Abstract
Small fiber neuropathy is common in a number of systemic diseases and is often challenging to diagnose. Laser-Doppler Imaging (LDI) is a test of small fiber neurovascular function that can quantify the integrity of the vasomotor C-fiber mediated axon-reflex, but no standardized method of analysis exists. We developed a novel LDI analysis technique and tested it in a human model of small fiber neuropathy. Eighteen healthy subjects (age 24±3 years) underwent LDI testing to assess the axon-mediated flare area in response to 10% acetylcholine iontophoresis. LDI measurements were taken before and longitudinally after a 48-hour application of 0.1% capsaicin (to cause a transient small fiber neuropathy) on the skin of the thigh; placebo cream was placed on the contralateral thigh as a control. We compared our new LDI image analysis technique to two previously published methods. The new LDI analysis technique was the only method to show a consistent difference in axon-reflex area between capsaicin treated and placebo treated skin on all testing days (p<0.05) with maximum attenuation of the flare area immediately post-application (438 ±298 mm2 vs. 824 ±375 mm2, p<0.05). In conclusion, this study demonstrates that our novel flare area method for LDI analysis can detect neurovascular dysfunction in a model of small fiber neuropathy, is an improvement over existing methods, and may supplement clinical assessment of small fiber neuropathy.
Keywords: Laser Doppler Imaging, LDI, Vasomotor Axon-Reflex, Small Fiber Neuropathy, Capsaicin, Ionotophoresis
1. Introduction
Peripheral small fiber neuropathy is present in a number of diseases including amyloidosis, paraneoplastic syndromes, diabetes and other glucose dysregulated states (Freeman, 2005). Neuropathy may variably involve both somatic and autonomic small nerve fibers (Gibbons et al., 2010a; Tavee et al., 2009). The diagnosis of small fiber neuropathy is often challenging because standard electrodiagnostic tests, which primarily assess large fiber function, are frequently normal in these patients (Tavee et al., 2009). Laser-Doppler analysis of cutaneous blood flow is a novel test of small nerve fiber function (Berghoff et al., 2006; Caselli et al., 2006). Techniques include the use of single-point laser-Doppler flowmetry (LDF) or 2-dimensional laser-Doppler imaging (LDI) to investigate the integrity of the neurogenic axon-reflex when combined with iontophoresis of a cholinergic agonist (Berghoff et al., 2006; Krishnan et al., 2004).
Over the past several decades, a number of studies confirm that LDI and LDF are able to detect differences in vasomotor function between groups of individuals with and without neuropathy (Benarroch et al., 1991; Berghoff et al., 2006; Bickel et al., 2002; Gibbons et al., 2010b; Krishnan et al., 2004). However, most investigators agree that LDF is not sensitive enough to detect neuropathy in individual subjects (Benarroch et al., 1991; Caselli et al., 2006; Parkhouse et al., 1988). In contrast, many investigators feel LDI is more reliable, but there are several proposed methods for LDI analysis and the optimal approach is not known (Bickel et al., 2002; Green et al., 2009; Kramer et al., 2004).
In this paper, we report a novel LDI analysis technique and compare the results against two previously published LDI analysis methods (Green et al., 2009; Kramer et al., 2004).
2. Methods
2.1. Study design
This was a randomized double-blind placebo controlled longitudinal study of LDI in eighteen healthy subjects. Subjects had 0.1% capsaicin cream applied in a 48-hour occlusive dressing to one anterior thigh to create a standardized, reversible small fiber neuropathy as previously described (Gibbons et al., 2010b; Polydefkis et al., 2004). Subjects had placebo cream applied to the contralateral thigh. LDI was measured before and weekly for 4 weeks after capsaicin/placebo application.
