Abstract
The amygdala forms a crucial link between central pain and stress systems. There is much evidence that psychological stress affects amygdala activity, but it is less clear how painful stressors influence subsequent amygdala functional connectivity. In the present study, we used pulsed arterial spin labeling (PASL) to investigate differences in healthy male adults’ resting-state amygdala functional connectivity following a cold pressor versus control task, with the stressor and control conditions conducted on different days. During the period of peak cortisol response to acute stress (approximately fifteen to thirty minutes after stressor onset), participants were asked to rest for six minutes with their eyes closed during a PASL scanning sequence. The cold pressor task led to reduced resting-state functional connectivity between the amygdalae and orbitofrontal cortex (OFC) and ventromedial prefrontal cortex (VMPFC), which occurred irrespective of cortisol release. The stressor also induced greater inverse connectivity between the left amygdala and dorsal anterior cingulate cortex (dACC), a brain region implicated in the down-regulation of amygdala responsivity. Furthermore, the degree of post-stressor left amygdala decoupling with the lateral OFC varied according to self-reported pain intensity during the cold pressor task. These findings indicate that the cold pressor task alters amygdala interactions with prefrontal and ACC regions 15–30 minutes after the stressor, and that these altered functional connectivity patterns are related to pain perception rather than cortisol feedback.
Keywords: amygdala, stress, pain, functional connectivity, arterial spin labeling, cold pressor task
Pain is a potent stressor with immediate relevance to survival. Central regulatory circuits help orchestrate the body’s adaptive response to painful stressors, optimizing survival during threat and restoring homeostasis in its aftermath. Acute physical stressors activate the amygdala, an emotional processing center that signals the saliency of incoming sensory information (LeDoux, 2000; Anderson and Phelps, 2001) and triggers affective or emotional responses (LeDoux, 2003). As a result, the amygdala functions as part of an early alarm system that rapidly detects threat and coordinates defensive behaviors (Liddell et al., 2005). The amygdala also bridges stress and pain regulatory systems, suggesting that it plays an important role in balancing the response demands that painful stressors place on the brain.
While heightened amygdala activity helps mobilize the body’s response to threat, prolonged salience signals become unnecessary when pain is no longer relevant to behavior. To restore homeostasis, amygdala activity is instead reduced during the period after painful stimulation. Initially, the amygdala promotes activation of the hypothalamo-pituitary-adrenocortical (HPA) axis, which culminates in the release of the stress hormone cortisol from the adrenal medulla (Herman et al., 2005). While this hormone plays an essential role in the stress response by stimulating glucose production (de Kloet et al., 1999), excessively high levels of cortisol can also produce the breakdown of muscle, bone, and neural tissue (Sapolsky, 1996). To limit the duration and magnitude of HPA activation, cortisol regulates its own release via a negative feedback loop with the hypothalamus (de Kloet and Reul, 1987) and target limbic structures, such as the medial prefrontal cortex (MPFC; Herman et al., 2005). The MPFC regulates the stress response by inhibiting the HPA axis (Boyle et al., 2005; Diorio et al., 2003). The amygdala also receives inhibitory inputs from MPFC, and this top-down regulation appears to be partially mediated by endogenous cortisol (Veer et al., 2011a; Urry et al., 2006). A positron-emission tomography (PET) study in humans supports this MPFC-amygdala cortisol feedback mechanism, demonstrating that greater stress-related MPFC metabolic activity anti-correlates with cortisol increase and is associated with decreased activity in the amygdala (Kern et al., 2008). Hydrocortisone has been shown to reduce amygdala activity 20 minutes after pharmacological administration (Lovallo et al., 2010), which is consistent with the time when cortisol reaches peak concentration after acute stress induction (Dickerson and Kemeny, 2004). These results suggest that delayed cortisol feedback to the MPFC helps disable amygdala-driven HPA activation during latter stages of the stress response.
Importantly, the amygdala not only modulates HPA axis output but represents a major relay station for both afferent and efferent nociceptive information processing (Bernard & Besson, 1988). For instance, the degree of activation in the amygdala has been associated with subjective pain ratings (Bornhovd et al., 2002). In addition, successful pain regulation has been shown to alter activity in the amygdala (Lapate et al., 2012). Stressor-related amygdala deactivation likely depends on frontal cortical regions, which shape nociceptive processing through both direct and indirect influences on descending inhibitory pain circuits (Hutchison et al. 1999; Lenz et al. 1998; Xie et al., 2009, for a review). In particular, engagement of dACC and lateral orbitofrontal cortex (LOFC) exert an inhibitory influence on amygdala responsivity, especially during pain modulation and emotion regulation (Johnstone et al., 2007; Lee et al., 2012; Ochsner et al., 2004; Petrovic and Ingvar, 2002). A positron emission tomography (PET) study supports the notion of frontal control, demonstrating that longer pain exposure, wherein participants reported using more coping strategies, was associated with greater activity in the dACC and reduced activity in the amygdala (Petrovic et al., 2004). Thus, the efficacy of fronto-amygdala functional interactions likely contributes to recovery from physical stressors, though this possibility has received little attention in humans.
To date, most stress studies have focused on activity changes in the amygdala itself, as opposed to its interactions with other brain regions. Furthermore, previous observations of top-down modulation during the stress response have been limited to simple correlations between mean activity in the amygdala and a given frontal regulatory region (Kern et al., 2008). The few studies that have examined stress-related changes in amygdala functional connectivity are consistent with the notion of time-dependent shifts that reflect underlying response and recovery demands. For example, immediately after viewing aversive film clips, the amygdala shows enhanced functional connectivity with regions that amplify the stress response and aversive processing, such as dACC and locus coeruleus (van Marle et al., 2010). In men, corticosteroid administration reduces amygdala functional connectivity with these brain regions approximately 2 hours after oral intake (Henckens et al., 2011). A separate study using acute psychological stress demonstrated that amygdala activity covaries positively with activity in the MPFC one hour later, supporting the notion of homeostatic recovery once the stressor is no longer salient (Veer et al., 2011b). We aim to expand upon these findings by determining how an acute painful stressor - the cold pressor task (CPT) - affects subsequent amygdala functional connectivity.
During the cold pressor task, participants are asked to submerge their non-dominant hand in ice water for several minutes. This painful task involves a combination of acute physical and psychological stress (Blandini et al., 1995) and has been shown to increase salivary cortisol, at least outside the scanner environment (Buchanan et al. 2006; Lighthall et al., 2009, 2011, in press; Mather et al., 2009; Pascualy et al., 2000; Porcelli et al., 2008). However, there have also been reports of negative findings, with some studies demonstrating either a moderate increase in cortisol responsivity (al’ Absi et al., 2002) or no increase at all (Duncko et al., 2007; Duncko et al., 2009; McRae et al., 2006). This discrepancy could be attributed to the cold pressor task engaging both pain- and stress-processing systems. Numerous pain studies have demonstrated that exposure to stress not only triggers the release of cortisol but a host of stress-related neurotransmitters, including endogenous opioids (Drolet et al., 2001). Men, in particular, demonstrate greater µ-opioid receptor activation in the amygdala during sustained pain than women (Zubieta et al., 2002), suggesting that males engage analgesic mechanisms to a greater extent after painful stressors. Since common frontal regions regulate amygdala responses to stress, pain and emotion, it is possible that cortisol and pain modulation have separable contributions to amygdala-mediated stress adaptation. Thus, the goal of the present study is to distinguish how HPA axis responsivity and pain relate to amygdala functional connectivity during post-stressor recovery in men.
Pulsed arterial spin labeling (PASL) serves as an optimal method for examining brain functional connectivity during sustained physiological states, such as stress. Unlike radiotracers used in PET imaging, PASL is non-invasive and uses magnetic inversion to “tag” arterial blood water so it can be used as an endogenous tracer of cerebral blood flow (CBF). PASL also holds several advantages over more conventional resting-state functional magnetic resonance imaging techniques, because it can limit the influence of artifacts that contaminate the blood oxygen level-dependent (BOLD) signal. Some of these benefits include improved inter-session reliability (Aguirre et al., 2002; Wang et al., 2003), lower sensitivity to magnetic field inhomogeneity effects (Wang et al., 2004), and less sensitivity to subject motion and scanner drift artifacts (Aguirre et al., 2002). In addition, PASL targets cerebral blood flow in arterioles and capillaries as opposed to venules, thereby acquiring signal that is better localized to the sites of neural activity than BOLD signal (Zappe et al., 2008).
Here, we used PASL and seed-based resting-state functional connectivity (rsFC) to observe coherent perfusion signal fluctuations between the amygdala and other brain regions approximately 15 to 30 minutes after a cold pressor task. Seventeen healthy male participants were scanned during two resting-state PASL sessions to acquire a within-subject measure of stressor-related changes in amygdala rsFC. Only males were recruited for this study given suggestions in the literature that they show stronger analgesic mechanisms after painful stimulation than do females (Zubieta et al., 2002), and to avoid confounding interactions between estradiol and cortisol responsivity (Kudielka and Kirchbaum, 2005) and cold pressor pain threshold and menstrual cycle phase (Aloisi and Bonifazi, 2006; Hapidou and Catanzaro, 1988; Hellstrom and Lundberg, 2000). We hypothesized that the cold pressor task would significantly alter functional coupling between the amygdala and regions that contribute to HPA axis regulation and pain perception. Moreover, we predicted that distinct patterns of amygdala rsFC would be associated with individual differences in stressor-induced cortisol elevation and self-reported pain intensity, thereby elucidating how overlapping stress and pain regulatory networks interact with the amygdala to restore homeostasis.
Methods
Participants
Seventeen healthy right-handed males (age range = 18–31; mean = 23.5) participated in the two-day study. On each day, participants were scanned between 2 and 5 p.m. when cortisol levels are relatively stable in order to limit inter-subject variability. Participants also refrained from eating, caffeine intake, and exercise for at least one hour and sleeping for at least two hours prior to arrival. All participants provided written informed consent approved by the University of Southern California (USC) Institutional Review Board.
Procedure
Upon arrival, participants gave informed consent and drank 8 oz. of water. The first 45 minutes of the study involved completing various questionnaires to assess baseline mood and subjective self-reports of overall and relative stress level. This portion of the experiment also served as an acclimation period before the stressor. After finishing the assessments, participants were asked to provide a 1mL baseline saliva sample by passively drooling into a test tube. In this within-subject design, participants underwent scan sessions on two separate days and were randomly assigned to the cold pressor task or control condition on the first day, and were run in the other condition during the second session. Participants were not informed of the water temperature condition beforehand, and the experimenter emphasized that the ice water (stress condition) could be administered on one or both days of the experimental procedure. During the cold-pressor task, participants were instructed to immerse their left, non-dominant hand in ice water (0–3 degrees Celsius) for at least 1 minute and up to 3 minutes. For the control condition, participants immersed their left hand in warm water (37–40 degrees Celsius) for up to 3 minutes. One experimenter remained in the room to watch the participant during the hand immersion manipulation. Previous research suggests that a combination of social-evaluative threat and the cold pressor elicits greater HPA activation than the cold pressor alone (Schwabe et al., 2008). Following the hand-immersion task, participants were given additional instruction about the scanning procedure then entered the scanner. Approximately 15 to 30 minutes after the onset of the hand-immersion task (mean = 18 min, standard deviation = 4 min., min = 10 min., max = 30 min.), a brief localizer scan was conducted to ensure the participant’s head was centered for the PASL resting-state scan. Participants were instructed to lay still with their eyes open during the 6-minute and 20 second resting-state sequence but not fall asleep. Immediately following the scan, participants were instructed to remain still while a second saliva sample was collected using a Sorbette, which they carefully placed in their mouth for 2 minutes (Salimetrics, LLC, State College, PA, USA). After performing an unrelated fMRI task for approximately 25 minutes, a high-resolution T1-weighted anatomical scan was acquired.
Data Collection
Salivary cortisol collection and assay
Salivary cortisol collection times were based on previous findings that the cortisol response peaks approximately 21– 40 minutes following the onset of an acute stressor (Dickerson and Kemeny, 2004). Since saliva sample 3 was acquired during the subsequent unrelated fMRI task (mean = 59 min., standard deviation = 6 min., min = 49 min., max = 78 min.) only the difference from baseline (sample 1) to post-PASL scan (sample 2) was used as a measure of cortisol change in subsequent analyses. Following the PASL scan, saliva samples were stored in a laboratory freezer at −30 degrees Celsius. Once the study was completed, the frozen samples were sent to analytical laboratories (Salimetrics, LLC, State College, Pennsylvania, USA) and stored at −80 degrees Celsius until assayed. Samples were centrifuged at 3000 rpm for 15 min to remove mucins. Duplicate assays were conducted for each saliva sample, and the mean of the two values was used in the final analysis. The intra-assay coefficient of variation (CV) between duplicates was determined to be 4.85%. Three participants were excluded from the cortisol analyses, because the quantities of their saliva samples were not sufficient to yield an accurate measure of cortisol concentration. Specifically, cortisol concentrations were unable to be determined for both the baseline (before hand immersion) and post hand-immersion samples in one participant, whereas two participants were missing concentration values for just the post hand-immersion saliva samples; since we required both samples to assess stressor-related cortisol change, both of these participants had to be excluded from further analyses involving cortisol.
