Abstract
Purpose
The purpose of this study was to establish a relationship between the lengthening of the Achilles tendon post rupture and surgical repair to muscle activation patterns during walking in order to serve as a reference for post-surgical assessment.
Method
The Achilles tendon lengths were collected from 4 patients with an Achilles tendon rupture 6 and 12 month post-surgery along with 5 healthy controls via ultrasound. EMG was collected from the triceps surae muscles and tibialis anterior during over-ground walking.
Results
Achilles lengths at 6 and 12 months post-surgery were significantly longer (p < 0.05) on the involved side compared to the uninvolved side but there were no side to side differences in the healthy controls. The integrated EMG (iEMG) of the involved side was significantly higher than the uninvolved side in the lateral gastrocnemius at 6 months and for the medial gastrocnemius at 12 months in the patients with Achilles tendon rupture; no side to side difference was found in the healthy controls. The triceps surae muscles’ activations were fair to moderately correlated to the Achilles lengths (0.38 < r < 0.52).
Conclusions
The increased Achilles tendon length and iEMG from the triceps surae muscles indicate that loss of function is primarily caused by anatomical changes in the tendon and the appearance of muscle weakness is due to a lack of force transmission capability. This study indicates that when aiming for full return of function and strength an important treatment goal appears to be to minimize tendon elongation.
Level of evidence
Prognostic prospective case series. Level IV.
Keywords: EMG, gait, neural inhibition, surgery, ankle
Introduction
Achilles tendon ruptures are common, life altering injuries that have compounding long term effects. It has been shown that gait abnormalities are present greater than a year after surgery including increased dorsiflexion range of motion, co-activation of the lower leg muscles, and decreased step length [2]. There also have been displays of plantar flexion weakness at the end range of motion in Achilles tendon repaired subjects [11]. The persistence of decreased step length and plantar flexion weakness induces a decrease in physical activity and overall quality of life [13]. The decreases in force and functionality have been linked to atrophy of the calf muscles in longitudinal studies [5,4]. Along with muscular changes, tendon lengthening has been associated with Achilles tendon rupture, which is directly linked to the increased dorsiflexion range of motion seen in the long term [1]. Though there are multiple possible causes of plantar flexor weakness, it is uncertain if weakness is due to anatomical or neuromuscular deficits. In order to design improved treatments and rehabilitation protocols, with the purpose of minimizing the long term deficits seen in these patients, it is of interest to discern whether the remaining functional deficits and gait abnormalities are due to physical alteration of the tendon or neural inhibition of the muscle.
The purpose of this study is to understand the relationship between Achilles tendon lengthening and its effect on muscle contraction activity during gait. It was hypothesized that the Achilles tendon of the involved side will be significantly longer in patients with an Achilles tendon rupture. The lengthened Achilles tendon will result in an increased muscle activity via iEMG, compensating for the reduced force transmission across the joint caused by the additional slack in the tendon, and no sign of muscle inhibition. Understanding the connection between the biomechanical changes with the functional limitations will direct the therapeutic interventions toward targeting the root of the remaining issues seen both in the short and long term following an Achilles tendon rupture.
Materials and Methods
Four patients (3 men and 1 woman) with a mean (SD) age of 48 (8) years whom had experienced a complete Achilles tendon rupture and were treated surgically were included in this study. 5 healthy controls (3 men and 2 women) with a mean (SD) age of 26 (6) years were included for comparison. The patients’ mean (SD) height was 180 (4) cm and weight 86 (9) kg and the healthy controls’ mean (SD) height was 176 (10) cm and weight 81 (20) kg. The inclusion criteria for the patients with Achilles tendon rupture were being between 20–80 years of age and having sustained a unilateral Achilles tendon rupture with no re-rupture or any other Achilles tendon injury to the contralateral side. The inclusion criteria for the healthy controls were 18–50 years of age and participated in at least 50 hours per year of Level I, II or III activities as described by the International Knee Documentation Committee. Subjects were excluded from the study if they had any physical or neurological condition preventing them from walking normally. The patients were recruited through the outpatient Physical Therapy clinic at University of Delaware and through one of the authors. The healthy subjects were recruited from the student population of the University of Delaware. The patients were evaluated on two occasions, once 6 months after injury and then again 12 months after injury. The healthy controls were evaluated once.
