Abstract
The presence of infectious microorganisms in urinary stones is commonly inferred from stone composition, especially by the presence of struvite in a stone. The presence of highly carbonated apatite has also been proposed as a marker of the presence of bacteria within a stone. We retrospectively studied 368 patients who had undergone percutaneous nephrolithotomy, and who also had culture results for both stone and urine. Urine culture showed no association with stone mineral content, but stone culture was more often positive in struvite containing stones (73% positive) and majority apatite stones (65%) than in other stone types (54%, lower than the others, P<0.02). In 51 patients in which the carbonate content of apatite could be measured, carbonate in the apatite was weakly predictive of positive stone culture with an optimal cutoff value of 13.5% carbonate (sensitivity 0.61, specificity 0.80). In positive cultures of stones (all mineral types combined), organisms that characteristically produce urease were present in 71% of the cases, with no difference in this proportion among different types of stone. In summary, the type of mineral in the stone was predictive of positive stone culture, but this correlation is imperfect, as over half of non-struvite, non-apatite stones were found to harbor culturable organisms. We conclude that mineral type is an inadequate predictor of whether a stone contains infectious organisms, and that stone culture is more likely to provide information useful to the management of patients undergoing percutaneous nephrolithotomy.
Keywords: nephrolithiasis, infection
Introduction
Historically, renal calculi associated with urinary tract infection have been identified by the presence of struvite (magnesium ammonium phosphate) in the mineral composition of the stone [1,2, 3]. This identification reflects the generally accepted relationship between the formation process of struvite stones and urea-splitting bacteria. The activity of the bacteria-produced enzyme urease results in an alkaline environment high in ammonia, and thus highly favorable for the precipitation of struvite crystals [4, 5].
Treatment of renal calculi associated with infection most often consists of surgical removal and appropriate administration of antimicrobial agents [6]. Percutaneous nephrolithotomy (PCNL) has become the treatment of choice for large, complex, and multiple renal calculi, [7] and infection stones often fall into this group. Although reported rates of sepsis with PCNL vary widely, systemic infection is certainly one of the most serious clinical problems that can follow PCNL treatment [8, 9]. Greatly complicating these cases is the fact that fever and bacteremia can present despite antibiotic treatment and negative preoperative urine culture [10]. Thus, simply equating post-procedure infection risk with infected bladder urine is not adequate for predicting that risk.
In addition, some studies show that post-operative development of these symptoms does not correlate with stone composition [10, 11]. That is, in these studies the occurrence of sepsis related symptoms was not significantly different in patients presenting with struvite or non-struvite renal calculi. One explanation of this observation is that stones may harbor non-urease-producing organisms (and thus do not provide the milieu necessary for struvite formation [12]) that can lead to sepsis related symptoms after surgery. The other aspect of the disconnect between the presence of struvite in the stone and infection risk may be that some struvite stones formed during infection no longer harbor culturable bacteria [13, 14]. Thus, it seems that urease-producing organisms must be present for the formation of struvite, but that a struvite stone can later be rendered sterile, so that it is not a source of infectious organisms for post-surgical complications.
However, many urinary stones—including struvite stones—also contain calcium phosphate in the mineral form of apatite, and this mineral retains imprints of bacteria [15], suggesting that it would be a likely source for retention of infectious agents that could be released during surgery. Moreover, the number of bacterial imprints in apatite has been shown to correlate with the carbonate content of the mineral [15], and high carbonate content in apatite is consistent with the mineral being formed in the presence of infection [12].
The purpose of the present study was to see if the carbonation level of apatite in stones might be an indicator of the presence of infectious organisms in the stone—as measured by stone culture—so that the apatite carbonation level could be used as an indicator for risk of infection in patients undergoing percutaneous removal of their stones.
Methods
We performed a retrospective study of percutaneous nephrolithotomy patients treated for renal calculi at the treatment site of the Methodist Hospital Institute for Kidney Stone Disease, in Indianapolis, Indiana. All consenting patients treated at this site between 1999 and 2009 formed the Institutional Review Board-approved database from which patients were selected for this study. The database contained all relevant clinical data including bacteriological findings from renal stone and urine cultures (all cultures done using both CNA and MacConkey agar methods) and results from the infrared spectroscopic stone analysis provided by the commercial stone laboratory (Beck Analytical Laboratories, Inc., Indianapolis, IN, USA). In all there were 368 patients for which there were stone analysis results along with data for stone and urine cultures.
