Abstract
In obstructive sleep apnea (OSA) patients, inspiratory activation (IA) of lingual muscles protects the upper airway from collapse. We aimed to determine when rats’ lingual muscles exhibit IA. In 5 Sprague-Dawley and 3 Wistar rats, we monitored cortical EEG and lingual, diaphragmatic and nuchal electromyograms (EMGs), and identified segments of records when lingual EMG exhibited IA. Individual segments lasted 2.4–269 s (median: 14.5 s), most (89%) occurred during slow-wave sleep (SWS), and they collectively occupied 0.3–6.1% of the total recording time. IA usually started to increase with a delay after SWS onset and ended with an arousal, or declined prior to rapid eye movement sleep. IA of lingual EMG was not accompanied by increased diaphragmatic activity or respiratory rate changes, but occurred when cortical EEG power was particularly low in a low beta-1 frequency range (12.5–16.4 Hz). A deep SWS-related activation of upper airway muscles may be an endogenous phenomenon designed to protect the upper airway against collapse.
Keywords: EEG power, genioglossus, hypoglossal motoneurons, sleep, sleep spindles, obstructive sleep apnea, upper airway
1. INTRODUCTION
In obstructive sleep apnea syndrome (OSA), recurrent hypoventilations and apneas occur during sleep as a result of a narrowing of the airway in the oropharyngeal region. Flow limitations and upper airway obstructions are caused by sleep-related decrements of upper airway muscle tone (Sauerland and Harper, 1976; Remmers et al., 1978).
When compared to healthy subjects, OSA patients have increased pharyngeal muscle activity during wakefulness (W), and a portion of this increase is carried over into slow-wave sleep (SWS) (Suratt et al., 1988; Mezzanotte et al., 1992; Henke, 1998). However, the augmentation only partially compensates for altered upper airway anatomy, with often precipitous decrements of upper airway muscle tone at the onset of SWS (Hendricks et al., 1993; Okabe et al., 1994; Mezzanotte et al., 1996; Katz and White, 2003; Wilkinson et al., 2008; reviewed by Kubin and Davies, 2002). Thus, the level and pattern of activity in upper airway muscles during W and the pattern of its changes with sleep depend on the propensity of the upper airway to collapse. Activity of the genioglossus, a major extrinsic muscle of the tongue and an important upper airway dilator innervated by the hypoglossal (XII) motor nucleus, is low or absent and has no, or minimal, respiratory modulation during quiet W in subjects with fully patent upper airway. In contrast, OSA patients typically exhibit some tonic activity with additional inspiratory activation. This difference is apparent when one compares healthy children with children who have compromised upper airway (Katz and White, 2003, 2004).
Inspiratory activation is of particular interest for studies of upper airway control across sleep-wake states because of its role to oppose the negative (collapsing) pressure in the upper airway during inspiration. In humans with sub-clinical propensity for sleep-disordered breathing and OSA patients, inspiratory activation of upper airway muscles occurs spontaneously under the baseline conditions and, if absent, can be elicited by experimental maneuvers that increase airway resistance, or by chemical stimulation of breathing (e.g., Horner et al., 1991; Pillar et al., 2000; Stanchina et al., 2002). In rats, a species that is widely used to study the mechanisms of sleep-wake and respiratory control, inspiratory modulation of upper airway muscle tone can be experimentally augmented by vagotomy (Taguchi et al., 1992; see Kubin and Davies, 1995 for a review), increased chemical drive for breathing (e.g., Hwang et al., 1983; Peever et al., 2001; Horner et al., 2002; Bailey et al., 2005), or microinjections of various excitatory compounds into the XII nucleus (e.g., Jelev et al., 2001; Morrison et al., 2003; Stettner et al., 2012). However, in contrast to humans, rodents have fully patent upper airway and can maintain adequate breathing across sleep-wake states.
We previously reported that, in normal rats who have no need for elevated tonic or phasic inspiratory activation of upper airway muscles, lingual electromyographic activity (EMG) is lowest during SWS (nearly atonic) and then gradually increases during rapid eye movement sleep (REMS) due to the appearance of progressively larger and more frequent, non-respiratory phasic twitches (Lu et al., 2005; Rukhadze et al., 2011). In another study, we conducted limited observations of inspiratory activation of lingual muscles across sleep-wake states, and noted that inspiratory modulation rarely occurred in lingual muscles of freely behaving rats (Lu and Kubin, 2009). However, the observations were based on a total of 8 h of recordings obtained from 4 animals, which was insufficient to determine which conditions favored the appearance of inspiratory activation of the muscles of the tongue. The goal of our present study was to systematically examine the incidence of respiratory modulation (RM) of lingual EMG in chronically instrumented, freely behaving rats across sleep-wake states, and identify the conditions under which RM of lingual EMG is most likely to occur. In two rat strains, Sprague-Dawley and Wistar, we found that RM occurs mainly during deep SWS, rather than during W, REMS, or less deep SWS. We propose that deep SWS-related inspiratory activation of lingual EMG represents a central phenomenon designed to protect the upper airway from collapse (Rukhadze et al., 2011).
2. MATERIAL AND METHODS
Experiments were conducted on 8 adult male rats obtained from Charles River Laboratories (Wilmington, MA). Their mean body weight was 363 g ±9 (SE) on the day of instrumentation, and about 450 g on the day when data were collected for this report. Five animals were Sprague-Dawley rats and 3 were Wistars. After instrumentation, the animals were housed individually under a 12 h light (7:00–19:00)/12 h dark cycle with freely available standard rodent chow diet (5001/AIN76, Nestle Purina, St. Louis, MO) and water. All animal procedures followed the American Physiological Society’s Guiding Principles in the Care and Use of Vertebrate Animals in Research and Training and were approved by the Institutional Animal Care and Use Committee of the University of Pennsylvania.
2.1. Instrumentation procedures
Prior to instrumentation, the animals were given atropine (0.04 mg/kg, i.m.) and were anesthetized with ketamine (60 mg/kg, i.m.) and xylazine (7.5 mg/kg, i.m.) followed by isoflurane administered through a nose mask (0.5–0.8%). They were instrumented for recording of cortical EEG and lingual, nuchal and diaphragmatic electromyograms (EMGs). For lingual EMG, we used 10-stranded, stainless steel, Teflon-coated wires (catalog number AS636; Cooner Wire, Chatsworth, CA) with tips exposed over a 0.5 mm distance. For the other muscles, we used a 64-stranded wire (AS631; Cooner Wire). One or two wires were inserted into the tongue close to its base, two into the sternal diaphragm, and two were sewn onto the dorsal neck muscles using the approach described previously (Lu et al., 2005; Lu et al., 2009). All leads were attached to a mini-socket (220–9 ABS Plug, Ginder Scientific, Ottawa, Canada) that was subsequently attached to the skull with dental acrylic and all skin openings were tightly sutured. At the conclusion of surgery, the animals were given gentamicin (5 mg/kg, i.m.) and an analgesic (Metacam, 2 mg/kg, p.o.), and their recovery was periodically observed for at least two subsequent days.
2.2. Habituation and recording procedures
On days 6–11 after instrumentation, the animals were placed in a ventilated, dimly illuminated and sound-attenuated recording chamber for 1–7 h/day on at least 4 separate days. On at least 3 of those days, the animals were connected to the recording amplifiers to habituate them to the recording procedures and establish the optimal recording conditions. The data for this report were derived from one recording from each of the 8 rats obtained from a 4–7 h-long session conducted between 9:30 am and 4:30 pm on day 12–27 after instrumentation (mean: 19.4 ±1.6 days). The nuchal and diaphragmatic EMGs were recorded differentially between the two wires implanted into the corresponding muscles. Lingual EMG was recorded between the wire implanted into the tongue and a reference electrode on the parietal bone. The cortical EEG, lingual, nuchal and diaphragmatic EMGs were amplified using Grass amplifiers with a bandwidth of 0.3–100 Hz for the EEG and 10–3,000 Hz for the EMGs. The gains were set to obtain maximal amplification without saturation of the A–D converters. All signals were continuously monitored and digitally stored using a sampling rate of 100 Hz and 1000/2000 Hz, respectively (Power-1401 and Spike-2 v.7 data acquisition hardware and software; Cambridge Electronics Design, Cambridge, UK). The animals were left undisturbed during the recordings.
