Abstract
We have previously reported that intratumoral injection of VRX-007—an Ad5-based vector overexpressing ADP (Adenovirus Death Protein)—can suppress the growth of subcutaneous HaK (hamster renal cancer) tumors. VRX-007 replication and tumor growth inhibition are enhanced when the hamsters are immunosuppressed by a high dose of cyclophosphamide (CP), an immunosuppressive and chemotherapeutic agent. Here we report that continuous immunosuppression with CP was not required for increased oncolytic activity of VRX-007 because short-term dosing or continuous dosing with the drug yielded similar antitumor results. Prolonged viral replication was found only in animals on continuous CP treatment. We used 007-Luc, a replication-competent, luciferase-expressing vector similar to VRX-007 to investigate the replication of the vector over time. Tumor growth inhibition was similar in hamsters given CP treatment either one week before or one week after 007-Luc injection, which suggests that CP exerts its antitumor efficacy independently of vector therapy. 007-Luc did not spread far from the inoculation site, even in immunosuppressed, CP-treated animals. Our results indicate that the enhanced effectiveness that is produced by the combination of VRX-007 and CP therapies is due to their two independent mechanisms and that they do not have to be given simultaneously for the improved outcome shown.
Keywords: tumor, adenovirus, hamster, cyclophosphamide, virotherapy
Introduction
Oncolytic viral vectors have been widely investigated for cancer gene therapy1. These viruses infect tumor cells, then viral replication and cell lysis release virus progeny to nearby cancer cells, in which the infection process continues. Adenoviruses (Ads) are often used as oncolytic vectors. Ads have a good safety profile, can be easily genetically modified, and can be produced in large stocks. Ad5 (a species C Ad) causes only subclinical or mild respiratory disease in immunocompetent individuals1, 2. Clinical trials have been conducted with many oncolytic Ad vectors. Although these vectors have been well-tolerated, their anticancer efficacy in patients has been limited compared to in vitro and preclinical studies1, 3-5.
We have constructed Ad5-based vectors that lack most of the E3 genes and overexpress ADP (Adenovirus Death Protein, formerly named E3-11.6K)—a key adenoviral protein involved in the lysis of infected cells6-9. One of these vectors, named VRX-0076, 10 (Fig. 1b), is currently being evaluated in a Phase I clinical trial for intratumoral treatment of solid tumors (Protocol #0510-732). This vector has been shown to suppress tumor growth in human xenograft tumors in nude mice10, in cotton rat tumors in cotton rats11, and hamster tumors in Syrian hamsters12-16. Human Ads can infect but do not replicate (or barely replicate) or go into late infection in murine cells and tissues. Because of this limitation, immunodeficient mice bearing human xenograft tumors are regularly used to examine the efficacy of oncolytic Ads. The Syrian (golden) hamster (Mesocricetus auratus) animal model has been established by ourselves and other groups to evaluate oncolytic Ad vectors due to its permissiveness to human Ad replication12, 17-20. With this model, we can study the effects of VRX-007 in immunocompetent animals, which may better mimic the state of human patients treated with virotherapy.
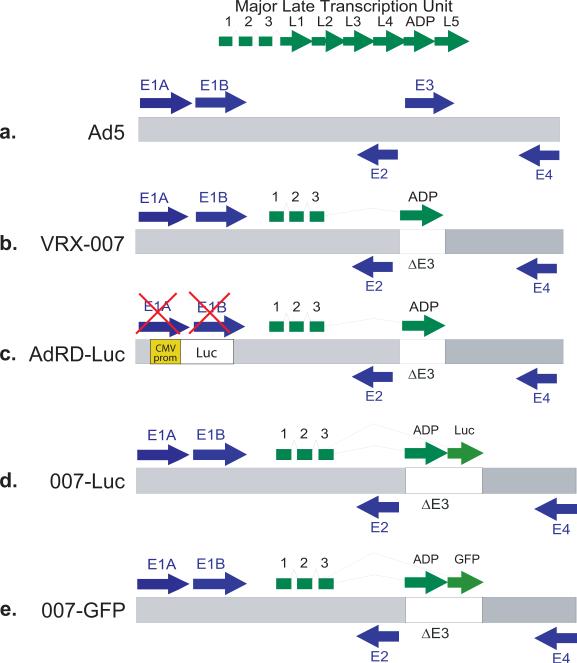
Figure 1. Schematics of the genomes of the vectors used in these studies.
(a) Ad5. The gray bar represents the double-stranded DNA genome, and the arrows represent transcription units. The major late transcription unit is expressed similarly by VRX-007, 007-Luc, and 007-GFP. (b) VRX-007. This vector is identical to Ad5 except that most of the E3 transcription unit is deleted and replaced with the adp gene. (c) AdRD-Luc. This vector is identical to VRX-007 except that the E1 transcription unit is deleted, rendering the virus replication-defective, and the luciferase gene is inserted instead, expressed from a CMV promoter. (d) 007-Luc. This vector is identical to VRX-007, except that the luciferase gene is inserted just downstream to adp. (e) 007-GFP. This vector is identical to VRX-007, except that the gfp gene is inserted just downstream to adp.
Combination therapy is common in cancer gene therapy3-5, 21. Cyclophosphamide (CP) is a chemotherapeutic drug22, 23 that can be used as a broad-spectrum immunosuppressive agent13. CP is administered as an inactive prodrug that is metabolized in the liver to become a phosphorylamide mustard, which is responsible for DNA alkylation and cell death24. Low doses of CP can increase antitumor immunity by reducing the number and activity of immunosuppressive regulatory T cells, resulting in greater potential for the development of an antitumor immune response25. High doses of CP are used for immunosuppression, particularly in transplant recipients26, 27. As an immunosuppressive agent, CP abrogates B cells28, impairs cytokine production, and inhibits T cell activation29. We have studied high dose CP combination treatment with VRX-007 and have reported enhanced antitumor efficacy as compared to CP or VRX-007 treatment alone13, 14; similar results have been reported with CP and other vectors29-33. We have found that intratumorally injected vectors persisted longer in CP-treated animals. Thus, we suggested that immunosuppression induced by CP prevents an antiviral immune response, permitting the virus more time to replicate and lyse tumor cells.
CP offers only transient inhibition of inflammatory cell infiltration, and to maintain immunosuppression, the drug must be given either continuously or with each vector treatment34. We chose to further investigate the role of CP in our hamster model. We found that long-term immunosuppression and the resulting prolonged vector persistence are not required for the enhanced antitumor activity of VRX-007 in HaK (hamster renal cancer) tumors, and that one week only of CP treatment before the vector injection yields similar tumor suppression as does continuous CP treatment. Hamsters dosed with CP for only one week before or one week after vector injection also showed similar tumor growth inhibition, suggesting that the VRX-007 and CP therapies do not need to interact or be present simultaneously for the beneficial effect. Furthermore, prolonged viral replication and intratumoral spread did not appear to determine efficacy.
