoking, age of daily smoking, highest number of cpd, or cessation confidence. To control for the general effects of race, ethnicity, and nicotine dependence on total puff volume, these variables were included as covariates in subsequent analyses. Two participants who did not report on either race or ethnicity were excluded from these analyses (final n = 64). FTND was chosen as a covariate because it is one of the best validated42 and widely used indices of nicotine dependence. Cigarettes per day and number of quit attempts were not included as covariates as they partially determine and are highly correlated with FTND.
Figure 1.
Mean ± SEM total puff volumes by racial and ethnic subgroup.
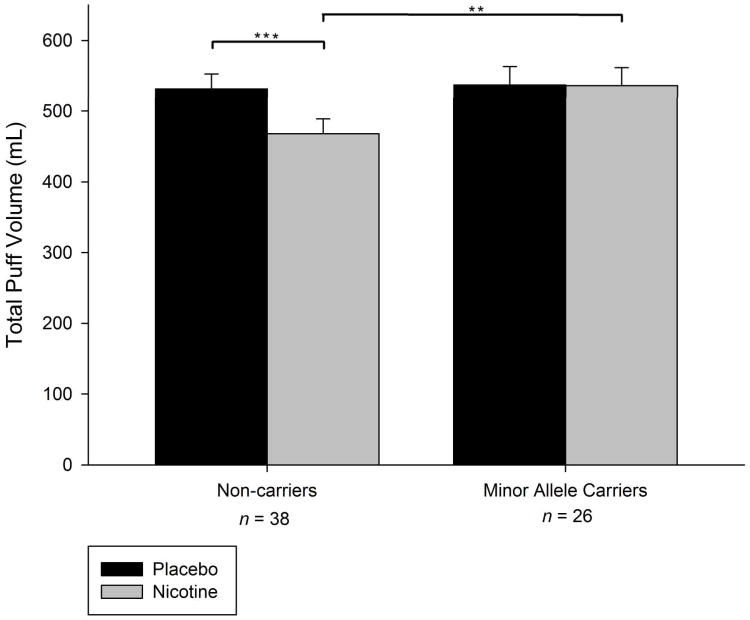
Primary Analyses: rs16969968
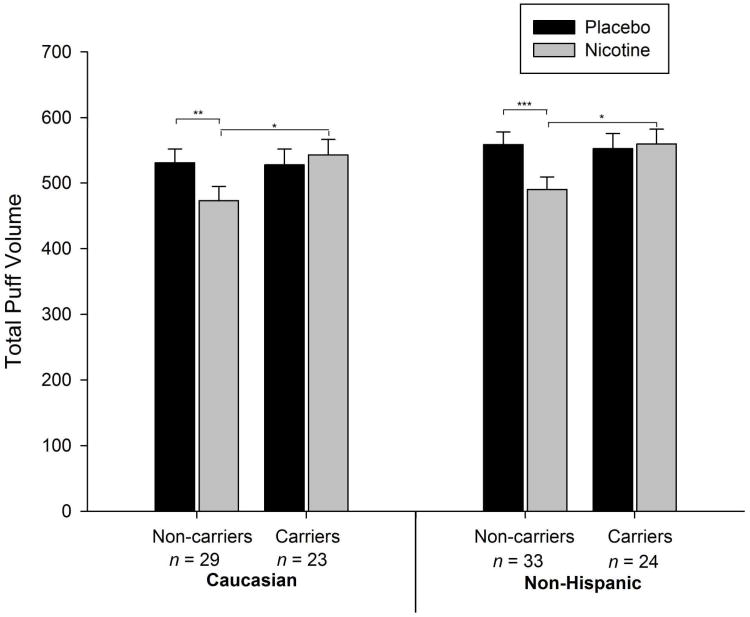
As depicted in Table 1, a significant nicotine effect (p = .006) and a significant genotype by nicotine content interaction was observed for rs16969968 (p = .008). Planned comparisons revealed that participants who did not carry a minor allele produced significantly reduced puff volumes when smoking nicotine-containing cigarettes relative to placebo cigarettes (12.01%, p < .001; see Figure 2 and Table 1). Total puff volume did not differ by nicotine content amongst carriers (p > .05). Ethnicity was a significant predictor in the model (p = .018), and both race and FTND trended towards significance (p = .081 and .086, respectively). To further examine the possibility that the observed interaction effects resulted from combining participants with different racial and ethnic backgrounds, separate analyses were also conducted on racial and ethnic subgroups. The effects observed in the combined sample were also observed in the Caucasian (n = 52) and Non-Hispanic (n = 57) subgroups (see Figure 3). Analyses in both groups yielded significant genotype by nicotine interactions (p = .017 and p = .014, respectively).