Baseline LDI measurements were collected on both anterior thighs of each subject on day 1. After LDI testing, subjects had a 48-hour application (days 1 & 2) of capsaicin cream (2.4 g of 0.1%, Chattem Inc., Chattanooga, USA) to one anterior thigh and placebo cream (Johnson & Johnson, New Brunswick, NJ, USA) to the contralateral thigh in occlusive dressings over a 50×80 mm2 area. Capsaicin and placebo creams were randomly assigned to the right or left anterior thigh using block randomization. LDI measurements were repeated on both thighs on days 3, 10, 17, 24 and 31. Three-millimeter punch skin biopsies were taken on day 17 in both capsaicin and placebo treated regions to measure intra-epidermal nerve fiber density.
2.2. Subjects
Eighteen healthy subjects (10 females, 8 males) ages 21 to 27 years (mean: 23 years) were enrolled. None of the subjects had evidence by history or exam of neuropathy, peripheral vascular disease, tobacco use, current medication use or other medical disease. The protocol was approved by the Beth Israel Deaconess Medical Center Institutional Review Board. Each subject signed a written informed consent.
2.3. Iontophoresis
Vasomotor axon-reflex mediated flare response was provoked through iontophoresis of 10% acetylcholine (Penta International Corporation, Fairfield, NJ, USA) at 0.4 mA for 140 seconds (Phoresor-PM850, IOMED, USA) using a drug delivery electrode with an internal diameter of 1.1 cm (LI 611, Perimed, Järfälla, Sweden) centered over the LDI scanning area as previously reported (Gibbons et al., 2010b).
2.4. Laser-Doppler imaging (LDI)
Blood flow measurements were performed in the capsaicin and placebo exposed area of subjects placed in a semi-recumbent position with standardized lighting in a temperature controlled room (25±1°C) at day 1 and on days 3, 10, 17, 24 and 31. The subjects’ legs were stabilized and immobilized through use of foam blocks. After a 20 minute acclimatization period, a laser-Doppler perfusion imager (Periscan PIM III, Perimed, Sweden) using a stable helium neon gas laser (λ = 632.8 nm) measured cutaneous blood flow over a 4.2 cm × 4.4 cm area at a distance of 30 cm from the skin surface. Images were recorded in repeated image mode (36 pixels × 37 pixels (18.5 cm2 image size), 1 mm step length, 28 pixels per second, 48 seconds per image). Five baseline images were obtained to quantify resting blood flow, followed by iontophoresis with acetylcholine to provoke an axon-reflex flare response and an additional 31 laser-Doppler images were obtained.
2.5 Blood Flow Analysis
The laser-Doppler imager records every image as a map of single point data cells with specific perfusion values. The values in each cell are reported in Perfusion Units (arbitrary blood flow values), but do not change across any analysis method. A perfusion ‘flare’ response is reported when the value of a cell exceeds a predetermined threshold. The selection of the appropriate threshold is what distinguishes each analysis method.
2.5.1. Analysis Method 1: Flare area method
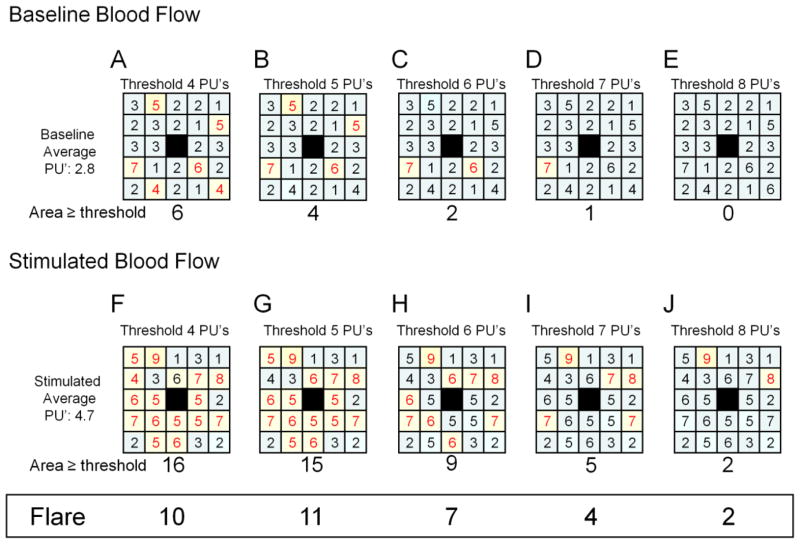
Our new method to quantify the axon-reflex area determined the optimal perfusion threshold by measuring the maximum axon-reflex flare in all 18 subjects on day 1. The raw data from the images were exported into an excel file. The 5 baseline images for all subjects from day 1 were averaged and the mean blood flow across all cells determined to be 111 PU’s. We then established a cutoff threshold and the flare area was defined as the number of cells above the threshold after iontophoresis subtracted from the number of cells above the threshold before iontophoresis [axon-reflex flare = (Flare Areaiontophoresis−Flare