Pain and stress ratings
Pain intensity during the cold-pressor task was assessed via participants’ self-report immediately before and after hand-immersion. The degree of pain was measured using two pain scales: the Faces Pain Scale – Revised (FPS-R) and a Pain Visual-Analogue Scale (VAS). For the FPS-R, participants made their ratings by circling the face that showed how much pain was experienced during hand immersion in ice or warm water. Similarly, participants also indicated their pain level on the Pain VAS via a hash mark on a line, which ranged from no pain on the left to worst possible pain on the right. These responses were codified by measuring the distance of the mark from no pain at 0 centimeters to worst possible pain at 10.16 centimeters. Subjective mood was assessed prior to hand immersion using the Positive and Negative Affect Schedule (PANAS; Watson et al., 1988). Participants also reported their overall and relative stress level on a scale from 1–10 prior to hand immersion, with 1 indicating no stress and 10 indicating extremely stressed.
Image acquisition
Perfusion images were acquired on a 3.0-T Siemens MAGNETOM TRIO scanner using a 12-channel head coil. Resting-state ASL was conducted with a pulsed-ASL sequence using the QUIPSS-II method (Wong et al., 1997; Wong et al., 1998). In addition, a Proximal Inversion with a Control for OFF-Resonance Effects (PICORE) mode was employed to provide high labeling efficiency (Luh et al., 1999). Eighty-eight interleaved label and control volumes were acquired during a 6-minute-and-20-second scan, along with one M0 image (axial slices = 20; TR/TE = 4000/30ms; TI1/TIs/TI2=700/1600/1800ms; matrix = 64 × 64; FOV = 224mm; flip angle = 90 degrees; in-plane resolution =3×3mm2; slice thickness = 4mm). The timing of the inversion pulses (TI) was optimized to reduce intravascular signal intensity at 3T (Donahue et al., 2006). The scanner’s built-in 3D online prospective acquisition correction (PACE) was used to correct for head motion artifact during the PASL scan. A high-resolution T1-weighted anatomical image (MPRAGE) was also obtained to aid with PASL image co-registration (slices = 208 coronal; TR/TE/TI=2530ms/3.09ms/800ms; FOV = 256mm × 256mm; in-plane resolution = 1mm2; slice thickness = 1mm with no gap; bandwidth = 220Hz/Px). The total acquisition time for the anatomical scan was 10 minutes and 42 seconds.
Statistical Analysis
Cortisol and pain measures
Cortisol data were analyzed using a 2 (condition) × 2 (saliva sample time) repeated-measures ANOVA, with condition order (stressor condition on day 1 or day 2) as a between-subjects factor. Similarly, hand immersion duration and the subjective pain measures from the Pain VAS and Faces Pain Scale were analyzed using separate 2 (condition) × 2 (rating time) repeated-measures ANOVAs, with condition order as a between-subjects factor. Post hoc t-tests were performed to assess significant main effects of condition and saliva sample or pain rating time. Paired t-tests were used to ensure that baseline positive and negative affect (PANAS questionnaire) and overall and relative stress level did not differ between conditions. A paired t-test was also used to verify that the mean time after the first and second salivary cortisol sample was comparable between the stressor and control conditions. Another paired t-test was used to test whether the mean time after the onset of hand immersion and the start of the PASL scan was comparable between the stressor and control condition.
Associations between pain and cortisol
Pearson’s correlations were performed between the stressor-induced changes in cortisol and pain measures (cold pressor duration, Pain VAS, and Faces Pain Scale). The duration of hand immersion in the ice water varied across participants, so partial correlations were also performed between the ROIs and the two pain measures after controlling for cold pressor duration. Since we identified a significant main effect of condition order on salivary cortisol levels, an additional partial correlation analysis was performed by controlling for condition order. Notably, only 14 participants had complete cortisol data. To take advantage of larger statistical power, the pain measures from the group of 17 participants were analyzed and reported. The strength of these associations was further validated by re-analyzing the pain rating data for the subgroup of 14 participants.
PASL Analysis
Image preprocessing
PASL images were preprocessed using FSL Version 4.1.6 (FMRIB’s Software Library, www.fmrib.ox.ac.uk/fsl). The first volume (M0), which is used to normalize quantification of perfusion maps, was discarded. The remaining eighty-eight interleaved control and tag images were preprocessed using the following steps: motion correction, removal of non-brain tissue, spatial smoothing using a Gaussian kernel of 6mm full-width at half-maximum (FWHM), grand-mean intensity normalization of the entire 4D dataset by a single multiplicative factor, and a high-pass temporal filter of 100 seconds (i.e., 0.01 Hz). PASL volumes were registered to the individual participant’s T1-weighted high-resolution anatomical volume using an affine registration with 12 degrees of freedom. The T1-weighted image was then linearly registered to the 2mm isotropic MNI-152 standard space image (T1-weighted standard brain averaged over 152 subjects; Montreal Neurological Institute, Montreal, QC, Canada). The resulting transformation matrices were then concatenated to create a functional to standard space transformation matrix, which was used to write the perfusion volumes into standard space.
Physiological and motion noise removal
A single-session independent component analysis (ICA) was performed to de-noise the 4D PASL data of subject motion and physiological artifact (e.g., cardiac and respiratory pulsation (Beckmann et al., 2005). Components were classified as noise if they met one or more of the following criteria: if signal was predominately located in white matter or ventricles; if the component’s power spectra showed erratic fluctuations; if there were enclosed rings of activation around the edges or superior slices of the brain; and if there were spikes in the principle eigenvector timeseries that exceeded 6 scale units, which is usually indicative of head motion. The components that were identified as noise were then filtered from each participant’s preprocessed PASL data.
To further reduce the influence of artifact, perfusion signal from white matter (WM) and cerebrospinal fluid (CSF) were modeled as nuisance regressors in the subsequent GLM. This noise removal technique relies on the assumption that CSF and WM carry physiological fluctuations that contain little contribution from neural activity (Fox et al., 2005). WM and CSF masks were produced by segmenting each individual’s T1-weighted structural image into tissue-type partial volume masks using FMRIB’s Automated Segmentation Tool (FAST). The CSF and WM masks were then co-registered to functional space resolution (2mm3) by applying the inverse transformation matrix produced by registering each participant’s T1 image to their mean perfusion volume. The re-sampled tissue masks were thresholded at 90% partial volume fraction and binarized in order to minimize overlap with grey matter. Finally, the resultant tissue masks were applied to each individual’s preprocessed perfusion data, and a mean timecourse was extracted by averaging the timeseries across all voxels within each mask.
Grey matter template
A study-specific grey matter (GM) mask was also created in order to further exclude non-grey matter contributions to the perfusion signal. To produce the GM template, each participant’s GM partial volume image was affine registered to the MNI152 standard space brain. The registered images were then merged into a single group 4D file, averaged. This group averaged GM image was spatially smoothed with a 8mm FWHM Gaussian kernel, thresholded at 30% tissue-type probability and binarized to constrain subsequent analyses to grey matter regions.
Amygdala seed-based correlation analysis
A seed-based correlation analysis was used to assess the effects of cold-pressor task on subsequent amygdala rsFC. Left and right amygdala seeds were defined using the Harvard-Oxford Atlas in FSL. To minimize overlap between the amygdala and neighboring brain regions, these seed masks were thresholded at 50% amygdala probability. For each participant, a lower-level, fixed-effects general linear model (GLM) analysis was used to identify statistically significant amygdala rsFC patterns within each session. A variation of FSL’s full perfusion signal modeling was used in order to extract perfusion-only signal from control-tag image pairs (Mumford et al., 2006). In this modeling technique, the control/label effect is built into the model implicitly. Specifically, the alternating control and label images are modeled as the “ON” and “OFF” periods of a square waveform, respectively, with each period equal to the duration of a single TR (4 seconds). Full perfusion modeling was chosen, because it provides optimal power for the analysis of ASL data and has been shown to be more sensitive than the standard approach of pairwise differencing (Mumford et al., 2006). A second regressor was used to control for the effects of the slow BOLD signal, which contaminates perfusion signal. The BOLD nuisance signal, modeled as the mean of the tag and control volumes, was convolved with the square perfusion waveform using a standard hemodynamic response function (HRF) convolution.
A seed physiological regressor was created by extracting the mean timeseries of activity across all of the voxels in both left and right amygdala. The mean left and right amygdala timeseries were used as temporal predictors in separate whole-brain multiple linear regressions. Additionally, WM and CSF nuisance variables were modeled in the GLM and regressed from the data. Amygdala rsFC maps were produced for each subject/session such that significant clusters represent synchronous perfusion signal fluctuations with the amygdala seed regions. Significant patterns of left and right amygdala rsFC were determined using cluster-correction and a Z-threshold of 2.3 (P = 0.05). The resulting contrast images from the session-level analysis were then processed in a higher-level random-effects group analysis in order to account for inter-subject variability. The study-specific GM group template was also applied to limit testing to GM-only voxels. To identify amygdala rsFC differences between the stressor and control conditions, a paired t-test was conducted on pairs of contrast images from each participant’s two scanning sessions. These spatial maps were also thresholded at a cluster size of Z > 2.3 and a whole-brain significance of P < 0.05. To examine whole-brain patterns of positive and negative left and right amygdala rsFC, group averages were also calculated using one-sample t-tests. Four separate whole-brain statistical parametric maps were produced in order to identify brain regions that were either positively or negatively correlated with perfusion signal fluctuations in the left and right amygdala seed regions, separately.
Amygdala connectivity-defined ROI analysis
A region-of-interest (ROI) analysis was conducted to determine the direction of functional connectivity between the right/left amygdala and significant clusters identified in the group-level seed-based correlation analysis. Significant mean group differences were only observed in the control - stressor contrast, so these ROIs represent brain regions that were functionally decoupled from the amygdala after the cold pressor task. The spatial location and extent of the stressor-induced amygdala rsFC changes was highly consistent between the group of 14 participants with cortisol data and the full group of 17 participants (Supplementary Figure 1). Thus, the amygdala functional connectivity data from the full group of 17 participants was used in subsequent analyses.
Several of the clusters were widely dispersed and covered brain regions with known functional heterogeneity. Therefore, smaller ROIs were defined as 8 mm-diameter spheres in MNI space, where peak coordinates corresponded to the four peak voxels from the control -stressor left and right amygdala rsFC contrasts. The four ROI spheres were centered about: left dACC (−8, 26, 34) and right lateral OFC (22, 28, −24) for the left amygdala seed region; and left mOFC (−8, 34, −28) and right posterior cingulate cortex (16, −20, 44) for the right amygdala seed region. Significant clusters from the left and right amygdala control - stressor contrast maps were thresholded at Z > 3 and intersected with their respective sphere ROIs to increase spatial specificity. The resulting masks were visually inspected to ensure that there was no overlap between any of the ROIs. The ROI masks were then binarized and applied to each session/subject’s lower-level amygdala rsFC maps to extract mean Z-scores. These Z-scores represent the strength of rsFC between the amygdala and the target brain regions; positive Z-scores signify a positive correlation between the ROI and temporal fluctuations in the amygdala perfusion signal, whereas negative Z-scores refer to negatively correlated perfusion signal changes with the amygdala seed regions. In addition, we performed two-tailed single-sample t-tests against zero to investigate whether functional coupling between the amygdala and target ROI regions was significantly positive or negative across the group within the stressor and control sessions.
Associations between stressor-altered amygdala functional connectivity, pain and cortisol outcomes
To determine whether the four stressor-related amygdala rsFC ROIs were associated with cortisol change or subjective pain measures, we correlated the mean connectivity Z-scores for each ROI with the following behavioral variables: 1) Pain VAS rating; 2) Faces Pain Scale rating; 3) Cold pressor hand immersion duration; and 4) Cortisol change. In addition, partial correlation analyses were performed by controlling for cold-pressor duration and condition order, separately.