Figure 2.
Mean ± SEM total puff volumes for nicotine (grey bars) versus placebo (black bars) cigarettes by dichotomized genotype at rs16969968. (*p<.05; ** p < .01; *** p < .001).
Figure 3.
Mean ± SEM total puff volumes for nicotine (grey bars) versus placebo (black bars) cigarettes by dichotomized genotype at rs16969968 amongst the majority racial and ethnic subsamples. (*p<.05; ** p < .01; *** p < .001).
Secondary Analyses: Non-coding SNPs
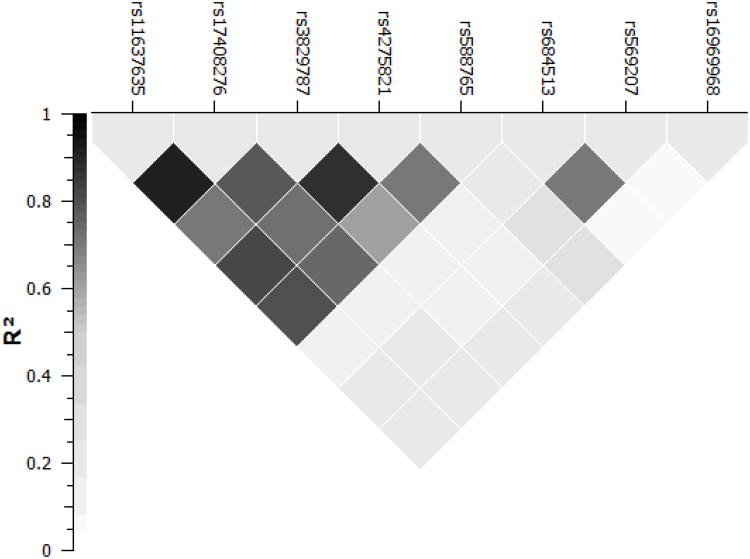
As shown in Table 1, no genotype × nicotine content interactions reached significance amongst the non-coding SNPs examined within the race, ethnicity and FTND controlled model (all ps > .05). However, a significant main effect of genotype was observed at rs3829787 (p = .027) and rs4275821 (p = .002). Only the effect for rs4275821 survived the bonferroni corrected significance level applied to the exploratory analysis of the non-coding SNPs (p < .007). In contrast to rs16969968, minor allele carriers at rs4275821 produced significantly lower puff volumes, irrespective of nicotine content. As depicted in Figure 4, rs4275821 was not strongly associated with rs16969968 (r2 = 0.214).
Figure 4.
Pairwise r2 of the included CHRNA5 SNPs. Boxes are shaded to display the degree of association (darker shades indicate greater r2).
Discussion
Recent studies have demonstrated the importance of using proximal and objective measures of smoking behavior to clarify the relationship between rs16969968, cigarette use, and respiratory diseases such as lung cancer2, 19. In keeping with this idea, the proposed study examined smokers' puff topography as a potential mechanism by which rs16969968 may influence toxicant exposure. However, several variables were associated with puff volume in our sample, most notably race, ethnicity, and nicotine dependence (FTND). Prior studies have generally not observed differences in smoking topography measures across racial groups 45-47 but see 48. Although, race differences might well be expected given that risk alleles for smoking intensity are not equally distributed across racial groups. The present study may have been more sensitive to subtle race effects given that multiple measurements of smoking topography were obtained from each participant and all participants were required to smoke the same cigarette brand. Prior studies also have not observed a relationship between smoking topography and subjective measures of nicotine dependence47, 48; However, smoking topography has been shown to predict other smoking phenotypes such as the number of cigarettes smoked per day, number of past quit attempts 49, and smoking cessation success 50, 51.