2.3. Signal processing and scoring of sleep-wake states
Cortical EEG was band pass filtered at 0.75–40 Hz. To eliminate state-dependent EEG signal from lingual EMGs, the latter was high pass-filtered at 125 Hz. The same filtering was applied to nuchal EMG, whereas diaphragmatic EMG was high-pass filtered at 175 Hz to minimize the ECG signal that is usually picked up by electrodes placed in the diaphragm. Filtered signals were displayed and behavioral states were scored using sleep-scoring software (Somnologica; Medcare, Buffalo, NY). Three behavioral states, W, SWS and REMS, were distinguished in successive 10 s epochs based on the appearance of the cortical EEG and nuchal EMG and simultaneous display of the EEG power spectrum within the scored interval. To reduce overestimation of EMG measurements during SWS or REMS by often large bursts of activity associated with awakenings, the epochs in which awakenings occurred were scored as SWS or REMS only when these states occupied at least 75% of the duration of the epoch; all other epochs were scored according to the state that occupied more than 50% of the epoch. After the initial scoring, the root mean squares (RMSs) of nuchal and lingual EMGs were calculated for each scoring interval and the measurements were exported to a spreadsheet together with concurrently determined cortical EEG powers in selected bands (delta-2: 0.75–2.0 Hz; delta-1: 2.0–4.5 Hz; theta: 5.5–8.0 Hz; alpha: 8.0–13.5 Hz; beta-1: 13.5–20 Hz and beta-2: 20–25 Hz). Two procedures were then used to verify the correctness of behavioral scoring and, if needed, make appropriate adjustments. First, the hypnogram was plotted together with the cortical EEG delta-1 power and beta-2/delta-2 power ratio and RMS values for nuchal EMG because these measures assume characteristic levels in the three distinguished behavioral states. Second, logarithmic scatter plots displaying the RMS of nuchal and lingual EMGs relative to the beta-2/delta-2 power ratio were examined because these plots yield a characteristic clustering of data scored as W, SWS and REMS (Lu and Kubin, 2009). For all animals, the first hour of recording was excluded from analysis to allow the animal to settle down after it was connected to the recoding system.
2.4. Data analysis
For quantification of lingual EMGs using RMS, we defined the recording baseline (signal level corresponding to electrical noise only) as the lowest RMS value among all 10 s-long scoring epochs within the entire record (cf., Lu et al., 2005; Lu and Kubin, 2009). This value was then subtracted from all EMG levels measured within each data set. Subsequently, the data were sorted by behavioral state, the mean RMS levels of the lingual and nuchal EMGs were calculated for all epochs scored as W, and the mean activity during W was used to normalize EMG levels during SWS and REMS.
To identify the segments of records in which lingual EMG exhibited respiratory modulation (RM), lingual and diaphragmatic EMGs were full-wave rectified and integrated with 0.1 s time constant. The integrated lingual and diaphragmatic records were systematically reviewed in 30 s increments using the maximum gain appropriate for each displayed segment. Lingual EMG was deemed to exhibit RM when its integrated amplitude changed in synchrony with integrated diaphragmatic activity for at least 5 successive respiratory cycles and there was a constant phase relationship between the two signals. Subsequently, the times of onset and offset of RM were combined with separately generated information about changes in sleep-wake states derived from behavioral scoring conducted as described in the preceding section. Although the RM segments, as defined here, could include periods when the two signals were out of phase (i.e., expiratory modulation of lingual EMG), in practice, the expiratory-modulated segments were very few and short, and occurred mainly during active W or REMS.
At the conclusion of the last recording session, the animals were decapitated under deep barbiturate anesthesia (Nembutal; 100 mg/kg, i.p.), the head was fixed in 10% formalin, and the tongue was sliced sagittally under microscopic observation to verify the location of the tips of the recording wires. The locations of the recording sites were then plotted onto a standard, sagittal cross-section of the tongue stained with Neutral red.
Following verification that the variables were normally distributed, within-subject (paired) Student’s t-tests were used to compare EMGs levels quantified as their RMS between SWS and REMS. The same approach was also applied to respiratory rate and mean integrated EMGs measured over behaviorally comparable periods with and without RM of lingual activity (Section 3.3). The variability of the means is characterized by the standard error (SE).
3. RESULTS
3.1. Lingual EMG recording sites
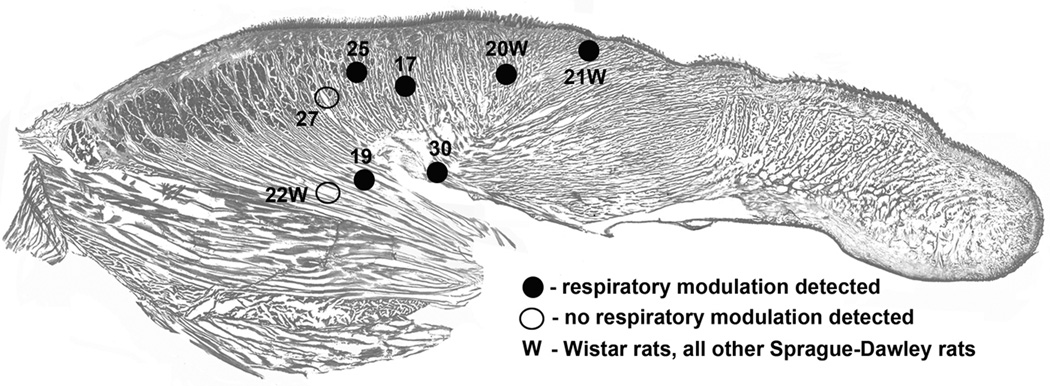
Figure 1 shows the distribution of the sites from which lingual EMG was recorded in the 8 rats used in this study. Bare tips of the recording wires were recovered post-mortem by gradually slicing formalin-fixed tongues in the sagittal plane. The sites are superimposed onto a standard sagittal cross-section of the rat’s tongue. All recordings were obtained from relatively proximal regions of the tongue, with some sites found in the area dominated by longitudinal fibers of the genioglossus muscle (rats 19 and 22W), and some located more dorsally and more anterior where genioglossal fibers are intermixed with intrinsic muscles. Segments of records during which lingual EMG exhibited RM were found in records from 6 rats (4 out of 5 Sprague-Dawley rats and 2 out of 3 Wistar rats), whereas in two rats no RM modulation of lingual EMG was present. There was no obvious relationship between the location of the recording sites and the presence or absence of RM of lingual EMG (see also Table 1).
Figure 1.
Distribution of the recording sites within the tongue, as recovered at the conclusion of each study and superimposed on a standard sagittal section of the rat tongue. Different symbols indicate whether respiratory modulation of lingual EMG was, or was not, detected in a given animal and numbers identify different rats and rat strains, as also listed in Table 1.
Table 1.
Subject-by-subject characteristics of the recording sessions from which data were collected for this report. When present, respiratory modulation (RM) of lingual EMG was mainly bound to the inspiratory phase of the respiratory cycle and occurred primarily during slow-wave sleep (SWS), whereas the segments of records with RM of lingual EMG were extremely rare during wakefulness (W) or rapid eye movement sleep (REMS).
| Subject ID* |
Days after instrumen- tation |
Record duration [h] |
% of time spent in different sleep-wake states |
Nuchal EMG level [% of mean in W] |
Lingual EMG level [% of mean in W] |
Total duration of lingual EMG with RM [s] |
Number of lingual EMG segments with RM |
% of recording time with RM of lingual EMG |
% of RM segment duration that occurred during SWS |
||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| W | SWS | REMS | SWS | REMS | SWS | REMS | |||||||
| 17 | 21 | 3.5 | 48 | 41 | 11 | 7.7 | 3 | 2.5 | 29 | 403 | 30 | 3.2 | 87 |
| 19 | 22 | 3 | 13 | 62 | 25 | 7.8 | 8.5 | 4.8 | 36.2 | 213 | 10 | 2 | 80 |
| 25 | 27 | 6 | 31 | 48 | 21 | 10.4 | 15.8 | 3.1 | 40.3 | 704 | 16 | 3.3 | 100 |
| 27 | 15 | 4.8 | 25 | 56 | 19 | 23.7 | 4.4 | 3.1 | 34.5 | 0 | 0 | 0 | --- |
| 30 | 22 | 3.5 | 44 | 40 | 16 | 21 | 5.5 | 2.2 | 13.8 | 40 | 7 | 0.3 | 41 |
| 20W | 12 | 5 | 27 | 55 | 18 | 6.4 | 6.6 | 8.9 | 28.2 | 1099 | 30 | 6.1 | 95 |
| 21W | 16 | 5 | 20 | 58 | 22 | 8.2 | 9.2 | 6.5 | 23.1 | 1016 | 21 | 5.6 | 100 |
| 22W | 20 | 5 | 27 | 60 | 13 | 34.3 | 10.7 | 10.1 | 20.5 | 0 | 0 | 0 | --- |
| Mean ±SE |
19.4 ±1.6 |
4.5 ±0.4 |
29 ±4 |
53 ±5 |
18 ±2 |
15 ±4 |
8 ±1 |
5 ±1 |
28# ±3 |
434 ±160 |
14 ±4 |
2.6 ±0.9 |
84 ±9 |
W at the end of subject identifier indicates Wistar rats, all others are Sprague-Dawley rats;
p=0.0003 when compared to SWS.
3.2. Distribution of behavioral states and incidence of respiratory modulation of lingual EMG
Table 1 provides information about recording durations, percentage amounts of sleep-wake states, mean levels of lingual and nuchal EMGs in different sleep states, and the amounts of time when RM of lingual EMG was present on the subject-by-subject basis. All recordings were obtained after a thorough habituation of the animals to the recording conditions, with the average period between instrumentation and the recording day on which data were collected for this report being 19.4 days. The average distribution of sleep-wake states was typical of recordings conducted during the rest period, with SWS representing the prevailing state (53% of the recording time). Similar to our previous reports (Lu et al., 2005; Lu and Kubin, 2009; Rukhadze et al., 2011), the average lingual EMG level quantified as RMS of lingual EMG during all scoring epochs classified as SWS was 5% of the mean during W. This was significantly less than the average level of lingual EMG during REMS (28%), when frequent and large twitches emerge from the otherwise atonic muscle.