As a test of our main hypotheses, we performed a t-test to test for any significant differences between the two dependent correlations, i.e., pain and amygdala rsFC and cortisol and amygdala rsFC, with 11 (n-3) degrees of freedom (Blalock, 1972). Only the 14 participants with complete pain and cortisol data were used in order to allow for a direct comparison between stressor-induced amygdala rsFC, cortisol responsivity and pain measures.
Results
Cortisol
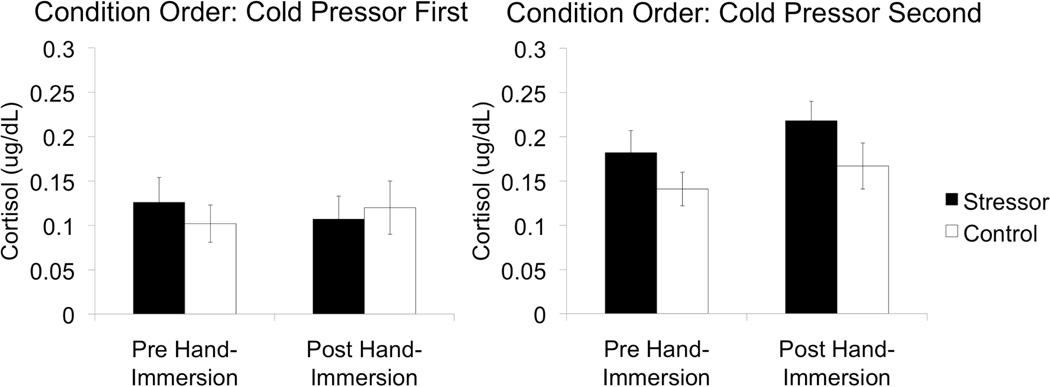
The results for the cortisol analyses are displayed in Figure 1. Salivary cortisol concentration was not significantly affected by the cold pressor task, as indicated by no significant main effects of condition, F(1,12) = 1.0, p = .26, time of the saliva sample, F(1,12) = 0.9, p = .36, or condition x time interaction, F(1,12) = 0.20, p = .66. Interestingly, there was a significant main effect of condition order on salivary cortisol, F(1,12) = 14.71, p = .002, indicating that cortisol levels were generally higher in the control-condition-first participants (see Fig. 1). There were no condition x order, F(1,12) = 0.90, p = .36, nor condition x time x order, F(1,12) = 0.55, p = .47, effects on cortisol concentration.
Figure 1.
Comparison of salivary cortisol between the stressor and control conditions pre hand-immersion (sample 1) and post hand-immersion (sample 2; measured after the PASL scan). To illustrate the main effect of condition order on cortisol level, the measurements for participants who performed the cold pressor task on day 1, i.e., stressor-condition-first, are presented separately from participants who performed the cold pressor task on day 2, i.e., control-condition-first.
Follow-up t-tests revealed a significant difference between the stressor-condition-first and control-condition-first groups only in the post-stressor cortisol sample (p = .007). Pre-stressor (baseline in the stress condition) cortisol samples did not significantly differ between these two groups (p = .40). Thus, elevated cortisol levels in the control-condition-first participants appeared to be driven by differences in the post-stressor sample as opposed to the pre-stressor sample. This makes it unlikely that anticipation of the stressor on day 2 can account for the main effect of order observed in the ANOVA.
Separate follow-up repeated-measures ANOVAs were conducted to determine the effects of time and condition on cortisol levels for the stressor-condition-first and control-condition-first groups. There were no significant time, condition, or time x condition interaction effects on salivary cortisol level in either of these two groups (p’s > 0.1). Thus, regardless of condition order, no significant effects of stressor condition were seen on cortisol levels.
The amount of time between stressor onset and the beginning of the PASL scan did not significantly affect cortisol concentration, F(1,15) = 1.77, p = .20, thereby verifying that variability in the measurement window could not account for differences in salivary cortisol concentration. The mean time between the two saliva samples also did not differ between the stressor and control conditions (p = .79).
Subjective Pain and Stress
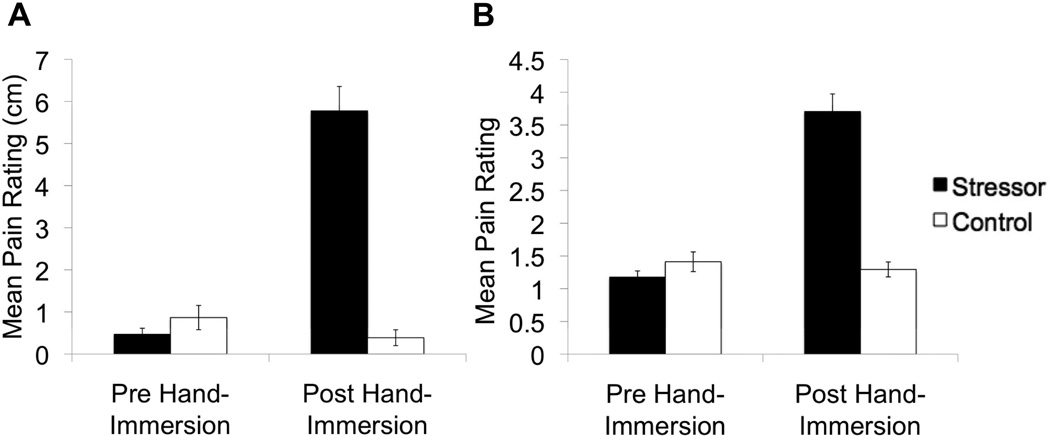
The results for the subjective pain measures are displayed in Figure 2. Though a reliable elevation in stressor-induced cortisol level was not observed, the cold pressor task did elicit a statistically significant increase between pre- and post-stressor ratings on the Pain VAS, F(1,15) = 61.01, p < .001, and Faces Pain Scale, F(1,15) = 73.30, p < .001. Subjective pain intensity was also highly significant when analyzing both pain measures for the group of 14 participants with cortisol data (p’s < .001). The condition order also marginally affected Faces Pain Scale ratings, with mean pain ratings being higher for control-condition-first participants, F(1,15) = 4.2, p = .058. This condition order effect was not observed for the Pain VAS, F(1,15) = 1.29, p < .274. The duration of hand immersion was significantly shorter for the ice water (M = 114 seconds +/− 14 seconds) than the warm water (M = 180 seconds +/− 0 seconds; p < .001) and did not differ by condition order (p = .39). Paired t-tests verified that participants’ baseline self-report of overall stress level, relative stress level and mood did not differ between conditions (p’s > .05).
Figure 2.
(A) Comparison of mean self-reported pain on the Pain VAS between stressor and control conditions. The means represent the post-pre hand immersion ratings, which were based on the distance of the hash mark on the line spectrum in centimeters. (B) Comparison of mean self-reported pain on the Faces Pain Scale between stressor and control conditions. The means represent the post-pre hand immersion ratings, thereby indicating the effects of water temperature on perceived pain. Error bars represent standard error of the mean (SEM).
Correlations Between Pain and Cortisol Outcomes
Results of the Pearson bivariate and partial correlations between stressor-related cortisol and pain ratings are presented in Table 1. As indicated in the table, all correlations that were significant for the full sample were also significant for the subsample of 14 participants who had complete cortisol data. The stressor-related (stressor – control) changes in self-reported pain intensity were highly consistent between the two pain measures (r = .76, p < .001). Increased stressor-related Pain VAS ratings were negatively associated with cold pressor duration time (r = −.57, p = .018), indicating that participants who were better able to endure the cold pressor task, or maintain ice water hand immersion, were also more likely to report experiencing less pain. While this association between subjective pain intensity and cold pressor duration was only marginally significant for the Faces Pain Scale with the full sample (r = −.42, p = .094), it was significant for the 14 participants who had complete cortisol data (r = −.56, p = .036).
Table 1.
Associations between stressor-related changes in pain and cortisol outcome measures
| aBivariate Correlations | N | Change in Pain VAS |
Change in Faces Pain |
Change in Cortisol |
|---|---|---|---|---|
| bChange in Pain VAS | 17 | ---- | ---- | ---- |
| Change in Faces Pain Scale | 17 | .76***† | ---- | ---- |
| Change in Cortisol | 14 | −.41 | −.28 | ---- |
| Change in CPT Duration | 17 | −.57*† | −.42† | .11 |
| cControlling for Condition Order | Change in Pain VAS |
Change in Faces Pain |
Change in Cortisol |
|
| Change in Pain VAS | 17 | ---- | ---- | ---- |
| Change in Faces Pain Scale | 17 | .72**† | ---- | ---- |
| Change in Cortisol | 14 | −.53 | −.38 | ---- |
| Change in CPT Duration | 17 | −.54*† | −.37 | .26 |
| cControlling for Change in CPT Duration | Change in Pain VAS |
Change in Faces Pain |
Change in Cortisol |
|
| Change in Pain VAS | 17 | ---- | ---- | ---- |
| Change in Faces Pain Scale | 17 | .69**† | ---- | ---- |
| Change in Cortisol | 14 | −.47 | −.27 | ---- |
Notes: CPT = cold pressor task; VAS = visual analogue scale.
Pearson’s correlation coefficient (r).
Change refers to post-pre difference scores for the stressor condition minus post-pre difference scores for the control condition.
Pearson’s partial correlation coefficients.
p < .05,
p < .01,
p < .001,
p-value is also significant (p < .05) for the group of 14 participants with cortisol data.
A marginally significant negative association was found between stressor-related cortisol change and Pain VAS ratings (r = −.41, p = .14). Interestingly, this trend was almost significant after controlling for condition order (r = −.53, p = .064). However, the association between cortisol change and stressor-related Faces Pain Scale rating change was weaker (p’s > .05).
Amygdala Seed-Based Correlation and ROI Analysis
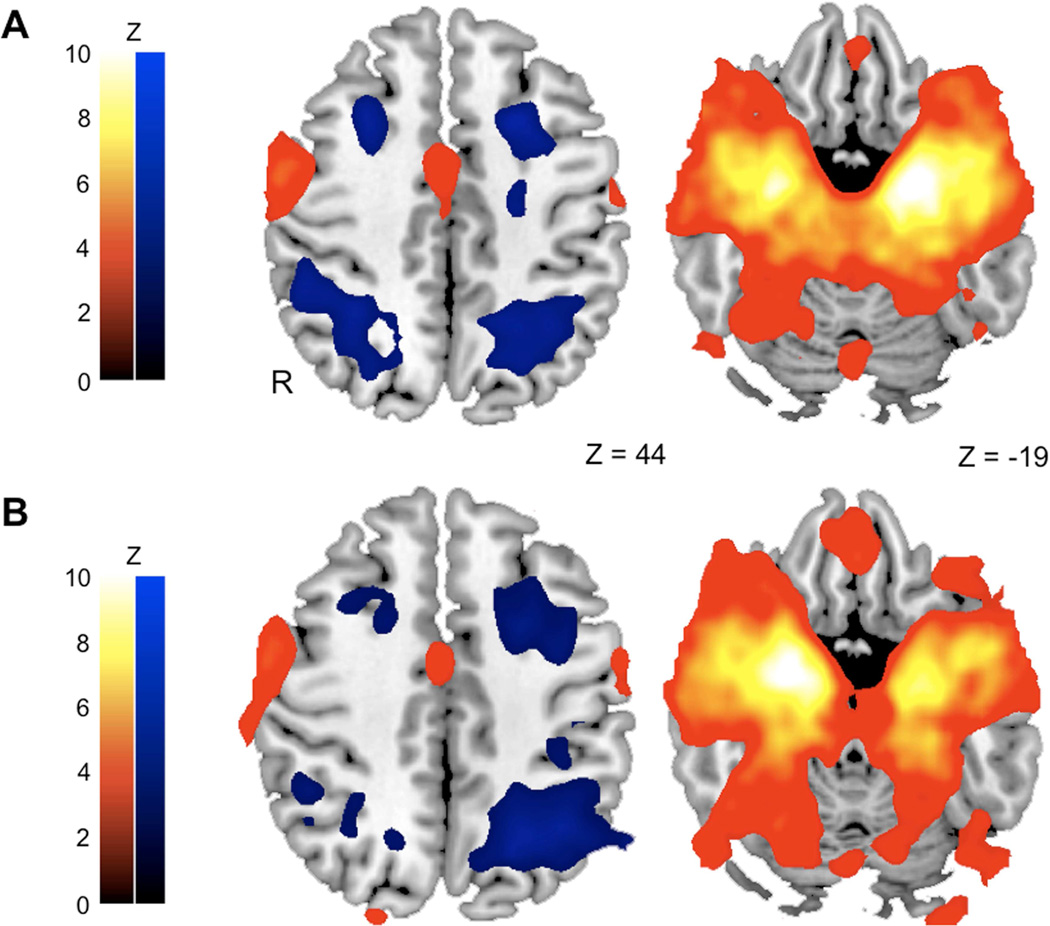
The group random effects rsFC analysis revealed distributed amygdala functional networks across the brain (Figure 3). These whole-brain patterns of amygdala rsFC are consistent with previous studies (Roy et al., 2009; Robinson et al., 2010), which describe a dorsal-ventral dissociation between two amygdala-based circuits: positive correlations with regions implicated in emotion-generation and regulation, such as MPFC and thalamus versus negative correlations with regions involved in cognitive processes, such as middle frontal gyrus and precuneus. We also observed amygdala rsFC differences along the medial-lateral axis such that the amygdala was positively correlated with medial brain regions and negatively correlated with more lateral cortical regions.
