Abstract
Calcineurin (protein phosphatase 3) regulates synaptic plasticity in the brain. The development of neuropathic pain appears dependent upon some of the same mechanisms that underlie brain synaptic plasticity. In this study we examined whether calcineurin regulates chronic constriction injury (CCI)-elicited plasticity in the spinal dorsal horn.
CCI animals exhibited mechanical and thermal hypersensitivity seven days post- ligation of the sciatic nerve. Neither control uninjured nor sham-operated animals exhibited pain behavior. Calcineurin activity and content of its Aα isoform were significantly decreased in the ipsilateral post-synaptic density (PSD) of dorsal horn neurons in CCI animals. Calcineurin activity and content in the contralateral PSD of CCI animals or either side of the dorsal horn in sham animals were not modified. The pain behavior in CCI animals was attenuated by intrathecal application of exogenous calcineurin. The treatment was long-lasting as a single injection provided analgesia for four days by restoring the phosphatase’s activity and Aα content in the PSD. No signs of toxicity were detected up to 14 days after the single intrathecal injection. Intrathecal application of the calcineurin inhibitor FK-506 elicited pain behavior in control uninjured animals and significantly reduced calcineurin activity in the PSD.
CCI may elicit neuropathic pain at least in part as a result of the loss of calcineurin-mediated dephosphorylation in the dorsal horn. Addition of the phosphatase by intrathecal injection reverses the injury-elicited loss and provides prolonged pain relief. Clinical therapy with calcineurin may prove to be a novel, effective and safe approach in the management of well-established neuropathic pain.
Keywords: Central sensitization, Chronic constriction injury, Neuropathic Pain, Nociception, Post-synaptic Density, Rat, Spinal Dorsal Horn, Phosphatase, Synaptic plasticity
1. Introduction
Injury-elicited plasticity accompanies peripheral nerve injury to ultimately lead to the development of neuropathic pain [11,18]. The development of neuropathic pain is dependent upon some of the same mechanisms that give rise to activity-dependent synaptic plasticity in the brain [5]. Like synaptic plasticity, neuropathic pain exhibits early and late phases that may be mediated by different mechanisms [5,11,18].
The balance between protein kinase and phosphatase activity at the synapse can critically determine overall synaptic strength [12]. As a result the loss of either one of these activities can engender long-lasting changes in synaptic function, i.e., long-lasting plasticity.
More than a decade ago, Kandel and colleagues described how the interplay between protein kinase A (PKA) and calcineurin (protein phosphatase 3, also protein phosphatase 2B) was essential in initiating and maintaining long-lasting enhancement of synaptic function in Aplysia, Drosophila, mice and rats [1]. Activation of PKA by cyclic AMP, and the subsequent phosphorylation of target proteins, resulted in long-term memory storage. In contrast, activation of calcineurin promoted the dephosphorylation of these target proteins to prevent the transition from short to long-term memory.
Later studies confirmed the general role of calcineurin in negatively constraining the acquisition of spatial or aversive memory, or of long-lasting plasticity in ocular dominance, cocaine addiction, and vestibular compensation [2,13,17,21,22].
Little is presently known about the role of calcineurin in injury-elicited plasticity in the spinal dorsal horn. Somatic and terminal immunoreactive staining for the phosphatase is heavy and highly restricted to the superficial dorsal horn [6,20]. The terminal staining is of dorsal horn origin due to the lack of staining in the dorsal root ganglion or dorsal root axons [20].
We reported previously that chronic constriction injury (CCI) of the rat sciatic nerve was associated with the loss of calcineurin content in the spinal dorsal horn [14]. In this study we extended our investigation by examining changes in calcineurin activity and content of its Aα isoform specifically in the post-synaptic density (PSD) of dorsal horn neurons. We concentrated on calcineurin Aα because this isoform is the most abundant in the dorsal horn [20]. We also focused on the PSD because dynamic remodeling of excitatory synapses appears to play an important role in regulating synaptic efficacy [10], and we reported recently that pain behavior due to CCI was associated with changes in the protein matrix of the PSD [15].
The PSD is deserving of attention because its function may represent a final common reflection of the many injury-elicited changes in receptors, pathways, transcription factors and genes that have been reported to accompany chronic pain. In addition, the localization of changes to the post-synaptic structure reduces difficulties in data interpretation with respect to the origin or site of action of the protein under investigation.
Given the already established role of calcineurin in the transition to long-term memory we hypothesized a similar association between calcineurin and neuropathic pain, i.e., that the development of the pain is a pivotal consequence of the loss of calcineurin activity in the PSD of spinal dorsal horn neurons.
2. Methods
2.1. Animals
Male Harlan-Sprague-Dawley rats (200–300g) were randomly assigned to control, sham-operated or CCI groups. All experiments were conducted in accordance with guidelines accepted by the International Association for the Study of Pain [23]. The animal protocol was approved by the Animal Care and Use Committee of the School of Medicine and Public Health at the University of Wisconsin-Madison.
2.1. Behavioral tests
For assessment of mechanical thresholds we used the Dynamic Plantar Aesthesiometer (Ugo Basile North America, Collegeville, PA) to record the force that resulted in an animal’s paw withdrawal. Thermal hyperalgesia was assessed with the hind paw withdrawal latency test using a plantar analgesia instrument (Ugo Basile North America, Collegeville, PA). The mechanical testing preceded thermal testing.
Animals were acclimated for 15–20 min. Each test consisted of four trials which were averaged to obtain a mean value. Each trial was separated by at least 5 min. In sham or CCI animals the ipsilateral, injured, paw was tested. Mechanical thresholds and thermal withdrawal latencies were obtained for all animals both at baseline and later. After the last behavioral test, animals were anesthetized, euthanized, and their lumbar spinal dorsal horns collected for enzyme activity, Western immunoblot or RT-PCR assays.
2.2. Anesthesia, sciatic ligation, intrathecal drug application, tissue collection
Animals were anesthetized with isoflurane. Body temperature was kept at 37°C with a homeothermic blanket system. Anesthesia was sufficiently deep to prevent arousal but light enough to permit spontaneous respiration. Adequate anesthesia was assessed by monitoring blink or ear reflexes, withdrawal to toe pinches, respiratory rate, and absence of spontaneous movements.
Loose ligation of the sciatic nerve (CCI) was performed using the Bennett and Xie [3] procedure as described previously [14]. Briefly, the sciatic nerve was exposed and loosely ligated with 4 simple interrupted 4–0 chromic gut sutures placed about 1mm apart. In sham-operated animals the sciatic nerve was exposed but not ligated. Control animals were anesthetized but were not subject to surgery.
Calcineurin (5 or 10 enzyme units/μl, Enzo Life Sciences, Plymouth Meeting, PA), FK-506 (1μg/μl, Sigma-Aldrich, St. Louis, MO) or saline were injected intrathecally in a volume of 10μl as described previously [15]. For all intrathecal injections animals were briefly anesthetized with isoflurane. Persons performing behavioral tests were blinded to drug treatment.