Materials and Methods
Cell culture
The Syrian hamster cell line HaK (hamster kidney) and the human cell lines HEK293 and A549 were cultured in Dulbecco's modified Eagle's medium (DMEM) (Sigma-Aldrich, St. Louis, MO) with 10% fetal bovine serum (FBS) at 37°C35. The HaK and A549 cell lines were obtained from American Type Culture Collection (ATCC, Manassas, VA), and the HEK293 cell line was purchased from Microbix (Toronto, Ontario, Canada). The SHPC6 (Syrian hamster pancreatic cancer) cell line16 was maintained in DMEM with 15% FBS, 1 mM sodium pyruvate, and 1 mM nonessential amino acids (Invitrogen, Carlsbad, CA).
Viruses
VRX-007 is an replication-competent vector similar to Ad5 except that it lacks most of the E3 region and overexpresses the Adenovirus Death Protein (ADP)6, 8, 36. 007-Luc and 007-GFP are replication-competent viruses identical to VRX-007 except that genes for luciferase or Enhanced Green Fluorescent Protein (GFP) were added downstream of adp. The replication-defective vector AdRD-Luc expresses luciferase from a CMV promoter.
Construction of 007-Luc and 007-GFP
The pL2L1TRAIL plasmid, which contains the full length TRAIL cDNA inserted downstream of the adp gene, was used to construct the shuttle plasmids pL2L1Luc and pL2L1EGFP. The parental pL2L1 plasmid, which consists of sequences of the Ad5 genome from 60-100 map units with the E3 region deleted at XbaI sites (Ad5 bp 29598 to 30469), was used to clone PCR fragments encoding the ADP open reading frame, a linker region (Ad5 bp 29397-29489), and the TRAIL cDNA subsequently into the XbaI site of the deletion. The resulting plasmid pL2L1TRAIL contains TRAIL cDNA located downstream of the adp gene and flanked with unmethylated XbaI sites. The unmethylated XbaI sites were used to replace the TRAIL cDNA with the luciferase or GFP transgenes. To generate the shuttle plasmids pL2L1Luc and pL2L1EGFP, the fragment encoding either luciferase or GFP cDNA was removed from plasmid pGL3 (Promega, Madison, WI) or pEGFP-C2 (Clontech, Mountain View, CA) at NheI/XbaI sites and was used to replace the TRAIL cDNA at unmethylated XbaI sites in pL2L1TRAIL. The resulting plasmid pL2L1Luc or pL2L1EGFP was cotransfected into 293 cells with H5dl327 virion DNA digested with EcoRI/SpeI to make 007-Luc or 007-GFP, respectively. All of the resulting vectors were screened for the expected genome structure by restriction enzyme digestion, and the regions across the inserted genes were sequenced. The vectors were plaque purified three times on 293 cells and titered on A549 cells.
Animals
Female Syrian hamsters (5-6 weeks old) were purchased from either Charles River Laboratories (Wilmington, MA) or Harlan Sprague Dawley (Indianapolis, IN). All of these studies were approved by The Institutional Animal Care and Use Committee of Saint Louis University and were conducted according to federal and institutional regulations.
Cyclophosphamide Treatment
Cyclophosphamide was purchased from Sigma-Aldrich (St, Louis, MO) and was diluted in phosphate buffered saline (PBS) at 20 mg/ml. CP was administered biweekly starting one week prior to the first vector injection13. The first dose was 140 mg/kg, and all subsequent doses were 100 mg/kg. In some experiments, CP was administered for only one week (Figs. 3 and 4).
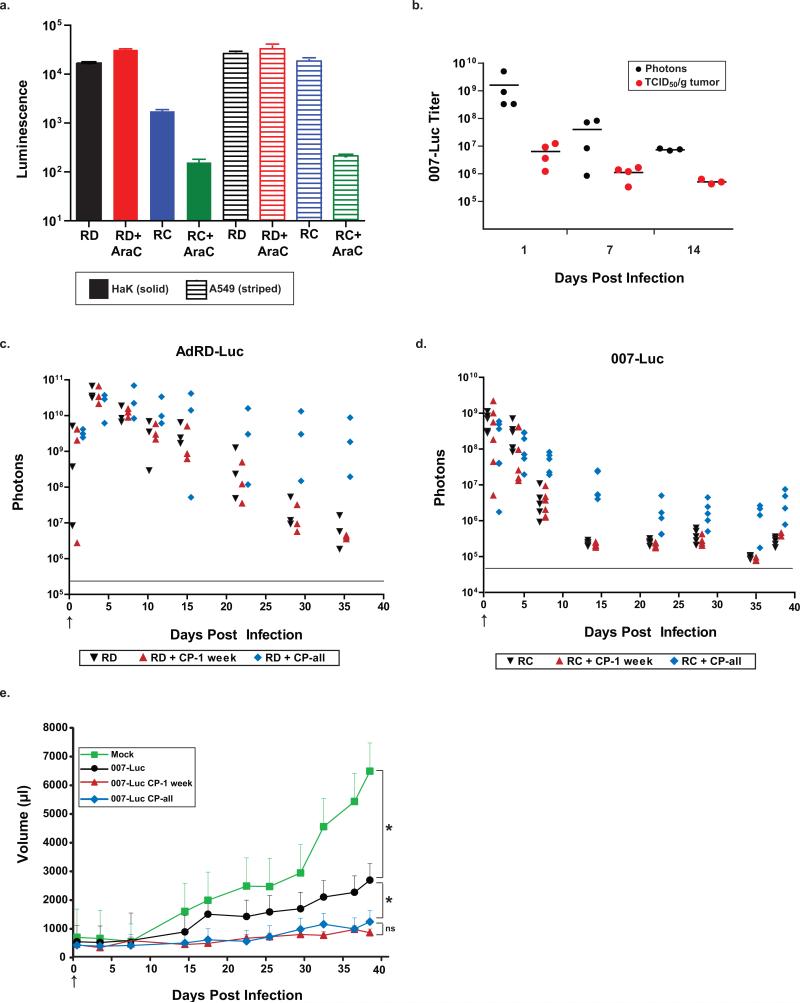
Figure 3. Cyclophosphamide does not increase the infectivity or replication of HaK cells in vivo.