For the 6 rats which had segments of records with RM of lingual EMG, the cumulative duration of all such segments ranged in individual rats from 40 to 1099 s, which corresponded to 0.3–6.1% of the total recording time. The overwhelming majority of RM segments coincided with the periods of SWS, with the average percentage of time with RM of lingual EMG occurring during SWS relative to the total time with RM being 84% (Table 1).
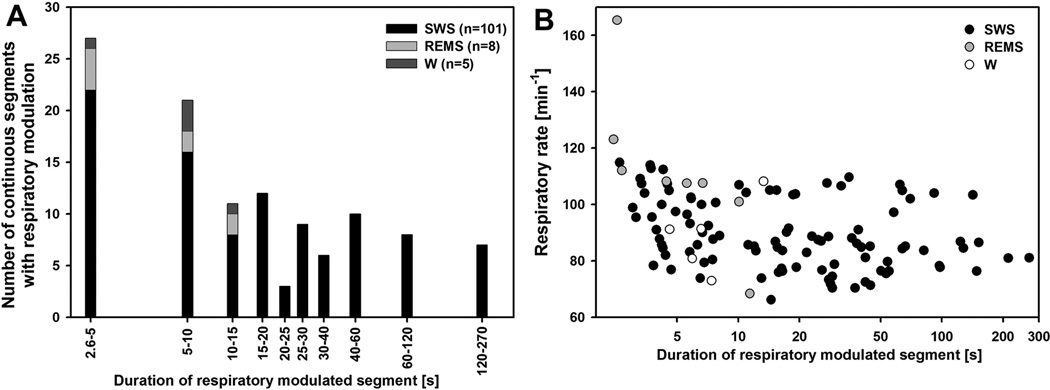
Figure 2A shows the distribution of the durations of RM segments. The distribution is exponential, with a median of 14.5 s. Of note here is that all RM segments that occurred in states other than SWS were located in the lower half of the histogram, whereas all segments longer than 15 s (a total of 57, with the longest one lasting 269 s) occurred exclusively during SWS. The great majority of the short segments were also characterized by low, or very low, amplitude of RM of lingual EMG, but nevertheless they met our criterion of a constant phase relationship relative to diaphragmatic activity over at least 5 successive respiratory cycles (see Methods). In contrast, some of the segments longer than 15 s included periods with remarkably large amplitude of RM of lingual EMG (Figs. 3 and 4). Common to all RM segments occurring during SWS was that they usually started with a considerable delay after SWS onset (25–75% interquartile range: 30–135 s). In the case of the short segments, RM of lingual EMG often emerged and then disappeared several times within the same SWS episode, without ever reaching large amplitude. In contrast, for most long segments, the amplitude of RM gradually increased with the duration of the SWS episode until the process was abruptly terminated by a sigh or arousal. If the SWS episode with a long segment of RM of lingual EMG progressed into REMS, the amplitude of RM declined during the pre-REMS period (Fig. 3).
Figure 2.
Distribution of the durations of separate segments of records in which lingual EMG exhibited respiratory modulation (RM), and the relationship between the duration of respiratorymodulated segments and respiratory rate. A: most segments of records in which RM of lingual EMG was present, and especially all those longer than 15 s, occurred during slow-wave sleep (SWS). B: with the exception of some very short segments of records, including a few in which RM of lingual EMG occurred during states other than SWS, there was no distinct relationship between RM segment duration and respiratory rate. For segments longer than 15 s, there was no relationship between the duration of the segment and respiratory rate, but data points diverged into two distinct levels due to a generally slower respiratory rate during SWS in Wistar rats than in Sprague-Dawley rats.
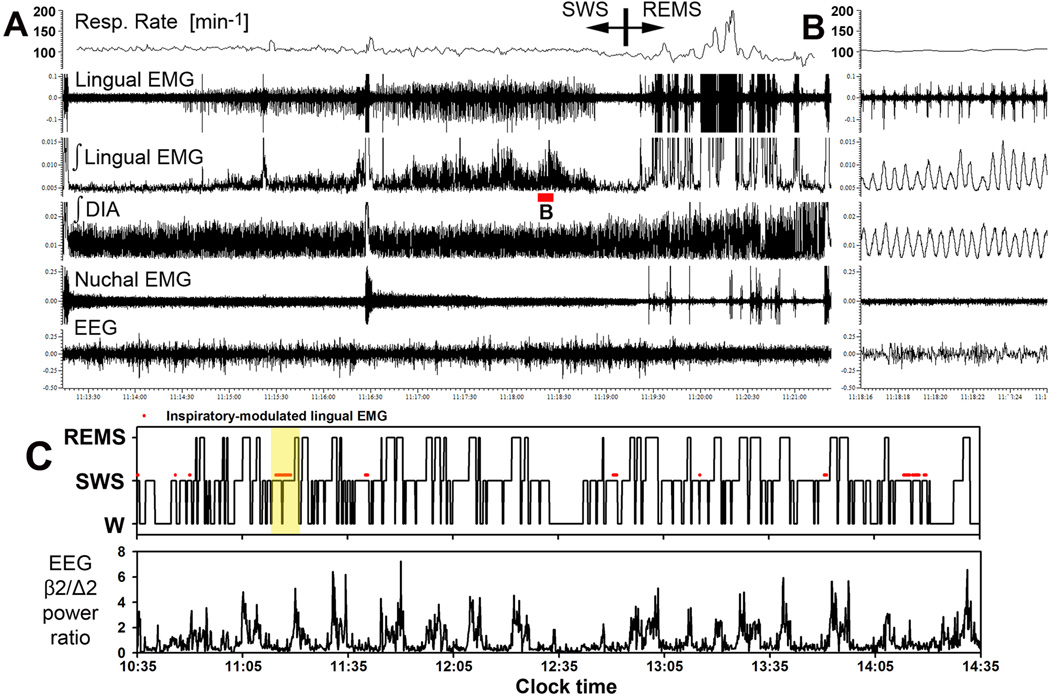
Figure 3.
Example of a record encompassing one full sleep cycle in which prominent inspiratory modulation of lingual EMG occurred during SWS. A: compressed record spanning 12 min and 10 s, with inspiratory activity appearing in lingual EMG with a delay following the onset of SWS and then growing in amplitude (Sprague-Dawley rat 27 in Fig. 1 and Table1). The respiratory rate trace represents the mean respiratory rate in successive 3 s intervals. Respiratory modulation (RM) of lingual EMG characteristically declines and ultimately disappears during pre-REMS period. B: expanded portion of the record in A corresponding to the part of the record marked by red bar under the integrated lingual EMG trace in A. The raw lingual EMG trace shows that multiple motor units contributed to the total inspiratory activity. Lingual EMG shows considerable breath-to-breath variability, whereas integrated diaphragmatic (DIA) activity is quite regular. C: hypnogram for the first 4 h of the recording from this rat, and the associated changes in beta-2/delta-2 ratio of powers in cortical EEG. Yellow shading shows the part of the record illustrated in A. Red marks above the level in the hypnogram corresponding to SWS show when RM of lingual EMG occurred in this recording session (no RM was present in the subsequent 2 h -- not illustrated). Segments of record with RM of lingual EMG are bound to the occurrence of SWS.
