Abstract
The present study examined age-related differences in multisensory integration and the role of attention in age-related differences in multisensory integration. The sound-induced flash illusion---the misperception of the number of visual flashes due to the simultaneous presentation of a different number of auditory beeps---was used to examine the strength of multisensory integration in older and younger observers. The effects of integration were examined when discriminating 1–3 flashes, 1–3 beeps, or 1–3 flashes presented with 1–3 beeps. Stimulus conditions were blocked according to these conditions, with baseline (unisensory) performance assessed during the multisensory block. Older participants demonstrated greater multisensory integration--a greater influence of the beeps when judging the number of visual flashes--than younger observers. In a second experiment, the role of attention was assessed using a go/no-go paradigm. The results of Experiment 2 replicated those of Experiment 1. In addition, the strength of the illusion was modulated by the sensory domain of the go/no-go task, though this did not differ by age group. In the visual go/no-go task we found a decrease in the illusion, while in the auditory go/no-go task we found an increase in the illusion. These results demonstrate that older individuals exhibit increased multisensory integration compared to younger individuals. Attention was also found to modulate the strength of the sound-induced flash illusion. However, the results also suggest that attention was not likely to be a factor in the age-related differences in multisensory integration.
Keywords: multisensory integration, aging, attention, vision, audition
Age-related sensory declines can have a profound effect on the health and well-being of older individuals. Epidemiological studies have shown that age-related declines in vision are associated with increased crash risk during driving and are a contributing factor to the increased likelihood of falls that occur with age (Ivers, Cumming, Mitchell, & Attebo, 1998; Owsley et al., 1998). Research also suggests that age-related declines in audition can lead to decreased quality of life (Appollonio, Carabellese, Frattola, & Trabucchi, 1996). However, perceptual processing is not solely based on the information from one sensory modality. Research has shown that this processing is based on combined information from multiple sensory inputs or multisensory integration (Massaro & Friedman, 1990; Massaro, 1987; McGurk & MacDonald, 1976; Meredith & Stein, 1986; Watkins, Shams, Josephs, & Rees, 2007). An important issue is whether age-related differences exist in the integration of multisensory sensory information that cannot be assessed in studies examining vision or audition in isolation. The experiments we report here aim to address this issue.
While early research on sensory processing proposed modularity of the senses until later stages of processing, more recent research has shown that this is unlikely to be the case and that cortical processing may be primarily multisensory (Ghazanfar & Schroeder, 2006; Schroeder & Foxe, 2005; Watkins et al., 2007). The integration of information from multiple sensory modalities into a unified multisensory percept may lead to more accurate processing of stimuli in the environment. Multisensory integration may prove to be of greater importance for older individuals due to a wide array of age-related declines in both vision and audition.
Age-related declines in vision have been observed for a wide range of different types of visual processing. These declines include decrements in visual acuity (Weale, 1975), discriminating luminance (Crassini, Brown, & Bowman, 1988), the detection and discrimination of motion (Andersen & Atchley, 1995; Atchley & Andersen, 1998; Bennett, Sekuler, & Sekuler, 2007; Trick & Silverman, 1991), the detection of an impending collision (Andersen & Enriquez, 2006), the perception of depth (Norman et al., 2008; Norman, Clayton, Shular, & Thompson, 2004; Norman, Crabtree, Bartholomew, & Ferrell, 2009), the discrimination of texture (Andersen, Ni, Bower, & Watanabe, 2010), and the discrimination of the orientation of stimuli (Betts, Sekuler, & Bennett, 2007). In addition, physiological studies have found age-dependent reductions in the number of photoreceptors (Curcio, Millican, Allen, & Kalina, 1993), loss of retinal ganglion cells (Curcio & Drucker, 1993), and decreases in retinal illuminance (Weale, 1988). These perceptual and physiological changes are likely to be underlying factors in age-related declines in visual performance (see G. J. Andersen, 2012 and Owsley, 2011 for a more thorough review of the vision and aging literature). Age-related declines in audition have also been observed for older individuals. These declines include pure-tone hearing thresholds, particularly at higher frequencies (Brant & Fozard, 1990), frequency and intensity discrimination (He, Dubno, & Mills, 1998), temporal gap detection (Schneider & Hamstra, 1999; Snell, 1997), and speech processing (Pichora-Fuller, Schneider, & Daneman, 1995). (For more detailed reviews of age-related declines in audition see Fitzgibbons & Gordon-Salant, 2010 and B. A. Schneider, Daneman, Pichora-Fuller, & Columbia, 2002.)
However, one possible way to reduce the negative effects of these declines is to use redundant information from multiple sensory modalities that can help to reduce false alarms. This would allow the system to maintain high levels of sensitivity while simultaneously reducing variance (Ernst & Bülthoff, 2004; Spence & Driver, 2004). This capacity of multisensory integration to reduce uncertainty and improve accuracy is one of the many reasons why an investigation of how multisensory integration changes with advancing age is important.
Recently several studies have examined age-related differences in multisensory integration. Research has found faster reaction times (RT) for older and younger participants when presented with multisensory as compared to unisensory stimuli (Mahoney, Li, Oh-Park, Verghese, & Holtzer, 2011) or multisensory as compared to unisensory orienting cues (Mahoney, Verghese, Dumas, Wang, & Holtzer, 2012). Studies have also found that older individuals demonstrate greater facilitation than younger individuals when multisensory information is present (Laurienti, Burdette, Maldjian, & Wallace, 2006; Peiffer, Mozolic, Hugenschmidt, Laurienti, & Pei, 2007). Studies have found that older observers demonstrate greater integration of auditory and visual stimuli, and greater reliance on visual information for spoken syllables (Thompson, 1995). The greater reliance on visual information was also found to be associated with the extent of visual-spatial attention (Thompson & Malloy, 2004). Other studies have found that presentation of multisensory information (visual presentation of a color and the spoken word of a color) in a simple color discrimination task resulted in faster performance for older as compared to younger observers (Laurienti et al., 2006). Even in tasks in which baseline age-related differences in reaction times are not present, older individuals still show greater facilitation for multisensory over unimodal stimuli than younger individuals (Peiffer et al., 2007). These results indicate a greater effect of multisensory stimuli in older as compared to younger individuals. Other research has found evidence of age-related differences in the temporal window of integrating multisensory information (Colonius & Diederich, 2011; Diederich, Colonius, & Schomburg, 2008) and suggested that a greater window of integration is associated with a greater risk of falls (Setti, Burke, Kenny, & Newell, 2011).
A general conclusion of these studies, considered together, is that older individuals as compared to younger individuals show greater multisensory integration suggesting that age-related differences exist in early levels of sensory processing. Other research has found evidence of age-related differences in higher-level factors in multisensory processing. For example, previous functional imaging research has shown that older individuals have difficulty inhibiting task-irrelevant information, and that increased processing of this task-irrelevant information also predicted distractibility in a cross-sensory behavioral task (Hugenschmidt, Mozolic, Tan, Kraft, & Laurienti, 2009). This increased difficulty in ignoring irrelevant information from other modalities may influence judgments of older individuals in multisensory tasks, particularly when using measures, such as reaction times, that are sensitive to these decisional components.