Figure 3.
Group average (A) left and (B) right amygdala resting-state functional connectivity (N =17). Patterns of activation reflect grey matter regions where dynamic perfusion signal changes were either significantly positively correlated (red) or negatively correlated (blue) with perfusion signal changes in the amygdala seed regions. The amygdala rsFC spatial maps were collapsed across stressor and control sessions and whole-brain statistical parametric maps were calculated using single-sample t-tests. Significant clusters were corrected for multiple comparisons using a cluster threshold of Z > 2.3, P = 0.05.
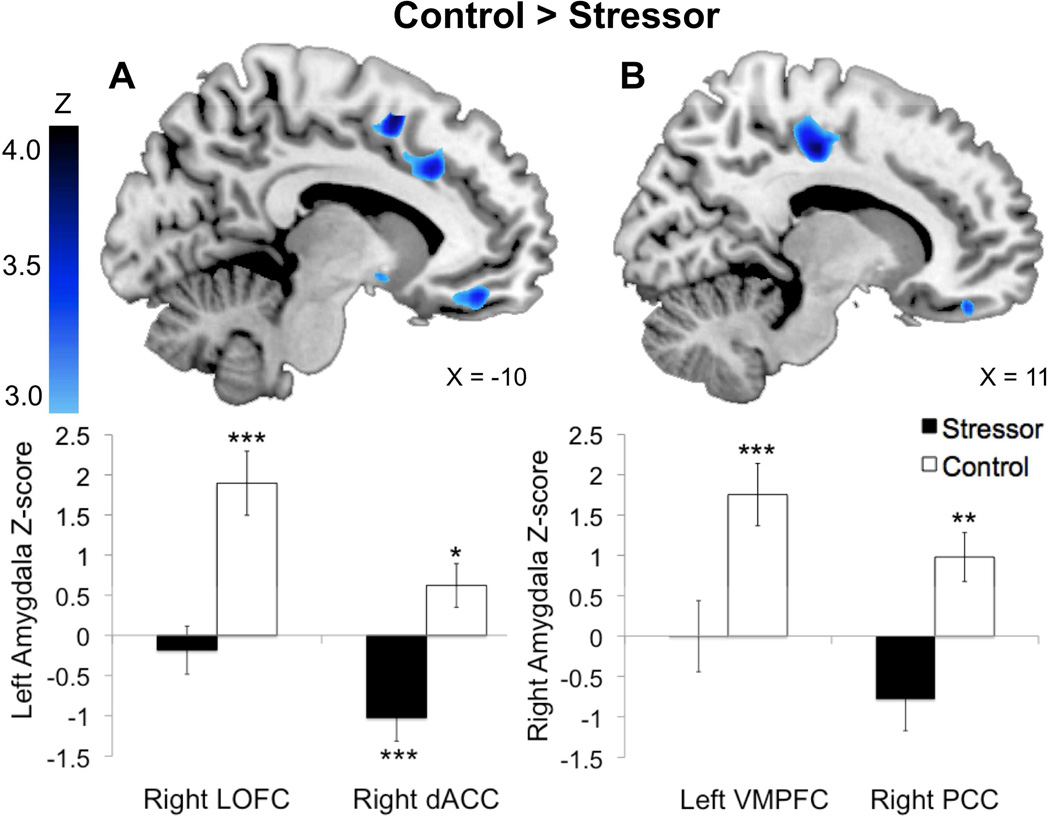
Results of the paired t-test comparison between the stressor and control conditions are presented in Figure 4. We observed greater left amygdala positive functional connectivity in the control versus stressor condition with the right LOFC, left VMPFC, right frontal pole, right VMPFC and a distributed cluster spanning the left dACC, left supplementary motor area (SMA), and left superior frontal gyrus (SFG). The stressor - control contrast yielded no significant connectivity differences. Extraction of the mean Z-scores from the dACC cluster revealed that this region was significantly positively coupled with left amygdala during the control condition (p = .047). In contrast, this dACC cluster was negatively correlated with left amygdala activity during the stressor condition (p = .0016). In addition, we found significant positive functional coupling between the left amygdala and right OFC during the control condition (p < .001). The inverse left amygdala functional coupling with right OFC during the stressor condition was not significantly different than zero (p > .05).
Figure 4.
(A) Left amygdala and (B) right amygdala resting-state functional connectivity clusters from the group-level random effects analysis (control – stressor) contrast, cluster-corrected at Z > 2.3, P = 0.05). Clusters are thresholded at Z > 3 for greater spatial specificity. Mean connectivity Z-scores were extracted for each of the target brain regions from each subject/session’s lower-level stressor and control session spatial maps. Two-tailed, single t-tests were performed against zero to determine whether amygdala coupling with each connectivity-defined target region was significantly positive or negative for the stressor or control condition. Overall, the cold pressor task led to significant reductions in functional connectivity between the amygdalae and prefrontal/cingulate cortex. The stressor also induced a significant negative association between perfusion signal fluctuations in the left amygdala and right dorsal anterior cingulate cortex (dACC). Error bars represent standard error of the mean (SEM). LOFC = lateral orbitofrontal cortex; VMPFC = ventromedial prefrontal cortex; PCC = posterior cingulate cortex. *p < .05, **p < .01, ***p < .005.
Compared with the stressor condition, the control condition showed greater positive right amygdala rsFC with left paracentral lobule/posterior cingulate cortex (PCC), right SMA, left VMPFC/OFC, right MPFC, left MPFC, left frontal pole, and right subcallosal cortex. There were no significant connectivity differences in the opposite contrast (stressor - control). The right amygdala was significantly positively coupled with both the PCC and left VMPFC during the control session (p = .0054 and p = .0034, respectively). During the stressor condition, inverse functional connectivity between the right amygdala and right PCC ROI was marginally significant (p = .06), but right amygdala coupling with the left VMPFC was not significant (p > .05). Thus, the cold pressor task functionally decoupled the right amygdala from these target brain regions. We also found three small regions in VMPFC/OFC (31 voxels total) where stressor-related left and right amygdala functional decoupling overlapped.
Relationship Between Stressor-Altered Amygdala Functional Connectivity, Pain and Cortisol
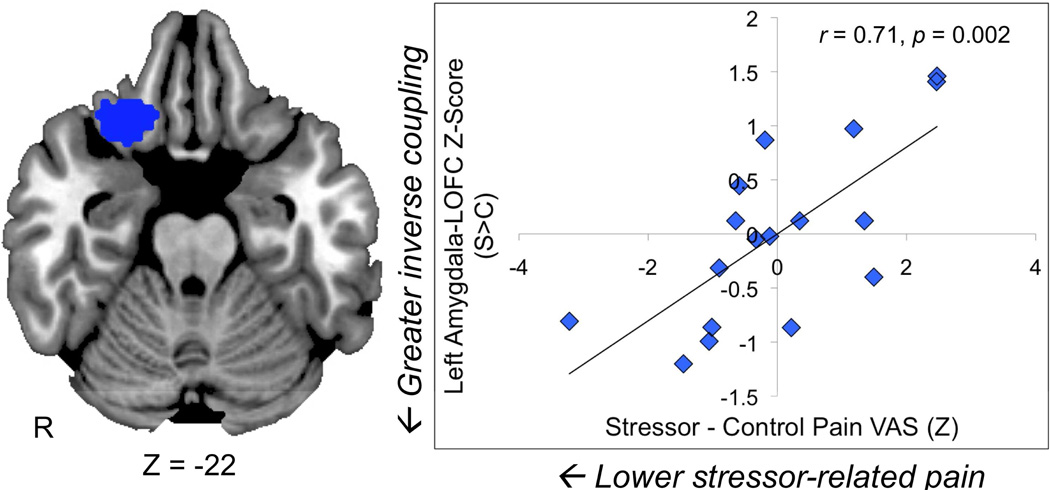
We performed Pearson bivariate and partial correlations to determine the functional significance of cortisol and pain outcomes on stressor-induced amygdala decoupling. The results of these correlations are presented in Table 2. We found a significant positive association between stressor-related Pain VAS ratings and functional coupling between the left amygdala and right LOFC (r = .5, p = .042). This indicates that participants who reported experiencing greater pain during the cold pressor also exhibited enhanced amygdala-LOFC functional connectivity approximately 15–30 minutes later. The strength of this association increased after controlling for cold pressor duration time (r = .71, p = .002; Figure 5). A positive association between stressor-induced changes in Faces Pain Scale ratings and left amygdala-LOFC functional connectivity also emerged after controlling for cold pressor duration (r = .54, p = .03). The relationship between Pain VAS and left amygdala-LOFC coupling was still significant for the subgroup of 14 participants with cortisol data (r = .59, p = .027), including after controlling for cold pressor duration (r = .61, p = .028).
Table 2.
Associations between stressor-related changes in amygdala resting-state functional connectivity regions-of-interest, pain measures and cortisol
| N | Pearson’s r |
c Controlling for Condition Order |
c Controlling for Change in CPT Duration |
|
|---|---|---|---|---|
| a Change in Pain VAS with | ||||
| c Change in Left Amygdala-LOFC | 17 | .50*† | .44† | .71**† |
| Change in Left Amygdala-dACC | 17 | .26 | .17 | .09 |
| Change in Right Amygdala-PCC | 17 | −.4 | −.31 | −.32 |
| Change in Right Amygdala-MPFC | 17 | −.05 | −.17 | −.12 |
| Change in Faces Pain Scale with | ||||
| Change in Left Amygdala -LOFC | 17 | .43 | .34 | .54* |
| Change in Left Amygdala-dACC | 17 | .01 | −.15 | −.16 |
| Change in Right Amygdala-PCC | 17 | −.39 | −.27 | −.31 |
| Change in Right Amygdala-MPFC | 17 | .17 | .05 | .15 |
| Change in CPT Duration with | ||||
| Change in Left Amygdala-LOFC | 17 | .15 | .24 | ---- |
| Change in Left Amygdala-dACC | 17 | −.35 | −.29 | ---- |
| Change in Right Amygdala-PCC | 17 | .26 | .19 | ---- |
| Change in Right Amygdala-MPFC | 17 | −.09 | −.02 | ---- |
| Change in Cortisol with | ||||
| Change in Left Amygdala-LOFC | 14 | −.2 | −.23 | −.18 |
| Change in Left Amygdala-dACC | 14 | .01 | −.14 | .04 |
| Change in Right Amygdala-PCC | 14 | −.15 | −.09 | −.25 |
| Change in Right Amygdala-MPFC | 14 | .41 | .37 | .45 |
Notes: LOFC = lateral orbitofrontal cortex; dACC = dorsal anterior cingulate cortex; PCC = posterior cingulate cortex; MPFC = medial prefrontal cortex; CPT = cold pressor task; VAS = visual analogue scale.
Partial correlation coefficients.
Change refers to post-pre difference scores for the stressor condition minus post-pre difference scores for the control condition.
Change refers to stressor minus control condition mean connectivity z-scores.
p < .05,
p < .01,
p-value was also significant (p < .05) for the group of 14 participants with cortisol data.
Figure 5.
Individuals who reported lower pain intensity during the cold pressor task also demonstrated greater inverse resting-state functional connectivity (rsFC) between the left amygdala and right lateral orbitofrontal cortex (LOFC). The blue cluster represents a functionally-defined region-of-interest (ROI) identified by the left amygdala seed-based correlation analysis. Mean connectivity Z-scores were extracted for this ROI from each subject/session’s lower-level stressor and control left amygdala rsFC maps. The scatter plot depicts the association between stressor-induced amygdala-LOFC functional coupling (stressor -control; y-axis) and Pain VAS residuals (stressor – control values; x-axis) after controlling for cold pressor duration time.
After controlling for condition order, stressor-induced changes in Pain VAS ratings and left amygdala-LOFC coupling became significant in the group of 14 participants (r = .59, p = .033), but not in the full sample (r = .44, p = .09). We did not observe any significant associations between cortisol or subjective pain and the left amygdala-dACC, right amygdala-VMPFC, or right amygdala-PCC ROIs.