Areabaseline)]. We tested all possible threshold values to determine the maximal flare response as demonstrated in Figure 1 (this was performed using if/then logic values in the excel spreadsheet – i.e. if value >X PU’s then count =1). Our method defined 125 PU’s as the threshold that measured the largest flare response on day 1 after iontophoresis. Therefore, in all additional testing days in all subjects, values of data cells that exceeded 125 PU were counted and flare area size was calculated based on the total number of cells that exceeded 125 PU after iontophoresis minus the area calculated before iontophoresis (the actual area = 1.39 mm2/cell).
Figure 1. Flare area method.
This is an example of the flare area method. The top row of squares (A–E) shows the same baseline perfusion units (PU’s) for a sample series of scanned images (this example shows a 5×5 grid for simplicity, the actual image would be 36×37 pixels). The center box of each square (shown in black) is the region of iontophoresis and is not included in the analysis. The values in each square that are ≥ to the perfusion threshold (listed at the top of each square – the threshold is 4 PU’s in A etc.) are highlighted in yellow and shown in red text. Thus, in square A, six boxes have values ≥4 PU’s and are highlighted. The total number of highlighted boxes are counted and reported below each box (in A, the area exceeding the perfusion threshold is 6). The bottom row of squares (F–J) is the perfusion values post-iontophoresis. The data values are tested against a series of increasing thresholds ranging (in this example) from 4 to 8 (A–E and F–J). The difference in flare area between the baseline (A–E) and stimulated (F–J) region is listed at the bottom of the figure in the box entitled “Flare”. In this example, the largest Flare response is with the threshold set to 5 PU’s where baseline flare area of 4 (B) and the stimulated flare area of 15 (G). The difference between baseline and stimulated areas is a flare area of 11.
2.5.2. Analysis Method 2: Baseline perfusion method
This method detects a change in axon-reflex blood flow as an increase of two standard deviations above baseline blood flow (Kramer et al., 2004). For each subject and each test day, the average value of the baseline images was calculated and a perfusion threshold was identified two standard deviations above the average. This method changed the perfusion threshold for every subject on every test to account for the variable baseline. Cells that increased two standard deviations above the baseline average value were counted as active (i.e. an axon-reflex response was detected). The total flare area was calculated based on total number of cells that exceeded the two standard deviation threshold (area of 1.39 mm2/cell).
2.5.3. Analysis Method 3: Resting blood flow method
This method detects a change in axon-reflex blood flow as an increase of three times over the baseline value (Green et al., 2009). Axon-reflex mediated flare response was defined as values exceeding 3x resting blood flow. The baseline perfusion values for all subjects and all testing days were averaged. The total average was 111 perfusion units (PU) and therefore the perfusion threshold was set at 333 PU. The data in the cells that exceeded 333 PU was counted as active (i.e. an axon-reflex response was detected) and flare area size was calculated based on the total number of cells that exceeded 333 PU (area of 1.39 mm2/cell).
2.6 Flare latency
The time to the largest flare was determined for each threshold analysis method and reported as the flare latency. Flare latency was reported as the average time across all 18 subjects for each method.