The study was approved by the institutional review board at the University of Delaware. All the subjects were informed of the study and gave informed consent before participation.
Procedure
Data were collected bilaterally on all the subjects. The subjects lay in the prone position during the ultrasound imaging with their foot hanging off the edge of a plinth in a relaxed position. Following the ultrasound measurement protocol, EMG electrodes were applied and maximum voluntary isometric contractions were performed on a Biodex 3 system (Biodex Medical Systems, Shirley, NY). The walking trials were performed over-ground at the subject’s self-selected speed across a 20-m walkway with a floor level force plate. Five (5) trials of each a single right and a single left foot strike were collected.
Patient-reported outcome and physical activity level
The patients were evaluated concerning their symptoms and physical activity level. The Achilles tendon Total Rupture Score (ATRS) [12] was used to evaluated patient reported symptoms and the Physical Activity Scale [16] was used to evaluate the physical activity level prior to injury and at 6 and 12 months after injury. The ATRS score range from 0–100 where a lower score indicates more symptoms and 100 is considered fully recovered. The Physical Activity Scale ranges from 1–6 where 1 is no physical activity and 6 is heavy physical exercise several times per week where the patient also considers work activity.
Achilles Tendon Length
Tendon length was measured with a B-Mode, 10 MHz, ultrasound image was taken with a 6.0cm transducer using an Aloka SSD-5000 system (Aloka, Tokyo, Japan). The 6.0cm transducer was not sufficiently wide to collect then entire length of the Achilles tendon requiring a hybrid method of ultrasound and motion capture to collect the length of the tendon. This method has been previously described with a reported error of <1.0% [18]. The transducer was fitted with retro-reflective markers directly over the edges of the sonogram scan area. The markers were used to track the position of the probe in a fixed laboratory reference frame. An 8 camera arrangement was used for motion capture collecting at 50hz (Qualisys Motion Capture System, Gothenburg, Sweden). The Achilles tendon length was defined as the distance between the musculotendinous junction and the calcaneal osteotendinous junction as previously described in the literature [15]. The images from the ultrasound and the motion capture were used in tandem to determine the overall length of the Achilles tendon. For each Achilles tendon two images of the osteotendinous junction and two images of the musculotendinous junction were taken. Since the location of the probe was known in the fixed laboratory reference, the location of the origin and insertion of the Achilles tendon were therefore also known relative to the laboratory reference. These four images were combined so each tendon had two length measurements. The mean of these measurements was used for analysis. Test-retest reliability of this technique has previously been established indicating good reliability with an ICC = 0.97 [18].
EMG
In order to establish muscle activation patterns during walking, EMG signals were collected from the medial and lateral gastrocnemii (MG, LG), soleus (SL), and tibialis anterior (TA). The EMG locations were shaved and lightly abraded with alcohol pads. Bipolar silver/silver chloride surface electrodes were placed on each of the muscles of both legs in accordance with the Anatomical Guide for the Electromyographer [14]. Wires transmitting from the electrodes were connected to a receiver which was then connected to a long coaxial tether leading back to the data acquisition system. Data were collected using a MA-300 system sampling at 1200hz. (Motion Lab System, Baton Rouge, LA). Once applied, the electrodes and wires were wrapped with elastic athletic wraps to prevent EMG motion artifact. The Biodex system was used to fix the ankle at 90°. The subject performed two maximum effort, isometric dorsiflexion and plantar flexion contractions for three seconds each with a minimum of 1 minute rest to prevent fatigue. The MVIC trials were followed by the walking trials at the subjects’ self-selected walking speed.