Though stressor-related left amygdala-LOFC functional coupling was not significantly related to cold pressor-induced cortisol increase (r = −.21, p = .49), this trend was in the opposite direction than Pain VAS. A t-test revealed a marginally significant difference between the left amygdala-LOFC pathway’s associations with cortisol versus with pain intensity (t = 1.93, p = .08), suggesting that stressor-induced amygdala-LOFC rsFC was more closely related to previously experienced pain than cortisol responsivity.
Discussion
Using pulsed arterial spin labeling (PASL), we found that an acute painful stressor alters amygdala functional connectivity with frontal and ACC regions 15 to 30 minutes after stressor onset. This study highlights the utility of PASL in detecting activation differences in brain regions that are vulnerable to signal loss, such as the OFC (Wang et al., 2004). Our functional connectivity results extend earlier findings that higher-order cortical regions modulate amygdala activity following an aversive experience.
Our first main finding was that exposure to the cold pressor reduced subsequent rsFC between the amygdalae and large regions spanning the VMPFC and OFC. This stressor-induced shift in amygdala rsFC is supported by evidence of dense reciprocal anatomical connections between the amygdala and the VMPFC/OFC (Ghashghaei and Barbas, 2002; Amaral and Price, 1984). Moreover, tracing studies in rodents have uncovered anatomical pathways linking the left and right amygdala to contralateral prefrontal cortex (Cavada et al., 2000; McDonald et al., 1996; Vertes, 2004). The posterior region of OFC receives and integrates polymodal sensory inputs, which enables encoding of the aversive value of a threatening stimulus through connections with the amygdala (Kringelbach and Rolls, 2004). Evidence from human lesion studies corroborates this role of the OFC, showing that lesions in this region lead to deficits in the ability to label sensory events as either pleasant or unpleasant (Rule et al., 2002). In the wake of physical insult, this disruption in amygdalo-frontal functional coupling could reflect attenuated processing of the aversive stressor. This aligns with evidence that prefrontal lobotomy patients’ show decreased spontaneous concern or rumination about their pain (Hardy et al., 1952). In the period after the stressor is removed, response priorities shift away from threat-related processing. As a result, decreased functional connectivity in this pathway could be a mechanism for reducing any unpleasantness associated with acute pain, thereby enabling the re-institution of homeostasis.
It is important to note that a previous study also showed reduced amygdala-MPFC coupling after an aversive event, but proposed that this decoupling instead reflects persistent negative interpretations and rumination evoked by the unpleasant stimuli (Eryilmaz et al., 2011). Our differing interpretations may be due to several important design differences. While our study only involved a single exposure to an aversive stimulus, the intermittent presentation of fearful movie excerpts by Eryilmaz and colleagues likely enhanced anticipatory anxiety and sustained fear. Furthermore, their resting-state scans were performed immediately after participants viewed each film clip, whereas we examined brain perfusion patterns approximately 15 to 30 minutes after stressor offset. Previous studies have linked rumination to increased cortisol levels after a stressor (Roger and Najarian, 1998; Zoccola et al., 2008), but we did not observe a reliable cortisol increase after the cold pressor task. Moreover, we found that individual differences in cortisol response were not related to neocortical interactions with the amygdala. Thus, in our study MPFC-amygdala decoupling did not appear to be due to enhanced ruminative processes.
Exposure to the cold pressor task also led to anti-correlated functional connectivity between the left amygdala and right dACC. This functional interaction is supported by robust reciprocal anatomical connections between these two regions (Vogt and Pandya, 1987). The anterior portion of dACC, in particular, receives direct input from the amygdala, which plays a central role in both fear and nociception (Bernard et al., 1992; Whalen et al., 1998). In accord with our results, increased activity in this region is often inversely correlated with amygdala response (Etkin et al., 2006; Hariri et al., 2003). Because we used a seed-based correlation approach, we were unable to infer any causal relationship between these regions. However, models of effective connectivity suggest that the dACC exerts top-down influences over the amygdala (Stein et al., 2007). It should be noted that the strength of this coupling was not associated with participants’ self-reported pain intensity during the cold pressor task. In pain manipulation studies, the dACC is primarily implicated in apprasing the affective-motivational component of nociception (Coghill et al., 2003; Maihofner and Handwerker, 2005; Rainville et al., 1997). Other studies have shown that the reappraisal of aversive events is often associated with increased activity in the dACC and decreased activity in the amygdala (Ochsner et al., 2002; Modinos et al., 2010), despite evidence that regulation is primarily localized to more ventral ACC regions (Etkin et al., 2011). Taking this into account, left amygdala-dACC decoupling after the cold pressor task could be indicative of negative emotion reappraisal.
Beyond group-level changes in stressor-induced amygdala functional connectivity, left amygdalar recruitment of the right posterior LOFC was negatively associated with self-reported pain intensity during the cold pressor task, especially after controlling for cold pressor duration. In a study of frontal-lobe-damaged patients, ratings for cold pressor pain intensity and unpleasantness were found to be significantly lower than controls (Talbot et al., 1995). Increased activation of the posterior OFC has also been linked to lower pain intensity ratings (Derbyshire et al., 1997), and electrical stimulation of the VMPFC/OFC produces analgesia in both primates and non-primates (Thorpe et al., 1983; Zhang et al., 1997). Neuroimaging studies in humans are in accord with these findings, revealing that greater activity in this region is inversely related to subjective pain intensity (Salomons et al., 2007; Wiech et al., 2006). However, the OFC is not the only brain region associated with shaping pain perception. Using a thermal pain paradigm, Lapate and colleagues showed that amygdala activity varies as a function of pain regulation success (Lapate et al., 2012). In addition, suppression of the startle reflex, an autonomic reflex that is dependent on the amygdala (Davis et al., 2010), has been observed during the cold pressor task, suggesting that amygdala activity is regulated while participants are forced to endure acute cold pain (Tavernor et al., 2000). Here, we reconcile findings from studies examining individual brain regions by showing that the relationship between posterior OFC and pain intensity may, at least indirectly, be mediated by altered OFC functional coupling with the left amygdala.
Interestingly, individuals who reported greater pain intensity during the cold pressor task also demonstrated a marginally significant decrease in salivary cortisol after the stressor. Exposure to stressors not only triggers the release of cortisol but a host of stress-related neurotransmitters, including endogenous opioids (Drolet et al., 2001). Growing evidence suggests that there is an antagonistic relationship between cortisol and opioids, as the endogenous opioid system has been shown to inhibit HPA axis activation (al’ Absi et al., 2004; Drolet et al., 2001). Thus, one intriguing possibility is that individuals who felt more pain during the cold pressor also triggered greater opioid release (Frew and Drummond, 2007). This is consistent with the notion that the endogenous opioid system underlies an “opponent process,” whereby greater pain intensity corresponds with greater pain relief during stressor offset (Leknes et al., 2008). Several limbic regions, including dACC, OFC and amygdala, are central target sites for opioid binding, making them prime candidates for the modulation and processing of pain (Fields, 2004; Zubieta et al., 2005). Furthermore, endogenous opioids typically have a delayed onset of several minutes to maximum effect (Price and Barrell, 2000). These delayed effects are slow to dissipate, which agrees with our finding of residual rsFC changes between the amygdala and higher-cortical nodes of this opiate-sensitive network 15 to 30 minutes after the stressor. Thus, variability in stressor-induced cortisol increase may have been a function of opioid-mediated engagement of a descending pain network (Bingel and Tracey, 2008; Watkins and Mayer, 1982). Our study design did not allow for a direct assessment of opioid release, so inclusion of such measures in future studies may help clarify whether the patterns of amygdala rsFC we observed were driven by opiodergic systems.
One limitation of our study was that we only used male participants. Thus, it is not clear if the lack of a reliable stressor-related increase in cortisol was a sex-specific effect. Previous studies show mixed results regarding whether men or women show greater HPA activation to the cold pressor task, with some indicating men (e.g., Dixon et al., 2004) and others women (e.g., Lighthall et al., 2009). Considering the anti-correlated trend between cortisol and pain intensity ratings, these mixed results could be attributed to individual differences in pain sensitivity or opioid release. Men demonstrate greater µ-opioid receptor activation in the amygdala during sustained pain than women (Zubieta et al., 2002), suggesting that different mechanisms are activated in women in response to painful stressors. Animal studies also indicate that acute stress affects the amygdala differently in males and females (Mitsushima et al., 2006; Wang et al., 2009). In humans, amygdala functional connectivity differs between males and females at rest (Kilpatrick et al., 2006), and the cold pressor task affects amygdala functional connectivity differently in males and females while participants view emotional faces (Mather et al., 2010). Similar to our findings, Mather et al. found that the cold pressor task reduced amygdalar recruitment of emotion processing regions in men, but not in women. Together these findings suggest there are sex differences in pain and emotion processing regions during stress, with men showing greater engagement of endogenous analgesic systems. Future stress studies should include female participants to elucidate how gender differences in amygdala reactivity influence recovery from painful stressors.
To our knowledge, this is the first study to show that the effects of an acute physical stressor can be detected in cognitive-affective circuits during rest, as opposed to single brain regions, approximately 15 to 30 minutes after its onset/offset. This finding complements emerging evidence that the amygdala plays an important role in persistent pain states (Neugebaur et al., 2004). Chronic inflammatory pain causes changes in the excitability and synaptic strength of noxious-detecting neurons in the amygdala (Neugebauer and Li, 2003). Similarly, enhanced activity in the amygdala can occur one week after the onset of pain, supporting a pain-producing or pain-enhancing role of the amygdala (Ikeda et al., 2007). Observations of pain-related structural changes are also in accord with functional studies. Specifically, the experience of chronic pain has been characterized by grey matter density declines in the amygdala, ACC and OFC/MPFC, suggesting that re-organization of pain modulation networks may contribute to the chronification of pain (Burgmer et al., 2009; see May, 2011 for a review). While speculations on chronicity are beyond the scope of this study, our observation of stressor-induced amygdala rsFC changes 15 to 30 minutes later suggests that pain-related modulation of amygdala activity persists even after the physical sensations have dissipated.
While we obtained pain intensity ratings immediately before and after the hand-immersion task, no further pain scales were completed during the PASL scan. As a direct follow-up to this study, future experiments could examine how pain and stress influence the temporal dynamics of amygdala functional connectivity by administering assessments of coping strategy, pain intensity and stress level throughout the PASL scan. Furthermore, an evaluation negative affect could help determine whether post-stressor amygdala functional connectivity relates to emotion regulation, particularly with the dACC. Acquiring concurrent measures of stress reactivity and pain could help dissociate the neural mechanisms that facilitate post-stressor recovery and help identify aberrant amygdala functional connectivity patterns that predict vulnerability to pathological pain and emotion states, such as depression, post-traumatic stress disorder, anxiety disorders, and chronic pain.
Supplementary Material
Table 3.
