Abstract
Background & Aims
There is an unclear relationship among bowel symptoms, excretion of unconjugated fecal bile acid (UBA), and colonic transit in irritable bowel syndrome (IBS). We measured total and main individual UBA in fecal samples of patients with IBS, and assessed relationships among stool frequency or consistency, fecal UBA (total and individual), and colonic transit.
Methods
In a study of 30 healthy volunteers (controls), 31 subjects with IBS with diarrhea (IBS-D), and 30 with IBS with constipation (IBS-C) were placed on 4-day diets containing 100 g fat; we measured stool characteristics, total fecal UBA and fat levels, and overall colonic transit. We assessed univariate associations of total and individual levels of fecal UBA with phenotype (controls, IBS-D, IBS-C) using the Kruskal-Wallis test; associations between endpoints were assessed using Spearman correlations. With response surface regression models, we assessed relationships between stool, colonic transit, and fecal total and secretory UBA.
Results
There was a significant association between total fecal UBA and phenotype (P=.029); the association was greater for IBS-D than IBS-C, compared with controls. Fecal levels of primary UBAs (cholic and chenodeoxycholic acids) were higher in subjects with IBS-D, compared with controls (both P<.01). Levels of fecal secretory UBAs (chenodeoxycholic acids, P=.019; deoxycholic acid, P=.025) were lower in subjects with IBS-C compared with controls, whereas levels of the nonsecretory UBA, lithocholic acid, were higher (P=.020). There were significant univariate associations between stool number and form and total fecal UBA (including percentages of lithocholic acid, chenodeoxycholic acids, and cholic acid), fecal fat, and colonic transit at 24 and 48 h after eating. In the regression models, the relative contribution of colonic transit was consistently greater, and largely independent of the contribution of bile acids.
Conclusions
Measurements of individual UBAs identify changes associated with stool characteristics in patients with IBS; these effects are independent of the effects of colonic transit.
Keywords: diarrhea, constipation, secretory, abdominal pain
Introduction
The pathophysiology of irritable bowel syndrome (IBS) is multi-factorial and may include altered sensation, psychosocial factors, mucosal defense, and motility.1 Colonic transit (CT) is accelerated in up to 50% of patients with diarrhea-predominant IBS (IBS-D) and delayed in up to 20% of patients with constipation-predominant IBS (IBS-C).2 Mechanisms contributing to the pathophysiology of IBS include intraluminal and mucosal factors such as bile acids (BA) that may induce changes in mucosal, motor, and sensory functions.1
In a systematic review, BA malabsorption (BAM) was reported in 32% of patients with IBS-D type symptoms and there is a dose-response relationship to treatment with BA binders based on severity of BAM.3 In patients with IBS-D, treatment with a BA sequestrant was associated with slower ascending colonic emptying and firmer stool.4
Conversely, deficiency or sulfation of BAs may be associated with constipation.5,6 BA supplementation with CDCA7 and the ileal BA transporter inhibitor, elobixibat,8 accelerate CT in chronic constipation, and rectal infusion of CDCA induces colonic propulsive motility.9.
BA species are differentiated by their hydroxylation and conjugation status. CDCA and cholic acid (CA) are primary BAs synthesized in the liver from cholesterol and conjugated with taurine and glycine; in the colon, bacteria deconjugate the BAs. CDCA and CA comprise a minor percentage of fecal BA pool because they are dehydroxylated by colonic bacteria to form the secondary BAs, lithocholic (LCA) and deoxycholic acid (DCA) respectively which are predominant BAs in feces.10
In a previous study, our group showed that total fecal BA excretion was significantly higher in patients with IBS-D than IBS-C.11 In addition to alterations in total fecal BAs, our hypothesis is that IBS may be characterized by changes in the profile of fecal BAs with an increased proportion of primary fecal BAs in patients with IBS-D.12
The primary aim of this study was to perform a quantitative assessment of total, primary and secondary fecal unconjugated BAs (UBA) in health and IBS with constipation or diarrhea, and to assess the inter-relationships between fecal total BA excretion, fecal UBA profile, IBS phenotype, CT, and stool characteristics in multiple variable response surface regression analyses.
Methods
Participants
In a prospective study of 91 participants, we enrolled 30 healthy volunteers (HV), 31 patients with IBS-D or functional diarrhea and 30 patients with IBS-C from within a 150-mile radius of Rochester, MN. HV were recruited by advertisement at Mayo Clinic and were screened for bowel symptoms using a brief questionnaire. Detailed history was obtained by medical staff during the screening evaluation to confirm eligibility of asymptomatic controls and the absence of lower functional gastrointestinal disease (FGID). All participants completed a validated bowel disease questionnaire13 and Hospital Anxiety & Depression Scale14 (HAD) at baseline. In symptomatic patients, a positive diagnosis of FGID was defined by Rome III criteria. Volunteers were 18–65 years old, had no prior abdominal surgeries (except appendectomy and/or cholecystectomy) and could not be taking GI medications known to alter motility within 6 months for HV and within the 48 hours prior to active study initiation for patients with IBS. Patients were excluded if they had a bleeding disorder, liver disease, or were pregnant or tobacco users.