Patients were then divided into three groups based on the analysis of stone mineral content: struvite patients, apatite patients, and non-struvite non-apatite patients. Struvite patients were defined as those patients with any amount of struvite reported in their stone analysis. The apatite group contained patients having renal stones with apatite content >50% in the absence of struvite. Lastly, the non-struvite, non-apatite group contained patients having renal stones with apatite content 50% in the absence of struvite.
Patients presenting with a positive culture associated with either the urine or stone, or both, were further classified as having a urease or non-urease producing bacterial infection. The presence or absence of urease was inferred by cross-referencing the identified bacteria with a reference table of organisms known to produce urease [16].
Patients within the apatite group for whom additional fragments of stone were available from the analytical company were further classified by measurement of carbonation level as previously described [17, 18]. These additional fragments were scanned by micro CT, using a SkyScan 1172 system with voxel sizes ranging from 14-18 μm and subsequently dissected, using the micro CT scans to find regions high in apatite mineral [19]. These dissected regions were analyzed by Fourier transform infrared spectroscopy (FT-IR) using the KBr pellet method and a Bruker Alpha-T Spectrometer [20]. Only those samples showing nearly pure apatite were used for measuring carbonate level (specifically, small quantities of proteins or calcium oxalate evident in the spectrum were deemed not to interfere with the measurement of carbonate content). Additionally, great care was taken to be sure that no struvite was present in those samples used for measuring carbonate levels, looking especially for evidence of the 1435 cm−1 peak within the carbonate region of apatite, and for a shift of the 1037 cm−1 phosphate peak toward lower values, as occurs when struvite is present in small amounts [21].
Statistical analysis was carried out using JMP software (SAS, Inc., Cary, NC.). For categorical data, differences were tested by the Chi-square method (two groups) or by analysis of means for proportions (for more than two groups). For numerical data, groups were compared by t-test (assuming unequal variances) or with analysis of variance and the Tukey-Kramer HSD test.
Results
Overall, of 368 patients meeting the study criteria, 304 (83%) presented with a positive culture associated with either the urine or stone, or both. Urine culture showed no association with stone mineral content (Table 1), but stone culture showed a higher proportion of positive results in struvite-containing and majority apatite stones (73% and 65% positive, respectively) than in other types (54% positive; analysis of means for proportions test, P<0.05). Lumping majority apatite and struvite-containing stones together averaged 68% positive stone cultures, significantly greater than the 54% positive rate with other stone types (chi-square, P=0.005), but no difference with urine culture results (P=0.06).
Table 1.
Culture results of PCNL patients grouped by stone mineral content.
| Stone Mineral Content | Both Negative | Positive Urine Culture | Positive Stone Culture | Both Urine and Stone Culture Positive |
|---|---|---|---|---|
| Struvite | 6 (9.4) | 11 (17.2) | 7 (10.9) | 40 (62.5) |
| Apatite | 21 (16.9) | 22 (17.7) | 19 (15.3) | 62 (50) |
| Other | 37 (20.6) | 46 (25.6) | 30 (16.7)* | 67 (37.2)* |
| Total | 64 (17.4) | 79 (24.5) | 56 (15.2) | 169 (45.9) |
PCNL: percutaneous nephrolithotomy. Struvite includes any patient with any struvite reported in the stone analysis, regardless of the percentage. Apatite includes patients with no struvite and >50% apatite (either reported as hydroxyapatite or carbonate apatite). Other is all non-struvite, non-apatite patients.
Fraction of stone cultures that were positive (summing all positive stone culture results, ignoring urine culture results) was significantly less than for Struvite and Apatite groups, p<0.05.
So, majority apatite content or struvite presence in a stone correlated with a positive stone culture result, but note that 27% of struvite patients and 35% of apatite stone patients showed no culturable organism in their stones, so stone composition alone was not a perfect indicator of culturable organisms in the stones of PCNL patients.