List of significant clusters from the group-level left and right amygdala seed-based resting-state functional connectivity analysis
|
Left Amygdala Group Average |
||||||
|---|---|---|---|---|---|---|
| Positive Functional Connectivity | Cluster Index |
Z-Stat | X | Y | Z | Cluster Size (voxels) |
| L Amygdala | 2 | 12.4 | −24 | −4 | −22 | 54444 |
| R Amygdala | 2 | 9.63 | 30 | −4 | −20 | |
| L Temporal Pole | 2 | 9.11 | −42 | 4 | −22 | |
| L Middle Temporal gyrus | 2 | 7.98 | −50 | −6 | −18 | |
| R Planum Temporale | 2 | 7.95 | 42 | −2 | −18 | |
| R Hippocampus | 2 | 7.58 | 40 | −18 | −16 | |
| L Frontal Pole | 1 | 3.8 | −10 | 72 | 12 | 1803 |
| L Medial Prefrontal Cortex | 1 | 3.54 | −2 | 50 | −18 | |
| L Frontal Pole | 1 | 3.43 | −2 | 62 | −14 | |
| Medial Prefrontal Cortex | 1 | 3.4 | 0 | 42 | −14 | |
| Left Frontal Pole | 1 | 3.2 | −4 | 68 | 18 | |
| Frontal Pole | 1 | 3.06 | 0 | 64 | 34 | |
|
Left Amygdala Group Average |
||||||
| Negative Functional Connectivity | Cluster Index |
Z-Stat | X | Y | Z | Cluster Size (voxels) |
| L Middle Frontal Gyrus | 2 | 4.8 | −30 | 12 | 34 | 7591 |
| L Superior Frontal Gyrus | 2 | 4.66 | −24 | 16 | 46 | |
| L Precuneus | 2 | 4.65 | −20 | −62 | 30 | |
| L Middle Frontal Gyrus | 2 | 4.62 | −26 | 14 | 38 | |
| L Lateral Occipital Cortex | 2 | 4.54 | −20 | −62 | 36 | |
| L Supracalcarine Cortex | 2 | 4.44 | −24 | −62 | 22 | |
| R Superior Parietal Lobule | 1 | 5.52 | 30 | −44 | 36 | 5785 |
| R Cererbral White Matter | 1 | 5.38 | 34 | −2 | 34 | |
| R Middle Frontal Gyrus | 1 | 4.94 | 30 | 22 | 38 | |
| R Angular Gyrus | 1 | 4.83 | 52 | −48 | 34 | |
| R Middle Frontal Gyrus | 1 | 4.56 | 28 | 12 | 32 | |
| R Precentral Gyrus | 1 | 4.32 | 38 | −8 | 34 | |
|
Right Amygdala Group Average |
||||||
| Positive Functional Connectivity | Cluster Index |
Z-Stat | X | Y | Z | Cluster Size (voxels) |
| R Amygdala | 1 | 11.8 | 24 | −4 | −20 | 64563 |
| L Hippocampus | 1 | 9.14 | −24 | −8 | −22 | |
| R Superior Temporal Gyrus | 1 | 7.65 | 50 | 2 | −18 | |
| L Planum Temporale | 1 | 7.64 | −40 | 0 | −22 | |
| R Temporal Pole | 1 | 7.4 | 34 | 10 | −30 | |
| R Temporal Pole | 1 | 7.38 | 40 | 16 | −28 | |
|
Right Amygdala Group Average |
||||||
| Negative Functional Connectivity | Cluster Index |
Z-Stat | X | Y | Z | Cluster Size (voxels) |
| L Lateral Occipital Cortex | 2 | 5.64 | −22 | −64 | 42 | 13330 |
| L Middle Frontal Gyrus | 2 | 5.59 | −38 | 12 | 30 | |
| L Lateral Occipital Cortex | 2 | 5.38 | −26 | −58 | 42 | |
| L Middle Frontal Gyrus | 2 | 5.25 | −34 | 28 | 32 | |
| L Angular Gyrus | 2 | 5.21 | −38 | −56 | 42 | |
| L Lateral Occipital Cortex | 2 | 4.81 | −58 | −60 | 36 | |
| R Angular Gyrus | 1 | 4.79 | 44 | −46 | 36 | 1557 |
| R Precuneus | 1 | 4.03 | 18 | −60 | 40 | |
| R Angular Gyrus | 1 | 4.02 | 32 | −46 | 34 | |
| R Lateral Occipital Cortex | 1 | 3.84 | 30 | −58 | 40 | |
Notes: All reported brain regions were cluster-corrected for multiple comparisons at Z > 2.3, P = 0.05. Peak voxel coordinates are in MNI space (mm3). R = right, L = left.
Table 4.
List of significant clusters from the random effects group analysis showing stressor-induced left and right amygdala resting-state functional connectivity (rsFC) changes with other brain regions
|
Right Amygdala Functional Connectivity |
||||||
|---|---|---|---|---|---|---|
| Regions: Control - Stressor | Cluster Index | Z-Stat | X | Y | Z | Cluster Size (voxels) |
| R Posterior Cingulate Cortex | 2 | 3.69 | 16 | −20 | 44 | 715 |
| L Precentral Gyrus | 2 | 3.67 | −6 | −30 | 50 | |
| L Precentral Gyrus | 2 | 3.45 | −2 | −22 | 52 | |
| L Supplementary Motor Area/Precentral Gyrus |
2 | 3.45 | −6 | −16 | 50 | |
| R Premotor Cortex | 2 | 3.38 | 16 | −20 | 58 | |
| R Supplementary Motor Area | 2 | 3.35 | 10 | −10 | 50 | |
| L Medial Orbitofrontal Cortex | 1 | 3.7 | −8 | 34 | −28 | 118 |
| R Medial Prefrontal Cortex | 1 | 3.67 | 10 | 42 | −18 | |
| L Medial Prefrontal Cortex | 1 | 3.58 | −6 | 42 | −18 | |
| L Frontal Pole | 1 | 3.37 | −20 | 44 | −18 | |
| R Subcallosal Cortex | 1 | 3.31 | 4 | 18 | −18 | |
| R Subcallosal Cortex | 1 | 3.21 | 6 | 22 | −18 | |
|
Left Amygdala Functional Connectivity |
||||||
| Regions: Control - Stressor | Cluster Index | Z-Stat | X | Y | Z | Cluster Size (voxels) |
| R Orbitofrontal Cortex | 2 | 4.29 | 22 | 28 | −24 | 766 |
| R Orbitofrontal Cortex | 2 | 4.26 | 24 | 16 | −26 | |
| L Medial Prefrontal Cortex | 2 | 4.22 | −10 | 40 | −16 | |
| R Medial Prefrontal Cortex | 2 | 4.19 | 26 | 18 | −22 | |
| R Medial Prefrontal Cortex/Frontal Pole |
2 | 4.12 | 10 | 46 | −20 | |
| R Medial Prefrontal Cortex | 2 | 4.09 | 8 | 42 | −16 | |
| L Supplementary Motor Area/Superior Frontal Gyrus |
1 | 3.67 | −10 | 10 | 54 | 322 |
| L Paracingulate | 1 | 3.63 | −8 | 24 | 34 | |
| L Superior Frontal Gyrus | 1 | 3.53 | −16 | 22 | 46 | |
| L Paracingulate/Anterior Cingulate Cortex |
1 | 3.1 | −8 | 14 | 38 | |
| L Supplementary Motor Area | 1 | 3.01 | −8 | 2 | 50 | |
| L Supplementary Motor Area | 1 | 2.95 | −2 | −14 | 58 | |
Notes: All reported brain regions were cluster−corrected for multiple comparisons at Z > 2.3, P = 0.05. Peak voxel coordinates are in MNI space (mm3). R = right, L = left.
Acknowledgments
We thank Zara Abrams for her assistance with scanning and Rico Velasco for his assistance with creating the figures.
This research was supported by NIA grants R01AG038043 and K02AG032309.
References
- Aguirre GK, Detre JA, Zarahn E, Alsop DC. Experimental design and the relative sensitivity of BOLD and perfusion fMRI. Neuroimage. 2002;15:488–500. doi: 10.1006/nimg.2001.0990. [DOI] [PubMed] [Google Scholar]
- al'Absi M, Hugdahl K, Lovallo WR. Adrenocortical stress responses and altered working memory performance. Psychophysiology. 2002;39:95–99. doi: 10.1017/S0048577202001543. [DOI] [PubMed] [Google Scholar]
- al’ Absi M, Wittmers LE, Ellestad D, Nordehen G, Do SWK, Kirschbaum C, Grant JE. Sex differences in pain and hypothalamic-pituitary-adrenocortical responses to opioid blockade. Psychosomatic Medicine. 2004;66(2):198–206. doi: 10.1097/01.psy.0000116250.81254.5d. [DOI] [PubMed] [Google Scholar]
- Aloisi AM, Bonifazi M. Sex hormones, central nervous system and pain. Hormones and behavior. 2006;50(1):1–7. doi: 10.1016/j.yhbeh.2005.12.002. [DOI] [PubMed] [Google Scholar]
- Amaral DG, Price JL. Amygdalo-cortical projections in the monkey (macaca fascicularis) Journal of Comparative Neurology. 1984;230:465–496. doi: 10.1002/cne.902300402. [DOI] [PubMed] [Google Scholar]
- Anderson AK, Phelps EA. Lesions of the human amygdala impair enhanced perception of emotionally salient events. Nature. 2001;411:305–309. doi: 10.1038/35077083. [DOI] [PubMed] [Google Scholar]
- Beckmann CF, DeLuca M, Devlin JT, Smith SM. Investigations into resting-state connectivity using independent component analysis. Philosophical Transactions of the Royal Society of London. Series B, Biological Sciences. 2005;360(1457):1001–1013. doi: 10.1098/rstb.2005.1634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bernard JF, Besson JM. Convergence of nociceptive information on the parabrachio-amygdala neurons in the rat. Comptes rendus de l’Acadamie des sciences. Serie III, Sciences de la vie. 1988;307(19):841–847. [PubMed] [Google Scholar]
- Bernard JF, Huang GF, Besson JM. Nucleus centralis of the amygdala and the globus pallidus ventralis: Electrophysiological evidence for an involvement in pain processes. Journal of Neurophysiology. 1992;68:551–569. doi: 10.1152/jn.1992.68.2.551. [DOI] [PubMed] [Google Scholar]
- Bingel U, Tracey I. Imaging CNS modulation of pain in humans. Physiology. 2008;23(3):371–380. doi: 10.1152/physiol.00024.2008. [DOI] [PubMed] [Google Scholar]
- Blalock H. Social Statistics. NY: McGraw-Hill; 1972. p. 407. [Google Scholar]
- Blandini F, Martignoni E, Sances E, Bono G, Nappi G. Combined response of plasma and platelet catecholamines to different types of short-term stress. Life Sciences. 1995;56(13):1113–1120. doi: 10.1016/0024-3205(95)00048-b. [DOI] [PubMed] [Google Scholar]
- Boyle MP, Brewer JA, Fanatsu M, Wozniak DF, Tsien JZ, Izumi Y, Muglia LJ. Acquired deficit of forebrain glucocorticoid receptor produces depression-like changes in adrenal axis regulation and behavior. Proceedings of the National Academy of Sciences of the United States of America. 2005;102:473–478. doi: 10.1073/pnas.0406458102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bornhovd K, Quante M, Glauche V, Bromm B, Weiller C, Buchel C. Painful stimuli evoke different stimulus-response functions in the amygdala, prefrontal, insula and somatosensory cortex: A single-trial fMRI study. Brain. 2002;125:1326–1336. doi: 10.1093/brain/awf137. [DOI] [PubMed] [Google Scholar]
- Buchanan TW, Tranel D, Adolphs R. Impaired memory retrieval correlates with individual differences in cortisol response but not autonomic response. Learning & memory. 2006;13(3):382–387. doi: 10.1101/lm.206306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burgmer M, Gaubitz M, Konrad C, Wrenger M, Hilgart S, Heuft G, Pfleiderer B. Decreased gray matter volumes in the cingulo-frontal cortex and the amygdala in patients with fibromyalgia. Psychosomatic Medicine. 2009;71:566–573. doi: 10.1097/PSY.0b013e3181a32da0. [DOI] [PubMed] [Google Scholar]
- Cavada C, Tejedor J, Cruz-Rizzolo RJ, Reinoso-Suárez F. The anatomical connections of the macaque monkey orbitofrontal cortex. A review. Cerebral Cortex. 2000;10(3):220–242. doi: 10.1093/cercor/10.3.220. [DOI] [PubMed] [Google Scholar]
- Coghill RC, McHaffie JG, Yen YF. Neural correlates of interindividual differences in the subjective experience of pain. Proceedings of the National Academy of Sciences of the United States of America. 2003;100:8538–8542. doi: 10.1073/pnas.1430684100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davis M, Walker DL, Miles L, Grillon C. Phasic vs. sustained fear in rats and humans: Role of the extended amygdala in fear vs. anxiety. Neuropsychopharmacology. 2010;35:105–135. doi: 10.1038/npp.2009.109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Kloet ER, Oitzl MS, Joëls M. Stress and cognition: Are corticosteroids good or bad guys? Trends in Neurosciences. 1999;22(10):422–426. doi: 10.1016/s0166-2236(99)01438-1. [DOI] [PubMed] [Google Scholar]
- de Kloet ER, Reul JM. Feedback action and tonic influence of corticosteroids on brain function: A concept arising from the heterogeneity of brain receptor systems. Psychoneuroendocrinology. 1987;12:83–105. doi: 10.1016/0306-4530(87)90040-0. [DOI] [PubMed] [Google Scholar]
- Derbyshire SW, Jones AK, Gyulai F, Clark S, Townsend D, Firestone LL. Pain processing during three levels of noxious stimulation produces differential patterns of central activity. Pain. 1997;73:431–445. doi: 10.1016/S0304-3959(97)00138-3. [DOI] [PubMed] [Google Scholar]
- Dickerson SS, Kemeny ME. Acute stressors and cortisol responses: a theoretical integration and synthesis of laboratory research. Psychological Bulletin. 2004;130(3):355–391. doi: 10.1037/0033-2909.130.3.355. [DOI] [PubMed] [Google Scholar]