HaK tumors were injected once with 1×1010 PFU of virus or PBS (mock group). The arrows represent days on which the virus was injected. (a) 007-Luc expresses luciferase primarily in the late phase of infection. HaK (solid bars) and A549 (striped bars) cells were grown on 6-well plates and infected with either AdRD-Luc (RD) or 007-Luc (RC) at 25 PFU/cell. AraC was added every 12 h to prevent viral replication. After 54 h post infection, the cells were lysed and luminescence was measured. Error bars represent mean + SD. (b) Comparison between TCID50 and IVIS assays. HaK tumors were injected once with 1×1010 PFU of 007-Luc and imaged via IVIS at days 1, 7, and 14 post infection. Hamsters were sacrificed immediately after imaging and TCID50 assays were performed on the tumors. In panels c and d, luciferase expression in HaK tumors was measured by total flux of photons. The gray lines indicate background intensity. (c) AdRD-Luc replication-defective virus (RD). There were 3 animals per group. (d) 007-Luc replication-competent virus (RC). There were 5-6 animals per group. (e) Mean tumor volume. The number of animals for the 007-Luc group was 5, and the number of animals for all other groups was 4. Error bars represent mean + SE. (d) and (e) represent data from the same set of animals. The tumors from 007-Luc + CP-1 week vs 007-Luc + CP-all groups were never significantly different in size (P>0.35). At 38 days post infection, the tumors from the mock group were significantly larger than all other groups. Animals given virus alone had statistically significant larger tumors than did CP-treated animals starting on day 17. (* P<0.04)
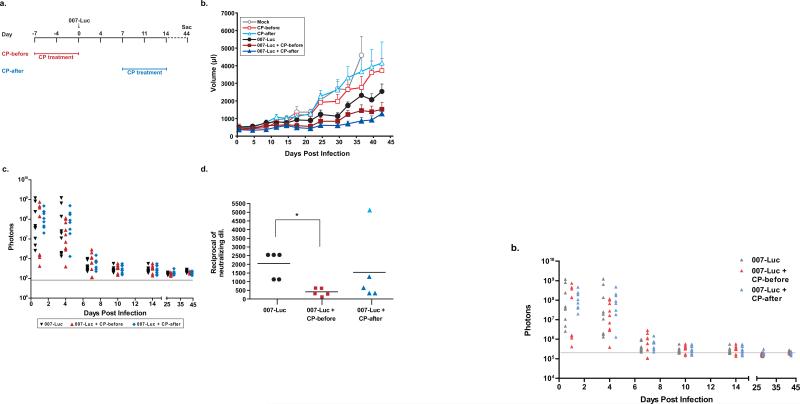
Figure 4. Cyclophosphamide and vector treatments work independently to inhibit tumor growth.
HaK tumors were injected once with 1×1010 PFU of 007-Luc or PBS (mock group). The arrows represent days on which the virus was injected. CP-treated groups were given CP treatment for one week, consisting of 2 doses either one week before infection (CP-before) or one week after infection (CP-after). Animals in the mock group were sacrificed on day 36 due to tumor burden. (a) Timeline for the study. The schedule for the CP-before groups is shown in red, and the schedule for the CP-after groups is shown in blue. (b) Mean tumor volume. Error bars represent mean + SE, and the numbers of animals per group were as follows: 9 (CP-before, CP-after, 007-Luc); 8 (mock, 007-Luc + CP-before); 7 (007-Luc + CP-after). Mean tumor volume in the mock group was significantly greater than in the 007-Luc group starting at day 24 (P=0.04) and in both 007-Luc + CP groups starting at day 18 (P<0.05). The 007-Luc + CP-before and 007-Luc + CP-after groups did not have significantly different tumor sizes (P<0.05). Double therapy produced more tumor suppression than CP treatment alone: CP-before vs 007-Luc + CP-before (P=0.01) and CP-after vs 007-Luc + CP-after (P=0.05). (c) Luciferase expression in tumors, measured by total flux of photons. The gray line indicates the background intensity. (d) Neutralizing antibody titers in the serum at time of death (44 days). *P=0.0079.
Antitumor efficacy
Subcutaneous tumors were formed by injecting 2×107 HaK cells or 1.5×107 SHPC6 cells (in 200 μl PBS) into the right side of the hamsters35. Digital calipers were used to measure the tumor volume (calculated as 0.5 × length × width2) biweekly. When the tumors were 200-300 μl in size, the hamsters were randomized into groups based on tumor size. The tumors were injected with vehicle (PBS), VRX-007, or 007-Luc. The hamsters were sacrificed and the organs and tumors were harvested at the designated time points. Animals were euthanized when tumor volume became greater than 10,000 μl. In the tumor growth studies presented in Figs. 2-4, the experiment had to be terminated at the days shown because of ethical considerations (e.g. tumors became ulcerated, the animals were anemic).
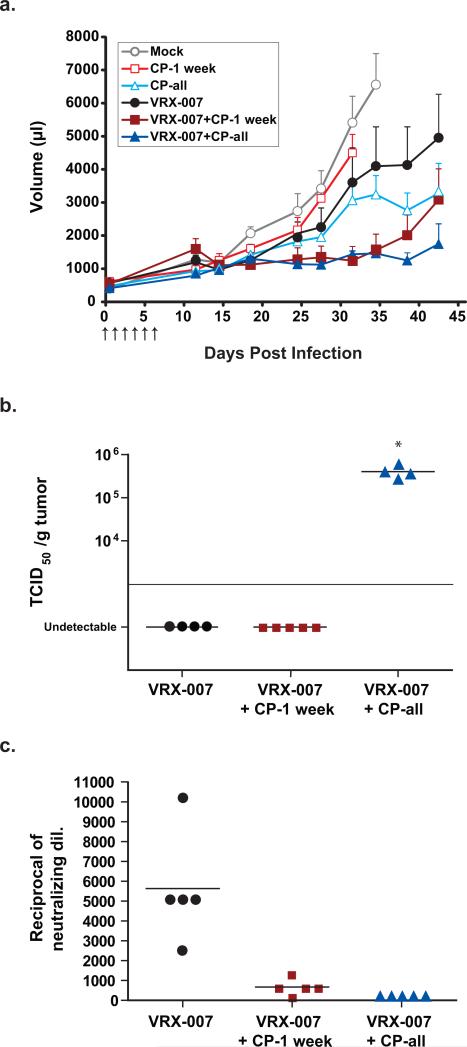
Figure 2. Long term immunosuppression with CP is not required for the combined effect of VRX-007 and CP against tumor growth.
HaK tumors were injected with 1×1010 PFU of VRX-007 or PBS (mock group) for 6 consecutive days. The arrows in panel a represent days on which the virus was injected. CP was given intraperitoneally biweekly starting one week before infection. The CP-1 week groups were taken off CP at the time of infection, and the CP-all groups were given CP biweekly for the duration of the study. The mock and CP-1 week groups were sacrificed on days 31 and 34, respectively, due to excessive tumor burden. The experiment was terminated at 44 days post infection because of animal welfare considerations. (a) Mean tumor volume. Error bars represent mean + SE, and the numbers of animals per group were as follows: 9 (mock); 8 (VRX-007+CP-1 week); 7 (CP-1 week); 6 (VRX-007 and CP-all); 4 (VRX-007 + CP-all). There was a significant difference in tumor growth suppression between the mock group and either of the VRX-007 + CP groups (P<0.003). There was no significant difference between VRX-007 + CP-1 week and VRX-007 + CP-all at any time throughout the study (P>0.10 throughout). (b) Virus titers in the tumor. Tumor samples for TCID50 assay were taken at time of death, 44 days post infection. (*P=0.0286) (c) Serum neutralizing antibody titers. An anti-Ad neutralizing antibody assay was performed for the serum collected at 44 days post infection (P < 0.02 for all groups compared to each other).