Experimental Protocol
After approval of the protocol by the Mayo Clinic Institutional Review Board and, following signed written informed consent, patient study eligibility was confirmed by medical history, physical examination, concomitant medication review, and clinical laboratory tests. Participants received instructional pamphlets to follow a 100g fat diet per day for four days. A food diary was used to confirm compliance with the high fat diet, and stool samples were collected during the last 48 hours of high fat intake for assessment of fecal fat, total fecal UBAs and individual fecal UBAs [CDCA, CA, LCA, DCA and ursodeoxycholic (UDCA)].
Measurement of Fecal Fat
Fecal fat was measured by Mayo Clinic Department of Laboratory Medicine using an adaptation of the van der Kamer test on an aliquot from the 48hour stool collection. The result was expressed as average excretion per 24hours by dividing the estimated fecal fat excretion by 2; the normal fecal fat is <6 g per 24h.
Measurement of Unconjugated Fecal Bile Acids
The extraction, LC/MS-MS analysis, calculations and analytical performance of the fecal BA assay (total and main UBAs) are detailed in the Appendix.
Assessment of Gastrointestinal and Colonic Transit
The validated method15 is described briefly in the Appendix.
Assessment of Bowel Symptoms
Over a two-week period that overlapped with the fecal collections and transit measurements, bowel symptoms were collected using a standard validated bowel pattern diary including the Bristol stool form scale,16 and ease of passage using a scale ranging in score from 1 = manual disimpaction to 7 = incontinence, used extensively in prior studies.17
Statistical Analysis
Primary endpoints in the analysis were CT (geometric center, GC) at 24 hours and total fecal BA excretion. Secondary endpoints were CT at 48h (GC48), ascending colon emptying T1/2, bowel symptoms (stool number, form, ease of passage, and completeness of evacuation), and total fecal fat and fecal BA profile.
The associations of the distributions of the levels of fecal UBAs with the three symptom phenotype groups (HV, IBS-D, IBS-C) were assessed using the Kruskal-Wallis test for a shift in location of the distributions, and we assessed univariate pairwise associations between other study endpoints using Spearman correlations (rs). Relationships between stool characteristics, CT and fecal UBA excretion were assessed using response surface regression models, including body mass index (BMI) as a covariate and linear, quadratic and cross product terms for the CT and UBA measurements.
Results
Demographics
Baseline characteristics of study participants are shown in Table 1A for all 3 groups (HV, IBS-D, IBS-C). BMI was associated with group (overall p=0.064), being highest in the IBS-D group. All volunteers were females in the IBS groups and there were 8 males among the 30 HV. History of prior cholecystectomy was present in 2/30 HV, 5/31 IBS-D, and 3/30 IBS-C. There was no significant association between baseline anxiety and group based on the HAD scores. Baseline depression scores were significantly associated with group (overall p=0.046) and were highest in the IBS-D group.