In the present study we examined age-related differences in perceptual multisensory integration using the sound-induced flash illusion (Shams, Kamitani, & Shimojo, 2000, 2002). In the sound-induced flash illusion paradigm, observers are simultaneously presented flashes and beeps. In some conditions, the number of flashes and beeps are identical (e.g., one, two or three flashes and beeps). In other conditions, the number of flashes and beeps are discrepant (e.g., one flash with either two or three beeps; two flashes with either one or three beeps, or three flashes with one or two beeps). An illusion is reported for discrepant trials when observers report a number of flashes that is different than the actual number of presented flashes, due to the discrepant number of auditory beeps (T. S. Andersen, Tiippana, & Sams, 2004; Shams, Ma, & Beierholm, 2005). Specifically, a greater number of flashes are reported than were presented when a greater number of beeps are presented. In addition, evidence of a fusion illusion has also been demonstrated, in which participants report seeing fewer flashes than were presented when the number of beeps is fewer than the presented number of flashes (Andersen et al., 2004; Shams, Iwaki, Chawla, & Bhattacharya, 2005). The illusion has been shown to be perceptual in nature and not merely due to a criterion shift (McCormick & Mamassian, 2008). Research has also demonstrated a flash-induced sound illusion in which the perceived number of beeps is influenced by the number of flashes presented (Andersen et al., 2004). Furthermore, research has suggested that the illusion may be due to feed-forward processes as indicated by early modulation of visual cortex (Shams, Iwaki, Chawla, & Bhattacharya, 2005; Watkins et al., 2007). Unlike previous studies examining age-related changes in multisensory integration using reaction times as an indicator of multisensory interactions (Laurienti et al., 2006; Peiffer et al., 2007), the sound-induced flash illusion can be used to assess changes in the visual percept. This avoids some of the complexity of speeded tasks that involve multiple stages of processing beyond perception, that may include cognition (Ratcliff, Thapar, & McKoon, 2001).
Although previous research has demonstrated age-related differences in the facilitation of reaction times in the presence of multisensory information (Laurienti et al., 2006; Peiffer et al., 2007), relatively few studies have examined adult age-related differences in multisensory perceptual processing. There are two possible outcomes regarding aging and multisensory integration. One possibility is that age-related declines in multisensory integration will occur due to declines in vision and audition. This outcome would be consistent with a model in which the noise or error present in either vision or audition are combined when information is present in both modalities. However, a second possibility is that integration will be better for older participants as compared to younger participants because older individuals may rely on multisensory information because of degraded unisensory processing. Previous research has suggested a role of overactivation and neural recruitment as a method of compensation for age-related declines in cognition (see Reuter-Lorenz & Cappell, 2008 for a discussion). The greater reliance on multisensory information, and thus greater integration, may thus be another type of compensatory process for age-related declines in unimodal sensory processing.
Experiment 1
Experiment 1 examined whether there were unimodal age-related differences in the ability to detect 1–3 visual flashes or 1–3 auditory beeps. Once unimodal differences between the groups were examined, we explored the existence of perceptual age-related differences in multisensory integration as assessed by the strength of the sound-induced flash illusion.
Methods
Participants
Twelve college students, 7 male and 5 female (M age = 20.75 years, SD = 0.62 years), from the University of California, Riverside and twelve older participants, 6 male and 6 female (M age = 75.67 years, SD = 5.07 years), from the surrounding community participated in the experiment. Older participants were required to be 65 years of age or older. All observers were naïve concerning the experimental purpose and were paid for their participation in the experiment. All subjects were pre-screened for self-reported eye disease (e.g. macular degeneration, glaucoma, retinitis pigmentosa), neurological disorders (e.g. Alzheimers disease, Parkinson’s disease, stroke), as well as for any significant hearing loss. After passing this pre-screening participants were then assessed using an array of visual (acuity and contrast sensitivity) and cognitive (forward and backward digit span; KBIT vocabulary) tests (see Table 1). Participants were required to have a log minimum angle of resolution of 0.40 or better, as well as log contrast sensitivity of 1.00 or better. Participants were also required to have digit-span scores no more than one standard deviation below the standards for cognitively normal individuals published by the Alzheimer’s Disease Center (Weintraub et al., 2010). Participants were allowed to wear hearing aids (however none of the participants came to the lab with hearing aids), and hearing was tested using the pre-test described in the experimental methods.
Table 1.
Means and standard deviations of participant demographics and results from cognitive and perceptual tests for Experiment 1.
| Experiment 1 | Younger | Older | ||
|---|---|---|---|---|
|
| ||||
| Variable | M | SD | M | SD |
| Age(years)1 | 20.75 | 0.62 | 75.67 | 5.07 |
| Years of Education | 15.08 | 0.67 | 14.92 | 1.88 |
| Log Contrast Sensitivity2 | 1.48 | 0.15 | 1.30 | 0.44 |
| LogMAR Acuity1 | 0.01 | 0.11 | 0.15 | 0.12 |
| Digit Span Forward | 12.25 | 1.91 | 10.50 | 2.43 |
| Digit Span Backwards1 | 8.83 | 2.62 | 6.83 | 1.90 |
| WAIS – Matrix Reasoning1 | 20.33 | 3.09 | 16.67 | 4.91 |
Note:
Differences between age groups were significant as indicated by a two-tailed t-test (p < 0.05).
Contrast sensitivity measured using the Pelli Robson Test (Pelli, Robson, & Wilkins, 1988).
Apparatus
Stimuli were presented on a 22″ Viewsonic PF817 CRT monitor at a resolution of 1024×768 at 100Hz (non-interlaced) driven by a Alienware Area-51 ALX equipped with an Intel Core i7 960 processor, and an NVIDIA Geforce GTX 480 graphics card, using the Microsoft Windows 7 (Service Pack 1) operating system. The background luminance of the display was 0.06 cd/m2. Custom experimental software was written in MATLAB (The Mathworks, Inc., version 7.8.0.347); the Psychophysics Toolbox extensions were also utilized (Brainard, 1997; Pelli, 1997). Calibration and measurements of the monitor were performed using a ColorCal2 colorimeter (Cambridge Research Systems).
Stimuli
Stimuli consisted of 1–3 flashes of a uniform white disc paired with 0–3 auditory beeps. The radius of the flashed disc was 0.75° in visual angle in size and was presented at 127.97 cd/m2. The flash duration was 10 milliseconds (ms) with a 70 ms inter-flash interval. Auditory beeps were 3.5 kHz sine wave tones. Beeps were 10 ms in length with 58 ms inter-beep intervals, and were presented at 74.2 dB. On trials that included both flashes and beeps, the onset of the first beep began 23 ms after the onset of the first flash. Previous research (Shams et al., 2002) has found no significant effect on the illusion with timing offsets of up to 70 ms. The stimulus parameters were identical to those used by (Shams, Ma, et al., 2005).
Experimental task and procedure
The monitor was viewed at a distance of 94 centimeters. Head position was stabilized with the use of a chin rest and stimuli were viewed binocularly. Any corrective lenses or contacts normally worn by the participants were allowed during the experiment. The only light source in the room during the experiment was the monitor.
At the beginning of the study, participants were required to pass a pre-test to determine whether they understood the task and were able to discriminate the beeps or flashes in isolation. The pre-test consisted of two sections, the first assessed their ability to discriminate 1 to 3 beeps, and the second assessed their ability to discriminate 1 to 3 flashes. Participants fixated a 0.33° fixation crosshair, which was centered horizontally, presented 3.12° below the top of the screen. Participants then pressed any key to advance each trial. This was followed by the presentation of the stimuli; the flashes or beeps were then presented. During the flash pre-test the flashes were presented 12° below fixation. At the end of the trial participants were shown a blank response screen and entered the number of flashes or beeps perceived using the number row of the keyboard. Response periods ended if the participant failed to respond within 6000 ms. Participants were given a maximum of five 30-trial blocks in each section to get eight trials in a row correct to be eligible for the experimental portion. No significant differences (p > 0.05) in the number of trials to completion were observed between age groups for either pre-test.