For tissue collection animals were anesthetized with isoflurane and while still deeply anesthetized, they were euthanized with an intracardiac injection of supersaturated potassium chloride (>350 mg/ml). A laminectomy rapidly (<2 min) exposed the lumbar spinal cord at L5, and about 1cm of the cord was excised and cut into dorsal and ventral halves and the dorsal half further divided into ipsilateral and contralateral quadrants. All tissues were stored at −80°C until use.
2.3. Fractionation, calcineurin activity assay, immunoblots, RT-PCR
Tissues were homogenized and sequentially centrifuged to yield the PSD-containing LP1 fraction [15]. After assaying for total protein content the fractions were used in the biochemical assays. Calcineurin activity was assayed with a commercial kit (Enzo Life Sciences, Plymouth Meeting, PA) and expressed as nmol of phosphate released/min/mg of protein. Western immunoblots were performed as described previously [14]. Antibodies to calcineurin Aα, PSD-95 and green fluorescent protein (GFP, Aequorea victoria) were purchased from Millipore (Billerica, MA) and used at a dilution of 1:1000, 1:1000 and 1:500 respectively. GFP itself was also purchased from Millipore. Developed membranes were stripped and re-probed with beta III tubulin (1:1000; Promega, Madison, WI) as the loading control. Protein levels were estimated from optical density measurements using the BioSpectrum 500 Image Analysis System (UVP, Upland, CA). Calcineurin Aα levels within a gel were expressed over the beta III tubulin levels, and then the levels in sham-operated or CCI animals were normalized to those in control, uninjured animals. A similar normalization procedure was used for the enzyme activity assays.
Calcineurin mRNA levels were determined by monitoring in real time the increase in fluorescence of SYBR-GREEN dye with the ABI Prism 7300 Sequence Detection System (Applied Biosystems, Foster City, CA) [16]. Relative expression levels of calcineurin in each sample were determined using a standard curve of 3-fold serial dilutions. Average fold induction relative to control animals was determined after normalizing to the amount of 18S rRNA in each sample. A 2-fold or greater change was considered significant.
Calcineurin primer sequences were GCAGCAATATTCAGTGACCACTTC (forward) and AACATCCAACTGCTGAGATGCA (reverse). The primers were designed with Primer Express software (Applied Biosystems) using the rat calcineurin mRNA sequence NM_017041.1 and were chosen for their uniqueness in the genome. Primers for 18s rRNA (reference controls) were AACGAGACTCTCGGCATGCTAA (forward) and CCGGACATCTAAGGGCATCA (reverse). All primers were purchased from Integrated DNA Technology, Coralville, IA. Criteria for primer selectivity included: about 20 bases in length, fall within the last 600bp of the sequence, contained higher GC content, and did not include runs of 4 or more G bases.
2.4. Statistical analysis
ANOVA was used for the statistical data analysis. The main emphasis was on detecting differences in mechanical thresholds, thermal latencies, calcineurin activity or calcineurin Aα protein content between control, sham-operated and ligated animals, or vehicle and drug-treated animals. Significant effects were further analyzed with Scheffe’s post-hoc test, and statistical difference was inferred at p ≤ 0.05. All data are expressed as mean±SEM.
3. Results
We focused on the 7 days post-ligation period because this is the time of maximal thermal hyperalgesia for CCI animals in our lab [14].
3.1. CCI animals developed mechanical and thermal hypersensitivity 7 days post-CCI
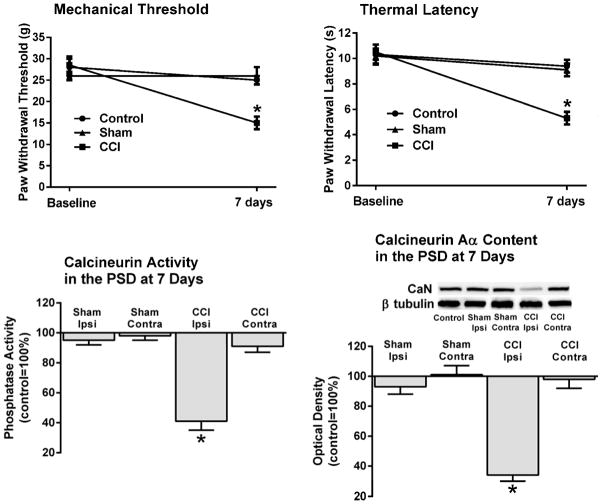
CCI animals (n=6) exhibited lower mechanical withdrawal thresholds in their injured, ipsilateral paws 7 days post-ligation (15±2g) when compared to baseline (29±2g). In contrast, neither uninjured control (28±2g vs. 25±1g; n=6), nor sham-operated animals (26±1g vs. 26±2g; n=6) showed a change in mechanical sensitivity (Fig. 1). ANOVA indicated a significant difference among groups, F(2,15)=17.4, p<0.001. Scheffe’s post-hoc test confirmed that the difference was due to the lowered thresholds in CCI animals.
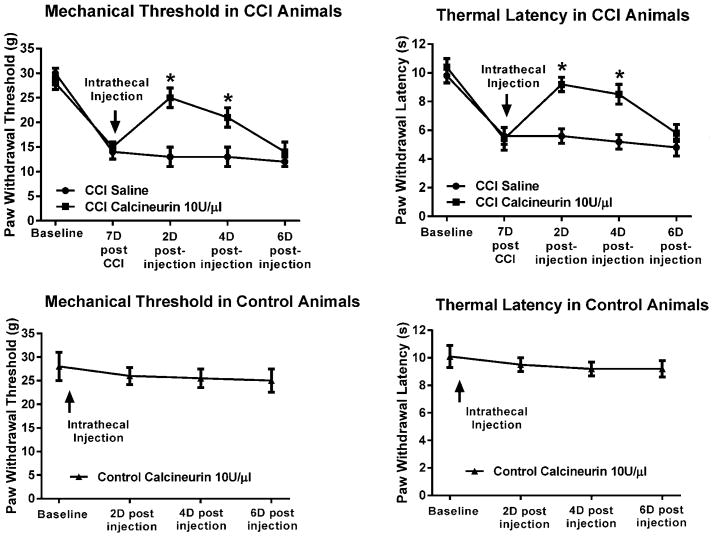
Figure 1. Loss of calcineurin activity and protein content in the ipsilateral PSD of spinal dorsal horn neurons were associated with pain behavior following CCI.
Mechanical and thermal hypersensitivity seven days post-CCI were accompanied by a significant loss of calcineurin activity and content of its Aα isoform (~58kDa) in the ipsilateral PSD. In contrast, calcineurin activity or content in the contralateral PSD of CCI animals or either side in sham-operated animals were not different from controls. Neither control uninjured nor sham-operated animals exhibited pain behavior. CaN=calcineurin. *p<0.001.