- Diorio D, Viau V, Meaney MJ. The role of the medial prefrontal cortex (cingulate gyrus) in the regulation of hypothalamic-pituitary-adrenal responses to stress. The Journal of Neuroscience. 1993;13(9):3839–3847. doi: 10.1523/JNEUROSCI.13-09-03839.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dixon KE, Thorn BE, Ward LC. An evaluation of sex differences in psychological and physiological responses to experimentally-induced pain: a path analytic description. Pain. 2004;112(1–2):188. doi: 10.1016/j.pain.2004.08.017. [DOI] [PubMed] [Google Scholar]
- Donahue MJ, Lu H, Jones CK, Pekar JJ, van Zijl PC. An account of the discrepancy between MRI and PET cerebral blood flow measures. A high-field MRI investigation. NMR in Biomedicine. 2006;19(8):1043–1054. doi: 10.1002/nbm.1075. [DOI] [PubMed] [Google Scholar]
- Drolet G, Dumon EC, Gosselin I, Kinhead R, Laforest S, Trottier JF. Role of endogenous opioid system in the regulation of the stress response. Science. 2001;25:729–741. doi: 10.1016/s0278-5846(01)00161-0. [DOI] [PubMed] [Google Scholar]
- Duncko R, Cornwell B, Cui L, Merikangas KR, Grillon C. Acute exposure to stress improves performance in trace eyeblink conditioning and spatial learning tasks in healthy men. Learning & Memory. 2007;14(5):329–335. doi: 10.1101/lm.483807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duncko R, Johnson L, Merikangas K, Grillon C. Working memory performance after acute exposure to the cold pressor stress in healthy volunteers. Neurobiology of Learning and Memory. 2009;91(4):377–381. doi: 10.1016/j.nlm.2009.01.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eryilmaz H, Van De, Ville D, Schwartz S, Vuilleumier P. Impact of transient emotions on functional connectivity during subsequent resting state: a wavelet correlation approach. Neuroimage. 2011;54(3):2481–2491. doi: 10.1016/j.neuroimage.2010.10.021. [DOI] [PubMed] [Google Scholar]
- Etkin A, Egner T, Peraza DM, Kandel ER, Hirsch J. Resolving emotional conflict: a role for the rostral anterior cingulate cortex in modulating activity in the amygdala. Neuron. 2006;51:871–882. doi: 10.1016/j.neuron.2006.07.029. [DOI] [PubMed] [Google Scholar]
- Etkin A, Egner T, Kalisch R. Emotional processing in anterior cingulate and medial prefrontal cortex. Trends in cognitive sciences. 2011;15(2):85–93. doi: 10.1016/j.tics.2010.11.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fields HL. State-dependent opioid control of pain. Nature Reviews Neuroscience. 2004;5(7):565–575. doi: 10.1038/nrn1431. [DOI] [PubMed] [Google Scholar]
- Fox MD, Snyder AZ, Vincent JL, Corbetta M, Van Essen DC, Raichle ME. The human brain is intrinsically organized into dynamic, anticorrelated functional networks. Proceedings of the National Academy of Sciences of the United States of America. 2005;102:9673–9678. doi: 10.1073/pnas.0504136102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frew AK, Drummond PD. Negative affect, pain and sex: The role of endogenous opioids. Pain. 2007;132(1):77–85. doi: 10.1016/j.pain.2007.04.010. [DOI] [PubMed] [Google Scholar]
- Ghashghaei HT, Barbas H. Pathways for emotion: interactions of prefrontal and anterior temporal pathways in the amygdala of the rhesus monkey. Neuroscience. 2002;115:1261–1279. doi: 10.1016/s0306-4522(02)00446-3. [DOI] [PubMed] [Google Scholar]
- Hapidou EG, De Catanzaro D. Sensitivity to cold pressor pain in dysmenorrheic and non-dysmenorrheic women as a function of menstrual cycle phase. Pain. 1988;34(3):277–283. doi: 10.1016/0304-3959(88)90123-6. [DOI] [PubMed] [Google Scholar]
- Hardy JD, Wolff HG, Goodell H. Pain Sensations and Reactions. Baltimore, MD: Williams & Wilkins; 1952. [Google Scholar]
- Hariri AR, Mattay VS, Tessitore A, Fera F, Weinberger DR. Neocortical modulation of the amygdala response to fearful stimuli. Biological Psychiatry. 2003;53(6):494–501. doi: 10.1016/s0006-3223(02)01786-9. [DOI] [PubMed] [Google Scholar]
- Hellström B, Lundberg U. Pain perception to the cold pressor test during the menstrual cycle in relation to estrogen levels and a comparison with men. Integrative Physiological and Behavioral Science. 2000;35(2):132–141. doi: 10.1007/BF02688772. [DOI] [PubMed] [Google Scholar]
- Henckens MJ, van Wingen GA, Joëls M, Fernández G. Corticosteroid induced decoupling of the amygdala in men. Cerebral Cortex. 2011 doi: 10.1093/cercor/bhr313. [DOI] [PubMed] [Google Scholar]
- Herman JP, Ostrander MM, Mueller NK, Figueiredo H. Limbic system mechanisms of stress regulation: hypothalamo-pituitary-adrenocortical axis. Progress in Neuro-Psychopharmacology and Biological Psychiatry. 2005;29(8):1201–1213. doi: 10.1016/j.pnpbp.2005.08.006. [DOI] [PubMed] [Google Scholar]
- Hutchinson WD, Davis KD, Lozano AM, Tasker RR, Dostrovsky JO. Pain-related neurons in the human cingulated cortex. Nature Neuroscience. 1999;2:403–405. doi: 10.1038/8065. [DOI] [PubMed] [Google Scholar]
- Ikeda R, Takahashi Y, Inoue K, Kato F. NMDA receptor-independent synaptic plasticity in the central amygdala in the rat model of neuropathic pain. Pain. 2007;127(1):161–172. doi: 10.1016/j.pain.2006.09.003. [DOI] [PubMed] [Google Scholar]
- Johnstone T, van Reekum CM, Urry HL, Kalin NH, Davidson RJ. Failure to regulate: counterproductive recruitment of top-down prefrontal-subcortical circuitry in major depression. The Journal of Neuroscience. 2007;27(33):8877–8884. doi: 10.1523/JNEUROSCI.2063-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kern S, Oakes TR, Stone CK, McAuliff EM, Kirschbaum C, Davidson RJ. Glucose metabolic changes in the prefrontal cortex are associated with HPA axis response to a psychosocial stressor. Psychoneuroendocrinology. 2008;33(4):517–529. doi: 10.1016/j.psyneuen.2008.01.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kilpatrick La, Zald DH, Pardo JV, Cahill LF. Sex-related differences in amygdala functional connectivity during resting conditions. Neuroimage. 2006;30(2):452–461. doi: 10.1016/j.neuroimage.2005.09.065. [DOI] [PubMed] [Google Scholar]
- Kringelbach ML, Rolls ET. The functional neuroanatomy of the human orbitofrontal cortex: evidence from neuroimaging and neuropsychology. Progress in Neurobiology. 2004;72(5):341–372. doi: 10.1016/j.pneurobio.2004.03.006. [DOI] [PubMed] [Google Scholar]
- Kudielka BM, Kirschbaum C. Sex differences in HPA axis responses to stress: a review. Biological psychology. 2005;69(1):113–132. doi: 10.1016/j.biopsycho.2004.11.009. [DOI] [PubMed] [Google Scholar]
- Lapate RC, Lee H, Salomons TV, van Reekum CM, Greischar LL, Davidson RJ. Amygdalar function reflects common individual differences in emotion and pain regulation success. Journal of Cognitive Neuroscience. 2012;24(1):148–158. doi: 10.1162/jocn_a_00125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LeDoux JE. Emotion circuits in the brain. Annual Review of Neuroscience. 2000;23:155–184. doi: 10.1146/annurev.neuro.23.1.155. [DOI] [PubMed] [Google Scholar]
- LeDoux J. The emotional brain, fear, and the amygdala. Cellular and Molecular Neurobiology. 2003;23(4–5):727–738. doi: 10.1023/A:1025048802629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee H, Heller AS, van Reekum CM, Nelson B, Davidson RJ. Amygdala-prefrontal coupling underlies individual differences in emotion regulation. Neuroimage. 2012;62(3):1575–1581. doi: 10.1016/j.neuroimage.2012.05.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leknes S, Brooks JC, Wiech K, Tracey I. Pain relief as an opponent process: a psychophysical investigation. European journal of neuroscience. 2008;28(4):794–801. doi: 10.1111/j.1460-9568.2008.06380.x. [DOI] [PubMed] [Google Scholar]
- Lenz FA, Rios M, Zirh A, Chau D, Krauss G, Lesser RP. Painful stimuli evoke potentials recorded over the human anterior cingulate gyrus. Journal of Neurophysiology. 1998;79:2231–2234. doi: 10.1152/jn.1998.79.4.2231. [DOI] [PubMed] [Google Scholar]
- Liddell BJ, Brown KJ, Kemp AH, Barton MJ, Das P, Peduto A, Williams LM. A direct brainstem-amygdala-cortical “alarm” system for subliminal signals of fear. Neuroimage. 2005;24(1):235–243. doi: 10.1016/j.neuroimage.2004.08.016. [DOI] [PubMed] [Google Scholar]
- Lighthall NR, Mather M, Gorlick MA. Acute stress increases sex differences in risk seeking in the Balloon Analogue Risk Task. PLoS ONE. 2009;4(7):6002. doi: 10.1371/journal.pone.0006002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lighthall NR, Sakaki M, Vasunilashorn S, Nga L, Somayajula S, Chen, Mather M. Gender differences in reward-related decision processing under stress. Social Cognitive and Affective Neuroscience. 2011;7(4):476–84. doi: 10.1093/scan/nsr026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lighthall NR, Gorlick MA, Schoeke A, Frank MJ, Mather M. Stress modulates reinforcement learning in younger and older adults. Psychology and Aging. doi: 10.1037/a0029823. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lovallo WR, Robinson JL, Glahn DC, Fox PT. Acute effects of hydrocortisone on the human brain: an fMRI study. Psychoneuroendocrinology. 2010;35(1):15–20. doi: 10.1016/j.psyneuen.2009.09.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luh WM, Wong EC, Bandettini PA, Hyde JS. QUIPSS II with thin-slice TI1 periodic saturation: a method for improving accuracy of quantitative perfusion imaging using pulsed arterial spin labeling. Magnetic Resonance in Medicine. 1999;41(6):1246–1254. doi: 10.1002/(sici)1522-2594(199906)41:6<1246::aid-mrm22>3.0.co;2-n. [DOI] [PubMed] [Google Scholar]
- Maihofner C, Handwerker HO. Differential coding of hyperalgesia in the human brain: a functional MRI study. Neuroimage. 2005;28(4):996–1006. doi: 10.1016/j.neuroimage.2005.06.049. [DOI] [PubMed] [Google Scholar]
- Mather M, Gorlick MA, Lighthall NR. To brake or accelerate when the light turns yellow? Stress reduces older adults' risk taking in a driving game. Psychological Science. 2009;20:174–176. doi: 10.1111/j.1467-9280.2009.02275.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mather M, Lighthall NR, Nga L, Gorlick MA. Sex differences in how stress affects brain activity during face viewing. Neuroreport. 2010;21(14):933–937. doi: 10.1097/WNR.0b013e32833ddd92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- May A. Structural brain imaging: a window into chronic pain. The Neuroscientist. 2011;17(2):209–220. doi: 10.1177/1073858410396220. [DOI] [PubMed] [Google Scholar]
- McDonald AJ, Mascagni F, Guo L. Projections of the medial and lateral prefrontal cortices to the amygdala: a phaseolus vulgaris leucoagglutinin study in the rat. Neuroscience. 1996;71(1):55–75. doi: 10.1016/0306-4522(95)00417-3. [DOI] [PubMed] [Google Scholar]
- McRae AL, Saladin ME, Brady KT, Upadhyaya H, Back SE, Timmermann MA. Stress reactivity: biological and subjective responses to the cold pressor and trier social stressors. Human Psychopharmacology. 2006;21:377–385. doi: 10.1002/hup.778. [DOI] [PubMed] [Google Scholar]
- Mitsushima D, Yamada K, Takase K, Funabashi T, Kimura F. Sex differences in the basolateral amygdala: the extracellular levels of serotonin and dopamine, and their responses to restraint stress in rats. European Journal of Neuroscience. 2006;24:3245–3254. doi: 10.1111/j.1460-9568.2006.05214.x. [DOI] [PubMed] [Google Scholar]