Quantification of bioluminescence imaging
Hamsters were given one injection of either AdRD-Luc or 007-Luc intratumorally. The animals were injected intraperitoneally with D-luciferin (Gold Biotechnology, St. Louis, MO) dissolved in PBS (15 mg/ml) at 15 min before each image. They were anesthetized using isoflurane and placed in the IVIS Spectrum optical imaging system (PerkinElmer, Waltham, MA). To quantify photon emission, Living Image software was used. Luminosity was calculated as follows: photons/sec/cm2/steradian.
Virus quantification in tissues
Tumor and organ samples were collected and snap frozen in liquid nitrogen. The samples were homogenized in PBS using the TissueLyser (Qiagen, Valencia, CA). The homogenates were freeze-thawed thrice and sonicated. The samples were diluted 10-fold across a 96-well plate in an end-point dilution assay on HEK293 cells (DMEM 5%FBS). After 14 days, the 50% tissue culture infective dose (TCID50) was calculated. Samples were marked as “undetectable” when no viral cytopathic effect was detected. Samples were marked “unquantifiable” when at least one positive well was detected under the limit of quantifiability.
Determination of neutralization antibody titers
Serum samples were incubated for 30 min at 56°C in order to inactivate complement. Two serum samples were assayed per plate. Serum samples (in four replicate wells) were diluted twofold across a 96-well plate in DMEM containing 10% FBS. VRX-007 was added at 100 PFU per well, and the dilutions of sera were incubated for 1 h at 37°C. A549 cells were then added at 5×105 cells/plate, and the plates were incubated at 37°C for 10 days. The media was replaced with 200μl of neutral red (30μl/ml in PBS) and incubated at 37°C for 1 h, after which the plates were washed with PBS twice. Next, 100 μl of acidified ethanol solution (50% ethanol, 1% acetic acid in H2O) was added. The absorbance was measured 10 min later at 550nm, and the cell viability determined by colorimetric assay on a Synergy 4 microplate reader (Biotek, Winooski, VT).
Luciferase expression in the presence of arabinosyl cytosine (AraC)
HaK and A549 cells were plated at 5.25×105 cells/well in a 6-well plate. Cells were infected at 25 PFU/cell of AdRD-Luc or 007-Luc (DMEM 5%FBS). At 5 h post infection, AraC was added (40 μg/ml) and was replaced every 12 h. After 54 h, cells were washed with PBS, and Reporter Lysis Buffer (1X) was added to the cells (Promega, Madison, WI). Plates were frozen at −80°C overnight. Once thawed, 20 μl of each sample was added to a Nunclon black, flat-bottomed 96-well plate, in four replicate wells (Sigma, St. Louis, MO). Luciferin substrate contained 1 mM D-luciferin potassium salt (Gold Biotechnology, St. Louis, MO), 3 mM ATP disodium salt, 30 mM HEPES buffer (Sigma, St. Louis, MO), and 15 mM MgCl2, and 100 μl was added per well. Luminescence was determined on a Synergy 4, microplate reader (Biotek, Winooski, VT).
Statistical Analyses
GraphPad Prism 4 for Windows was used to calculate statistical tests (GraphPad Software, Inc., San Diego, CA), and nonparametric tests were used to analyze the data. The Kruskal-Wallis test was used to deteremine the effects of treatment, and the Mann-Whitney U test was used to examine pairwise comparisons. P≤0.05 was considered to be significant. Statistical significance was calculated using the limit of quantification for the TCID50 assay.
Results
Continuous administration of CP may not be necessary for the enhanced antitumor efficacy that occurs with combined therapies
Previous studies in our laboratory have examined the role of high dose CP in enhancing VRX-007 therapy in hamster tumors13, 15. Hamsters that have been immunosuppressed with CP have increased viral titers in their tumors (through 40 days post infection), and this protracted replication of this virus was presumed to be responsible for the reduction in tumor growth. However, long-term CP treatment has serious side effects—most prominently anemia and weight loss. We decided to further investigate the role of CP by determining its effect on vector therapy and antitumor efficacy when administered for a short-term, one week prior to vector injection vs. continuous CP administration for the duration of the experiment.
HaK tumor-bearing Syrian hamsters were immunosuppressed with CP or left with an intact immune system, and their tumors were injected intratumorally with VRX-007 or vehicle (PBS). With our immunosuppressive protocol, CP treatment is administered biweekly, starting one week prior to vector injection. This dosing regimen abrogated the immune response within a week by depleting leukocytes, and that is when vector was injected intratumorally13. In one group, the drug treatment was terminated after one week (i.e. right after vector injection) (CP-1 week). We know from previous studies that the immune system rebounds within 10-14 days following termination of CP treatment (D. Dhar, unpublished results). In the other group of hamsters, CP was given biweekly, continuously for the duration of the study (CP-all). As shown in Fig. 2a, tumors in the mock group (injected with PBS) grew well, as did the tumors in hamsters treated with CP for one week (CP-1 week). Tumor growth was moderately decreased as compared to the mock group in hamsters treated with CP continuously (CP-all) (P= 0.066 at 34 days for both groups) and in hamsters in which VRX-007 was injected into tumors (P= 0.224 at 34 days). The greatest antitumor efficacy was obtained in the two groups with VRX-007 + CP (CP-1 week or CP-all) as compared to mock (P<0.003 at 34 days), indicating that the combination of VRX-007 + CP is more effective at suppressing tumor growth than either treatment alone. Interestingly, there was no significant difference in tumor volume (P=0.8081 after 44 days) between the VRX-007 + CP-1 week and the VRX-007 + CP-all groups. The experiment had to be terminated after 44 days for ethical reasons (i.e. tumors became ulcerated, animals developed anemia, etc.)
However, there was a small increase in tumor volume after 40 days in the VRX-007 + CP-1 week group. Infectious virus was detected in the tumors of the CP-all group (Fig. 2b), and this virus present in the tumors could have inhibited tumor growth in the latter part of the study (days 38-44 post infection). These hamsters had no serum neutralizing antibodies, which confirms the immunosuppression by CP (Fig. 2c). In contrast, no virus was extracted from the tumors of the VRX-007 + CP-1 week group (Fig. 2b), possibly due to the antiviral immune response that may have recovered after CP treatment was terminated (Fig. 2c, mean neutralizing antibody titer of 1:670). Altogether, these results suggest that long-term immunosuppression leading to continuous viral replication may not be the reason for the enhanced antitumor effect of CP and vector therapies combined, at least during the first 40 days of treatment. Further, as CP is a chemotherapeutic drug and it reduced the growth of tumors when given alone (Fig. 2a), an alternative explanation can be offered for the uptick in tumor growth in the VRX-007 + CP-1 week group at end of the study: it could be due to the absence of long-term CP chemotherapy.
The TCID50 analysis was performed on tumors taken at time of sacrifice, but that measurement does not account for viral replication that occurred prior to that time point, meaning we would not detect a virus “spike” at an earlier time point in the study. Because of these results, we chose to further investigate what effect CP treatment has on viral replication in tumor therapy.