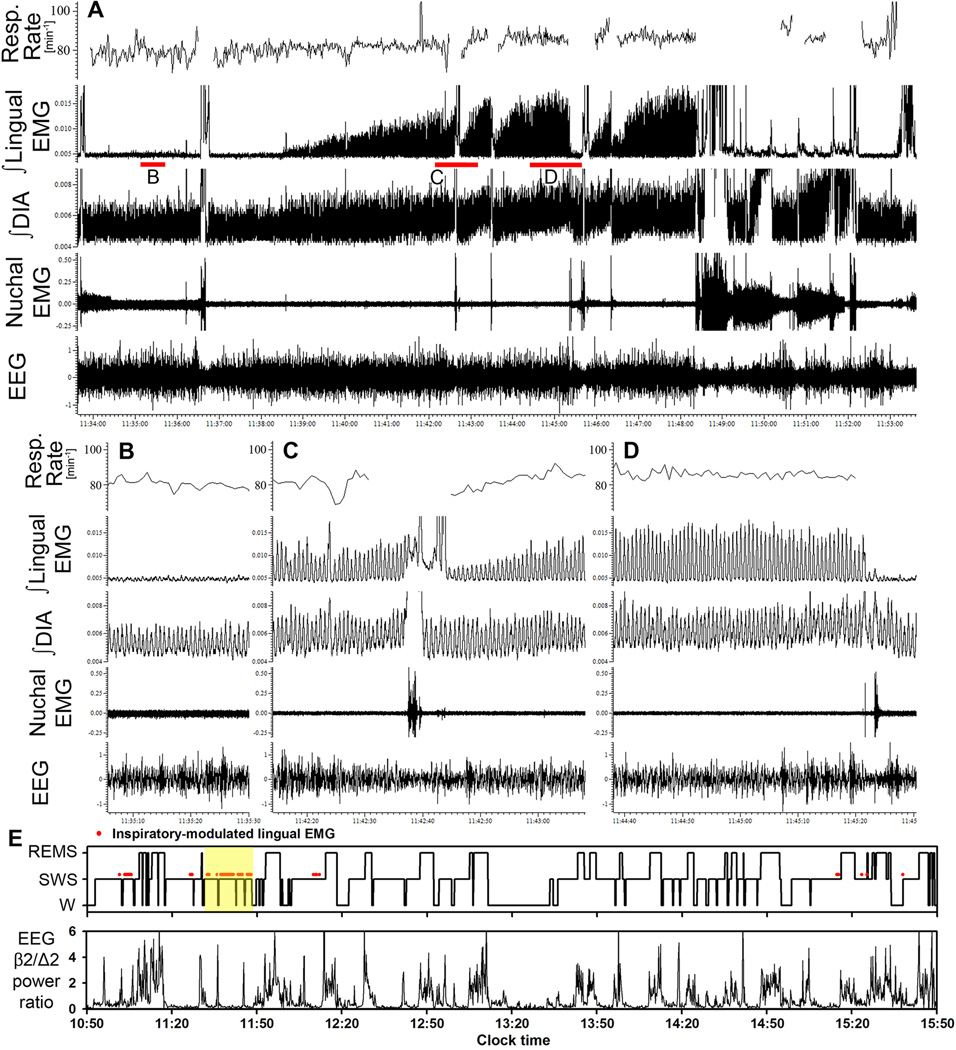
Figure 4.
Example of a 20 min-long segment of record containing several intervals during which inspiratory modulation appeared in lingual EMG. A: compressed record, with the position of its expanded portions illustrated in B–D indicated by red bars under the integrated lingual EMG trace. The respiratory rate trace represents the mean respiratory rate in successive 3 s intervals, with parts of the trace blanked out when accurate measurements could not be derived from integrated diaphragmatic (DIA) EMG due to contamination by postural activity. E: hypnogram for the entire recording session with this rat (Wistar rat 21W in Fig. 1 and Table 1), and the associated changes in beta-2/delta-2 ratio of powers in cortical EEG, shown using the same format as in Fig. 3C. Yellow shading shows the part of the record illustrated in A. See text for additional comments on the characteristic features of inspiratory modulation of lingual EMG illustrated by this record.
Figure 2B shows the relationship between the respiratory rate and duration of the segments of records with RM of lingual EMG. Within the lower half of the range of segment durations (less than 15 s), the plot suggests an inverse relationship but for longer durations the relationship is flat. Of note here is that many of the segments shorter than 15 s were identified during states other than SWS and mainly these contribute to the impression of an inverse relationship between the segment duration and respiratory rate. For the RM segments longer than 15 s, the distribution points to two distinct levels of respiratory rate. This is caused by generally higher respiratory rates during SWS in Sprague-Dawley rats than in Wistar rats. Other than that, there was no relationship between respiratory rate and segment duration. Although we initially hypothesized that slower respiratory rate would favor the appearance of RM in lingual EMG by allowing more time during inspiration for such a modulation to develop, the flat relationship in Fig. 2B suggested a rather weak dependence of RM in lingual EMG on the activity in the brainstem respiratory rhythm-generating network.
Figures 3 and 4 illustrate two examples of some of the most prominent cases of RM of lingual EMG found in the present study. The recording in Fig. 3, lasting 12 min and 10 s, shows one sleep cycle that starts from a brief arousal followed by an entry into SWS and then into REMS (Sprague-Dawley rat 25 in Fig. 1 and Table 1). The characteristic features of RM of lingual EMG illustrated in this record include: the gradual development of RM in lingual EMG that begins with a delay after SWS onset, the transient loss of RM during a brief arousal, and the decline of RM just before the entry into REMS. Of note is also that neither the respiratory rate nor the amplitude of inspiratory activity of the diaphragm change during the period when the amplitude of RM of lingual EMG gradually increases. Thus, as with the data shown in Fig. 2B, this record suggests that RM of lingual EMG occurs and develops independently of changes in activity of the central respiratory network. It is also of note that RM of lingual EMG entirely disappears during REMS, and it is replaced by large muscle twitches that occur independently of the respiratory rhythm, as we documented previously (Lu and Kubin, 2009). Panel C of Fig. 3 shows the hypnogram for the recording from this rat together with the time course of the ratio of EEG powers in beta-2 and delta-2 frequencies (the ratio is one of the indices that we use as an aid in behavioral scoring of sleep-wake states; it peaks during REMS and brief arousals and maintains very low level during SWS). The red lines and dots placed above the lines corresponding to SWS in the hypnogram mark the periods when RM of lingual EMG occurred (in an additional 2 h of this recording that are not illustrated, there was no RM of lingual EMG). The intermittent appearance of RM segments and their preferential occurrence during SWS corroborate the quantitative data for this rat listed in Table 1.
Figure 4 presents another example of a 20 min-long segment of recording from a Wistar rat (rat 21W in Fig. 1 and Table 1) that illustrates some of the features of RM of lingual EMG similar to those illustrated for the Sprague-Dawley rat of Fig. 3. Additionally, the record includes a period of short and low-amplitude RM that occurred during the first SWS episode that preceded subsequent SWS periods in which RM of lingual EMG was very prominent. The record ends with a full awakening, after which another segment with RM of lingual EMG did not occur for at least another 30 min (see hypnogram in panel E). As in the example shown in Fig. 3, RM of lingual EMG develops gradually and is interrupted by transient arousals. In addition, in this particular case, the tonic activity recorded from the diaphragmatic leads tends to increase together with the increase of RM of lingual EMG, whereas the respiratory modulation of diaphragmatic activity and the respiratory rate show minimal changes. Although such a concurrent increase of postural activity and RM of lingual activity is not typical of most of our records (e.g., absent in the example shown in Fig. 3), this case suggests that postural activity and the transmission of inspiratory drive to XII motoneurons can be influenced by a common mechanism.
In the example shown in Fig. 4, the peak magnitude of RM of lingual EMG was as high as 15% of the maximal lingual EMG amplitude measured during W using the same metrics. For the record illustrated in Fig. 3, the peak amplitude of RM of lingual EMG represented 4.5% of the maximal amplitude encountered anywhere in that entire recording session. These, however, are some of the most prominent examples. Due to the low frequency of occurrence of periods with RM of lingual EMG (Figs. 3C and 4E), and the amplitude of RM being usually lower than in these two examples, the intermittent presence of inspiratory activity in lingual EMG had small impact on the average level of lingual EMG during SWS, which corresponded on the average to only 5% of the mean level of activity during W (Table 1).