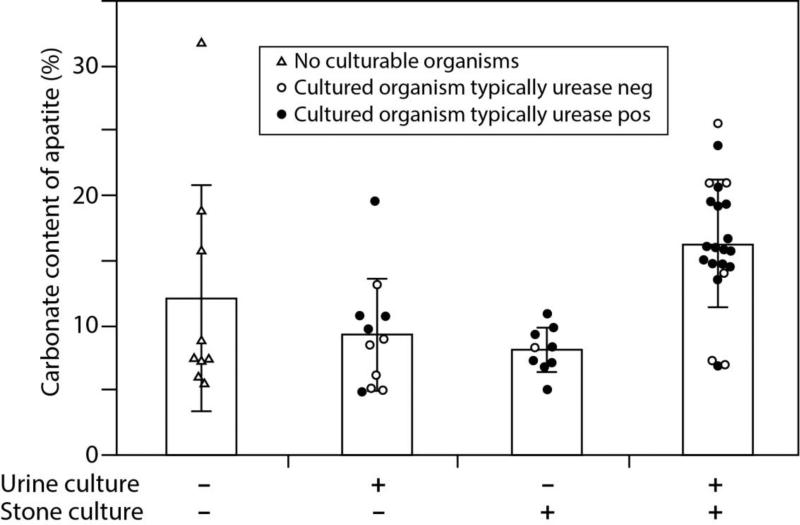
Looking specifically at the patients with majority apatite stones, sufficient additional material was available to measure the carbonate content of the apatite in the stone samples of 51 of the patients. Each of these specimens was imaged by micro CT for guiding the stone dissection, and carbonate content was calculated only for those specimens in which an infrared spectrum could be obtained showing nearly pure apatite. The results of these measurements are shown in Figure 1, with the data distributed by the results of urine and stone cultures for each patient.
Figure 1.
Carbonation rate of apatite in apatite stones grouped according to level of infection. Minus sign indicates a negative culture, and plus sign indicates a positive culture. Dark circles indicate urease positive infection, and open circles indicate urease negative infection. Bars show standard deviation. Analysis of variance suggests that the groups are not the same (P<0.002); Tukey-Kramer HSD test shows +/+ group to be greater than +/− or −/+ (P<0.05), but none of these is different from the no infection (−/−) group.
Overall, the average values for carbonate content of the apatite stones did not differ between those that were negative (10.5±6.6% carbonate, n=20) or positive for stone culture (13.8±5.7% carbonate, n=31; P=0.07 by t-test). However, as shown in Figure 1, when patients were grouped by both urine and stone cultures, those with positive culture results in both had a significantly higher rate of apatite carbonation (16.2±4.9% carbonate, n=22) than did those patients with only a positive urine culture (9.2±4.4%, n=11) or only a positive stone culture (8.0%±1.8%, n=9). Note, though, that none of these groups differed from the apatite carbonate content of patients with no positive culture results (12.0±8.7%, n=9); this lack of difference is likely because the patient with the highest measured value of apatite carbonate (32%) showed both sterile urine and sterile stone, so that the standard deviation in this group was quite high.
This high value of carbonate content in the specimen from a patient with no positive culture was apparently real, as two samples of apatite were able to be measured on this patient's stone sample, with carbonate measurements of 28.4% and 35.2%, yielding a mean carbonate value for this specimen of 31.8±4.8%. Overall the measures of carbonate content of apatite were rather consistent; carbonate was determined in 2 or more samples in 30 of the apatite specimens, with an average standard deviation of carbonate content of only 1.8±1.7% (median of 1.3%, range 0.1-7.1).