Luciferase-expressing vectors can be utilized to measure vector infection and replication
Further experiments addressed the effect of CP on viral replication in our hamster cancer model. We know that continuous CP treatment prolongs viral replication, but we do not know if it increases viral infection or replication at all time points in this study. CP may increase the infectivity of tumor cells, increase viral replication directly, or promote viral persistence due to immunosuppression. To examine this question, an experiment was performed utilizing in vivo imaging, which allows us to monitor virus replication within the same animals at multiple time points.
We introduced two Ad5-based virus constructs with the luciferase gene present and used luciferase activity as a marker to locate virus-infected cells. The AdRD-Luc virus (Fig. 1c) is replication-defective, and the luciferase gene is transcribed from a CMV promoter upon infection, resulting in all infected cells expressing luciferase. The 007-Luc virus (Fig. 1d) is replication-competent, and luciferase is produced from the major late promoter, as is expression of ADP. This ensures that luciferase will be expressed only in cells in which the virus reached the late stage of infection37.
To verify that luciferase expression acts as a surrogate for 007-Luc viral replication, hamster HaK cells were infected with virus in vitro and then incubated with arabinosyl cytosine (AraC). AraC is a very potent inhibitor of adenoviral DNA replication and synthesis of late viral proteins in both human and hamster cancer cell lines 37, 38. If luciferase is expressed only after viral DNA has been synthesized, then no luciferase activity should be present in AraC-treated cells. A549 (human lung carcinoma) cells were used as a control to compare to the hamster cell line. As shown in Fig. 3a, AraC significantly reduced luciferase expression by 007-Luc in both hamsters and human cell lines (a 1- and 2-logfold decrease in luminescence, respectively). In contrast, luciferase expression by the replication-deficient (RD) AdRD-Luc was at similar high levels in both cell lines in the absence and presence of AraC, as expected (Fig. 3a). This result indicates that most of the luciferase expression occurs at late stages of infection, and therefore is likely a surrogate for virus replication. Less viral replication occurred in HaK cells, which was expected inasmuch as hamster cells are semi-permissive to Ad5 replication when compared to human cells 12. AraC did not suppress all luciferase expression by 007-Luc, as a low level of luciferase activity was still seen in treated cells. The low levels of luciferase synthesis in the AraC-treated cells may be the result of expression from the early E3 promoter.
To determine how in vivo bioluminescence compares to infectious titers calculated by TCID50 assays, the subcutaneous HaK tumors in the hamsters were injected with 007-Luc and imaged on the IVIS Spectrum, at days 1, 7, and 14 post infection; the tumors were excised, and the infectious virus titer in the tumors was determined. Luciferase expression in the tumors correlated with the amount of infectious virus extracted from the tumors (Fig. 3b), which confirmed the earlier findings by other groups shown in Fig. 3b38, 39. There was a relative decrease in titers over time that correlated with both measurement protocols. Thus, we conclude that luciferase expression from 007-Luc-infected cells or tumors is a surrogate for infectious titers. Luciferase needs ATP for its activity, and the half-life of the enzyme is approximately 2-3 h in mammalian cells40. Thus, luciferase activity indicates a cell in which the vector is currently replicating, and differences in infection and replication inside the tumor can be determined via luciferase expression.
CP does not enhance but prolongs vector persistence and replication
HaK tumor-bearing hamsters were injected with AdRD-Luc (RD) or 007-Luc (RC) in the absence of CP. Another group of hamsters was given short-term CP treatment, one week only, prior to intratumoral injection of AdRD-Luc (RD + CP-1 week) or 007-Luc (RC + CP-1 week). Another group of hamsters was administered continuous CP treatment, starting one week before intratumoral injection of AdRD-Luc (RD + CP-all) or 007-Luc (RC + CP-all). This experiment is similar to that in Fig. 2 except that the luciferase-expressing vectors were injected only once so that we could monitor luciferase expression starting on day 1. As shown in Fig. 3a for the replication-defective vector, luciferase expression was similar in CP-treated and untreated groups for the first week following infection. This result indicates that CP treatment does not affect infectivity of the tumor cells. CP-induced chronic immunosuppression in the AdRD-Luc + CP-all group allowed for persistence of the virus through 36 days post infection (Fig. 3c), while luciferase expression declined at a similar rate in the CP-1 week and non-CP groups, likely due to the immune response to the vector.
As shown in Fig. 3d, CP treatment does not change the ability of 007-Luc to infect tumor cells or to move into the late stage of infection inasmuch as the luciferase expression was similar in all three groups for days 1 and 3 post infection. Long-term immunosuppression with CP permitted persistent replication of 007-Luc, though there was never an increase in luciferase expression. The overall expression of 007-Luc was lower than that of AdRD-Luc. Presumably, the decline in luciferase expression by 007-Luc to low levels (baseline is approximately 105 photons) at 25-40 days could be due to lysis of the originally infected cells and lack of infection of neighboring cells in the tumor.
The 007-Luc vector alone (without CP) reduced tumor growth compared to vehicle-trated tumors (P<0.05). The two groups treated with 007-Luc plus CP had less tumor growth compared to the 007-Luc alone group (P<0.04). Importantly, we found no significant difference in antitumor efficacy between the 007-Luc + CP-1 week and 007-Luc + CP-all groups at any time during the study (P>0.35) (Fig. 3e), even though after 14 days, virus was replicating only in the 007-Luc + CP-all group (Fig. 3d). Viral replication decreased to almost baseline levels between days 7 and 14 in the 007-Luc and 007-Luc + CP-1 week groups. However, there was no correlation between the drop in vector replication in the 0070-Luc + CP-1 week and tumor size.
We conclude that CP treatment does not increase vector replication at early times (the first 5 days post injection) of the study, and that the beneficial effect of CP on antitumor efficacy may not be due to prolonged viral replication.
Enhanced tumor control obtained in 007-Luc and CP therapy is due to the combined effects of independent treatments
The next question addressed was whether an interaction of 007-Luc and CP was necessary for the enhanced antitumor effect. CP suppresses the immune system, allowing the virus to continue to replicate longer than in animals with competent immune responses. However, CP, being especially effective against rapidly dividing cells, is also a chemotherapeutic drug used to treat cancer. In order to determine how CP affects the tumor cell or virus replication, the dosing regimen was altered to include animal groups in which the administration of CP and the vector was separated in time, which distinguishes the independent chemotherapeutic effect of the drug from its potential vector-dependent response. The protocol is outlined in Fig. 4a. One group of hamsters bearing HaK tumors was treated with CP for one week starting 7 days prior to intratumoral injection with 007-Luc, and CP treatment was terminated at time of infection (007-Luc + CP-before). We know from earlier experiments that the hamsters are immunosuppressed after one week of CP treatment. One week of CP therapy includes 2 doses, one at 7 days before injection and the other at 3-4 days after the first. A second group of hamsters was injected intratumorally on day 0 and were treated with CP for one week starting at day 7 (007-Luc + CP-after) (Fig. 4a). We know from previous studies with VRX-007 and Ad5 that a neutralizing antibody response to the virus develops by day 7. Thus, we expect little if any vector present at this time. The CP dosing was administered the same way in uninfected animals.