The experiment consisted of three experimental blocks, a flash only block, a beep only block, and a multisensory block. These unimodal blocks during the experiment were separate from the unimodal pre-tests. The order of these blocks was counter-balanced across participants, with two younger and two older participants assigned to each possible block ordering. Each block was separated by a mandatory five-minute break. The entire experiment took approximately 1.5 hours. The flash-only block assessed participants’ ability to discriminate 1 to 3 visual flashes. Each possible number of flashes was presented for 100 trials for a total of 300 trials in the flash-only block. The presentation order was randomized for each participant. The task of the participant was to report the perceived number of flashes (or the number of beeps if in a beep only block) using the number row of the keyboard. Participants were informed that the upcoming trials would occasionally be accompanied by a series of beeps, and that while these beeps may be distracting to remember to respond only to the number of flashes presented. On each trial participants fixated on a 0.33° white crosshair presented 3.12° below the top of the screen centered horizontally that was presented for 1000 ms. Then 1–3 visual flashes 12° below the fixation point were presented. The crosshair remained on the screen and participants entered their response. As in the pre-test, the response period ended if the participant failed to respond within 6 sec. Subsequent to their response or after 6 sec had elapsed, the crosshair vanished and a prompt instructed participants to press any key to continue to the next trial. The beep-only block was identical to the flash-only block, except that participants were presented and asked to respond to 1–3 beeps. Again, each number-of-beeps condition was presented for 100 trials for a total of 300 trials. The multisensory block was the same as the flash-only, except that each visual presentation was also paired with zero to three beeps. Trials with no beeps during the multisensory block were used in later analyses to examine whether congruent multisensory information improved performance over unimodal trials. Participants received 25 trials for each possible combination of flashes (1,2 or 3) and beeps (0, 1, 2 or 3), for a total of 300 trials in the multisensory block.
Results
The results for each block were analyzed separately. Original degrees of freedom are reported, and Greenhouse-Geisser corrections were used where appropriate. Data from the flash-only block were analyzed using a 2 (age) by 3(flashes) mixed repeated-measures analysis of variance (ANOVA). As expected, the number of flashes influenced the perceived number of flashes, F(2, 44) = 384.87, MSE = 13.53, p < 0.001. A significant age by flash interaction, F(2, 44) = 3.90, MSE = 0.14, p < 0.05, was also observed (see Figure 1A). Simple effects analyses were conducted using localized independent-samples t-tests. These indicated that the only significant difference between older and younger individuals occurred in the 2-flash condition, t(23) = 3.61, p = 0.001. Older participants tended to underestimate the number of flashes (M = 1.89, SD = 0.23), though this effect did not reach significance, t(11) = 1.628, p = 0.132, while younger participants significantly overestimated the number of flashes (M = 2.17, SD = 0.16), t(11) = 3.659, p = 0.004.
Figure 1.
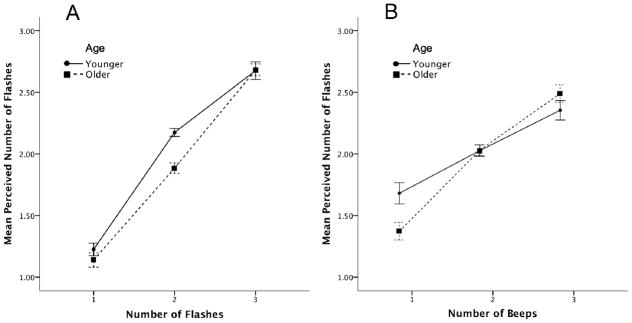
Panel A shows the mean perceived number of flashes as indicated by age group and number of flashes for the flash only block for Experiment 1. Panel B shows the mean perceived number of flashes as indicated by age group and number of beeps for the multisensory block for Experiment 1. Error bars indicate ±1 SEM.. Error bars indicate ±1 SEM.
Data from the beep-only block were analyzed using a 2 (age) by 3 (beeps) mixed repeated-measures ANOVA. There was a significant main effect of beeps, F(2, 44) = 1798.46, MSE = 21.38, p < 0.001. Older and younger individuals exhibited nearly veridical performance at all beep levels. No significant interaction between the number of beeps and age were observed.
The multisensory block was analyzed using a 2 (age) by 3(flashes) by 3(beeps) mixed repeated-measures ANOVA. The zero-beep conditions were excluded from this analysis because these trials provide no information concerning multisensory integration. The perceived number of flashes was influenced by the displayed number of flashes, F(2,44) = 103.12, MSE = 10.23, p < 0.001. A significant main effect of beeps was also observed, F(2,44) = 132.13, MSE = 14.47, p < 0.001. This finding suggests that the perceived number of flashes was influenced by the auditory information. A significant age by beep interaction was also found, F(2, 44) = 8.51, MSE = 0.93, p < 0.01. Older individuals exhibited greater integration of the cross-modal auditory information, as their average responses were lower than that of younger individuals in the one-beep condition, and higher than that of younger participants in the three beep condition (see Figure 1B). One way to analyze this interaction is through an analysis of individual slopes obtained through a linear regression predicting the perceived number of flashes from the number of beeps for each participant. This allows examination of the strength of the illusion. If the presence of beeps had no effect on the perceived number of flashes then we would expect a slope of zero with a mean of two (due to an equal number of trials at each possible level of 1–3 flashes). A greater slope indicates a larger effect of the illusion as the 1-beep and 3-beep conditions are respectively greater than or less than the mean. According to these results, older individuals showed a significantly steeper slope (M=0.66, SD=0.15) compared to younger individuals (M=0.48, SD=0.12), as indicated by a two-tailed independent-samples t-test, t(22)=3.187, p=0.004. A flash by beep interaction was also observed, F(4, 88) = 17.03, MSE = 0.48, p < 0.001. As incongruence between the actual number of flashes and beeps increased, the effect of the beeps on the perceived number of flashes decreased (see Figure 2).
Figure 2.
Mean perceived number of flashes as indicated by number of flashes and number of beeps for the multisensory block for Experiment 1. Error bars indicate ±1 SEM.
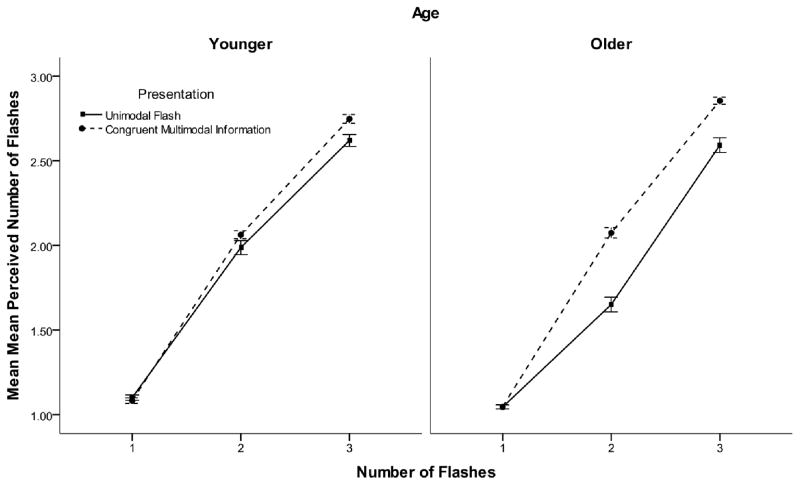
A 2 (age) by 3 (number of flashes) by 2 (congruent multisensory information versus unimodal flashes) mixed repeated-measures ANOVA was conducted on the response data to determine whether age-related differences exist for congruent beep information during the multisensory block as compared to the unimodal flash trials during the multisensory block. We found a significant main effect of congruency, F(1, 22) = 627.67, MSE = 41.004, p < 0.001), in which performance was more veridical on trials with congruent multisensory information. The actual number of flashes significantly influenced the perceived number of flashes, F(2,44) = 236.33, MSE = 6.48, p < 0.001. We also found a significant congruency by flash interaction, F(2,44) = 31.01, MSE = 0.80, p < 0.001). Specifically, congruent beep information resulted in improved performance as the number of flashes was increased. Finally, we found a congruency by flash by age interaction, F (2, 44) = 7.12, MSE = 0.18, p = 0.002. Older individuals showed a greater improvement in performance with congruent multisensory information than younger individuals. Older participants showed significant improvement (p < 0.05) in the 2 and 3-flash conditions, in which the mean reported number of flashes was closer to veridical when congruent multisensory information was present. Younger participants only showed a significant improvement in the 2-flash condition (see Figure 3).