The present results also showed that rs16969968 was associated with total puff volumes produced during the smoking of nicotine-containing, but not placebo cigarettes. Specifically, puff volumes were not different across nicotine-containing and placebo cigarettes amongst minor allele carriers (A:G or A:A), but were significantly reduced for nicotine-containing relative to placebo cigarettes (12% reduction) amongst non-carriers (G:G). None of the self-report measures of smoking behavior (e.g., cpd, age of first cigarette, age of daily smoking initiation) or nicotine dependence (FTND) were significantly predicted by genotype at rs16969968.
The genotype × nicotine interaction observed is consistent with pre-clinical work; α5 knock-out mice do not reduce self-administration rates in response to increasing nicotine dose concentrations as is observed in wild-type controls24. Of course, in order to make a more meaningful comparison with animal models, smokers' puff topography must be assessed across a wide range of nicotine doses. Until recently, research cigarettes were not readily available for this purpose. A new line of cigarettes (22nd Century Group, Inc. Clarence, NY), now available from the National Institute on Drug Abuse, might be used in future work to replicate and extend the findings reported here.
Another important consideration is that the rs16969968 polymorphism does not prohibit α5 subunit expression as does a null mutation in mice. However, as an accessory subunit, the α5 protein substitutes for other receptor subunits to alter receptor properties. The rs16969968 variant reduces the functioning of nicotinic receptors incorporating the α5 subunit and thus may produce effects similar to reduced expression within certain neural pathways. In mice, selective knockdown of Chrna5 expression within projections from the medial habenula (MHb) to the interpeduncular nucleus (IPN) produces the self-administration abnormalities previously described, and localized “rescue” of the α5 subunit in knockouts (e.g., via injection of lentivirus delivering the Chrna5 gene) normalizes self-administration24, 30. It has been suggested that activation of MHb-IPN pathway by high doses of nicotine serves to reduce the reward value of nicotine and thus decreases self-administration24, 30. In humans, the rs16969968 polymorphism may similarly influence nicotine titration by moderating the MHb-IPN response to nicotine4, 5.
It should also be noted that the α5 receptor is expressed in multiple regions in the brain and periphery, and may impact processes outside of the MHb-IPN tract that are involved in smoking behavior. For example, human imaging studies have suggested that functional connectivity between the anterior cingulate cortex and ventral striatum is associated with the smoking risk conferred by the risk allele of rs1696996852. In mice, the α5 receptor has been linked to performance on tasks of attention, such as the 5-choice serial reaction time task, and has been shown to play a critical role in cholinergic signaling within pre-frontal regions involved in attention processes53. In both humans and rodents, nicotine has been shown to enhance certain forms of attention54, 55 and it has been suggested that cognitive enhancements may reinforce smoking behavior, particularly amongst those with cognitive impairments45. Thus, variation in α5 receptor gene may impact multiple neuronal circuits and cognitive processes that moderate smoking behavior.
There are also likely multiple variations within CHRNA5 that affect smoking behavior. Additional work is needed particularly with regard to characterizing non-coding polymorphisms in CHRNA5. Although a host of non-coding SNPs have been identified that associate with smoking phenotypes, their effects are difficult to interpret because many are in strong linkage disequilibrium and because much less is known about the functional effects of these polymorphisms. A main effect of gene on puff volume was detected at rs4275821, which reached bonferroni corrected significance while controlling for nicotine dependence, race, and ethnicity. This SNP was not associated with any other measure of dependence or smoking behavior. A significant association between genotype at rs4275821 and cpd has been previously observed in European smokers31. Unlike rs16969968, nicotine was not a significant moderator of the associations observed with rs4275821. Characterizing the functional effects of candidate SNPs within the non-coding regions of CHRNA5 could shed light on regulatory mechanisms related to α5 subunit expression. To the extent that expression and function of this subunit plays a role in nicotine self-administration, such mechanisms may serve as targets for the development of allosteric α5 modulators.