3.3. Behavioral state, as characterized by spectral analysis of cortical EEG, is a significant determinant of the occurrence of RM of lingual EMG
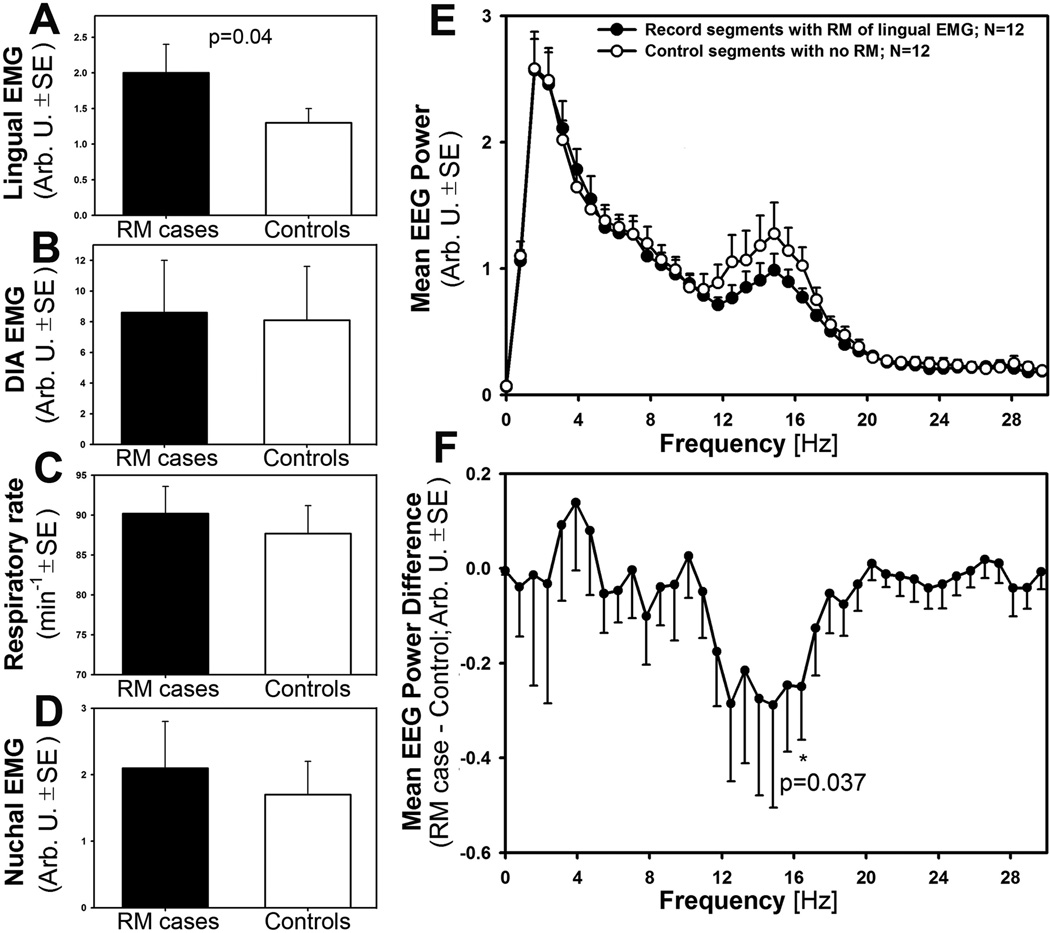
To identify possible determinants of the appearance of RM in lingual EMG, we subjected our records to a “case-control” analysis in which segments of records with prominent and lasting longer than 20 s RM of lingual EMG were compared to adjacent, matching segments of records from the same animal in which no RM was present. The analysis was limited to SWS because only SWS segments were of sufficient duration to allow for accurate and representative measurements. Our criteria for matching the “RM cases” with their “Control” segments included: (1) identical durations of the compared segments and the same onset times for the “RM case” and “Control” segment relative to the onset of their corresponding SWS episodes; (2) the interval between the “RM case” and “Control” segment of less than ±1 h; (3) similar durations and patterns of sleep-wake activity around the “RM case” and “Control” segments (i.e., the “RM case” segments that ended with arousal were matched with “Control” segments in which the total duration of SWS was similar and the segment also ended with arousal; when the “RM case” segment ended with an entry into REMS, it was matched with a “Control” segment that also ended with REMS). With all these restraining criteria, we could select for this analysis 12 “case-control” pairs of recordings derived from 5 rats (3 Sprague-Dawleys and 2 Wistars). The average duration of these segments was 51 ±3 s (range: 20–60 s). We then derived the following parameters from the matched pairs of the selected segments of records: mean amplitude of rectified and integrated lingual EMG relative to baseline, mean amplitude of respiratory modulation of rectified and integrated diaphragmatic activity, respiratory rate, mean amplitude of nuchal EMG relative to baseline, and EEG power spectrum across the entire segments calculated within 0–50 Hz range divided into 128 frequency intervals (Hanning filter). All measures were then compared pairwise between the matching segments of records using arbitrary units, or min−1 for the respiratory rate.
Figure 5 summarizes the results of our “case-control” analysis. The amplitude of lingual EMG (panel A) was significantly larger in the RM segments. This, albeit it may seem an expected outcome given that the “RM cases” included RM of lingual EMG whereas the “Controls” did not, also indicates that RM emerged from very low level of baseline activity rather than being replaced in the “Control” segments by elevated levels of tonic activity. The amplitude of respiratory modulation of diaphragmatic activity (panel B) was only marginally larger in the RM segments (not significant) and so were the respiratory rate (panel C) and tonic nuchal activity (panel D). In contrast, a significant difference emerged from the comparison of the EEG power spectra between “RM cases” and “Controls.” Panel E shows the average “RM case” and “Control” EEG power spectra in which a visible difference distinctly occurred in the frequency range from 11.7 to 16.4 Hz. The difference was significant at 16.4 Hz when compared pairwise within each frequency bin (p=0.037; panel F), and when EEG power was calculated for a larger frequency range spanning from 12.5 to 16.4 Hz. This range represents the low end of beta-1 frequencies (or an intermediate band between high sigma and low beta frequencies), and the direction of the difference is such that the records in which RM of lingual EMG occurred contained lower EEG power in this band than the records in which RM of lingual EMG was absent. Thus, the presence of RM was associated with reduced activation of cortical EEG in this particular frequency range.
Figure 5.
The “case-control” analysis revealed that RM of lingual EMG is associated with deep SWS, as indicated by decreased cortical EEG power in a low beta-1 frequency band. In panels A–E, measures of lingual and diaphragmatic EMG, respiratory rate, postural tone, and EEG power are compared pairwise between adjacent episodes of SWS in which RM of lingual EMG occurred (RM cases) or was absent (Controls). As expected, lingual EMG amplitude was significantly higher in RM cases than in Controls (A), but other measures such as the amplitude of inspiratory modulation of diaphragmatic EMG (B), respiratory rate (C) and postural tone measured from nuchal EMG (D) did not differ. In contrast, the power spectra of cortical EEG differed between RM cases and Controls within one distinct frequency band (E). This band, 12.5–16.7 Hz, is designated as a low beta-1 or sigma in rats. F: expanded plot of the mean cortical EEG power difference between RM cases and Controls, with significant differences present at 16.7 Hz and across the entire 12.5–16.7 Hz range.
4. DISCUSSION
Our main finding is that, in both Sprague-Dawley and Wistar rats, RM of lingual EMG occurs intermittently and relatively infrequently, but its appearance is not random. Rather, SWS is the state when RM of lingual EMG is most likely to occur, and especially when the EEG power in a low beta-1 frequency range is particularly low. On the other hand, our measurements and observations do not point to changes in central respiratory drive, as measured by the amplitude of diaphragmatic activity or respiratory rate, as major determinants of the intermittent occurrence of RM of lingual EMG. Collectively, our data lead us to postulate that transmission of inspiratory input to XII motoneurons is facilitated during deep SWS, identified as SWS periods with diminished power of beta-1 frequency in cortical EEG. As such, the appearance of RM in lingual EMG is a state-dependent phenomenon that may be endogenously designed to support airway patency during deep sleep. In rodents, the functional significance of this mechanism may be negligible, because rodents do not experience sleep-related upper airway obstructive events. However, such an endogenous mechanism may have an important facilitatory role in OSA patients who need inspiratory activation of lingual muscles during sleep in order to maintain adequate ventilation.
The intermittent appearance of RM of lingual EMG is puzzling and could be taken to suggest that movements of the recording wires in the tongue, combined with scarcity of lingual motor units with inspiratory-modulated activity could explain the phenomenon. This, however, we find very unlikely for several reasons. First, our recordings were obtained at a relatively long time after instrumentation (19 days on the average), thus allowing the implants to firmly settle within the tissue. In fact, when the tongues were dissected to recover the location of the tips of the recording wires, we verified that there was no evidence of inflammation around the recording sites, and the wires were tightly embedded in the muscle, with no evidence of their movement against the surrounding tissue. Second, most periods when lingual EMG exhibited RM coincided with SWS, they usually started with a delay from the SWS onset, and the amplitude of RM often exhibited a distinctly increasing pattern with the duration of the SWS episode. Thus, RM of lingual EMG, albeit not frequent, did not occur at random. Rather, it was associated primarily with just one behavioral state (SWS) and, within this state, followed a characteristic pattern of onset and gradual increase. Importantly, since both the tongue and the entire animal were motionless when RM started and then increased in amplitude, movements of the recording wires within the tongue cannot explain the progressive increase of RM of lingual EMG. Third, when RM of lingual EMG occurred, inspection of the raw lingual EMG usually revealed that it originated in multiple distinct motor units that were gradually recruited and then gradually increased their inspiratory-modulated activity. Thus, the appearance of RM was not a phenomenon caused by a sudden appearance and then disappearance of activity generated in just one motor unit.
We consistently observed that RM of lingual EMG was transiently suppressed following brief arousals, swallows or sighs that involved movements of the tongue, whereas overt awakenings, with or without prior transition into REMS, often suppressed the occurrence of RM for prolonged periods lasting several sleep-wake cycles. The latter could suggest that the appearance of RM of lingual EMG was associated with certain positions of the head and body during SWS that may vary from one consolidated period of sleep to another. Indeed, Megirian et al. (1985) reported that inspiratory modulation of lingual EMG is increased in rats when they sleep in a fully curled up position. They also noted that the magnitude of RM of lingual EMG varies during SWS from non-existent to very prominent, at least when rats sleep in less curled up positions, but no quantitative data were provided for the relative incidence of RM of lingual EMG either during SWS or W. Nevertheless, the observations conducted during SWS have led these authors to suggest that the emergence of RM of lingual EMG is driven by the need to maintain adequate patency of the upper airway. However, in our study, the appearance of RM of lingual EMG during SWS was not associated with any evidence of respiratory problems, as indicated by insignificant changes in respiratory rate or respiratory modulation of diaphragmatic activity. Rather, in most instances, these measures remained constant while the amplitude of RM of lingual EMG gradually increased. This makes it unlikely that increased airway resistance and flow limitation reflexly elicited RM of lingual EMG during some SWS episodes.