Once the signals were collected from the MVIC and walking trials, linear envelopes were created by filtering with a Butterworth filter with a high pass limit of 30hz, full-wave rectifying, a low pass limit of 4hz, and normalizing to the maximum EMG of each muscle, determined during an MVIC test. The integrated EMG (iEMG) was determined from the area under the linear envelope created using Visual 3D (C-Motion Inc., Bethesda, MD).
Statistical Analysis
Analyses of the data were performed with the Statistical Package for the Social Sciences version 19. The descriptive data are reported as mean (SD). Each walk trial was analyzed for one gait cycle. Data for the involved side were taken from the trials in which the involved leg was making contact with the force plate. The iEMG of each trial was collected from the linear envelope and was averaged within the 5 trials for each leg. A linear fit was applied, using pearson’s r (significance p), to the data correlating the iEMG during one gait cycle to the length of the Achilles tendon for both the injured and healthy populations. A one-tailed students paired t-test (p<0.05) was used to compare the iEMG of the involved and uninvolved sides at 6 months. This test was repeated for the 12 months case. The signals from the left and right legs were compared for the healthy control group. A paired ttest (p < 0.05) was also used to determine a difference in Achilles tendon length at six months and also determined a difference at 12 months.
Results
Achilles tendon length
No significant length differences were found between the right and left Achilles tendon within the healthy controls (n.s.) (Table 1). However, significant differences were found between the tendon lengths of the involved and uninvolved sides at 6 month, 3.6± 0.8cm (p<0.01) and at 12 months, 3.1±0.1cm (p=0.01) for the patients with Achilles tendon rupture (Table 1).
Table 1.
The mean Achilles tendon lengths (cm) of the subjects at 6 and 12 months along with 5 healthy controls.
| Mean AT Lengths |
Involved | Uninvolved | Difference |
|---|---|---|---|
| 6 Months | 24.5 | 20.9 | 3.6* |
| 12 Months | 23.8 | 20.7 | 3.1* |
| Healthy | 20.3 | 19.8 | 0.5 |
indicates p<0.05
Patient reported outcomes
The mean (SD) ATRS score was 82 (9) at 6 months and 89 (4.5) at 12 months after surgery. The mean (SD) Physical Activity level was 3.8 (1) at 6 months and 4.3 (1) at 12 months. As part of the patient reported outcomes, the subjects reported no pain during the MVIC contractions.
EMG activity
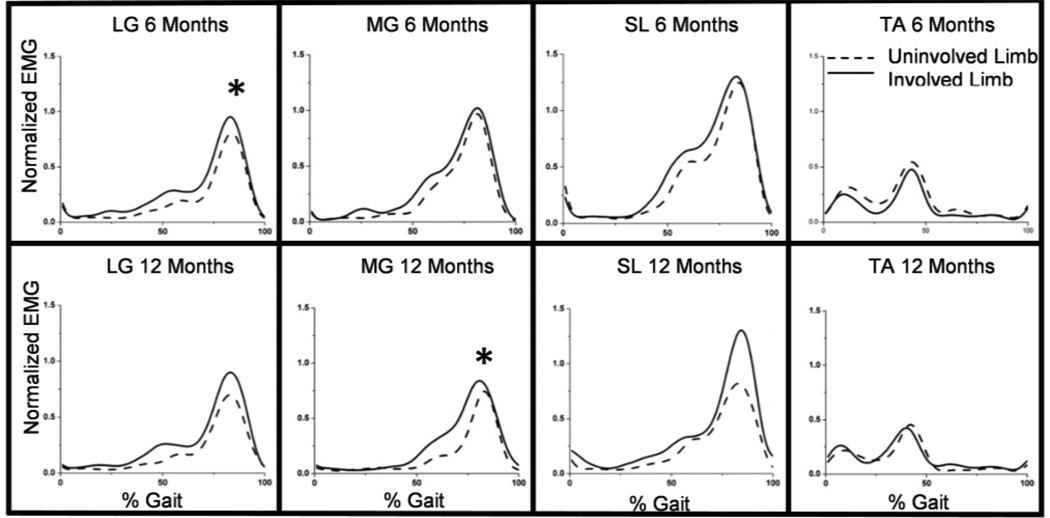
No significant side to side differences existed between iEMG signals of the healthy subjects. Significant differences were found in muscle activation between the involved and uninvolved sides in the patients with an Achilles tendon rupture. The LG at 6 months (p=0.02) and the MG at 12 months (p=0.04) had significant increases of EMG activation of the involved side during gait (Figure 1). There were no significant differences between the TA muscles for the 6 months, 12 months, or healthy cases.