Figure 3.
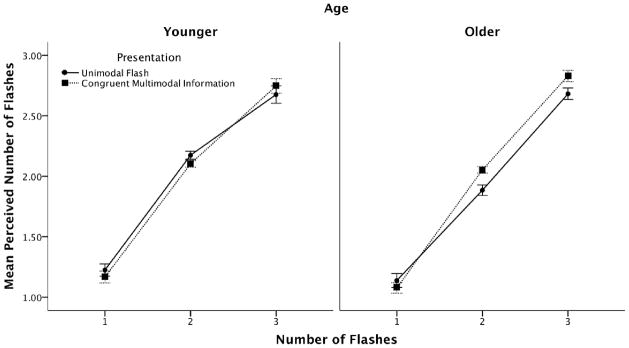
Mean perceived number of flashes as indicated by number of flashes, modality and age for the multisensory block for Experiment 1. Error bars indicate ±1 SEM.
Experiment 2
The results of Experiment 1 indicated a greater effect of the sound-induced flash illusion in older as compared to younger observers. Previous research (Alsius, Navarra, Campbell, & Soto-Faraco, 2005; Mishra, Martínez, & Hillyard, 2010; Talsma, Doty, & Woldorff, 2007) has found evidence that attention is a critical factor in multisensory integration. In addition, research has found that older individuals have a decreased capacity to ignore irrelevant information within, and across modalities, and this may be an alternative explanation for the age-related differences in integration (Poliakoff, Ashworth, Lowe, & Spence, 2006; Schmitz, Cheng, & De Rosa, 2010). Specifically, if there are age-related differences in the ability to ignore irrelevant auditory information, then greater encoding of the auditory stimuli by older individuals for multisensory trials may have led to an increased effect of the illusion for older as compared to younger observers.
To examine whether these age-related differences may be due to an age-related difference in the capacity to ignore the irrelevant auditory stimuli, a go/no-go task was used. Three conditions were examined---a condition including an auditory go/no-go cue, a condition with a visual go/no-go cue, and a condition with no cue condition. The no-cue condition was a replication of the conditions in Experiment 1. For the auditory go/no-go cue condition participants were instructed to withhold a response if the beeps were presented at a lower frequency. For the visual go/no-go cue condition participants were instructed to withhold a response if the flashed disk was increased in size. If the age-related results of Experiment 1 were due to differences in attending to irrelevant information, then older observers may show little or no change in the sound-induced flash illusion when an auditory cue is used or no cue is present. This outcome would suggest that older participants are attending to auditory information in both of these conditions. In contrast, cuing younger individuals to attend to the auditory information with an auditory cue should result in a greater sound-induced flash illusion than in trials without the go/no-go task because participants are prompted to attend to auditory information. We also included a go/no-go task in the visual modality with a visual cue for comparison purposes.
Methods
Participants
Twenty-four college students, 11 male and 13 female, from the University of California, Riverside (M age = 21.96 years, SD = 0.75 years) and twenty-seven older participants, 15 male and 12 female, from the surrounding community (M age = 72.50 years, SD = 4.30 years) participated in the experiment. Three older participants were unable to pass the pre-test (see methods below) and were not included in the study. Older participants were required to be 65 years of age or older. All observers were paid for their participation in the experiment, were naïve concerning the purpose of the Experiment and had not participated in Experiment 1. Subjects were screened using the same procedures as those used in Experiment 1 (see Table 2).
Table 2.
Means and standard deviations of participant demographics and results from cognitive and perceptual tests for Experiment 2.
| Experiment 2 | Younger | Older | ||
|---|---|---|---|---|
|
| ||||
| Variable | M | SD | M | SD |
| Age(years)1 | 21.96 | 0.75 | 72.50 | 4.30 |
| Years of Education | 15.50 | 1.02 | 16.71 | 4.96 |
| Log Contrast Sensitivity2 | 1.45 | 0.50 | 1.36 | 0.48 |
| LogMAR Acuity | 0.02 | 0.10 | 0.07 | 0.12 |
| Digit Span Forward | 10.54 | 2.47 | 10.75 | 2.77 |
| Digit Span Backwards | 7.50 | 1.96 | 7.75 | 2.44 |
| WAIS - Matrix Reasoning1 | 20.04 | 3.01 | 17.08 | 2.83 |
Note:
Differences between age groups were significant as indicated by a two-tailed t-test (p < 0.05).
Contrast sensitivity measured using the Pelli Robson Test (Pelli, Robson & Wilkins, 1988).
Apparatus
The apparatus used was the same as that in Experiment 1.
Stimuli
Stimuli were the same as those in the first experiment, with the following exceptions. The standard frequency for beeps during the go and control trials was 4.5 kHz. On visual no-go trials, the radius of the flashed disc was increased on 12% of the trials during the visual go/no-go block to 1.5° to indicate that the subject should not respond during that trial. On auditory no-go trials, the frequency of the tones was decreased to 2.5 kHz. This occurred on 12% of the trials in the auditory go/no-go block to indicate that the subject should not respond to the trial. The percentage of no-go trials (12%) was choosen to increase the attentional demand due to the low frequency of no-go trials.
Experimental task and procedure
The pre-test for inclusion in the study was the same as that used in Experiment 1. Experiment 2 consisted of three blocks, a multisensory block without any additional task, a multisensory block with a go/no-go task in the visual domain, and a multisensory block with a go/no-go task in the auditory domain. The order of the blocks was counter-balanced across participants such that two younger and two older participants were tested at each possible block order. Participants were allowed to take a break between each block. One of the blocks was the same as the multisensory block from Experiment 1. A block with a go/no-go task in the visual domain required participants to withhold their response when the flashes were presented at twice their standard size. One block with a go/no-go task in the auditory domain was also included. During this block, participants were to withhold their responses when the beeps were presented at a frequency 2 kHz lower (2.5 kHz) than the standard beep frequency (4.5 kHz). Similar to Experiment 1, if the participant failed to respond the response period ended after 6000 ms in all three blocks. Participants received 25 trials of each combination of flashes and beeps in random order, for a total of 300 trials within each block. The duration of the experiment was approximately 1.5 hours.