In the same CCI animals there was also a significant reduction in their withdrawal latency to the thermal stimulus (5.3±0.5s) when compared to their pre-surgery baseline (10.5±0.6s). In contrast, there was no significant reduction between baseline and the thermal withdrawal latencies in either control or sham-operated animals (10.3±0.8s vs. 9.4±0.5s, and 10.2±0.6s vs. 9.1±0.5s, respectively). ANOVA confirmed a significant difference among the groups which was due to the decreased latencies of 7 days post-CCI animals, F(2,15)=36.5, p<0.001.
3.2. Decreases in calcineurin activity and content of its Aα isoform in the PSD of dorsal horn neurons accompanied the pain behavior
After the last behavioral test the animals were euthanized and their tissues collected for the biochemical assays.
Calcineurin activity in the ipsilateral PSD-containing LP1 fraction of 7 days CCI animals was significantly lower than in control animals, 41±6%, p<0.001. In contrast, calcineurin activity in the contralateral LP1 fraction of these CCI animals (91±4%), or either the ipsilateral (95±3%) or contralateral (98±3%) LP1 fraction in sham-operated animals was not significantly different from controls (Fig. 1).
Similarly, the protein content of calcineurin Aα in the ipsilateral LP1 fraction of CCI animals was significantly lower than in control animals, 34±4%, p<0.001. Calcineurin Aα levels in the contralateral fraction of these CCI animals (98±6%), or either the ipsilateral (93±5%) or contralateral (101±6%) LP1 fractions in sham-operated animals were not significantly different from controls.
ANOVA confirmed significant differences among groups due to the loss of activity and Aα content in the ipsilateral PSD of CCI animals, F(4,25)=41.7, p<0.001 and F(4,25)=135.2, p<0.001, respectively.
3.3. Intrathecal treatment with calcineurin alleviated neuropathic pain in CCI animals
Given that the pain behavior in CCI animals was associated with a loss of both calcineurin activity and Aα content in their ipsilateral PSD we next sought to examine whether addition of exogenous enzyme by intrathecal injection would alleviate the pain by perhaps substituting for the CCI-associated loss in the PSD.
CCI animals exhibited typical pain behavior 7 days post-ligation (Fig. 2). After the behavioral testing, saline (n=3) or calcineurin (n=9) were injected intrathecally, and the animals were behaviorally retested 90 min after the injection. Three of the calcineurin injected animals were treated with 5 enzyme units per microliter, while the other six were injected with 10 enzyme units per microliter. Both the mechanical and the thermal hypersensitivity were significantly attenuated in the CCI animals injected with the higher calcineurin dose. In other words, mechanical thresholds improved to 27±2g after having decreased to 13±2g at 7 days from 32±1g at baseline. Similarly, latencies progressed from 8.9±0.6s at baseline to 5.9±0.4s at 7 days to 7.2±0.6s after the calcineurin injection. ANOVA confirmed a significant effect of treatment for both the mechanical, F(2,9)=81.9, p<0.001, and thermal behavior, F(2,9)=15.8, p<0.01. The pain behavior of CCI animals injected with saline or with the lower dose of calcineurin was not attenuated.
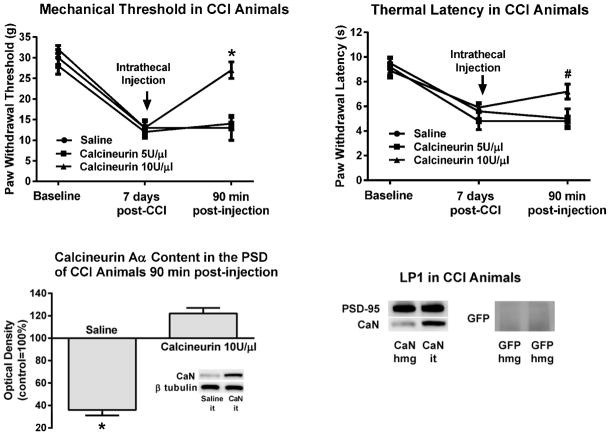
Figure 2. Intrathecal application of calcineurin alleviated the mechanical and thermal hypersensitivity following CCI.
CCI animals exhibited pain behavior seven days post-ligation. After the behavioral testing, saline or calcineurin were injected intrathecally and the animals were behaviorally retested 90 min post-injection. Both the mechanical and the thermal hypersensitivity were significantly attenuated by the higher dose of calcineurin. In these same animals there was significantly more calcineurin Aα in their ipsilateral PSD when compared to the saline injected animals. The PSD-containing LP1 fraction was enriched in PSD-95. Addition of exogenous calcineurin to homogenates of the spinal dorsal horn did not produce increased levels of the enzyme in the LP1 fraction of CCI animals. On the other hand, intrathecal injection of the enzyme resulted in its accumulation in the PSD. GFP added to the homogenates was not detected in the LP1 fraction. CaN=calcineurin, GFP=green fluorescent protein, hmg=added to homogenate, it=injected intrathecally. #p<0.01, *p<0.001.
At the completion of the behavioral testing the three animals injected with saline and the six animals injected with the effective dose of calcineurin were anesthetized, euthanized and their tissue collected for immunoblot processing. In the saline-treated CCI animals there was the expected decrease in calcineurin Aα content in the PSD-containing LP1 fraction of the spinal dorsal horn, 36±5% (Fig. 2). In contrast, the calcineurin Aα PSD content in the calcineurin-injected CCI animals was greater than in controls, 122±5%. ANOVA confirmed a significant treatment-associated difference, F(1,7)=194.9, p<0.001. These data suggested that the exogenously added calcineurin was accumulating in the PSD, and that the attenuated pain behavior may have been the result of this accumulation.
To assess whether the increased content of calcineurin in the LP1 fraction was not the result of a homogenization or fractionation-elicited nonspecific migration of the phosphatase to the PSD but rather a specific outcome of the intrathecal injection we performed some additional control experiments (Fig. 2). As previously shown [15], our LP1 fractions are enriched in PSD-95, a well-established marker of the PSD protein matrix [10]. Addition of calcineurin to homogenates of the spinal dorsal horn of CCI animals did not increase the levels of the enzyme in the LP1 fraction. These fractions still showed the typical CCI-associated loss of the phosphatase. On the other hand, an intrathecal injection of the enzyme resulted in its accumulation in the PSD. Addition of soluble GFP to homogenates of the spinal dorsal horn also did not result in the detection of this non-mammalian protein in LP1 fractions. Taken together, these results suggested that the injected calcineurin was accumulating specifically in the PSD.