As shown in Fig. 4b, all non-virus-treated groups had the largest tumors and most tumor growth. Notably, the 007-Luc + CP-before and the 007-Luc + CP-after groups had similar tumor growth suppression to each other (P=0.54), and were significantly better than 007-Luc alone (P<0.05 starting at day 34 post infection). Luciferase expression did not differ significantly among any 007-Luc groups at any time point (Fig. 4c). Most of the vector was eliminated after 7 days post infection, and luminosity was decreased to near background levels starting at approximately 10 days post infection. Adding CP one week after vector injection did not alter the course of the infection (i.e. there was no peak in luciferase expression in response to CP treatment). All hamsters given CP had lower neutralizing antibody levels against Ad than their immunocompetent counterparts, though they did produce antibodies when their immune systems rebounded (Fig. 4d). Thus, CP does not need to be present at the time of infection for enhancing the 007-Luc-mediated inhibition of tumor growth. These data provide strong evidence that CP and vector therapy impart two independent antitumor effects, and that their direct interaction is not required for their combined efficacy. This enhanced efficacy is not due to CP-induced prolonged viral replication.
The distribution of luciferase-expressing vectors within solid tumors is confined to the site of injection
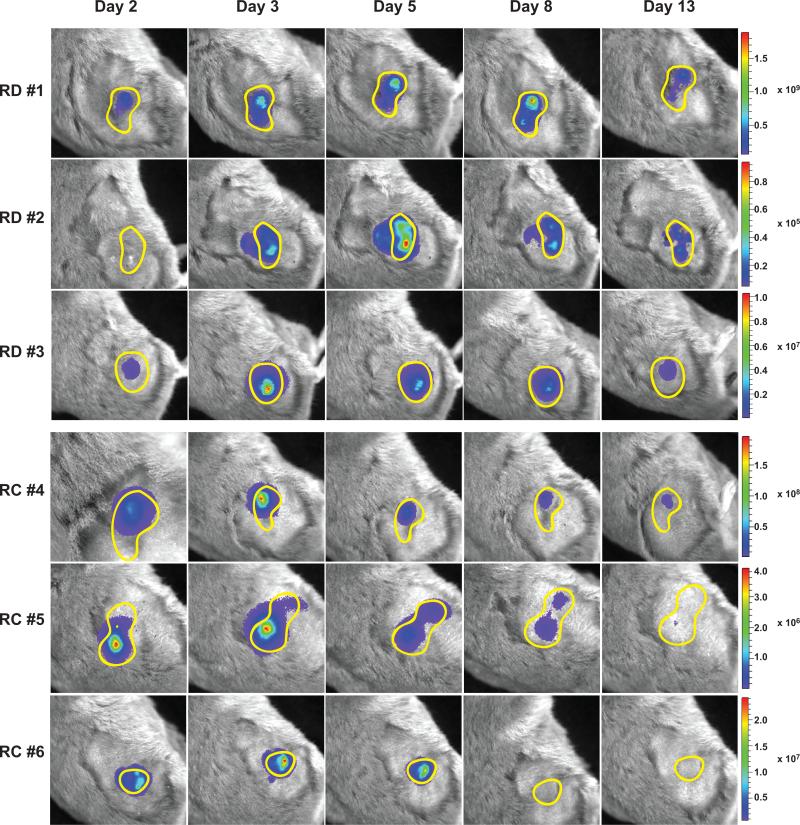
Although the combination therapy with continuous CP treatment and 007-Luc does not prolong virus replication and has better antitumor efficacy than either single treatment, it still does not completely eliminate the HaK tumors. We wanted to address the question of whether the vector is sufficiently spreading throughout the tumors. Others have reported that physical barriers exist in solid tumors that can hinder the spread of the virus, including dense intratumoral connective tissue and necrotic cells41, 42. To ensure better vector distribution throughout the tumor, vectors are usually administered in multiple injections placed in various places on the tumor43, 44. To visualize the distribution of the vector within tumors, subcutaneous HaK tumors were injected once with a small volume (50μl) of either AdRD-Luc (replication-defective) or 007-Luc (replication-competent). Care was taken to inject the vector to only one location in the tumor. We used this small injection volume so that we could better determine the natural spread of the vector, and tumor efficacy was not studied in this experiment. All animals were treated with CP continuously so that the virus could continue to persist/replicate. In vivo imaging was used to follow the distribution of each virus over a 2-week period post infection. Images are shown for 3 animals per group treated with AdRD-Luc or 007-Luc. As shown in Fig. 5, top panel, the level of luciferase activity remained more consistent in the tumors infected with AdRD-Luc. The luciferase expression decreased over time in tumors infected with 007-Luc, presumably because this vector can replicate and cause cell lysis, decreasing the number of infected cells over time. Luciferase expression (i.e. vector infection) was confined to the original injection site and did not progress into new areas of the tumor for either the replication-competent or replication-defective vector. A similar experiment was performed using SHPC6 tumors and yielded comparable results (Fig. S1).
Figure 5. Vectors remain confined near the injection site in HaK tumors.
HaK tumors were injected once with either 1.8×109 PFU of AdRD-Luc (RD) or 1.5×109 PFU of 007-Luc (RC) in 50 μl volume). Images were taken of 6 different animals (3 per group) on the days specified. Every animal was treated with CP biweekly starting one week before infection. A yellow line demarcates the approximate location of the subcutaneous tumor. Intensity scales are added for each animal; the range of expression was normalized for each animal individually, which is reflected in individual scale bars.
From these results, we conclude that, even in immunosuppressed animals, the replication of an intratumorally-injected oncolytic Ad vector is confined to the site of original delivery.