Possibly relevant to our present observations are earlier data from paralyzed and artificially ventilated cats showing that a transient electrical stimulation of the superior laryngeal nerve can cause a prolonged augmentation of respiratory modulation of XII motoneurons (Jiang et al., 1991). In our studies of this phenomenon, we additionally noted that a brief electrical stimulation of the lingual nerve could immediately re-set the prolonged augmentation elicited by prior stimulation of the superior laryngeal nerve (Fig. 6 in Kubin and Davies, 2002). These findings, obtained from tightly controlled acute experiments, suggest that stimulation of certain afferent pathways in relevant cranial sensory nerves can induce sudden and prolonged changes in the level of inspiratory activity recorded from XII motoneurons. Accordingly, it is conceivable that, in chronically instrumented rats, natural behaviors such a swallows, sighs or major changes in head and neck position may elicit prolonged changes in the excitability of XII motoneurons and influence the efficiency of transmission of inspiratory input to these motoneurons.
Rats exhibit no propensity for sleep-related airway obstructions. Therefore, it is not surprising that their lingual EMG rarely exhibits inspiratory-modulation under the baseline conditions, or that it has minimal or no tonic activity during SWS. In contrast, human subjects often have some tonic and some respiratory-modulated activity in the muscles of the tongue, even when they do not present with clinically significant symptoms of sleep-disordered breathing. The reasons for more frequent occurrence of RM of lingual EMG in humans probably include the distinct anatomy of the hyoid bone and particular patterns of the fat and soft tissue distributions in the pharyngeal region in humans when compared to rodents. In humans, the hyoid bone is relatively mobile, which allows the upper airway muscles that use it as an attachment (e.g., hyoglossus, geniohyoid) to shift with changes in airway pressure and body position. In contrast, in rats, hyoid bone is rigidly connected to the trachea, which stabilizes the position of upper airway walls and airway patency. Increased fat accumulation in upper airway walls is a known factor exacerbating the propensity for OSA in humans (Schotland et al., 1999; O'Donnell et al., 2000; Kirkness et al., 2008), and obese rodents have less negative upper airway critical pressure and narrower upper airway than lean control animals (Nakano et al., 2001; Tagaito et al., 2001; Brennick et al., 2009). However, in contrast to humans, obesity does not lead to sleep-related upper airway obstructions in rodents. Indeed, unlike humans whose upper airway muscle tone increases with increasing body mass index, genetically obese rats do not exhibit any obvious elevation of lingual EMG during W or sleep (Sood et al., 2007). Thus, the differences between the sleep-wake patterns of lingual EMG between humans and rodents can be explained on the grounds of different upper airway anatomies and the associated difference in the propensity for flow limitation in a passive upper airway.
Inspiration-related and tonic activations of lingual EMG are of particular clinical interest because they stiffen and protrude the tongue, thereby preventing its collapse into the pharyngeal lumen (Fuller et al., 1999). In individuals with compromised upper airway, retraction of the tongue increases upper airway resistance and may result in airway occlusion (Remmers et al., 1978; Brouillette and Thach, 1979). Consequently, these two components of lingual activity have been extensively analyzed. Some tonic activity, and a degree of inspiratory modulation, are frequently present in records from the genioglossus, as well as other pharyngeal muscles, during quiet W and SWS in humans (e.g., Sauerland and Harper, 1976; Mezzanotte et al., 1996; Henke, 1998). However, in chronically instrumented, behaving rats, the proportion of XII motoneurons with tonic activity during quiet W is low, and phasic activity occurs primarily in association with distinct ingestive behaviors (Travers and Jackson 1992). These findings are consistent with our quantitative data from rats obtained in the absence of any experimental manipulations that could affect the normal levels and patterns of lingual EMG.
Our case-control analysis revealed that the occurrence of RM of lingual EMG was associated with a decline of low beta-1 frequency in cortical EEG (Fig. 5E and F). Cortical beta frequencies are most commonly studied in the awake brain, because they are enhanced by sensory stimulation, during attentive states, and in association with sensorimotor integration (Wróbel et al., 2007; Baker, 2008). However, beta frequencies also occur during sleep, and exhibit distinct changes with sleep stages and sleep pathology. During non-REMS, cortical activity in this frequency band, designated as sigma in humans and either sigma or beta-1 in rats, is associated with sleep spindles, and it is increased in patients with insomnia (Uchida et al., 1994; Sanacora et al., 2000; Spiegelhalder et al., 2012). In rats, EEG power in a low beta frequency range (11–16 Hz) is higher during SWS when the episode is more likely to end with a transition into REMS than into awakening (Capitani et al., 2005). Collectively, these data associate beta-1 frequency with a relatively activated cortex and, conversely, a reduced power in this frequency range is consistent with the occurrence of deep SWS. Accordingly, we interpret the association between a decline in cortical beta-1 frequency and the appearance of RM in lingual EMG as indicative of a facilitatory effect of deep SWS on transmission of inspiratory drive to XII motoneurons.
Increased RM of upper airway muscle tone (genioglossus and geniohyoid) during SWS has been previously reported in humans (Basner et al., 1991; Tangel et al., 1992; Shea et al., 1999; McSharry et al., 2013). While Basner et al. (1991) found no increase of pharyngeal resistance associated with transitions from non-REMS stage N2 to SWS, several other studies in humans suggested a trend towards an increased resistance (Wiegand et al., 1990; Trinder et al., 1997; Pillar et al., 2000). Consequently, SWS-related increases of upper airway muscle tone tend to be attributed to a reflex activation of upper airway muscles initiated by an increase in upper airway resistance and reduced ventilation, although a recent study in healthy humans also offered an alternative suggestion that they are driven by a central respiratory activation (McSharry et al., 2013). Possibly relevant to our findings is the report by Ondze et al. (2003) that mild OSA patients (who have a higher level of upper airway muscle tone during sleep than healthy subjects) exhibit reduced EEG power in the sigma range, and a lower spindle activity, during SWS than healthy persons. Furthermore, the observation that some OSA patients can resume stable breathing if they do not wake up as a result of an initial decline of upper airway muscle tone at the onset of non-REMS and the concomitant flow limitation has been the basis to suggest that a suppression of the arousal threshold can offer an effective means of preserving sleep continuity in selected individuals with OSA (Younes, 2004). Collectively, these reports support the notion that deep SWS with reduced cortical EEG power in a low beta/sigma range is associated with increased inspiratory activation of the upper airway.
Our data are consistent with the observations in humans that RM of lingual EMG can increase during SWS, but our analysis leads us to suggest that the increase is, at least in part, facilitated by a central endogenous sleep mechanism. Specifically, our finding that RM increased during SWS without evidence of an increased activation of the central respiratory network, as inferred from the absence of changes in respiratory rate or inspiratory activation of the diaphragm, but in association with de-activation of cortical EEG, leads us to postulate that the state of deep SWS itself facilitates transmission of inspiratory input to XII motoneurons. The source of this input has been localized in rats to inspiratory-modulated neurons scattered in the reticular formation ventrolateral to the XII motor nucleus (Woch et al., 2000; Peever et al., 2002; Chamberlin et al., 2007; Koizumi et al., 2008). Importantly, at least two studies on anesthetized rats support our suggestion that transmission of inspiratory input to XII motoneurons is statedependent and facilitated during SWS-like state when compared to other states. In one study, inspiratory activation of medullary XII premotor inspiratory neurons was unchanged or increased during pharmacologically-induced REMS-like state while inspiratory activation of XII motoneurons was reduced (Woch et al., 2000). In another study, RM of lingual EMG periodically increased in association with spontaneously occurring de-activation (slowing) of cortical EEG and declined when cortical EEG was activated with a REMS-like or less deep SWS-like pattern (Pagliardini et al., 2012). Thus, we propose that the facilitation of RM of lingual EMG during deep SWS is an endogenous process that has evolved to maintain the patency of the upper airway at times when there is a potential for collapse. We further suggest that an additional reflex inspiratory activation of upper airway muscles can be superimposed onto this endogenous process under those conditions, and in those species, in which sleep leads to a functionally significant airway narrowing and flow limitation.
The population of rats included in our study comprised 5 Sprague-Dawley and 3 Wistar rats. In both strains, the key features of RM of lingual EMG, such as its association with deep SWS and its gradual growth with time within the SWS episodes, were similar. In the 2 Wistar rats in which RM occurred, the percentage of total recording time when RM was present tended to be higher than in any of the Sprague-Dawley rats. However, the third Wistar rat had no RM of lingual EMG and in 2 of the 5 Sprague-Dawley rats RM was entirely absent or minimal. It is possible that the slower respiratory rate during SWS of Wistar than Sprague-Dawley rats presents more favorable conditions for inspiratory input to “break through” to XII motoneurons, but more animals from these two strains and examination of additional strains will be needed to establish whether there are significant strain differences in the expression of RM of lingual EMG.
In summary, our data from two rat strains demonstrate that inspiratory activation of lingual muscles is a rare phenomenon, but when it occurs, it is closely associated with deep SWS. As such, the inspiratory activation appears to represent an endogenous and state-dependent mechanism designed for protection of the upper airway against airway collapse that is most likely to occur during deep SWS.