Table 1.
| A. Baseline characteristics [age, gender, body mass index], total fecal fat, total and individual fecal unconjugated bile acids (UBA), mean stool number, mean stool form, and anxiety and depression scores in healthy volunteers (HV) and diarrhea-predominant (IBSD) and constipation-predominant (IBS-C) irritable bowel syndrome groups | |||
|---|---|---|---|
|
Group/ Data median (IQR) |
HV | IBS-C | IBS-D |
| Age, y | 40.5 (31.0, 48.0) | 47.5 (39.0, 50.0) | 36.0 (31.0, 43.0) |
| Number, gender | 30; 22F:8M | 30; all F | 31; all F |
| BMI, kg/m2 | 25.7 (22.0, 28.2) | 26.4 (23.7, 29.2) | 28.2 (23.7, 33.5) |
| Fecal fat, g/24h* | 6.0 (4.0, 7.0) | 3.0 (2.0, 6.0) | 6.0 (3.0, 11.0) |
| Fecal UBAs, µM/48h# | 511.7 (201.0, 1586.3) | 317.1 (167.6, 762.0) | 1204.9 (421.3, 2169.3) |
| % CA^ | 0.3 (0.3, 0.6) | 0.3 (0.2, 0.5) | 1.4 (0.4, 7.6) |
| % CDCA $ | 0.2 (0.2, 0.6) | 0.1 (0.1, 0.2) | 1.7 (0.4, 8.6) |
| % DCA | 61.4 (49.5, 68.9) | 47.7 (39.3, 62.1) | 56.3 (50.7, 64.1) |
| % LCA** | 37.3 (30.3, 46.6) | 49.3 (37.5, 60.5) | 31.5 (21.1, 38.9) |
| % UDCA | 0.1 (0, 0.4) | 0.1 (0, 0.1) | 2.6 (0.7, 3.5) |
| Mean stool number | 1.1 (0.9, 1.4) | 0.8 (0.5, 1.0) | 1.9 (1.6, 2.6) |
| Mean stool form | 3.8 (3.4, 4.2) | 2.8 (2.0, 3.4) | 5.0 (4.4, 5.3) |
| Anxiety score | 2.0 (1.0, 4.0) | 2.0 (1.0, 5.0) | 2.0 (0, 6.0) |
| Depression score! | 0 (0, 1.0) | 0.5 (0, 1.0) | 1.0 (0, 2.0) |
| B. Gastric emptying and colonic transit in irritable bowel syndrome (IBS) groups and male (M) and female (F) healthy volunteers | ||||
|---|---|---|---|---|
|
Group/ Data mean (IQR) |
IBS-C | IBS-D | HV (M and F) | HV (F |
| Gastric emptying T1/2 min | 114.0 (99.0, 133.0) | 116.0 (98.0, 143.0) | 116.0 (100.0, 137.0) | 122.5 (106.0, 142.9) |
| Colonic filling at 6h | 50.5 (39.0, 76.0) | 68.0 (39.0, 82.0) | 59.0 (35.0, 70.0) | 59.0 (35.0, 69.0) |
| CT GC24h | 2.0 (1.7, 2.8) | 3.1 (1.9, 3.9) | 2.2 (1.9, 3.0) | 2.1 (1.8, 2.7) |
| CT GC48h@, β | 3.0 (2.6, 3.8) | 4.7 (3.8, 5.0)γ | 4.2 (3.3, 4.5) | 4.1 (3.1, 4.5) |
Statistical analysis by Kruskal-Wallis test assessing overall group differences:
p=0.022;
p=0.029;
p=0.0005;
p<0.001;
p=0.0013;
p=0.0002;
p=0.0463
BMI = body mass index; CA = cholic acid; CDCA = chenodeoxycholic acid; DCA = deoxycholic acid; LCA = lithocholic acid; UDCA = ursodeoxycholic acid; CT = colonic transit; GC = geometric center; IQR = interquartile range
Statistical analysis by Kruskal-Wallis test assessing overall group differences:
p=0.0002 (IBSC, IBS-D, and all HV);
p=0.0003 (IBS-C, IBS-D, and female HV).
Statistical analysis by Wilcoxon rank sum test for pairwise comparison between female HV and IBS-D patients (all female):
p=0.045