Results
Results were analyzed using a 2 (age) by 3 (block) by 3 (flashes) by 3 (beeps) mixed repeated-measures ANOVA. Focused analyses that examined whether age-related differences could be influenced by manipulating selective attention were also conducted. Trials in which participants withheld their response were removed from the primary analysis. Separate analyses were conducted examining accuracy for correctly withheld responses, as well as false alarms for blocks including the go/no-go tasks. All analyses report original degrees of freedom and Greenhouse-Geisser corrections were used where appropriate. The zero beep conditions were dropped from this analysis, as they provide no information concerning multisensory integration. They were included in the blocks to ensure participants were continuing to perform the task, and were used to assess whether congruent multisensory information improved performance over unimodal trials and whether this was dependent on the attentional manipulation. A significant main effect of flashes was found, F(2,92) = 164.26, MSE = 152.23, p < 0.001, indicating that the number of flashes influenced the number of perceived flashes. A significant main effect of beeps, F(2,92) = 156.80, MSE = 127.32, p < 0.001, was also observed, indicating that the beeps had an effect on the perceived number of flashes. We also found a significant age by beep interaction, F(2,92) = 9.42, MSE = 7.65, p = 0.002. Similar to Experiment 1, older individuals showed a greater effect of auditory information on visual judgments. An analysis of the slopes (number of beeps relative to perceived number of flashes) indicated that older participants had a significantly greater slope (M=0.52, SD=0.26) compared to younger participants (M=0.32, SD=0.17), as indicated by a two-tailed t-test, t(46) = 3.16, p = 0.003. A significant interaction between flashes and beeps, F(4,184) = 18.44, MSE = 2.65, p < 0.001, was also found, indicating that the effect of the beeps on the perceived number of flashes decreased as the number of beeps became more incongruent with the number of presented flashes.
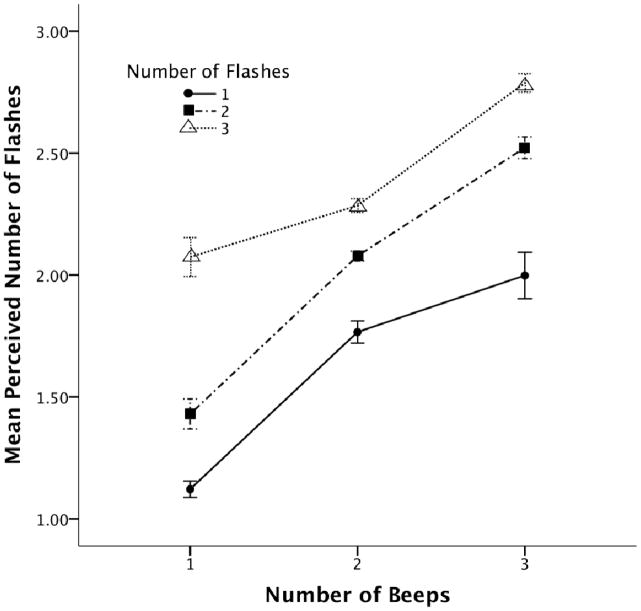
In addition, we also found that the attentional manipulation did influence the sound-induced flash illusion as indicated by a block by beep interaction, F(4, 184) = 3.57, MSE = 0.18, p = 0.02, and a block by flash by beep interaction, F(8, 368) = 3.89, MSE = 0.06, p = 0.001. The interaction between age and cue condition was not significant, F(2, 92) = 1.05, MSE = 0.10, p = 0.354. However, we found that the modality of the attentional manipulation did influence the strength of the illusion (see Figure 4). A repeated-measures mixed ANOVA--analyzing the slopes (i.e. examining the perceived number of flashes relative to number of beeps) of the two attention manipulations and age--indicated an increase in slope for the auditory go/no-go task (M=0.44, SD=0.26) as compared to the visual go/no-go task (M=0.38, SD=0.24), F(2,46) = 8.41, p = 0.006. Though, this change in the degree of integration by modality of the go/no-go cue did not significantly differ with age, F(1, 46) = 0.41, p = 0.53. The presence of the auditory go/no-go task increased the strength of the illusion, while the presence of the visual go/no-go task resulted in a decrease in the strength of the illusion. This finding suggests that an attentional cue can alter the illusion, with the increase in illusion strength for the auditory go/no go task suggesting greater integration whereas the decrease in illusion strength for the visual go/no-go task suggesting a decrease in integration. However, we failed to find evidence that the impact of the go/no-go cue varied with age.
Figure 4.
Mean perceived number of flashes as indicated by number of beeps and block for Experiment 2. Error bars indicate ±1 SEM.
No-go trials were analyzed using the proportion of correctly withheld responses with a 2 (go/no-go signal domain) by 2 (age) mixed repeated-measures ANOVA. A significant effect of block was found, F(1, 46) = 40.96, MSE = 0.31, p < 0.001. Participants showed a significant decrease in performance in their ability to withhold responses for the visual go/no-go task (M = 0.86, SD = 0.13) compared to the auditory go/no-go task (M = 0.97, SD = 0.05). No main effect or interaction of age was found. An analysis of the proportion correct of go trials that participants correctly responded showed no significant main effect of block or age. This indicates that both older and younger individuals were correctly performing the go/no-go task in both conditions, and that high performance in the blocks including the attention manipulation was not merely due to a high rate of withholding responses by either group.
An analysis of the effect of congruent multisensory versus unimodal information on responses was performed using a 2 (age) by 3 (block) by 3 (flashes) by 2 (congruency) mixed repeated-measures ANOVA. A significant effect of flashes F(2,92) = 1275.23, MSE = 194.77, p < 0.001 was found. We also found a significant effect of congruency F(1,46) = 47.68, MSE = 4.58, p < 0.001, indicating that performance was closer to veridical when congruent multisensory information was available. A significant congruency by age interaction, F(1,46) = 15.71, MSE = 1.51, p < 0.001, was also observed, with a greater improvement in performance with congruent multisensory information for older observers. The block by flashes interaction F(4,184) = 5.65, MSE = 0.10, p < 0.001 was also significant. As previously described the go/no-go task altered responses such that performance in the primary task was greater in the visual go/no-go condition compared to the auditory go/no-go condition. We also observed a significant, F(2,92) = 32.32, MSE = 1.44, p < 0.001, flash by congruency effect. As the number of flashes increased so did the benefit of the congruent multisensory information. Finally, we found a significant, F(2,92) = 12.15, MSE = 0.54, p < 0.001, age by flash by congruency effect. As the number of flashes increased older participants showed a greater benefit of congruent multisensory information than younger participants (see Figure 5). Older participants again showed a significant improvement (p < 0.05) in the 2 and 3-flash conditions whereas younger participants only showed a significant improvement in the 3-flash condition.
Figure 5.
Mean perceived number of flashes as indicated by age and presentation type for Experiment 2. Error bars indicate ±1 SEM.
General Discussion
In the present experiments, we found evidence of age-related perceptual differences in multisensory integration. In Experiment 1, we examined whether age-related differences in multisensory integration exist using the sound-induced flash illusion. In addition to completing a multisensory sound-induced flash illusion block, participants also completed beep-only and flash-only blocks. We found that the auditory beeps had a greater influence on the perceived number of flashes for older as compared to younger participants. We also examined the unimodal data to determine whether this difference could be explained by age-related differences in vision or audition. There were no significant age-related differences in accuracy for the beep-only condition. However, we did find a significant effect of age in the 2-flash condition of the flash-only block. Older participants tended to overestimate the number of flashes while younger participants significantly underestimated the number of flashes. Nevertheless, the observed age-related differences in the multisensory effects were found to be the greatest in the 1-flash and 3-flash conditions — conditions in which the difference between the presented number of flashes and beeps was the largest. Therefore, it is unlikely that the observed age-related differences in multisensory integration could be due to age-related differences in vision or audition alone. This conclusion is supported by the finding that the greatest age-related differences were found in different conditions of the multisensory blocks as compared to the single significant age effect in the 2-flash condition.
In addition, we found that fusion, the combining of flashes due to a smaller number of auditory beeps, occurred for both age groups. We also found that fission, the splitting of a single flash due to a larger number of auditory beeps, occurred for both age groups (see Andersen et al. 2004 for similar findings of fission and fusion for younger observers). In the unimodal flash condition, we only found a decrease in the reported number of flashes for older individuals. We did not find an increase in the 3-flash condition of the multisensory block. The decrease for the 1-flash condition of the multisensory condition was also much greater than that seen in the unimodal condition. For these reasons, we do not believe that unimodal differences in vision or audition contributed to the results found in the present study.