Cells isolated from excised HaK tumors are not resistant to 007-GFP infection in vitro
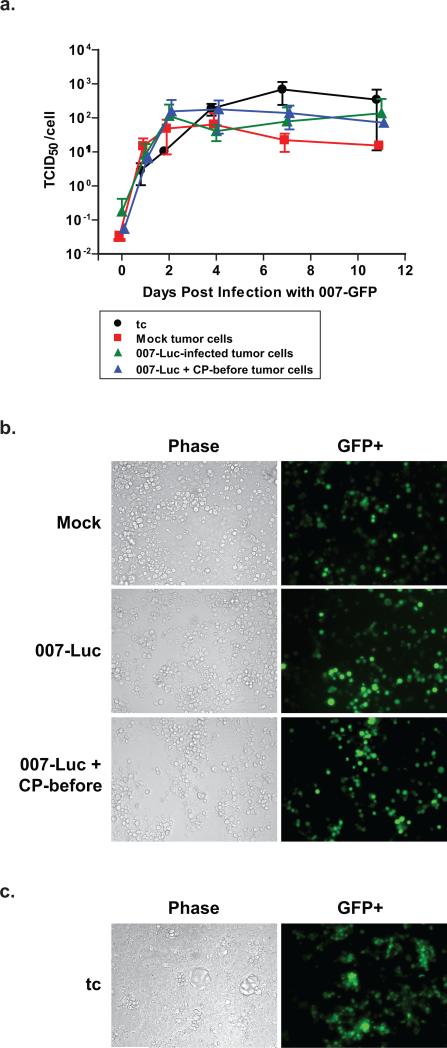
The replication-competent vector, 007-Luc, was unable to spread throughout the tumor, so we questioned whether the remaining cells in virus-treated HaK tumors were still susceptible to infection or if they had developed resistance to the virus. Tumors were removed from the animals and homogenized into single cell suspensions using trypsin/EDTA. (Three tumors per group were randomly selected from the mock, 007-Luc, and 007-Luc + CP-before groups discussed in Fig. 4). The resulting ex vivo HaK cells, along with HaK cells grown from tissue culture (tc), were infected with 007-GFP (Fig. 1e), and a single step growth curve study was completed (Fig. 6a). The 007-GFP virus was used so that we could evaluate the percentage of cells infected. 007-GFP is similar to 007-Luc except that the gene for the green fluorescent protein (GFP) replaces the luciferase gene (Figs. 1d,e). The cells from all three animal groups were still permissive to virus infection ex vivo (Fig. 6a). Images were taken of the cells to determine the relative percentage of GFP+ cells per group (Fig. 6b,c). (GFP is produced after the virus has replicated and entered into late infection.) Almost all cells were expressing GFP in each group, showing no substantial difference per treatment. None of the groups were significantly different statistically (P>0.05) from one another in either infectious virus yields or percentage of GFP-positive cells. Vector and CP treatment did not create a significant alteration in the tumor cells’ ability to be infected and produce infectious virus, and HaK tumors did not develop resistance to VRX-007 in vivo.
Figure 6. Cells from excised HaK tumors are not resistant to 007-GFP infection in culture.
Three hamsters from the mock, 007-Luc, and 007-Luc + CP-before groups from the experiment in Fig. 4 were selected at random. The tumors from each group were excised and homogenized into single cell suspensions by digestion in trypsin/EDTA for 30 min. 5×104 cells were plated per well in a 12-well plate. Three samples from HaK tissue culture cells were used as well (designated as tc). (a) Single step growth curve. Cells were infected 2 days after isolation with 007-GFP (50 PFU/cell) and then washed with DMEM 5%FBS. At each time point, one 12-well plate with all 12 samples was frozen away. A TCID50 assay was performed. Graphs represent virus yield produced per cell. (P>0.100 between each group on every day). GFP expression in (b) ex vivo tumor cells and (c) tissue culture cells. Images were taken of the tumor cells to determine the presence of GFP+ cells. Images above are representatives of each group, taken 4 days post infection. The number of cells per well at time of infection were as follows: Mock, 1.8×105; 007-Luc, 1.8×105; 007-Luc + CP-before, 1.1×105; tc, 4.4×105.
Discussion
CP is a chemotherapeutic drug often used in combination with oncolytic viruses. The mechanisms by which the drug increases the activity of oncolytic vectors are dependent on the model system and dosing regimen used. Low doses of CP lead to the suppression of regulatory T cells, and thus increase antitumor immune responses25. The research group of A. Chiocca has reported that high doses of CP administered before the injection of an oncolytic herpesvirus cause a decrease in antiviral cytokine production and inhibit the activity of natural killer cells, increasing vector replication and persistence34. We have previously concluded that the continuous inhibition of the antiviral immune response by high doses of CP permits prolonged oncolytic viral vector replication and increased antitumor efficacy13, 14.
Our current studies were designed to investigate the mechanisms by which high dose CP enhances VRX-007 antitumor therapy in the semi-permissive hamster HaK tumor model. In one set of experiments, we asked whether short-term CP dosing would be as effective as continuous dosing. We found that for about 40 days post injection, hamsters that were given CP for only one week immediately before vector injection had similar antitumor efficacy as did hamsters given continuous CP treatment (Figs. 2a and 3c). Extended viral replication only occurred in continuously immunosuppressed animals (Figs. 2b and 3b). We argue that this improved tumor suppression by the combination of VRX-007 and long-term CP treatment is due to the chemotherapeutic effect of CP, rather than the prolonged replication of VRX-007. Virus replication is mostly lost by days 7 to 14 post injection in the animals that received only one week of immunosuppression, yet the tumors started growing only 40 days post injection. For a part of this time period, extending from the loss of virus replication in the CP-1 week groups to the end of the study, there was 100-fold increase in virus replication that occurred in the continuously immunosuppressed animals, yet there was still no difference in antitumor efficacy at any time. We have not found any difference in the replication of our oncolytic Ad vector immediately following injection, either. CP did not increase the infectivity of AdRD-Luc or 007-Luc in HaK tumors or induce increased replication of 007-Luc at early time points (Figs. 3c-d and 4c). The only effect by long-term CP treatment on virus replication was the prolongation of a low-level (approximately 1000-fold less than one day post infection) persistence of the virus for the duration of the study. These results suggest that the antitumor efficacy seen with CP and vector treatments is not likely due to CP-induced immunosuppression prolonging viral replication. The differences in the duration of infectious virus replication are not reflected in tumor growth.
Hallden et al. reported that they found no correlation between high-level viral replication and tumor growth when Ad5 was injected into mice with subcutaneous tumors formed from multiple murine and human cancer cell lines (i.e. Ad5 produced the most tumor suppression in the tumors that were least permissive to Ad5 replication)45. However, they and others have found that UV-inactivated vectors did not inhibit tumor growth11, 44, 45. Similarly, UV-inactivated VRX-007 did not inhibit HaK tumor growth in Syrian hamsters 12. Therefore, gene expression from VRX-007 and other oncolytic vectors, and possibly replication, is required for retarding tumor growth.
If CP-induced immunosuppression enhances antitumor activity by preventing early clearance of the virus, then the greatest tumor growth suppression should be observed in animals treated with CP prior to infection. H. Wakimoto et al., reported that CP administration at 24 h before infection—but not when given simultaneously to infection—enhanced viral oncolysis by suppressing the innate antiviral response29. We chose to examine if CP pretreatment is necessary for the enhanced oncolytic activity seen in hamster tumors treated with CP and Ad vectors, and we performed experiments to compare if these two modalities required direct interaction for improved efficacy. One week of CP therapy, including 2 separate doses, was initiated either 7 days before infection (007-Luc + CP-before)—such that its effects were present at the time of infection—or 7 days after infection (007-Luc + CP-after)—such that the virus had already been mostly eliminated and thus there should be only minimal interaction between vector and CP. Similar tumor suppression was observed in both groups, and CP did not result in an increase in virus production when administered one week after infection (Fig. 4). These results and conclusions differ from our previous assumptions that tumor growth is inhibited due to the immunosuppressive effects of CP leading to increased viral replication and oncolytic activity. We conclude that, in the current study, the combined antitumor efficacy does not result from increased vector replication in animals immunosuppressed with CP.