Quantification of Fecal UBA Excretion and Stool Fat
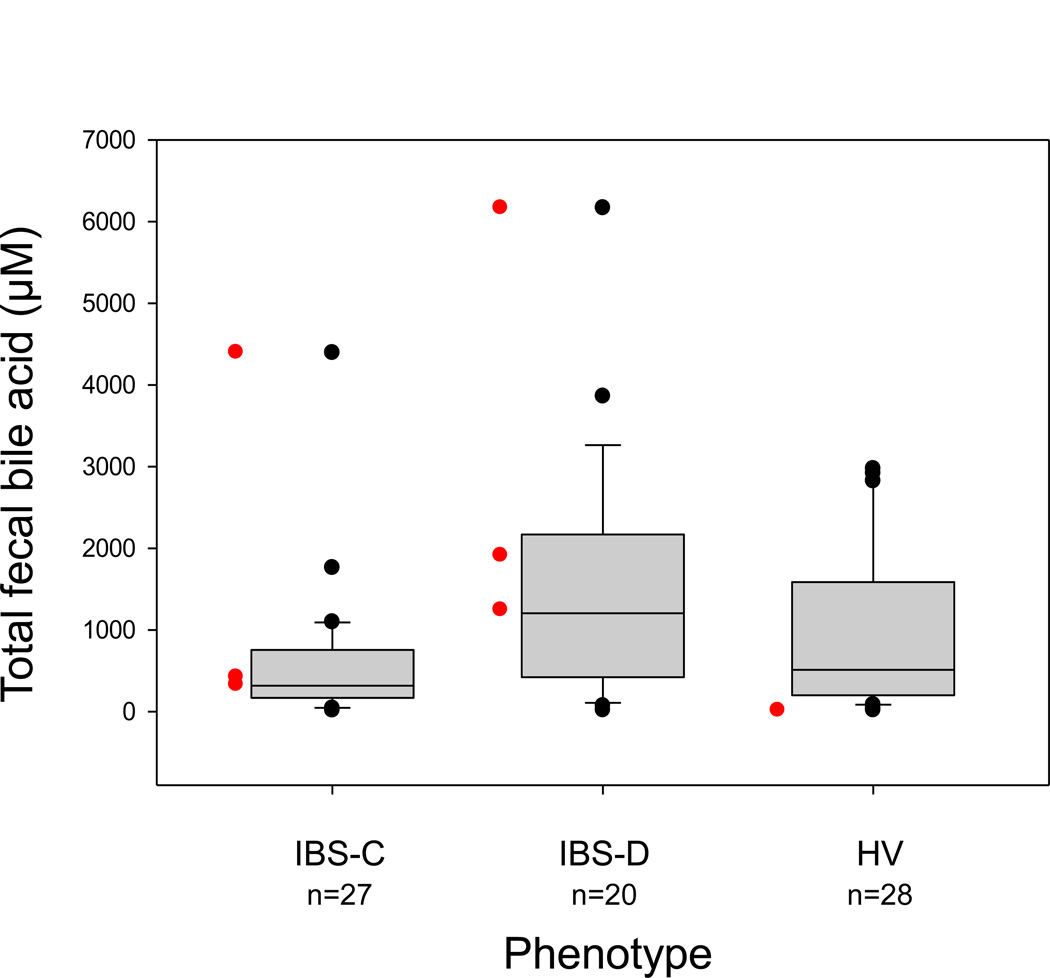
Fecal fat and total and individual UBAs for all 3 groups are summarized in Table 1A. Data on fecal fat and UBAs were missing (sample not obtained or incorrectly processed) in 4 participants for fecal fat and 16 for fecal BAs. There was a significant correlation in overall group between fecal fat and fecal total UBAs (rs= 0.48, p<0.001). Lower fecal fat but not fecal total UBAs (Figure 1) were significantly associated with the IBS-C group (p=0.013 and p=0.20, respectively) relative to the HV. Total fecal UBA and fat excretion were greater in the IBS-D group than the normal stool donors, although the differences were not statistically significant. Percentage of primary UBA excretions in stool was significantly associated with group, being higher in IBS-D compared to HV (CA [p= 0.0014], CDCA [p=0.0007]). Percentages of fecal excretion of two of the secretory UBAs, CDCA and DCA, were lower in IBS-C (p=0.015 and 0.025 respectively) and percent of fecal excretion of the non-secretory secondary UBA, LCA, was significantly higher in IBS-C (p=0.020) compared to HV. Three group differences in UDCA (p<0.001) were of unclear significance given the mean <2.2% total excretion in all groups.
Figure 1.
Comparison of total 48-hour stool unconjugated bile acids (UBA) in the three phenotype subgroups [constipation-predominant irritable bowel syndrome (IBS-C), diarrhea-predominant irritable bowel syndrome (IBS-D), and healthy volunteers (HV)]. Data show median, interquartile range and 5–95th percentile range, as well as outliers. There was a significant three-group (overall) association between total fecal UBA and phenotype group, but no significant differences between IBS-D or IBS-C and controls. Red data points denote patients with prior cholecystectomy.
Gastric, Small Bowel and Colonic Transit
These data appear in Table 1B. There were no significant group differences in gastric emptying and colonic filling at 6h, but there was an overall difference in CT at 48h (p=0.0002) with faster transit in IBS-D. Faster CT at 24h in IBS-D was of borderline statistical significance (p=0.118).
Relationships between Stool Characteristics, Colonic Transit and Fecal BAs
There were significant univariate associations for the overall group (Table 2 showing Spearman correlation coefficients [rs]) between stool number and stool form and total fecal UBAs (including % LCA, % CDCA, and % CA), fecal fat, and CT at 24 and 48h. GC48 was significantly associated with total fecal UBAs (including % CDCA, %LCA, and %DCA) and fecal fat (all p<0.01). All associations between UBAs (total and individual), stool characteristics and transit showed positive correlations with the exception of LCA. No significant associations were observed with baseline anxiety and depression scores and the stool number or form, CT or fecal UBAs.
Table 2.