As a secondary analysis, the present study was limited by a modest sample size. Larger scale replications will be necessary to determine the generalizability of these findings. Additionally, biological markers of smoke exposure (e.g., expired air carbon monoxide, plasma nicotine concentration) were not collected in the present study, preventing a direct comparison between puff volume and toxicant exposure. Finally, while we present an association between smoking behavior and the non-coding SNP, rs4275821, further work is needed with regard to the mechanisms underlying the observed relationship.
In conclusion, we report that a coding SNP in CHRNA5 (rs16969968) is associated with total puff volumes produced when smoking nicotine-containing cigarettes. Specifically, minor-allele carriers do not appear to reduce the volume of their puffs in response to increased nicotine content as was observed with non-carriers. In contrast to the measure of smoking topography, self-report measures of smoking and nicotine dependence were not significantly associated with rs16969968. Moreover, genotype remained predictive of puff volume even after controlling for nicotine dependence, ethnicity and race. Thus, as a proximal and objective measure of smoking behavior, puff topography measures may serve as an endophenotype for exploring the relationship between genetic variation, smoking, and subsequent health consequences. In addition, topography measures may be useful for testing hypotheses developed from preclinical investigations regarding the functional effects of candidate SNPs. As preclinical investigations continue to explore the function of candidate genes identified from GWAS, human experimental investigations of gene × drug/environment interactions will become increasingly necessary to develop and assess novel treatments for tobacco dependence56.
Acknowledgments
This study was funded by NIH grants R21 DA027001, R21 DA024226 and R25 CA090314. The authors would like to thank Renee Ornduff and Natasha Garcia for their work on the project.
Footnotes
Conflicts of Interest: None.
References
- 1.The Tobacco and Genetics Consortium. Genome-wide meta-analyses identify multiple loci associated with smoking behavior. Nat Genet. 2010;42(5):441–447. doi: 10.1038/ng.571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bierut LJ. Convergence of genetic findings for nicotine dependence and smoking related diseases with chromosome 15q24-25. Trends Pharmacol Sci. 2010;31(1):46–51. doi: 10.1016/j.tips.2009.10.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Saccone SF, Hinrichs AL, Saccone NL, Chase GA, Konvicka K, Madden PA, et al. Cholinergic nicotinic receptor genes implicated in a nicotine dependence association study targeting 348 candidate genes with 3713 SNPs. Hum Mol Genet. 2007;16(1):36–49. doi: 10.1093/hmg/ddl438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bierut LJ, Stitzel JA, Wang JC, Hinrichs AL, Grucza RA, Xuei X, et al. Variants in nicotinic receptors and risk for nicotine dependence. Am J Psychiatry. 2008;165(9):1163–1171. doi: 10.1176/appi.ajp.2008.07111711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kuryatov A, Berrettini W, Lindstrom J. Acetylcholine receptor (AChR) alpha5 subunit variant associated with risk for nicotine dependence and lung cancer reduces (alpha4beta2)alpha5 AChR function. Mol Pharmacol. 