HIGHLIGHTS.
-
-
Inspiratory activation (IA) of upper airway muscles protects the airway from collapse.
-
-
IA of upper airway muscle tone is rare in rats, and its determinants are unknown.
-
-
In Sprague-Dawley and Wistar rats, IA occurs mainly during deep slow-wave sleep.
-
-
State-dependence of IA suggests that it is a central, airway-protecting mechanism.
Acknowledgments
The study was supported by grants HL-092962 and HL-047600 from the National Institutes of Health and a research fellowship from the Deutsche Forschungsgemeinschaft (DFG Ste1899/1-1) to GMS.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- Bailey EF, Janssen PL, Fregosi RF. PO2-dependent changes in intrinsic and extrinsic tongue muscle activities in the rat. Am. J. Respir. Crit. Care Med. 2005;171:1403–1407. doi: 10.1164/rccm.200411-1550OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baker SN. Oscillatory interactions between sensorimotor cortex and the periphery. Cur. Op. Neurobiol. 2007;17:649–655. doi: 10.1016/j.conb.2008.01.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Basner RC, Ringler J, Schwartzstein RM, Weinberger SE, Weiss JW. Phasic electromyographic activity of the genioglossus increases in normals during slow-wave sleep. Respir. Physiol. 1991;83:189–200. doi: 10.1016/0034-5687(91)90028-h. [DOI] [PubMed] [Google Scholar]
- Brennick MJ, Pack AI, Ko K, Kim E, Pickup S, Maislin G, Schwab RJ. Altered upper airway and soft tissue structures in the New Zealand obese mouse. Am. J. Respir. Crit. Care Med. 2009;179:158–169. doi: 10.1164/rccm.200809-1435OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brouillette RT, Thach BT. A neuromuscular mechanism maintaining extrathoracic airway patency. J. Appl. Physiol. 1979;46:772–779. doi: 10.1152/jappl.1979.46.4.772. [DOI] [PubMed] [Google Scholar]
- Capitani P, Cerri M, Amici R, Baracchi F, Jones CA, Luppi M, Perez E, Parmeggiani PL, Zamboni G. Changes in EEG activity and hypothalamic temperature as indices for non-REM sleep to REM sleep transitions. Neurosci. Lett. 2005;383:182–187. doi: 10.1016/j.neulet.2005.04.009. [DOI] [PubMed] [Google Scholar]
- Chamberlin NL, Eikermann M, Fassbender P, White DP, Malhotra A. Genioglossus premotoneurons and the negative pressure reflex in rats. J. Physiol. (Lond.) 2007;579:515–526. doi: 10.1113/jphysiol.2006.121889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fuller DD, Williams JS, Janssen PL, Fregosi RF. Effect of co-activation of tongue protrudor and retractor muscles on tongue movements and pharyngeal airflow mechanics in the rat. J. Physiol. (Lond.) 1999;519:601–613. doi: 10.1111/j.1469-7793.1999.0601m.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hendricks JC, Petrof BJ, Panckeri K, Pack AI. Upper airway dilating muscle hyperactivity during non-rapid eye movement sleep in English bulldogs. Am. Rev. Respir. Dis. 1993;148:185–194. doi: 10.1164/ajrccm/148.1.185. [DOI] [PubMed] [Google Scholar]
- Henke KG. Upper airway muscle activity and upper airway resistance in young adults during sleep. J. Appl. Physiol. 1998;84:486–491. doi: 10.1152/jappl.1998.84.2.486. [DOI] [PubMed] [Google Scholar]
- Horner RL, Innes JA, Murphy K, Guz A. Evidence for reflex upper airway dilator muscle activation by sudden negative airway pressure in man. J. Physiol. (Lond.) 1991;436:15–29. doi: 10.1113/jphysiol.1991.sp018536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horner RL, Liu X, Gill H, Nolan P, Liu H, Sood S. Effects of sleep-wake state on the genioglossus vs.diaphragm muscle response to CO2 in rats. J. Appl. Physiol. 2002;92:878–887. doi: 10.1152/japplphysiol.00855.2001. [DOI] [PubMed] [Google Scholar]
- Hwang J-C, Bartlett D, Jr, St John WM. Characterization of respiratory-modulated activities of hypoglossal motoneurons. J. Appl. Physiol. 1983;55:793–798. doi: 10.1152/jappl.1983.55.3.793. [DOI] [PubMed] [Google Scholar]
- Jelev A, Sood S, Liu H, Nolan P, Horner RL. Microdialysis perfusion of 5-HT into hypoglossal motor nucleus differentially modulates genioglossus activity across natural sleep-wake states in rats. J. Physiol. (Lond.) 2001;532:467–481. doi: 10.1111/j.1469-7793.2001.0467f.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiang C, Mitchell GS, Lipski J. Prolonged augmentation of respiratory discharge in hypoglossal motoneurons following superior laryngeal nerve stimulation. Brain Res. 1991;538:215–225. doi: 10.1016/0006-8993(91)90433-v. [DOI] [PubMed] [Google Scholar]
- Katz ES, White DP. Genioglossus activity in children with obstructive sleep apnea during wakefulness and sleep onset. Am. J. Respir. Crit. Care Med. 2003;168:664–670. doi: 10.1164/rccm.200301-092OC. [DOI] [PubMed] [Google Scholar]
- Katz ES, White DP. Genioglossus activity during sleep in normal control subjects and children with obstructive sleep apnea. Am. J. Respir. Crit. Care Med. 2004;170:553–560. doi: 10.1164/rccm.200403-262OC. [DOI] [PubMed] [Google Scholar]
- Kirkness JP, Schwartz AR, Schneider H, Punjabi NM, Maly JJ, Laffan AM, McGinley BM, Magnuson T, Schweitzer M, Smith PL, Patil SP. Contribution of male sex, age, and obesity to mechanical instability of the upper airway during sleep. J. Appl. Physiol. 2008;104:1618–1624. doi: 10.1152/japplphysiol.00045.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koizumi H, Wilson CG, Wong S, Yamanishi T, Koshiya N, Smith JC. Functional imaging, spatial reconstruction, and biophysical analysis of a respiratory motor circuit isolated in vitro . J. Neurosci. 2008;28:2353–2365. doi: 10.1523/JNEUROSCI.3553-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kubin L, Davies RO. Central pathways of pulmonary and airway vagal afferents. In: Dempsey JA, Pack AI, editors. Regulation of Breathing. New York: Marcel Dekker; 1995. pp. 219–284. [Google Scholar]
- Kubin L, Davies RO. Mechanisms of airway hypotonia. In: Pack AI, editor. Sleep Apnea. Pathogenesis, Diagnosis, and Treatment. New York: Dekker; 2002. pp. 99–154. [Google Scholar]
- Lu JW, Kubin L. Electromyographic activity at the base and tip of the tongue across sleep-wake states in rats. Respir. Physiol. Neurobiol. 2009;167:307–315. doi: 10.1016/j.resp.2009.06.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu JW, Mann GL, Ross RJ, Morrison AR, Kubin L. Differential effect of sleepwake states on lingual and dorsal neck muscle activity in rats. Respir. Physiol. Neurobiol. 2005;147:191–203. doi: 10.1016/j.resp.2005.05.007. [DOI] [PubMed] [Google Scholar]
- McSharry DG, Saboisky JP, DeYoung P, Matteis P, Jordan AS, Trinder J, Smales E, Hess L, Guo M, Malhotra A. A mechanism for upper airway stability during slow wave sleep. Sleep. 2013;36:555–563. doi: 10.5665/sleep.2544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Megirian D, Hinrichsen CFL, Sherrey JH. Respiratory roles of genioglossus, sternothyroid, and sternohyoid muscles during sleep. Exp. Neurol. 1985;90:118–128. doi: 10.1016/0014-4886(85)90045-7. [DOI] [PubMed] [Google Scholar]
- Mezzanotte WS, Tangel DJ, White DP. Waking genioglossal electromyogram in sleep apnea patients versus normal controls (a neuromuscular compensatory mechanism) J. Clin. Invest. 1992;89:1571–1579. doi: 10.1172/JCI115751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mezzanotte WS, Tangel DJ, White DP. Influence of sleep onset on upper-airway muscle activity in apnea patients versus normal controls. Am. J. Respir. Crit. Care Med. 1996;153:1880–1887. doi: 10.1164/ajrccm.153.6.8665050. [DOI] [PubMed] [Google Scholar]