Univariate associations of stool form, stool number and colonic transit at 24 (GC24) and 48 (GC48) hours, fecal fat, and total and individual (CDCA, LCA, CA, DCA) fecal bile acids using Spearman correlation
| Data show rs | Stool number |
Stool form |
GC24 | GC48 | Fecal fat (g/24h) |
Total fecal BA (µM) |
|---|---|---|---|---|---|---|
| Stool number | XXX | XXX | XXX | XXX | XXX | XXX |
| Stool form | 0.57** | XXX | XXX | XXX | XXX | XXX |
| GC24 | 0.29** | 0.28** | XXX | XXX | XXX | XXX |
| GC48 | 0.54** | 0.53** | 0.75** | XXX | XXX | XXX |
| Fecal fat (g/24h) | 0.35** | 0.24* | 0.23* | 0.36** | XXX | XXX |
| Total fecal UBA (µM) | 0.25* | 0.25* | 0.16 | 0.41** | 0.47** | XXX |
| % CDCA | 0.49** | 0.49** | 0.22 | 0.48** | 0.30** | 0.41** |
| % LCA | −0.34** | −0.48** | −0.22 | −0.48** | −0.26* | −0.58** |
| % CA | 0.33** | 0.46** | 0.02 | 0.14 | 0.03 | 0.02 |
| % DCA | 0.14 | 0.28* | 0.14 | 0.32** | 0.17 | 0.43** |
| Anxiety | 0.15 | 0.11 | −0.04 | 0.04 | −0.04 | −0.09 |
| Depression | 0.19 | 0.10 | 0.08 | 0.15 | 0.05 | −0.05 |
p<0.05;
p<0.01
UBA = unconjugated bile acids; GC = geometric center; CDCA = chenodeoxycholic acid; LCA = lithocholic acid; CA = cholic acid; DCA = deoxycholic acid
Three-way Associations between Bowel Functions, Fecal UBAs and Colon Transit
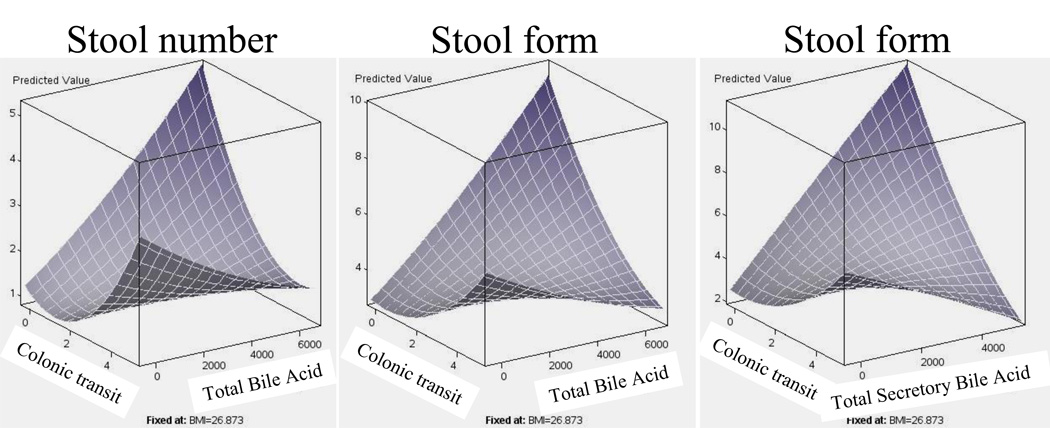
These were explored using multiple variable response surface regression analysis and results are shown in surface plots. The response surface model for stool number (Figure 2, left panel) with total fecal UBAs and CT (GC24) explained 29% of the variation (r=0.54, p<0.001); similarly, the model for stool form (Figure 2, middle panel) with fecal UBAs and CT (GC24) explained 20% of the variation (r=0.45, p=0.021). Using only total (expressed in µM) secretory UBAs (CA+DCA+CDCA), the response surface model for stool form (Figure 2, right panel) explained 23% of the variation (r=0.49, p=0.006).
Figure 2.
Surface plots of relationship of stool number or form with total and secretory fecal unconjugated bile acids (BA) and colonic transit (CT) at 24 hours (GC24). Note that stool number and form increase with increased CT measurement (x axis) and with increased fecal unconjugated total or secretory BA (z axis) at low CT measurements. However, the surface plot shows that both stool number and form were lower when the CT was fastest and fecal BA highest.
In these models, the relative contribution of CT was consistently greater than that for fecal UBAs, with the coefficients corresponding to CT being statistically significant (p<0.05) in all models, while those for UBAs were borderline (p=0.073 for the coefficient for secretory UBAs and stool form and p=0.227 for stool number; p=ns for both coefficients for total UBAs and stool form and stool number). On the other hand, the surface plots also show that for patients with lower CT measurements, increased levels of fecal UBAs (total and secretory) are associated with increased stool number and looser consistency.