2011;79(1):119–125. doi: 10.1124/mol.110.066357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Johnson EO, Chen LS, Breslau N, Hatsukami D, Robbins T, Saccone NL, et al. Peer smoking and the nicotinic receptor genes: an examination of genetic and environmental risks for nicotine dependence. Addiction. 2010;105(11):2014–2022. doi: 10.1111/j.1360-0443.2010.03074.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Sherva R, Wilhelmsen K, Pomerleau CS, Chasse SA, Rice JP, Snedecor SM, et al. Association of a single nucleotide polymorphism in neuronal acetylcholine receptor subunit alpha 5 (CHRNA5) with smoking status and with ‘pleasurable buzz’ during early experimentation with smoking. Addiction. 2008;103(9):1544–1552. doi: 10.1111/j.1360-0443.2008.02279.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Saccone NL, Culverhouse RC, Schwantes-An TH, Cannon DS, Chen X, Cichon S, et al. Multiple independent loci at chromosome 15q25.1 affect smoking quantity: a meta-analysis and comparison with lung cancer and COPD. PLoS Genet. 2010;6(8) doi: 10.1371/journal.pgen.1001053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Chen X, Chen J, Williamson VS, An SS, Hettema JM, Aggen SH, et al. Variants in nicotinic acetylcholine receptors alpha5 and alpha3 increase risks to nicotine dependence. Am J Med Genet B Neuropsychiatr Genet. 2009;150B(7):926–933. doi: 10.1002/ajmg.b.30919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hong LE, Yang X, Wonodi I, Hodgkinson CA, Goldman D, Stine OC, et al. A CHRNA5 allele related to nicotine addiction and schizophrenia. Genes Brain Behav. 2011;10(5):530–535. doi: 10.1111/j.1601-183X.2011.00689.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Saccone NL, Saccone SF, Hinrichs AL, Stitzel JA, Duan W, Pergadia ML, et al. Multiple distinct risk loci for nicotine dependence identified by dense coverage of the complete family of nicotinic receptor subunit (CHRN) genes. Am J Med Genet B Neuropsychiatr Genet. 2009;150B(4):453–466. doi: 10.1002/ajmg.b.30828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Xie P, Kranzler HR, Zhang H, Oslin D, Anton RF, Farrer LA, et al. Childhood adversity increases risk for nicotine dependence and interacts with alpha5 nicotinic acetylcholine receptor genotype specifically in males. Neuropsychopharmacology. 2012;37(3):669–676. doi: 10.1038/npp.2011.240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lips EH, Gaborieau V, McKay JD, Chabrier A, Hung RJ, Boffetta P, et al. Association between a 15q25 gene variant, smoking quantity and tobacco-related cancers among 17 000 individuals. Int J Epidemiol. 2010;39(2):563–577. doi: 10.1093/ije/dyp288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Young RP, Hopkins RJ, Hay BA, Epton MJ, Black PN, Gamble GD. Lung cancer gene associated with COPD: triple whammy or possible confounding effect? Eur Respir J. 2008;32(5):1158–1164. doi: 10.1183/09031936.00093908. [DOI] [PubMed] [Google Scholar]
- 15.Zienolddiny S, Skaug V, Landvik NE, Ryberg D, Phillips DH, Houlston R, et al. The TERT-CLPTM1L lung cancer susceptibility variant associates with higher DNA adduct formation in the lung. Carcinogenesis. 2009;30(8):1368–1371. doi: 10.1093/carcin/bgp131. [DOI] [PubMed] [Google Scholar]