- Morrison JL, Sood S, Liu H, Park E, Nolan P, Horner RL. GABAA receptor antagonism at the hypoglossal motor nucleus increases genioglossus muscle activity in NREM but not REM sleep. J. Physiol. (Lond.) 2003;548:569–583. doi: 10.1113/jphysiol.2002.033696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakano H, Magalang UJ, Lee SD, Krasney JA, Farkas GA. Serotonergic modulation of ventilation and upper airway stability in obese Zucker rats. Am. J. Respir. Crit. Care Med. 2001;163:1191–1197. doi: 10.1164/ajrccm.163.5.2004230. [DOI] [PubMed] [Google Scholar]
- O'Donnell CP, Schwartz AR, Smith PL. Upper airway collapsibility: the importance of gender and adiposity. Am. J. Respir. Crit. Care Med. 2000;162:1606–1607. doi: 10.1164/ajrccm.162.5.ed11-00b. [DOI] [PubMed] [Google Scholar]
- Okabe S, Hida W, Kikuchi Y, Taguchi O, Takishima T, Shirato K. Upper airway muscle activity during REM and non-REM sleep of patients with obstructive apnea. Chest. 1994;106:767–773. doi: 10.1378/chest.106.3.767. [DOI] [PubMed] [Google Scholar]
- Ondze B, Espa F, Dauvilliers Y, Billiard M, Besset A. Sleep architecture, slow wave activity and sleep spindles in mild sleep disordered breathing. Clin. Neurophysiol. 2003;114:867–874. doi: 10.1016/s1388-2457(02)00389-9. [DOI] [PubMed] [Google Scholar]
- Pagliardini S, Greer JJ, Funk GD, Dickson CT. State-dependent modulation of breathing in urethane-anesthetized rats. J. Neurosci. 2012;32:11259–11270. doi: 10.1523/JNEUROSCI.0948-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peever JH, Mateika JH, Duffin J. Respiratory control of hypoglossal motoneurones in the rat. Pflügers Arch. 2001;442:78–86. doi: 10.1007/s004240000502. [DOI] [PubMed] [Google Scholar]
- Peever JH, Shen L, Duffin J. Respiratory pre-motor control of hypoglossal motoneurons in the rat. Neuroscience. 2002;110:711–722. doi: 10.1016/s0306-4522(01)00594-2. [DOI] [PubMed] [Google Scholar]
- Pillar G, Malhotra A, Fogel RB, Beauregard J, Slamowitz DI, Shea SA, White DP. Upper airway muscle responsiveness to rising PCO2 during NREM sleep. J. Appl. Physiol. 2000;89:1275–1282. doi: 10.1152/jappl.2000.89.4.1275. [DOI] [PubMed] [Google Scholar]
- Remmers JE, DeGroot WJ, Sauerland EK, Anch AM. Pathogenesis of upper airway occlusion during sleep. J. Appl. Physiol. 1978;44:931–938. doi: 10.1152/jappl.1978.44.6.931. [DOI] [PubMed] [Google Scholar]
- Rukhadze I, Kamani H, Kubin L. Quantitative differences among EMG activities of muscles innervated by subpopulations of hypoglossal and upper spinal motoneurons during non-REM sleep - REM sleep transitions: a window on neural processes in the sleeping brain. Arch. Ital. Biol. 2011;149:499–515. doi: 10.4449/aib.v149i4.1385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rukhadze I, Stettner GM, Kubin L. Inspiratory modulation of lingual EMG is rare in rats and occurs predominantly during slow-wave sleep. Sleep. 2011;34(Suppl.):A31. Abstract. [Google Scholar]
- Sanacora G, Mason GF, Krystal JH. Impairment of GABAergic transmission in depression: new insights from neuroimaging studies. Crit. Rev. Neurobiol. 2000;14:23–45. doi: 10.1615/critrevneurobiol.v14.i1.20. [DOI] [PubMed] [Google Scholar]
- Sauerland EK, Harper RM. The human tongue during sleep: electromyographic activity of the genioglossus muscle. Exp. Neurol. 1976;51:160–170. doi: 10.1016/0014-4886(76)90061-3. [DOI] [PubMed] [Google Scholar]
- Schotland HM, Insko EK, Schwab RJ. Quantitative magnetic resonance imaging demonstrates alterations of the lingual musculature in obstructive sleep apnea. Sleep. 1999;22:605–613. doi: 10.1093/sleep/22.5.605. [DOI] [PubMed] [Google Scholar]
- Shea SA, Edwards JK, White DP. Effect of wake-sleep transitions and rapid eye movement sleep on pharyngeal muscle response to negative pressure in humans. J. Physiol. (Lond.) 1999;520:897–908. doi: 10.1111/j.1469-7793.1999.00897.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sood S, Liu X, Liu H, Horner RL. Genioglossus muscle activity and serotonergic modulation of hypoglossal motor output in obese Zucker rats. J. Appl. Physiol. 2007;102:2240–2250. doi: 10.1152/japplphysiol.01229.2006. [DOI] [PubMed] [Google Scholar]
- Spiegelhalder K, Regen W, Feige B, Holz J, Piosczyk H, Baglioni C, Riemann D, Nissen C. Increased EEG sigma and beta power during NREM sleep in primary insomnia. Biol. Psychol. 2012;91:329–333. doi: 10.1016/j.biopsycho.2012.08.009. [DOI] [PubMed] [Google Scholar]
- Stanchina ML, Malhotra A, Fogel RB, Ayas N, Edwards JK, Schory K, White DP. Genioglossus muscle responsiveness to chemical and mechanical stimuli during non-rapid eye movement sleep. Am. J. Respir. Crit. Care Med. 2002;165:945–949. doi: 10.1164/ajrccm.165.7.2108076. [DOI] [PubMed] [Google Scholar]
- Stettner GM, Fenik VB, Kubin L. Effect of chronic intermittent hypoxia on noradrenergic activation of hypoglossal motoneurons. J. Appl. Physiol. 2012;112:305–312. doi: 10.1152/japplphysiol.00697.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suratt PM, McTier RF, Wilhoit SC. Upper airway muscle activation is augmented in patients with obstructive sleep apnea compared with that in normal subjects. Am. Rev. Respir. Dis. 1988;137:889–894. doi: 10.1164/ajrccm/137.4.889. [DOI] [PubMed] [Google Scholar]
- Tagaito Y, Polotsky VY, Campen MJ, Wilson JA, Balbir A, Smith PL, Schwartz AR, O'Donnell CP. A model of sleep-disordered breathing in the C57BL/6J mouse. J. Appl. Physiol. 2001;91:2758–2766. doi: 10.1152/jappl.2001.91.6.2758. [DOI] [PubMed] [Google Scholar]
- Taguchi O, Kubin L, Pack AI. Evocation of postural atonia and respiratory depression by pontine carbachol in the decerebrate rat. Brain Res. 1992;595:107–115. doi: 10.1016/0006-8993(92)91458-q. [DOI] [PubMed] [Google Scholar]
- Tangel DJ, Mezzanotte WS, Sandberg EJ, White DP. Influences of NREM sleep on the activity of tonic vs. inspiratory phasic muscles in normal men. J. Appl. Physiol. 1992;73:1058–1066. doi: 10.1152/jappl.1992.73.3.1058. [DOI] [PubMed] [Google Scholar]
- Travers JB, Jackson LM. Hypoglossal neural activity during licking and swallowing in the awake rat. J. Neurophysiol. 1992;67:1171–1184. doi: 10.1152/jn.1992.67.5.1171. [DOI] [PubMed] [Google Scholar]
- Trinder J, Kay A, Kleiman J, Dunai J. Gender differences in airway resistance during sleep. J. Appl. Physiol. 1997;83:1986–1997. doi: 10.1152/jappl.1997.83.6.1986. [DOI] [PubMed] [Google Scholar]
- Uchida S, Maloney T, Feinberg I. Sigma (12–16 Hz) and beta (20–28 Hz) EEG discriminate NREM and REM sleep. Brain Res. 1994;659:243–248. doi: 10.1016/0006-8993(94)90886-9. [DOI] [PubMed] [Google Scholar]
- Wiegand DA, Latz B, Zwillich CW, Wiegand L. Upper airway resistance and geniohyoid muscle activity in normal men during wakefulness and sleep. J. Appl. Physiol. 1990;69:1252–1261. doi: 10.1152/jappl.1990.69.4.1252. [DOI] [PubMed] [Google Scholar]
- Wilkinson V, Malhotra A, Nicholas CL, Worsnop C, Jordan AS, Butler JE, Saboisky JP, Gandevia SC, White DP, Trinder J. Discharge patterns of human genioglossus motor units during sleep onset. Sleep. 2008;31:525–533. doi: 10.1093/sleep/31.4.525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woch G, Ogawa H, Davies RO, Kubin L. Behavior of hypoglossal inspiratory premotor neurons during the carbachol-induced, REM sleep-like suppression of upper airway motoneurons. Exp. Brain Res. 2000;130:508–520. doi: 10.1007/s002219900244. [DOI] [PubMed] [Google Scholar]
- Wróbel A, Ghazaryan A, Bekisz M, Bogdan W, Kaminski J. Two streams of attention-dependent beta activity in the striate recipient zone of cat's lateral posteriorpulvinar complex. J. Neurosci. 2007;27:2230–2240. doi: 10.1523/JNEUROSCI.4004-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Younes M. Role of arousals in the pathogenesis of obstructive sleep apnea. Am. J. Respir. Crit. Care Med. 2004;169:623–633. doi: 10.1164/rccm.200307-1023OC. [DOI] [PubMed] [Google Scholar]