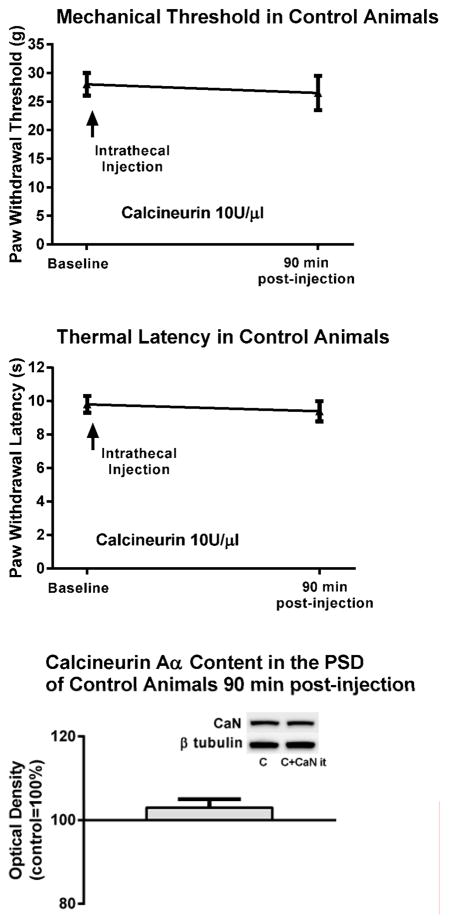
To examine whether intrathecal treatment with calcineurin would affect the behavior of control, otherwise uninjured animals we obtained baseline mechanical thresholds and thermal withdrawal latencies of six of control animals and then injected them intrathecally with the effective dose of calcineurin (10 units/μl). Their mechanical thresholds and thermal latencies 90 min post-injection of the phosphatase were unchanged from baseline, 27±3g vs. 28±2g, and 9.4±0.6s vs. 9.8±0.5, respectively. The content of calcineurin Aα in the PSD of spinal dorsal horn neurons in these animals was also unchanged (103±5%), i.e., it was the same as in control animals that were not injected with calcineurin (Fig. 3).
Figure 3. Intrathecal application of calcineurin did not modify the behavior or the content of calcineurin protein in the PSD of control uninjured animals.
The mechanical and thermal behavior of control, otherwise uninjured animals was not modified 90 min after a single intrathecal injection of the effective dose of calcineurin. The content of calcineurin Aα in the PSD of spinal dorsal horn neurons in these animals was also not modified, i.e., it was the same as in control animals not injected with calcineurin. C=control no calcineurin injection. C+CaN it=control calcineurin injected intrathecally.
3.4. A single intrathecal injection of the calcineurin inhibitor FK-506 elicited signs of pain behavior in control, uninjured, animals and reversed calcineurin-associated analgesia in CCI animals
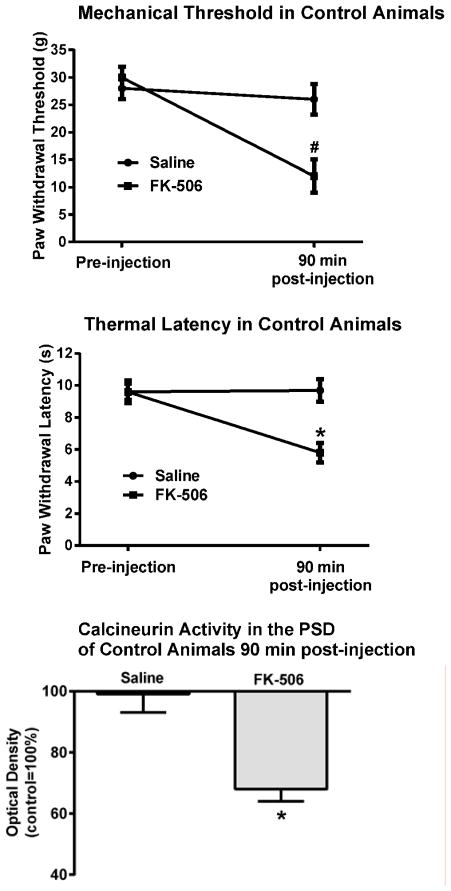
In these experiments we focused on the consequences of the pharmacological inhibition of calcineurin activity in control, otherwise uninjured animals, and in CCI animals showing calcineurin-associated analgesia. The control uninjured animals were behaviorally tested to establish baseline responses and then injected intrathecally with either the calcineurin inhibitor FK-506 (n=6) or saline (n=3). Their behavior was retested 90 min after the injection. Animals treated with FK-506 exhibited behavioral signs of pain as their mechanical thresholds significantly decreased from 30±2g to 12±3g, F(1,7)=29.0, p<0.005, and their thermal latencies from 9.6±0.7s to 5.8±0.6s, F(1,7)=54.3, p<0.001. In contrast, saline-injected animals did not show signs of pain behavior (Fig. 4).
Figure 4. Intrathecal application of FK-506 elicited mechanical and thermal hypersensitivity in control, uninjured animals.
Ninety minutes after an intrathecal injection of the calcineurin inhibitor FK-506 there was a significant decrease in withdrawal thresholds and latencies in control, uninjured, animals. In these same animals there was also a significant decrease in calcineurin activity in their PSD #p<0.005, *p<0.001.
After the behavioral tests were completed the animals were anesthetized, euthanized and their spinal dorsal horn tissue collected for the calcineurin enzyme activity assay. In FK-506 treated animals there was a substantial decrease in calcineurin activity in their LP1 fraction, (68±4%). No such decrease was seen in saline-injected animals as their enzyme activity was identical to that of control untreated animals. ANOVA confirmed a significant effect of the FK-506 treatment, F(1,7)=85.9, p<0.001.
In CCI animals we sought to determine whether an injection of FK-506 would reverse calcineurin-associated analgesia. We injected six CCI animals exhibiting pain behavior 7 days post-CCI with the effective dose of calcineurin and then assessed their behavior 90 min after the intrathecal injection. As seen before (Fig. 2), both the mechanical and the thermal hypersensitivity were significantly attenuated by calcineurin (Fig. 5). Thresholds recovered to 23±2g from 14±2g at 7 days post-CCI p<0.005, and thermal latencies to 7.4±0.6s from 5.9±0.5s at 7 days post-CCI, p<0.001. Following the post-calcineurin behavioral tests, the same animals were injected intrathecally with FK-506, and the behavioral tests repeated once more 90 min after the FK-506 injection. All six animals exhibited now signs of hypersensitivity. Mechanical thresholds decreased to 14±1g, p<0.005 and thermal latencies to 5.4±0.5, p<0.005.
Figure 5. Intrathecal application of FK-506 reversed calcineurin-associated analgesia in CCI animals.