- 16.Yang P, Li Y, Jiang R, Cunningham JM, Zhang F, de Andrade M. A rigorous and comprehensive validation: common genetic variations and lung cancer. Cancer Epidemiol Biomarkers Prev. 2010;19(1):240–244. doi: 10.1158/1055-9965.EPI-09-0710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Sakoda LC, Loomis MM, Doherty JA, Neuhouser ML, Barnett MJ, Thornquist MD, et al. Chromosome 15q24-25.1 variants, diet, and lung cancer susceptibility in cigarette smokers. Cancer Causes Control. 2011;22(3):449–461. doi: 10.1007/s10552-010-9716-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.VanderWeele TJ, Asomaning K, Tchetgen Tchetgen EJ, Han Y, Spitz MR, Shete S, et al. Genetic variants on 15q25.1, smoking, and lung cancer: an assessment of mediation and interaction. Am J Epidemiol. 2012;175(10):1013–1020. doi: 10.1093/aje/kwr467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Munafo MR, Timofeeva MN, Morris RW, Prieto-Merino D, Sattar N, Brennan P, et al. Association Between Genetic Variants on Chromosome 15q25 Locus and Objective Measures of Tobacco Exposure. J Natl Cancer Inst. 2012 doi: 10.1093/jnci/djs191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Benowitz NL, Jacob P, 3rd, Kozlowski LT, Yu L. Influence of smoking fewer cigarettes on exposure to tar, nicotine, and carbon monoxide. N Engl J Med. 1986;315(21):1310–1313. doi: 10.1056/NEJM198611203152102. [DOI] [PubMed] [Google Scholar]
- 21.Benowitz NL, Hall SM, Herning RI, Jacob P, 3rd, Jones RT, Osman AL. Smokers of low-yield cigarettes do not consume less nicotine. N Engl J Med. 1983;309(3):139–142. doi: 10.1056/NEJM198307213090303. [DOI] [PubMed] [Google Scholar]
- 22.Le Marchand L, Derby KS, Murphy SE, Hecht SS, Hatsukami D, Carmella SG, et al. Smokers with the CHRNA lung cancer-associated variants are exposed to higher levels of nicotine equivalents and a carcinogenic tobacco-specific nitrosamine. Cancer Res. 2008;68(22):9137–9140. doi: 10.1158/0008-5472.CAN-08-2271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Scherer G. Smoking behaviour and compensation: a review of the literature. Psychopharmacology (Berl) 1999;145(1):1–20. doi: 10.1007/s002130051027. [DOI] [PubMed] [Google Scholar]
- 24.Fowler CD, Lu Q, Johnson PM, Marks MJ, Kenny PJ. Habenular alpha5 nicotinic receptor subunit signalling controls nicotine intake. Nature. 2011;471(7340):597–601. doi: 10.1038/nature09797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Blank MD, Disharoon S, Eissenberg T. Comparison of methods for measurement of smoking behavior: mouthpiece-based computerized devices versus direct observation. Nicotine Tob Res. 2009;11(7):896–903. doi: 10.1093/ntr/ntp083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Herning RI, Jones RT, Bachman J, Mines AH. Puff volume increases when low-nicotine cigarettes are smoked. Br Med J (Clin Res Ed) 1981;283(6285):187–189. doi: 10.1136/bmj.283.6285.187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Zacny JP, Stitzer ML, Brown FJ, Yingling JE, Griffiths RR. Human cigarette smoking: effects of puff and inhalation parameters on smoke exposure. J Pharmacol Exp Ther. 1987;240(2):554–564. [PubMed] [Google Scholar]
- 28.MacQueen DA, Heckman BW, Blank MD, Janse Van, Rensburg K, Evans DE, Drobes DJ. Transient compensatory smoking in response to placebo cigarettes. Psychopharmacology (Berl) 2012 doi: 10.1007/s00213-012-2685-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Jackson KJ, Marks MJ, Vann RE, Chen X, Gamage TF, Warner JA, et al. Role of alpha5 nicotinic acetylcholine receptors in pharmacological and behavioral effects of nicotine in mice. J Pharmacol Exp Ther. 2010;334(1):137–146. doi: 10.1124/jpet.110.165738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Tuesta LM, Fowler CD, Kenny PJ. Recent advances in understanding nicotinic receptor signaling mechanisms that regulate drug self-administration behavior. Biochem Pharmacol. 2011;82(8):984–995. doi: 10.1016/j.bcp.2011.06.