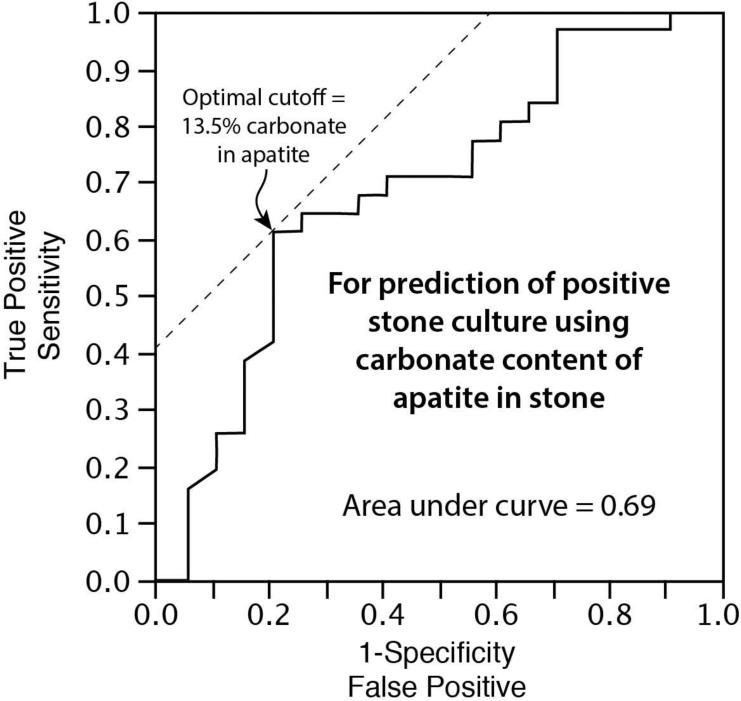
So, carbonate content in the apatite stones did not correlate perfectly with culturable organisms in the stones or urines of the patients. Using the value of mean carbonate content as a predictor of stone culture results yielded a receiver operating characteristic curve with an area under the curve of only 0.69 (Figure 2). Using 13.5% mean carbonate content as the diagnostic cutoff value, high carbonate content of apatite was weakly predictive of positive stone culture (sensitivity of 0.61, specificity of 0.80).
Figure 2.
Receiver operating characteristic curve showing prediction of positive stone culture on the basis of the carbonate content in apatite stones. Optimal cutoff of 13.5% carbonate indicates the value with the best combination of sensitivity and specificity.
In the 51 patients with apatite stones and carbonate measures, the values of carbonation corresponded reasonably well with the apatite designations from the clinical stone analysis, as either hydroxyapatite or carbonate apatite. The additional fragments of those stone specimens designated as hydroxyapatite (n=44) had an average carbonate content of 11.8±6.3%. Those specimens designated as carbonate apatite (n=7) had on average a greater carbonate content (16.9±2.9%, P<0.004 by t-test). All 7 of the patients designated as having carbonate apatite stones also had a positive stone culture, compared with only 24 of the 44 designated as hydroxyapatite (P<0.05, Fisher's exact test).
Regarding the organisms cultured in these PCNL patients, the percentages of positive cultures with organisms typically found to be urease-positive showed little variation among the different stone formers: Lumping urine or stone cultures together, struvite stone formers with positive cultures had urease-producing genera or species in 76% of the cases, apatite stone formers 70%, and non-struvite, nonapatite stone formers, 69%. Similarly, there were no significant differences in the presence of urease producing organisms among patients with a positive stone culture when compared by stone type, regardless of the urine culture results.
Of patients presenting with both a positive urine and stone culture (n=169), 35% were found to have different culturable organisms between the urine and the stone cultures. There was no significant difference in the rate of organism discordance between urine and stone culture when patients were compared by stone type.
Discussion
The common use of the term ‘infection stone’ indicates a stone that is formed in the presence of infection, and such stones are commonly composed of struvite and/or apatite, as a urinary tract infection typically creates a physicochemical environment that favors the formation of these two minerals [12]. In thinking about the causes of post-operative complications following percutaneous nephrolithotomy, the concern is not so much that a stone initially formed in the presence of infection, but rather whether pathogenic organisms are present in the stone at the time of the procedure, when they could become a source of systemic infection [8].
Some studies have argued that stone cultures are the best indicator of whether a patient may be at risk for complications of infection after percutaneous nephrolithotomy, and that the results of stone culture should be used for directing antibiotic therapy following surgery [8, 22, 23]. However, not all surgeons regularly collect stone cultures, and so other indicators of the likelihood of post-operative infection are desirable [11, 24].