Fig 1.
The mean iEMG during one gait cycle for all injured subjects. The asterix (*) indicates a significant increase in iEMG. Note the significant increases existed in the triceps surae cases, but not in the TA
Correlation between tendon length and EMG activity
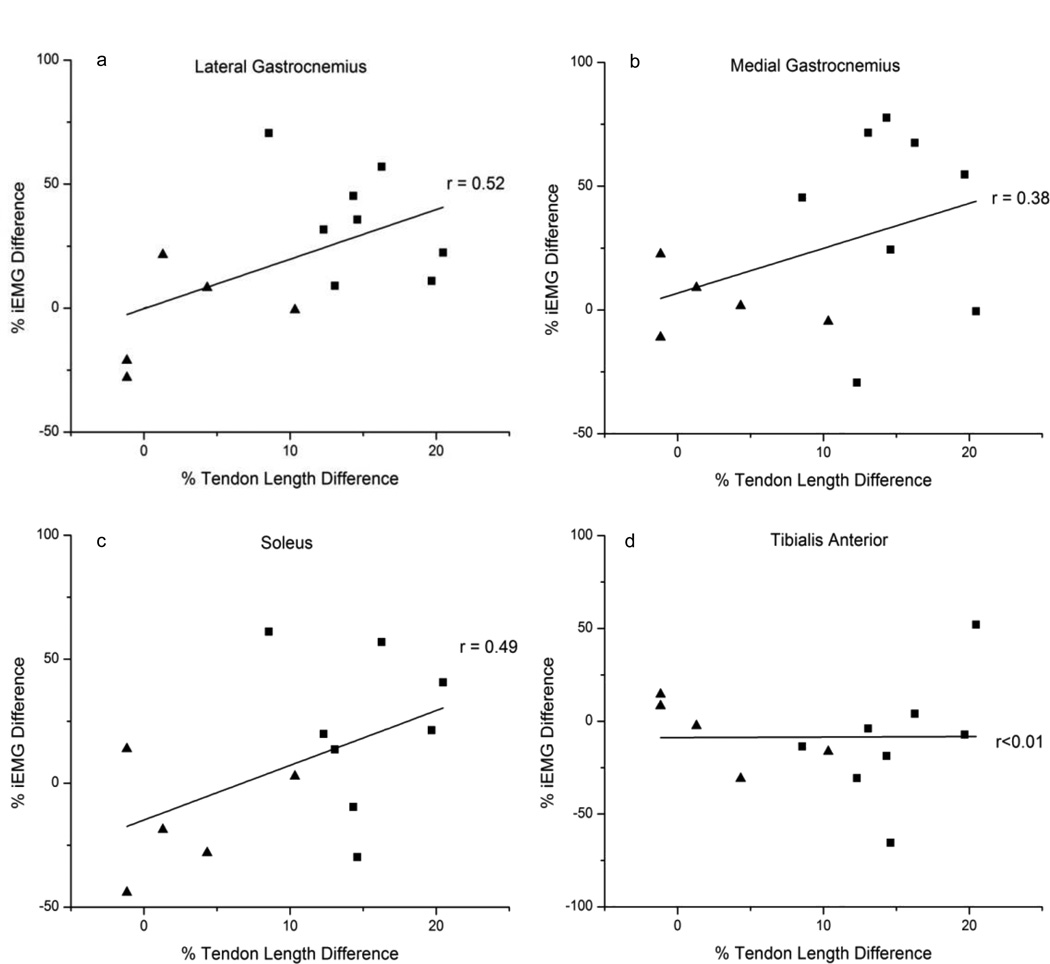
A moderate correlation exists between the length of the Achilles tendon and the iEMG of the LG (r = 0.52, ns) and a fair correlation exists for the MG and SL (r = 0.38, ns; r = 0.49, ns, respectively). No correlation existed between the Achilles tendon length and the iEMG of the TA (r < 0.01, ns) (Figure 2).