2.6. Skin biopsies
Two weeks after capsaicin and placebo application (day 17) one 3-mm punch skin biopsy was obtained from each anterior thigh to assess intra-epidermal nerve fiber density (IENFD). Skin biopsy specimens were fixed, processed, cut into 50 micrometer thick frozen sections and stained with protein gene product 9.5 (1:1,000 dilution, rabbit anti-PGP 9.5, Chemicon International, Temecula, CA) using standard techniques (Gibbons et al., 2006). Intra-epidermal nerve fibers were counted using light microscopy (Olympus BH-2, Olympus Corp., Tokyo, Japan) by a physician blinded to treatment allocation and data were expressed as a linear density (number of fibers per millimeter) as previously described (Gibbons et al., 2006; Lauria et al., 2010).
2.7. Statistical analysis
Statistical analysis was performed using SPSS v17 (IBM, Chicago, IL 60606). The primary outcome for the study was axon-reflex mediated flare area. Repeated measures ANOVA with Tamhane’s T2 post-hoc test was used for each of the LDI assessment methods to compare flare area in capsaicin and placebo treated skin. Secondary outcomes included latency to maximum spatial spread and nerve fiber densities. Intra-class correlation coefficients were measured in the placebo regions using all 3 flare analysis methods. A paired t-test was used to compare IENFD between capsaicin and placebo areas. Data are presented as mean ± standard deviation (SD); figures are presented as mean ± standard error of the mean (SEM). All tests were two-tailed and p-values less than 0.05 considered statistically significant.
3. Results
3.1. Skin biopsies
Skin biopsies taken on day 17 had significant reductions in intra-epidermal nerve fiber density in capsaicin treated regions compared to those from placebo treated regions (2.3 ±1.0 fibers/mm capsaicin vs. 12.4 ±2 fibers/mm placebo; p<0.001).
3.2. Longitudinal vasomotor function assessment
3.2.1. Flare area method
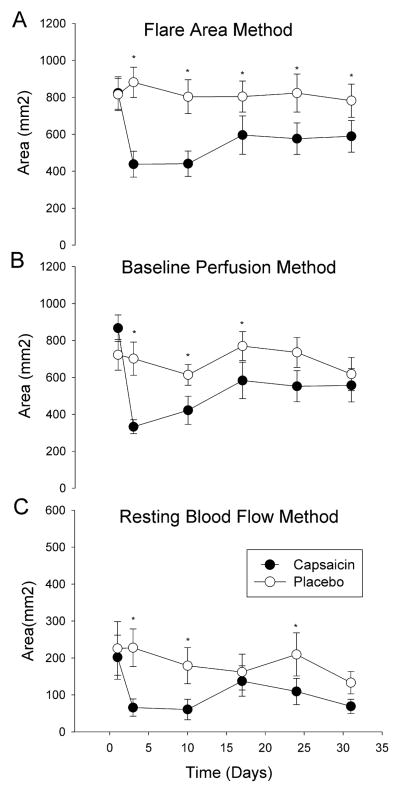
The axon-reflex area was reduced in capsaicin treated sites on all evaluation days compared to placebo sites (p<0.05, Figure 2A). The maximum reduction in flare area occurred immediately after capsaicin application (824 ±375 mm2 day 1 to 438 ±298 mm2 day 3; p<0.05).
Fig. 2. Flare analysis methods.
Comparison of the three methods for analyzing blood flow flare response. Capsaicin treated skin is shown with black circles, placebo control skin is shown in white circles. In (A) the flare area method detects a difference in blood flow across all testing days. In (B) the baseline perfusion method detects a difference in blood flow on days 3, 10 and 17. In (C) the resting blood flow method detects a change in blood flow on days 3, 10 and 24. *P<0.05. Values shown are mean ± SEM.
3.2.2 Baseline perfusion method
The axon-reflex area was reduced in capsaicin sites on day 3, 10 and 24 after capsaicin application compared to placebo sites (p<0.05, Figure 2B) but not on days 17 and 31. The maximum reduction in flare area occurred immediately after capsaicin application (867 ±304 mm2 day 1 to 333 ±159 mm2 day 3; p<0.05).