The results of the present study suggest that mineral type is associated with culturable organisms in the stone, as 68% of the patients with struvite containing or majority apatite stones also had a positive stone culture, as compared with only 54% positive cultures in patients with other stone mineral types. This result is consistent with results that show struvite as an indicator of post-operative fever symptoms [22]. However these numbers also show that mineral type is an imperfect predictor of culturable organisms in the stone, as a third of patients with struvite or apatite stones yielded a negative stone culture, and over half of the patients with non-infection type stones showed a positive stone culture. This kind of result is thus consistent with another study in which post-operative fever symptoms did not correlate with struvite in the stone [11].
One study has shown that the number of bacterial imprints found in apatite stones correlated positively with the carbonate content of the apatite [15]. All apatite in stones contains at least some carbonate [25], probably because the apatite crystal lattice is open to the inclusion of impurities [26], and carbonate/bicarbonate ions are always present in physiological fluids. Carpentier et al. reasoned that their results with bacterial imprints and the carbonate content of apatite related to the environment created by bacterial growth, so that a greater number of bacteria in the urine would yield a higher carbonate content in the apatite mineral formed there [15].
Our results on correlating the carbonate content of apatite with culture results support this overall concept, as patients with a positive stone culture had higher levels of carbonate in apatite stones. However, the overall predictive value for culture-positive stones of carbonate level in the apatite was not great (sensitivity of 0.61 and specificity of 0.80), suggesting that it is not a simple relationship between the presence of highly carbonated apatite in stones and the presence of culturable bacteria remaining in the kidney stone.
Highly carbonated apatite—which likely formed with infection—is not a perfect indicator of present infection in the stone. Similarly, the presence of struvite, which is thought to form only in the presence of infection [5], is an imperfect indicator of present infection, as in the present study where 27% of patients with struvite stones showed no culturable organism in their kidney stone. A possible explanation of these results is that stones that form in the presence of infection can later be rendered sterile by antibiotic treatment. This suggestion goes against long-held ideas that infective organisms in stones are encased in the mineral and thus highly resistant to chemical treatment [27]. However, it may be that in many cases the infecting organism can be eliminated from the stone, so that the stone no longer harbors the organism, and thus the stone pieces are not a source for systemic infection during surgery.
The present study has implicitly used a positive stone culture as the standard for indicating the danger of infection following percutaneous nephrolithotomy. This idea makes sense, and fits with clinical results [8]. However, it should be noted that the rate of post-operative complications was quite low in the present series. In 931 total patients consented for data collection with percutaneous nephrolithotomy, only 36 (3.9%) had post-operative fever and only 7 (0.75%) had sepsis. In contrast, another recent study reported 22% of their patients as having sepsis related symptoms [8]. The very low rate in the present series is likely due to the experience level of the surgeons and the regular use of prophylactic antibiotics before and after the surgery. It is also our practice to change the post-operative antibiotic if a positive stone culture indicates an organism that is insensitive to the antibiotic in use. Thus, in practice we use stone culture as the practical indicator of potential infective complications after surgery, and the results of the present study show that stone mineral type is probably not a good surrogate for stone culture.
It should be noted that the quantitation of carbonate level in stones can at present be done only with infrared spectroscopy [15]. The poorly crystalline nature of apatite makes the identification of carbonate content difficult with x-ray diffraction [28]. Although we have previously identified different morphologies of kidney stone apatite using micro CT [17], we did not in the present study see any correlation between apatite morphology and the measured level of carbonate. As to chemical analysis of stones—which can give a value for carbonate ion—such methods are notoriously inaccurate [29] and cannot indicate mineral content; that is, chemical analysis kits for kidney stones cannot indicate the presence of apatite at all.
In conclusion, the present study supports the concept that the mineral type of kidney stone is an indicator of culturable organisms in stones removed by percutaneous nephrolithotomy, but this indicator—even with the presence of struvite in a stone—is imperfect. Adding measurement of carbonate content to the analysis of apatite stones adds some ability to predict infected stones, but this measure is also imperfect. It seems probable that actual stone culture is the best practical measure in assessing infection risk for patients following percutaneous nephrolithotomy, but further direct study of this will be required.