In addition, we examined age-related differences in the advantages conveyed by congruent multisensory stimuli by comparing performance in the multisensory block on the flash-only trials to performance during the same block in which the number of flashes and beeps were congruent. In both experiments, we found that older participants showed a greater benefit of congruent multisensory information compared to younger individuals, particularly as the number of flashes increased. This finding is in accord with the results of other studies demonstrating that older individuals show greater performance gains in the presence of redundant multisensory information than younger individuals (Hugenschmidt, Peiffer, McCoy, Hayasaka, & Laurienti, 2009; Peiffer et al., 2007).
In the second experiment, we examined the effects of attention on the sound-induced flash illusion. Previous research has shown that older individuals have difficulties inhibiting task-irrelevant information (Hugenschmidt, Mozolic, Tan, et al., 2009; Schmitz et al., 2010; Sweeney, Rosano, Berman, & Luna, 2001). In our study, it was possible that older individuals were exhibiting differences in integration due to an inability to inhibit the auditory information. To examine this possibility we included a go/no-go component in the second experiment to encourage younger and older participants to attend to specific aspects of each stimulus domain. Similar to the results of Experiment 1, we found that older individuals as compared to younger individuals demonstrated greater integration of auditory and visual information. We found that congruent multisensory information was more beneficial to older as compared to younger individuals. In addition, we found that the go/no-go task had an effect on the strength of the illusion, and that this effect changed in accordance with the sensory domain of the go/no-go signal. Specifically, we found a decrease in the strength of the illusion when the go/no-go task was in the visual domain, and we found an increase in the strength of the illusion when the go/no-go task was in the auditory domain. This suggests that the strength of the illusion was modulated by attention. However, the effect of attention on the illusion did not vary with age. This suggests that attention was not the basis of the age-related difference in the magnitude of the illusion. This finding is consistent with previous research (Hugenschmidt, Mozolic, & Laurienti, 2009) that found that multisensory integration increased with age, with the effect modulated by directing attention to either visual or auditory information for both younger and older participants. In summary, these results suggest that age-related differences in multisensory integration are not the result of age-related differences in the ability to ignore irrelevant information or increased attention of older individuals under multisensory conditions.
The finding of an attention effect is interesting. While previous research has proposed that the sound-induced flash illusion may be driven by an early processing component through feed-forward connections to visual cortex (Shams, Iwaki, et al., 2005; Watkins et al., 2007), other studies have found that the illusion can be influenced by higher-level processes (Setti & Chan, 2011). The results of the present study also suggest the possible influence of higher-level processes. Evidence in support of higher-level factors has been previously suggested by research that found audio-visual interactions identified as temporally “moderately early” and “late” in frontal cortex (Shams, Iwaki, et al., 2005). The results of Experiment 2 further support the influence of higher-level factors (beyond sensory cortex) on the sound-induced flash illusion. While these results support prior research showing little age-related change in the ability to engage selective attention (Hugenschmidt, Peiffer, et al., 2009), further study will be needed to explore the mechanisms involved in these higher-level processes.
The results of the present experiments, considered together with the findings of other studies (e.g., Mahoney et al., 2011; 2012; Thompson, 1995; Laurienti et al., 2006), suggest that multisensory integration is maintained with increased age. In fact, older as compared to younger individuals showed greater integration of multisensory information. While this may indicate a compensatory mechanism to aid processing to offset unimodal processing declines, it may also be due to decreased selectivity in multisensory networks. This may be similar to selectivity losses seen in neurophysiological studies of animal visual cortex (Hua et al., 2006; Schmolesky, Wang, Pu, & Leventhal, 2000). While older participants were more susceptible to the illusion, our results also indicated greater facilitation for older participants in cases of congruent multisensory stimuli. In natural environments, it is unlikely that visual and auditory stimuli that are spatially collocated will provide conflicting information. Outside of artificial conditions, such as that used in the sound-induced flash illusion, this increased integration may be beneficial. For example, in other audio-visual illusions, such as the McGurk effect, research has shown that older participants demonstrate a trend towards the visual alternative while simultaneously exhibiting a decrease in lip-reading performance (Cienkowski & Carney, 2002; McGurk & MacDonald, 1976). This suggets that while there is a decline in visual lip-reading performance older observers are still integrating that visual information into the auditory stimuli. The literature considered together suggests an overall increase in multisensory integration in older individuals. However, an important issue for further research will be to examine whether these age-related differences in multisensory integration indicate increases in the sensitivity of multisensory networks, possibly to compensate for unimodal declines, or if this increased integration may reflect a decrease in temporal and spatial selectivity such that integration is occurring for a broader range of non-coincident stimuli.
Acknowledgments
This research was supported by NIH grants EY018334 and AG031941. Portions of the data were presented at the 12th annual meeting of the Vision Sciences Society and at the 2012 Cognitive Aging Conference.
References
- Alsius A, Navarra J, Campbell R, Soto-Faraco S. Audiovisual integration of speech falters under high attention demands. Current biology 3: CB. 2005;15(9):839–43. doi: 10.1016/j.cub.2005.03.046. [DOI] [PubMed] [Google Scholar]
- Andersen GJ. Aging and vision: changes in function and performance from optics to perception. Wiley Interdisciplinary Reviews: Cognitive Science. 2012;3(3):403–10. doi: 10.1002/wcs.1167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andersen GJ, Atchley P. Age-Related Differences in the Detection of Three-Dimensional Surfaces From Optic Flow. Psychology and Aging. 1995;10(4):650–8. doi: 10.1037//0882-7974.10.4.650. [DOI] [PubMed] [Google Scholar]
- Andersen GJ, Enriquez A. Aging and the Detection of Observer and Moving Object Collisions. 2006;21(1):74–85. doi: 10.1037/0882-7974.21.1.74. [DOI] [PubMed] [Google Scholar]