3.2.3 Resting blood flow method
The axon-reflex area was reduced in capsaicin sites on day 3, 10, 24 and 31 after capsaicin application compared to placebo sites (p<0.05, Figure 2C) but not on day 17. The maximum reduction in flare area occurred 1 week after capsaicin application (202 ±254 mm2 day 1 to 66 ±98 mm2 day 10; p<0.05).
3.3. Reliability of methods
The flare area method had an individual intra-class correlation (ICC) of 0.67 with an average ICC of 0.91. The baseline perfusion method had individual ICC of 0.54 with an average ICC of 0.85. The resting blood flow method had individual ICC of 0.50 with an average ICC of 0.83.
3.4. Flare latency
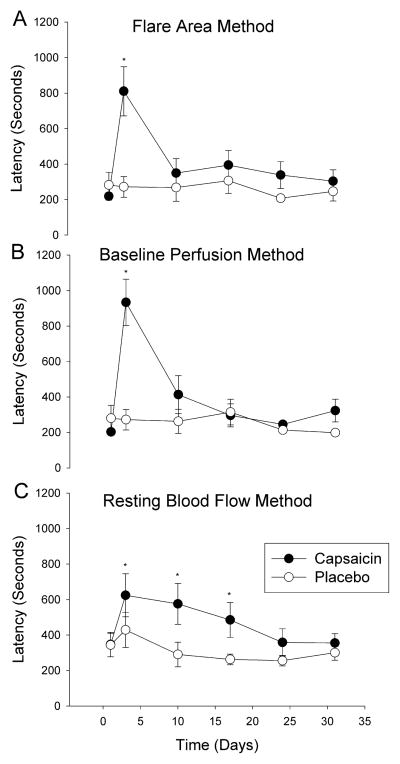
There was an increase in the axon-reflex latency on day 3 using the flare area method (Figure 3A: 811 ±587 seconds day 3 vs. 219 ±91 seconds day 1, p<0.01) that returned to baseline by day 10. There was an increase in the axon-reflex latency on day 3 using the baseline perfusion method (Figure 3B) (933 ±557 seconds day 3 vs. 203 ±63 seconds day 1, p<0.05) that returned to baseline by day 10. There was an increase in the axon-reflex latency on day 3, 10 and 17 using the resting blood flow method that returned to baseline by day 24 (Figure 3C) (P<0.05 days 3,10,17 vs. day 1). There were no changes in latency noted under placebo conditions for any method (Figure 3).
Fig. 3. Axon-reflex flare latency.
Comparison of the flare latency using the three analysis methods. Capsaicin treated skin is shown with black circles, placebo control skin is shown in white circles. In (A) the flare area method, there is an increase in flare latency on day 3 in the capsaicin treated region. In (B) the baseline perfusion method, there is an increase in flare latency on day 3 in the capsaicin treated region. In (C) the resting blood flow method, there is an increase on flare latency on days 3, 10 and 17 in the capsaicin treated region. *P<0.05. Values shown are mean ± SEM.
4. Discussion
In this study we demonstrate a novel LDI analysis technique that can: (1) detect a consistent change in laser-Doppler imaging flare response in chemically denervated skin, (2) improve the reliability of the flare measurement over previously reported methods, (3) identify neuropeptide depletion as a prolonged flare latency response and (4) is applicable to a number of different study paradigms through use of a calibration site to establish a baseline. These results suggest that neurovascular dysfunction due to small fiber neuropathy can be assessed by LDI analysis of the neurogenic axon-reflex flare area response.
We used a standardized nerve fiber injury paradigm to test our flare area analysis method. This model causes a reproducible nociceptive C-fiber injury with near complete loss of IENFD for 2 weeks, some limited regeneration over 1 month and gradual return to baseline levels over 2–3 months (Gibbons et al., 2010b; Polydefkis et al., 2004). This model allows us to test the different flare analysis methods in a known small fiber neuropathy. The skin biopsies taken on day 17 confirmed that the reduction in IENFD was consistent with our prior report (Gibbons et al., 2010b). Our flare area method measured a diminished flare size on all testing days after capsaicin application, confirming that the technique is reliable in detecting a difference in groups of individuals with small fiber neuropathy. In contrast, the baseline perfusion method and the resting blood flow method both measured a decrease in flare size on most, but not all testing days.