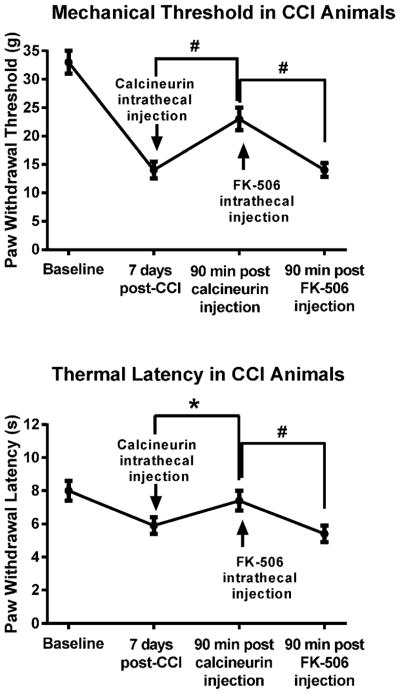
CCI animals exhibiting pain behavior 7 days post-CCI were intrathecally injected with the effective dose of calcineurin and behaviorally retested 90 min after the injection. Both signs of pain behavior were significantly attenuated. The same animals were then injected intrathecally with FK-506, and the behavioral tests repeated once more 90 min after the calcineurin inhibitor injection. The CCI animals exhibited again signs of hypersensitivity at this time. #p<0.005, *p<0.001.
These data supported the notion that inhibition of calcineurin activity in the PSD lead to the pain behavior. The data were also in agreement with multiple studies in humans in which FK-506, given before liver transplantation to depress immune responses, elicited chronic pain, i.e., the “calcineurin-inhibitor pain syndrome” [19].
3.5. A single intrathecal application of calcineurin elicited prolonged analgesia in CCI animals
To examine the duration of the analgesic action of calcineurin we repeatedly tested the behavior of CCI animals two, four and six days after a single injection.
As expected, all CCI animals exhibited mechanical and thermal hypersensitivity 7 days after the ligation (Fig. 6). These animals were then anesthetized and injected intrathecally with saline (n=6) or calcineurin at the effective dose of 10 enzyme units/μl (n=9). Two days later the animals were behaviorally retested. In the calcineurin-treated animals both the mechanical and the thermal hypersensitivity were significantly attenuated. In other words, mechanical thresholds recovered to 25±2g and thermal latencies to 9.2±0.5s. The calcineurin-treated CCI animals continued to exhibit analgesia four days after the injection (21±2g and 8.5±0.7s). It was only six days after the injection that the calcineurin-treated CCI animals exhibited again mechanical and thermal hypersensitivity (14±2g and 5.8±0.6s) suggesting that calcineurin analgesia had ended. Saline-treated CCI animals exhibited typical pain behavior throughout the testing period.
Figure 6. A single intrathecal application of calcineurin provided prolonged analgesia in neuropathic animals.
CCI animals exhibited pain behavior seven days post-ligation. After the behavioral testing, saline or calcineurin were injected intrathecally and the animals were behaviorally retested two, four, and six days after the single injection. Both mechanical and thermal hypersensitivity were significantly attenuated in the calcineurin injected animals two and four days post-injection. By six days the pain behavior returned. Saline-injected CCI animals exhibited pain behavior throughout the six day testing period. A single intrathecal injection of calcineurin did not modify the behavior of control uninjured animals two, four and six days post-injection. *p<0.001.
Repeated measures ANOVA confirmed an effect of time in the calcineurin-treated group for both mechanical thresholds, F(4,32)=44.3, p<0.001 and thermal latencies F(4,32)=72.1, p<0.001. The differences were due to the significant recovery in threshold and latency two and four days after the calcineurin injection. These data established that a single intrathecal injection of calcineurin provided pain relief for four days.
The mechanical and thermal behavior of control, uninjured, animals (n=6) was not modified by the intrathecal injection of calcineurin (10 units/μl) two, four and six days later, F(3,15)=4.7, p<0.1, and F(3,15)=5.0, p<0.1 (Fig. 6).
3.6. Calcineurin analgesia was associated with the restoration of calcineurin activity and content in the PSD
To investigate whether the prolonged analgesic action of calcineurin was associated with a restoration of activity or content of the enzyme in the PSD we repeated the above experiment in new groups of CCI animals. We injected saline or the effective dose of calcineurin (10 units/μl) intrathecally 7 days post-ligation and then euthanized individual animal groups two, four or six days after the injection to collect tissues for the biochemical assays.
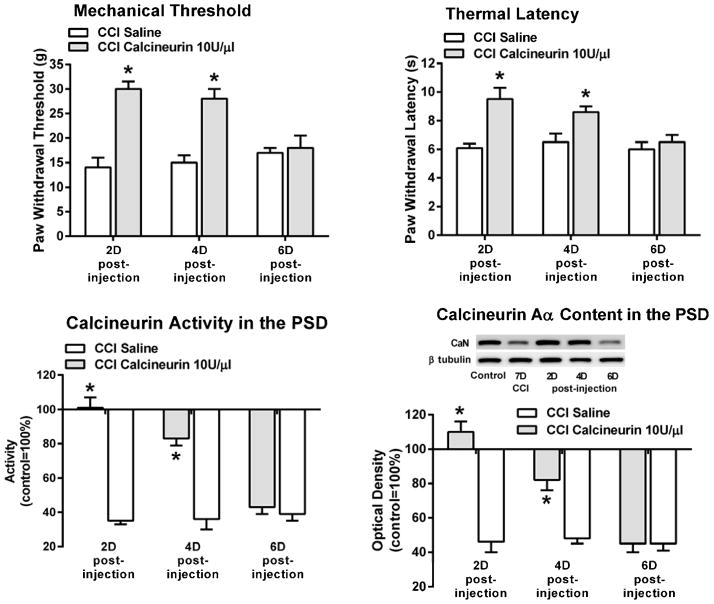
All the 7 days post-CCI animals exhibited mechanical and thermal hypersensitivity, 15±2g, F(1,25)=298.7, p<0.001, and 5.6±0.6s, F(1,25)=454.4, p<0.001 (Fig. 7). A single intrathecal injection of calcineurin provided pain relief in the two days (n=6) and four days (n=6) post-injection groups. In other words, both the mechanical and the thermal hypersensitivity were significantly attenuated, 30±2g and 9.5±0.8s at two days, and 28±2g and 8.6±0.4s at four days. Six days after the single intrathecal injection the analgesic action of calcineurin had terminated as these CCI animals (n=6) exhibited both mechanical and thermal hypersensitivity, 18±3g and 6.5±0.5s.
Figure 7. Calcineurin-elicited analgesia was associated with the restoration of calcineurin activity and Aα content in the ipsilateral PSD.
Saline or calcineurin were injected intrathecally seven days post-CCI and individual animal groups were euthanized two, four or six days after the injection. The calcineurin-injected two and four days CCI animal groups exhibited analgesia, and this was associated with a recovery in both calcineurin activity and Aα content in their PSD. In contrast, the six days calcineurin-injected CCI animal group exhibited pain behavior, and this was associated with significantly reduced levels in calcineurin activity and Aα content in their PSD. All saline-injected CCI animal groups exhibited pain behavior, and this was accompanied by a significant loss of activity and Aα content of calcineurin in the PSD of these animals. CaN=calcineurin. *p<0.001 when compared to the seven days post-CCI values.