- Andersen GJ, Ni R, Bower JD, Watanabe T. Perceptual learning, aging, and improved visual performance in early stages of visual processing. Journal of Vision. 2010;10(13):4. doi: 10.1167/10.13.4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andersen TS, Tiippana K, Sams M. Factors influencing audiovisual fission and fusion illusions. Brain research. Cognitive brain research. 2004;21(3):301–8. doi: 10.1016/j.cogbrainres.2004.06.004. [DOI] [PubMed] [Google Scholar]
- Appollonio I, Carabellese C, Frattola L, Trabucchi M. Effects of sensory aids on the quality of life and mortality of elderly people: a multivariate analysis. Age and ageing. 1996;25(2):89–96. doi: 10.1093/ageing/25.2.89. [DOI] [PubMed] [Google Scholar]
- Atchley P, Andersen GJ. The effect of age, retinal eccentricity, and speed on the detection of optic flow components. Psychology and aging. 1998;13(2):297–308. doi: 10.1037//0882-7974.13.2.297. [DOI] [PubMed] [Google Scholar]
- Bennett PJ, Sekuler R, Sekuler AB. The effects of aging on motion detection and direction identification. Vision research. 2007;47(6):799–809. doi: 10.1016/j.visres.2007.01.001. [DOI] [PubMed] [Google Scholar]
- Betts LR, Sekuler AB, Bennett PJ. The effects of aging on orientation discrimination. Vision Research. 2007;47(13):1769–80. doi: 10.1016/j.visres.2007.02.016. [DOI] [PubMed] [Google Scholar]
- Brainard DH. The Psychophysics Toolbox. Spatial Vision. 1997;10(4):433–436. doi: 10.1163/156856897X00357. [DOI] [PubMed] [Google Scholar]
- Brant LJ, Fozard JL. Age changes in pure-tone hearing thresholds in a longitudinal study of normal human aging. The Journal of the Acoustical Society of America. 1990;88(2):813–20. doi: 10.1121/1.399731. [DOI] [PubMed] [Google Scholar]
- Cienkowski KM, Carney AE. Auditory-visual speech perception and aging. Ear and hearing. 2002;23(5):439–49. doi: 10.1097/01.AUD.0000034781.95122.15. [DOI] [PubMed] [Google Scholar]
- Colonius H, Diederich A. Computing an optimal time window of audiovisual integration in focused attention tasks: illustrated by studies on effect of age and prior knowledge. Experimental brain research. Experimentelle Hirnforschung. Expérimentation cérébrale. 2011;212(3):327–37. doi: 10.1007/s00221-011-2732-x. [DOI] [PubMed] [Google Scholar]
- Crassini B, Brown B, Bowman K. Age-related changes in contrast sensitivity in central and peripheral retina. Perception. 1988;17(3):315–32. doi: 10.1068/p170315. [DOI] [PubMed] [Google Scholar]
- Curcio CA, Drucker DN. Retinal ganglion cells in Alzheimer’s disease and aging. Annals of Neurology. 1993;33(3):248–57. doi: 10.1002/ana.410330305. [DOI] [PubMed] [Google Scholar]
- Curcio CA, Millican CL, Allen Ka, Kalina RE. Aging of the human photoreceptor mosaic: evidence for selective vulnerability of rods in central retina. Investigative Ophthalmology & Visual Science. 1993;34(12):3278–3296. [PubMed] [Google Scholar]
- Diederich A, Colonius H, Schomburg A. Assessing age-related multisensory enhancement with the time-window-of-integration model. Neuropsychologia. 2008;46(10):2556–62. doi: 10.1016/j.neuropsychologia.2008.03.026. [DOI] [PubMed] [Google Scholar]
- Ernst MO, Bülthoff HH. Merging the senses into a robust percept. Trends in cognitive sciences. 2004;8(4):162–9. doi: 10.1016/j.tics.2004.02.002. [DOI] [PubMed] [Google Scholar]
- Fitzgibbons PJ, Gordon-Salant S. In: The Aging Auditory System. Gordon-Salant S, Frisina RD, Popper AN, Fay RR, editors. Vol. 34. New York, NY: Springer New York; 2010. pp. 111–34. [DOI] [Google Scholar]
- Ghazanfar AA, Schroeder CE. Is neocortex essentially multisensory? Trends in cognitive sciences. 2006;10(6):278–85. doi: 10.1016/j.tics.2006.04.008. [DOI] [PubMed] [Google Scholar]
- He N, Dubno JR, Mills JH. Frequency and intensity discrimination measured in a maximum-likelihood procedure from young and aged normal-hearing subjects. The Journal of the Acoustical Society of America. 1998;103(1):553–65. doi: 10.1121/1.421127. [DOI] [PubMed] [Google Scholar]
- Hua T, Li X, He L, Zhou Y, Wang Y, Leventhal AG. Functional degradation of visual cortical cells in old cats. Neurobiology of aging. 2006;27(1):155–62. doi: 10.1016/j.neurobiolaging.2004.11.012. [DOI] [PubMed] [Google Scholar]
- Hugenschmidt CE, Mozolic JL, Laurienti PJ. Suppression of multisensory integration by modality-specific attention in aging. Neuroreport. 2009;20(4):349–53. doi: 10.1097/WNR.0b013e328323ab07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hugenschmidt CE, Mozolic JL, Tan H, Kraft Ra, Laurienti PJ. Age-related increase in cross-sensory noise in resting and steady-state cerebral perfusion. Brain topography. 2009;21(3–4):241–51. doi: 10.1007/s10548-009-0098-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hugenschmidt CE, Peiffer AM, McCoy TP, Hayasaka S, Laurienti PJ. Preservation of crossmodal selective attention in healthy aging. Experimental Brain Research. 2009;198(2–3):273–85. doi: 10.1007/s00221-009-1816-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ivers RQ, Cumming RG, Mitchell P, Attebo K. Visual impairment and falls in older adults: the Blue Mountains Eye Study. Journal of the American Geriatrics Society. 1998;46(1):58–64. doi: 10.1111/j.1532-5415.1998.tb01014.x. [DOI] [PubMed] [Google Scholar]
- Laurienti PJ, Burdette JH, Maldjian JA, Wallace MT. Enhanced multisensory integration in older adults. Neurobiology of aging. 2006;27(8):1155–63. doi: 10.1016/j.neurobiolaging.2005.05.024. [DOI] [PubMed] [Google Scholar]
- Mahoney JR, Li PCC, Oh-Park M, Verghese J, Holtzer R. Multisensory integration across the senses in young and old adults. Brain research. 2011;1426:43–53. doi: 10.1016/j.brainres.2011.09.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mahoney JR, Verghese J, Dumas K, Wang C, Holtzer R. The effect of multisensory cues on attention in aging. Brain research. 2012;1472:63–73. doi: 10.1016/j.brainres.2012.07.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Massaro DW. Speech Perception by Ear and Eye: A Paradigm for Psychological Inquiry. Hillsdale, New Jersey: Erlbaum; 1987. [Google Scholar]
- Massaro DW, Friedman D. Models of integration given multiple sources of information. Psychological review. 1990;97(2):225–52. doi: 10.1037/0033-295x.97.2.225. [DOI] [PubMed] [Google Scholar]
- McCormick D, Mamassian P. What does the illusory-flash look like? Vision research. 2008;48(1):63–9. doi: 10.1016/j.visres.2007.10.010. [DOI] [PubMed] [Google Scholar]
- McGurk H, MacDonald J. Hearing lips and seeing voices. Nature. 1976;264(5588):746–8. doi: 10.1038/264746a0. [DOI] [PubMed] [Google Scholar]
- Meredith MA, Stein BE. Visual, auditory, and somatosensory convergence on cells in superior colliculus results in multisensory integration. Journal of neurophysiology. 1986;56(3):640–62. doi: 10.1152/jn.1986.56.3.640. [DOI] [PubMed] [Google Scholar]
- Mishra J, Martínez A, Hillyard Sa. Effect of attention on early cortical processes associated with the sound-induced extra flash illusion. Journal of cognitive neuroscience. 2010;22(8):1714–29. doi: 10.1162/jocn.2009.21295. [DOI] [PubMed] [Google Scholar]
- Norman JF, Clayton AM, Shular CF, Thompson SR. Aging and the perception of depth and 3-D shape from motion parallax. Psychology and aging. 2004;19(3):506–14. doi: 10.1037/0882-7974.19.3.506. [DOI] [PubMed] [Google Scholar]