We also investigated the individual and group intra-class correlation coefficients in the placebo treated regions. The placebo treated skin should theoretically provide similar results on each testing day, although environmental and other factors may play a role in individual responses. As seen in Figure 2, the flare size in the placebo treated region was stable using our flare area method, but did fluctuate more widely in the baseline perfusion and resting blood flow methods. These results visually confirm the higher intra-class correlation coefficients noted using the flare area method. Our results demonstrate an improvement in the consistency of group data over time compared to other methods. However, the individuals ICC’s were not sufficient for clinical use where diagnosis and monitoring of individual responses over time is of critical importance. Therefore, further refinement of this technique is necessary before this can be considered a clinical tool.
We did note that flare latency was transiently longer in the capsaicin-treated, but not placebo-treated regions. Capsaicin causes depletion of neuropeptides from nerve terminals and is the likely explanation for the transient delay in flare latency. In this study, flare latency did not correlate in any way with the reduced intra-epidermal nerve fiber density. Therefore, flare latency may prove useful in studies of neuropeptide depletion or novel therapeutic agents that alter neuropeptide function, but it is unclear if there is any utility in the study of small fiber neuropathy.
There are several limitations to our study. 1) Some subjects had a flare area with borders that exceeded our scanned region, thus underestimating the decrease in flare area after capsaicin treatment. Larger regions of scanning require significantly longer time per image, and therefore reduce the discriminatory capacity of the flare latency and may miss the period of maximal flare response. Further study is necessary to determine the relative balance between scanning area and frequency. 2) The perfusion threshold will change depending on scanned body area, distance between scanner head and skin and other testing parameters. Our study design allowed ‘calibration’ in the tested region prior to capsaicin application, a scenario not applicable to patients with neuropathy. We recommend calibration on an unaffected body part to determine perfusion thresholding values in studies of neuropathy. 3) The LDI technique is a very sensitive method prone to confounding environmental factors. Preparation should include regular laser calibration, controlled room humidity, temperature and lighting conditions and proper patient preparation (enough time to accommodate to the environment, identifying and avoiding areas of skin irritation, avoidance of movement artifacts). We detected an increase in resting blood flow in all subjects on day 17 that we believe was secondary to anxiety about an impending skin biopsy. This highlights the susceptibility of laser-Doppler studies to external factors. 4) For hypothesis testing purposes, we used the capsaicin model of small fiber neuropathy to avoid confounding parameters from an underlying disease and baseline physiologic differences in cutaneous blood flow. This allowed us to define the effect of a small fiber neuropathy with the change in flare area. Further study in patients with disease specific neuropathies is required to determine the utility of our technique.
In conclusion, the LDI axon-reflex flare area assessment may be a useful endpoint for the detection of neuropathic changes in vasomotor nerve function. Our flare area method offers improved reproducibility and sensitivity over previously reported methods, but further investigation is necessary to confirm this finding in patients with neuropathy.
Acknowledgments
The present study was supported by NIH K23 NS050209 (CHG) and the Langer Family Foundation.