Calcineurin-elicited analgesia in the two and four days CCI animal groups was associated with a significant recovery in the activity and Aα content of the phosphatase in the PSD both at two days, 101±6 and 110±6% and at four days, 83±4% and 82±6%. In contrast, the pain behavior in the six days post-calcineurin injection group was associated with significantly reduced levels of calcineurin activity and Aα content in the PSD, 43±4% and 45±5%.
The two, four or six days post-saline injection CCI groups (n=6 in each group) exhibited the expected pain behavior, and this was accompanied by a significant loss of both calcineurin activity and Aα content in the PSD of these animals (Fig. 7).
Taken together these data suggested that a single injection of the enzyme accumulated in the PSD for four days post-injection to alleviate well-established neuropathic pain during this entire time.
3.7. No change in calcineurin message was detected in CCI animals exhibiting analgesia two days post-calcineurin injection
We reported recently that there was no change in calcineurin message in the spinal dorsal horn of 7 days post-CCI animals [16]. These data suggested that the observed loss of protein in the PSD was not due to a down-regulation of calcineurin gene expression. In this study we again used RT-PCR to examine whether an up-regulation of calcineurin gene expression was associated with the observed increases in enzyme protein levels in the PSD of calcineurin-injected CCI groups (Fig. 7).
Six additional animals underwent CCI and exhibited mechanical and thermal hypersensitivity 7 days post-ligation (Fig. 8). They were then injected with the effective dose of calcineurin and behaviorally retested two days post-injection. In all six animals the mechanical and thermal hypersensitivity were attenuated. ANOVA confirmed a significant effect of treatment for both the mechanical, F(1,7)=31.4, p<0.005, and thermal behavior, F(1,7)=28.8, p<0.005. The animals were then euthanized and their tissues collected for RT-PCR. The levels of calcineurin message in these animals were the same as in control uninjured animals not injected with calcineurin, i.e., 1.2±0.2-fold. These data suggested that prolonged increases in calcineurin protein levels in the PSD were not a reflection of increased calcineurin gene expression.
Figure 8. No change in calcineurin message was detected in CCI animals exhibiting analgesia two days post-calcineurin injection.
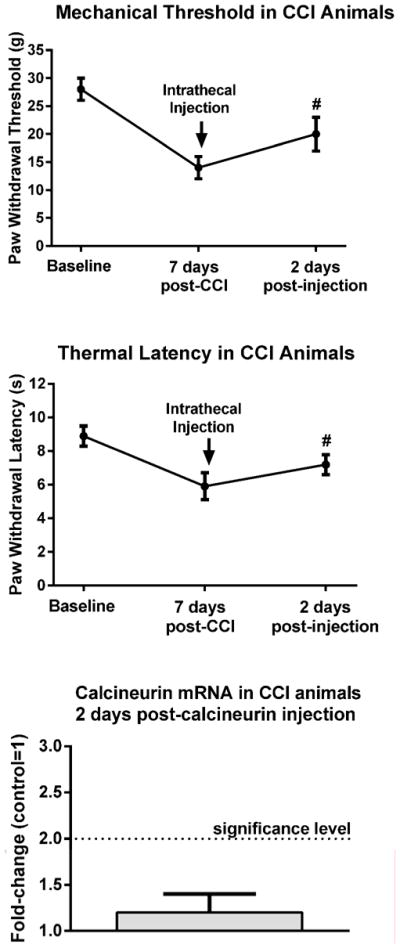
CCI animals exhibited pain behavior seven days post-ligation. After the behavioral testing, an effective dose of calcineurin was injected intrathecally and the animals were behaviorally retested two days post-injection. Both mechanical and thermal hypersensitivity were significantly attenuated at this time. In these same animals the levels of calcineurin message were the same as in control uninjured animals not injected with calcineurin. #p<0.005.
3.8. Intrathecal calcineurin treatments were not accompanied by signs of toxicity
To establish whether intrathecal injection of the phosphatase elicited signs of toxicity we injected six control uninjured animals with the effective dose of calcineurin (10 units/μl) and euthanized three animals two days post-injection and three animals 14 days post-injection. We also injected three control uninjured animals with saline and euthanized these two days post-injection. Three uninjured animals which were not injected at all served as a control comparison group.
Before euthanasia blood samples were obtained from each animal for routine clinical chemistry (small animal panel), hematology (complete blood count, white cell blood count) and serology (comprehensive panel) analysis. After euthanasia the animals underwent routine necropsy and representative sections of the kidney (right, left), liver (at least 5 lobes), brain (cortex, hippocampus, cerebellum, brain stem) and spinal cord (cervical, thoracic, lumbar) were processed for histopathology.
There were no differences in behavior (eating, grooming, motor activity) among any of the four animal groups. Similarly, there were no significant differences in any of the clinical chemistry, hematology and serology parameters examined, or in the necropsy or histopathology findings (Table 1). These data suggested that a single intrathecal injection of calcineurin provided prolonged analgesia without signs of toxicity.
Table 1.
Effects of a single intrathecal injection of saline or calcineurin (10 units/μl).
| 2 days post-saline | 2 days post-calcineurin | 14 days post-calcineurin | |
|---|---|---|---|
| Behaviora | No treatment effect | No treatment effect | No treatment effect |
| Clinical Chemistryb | No treatment effect | No treatment effect | No treatment effect |
| Hematologyc | No treatment effect | No treatment effect | No treatment effect |
| Serologyd | No treatment effect | No treatment effect | No treatment effect |
| Histopathologye | No treatment effect | No treatment effect | No treatment effect |
grooming, eating, motor activity
comprehensive small animal panel
complete blood count, white blood cell count
comprehensive profile
kidney, liver, brain, spinal cord
4. Discussion
In this study we investigated whether calcineurin plays a role in the development of neuropathic pain following peripheral nerve injury. Specifically, we examined the consequences of CCI on the activity and protein content of calcineurin in the PSD of spinal dorsal horn neurons. Once we established that a significant loss of the phosphatase was associated with CCI-elicited pain we sought to determine whether addition of calcineurin would alleviate the well-established pain behavior by restoring the enzyme’s activity and protein content without eliciting signs of toxicity.
In summary, our data revealed that there was significantly lower calcineurin activity and content in the ipsilateral PSD of spinal dorsal horn neurons in CCI animals exhibiting neuropathic pain. The pain behavior was attenuated by intrathecal application of exogenous calcineurin. The calcineurin analgesia was long-lasting as a single injection provided pain relief for four days by restoring the phosphatase’s activity and Aα content in the PSD of spinal dorsal horn neurons. No signs of toxicity were detected up to 14 days after the single intrathecal injection of calcineurin. An intrathecal application of the calcineurin inhibitor FK-506 elicited pain behavior in control, uninjured animals. The same injection significantly reduced calcineurin activity in the PSD. These results confirmed the similarity in some of the mechanisms underlying activity-dependent synaptic plasticity in the brain and injury-elicited plasticity in the spinal dorsal horn.