Fig 2.
The difference in iEMG during one gait cycle compared to the difference in length of the Achilles tendon for healthy and injured subjects. The right leg is positive for the healthy subjects and the involved side is graphed as positive for the injured subjects. The (▲) denote the healthy subjects and the (■) denote the injured subjects. Note the (a) LG, (b) MG, and (c) SL each have a moderate to fair correlation while the (d) TA has no correlation
Discussion
The most important finding of the present study was muscle activation of the triceps surae was greater in the affected side with a lengthened Achilles tendons post-tendon rupture. We studied Achilles tendon lengths at rest and iEMG during walking to understand remaining functional deficits following Achilles tendon rupture in subjects at 6 months and 12 months post-surgery compared to healthy controls. Our hypothesis was confirmed. The Achilles tendon was significantly longer on the involved side in patients with Achilles tendon rupture and this was not seen in the healthy controls. The iEMG was increased on the involved side during gait in several of the triceps surae muscles in the patients with an Achilles tendon rupture but in the healthy controls there were no side to side differences. The larger muscle activity seen at 6 and 12 months post-surgery indicate, following an Achilles tendon rupture, the calf muscles ability to contract during gait is not reduced. Instead the lengthened Achilles tendon and the increased iEMG of the MG and LG indicate that the remaining functional deficits are primarily due to anatomical changes of the tendon and not due to neural inhibition of the muscle, which is supported by fair to moderate liner regressions of the length versus iEMG plots (Figure 2). Since these patients all had significantly longer Achilles tendons on the injured side, the triceps surae could be contracting more in an attempt to compensate for the increase in tendon slack during walking (Figure 3). The difference in activations was confirmed to be related to the tendon lengthening and not to the normalization method through affirmation from each subjects of no pain during MVIC trials. This was supported by the high ATRS values at 6 and 12 months.
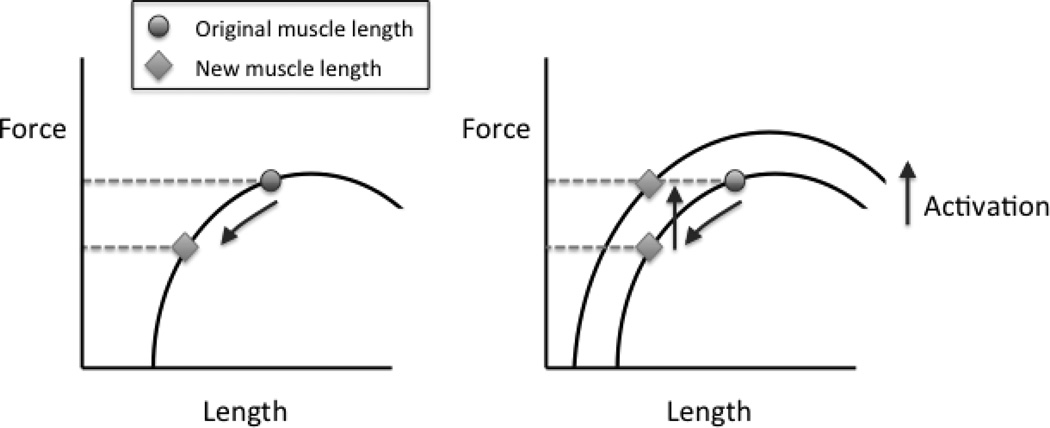
Fig 3.