Acknowledgements
We thank Molly Jackson for excellent technical work on this project, and Leslie Pillow for help in studying the apatite morphologies. This work was funded by NIH R01 DK059933.
Footnotes
Conflict of interest statement
The authors declare that they have no conflict of interest.
References
- 1.Miano R, Germani S, Vespasiani G. Stones and urinary tract infections. Urologia Internationalis. 2007;79:32–36. doi: 10.1159/000104439. [DOI] [PubMed] [Google Scholar]
- 2.Rieu P. Lithiases d'infection. Ann Urol (Paris) 2005;39:16–29. doi: 10.1016/j.anuro.2005.01.001. [DOI] [PubMed] [Google Scholar]
- 3.Abrahams HM, Stoller ML. Infection and urinary stones. Current Opinion in Urology. 2003;13:63–67. doi: 10.1097/00042307-200301000-00011. [DOI] [PubMed] [Google Scholar]
- 4.Griffith DP, Osborne CA. Infection (urease) stones. Miner Electrolyte Metab. 1987;13:278–285. [PubMed] [Google Scholar]
- 5.Griffith DP. Struvite stones. Kidney Int. 1978;13:372–382. doi: 10.1038/ki.1978.55. [DOI] [PubMed] [Google Scholar]
- 6.Thomas B, Tolley D. Concurrent urinary tract infection and stone disease: Pathogenesis, diagnosis and management. Nat Clin Pract Urol. 2008;5:668–675. doi: 10.1038/ncpuro1254. [DOI] [PubMed] [Google Scholar]
- 7.Miller NL, Lingeman JE. Management of kidney stones. BMJ. 2007;334:468–472. doi: 10.1136/bmj.39113.480185.80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Margel D, Ehrlich Y, Brown N, Lask D, Livne PM, Lifshitz DA. Clinical implication of routine stone culture in percutaneous nephrolithotomy--a prospective study. Urology. 2006;67:26–29. doi: 10.1016/j.urology.2005.08.008. [DOI] [PubMed] [Google Scholar]
- 9.Preminger GM, Assimos DG, Lingeman JE, Nakada SY, Pearle MS, Wolf JS. Chapter 1: Aua guideline on management of staghorn calculi: Diagnosis and treatment recommendations. J Urol. 2005;173:1991–2000. doi: 10.1097/01.ju.0000161171.67806.2a. [DOI] [PubMed] [Google Scholar]
- 10.Gonen M, Turan H, Ozturk B, Ozkardes H. Factors affecting fever following percutaneous nephrolithotomy: A prospective clinical study. J Endourol. 2008;22:2135–2138. doi: 10.1089/end.2008.0139. [DOI] [PubMed] [Google Scholar]
- 11.Cadeddu JA, Chen R, Bishoff J, Micali S, Kumar A, Moore RG, Kavoussi LR. Clinical significance of fever after percutaneous nephrolithotomy. Urology. 1998;52:48–50. doi: 10.1016/s0090-4295(98)00146-0. [DOI] [PubMed] [Google Scholar]
- 12.Griffith DP. Infection-induced renal calculi. Kidney Int. 1982;21:422–430. doi: 10.1038/ki.1982.40. [DOI] [PubMed] [Google Scholar]
- 13.Hugosson J, Grenabo L, Hedelin H, Pettersson S, Seeberg S. Bacteriology of upper urinary tract stones. J Urol. 1990;143:965–968. doi: 10.1016/s0022-5347(17)40152-2. [DOI] [PubMed] [Google Scholar]
- 14.Bazin D, André G, Weil R, Matzen G, Emmanuel V, Carpentier X, Daudon M. Absence of bacterial imprints on struvite-containing kidney stones: A structural investigation at the mesoscopic and atomic scale. Urology. 2012;79:786–790. doi: 10.1016/j.urology.2011.08.054. [DOI] [PubMed] [Google Scholar]
- 15.Carpentier X, Daudon M, Traxer O, Jungers P, Mazouyes A, Matzen G, Vèron E, Bazin D. Relationships between carbonation rate of carbapatite and morphologic characteristics of calcium phosphate stones and etiology. Urology. 2009;73:968–975. doi: 10.1016/j.urology.2008.12.049. [DOI] [PubMed] [Google Scholar]