Discussion
We performed a detailed quantitative assessment of total fecal UBA and main individual UBA excretion profiles in patients with IBS and HV. Our study shows that the percentages of primary UBAs are significantly increased in IBS-D in comparison to HV, while the percentages of the two most potent secretory UBAs (CDCA and DCA) were significantly decreased in IBS-C. In the latter group, the predominant fecal UBA is the non-secretory LCA. Fecal BA profiles are influenced by the bacterial dehydroxylation of the BAs. One of the factors that could impact the percentage of BAs appearing in stool is the duration of colonic transit; a second factor is the possible effects of the colonic microbial flora. Recently, Duboc et al.12 reported fecal BAs and microbial flora in 14 IBS-D and 18 healthy controls. Interestingly, fecal counts of all bacteria, lactobacillus, coccoides, leptum and Faecalibacterium prausnitzii, were similar; whereas, significant increase of Escherichia coli and significant decrease of leptum and bifidobacterium were observed in IBS-D patients.12
For the overall IBS group, we observed the highest values of total fecal fat in IBS-D (only modestly elevated over normal values) and total fecal UBAs (markedly but not significantly increased over normal healthy volunteers concurrently studied, consistent with prior studies showing UBA excess in ~25% of the entire IBS-D group). The lowest fecal UBA values were in IBS-C (Figure 1), as in our prior study.11 There was also a significant correlation found between fecal fat and total fecal UBAs, which may be due to other factors not investigated in our study. One hypothesis is that chronic increase in fecal UBA may eventually result in depletion of the BA pool and subsequent steatorrhea. These findings support prior observations of a significant response to BA sequestrants in patients without overt BAM4 and the paradoxically high levels of serum C4 (a surrogate of hepatic BA synthesis) in patients with slow-transit constipation.5 Given the observation that total fecal UBAs in IBS-D were not significantly greater than in HV, it appears that measurement in stool of individual primary and secondary UBAs, rather than total fecal UBAs, is necessary to identify changes in UBA in patients with bowel dysfunction in IBS. Goy et al. first described fecal BA profiles in healthy controls and patients with IBS-D and IBS-C (previously referred to as “spastic colon”), noting the presence of primary fecal BAs in the IBS-D group. Although no significant differences were noted between groups in total fecal BA output, the results appear to have been influenced by the presence of several outliers in the control and IBS-C groups, potentially obscuring the significance of their findings which showed skewed distribution.18 Duboc et al. recently showed a significantly increased proportion of primary BAs in patients with IBS-D compared to healthy subjects.12 Increased primary BAs and decreased secondary BAs in feces have also been described in patients with Crohn’s disease and/or ileal-resection,19 conditions that would be expected to have type I BAM [accelerated BA turnover rate with <10% retention of 75SeHCAT20]. Conversely, retardation of CT is associated with increased serum levels of the secondary BA, DCA, in acromegalic patients treated with octreotide secondary to prolongation of CT and greater 7 α-dehydroxylation of CA with a subsequent increase in colonic delivery and passive absorption of DCA21 into the systemic circulation.
To further investigate the relationship between bowel symptoms, fecal UBA excretion and CT, we used scintigraphy over 48h and recorded stool characteristics by 14-day bowel diary. We demonstrated significant associations between total UBA excretion, individual UBA profile, CT, and stool characteristics. There were significant positive correlations for stool number and form with CT at 24 and 48h, total fecal UBAs, % primary fecal UBAs and a significant negative correlation with % fecal LCA. Positive correlations were also observed between CT at 24 and 48h and total fecal UBAs and % fecal CDCA while a negative correlation was observed between CT at 48h and % fecal LCA. These findings are consistent with the known secretory properties of individual BAs22,23 and the effect of CDCA on accelerating CT in IBS-C.7
Multiple linear regression analyses revealed that, relative to total UBAs or total secretory UBA content in the stool, CT is the greater determinant of stool number and stool form. Increased total and secretory fecal UBAs (primary BAs and DCA) enhance the effects of CT on stool characteristics. The surface plots show there is an effect of fecal BAs on stool number and form that is modified by, but largely independent of, transit as increased fecal BAs is associated with increased stool number and looser consistency at lower CT measurements. Our hypothesis is that, in IBS-D or functional diarrhea, increased fecal BAs may result from either the rapid transit, or the reduced absorption (in the presence of rare mutation in the ASBT), or increased synthesis of BAs. The latter may be multifactorial, e.g. deficiency of FGF-19,24 genetic variation in KLB or FGFR425 (or both), or upregulation of CYP7A1. Conversely, the data available suggest that reduced fecal BAs in constipation may reflect mostly delayed CT or, rarely, metabolic changes in the BAs such as sulfation.
The relationship between fecal UBA excretion, fecal UBA profile, and CT may involve a complex interaction between factors in which rapid transit may be associated with decreased intestinal BA absorption and decreased biotransformation to secondary BAs through dehydroxylation; on the other hand, slow transit may be associated with increased intestinal BA absorption and biotransformation. Meanwhile, the changes in fecal BA excretion and composition may also modify effects of CT (e.g. CDCA causes high amplitude propagated contractions26), contributing to the clinical presentation of IBS.
Our findings provide evidence in support of the relationship between the individual fecal UBA profile and quantitative traits relevant to the pathophysiology of IBS. These data, and the previous studies showing normalization of bowel function with BA supplementation or sequestration in IBS-C or IBS-D respectively, confirm that characterization of fecal BA composition may be necessary in order to clarify the pathogenic role of BAs and optimize treatment in patients with FGID. When alternative measurements of BA retention (such as 75SeHCAT test) are not available, we recommend fecal total, primary and secondary UBAs are indicated in lower FGID patients and can be conveniently measured in the samples collected for 48h stool fat measurement.
No significant associations were observed between study endpoints and scores for anxiety and depression, suggesting that, although anxiety and depression are associated with IBS phenotype,27 they do not have a significant impact on quantitative traits and symptoms.