To our knowledge, this is the first demonstration of a nerve injury-associated loss of calcineurin activity and protein content in the PSD of spinal dorsal horn neurons which can be prevented by enzyme substitution to alleviate well-established neuropathic pain for a prolonged period of time without signs of toxicity.
4.1 Potential consequences of the loss of calcineurin dephosphorylating activity at the synapse
While our results suggested that there was a link between calcineurin and neuropathic pain it remains unclear how the loss of the phosphatase in the PSD was translated into the development of neuropathic pain. We postulate at least three negative consequences of calcineurin’s loss: (1) disruption of a complex between the A kinase anchoring protein (AKAP) and PKA with calcineurin [9], (2) persistent phosphorylation and insertion of AMPA receptors in the PSD membrane [4], and (3) the phosphorylation-dependent physical linking of AMPA, NMDA and metabotropic glutamate receptor families and their associated signaling pathways [15]. Individually or in combination, all three of these mechanisms would allow for the injury-elicited functional and structural changes in the PSD to persist and thus maintain enhanced synaptic function, i.e., neuropathic pain.
Calcineurin is anchored to the PSD by AKAP which also binds PKA to place both enzymes in close proximity to their substrates at the synapse [9]. Among those substrates are glutamatergic AMPA receptors. Phosphorylation of these receptors leads to their insertion in the membrane and to enhanced synaptic function. In contrast, dephosphorylation engenders their internalization and loss of synaptic strength [4]. The CCI-associated loss of calcineurin in spinal dorsal horn neurons may remove the enzyme from the complex with PKA and AKAP and prevent the recycling of AMPA receptors. This would permit the continued insertion of phosphorylated (activated) AMPA receptors in the membrane and maintain the injury-elicited enhancement of synaptic function, i.e., neuropathic pain.
Phosphorylation of scaffolding proteins in the PSD can also markedly influence synaptic function [10] by producing or enhancing the linking of AMPA, NMDA and metabotropic glutamate receptor complexes. The injury-associated loss of calcineurin dephosphorylating activity may allow for the continual maintenance of these links and thus for the coordinated activation of all PSD proteins associated with the three complexes even if only one of these receptors were stimulated. In other words, a low intensity peripheral stimulus that would ordinarily only activate metabotropic glutamate receptors could now influence NMDA receptor-linked proteins even if the NMDA receptors remained inactive due to an insufficient degree of membrane depolarization elicited by the low intensity stimulus. Behaviorally, this may be reflected then in exaggerated responses to both innocuous and noxious peripheral stimuli, e.g., allodynia and hyperalgesia.
It remains unclear why calcineurin protein was lost in the PSD. We reported recently that there was no decrease in calcineurin message in the spinal dorsal horn 7 days post-CCI [16]. So the loss was not a result of the down-regulation of calcineurin gene expression but possibly a consequence of the phosphatase’s redistribution from the PSD or local degradation at the synapse.
4.2 Calcineurin in the PSD and neuropathic pain
The relationship between calcineurin and neuropathic pain appears complex. Calcineurin-inhibitor pain syndrome (CIPS) is a well-described complication associated with the use of the calcineurin inhibitors cyclosporine and FK-506 (tacrolimus) in organ transplant patients [19]. This clinical outcome suggests that there is an interdependent link between the development of pain and the inhibition of calcineurin activity. On the other hand, several publications reported that calcineurin inhibitors prevented the calcineurin-dependent activation of the transcription factor NFAT which, upon translocation to the nucleus, elicited the expression of many genes including those considered pro-nociceptive such as nerve growth factor (NGF), brain-derived neurotrophic factor (BDNF) and cyclooxygenase-2 (COX-2) [7,8].
It is likely that the phosphatase assumes a differential role based on its cellular location. Calcineurin activity in glial cells or in the cell bodies of primary afferent neurons may facilitate NFAT-dependent gene expression. In contrast, calcineurin activity within the PSD of spinal dorsal horn neurons may prevent injury-associated long-lasting plasticity and thus block the development of neuropathic pain.
The loss of calcineurin activity in the PSD may critically permit nerve injury-elicited afferent activity to remodel the synapses of spinal dorsal horn neurons in such a way as to allow for the transition to long-lasting plasticity and thus the development of neuropathic pain. As stated above, a balance between kinase and phosphatase activity determines synaptic strength and consequently a loss of either one of these activities has the potential to create long-lasting changes in synaptic function. In our study, the injury-associated loss of calcineurin may have removed the negative constraint of the phosphatase on the phosphorylation of multiple target proteins including transcription factors involved in gene regulation such as cyclic AMP response element binding protein (CREB) [14]. This in turn may have initiated a series of steps that led to the strengthening (enhancement) of dorsal horn synaptic function. This enhancement would potentiate responses to primary afferent activity and the amplified input may then be manifested as allodynia to innocuous stimulation and hyperalgesia to noxious stimuli.
The addition of exogenous calcineurin by intrathecal injection restored the balance between kinase and phosphatase activity. This initiated a series of steps to ‘de-strengthen’ the synapse and restore synaptic function to a normal pre-injury de-potentiated state through the phosphatase-dependent dephosphorylation of the target proteins, and the subsequent reversal of the injury-remodeled PSD. This then may have elicited relief from the well-established pain by allowing innocuous stimuli to again be perceived as such, and preventing noxious stimuli from eliciting exaggerated responses. The analgesia persisted as long as the phosphatase was present within the PSD. Once calcineurin was lost through routine pharmacokinetics six days post-injection the analgesia disappeared as well. This suggested that the reappearance of the pain was dependent once again upon the loss of dephosphorylating activity in the PSD.
Our results have intriguing clinical implications. Intrathecal treatment with calcineurin provided prolonged analgesia without signs of toxicity. Clinical therapy with calcineurin may thus prove to be a novel, effective and safe approach in the management of well-established neuropathic pain.
Summary.
Loss of calcineurin activity and protein content in the post-synaptic density (PSD) of spinal dorsal horn neurons was associated with neuropathic pain following chronic constriction injury of the sciatic nerve. A single intrathecal injection of exogenous calcineurin accumulated at the synapse for four days and provided prolonged relief of well-established pain without signs of toxicity.
Acknowledgments
We wish to thank Georgia L. Bosscher and Jessie L. Hermes for their help with some of the behavioral testing, animal surgeries and biochemical procedures.
We also wish to thank Dr. Annette Gendron-Fitzpatrick for her help in obtaining and interpreting the clinical chemistry and histopathology data.
Supported in part by National Institutes of Health grants NS034870, NS055042 and NS075917.