- Modinos G, Ormel J, Aleman A. Individual differences in dispositional mindfulness and brain activity involved in reappraisal of emotion. Social Cognitive and Affective Neuroscience. 2010;5(4):369–377. doi: 10.1093/scan/nsq006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mumford JA, Hernandez-Garcia L, Lee GR, Nichols TE. Estimation efficiency and statistical power in arterial spin labeling fMRI. Neuroimage. 2006;33(1):103–114. doi: 10.1016/j.neuroimage.2006.05.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neugebauer V, Li W. Differential sensitization of amygdala neurons to afferent inputs in a model of arthritic pain. Journal of Neurophysiology. 2003;89:716–727. doi: 10.1152/jn.00799.2002. [DOI] [PubMed] [Google Scholar]
- Neugebauer V, Li W, Bird GC, Han JS. The amygdala and persistent pain. The Neuroscientist. 2004;10(3):221–234. doi: 10.1177/1073858403261077. [DOI] [PubMed] [Google Scholar]
- Ochsner KN, Bunge SA, Gross JJ, Gabrieli JD. Rethinking feelings: An fMRI study of the cognitive regulation of emotion. Journal of Cognitive Neuroscience. 2002;14(8):1215–1229. doi: 10.1162/089892902760807212. [DOI] [PubMed] [Google Scholar]
- Ochsner KN, Ray RD, Cooper JC, Robertson ER, Chopra S, Gabrieli JD, Gross JJ. For better or for worse: neural systems supporting the cognitive down- and up-regulation of negative emotion. Neuroimage. 2004;23:483–499. doi: 10.1016/j.neuroimage.2004.06.030. [DOI] [PubMed] [Google Scholar]
- Pascualy M, Petrie EC, Brodkin K, Peskind ER, Wilkinson CW, Raskind MA. Hypothalamic pituitary adrenocortical and sympathetic nervous system responses to the cold pressor test in Alzheimer's disease. Biological Psychiatry. 2000;48(3):247–254. doi: 10.1016/s0006-3223(00)00879-9. [DOI] [PubMed] [Google Scholar]
- Petrovic P, Ingvar M. Imaging cognitive modulation of pain processing. Pain. 2002;95:1–5. doi: 10.1016/s0304-3959(01)00467-5. [DOI] [PubMed] [Google Scholar]
- Petrovic P, Carlsson K, Petersson KM, Hansson P, Ingvar M. Context-dependent deactivation of the amygdala during pain. Journal of Cognitive Neuroscience. 2004;16(7):1289–1301. doi: 10.1162/0898929041920469. [DOI] [PubMed] [Google Scholar]
- Porcelli AJ, Cruz D, Wenberg K, Patterson MD, Biswal BB, Rypma B. The effects of acute stress on human prefrontal working memory systems. Physiology & Behavior. 2008;95:282–289. doi: 10.1016/j.physbeh.2008.04.027. [DOI] [PubMed] [Google Scholar]
- Price DD, Barrell JJ. Mechanisms of analgesia produced by hypnosis and placebo suggestions. Progress in Brain Research. 2000;122:255–271. doi: 10.1016/s0079-6123(08)62144-5. [DOI] [PubMed] [Google Scholar]
- Rainville P, Duncan GH, Price DD, Carrier B, Bushnell MC. Pain affect encoded in human anterior cingulate but not somatosensory cortex. Science. 1997;277:968–971. doi: 10.1126/science.277.5328.968. [DOI] [PubMed] [Google Scholar]
- Robinson JL, Laird AR, Glahn DC, Lovallo WR, Fox PT. Meta-analytic connectivity modeling: delineating the functional connectivity of the human amygdala. Human Brain Mapping. 2010;31(2):173–184. doi: 10.1002/hbm.20854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roger D, Najarian B. The relationship between emotional rumination and cortisol secretion under stress. Personality and Individual Differences. 1998;24(4):531–538. [Google Scholar]
- Roy AK, Shehzad Z, Margulies DS, Kelly MC, Uddin LQ, Gotimer K, Milham MP. Functional connectivity of the human amygdala using resting state fMRI. Neuroimage. 2009;45(2):614–626. doi: 10.1016/j.neuroimage.2008.11.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rule RR, Shimamura AP, Knight RT. Orbitofrontal cortex and dynamic filtering of emotional stimuli. Cognitive, Affective, and Behavioral Neuroscience. 2002;2(3):264–270. doi: 10.3758/cabn.2.3.264. [DOI] [PubMed] [Google Scholar]
- Salomons TV, Johnstone T, Backonja MM, Shackman AJ, Davidson RJ. Individual differences in the effects of perceived controllability on pain perception: critical role of the prefrontal cortex. The Journal of Cognitive Neuroscience. 2007;19:993–1003. doi: 10.1162/jocn.2007.19.6.993. [DOI] [PubMed] [Google Scholar]
- Sapolsky RM. Why stress is bad for your brain. Science. 1996;273:749–750. doi: 10.1126/science.273.5276.749. [DOI] [PubMed] [Google Scholar]
- Schwabe L, Haddad L, Schachinger H. HPA axis activation by a socially evaluated cold pressor test. Psychoneuroendocrinology. 2008;33(6):890–895. doi: 10.1016/j.psyneuen.2008.03.001. [DOI] [PubMed] [Google Scholar]
- Stein JL, Wiedholz LM, Bassett DS, Weinberger DR, Zink CF, Mattay VS, Meyer-Lindenberg A. A validated network of effective amygdala connectivity. Neuroimage. 2007;36(3):736–745. doi: 10.1016/j.neuroimage.2007.03.022. [DOI] [PubMed] [Google Scholar]
- Talbot JD, Villemure JG, Bushnell MC, Duncan GH. Evaluation of pain perception after anterior capsulotomy: a case report. Somatosensory and Motor Research. 1995;12(2):115–126. doi: 10.3109/08990229509101503. [DOI] [PubMed] [Google Scholar]
- Tavernor SJ, Abduljawad KAJ, Langley RW, Bradshaw CM, Szabadi E. Effects of pentagastrin and the cold pressor test on the acoustic startle response and pupillary function in man. Journal of Psychopharmacology. 2000;14(4):387–394. doi: 10.1177/026988110001400407. [DOI] [PubMed] [Google Scholar]
- Thorpe SJ, Rolls ET, Maddison S. The orbitofrontal cortex: neuronal activity in the behaving monkey. Experimental Brain Research. 1983;49:93–115. doi: 10.1007/BF00235545. [DOI] [PubMed] [Google Scholar]
- Urry HL, Van Reekum CM, Johnstone T, Kalin NH, Thurow ME, Schaefer HS, Davidson RJ. Amygdala and ventromedial prefrontal cortex are inversely coupled during regulation of negative affect and predict the diurnal pattern of cortisol secretion among older adults. The Journal of Neuroscience. 2006;26(16):4415–4425. doi: 10.1523/JNEUROSCI.3215-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Marle HJ, Hermans EJ, Qin S, Fernández G. Enhanced resting-state connectivity of amygdala in the immediate aftermath of acute psychological stress. Neuroimage. 2010;53:348–354. doi: 10.1016/j.neuroimage.2010.05.070. [DOI] [PubMed] [Google Scholar]
- Veer IM, Oei NY, Spinhoven P, van Buchem MA, Elzinga BM, Rombouts SA. Psychoneuroendocrinology. 2011a. Endogenous cortisol is associated with functional connectivity between the amygdala and medial prefrontal cortex. [DOI] [PubMed] [Google Scholar]
- Veer IM, Oei NYL, Spinhoven P, van Buchem MA, Elzinga BM, Rombouts SARB. Beyond acute social stress: Increased functional connectivity between amygdala and cortical midline structures. Neuroimage. 2011b;57(4):1534–1541. doi: 10.1016/j.neuroimage.2011.05.074. [DOI] [PubMed] [Google Scholar]
- Vertes RP. Differential projections of the infralimbic and prelimbic cortex in the rat. Synapse. 2004;51(1):32–58. doi: 10.1002/syn.10279. [DOI] [PubMed] [Google Scholar]
- Vogt BA, Pandya DN. Cingulate cortex of the rhesus monkey: II. Cortical afferents. The Journal of comparative neurology. 1987;262(2):271–289. doi: 10.1002/cne.902620208. [DOI] [PubMed] [Google Scholar]
- Wang J, Aguirre GK, Kimberg DY, Roc AC, Li L, Detre JA. Arterial spin labeling perfusion fMRI with very low task frequency. Magnetic Resonance in Medicine. 2003;49:796–802. doi: 10.1002/mrm.10437. [DOI] [PubMed] [Google Scholar]
- Wang J, Li L, Roc AC, Alsop DC, Tang K, Butler NS, Detre JA. Reduced susceptibility effects in perfusion fMRI with single-shot spin-echo EPI acquisitions at 1.5 Tesla. Magnetic Resonance Imaging. 2004;22(1):1–7. doi: 10.1016/S0730-725X(03)00210-8. [DOI] [PubMed] [Google Scholar]
- Wang Z, Guo Y, Bradesi S, Labus JS, Maarek JMI, Lee K, Holschneider D. Sex differences in functional brain activation during noxious visceral stimulation in rats. Pain. 2009;145(1–2):120–128. doi: 10.1016/j.pain.2009.05.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watkins LR, Mayer DJ. Organization of endogenous opiate and nonopiate pain control systems. Science. 1982;216(4551):1185–1192. doi: 10.1126/science.6281891. [DOI] [PubMed] [Google Scholar]
- Watson D, Clark LA, Tellegan A. Development and validation of brief measures of positive and negative affect: The PANAS scales. Journal of Personality and Social Psychology. 1988;54(6):1063–1070. doi: 10.1037//0022-3514.54.6.1063. [DOI] [PubMed] [Google Scholar]
- Whalen PJ, Rauch SL, Etcoff NL, McInerney SC, Lee MB, Jenike MA. Masked presentations of emotional facial expressions modulate amygdala activity without explicit knowledge. The Journal of Neuroscience. 1998;18:411–418. doi: 10.1523/JNEUROSCI.18-01-00411.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wiech K, Kalisch R, Weiskopf N, Pleger B, Stephan KE, Dolan RJ. Anterolateral prefrontal cortex mediates the analgesic effect of expected and perceived control over pain. The Journal of Neuroscience. 2006;26(44):11501–11509. doi: 10.1523/JNEUROSCI.2568-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wong EC, Buxton RB, Frank LR. Implementation of quantitative perfusion imaging techniques for functional brain mapping using pulsed arterial spin labeling. NMR in Biomedicine. 1997;10(4–5):237–249. doi: 10.1002/(sici)1099-1492(199706/08)10:4/5<237::aid-nbm475>3.0.co;2-x. [DOI] [PubMed] [Google Scholar]
- Wong EC, Buxton RB, Frank LR. Quantitative imaging of perfusion using a single subtraction (QUIPSS and QUIPSS II) Magnetic Resonance in Medicine. 1998;39(5):702–708. doi: 10.1002/mrm.1910390506. [DOI] [PubMed] [Google Scholar]
- Xie YF, Huo FQ, Tang JS. Cerebral cortex modulation of pain. Acta Pharmacologica Sinica. 2009;30(1):31–41. doi: 10.1038/aps.2008.14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zappe AC, Pfeuffer J, Merkle H, Logothetis NK, Goense JBM. The effect of labeling parameters on perfusion-based fMRI in nonhuman primates. Journal of Cerebral Blood Flow and Metabolism. 2008;28(3):640–652. doi: 10.1038/sj.jcbfm.9600564. [DOI] [PubMed] [Google Scholar]
- Zhang Y, Tang J, Yuan B, Jia H. Inhibitory effects of electrically evoked activation of ventrolateral orbital cortex on the tail-flick reflex are mediated by periaqueductal gray in rats. Pain. 1997;72(1–2):127–135. doi: 10.1016/s0304-3959(97)00025-0. [DOI] [PubMed] [Google Scholar]
- Zoccola PM, Dickerson SS, Zaldivar FP. Rumination and cortisol responses to laboratory stressors. Psychosomatic medicine. 2008;70(6):661–667. doi: 10.1097/PSY.0b013e31817bbc77. [DOI] [PubMed] [Google Scholar]
- Zubieta J, Smith YR, Bueller JA, Xu Y, Kilbourn MR, Jewett D, Stohler CS. Mu-opioid receptor-mediated antinociceptive responses differ in men and women. The Journal of Neuroscience. 2002;22(12):5100–5107. doi: 10.1523/JNEUROSCI.22-12-05100.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zubieta JK, Bueller JA, Jackson LR, Scott DJ, Xu Y, Koeppe RA, Stohler CS. Placebo effects mediated by endogenous opioid activity on mu-opioid receptors. The Journal of Neuroscience. 2005;25(34):7754–62. doi: 10.1523/JNEUROSCI.0439-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.