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Amos CI, Wu X, Broderick P, Gorlov IP, Gu J, Eisen T, et al. Genome-wide association scan of tag SNPs identifies a susceptibility locus for lung cancer at 15q25.1. Nat Genet. 2008;40(5):616–622. doi: 10.1038/ng.109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Hansen HM, Xiao Y, Rice T, Bracci PM, Wrensch MR, Sison JD, et al. Fine mapping of chromosome 15q25.1 lung cancer susceptibility in African-Americans. Hum Mol Genet. 2010;19(18):3652–3661. doi: 10.1093/hmg/ddq268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Hung RJ, McKay JD, Gaborieau V, Boffetta P, Hashibe M, Zaridze D, et al. A susceptibility locus for lung cancer maps to nicotinic acetylcholine receptor subunit genes on 15q25. Nature. 2008;452(7187):633–637. doi: 10.1038/nature06885. [DOI] [PubMed] [Google Scholar]
- 34.King DP, Paciga S, Pickering E, Benowitz NL, Bierut LJ, Conti DV, et al. Smoking cessation pharmacogenetics: analysis of varenicline and bupropion in placebo-controlled clinical trials. Neuropsychopharmacology. 2012;37(3):641–650. doi: 10.1038/npp.2011.232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Stevens VL, Bierut LJ, Talbot JT, Wang JC, Sun J, Hinrichs AL, et al. Nicotinic receptor gene variants influence susceptibility to heavy smoking. Cancer Epidemiol Biomarkers Prev. 2008;17(12):3517–3525. doi: 10.1158/1055-9965.EPI-08-0585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Wang JC, Bierut LJ, Goate AM. Variants weakly correlated with CHRNA5 D398N polymorphism should be considered in transcriptional deregulation at the 15q25 locus associated with lung cancer risk. Clin Cancer Res. 2009;15(17):5599. doi: 10.1158/1078-0432.CCR-09-1108. author reply 5599. [DOI] [PubMed] [Google Scholar]
- 37.Wang JC, Grucza R, Cruchaga C, Hinrichs AL, Bertelsen S, Budde JP, et al. Genetic variation in the CHRNA5 gene affects mRNA levels and is associated with risk for alcohol dependence. Mol Psychiatry. 2009;14(5):501–510. doi: 10.1038/mp.2008.42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Weiss RB, Baker TB, Cannon DS, von Niederhausern A, Dunn DM, Matsunami N, et al. A candidate gene approach identifies the CHRNA5-A3-B4 region as a risk factor for age-dependent nicotine addiction. PLoS Genet. 2008;4(7):e1000125. doi: 10.1371/journal.pgen.1000125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Winterer G, Mittelstrass K, Giegling I, Lamina C, Fehr C, Brenner H, et al. Risk gene variants for nicotine dependence in the CHRNA5-CHRNA3-CHRNB4 cluster are associated with cognitive performance. Am J Med Genet B Neuropsychiatr Genet. 2010;153B(8):1448–1458. doi: 10.1002/ajmg.b.31126. [DOI] [PubMed] [Google Scholar]
- 40.Zheng X, Duan W, Xu J, Nie C, Yang Z, Wang H, et al. Functionally significant nicotine acetylcholine receptor subunit alpha5 promoter haplotypes are associated with susceptibility to lung cancer in Chinese. Cancer. 2011 doi: 10.1002/cncr.26017. [DOI] [PubMed] [Google Scholar]
- 41.First MB, Spitzer RL, Gibbon M, Williams JBW. Structured Clinical Interview for Axis I DSM-IV Disorders - Patient Edition (SCID-I/P, version 2.0) Biometrics Research, New York State Psychiatric Institute; 1994. [Google Scholar]
- 42.Heatherton TF, Kozlowski LT, Frecker RC, Fagerstrom KO. The Fagerstrom Test for Nicotine Dependence: a revision of the Fagerstrom Tolerance Questionnaire. Br J Addict. 1991;86(9):1119–1127. doi: 10.1111/j.1360-0443.1991.tb01879.x. [DOI] [PubMed] [Google Scholar]