Footnotes
Disclosures:
This study was funded by NIH grant K23 NS050209 and the Langer Family Foundation. Dr. Illigens reports no disclosures. Dr. Siepmann is funded by German Research Foundation grant Si 1589/1-1. Mr. Roofeh reports no disclosures. Dr. Gibbons is funded by NIH grant K23 NS050209.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Benarroch EE, Low PA. The acetylcholine-induced flare response in evaluation of small fiber dysfunction. Ann Neurol. 1991;29:590–595. doi: 10.1002/ana.410290604. [DOI] [PubMed] [Google Scholar]
- Berghoff M, Kilo S, Hilz MJ, Freeman R. Differential impairment of the sudomotor and nociceptor axon-reflex in diabetic peripheral neuropathy. Muscle Nerve. 2006 doi: 10.1002/mus.20497. [DOI] [PubMed] [Google Scholar]
- Bickel A, Kramer HH, Hilz MJ, Birklein F, Neundorfer B, Schmelz M. Assessment of the neurogenic flare reaction in small-fiber neuropathies. Neurology. 2002;59:917–919. doi: 10.1212/wnl.59.6.917. [DOI] [PubMed] [Google Scholar]
- Caselli A, Spallone V, Marfia GA, Battista C, Pachatz C, Veves A, Uccioli L. Validation of the nerve axon reflex for the assessment of small nerve fibre dysfunction. J Neurol Neurosurg Psychiatry. 2006;77:927–932. doi: 10.1136/jnnp.2005.069609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Freeman R. Autonomic peripheral neuropathy. Lancet. 2005;365:1259–1270. doi: 10.1016/S0140-6736(05)74815-7. [DOI] [PubMed] [Google Scholar]
- Gibbons CH, Freeman R, Veves A. Diabetic neuropathy: a cross-sectional study of the relationships among tests of neurophysiology. Diabetes Care. 2010a;33:2629–2634. doi: 10.2337/dc10-0763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gibbons CH, Griffin JW, Polydefkis M, Bonyhay I, Brown A, Hauer PE, McArthur JC. The utility of skin biopsy for prediction of progression in suspected small fiber neuropathy. Neurology. 2006;66:256–258. doi: 10.1212/01.wnl.0000194314.86486.a2. [DOI] [PubMed] [Google Scholar]
- Gibbons CH, Wang N, Freeman R. Capsaicin induces degeneration of cutaneous autonomic nerve fibers. Ann Neurol. 2010b;68:888–898. doi: 10.1002/ana.22126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Green AQ, Krishnan ST, Rayman G. C-fiber function assessed by the laser doppler imager flare technique and acetylcholine iontophoresis. Muscle Nerve. 2009;40:985–991. doi: 10.1002/mus.21333. [DOI] [PubMed] [Google Scholar]
- Kramer HH, Schmelz M, Birklein F, Bickel A. Electrically stimulated axon reflexes are diminished in diabetic small fiber neuropathies. Diabetes. 2004;53:769–774. doi: 10.2337/diabetes.53.3.769. [DOI] [PubMed] [Google Scholar]
- Krishnan ST, Rayman G. The LDIflare: a novel test of C-fiber function demonstrates early neuropathy in type 2 diabetes. Diabetes Care. 2004;27:2930–2935. doi: 10.2337/diacare.27.12.2930. [DOI] [PubMed] [Google Scholar]
- Lauria G, Hsieh ST, Johansson O, Kennedy WR, Leger JM, Mellgren SI, Nolano M, Merkies IS, Polydefkis M, Smith AG, Sommer C, Valls-Sole J. European Federation of Neurological Societies/Peripheral Nerve Society Guideline on the use of skin biopsy in the diagnosis of small fiber neuropathy. Report of a joint task force of the European Federation of Neurological Societies and the Peripheral Nerve Society. Eur J Neurol. 2010;17:903–909. doi: 10.1111/j.1468-1331.2010.03023.x. [DOI] [PubMed] [Google Scholar]
- Parkhouse N, Le Quesne PM. Impaired neurogenic vascular response in patients with diabetes and neuropathic foot lesions. N Engl J Med. 1988;318:1306–1309. doi: 10.1056/NEJM198805193182005. [DOI] [PubMed] [Google Scholar]
- Polydefkis M, Hauer P, Sheth S, Sirdofsky M, Griffin JW, McArthur JC. The time course of epidermal nerve fibre regeneration: studies in normal controls and in people with diabetes, with and without neuropathy. Brain. 2004;127:1606–1615. doi: 10.1093/brain/awh175. [DOI] [PubMed] [Google Scholar]
- Tavee J, Zhou L. Small fiber neuropathy: A burning problem. Cleve Clin J Med. 2009;76:297–305. doi: 10.3949/ccjm.76a.08070. [DOI] [PubMed] [Google Scholar]