If Achilles tendon length increases, the muscle length must decrease an equal amount. The figure at left shows that, given a muscle force-length curve at a constant activation level, if the length of the muscle decreases, its corresponding force will decrease. The figure at right shows that to produce the same amount of force at that new length, the activation level must be increased (as marked by shifting to a new force-length curve). Hence, higher EMGs are needed for the same task if the muscle length shortens
Achilles tendon lengthening with tendon end separation has been reported to occur primarily during the first 4 months after surgery [6, 9, 17]. Of clinical relevance, the clinical outcome has been reported to correlate with the degree of tendon elongation [6, 8]. It is also of interest to note that the separation of tendon ends are similar regardless of if the tendon is sutured or not [17]. Instead it has been shown that early mobilization results in a lesser degree of tendon elongation [6]. Since this study indicated that the primary limitation for achieving full recovery might be the structural change of the tendon and not a neuromuscular change of the gastrocnemius muscle; future treatment should aim at minimizing the tendon elongation.
The average lengthening of the subjects’ Achilles tendons was ~15% in this study, compared to their contralateral side, which was within the range seen in previous studies [10]. This additional tendon length may require a stronger muscle contraction during the preparation of push-off in gait in order to create force at the joint because the muscle is potentially acting at a different position of its force-length curve. In response to the initial contraction increasing during the preparation, the force produced at peak contraction may also be weaker. Other studies demonstrated plantar flexor weakness at the end range of motion [11]. This can be explained through the SL, MG, and LG acting outside of their maximum force generating length. With the additional shortening of the muscle prior to producing tension on the tendon, the muscle, at maximum contraction, is functioning at a less advantaged region of the muscle’s length tension curve (Figure 3). The relative muscle force of the soleus has been shown to decrease greatly with as little as 0.5cm difference in fiber length [7]. In this study, the average length change of the Achilles tendon being between 3.0cm and 3.5cm showed force deficits of the triceps surae at maximum contraction are linked to the additional muscle activity needed to begin flexion at the ankle (Table 1). This lengthening not only affects the activation of the muscles prior to needing a strong contraction, but the maximum possible force when a maximum contraction is required.
The lengthening of the Achilles tendon contributes to anatomical variation over time as well. It has been shown that atrophy occurs on the involved side 10 years post-surgery [5]. The lengthened Achilles tendon could be responsible for this finding. Atrophy is marked by a lack of tension in the muscle. Though the muscle is contracting more, it is contracting without tension during the initial phase. In order to hypertrophy, muscles require an increased resistance during contraction [3]. The limited transmission of force through the Achilles tendon reduces the effectiveness of training protocols in the strengthening and recovery of the patients’ triceps surae muscles. The gastrocnemius muscle atrophy seen in patients with Achilles tendon rupture can therefore be explained by the elongated tendon which limits the impact of strengthening exercises.
The small sample size is a limitation of this study. However despite the limited sample we have found significant differences between the injured and uninjured side in the patients with an Achilles tendon rupture, which was not found in the healthy control group. This study also allowed for the correlation of Achilles tendon length to the required muscle activation for gait (Figure 2). The correlation included both pathologic and healthy subjects demonstrating a relationship between increasing tendon lengths and required activations in all subjects illustrating the small sample size having limited effects on the overall outcome of the study. This study therefore provides a piece of the puzzle in understanding a major step towards complete recovery. Future studies are needed to further our knowledge in how this can be minimized such as through improved suture techniques or through improved early rehabilitation. Furthermore, the patient demographics and patient reported outcomes in this study are similar as to what has been reported in other studies [12, 19]. We therefore have no reason to believe that the sample in this study is significantly different than this patient population in general.