- 43.Cappelleri JC, Bushmakin AG, Baker CL, Merikle E, Olufade AO, Gilbert DG. Confirmatory factor analyses and reliability of the modified cigarette evaluation questionnaire. Addict Behav. 2007;32(5):912–923. doi: 10.1016/j.addbeh.2006.06.028. [DOI] [PubMed] [Google Scholar]
- 44.Lee EM, Malson JL, Waters AJ, Moolchan ET, Pickworth WB. Smoking topography: reliability and validity in dependent smokers. Nicotine Tob Res. 2003;5(5):673–679. doi: 10.1080/1462220031000158645. [DOI] [PubMed] [Google Scholar]
- 45.Ahijevych K, Gillespie J. Nicotine dependence and smoking topography among black and white women. Res Nurs Health. 1997;20(6):505–514. doi: 10.1002/(sici)1098-240x(199712)20:6<505::aid-nur5>3.0.co;2-q. [DOI] [PubMed] [Google Scholar]
- 46.Ahijevych K, Gillespie J, Demirci M, Jagadeesh J. Menthol and nonmenthol cigarettes and smoke exposure in black and white women. Pharmacol Biochem Behav. 1996;53(2):355–360. doi: 10.1016/0091-3057(95)02034-9. [DOI] [PubMed] [Google Scholar]
- 47.Veilleux JC, Kassel JD, Heinz AJ, Braun A, Wardle MC, Greenstein J, et al. Predictors and sequelae of smoking topography over the course of a single cigarette in adolescent light smokers. J Adolesc Health. 2011;48(2):176–181. doi: 10.1016/j.jadohealth.2010.06.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Collins CC, Epstein DH, Parzynski CS, Zimmerman D, Moolchan ET, Heishman SJ. Puffing behavior during the smoking of a single cigarette in tobacco-dependent adolescents. Nicotine Tob Res. 2010;12(2):164–167. doi: 10.1093/ntr/ntp176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Wood T, Wewers ME, Groner J, Ahijevych K. Smoke constituent exposure and smoking topography of adolescent daily cigarette smokers. Nicotine Tob Res. 2004;6(5):853–862. doi: 10.1080/1462220042000282537. [DOI] [PubMed] [Google Scholar]
- 50.Franken FH, Pickworth WB, Epstein DH, Moolchan ET. Smoking rates and topography predict adolescent smoking cessation following treatment with nicotine replacement therapy. Cancer Epidemiol Biomarkers Prev. 2006;15(1):154–157. doi: 10.1158/1055-9965.EPI-05-0167. [DOI] [PubMed] [Google Scholar]
- 51.Strasser AA, Pickworth WB, Patterson F, Lerman C. Smoking topography predicts abstinence following treatment with nicotine replacement therapy. Cancer Epidemiol Biomarkers Prev. 2004;13(11 Pt 1):1800–1804. [PubMed] [Google Scholar]
- 52.Hong LE, Hodgkinson CA, Yang Y, Sampath H, Ross TJ, Buchholz B, et al. A genetically modulated, intrinsic cingulate circuit supports human nicotine addiction. Proc Natl Acad Sci U S A. 2010;107(30):13509–13514. doi: 10.1073/pnas.1004745107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Bailey CD, De Biasi M, Fletcher PJ, Lambe EK. The nicotinic acetylcholine receptor alpha5 subunit plays a key role in attention circuitry and accuracy. J Neurosci. 2010;30(27):9241–9252. doi: 10.1523/JNEUROSCI.2258-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Heishman SJ, Kleykamp BA, Singleton EG. Meta-analysis of the acute effects of nicotine and smoking on human performance. Psychopharmacology (Berl) 2010;210(4):453–469. doi: 10.1007/s00213-010-1848-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Balaban MT, Losito BDG, Simons RF, Graham FK. Off-line latency and amplitude scoring of the human reflex blink with Fortran IV. Psychophysiology. 1986;23:612. [Google Scholar]
- 56.Ware JJ, van den Bree M, Munafo MR. From Men to Mice: CHRNA5/CHRNA3, Smoking Behavior and Disease. Nicotine Tob Res. 2012 doi: 10.1093/ntr/nts106. [DOI] [PMC free article] [PubMed] [Google Scholar]