In summary, our data show that fecal fat elevation is unusual and relatively minor in patients with IBS-D, whereas fecal measurements of total and secretory UBAs are significantly different in the entire group, and the percentages of individual BAs can identify a subgroup with BA diarrhea, demonstrating the utility of the measurement of total and primary and secondary UBAs in a 48h fecal collection in patients with unexplained functional diarrhea. In addition, our results support the role of low fecal secretory UBAs in the pathogenesis of IBS-C, emphasizing the role of measurement of total fecal UBAs and the percentages of individual primary and secondary UBAs in both IBS-D and IBS-C.
Acknowledgment
We thank Mrs. Cindy Stanislav for secretarial support.
Funding: This study is supported by DK92179 (Drs. MC, ARZ) and Mayo CTSA grant UL1-TR000135
Abbreviations
- IBS
irritable bowel syndrome
- D
diarrhea
- C
constipation
- BA
bile acid(s)
- CDCA
chenodeoxycholic acid
- CA
cholic acid
- LCA
lithocholic acid
- DCA
deoxycholic acid
- UBA
unconjugated bile acids
- UDCA
ursodeoxycholic acid
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Disclosure: Mayo Clinic, Dr. M. Camilleri and Mr. D. Burton have filed a provisional patent on treatment of constipation with delayed release preparation of bile acid. The other authors have no conflicts of interest.
Authors’ contributions: Dr. M. Camilleri: principal investigator, concept development, study design, senior author; Dr. A. Shin: fellow co-investigator, co-author; P. Vijayvargiya: medical student investigator, co-author; I. Busciglio: patient recruitment, study coordinator; D Burton: transit data analysis; M. Ryks and D. Rhoten: technologists; Drs. A. Lueke, A. Saenger and A Girtman: analysis of total, primary and secondary bile acids in stool and co-authors; and Dr. AR Zinsmeister: study design, biostatistician, co-author
References
- 1.Camilleri M. Peripheral Mechanisms in Irritable Bowel Syndrome. N Engl J Med. 2012;367:1626–1635. doi: 10.1056/NEJMra1207068. [DOI] [PubMed] [Google Scholar]
- 2.Camilleri M, McKinzie S, Busciglio I, et al. Prospective study of motor, sensory, psychologic, and autonomic functions in patients with irritable bowel syndrome. Clin Gastroenterol Hepatol. 2008;6:772–781. doi: 10.1016/j.cgh.2008.02.060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Wedlake L, A'Hern R, Russell D, et al. Systematic review: the prevalence of idiopathic bile acid malabsorption as diagnosed by SeHCAT scanning in patients with diarrhoea-predominant irritable bowel syndrome. Aliment Pharmacol Ther. 2009;30:707–717. doi: 10.1111/j.1365-2036.2009.04081.x. [DOI] [PubMed] [Google Scholar]
- 4.Odunsi-Shiyanbade ST, Camilleri M, McKinzie S, et al. Effects of chenodeoxycholate and a bile acid sequestrant, colesevelam, on intestinal transit and bowel function. Clin Gastroenterol Hepatol. 2010;8:159–165. doi: 10.1016/j.cgh.2009.10.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Abrahamsson H, Ostlund-Lindqvist AM, Nilsson R, et al. Altered bile acid metabolism in patients with constipation-predominant irritable bowel syndrome and functional constipation. Scand J Gastroenterol. 2008;43:1483–1488. doi: 10.1080/00365520802321212. [DOI] [PubMed] [Google Scholar]
- 6.Hofmann AF, Loening-Baucke V, Lavine JE, et al. Altered bile acid metabolism in childhood functional constipation: inactivation of secretory bile acids by sulfation in a subset of patients. J Pediatr Gastroenterol Nutr. 2008;47:598–606. doi: 10.1097/MPG.0b013e31816920a6. [DOI] [PubMed] [Google Scholar]
- 7.Rao AS, Wong BS, Camilleri M, et al. Chenodeoxycholate in females with irritable bowel syndrome-constipation: a pharmacodynamic and pharmacogenetic analysis. Gastroenterology. 2010;139:1549–1558. doi: 10.1053/j.gastro.2010.07.052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Wong BS, Camilleri M, McKinzie S, et al. Effects of A3309, an ileal bile acid transporter inhibitor, on CT and symptoms in females with functional constipation. Am J Gastroenterol. 2011;106:2154–2164. doi: 10.1038/ajg.2011.285. [DOI] [PubMed] [Google Scholar]
- 9.Bampton PA, Dinning PG, Kennedy ML, et al. The proximal colonic motor response to rectal mechanical and chemical stimulation. Am J Physiol. 2002;282:G443–G449. doi: 10.1152/ajpgi.00194.2001. [DOI] [PubMed] [Google Scholar]