The increased Achilles tendon length and iEMG from the muscles used to stretch the tendon indicate that loss of function is primarily caused by anatomical changes in the tendon and the appearance of muscle weakness is due to reduced capacity for force transmission across the joint. During gait, patients’ compensate for an elongated Achilles tendon following surgical repair by greater activation of the gastrocnemii muscles to account for the additional tendon slack before motion is produced at the joint. Due to the anatomical nature of this condition, physical therapy may not have an effect in reducing plantar flexion weakness. Limiting tendon lengthening should be a primary concern for any Achilles tendon rupture treatment method.
Conclusion
This study confirmed our hypothesis that there would be an increase in triceps surae muscle activity on the side with an elongated Achilles tendon, during gait. Increased muscle activation in the triceps surae seem to be required to account for the additional stretch within the tendon before force is transmitted to the foot. The additional tendon slack may lead to decreased plantar flexion strength at maximum muscle activation.
Acknowledgements
The authors would like to acknowledge the Swedish Research Council for its financial support. This work was also supported by NIH grant P30-GM103333.
Footnotes
Conflict of Interest
The authors declare that they have no conflict of interest.
Contributor Information
Stephen M. Suydam, University of Delaware, 126 Spencer Lab, Newark, DE 19716-3140
Thomas S. Buchanan, University of Delaware, 126 Spencer Lab, Newark, DE 19716-3140
Kurt Manal, University of Delaware, 126 Spencer Lab, Newark, DE 19716-3140.
Karin Gravare Silbernagel, University of the Sciences, 600 S. 43rd Street, Philadelphia, PA 19104.
References
- 1.Costa M. The effect of Achilles Tendon lengthening on ankle dorsifelxion: A cadaver study. Foot Ankle Int. 2006;27:414–417. doi: 10.1177/107110070602700605. [DOI] [PubMed] [Google Scholar]
- 2.Don R, Ranavolo A, Cacchio A, Serrao M, Costabile F, Iachelli M, Camerota F, Frascarelli M, Santilli V. Relationship between recovery of calf-muscle biomechanical properties and gait pattern following surgery for Achilles tendon rupture. Clin Biomech. 2007;22:211–220. doi: 10.1016/j.clinbiomech.2006.10.001. [DOI] [PubMed] [Google Scholar]
- 3.Ferri A, Scaglioni G, Pousson M, Capodaglio P, Van Hoecke J, Narici MV. Strength and power changes of the human plantar flexors and knee extensors in response to resistance training in old age. Acta Physiol Scand. 2003;177:69–78. doi: 10.1046/j.1365-201X.2003.01050.x. [DOI] [PubMed] [Google Scholar]
- 4.Haggmark T, Eriksson E. Hypotrophy of the soleus muscle in man after Achilles tendon rupture: Discussion of findings obtained by computed tomography and morphologic studies. Am J Sport Med. 1979;7:121–126. doi: 10.1177/036354657900700208. [DOI] [PubMed] [Google Scholar]
- 5.Horstmann T, Lukas C, Mayer F, Winter E, Ambacher T, Heitkamp H, Dickhuth H. Isokinetic strength and strength endurance of lower limb musculature ten years after Achilles tendon repair. Isokinet and Exerc Sci. 2000;8:141–145. [Google Scholar]
- 6.Kangas J, Pajala A, Ohtonen P, Leppilahti J. Achilles tendon elongation after rupture repair: A randomized comparison of 2 postoperative regimens. Am J Sport Med. 2007;35:59–64. doi: 10.1177/0363546506293255. [DOI] [PubMed] [Google Scholar]