- 16.Griffith DP, Klein AS. Infection-induced urinary stones. In: Roth RA, Finlayson B, editors. Stones: Clinical management of urolithiasis. Williams & Wilkins; Baltimore: 1983. [Google Scholar]
- 17.Pramanik R, Asplin JR, Jackson ME, Williams JC., Jr. Protein content of human apatite and brushite kidney stones: Significant correlation with morphologic measures. Urol Res. 2008;36:251–258. doi: 10.1007/s00240-008-0151-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Maurice-Estepa L, Levillain P, Lacour B, Daudon M. Crystalline phase differentiation in urinary calcium phosphate and magnesium phosphate calculi. Scandinavian J Urol and Nephrology. 1999;33:299–305. doi: 10.1080/003655999750017365. [DOI] [PubMed] [Google Scholar]
- 19.Williams JC, Jr., Mcateer JA, Evan AP, Lingeman JE. Micro-computed tomography for analysis of urinary calculi. Urol Res. 2010;38:477–484. doi: 10.1007/s00240-010-0326-x. [DOI] [PubMed] [Google Scholar]
- 20.Krambeck AE, Khan NF, Jackson ME, Lingeman JE, Mcateer JA, Williams JC., Jr. Inaccurate reporting of mineral composition by commercial stone analysis laboratories: Implications for infection and metabolic stones. J Urol. 2010;184:1543–1549. doi: 10.1016/j.juro.2010.05.089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hesse A, Sanders G. Georg Thieme Verlag; Stuttgart: 1988. Atlas of infrared spectra for the analysis of urinary concrements. [Google Scholar]
- 22.Korets R, Graversen JA, Kates M, Mues AC, Gupta M. Post-percutaneous nephrolithotomy systemic inflammatory response: A prospective analysis of preoperative urine, renal pelvic urine and stone cultures. J Urol. 2011;186:1899–1903. doi: 10.1016/j.juro.2011.06.064. [DOI] [PubMed] [Google Scholar]
- 23.Mariappan P, Smith G, Bariol SV, Moussa SA, Tolley DA. Stone and pelvic urine culture and sensitivity are better than bladder urine as predictors of urosepsis following percutaneous nephrolithotomy: A prospective clinical study. J Urol. 2005;173:1610–1614. doi: 10.1097/01.ju.0000154350.78826.96. [DOI] [PubMed] [Google Scholar]
- 24.Draga RO, Kok ET, Sorel MR, Bosch RJ, Lock TM. Percutaneous nephrolithotomy: Factors associated with fever after the first postoperative day and systemic inflammatory response syndrome. J Endourol. 2009;23:921–927. doi: 10.1089/end.2009.0041. [DOI] [PubMed] [Google Scholar]
- 25.Hesse A, Heimbach D. Causes of phosphate stone formation and the importance of metaphylaxis by urinary acidification: A review. World Journal of Urology. 1999;17:308–315. doi: 10.1007/s003450050152. [DOI] [PubMed] [Google Scholar]
- 26.Nancollas GH. In vitro studies of calcium phosphate crystallization. In: Mann S, Webb J, Williams RJP, editors. Biomineralization. Chemical and biochemical perspectives. VCH Verlagsgesellschaft; Weinheim: 1989. [Google Scholar]
- 27.Fowler JE., Jr. Bacteriology of branched renal calculi and accompanying urinary tract infection. J Urol. 1984;131:213–215. doi: 10.1016/s0022-5347(17)50311-0. [DOI] [PubMed] [Google Scholar]
- 28.Wandt M, Rodgers A. Quantitative x-ray diffraction analysis of urinary calculi by use of the internal-standard method and reference intensity ratios. Clin Chem. 1988;34:289–293. [PubMed] [Google Scholar]
- 29.Kasidas GP, Samuell CT, Weir TB. Renal stone analysis: Why and how? Ann Clin Biochem. 2004;41:91–97. doi: 10.1258/000456304322879962. [DOI] [PubMed] [Google Scholar]