- 10.Ridlon JM, Kang DJ, Hylemon PB. Bile salt biotransformations by human intestinal bacteria. J Lipid Res. 2006;47:241–259. doi: 10.1194/jlr.R500013-JLR200. [DOI] [PubMed] [Google Scholar]
- 11.Wong BS, Camilleri M, Carlson P, et al. Increased bile acid biosynthesis is associated with irritable bowel syndrome with diarrhea. Clin Gastroenterol Hepatol. 2012;10:1009–1015. doi: 10.1016/j.cgh.2012.05.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Duboc H, Rainteau D, Rajca S, et al. Increase in fecal primary bile acids and dysbiosis in patients with diarrhea-predominant irritable bowel syndrome. Neurogastroenterol Motil. 2012;24:513–520. doi: 10.1111/j.1365-2982.2012.01893.x. [DOI] [PubMed] [Google Scholar]
- 13.Talley NJ, Phillips SF, Wiltgen CM, et al. Assessment of functional gastrointestinal disease: the bowel disease questionnaire. Mayo Clin Proc. 1990;65:1456–1479. doi: 10.1016/s0025-6196(12)62169-7. [DOI] [PubMed] [Google Scholar]
- 14.Zigmond AS, Snaith RP. The hospital anxiety and depression scale. Acta Psychiatr Scand. 1983;67:361–370. doi: 10.1111/j.1600-0447.1983.tb09716.x. [DOI] [PubMed] [Google Scholar]
- 15.Deiteren A, Camilleri M, Bharucha AE, et al. Performance characteristics of scintigraphic colon transit measurement in health and irritable bowel syndrome and relationship to bowel functions. Neurogastroenterol Motil. 2010;22:415–423. doi: 10.1111/j.1365-2982.2009.01441.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lewis SJ, Heaton KW. Stool form scale as a useful guide to intestinal transit time. Scand J Gastroenterol. 1997;32:920–924. doi: 10.3109/00365529709011203. [DOI] [PubMed] [Google Scholar]
- 17.Coulie B, Szarka LA, Camilleri M, et al. Recombinant human neurotrophic factors accelerate colonic transit and relieve constipation in humans. Gastroenterology. 2000;119:41–50. doi: 10.1053/gast.2000.8553. [DOI] [PubMed] [Google Scholar]
- 18.Goy JA, Eastwood MA, Mitchell WD, et al. Fecal characteristics contrasted in the irritable bowel syndrome and diverticular disease. Am J Clin Nutr. 1976;29:1480–1484. doi: 10.1093/ajcn/29.12.1480. [DOI] [PubMed] [Google Scholar]
- 19.Lapidus A, Akerlund JE, Einarsson C. Gallbladder bile composition in patients with Crohn 's disease. World J Gastroenterol. 2006;12:70–74. doi: 10.3748/wjg.v12.i1.70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Nyhlin H, Merrick MV, Eastwood MA. Bile acid malabsorption in Crohn's disease and indications for its assessment using SeHCAT. Gut. 1994;35:90–93. doi: 10.1136/gut.35.1.90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Thomas LA, Veysey MJ, Murphy GM, et al. Octreotide induced prolongation of CT increases faecal anaerobic bacteria, bile acid metabolising enzymes, and serum deoxycholic acid in patients with acromegaly. Gut. 2005;54:630–635. doi: 10.1136/gut.2003.028431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Keely SJ, Scharl MM, Bertelsen LS, et al. Bile acid-induced secretion in polarized monolayers of T84 colonic epithelial cells: Structure-activity relationships. Am J Physiol. 2007;292:G290–G297. doi: 10.1152/ajpgi.00076.2006. [DOI] [PubMed] [Google Scholar]
- 23.Chadwick VS, Gaginella TS, Carlson GL, et al. Effect of molecular structure on bile acidinduced alterations in absorptive function, permeability, and morphology in the perfused rabbit colon. J Lab Clin Med. 1979;94:661–674. [PubMed] [Google Scholar]
- 24.Walters JR, Tasleem AM, Omer OS, et al. A new mechanism for bile acid diarrhea: defective feedback inhibition of bile acid biosynthesis. Clin Gastroenterol Hepatol. 2009;7:1189–1194. doi: 10.1016/j.cgh.2009.04.024. [DOI] [PubMed] [Google Scholar]
- 25.Wong BS, Camilleri M, Carlson PJ, et al. A Klothoβ variant mediates protein stability and associates with colon transit in irritable bowel syndrome with diarrhea. Gastroenterology. 2011;140:1934–1942. doi: 10.1053/j.gastro.2011.02.063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Mekhjian HS, Phillips SF. Perfusion of the canine colon with unconjugated bile acids. Effect on water and electrolyte transport, morphology, and bile acid absorption. Gastroenterology. 1970;59:120–129. [PubMed] [Google Scholar]
- 27.Lydiard RB, Fossey MD, Marsh W, et al. Prevalence of psychiatric disorders in patients with irritable bowel syndrome. Psychosomatics. 1993;34:229–234. doi: 10.1016/S0033-3182(93)71884-8. [DOI] [PubMed] [Google Scholar]