- 7.Maganaris CN. Force-length characteristics of in vivo human skeletal muscle. Acta Physiol Scand. 2001;172:279–285. doi: 10.1046/j.1365-201x.2001.00799.x. [DOI] [PubMed] [Google Scholar]
- 8.Maquirriain J. Achilles tendon rupture: Avoiding tendon lengthening during surgical repair and rehabilitation. Yale J Biol Med. 2011;84:289–300. [PMC free article] [PubMed] [Google Scholar]
- 9.Mortensen NH, Saether J, Steinke H, Mikkelsen SS. Separation of tendon ends after Achilles tendon repair: A prospective, randomized, multicenter study. Orthopedics. 1992;15:299–903. doi: 10.3928/0147-7447-19920801-06. [DOI] [PubMed] [Google Scholar]
- 10.Mortensen NHM, Jensen PE. Early Motion of the ankle after operative treatment of a rupture of the Achilles tendon. J Bone Joint Surg Am. 1999;81:983–990. doi: 10.2106/00004623-199907000-00011. [DOI] [PubMed] [Google Scholar]
- 11.Mullaney MJ, McHugh MP, Tyler TF, Nicholas SJ, Lee SJ. Weakness in end-range plantar flexion after Achilles tendon repair. Am J Sport Med. 2006;34:1120–1125. doi: 10.1177/0363546505284186. [DOI] [PubMed] [Google Scholar]
- 12.Nilsson-Helander K, Thomeé R, Silbernagel KG, Grävare-Silbernagel K, Thomeé P, Faxén E, Eriksson BI, Karlsson J. The Achilles tendon Total Rupture Score (ATRS): development and validation. Am J Sport Med. 2007;35:421–426. doi: 10.1177/0363546506294856. [DOI] [PubMed] [Google Scholar]
- 13.Olsson N, Nilsson-Helander K, Karlsson J, Eriksson BI, Thomée R, Faxén E, Silbernagel KG. Major functional deficits persist 2 years after acute Achilles tendon rupture. Knee Surg Sports Traumatol Arthrosc. 2011;19:1385–1393. doi: 10.1007/s00167-011-1511-3. [DOI] [PubMed] [Google Scholar]
- 14.Perotto AO, Delagi EF, Iazzetti J, Morrison D. Anatomical Guide for the Electromyographer. Springfield: Charles C Thomas Pub Ltd; 2005. pp. 152–168. [Google Scholar]
- 15.Rees JD, Lichtwark GA, Wolman RL, Wilson AM. The mechanism for efficacy of eccentric loading in Achilles tendon injury; An in vivo study in humans. Rheumatology. 2008;47:1493–1497. doi: 10.1093/rheumatology/ken262. [DOI] [PubMed] [Google Scholar]
- 16.Roos EM, Brandsson S, Karlsson J. Validation of the foot and ankle outcome score for ankle ligament reconstruction. Foot Ankle Int. 2001;22:788–794. doi: 10.1177/107110070102201004. [DOI] [PubMed] [Google Scholar]
- 17.Schepull T, Kvist J, Andersson C, Aspenberg P. Mechanical properties during healing of Achilles tendon ruptures to predict final outcome: A pilot Roentgen stereophotogrammetric analysis in 10 patients. BMC Musculoskelet Disord. 2007;8:116. doi: 10.1186/1471-2474-8-116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Silbernagel KG, Steele R, Manal K. Deficits in heel-rise height and Achilles tendon elongation occur in patients recovering from an Achilles tendon rupture. Am J Sport Med. 2012;40:1564–1571. doi: 10.1177/0363546512447926. [DOI] [PubMed] [Google Scholar]
- 19.Willits K, Amendola A, Bryant D, Mohtadi NG, Giffin JR, Fowler P, Kean CO, Kirkley A. Operative versus nonoperative treatment of acute Achilles tendon ruptures: A multicenter randomized trial using accelerated functional rehabilitation. J Bone Joint Surg Am. 2010;92:2767–2775. doi: 10.2106/JBJS.I.01401. [DOI] [PubMed] [Google Scholar]