- Norman JF, Crabtree CE, Bartholomew AN, Ferrell EL. Aging and the perception of slant from optical texture, motion parallax, and binocular disparity. Attention, perception & psychophysics. 2009;71(1):116–30. doi: 10.3758/APP.71.1.116. [DOI] [PubMed] [Google Scholar]
- Norman JF, Norman HF, Craft AE, Walton CL, Bartholomew AN, Burton CL, Crabtree CE. Stereopsis and aging. Vision research. 2008;48(23–24):2456–65. doi: 10.1016/j.visres.2008.08.008. [DOI] [PubMed] [Google Scholar]
- Owsley C. Aging and vision. Vision research. 2011;51(13):1610–22. doi: 10.1016/j.visres.2010.10.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Owsley C, Ball K, McGwin G, Sloane ME, Roenker DL, White MF, Overley ET. Visual Processing Impairment and Risk of Motor Vehicle Crash Among Older Adults. JAMA: The Journal of the American Medical Association. 1998;279(14):1083–1088. doi: 10.1001/jama.279.14.1083. [DOI] [PubMed] [Google Scholar]
- Peiffer AM, Mozolic JL, Hugenschmidt CE, Laurienti PJ, Pei AM. Age-related multisensory enhancement in a simple audiovisual detection task. Neuroreport. 2007;18(10):1077–81. doi: 10.1097/WNR.0b013e3281e72ae7. [DOI] [PubMed] [Google Scholar]
- Pelli DG. The VideoToolbox software for visual psychophysics: transforming numbers into movies. Spatial Vision. 1997;10(6):437–442. doi: 10.1163/156856897X00366. [DOI] [PubMed] [Google Scholar]
- Pelli D, Robson J, Wilkins A. The design of a new letter chart for measuring contrast sensitivity. Clinical Vision Sciences. 1988;2(3):187–99. [Google Scholar]
- Pichora-Fuller MK, Schneider BA, Daneman M. How young and old adults listen to and remember speech in noise. The Journal of the Acoustical Society of America. 1995;97(1):593–608. doi: 10.1121/1.412282. [DOI] [PubMed] [Google Scholar]
- Poliakoff E, Ashworth S, Lowe C, Spence C. Vision and touch in ageing: crossmodal selective attention and visuotactile spatial interactions. Neuropsychologia. 2006;44(4):507–17. doi: 10.1016/j.neuropsychologia.2005.07.004. [DOI] [PubMed] [Google Scholar]
- Ratcliff R, Thapar A, McKoon G. The effects of aging on reaction time in a signal detection task. Psychology and Aging. 2001;16(2):323. [PubMed] [Google Scholar]
- Reuter-Lorenz Pa, Cappell Ka. Neurocognitive Aging and the Compensation Hypothesis. Current Directions in Psychological Science. 2008;17(3):177–82. doi: 10.1111/j.1467-8721.2008.00570.x. [DOI] [Google Scholar]
- Schmitz TW, Cheng FHT, De Rosa E. Failing to ignore: paradoxical neural effects of perceptual load on early attentional selection in normal aging. The Journal of neuroscience 3: the official journal of the Society for Neuroscience. 2010;30(44):14750–8. doi: 10.1523/JNEUROSCI.2687-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmolesky MT, Wang Y, Pu M, Leventhal AG. Degradation of stimulus selectivity of visual cortical cells in senescent rhesus monkeys. Nature neuroscience. 2000;3(4):384–90. doi: 10.1038/73957. [DOI] [PubMed] [Google Scholar]
- Schneider BA, Daneman M, Pichora-Fuller MK. Listening in aging adults: from discourse comprehension to psychoacoustics. Canadian journal of experimental psychology. 2002;56(3):139–52. doi: 10.1037/h0087392. [DOI] [PubMed] [Google Scholar]
- Schneider BA, Hamstra SJ. Gap detection thresholds as a function of tonal duration for younger and older listeners. The Journal of the Acoustical Society of America. 1999;106(1):371–80. doi: 10.1121/1.427062. [DOI] [PubMed] [Google Scholar]
- Schroeder CE, Foxe J. Multisensory contributions to low-level, “unisensory” processing. Current opinion in neurobiology. 2005;15(4):454–8. doi: 10.1016/j.conb.2005.06.008. [DOI] [PubMed] [Google Scholar]
- Setti A, Burke KE, Kenny RA, Newell FN. Is inefficient multisensory processing associated with falls in older people? Experimental brain research. Experimentelle Hirnforschung. Expérimentation cérébrale. 2011;209(3):375–84. doi: 10.1007/s00221-011-2560-z. [DOI] [PubMed] [Google Scholar]
- Setti A, Chan JS. Familiarity of objects affects susceptibility to the sound-induced flash illusion. Neuroscience letters. 2011;492(1):19–22. doi: 10.1016/j.neulet.2011.01.042. [DOI] [PubMed] [Google Scholar]
- Shams L, Iwaki S, Chawla A, Bhattacharya J. Early modulation of visual cortex by sound: an MEG study. Neuroscience letters. 2005;378(2):76–81. doi: 10.1016/j.neulet.2004.12.035. [DOI] [PubMed] [Google Scholar]
- Shams L, Kamitani Y, Shimojo S. Illusions. What you see is what you hear. Nature. 2000;408(6814):788. doi: 10.1038/35048669. [DOI] [PubMed] [Google Scholar]
- Shams L, Kamitani Y, Shimojo S. Visual illusion induced by sound. Brain research. Cognitive brain research. 2002;14(1):147–52. doi: 10.1016/S0926-6410(02)00069-1. [DOI] [PubMed] [Google Scholar]
- Shams L, Ma WJ, Beierholm U. Sound-induced flash illusion as an optimal percept. Neuroreport. 2005;16(17):1923–7. doi: 10.1097/01.wnr.0000187634.68504.bb. [DOI] [PubMed] [Google Scholar]
- Snell KB. Age-related changes in temporal gap detection. The Journal of the Acoustical Society of America. 1997;101(4):2214–20. doi: 10.1121/1.418205. [DOI] [PubMed] [Google Scholar]
- Spence C, Driver J. Crossmodal Space and Crossmodal Attention. Oxford University Press; 2004. p. 323. [Google Scholar]
- Sweeney JA, Rosano C, Berman RA, Luna B. Inhibitory control of attention declines more than working memory during normal aging. Neurobiology of Aging. 2001;22(1):39–47. doi: 10.1016/S0197-4580(00)00175-5. [DOI] [PubMed] [Google Scholar]
- Talsma D, Doty TJ, Woldorff MG. Selective attention and audiovisual integration: is attending to both modalities a prerequisite for early integration? Cerebral cortex (New York, NY 3: 1991) 2007;17(3):679–90. doi: 10.1093/cercor/bhk016. [DOI] [PubMed] [Google Scholar]
- Thompson LA. Encoding and memory for visible speech and gestures: a comparison between young and older adults. Psychology and aging. 1995;10(2):215–28. doi: 10.1037//0882-7974.10.2.215. [DOI] [PubMed] [Google Scholar]
- Thompson LA, Malloy D. Attention resources and visible speech encoding in older and younger adults. Experimental aging research. 2004;30(3):241–52. doi: 10.1080/03610730490447877. [DOI] [PubMed] [Google Scholar]
- Trick GLGL, Silverman SESE. Visual sensitivity to motion. Neurology. 1991;41(9):1437. doi: 10.1212/wnl.41.9.1437. [DOI] [PubMed] [Google Scholar]
- Watkins S, Shams L, Josephs O, Rees G. Activity in human V1 follows multisensory perception. NeuroImage. 2007;37(2):572–8. doi: 10.1016/j.neuroimage.2007.05.027. [DOI] [PubMed] [Google Scholar]
- Weale RA. Senile changes in visual acuity. Transactions of the ophthalmological societies of the United Kingdom. 1975;95(1):36–8. [PubMed] [Google Scholar]
- Weale RA. Age and the transmittance of the human crystalline lens. The Journal of physiology. 1988;395(1):577–87. doi: 10.1113/jphysiol.1988.sp016935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weintraub S, Salmon D, Mercaldo N, Ferris S, Graff-Radford NR, Chui H, Morris JC. The Alzheimer’s Disease Centers’ Uniform Data Set (UDS): the neuropsychologic test battery. Alzheimer disease and associated disorders. 2010;23(2):91–101. doi: 10.1097/WAD.0b013e318191c7dd. [DOI] [PMC free article] [PubMed] [Google Scholar]