Footnotes
Conflict of Interest
None of the authors have financial arrangements that could represent a possible conflict of interest. A patent for the intrathecal treatment approach is pending.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Reference List
- 1.Abel T, Kandel E. Positive and negative regulatory mechanisms that mediate long-term memory storage. Brain Res Rev. 1998;26:360–378. doi: 10.1016/s0165-0173(97)00050-7. [DOI] [PubMed] [Google Scholar]
- 2.Baumgartel K, Genoux D, Welzl H, Tweedie-Cullen RY, Koshibu K, Livingstone-Zatchej M, Mamie C, Mansuy IM. Control of the establishment of aversive memory by calcineurin and Zif268. Nature Neurosci. 2008;11:572–578. doi: 10.1038/nn.2113. [DOI] [PubMed] [Google Scholar]
- 3.Bennett GJ, Xie YK. A peripheral mononeuropathy in rat that produces disorders of pain sensation like those seen in man. Pain. 1988;33:87–107. doi: 10.1016/0304-3959(88)90209-6. [DOI] [PubMed] [Google Scholar]
- 4.Choi JI, Svensson CI, Koehrn FJ, Bhuskute A, Sorkin LS. Peripheral inflammation induces tumor necrosis factor dependent AMPA receptor trafficking and Akt phosphorylation in spinal cord in addition to pain behavior. Pain. 2010;149:243–253. doi: 10.1016/j.pain.2010.02.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Citri A, Malenka RC. Synaptic plasticity: multiple forms, functions, and mechanisms. Neuropsychopharmacol. 2008;33:18–41. doi: 10.1038/sj.npp.1301559. [DOI] [PubMed] [Google Scholar]
- 6.Goto S, Hirano A, Pearson J. Calcineurin and synaptophysin in the human spinal cord of normal individuals and patients with familial dysautonomia. Acta Neuropathol. 1990;79:647–652. doi: 10.1007/BF00294243. [DOI] [PubMed] [Google Scholar]
- 7.Groth RD, Dunbar RL, Mermelstein PG. Calcineurin regulation of neuronal plasticity. Biochem Biophys Res Comm. 2004;311:1159–1171. doi: 10.1016/j.bbrc.2003.09.002. [DOI] [PubMed] [Google Scholar]
- 8.Groth RD, Coicou LG, Mermelstein PG, Seybold VS. Neurotrophin activation of NFAT-dependent transcription contributes to the regulation of pro-nociceptive genes. J Neurochem. 2007;102:1162–1174. doi: 10.1111/j.1471-4159.2007.04632.x. [DOI] [PubMed] [Google Scholar]
- 9.Jeske NA, Diogenes A, Ruparel NB, Fehrenbacher JC, Henry M, Akopian AN, Hargreaves KM. A-kinase anchoring protein mediates TRPV1 thermal hyperalgesia through PKA phosphorylation of TRPV1. Pain. 2008;138:604–616. doi: 10.1016/j.pain.2008.02.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kreienkamp HJ. Scaffolding proteins at the postsynaptic density: Shank as the architectural framework. Handb Exp Pharmacol. 2008;186:365–380. doi: 10.1007/978-3-540-72843-6_15. [DOI] [PubMed] [Google Scholar]
- 11.Kuner R. Central mechanisms of pathological pain. Nature Med. 2010;16:1258– 1266. doi: 10.1038/nm.2231. [DOI] [PubMed] [Google Scholar]
- 12.Lee HK. Synaptic plasticity and phosphorylation. Pharmacol Therap. 2006;112:810–832. doi: 10.1016/j.pharmthera.2006.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Masumura C, Horii A, Mitani K, Kitahara T, Uno A, Kubo T. Unilateral vestibular deafferentation-induced changes in calcium signaling-related molecules in the rat vestibular nuclear complex. Brain Res. 2007;138:129–135. doi: 10.1016/j.brainres.2006.12.072. [DOI] [PubMed] [Google Scholar]
- 14.Miletic G, Pankratz MT, Miletic V. Increases in the phosphorylation of cyclic AMP response element binding protein (CREB) and decreases in the content of calcineurin accompany neuropathic pain following chronic constriction injury in rats. Pain. 2002;99:493–500. doi: 10.1016/S0304-3959(02)00242-7. [DOI] [PubMed] [Google Scholar]
- 15.Miletic G, Dumitrascu CI, Honstad CE, Micic D, Miletic V. Loose ligation of the rat sciatic nerve elicits early accumulation of Shank1 protein in the postsynaptic density of spinal dorsal horn neurons. Pain. 2010;149:152–159. doi: 10.1016/j.pain.2010.02.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Miletic G, Sullivan KM, Koos Dodson AM, Lippitt JA, Schneider JA, Miletic V. Changes in calcineurin message, enzyme activity and protein content in the spinal dorsal horn are associated with chronic constriction injury of the rat sciatic nerve. Neuroscience. 2011;188:142–147. doi: 10.1016/j.neuroscience.2011.05.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Pulipparacharuvil S, Renthal W, Hale CF, Taniguchi M, Xiao G, Kumar A, Russo SJ, Sikder D, Dewey CM, Davis MM, Greengard P, Nairn AC, Nestler EJ, Cowan CW. Cocaine regulates MEF2 to control synaptic and behavioral plasticity. Neuron. 2008;59:621–633. doi: 10.1016/j.neuron.2008.06.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Sandkuhler J. Models and mechanisms of hyperalgesia and allodynia. Prog Brain Res. 2009;89:707–758. doi: 10.1152/physrev.00025.2008. [DOI] [PubMed] [Google Scholar]
- 19.Smith HS. Calcineurin as a nociceptor modulator. Pain Physician. 2009;12:E309–E318. [PubMed] [Google Scholar]
- 20.Strack S, Wadzinski BE, Ebner FF. Localization of the calcium/calmodulin-dependent protein phosphatase, calcineurin, in the hindbrain and spinal cord of the rat. J Comp Neurol. 1996;375:66–76. doi: 10.1002/(SICI)1096-9861(19961104)375:1<66::AID-CNE4>3.0.CO;2-M. [DOI] [PubMed] [Google Scholar]
- 21.Wang GP, Huang LQ, Wu HJ, Zhang L, You ZD, Zhao ZX. Calcineurin contributes to spatial memory impairment induced by rapid eye movement sleep deprivation. NeuroReport. 2009;20:1172–1176. doi: 10.1097/WNR.0b013e32832f0772. [DOI] [PubMed] [Google Scholar]
- 22.Yang Y, Fischer QS, Zhang Y, Baumgartel K, Mansuy IM, Daw NW. Reversible blockade of experience-dependent plasticity by calcineurin in mouse visual cortex. Nature Neurosci. 2005;8:791–796. doi: 10.1038/nn1464. [DOI] [PubMed] [Google Scholar]
- 23.Zimmermann M. Ethical guidelines for investigations of experimental pain in conscious animals. Pain. 1983;16:109–110. doi: 10.1016/0304-3959(83)90201-4. [DOI] [PubMed] [Google Scholar]