Because long-term viral replication was not required for antitumor efficacy, we next studied viral distribution throughout the tumors. One of the major limiting factors in oncolytic virotherapy is the restriction of vector distribution due to the tumor microenvironment. The rate of spatial distribution of a replication-competent virus within a tumor is considered by some to be the most important factor in achieving tumor eradication43. Large established tumors (such as the HaK tumors we study), have connective tissue and necrotic cells that limit replication to the injection site41. Others have reported that CP pretreatment can increase vector distribution of vaccinia33 and herpesvirus29 oncolytic vectors in rat gliomas. We found that 007-Luc did not spread throughout subcutaneous HaK or SHPC6 tumors in hamsters pretreated with CP, and instead remained near the injection site after a single low volume vector injection. This lack of distribution may explain why many tumors have not been completely eradicated in our models6, 13-15.
We also questioned whether the tumor cells that remain, which were not killed by the virus, were still susceptible to virus infection. We excised HaK tumors from hamsters post treatment with or without 007-Luc and CP. We found that excised HaK tumors did not show resistance to 007-GFP (a version of 007-Luc that expresses GFP instead of luciferase) and were still equally susceptible to infection, regardless of the type of therapy they received in vivo. 007-Luc did not spread within the tumors, but those cells can be infected. It is important to understand why tumors are not fully eliminated, despite viral persistence46.
VRX-007 is in a Phase I clinical trial. Additional preclinical data will be important to better understand why combined therapies provide enhanced efficacy. Here, we report that VRX-007 and CP produce antitumor activities that are independent of each other and do not rely on CP-induced immunosuppression enhancing vector replication. Thus far, these findings have been limited to VRX-007 vector therapy in Syrian hamster tumors, and more data is needed to determine whether these results correlate with other models.
Supplementary Material
Acknowledgments
This work was supported by grant CA118022 from the National Institutes of Health. The VRX-007 vector was provided by VirRx, Inc., and W.S.M.W., K.T., and A.E.T. own stock in the company.
References
- 1.Toth K, Wold WS. Increasing the efficacy of oncolytic adenovirus vectors. Viruses. 2010;2(9):1844–66. doi: 10.3390/v2091844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Wold WSM, Ison MG. Chapter 56: Adenoviruses. In: Knipe DM, Howley PM, editors. Fields Virology. 6 edn Vol. 1. Lippincott Williams & Wilkins; Philadelphia: 2013. pp. 1732–1767. [Google Scholar]
- 3.Liu TC, Galanis E, Kirn D. Clinical trial results with oncolytic virotherapy: a century of promise, a decade of progress. Nat. Clin. Pract. Oncol. 2007;4(2):101–117. doi: 10.1038/ncponc0736. [DOI] [PubMed] [Google Scholar]
- 4.Pesonen S, Kangasniemi L, Hemminki A. Oncolytic adenoviruses for the treatment of human cancer: focus on translational and clinical data. Mol. Pharm. 2011;8(1):12–28. doi: 10.1021/mp100219n. [DOI] [PubMed] [Google Scholar]
- 5.Yamamoto M, Curiel DT. Current issues and future directions of oncolytic adenoviruses. Mol. Ther. 2010;18(2):243–250. doi: 10.1038/mt.2009.266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Doronin K, Toth K, Kuppuswamy M, Krajcsi P, Tollefson AE, Wold WSM. Overexpression of the ADP (E3-11.6K) protein increases cell lysis and spread of adenovirus. Virology. 2003;305:378–387. doi: 10.1006/viro.2002.1772. [DOI] [PubMed] [Google Scholar]
- 7.Doronin K, Toth K, Kuppuswamy M, Ward P, Tollefson AE, Wold WSM. Tumor-specific, replication-competent adenovirus vectors overexpressing the Adenovirus Death Protein. J. Virol. 2000;74(13):6147–6155. doi: 10.1128/jvi.74.13.6147-6155.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Tollefson AE, Scaria A, Hermiston TW, Ryerse JS, Wold LJ, Wold WSM. The adenovirus death protein (E3-11.6K) is required at very late stages of infection for efficient cell lysis and release of adenovirus from infected cells. J. Virol. 1996;70:2296–2306. doi: 10.1128/jvi.70.4.2296-2306.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Doronin K, Kuppuswamy M, Toth K, Tollefson AE, Krajcsi P, Krougliak V, et al. Tissue-specific, tumor-selective, replication-competent adenovirus vector for cancer gene therapy. J. Virol. 2001;75:3314–3324. doi: 10.1128/JVI.75.7.3314-3324.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Toth K, Kuppuswamy M, Shashkova EV, Spencer JF, Wold WSM. A fully replication-competent adenovirus vector with enhanced oncolytic properties. Cancer Gene Ther. 2010;17(11):761–770. doi: 10.1038/cgt.2010.33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Toth K, Spencer JF, Tollefson AE, Kuppuswamy M, Doronin K, Lichtenstein DL, et al. Cotton rat tumor model for the evaluation of oncolytic adenoviruses. Hum. Gene Ther. 2005;16(1):139–146. doi: 10.1089/hum.2005.16.139. [DOI] [PubMed] [Google Scholar]
- 12.Thomas MA, Spencer JF, La Regina MC, Dhar D, Tollefson AE, Toth K, et al. Syrian hamster as a permissive immunocompetent animal model for the study of oncolytic adenovirus vectors. Cancer Res. 2006;66(3):1270–1276. doi: 10.1158/0008-5472.CAN-05-3497. [DOI] [PubMed] [Google Scholar]
- 13.Thomas MA, Spencer JF, Toth K, Sagartz JE, Phillips N, Wold WSM. Immunosuppression enhances oncolytic adenovirus replication and anti tumor efficacy in the Syrian hamster model. Mol. Ther. 2008;16:1665–1673. doi: 10.1038/mt.2008.162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Dhar D, Spencer JF, Toth K, Wold WSM. Effect of preexisting immunity on oncolytic adenovirus vector INGN 007 antitumor efficacy in immunocompetent and immunosuppressed Syrian hamsters. J. Virol. 2009;83(5):2130–2139. doi: 10.1128/JVI.02127-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Dhar D, Spencer JF, Toth K, Wold WSM. Pre-existing immunity and passive immunity to adenovirus 5 prevents toxicity caused by an oncolytic adenovirus vector in the Syrian hamster model. Mol. Ther. 2009;17:1724–1732. doi: 10.1038/mt.2009.156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Spencer JF, Sagartz JE, Wold WSM, Toth K. New pancreatic carcinoma model for studying oncolytic adenoviruses in the permissive Syrian hamster. Cancer Gene Ther. 2009;16(12):912–922. doi: 10.1038/cgt.2009.36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Sonabend AM, Ulasov IV, Han Y, Rolle CE, Nandi S, Cao D, et al. Biodistribution of an oncolytic adenovirus after intracranial injection in permissive animals: a comparative study of Syrian hamsters and cotton rats. Cancer Gene Ther. 2009;16(4):362–372. doi: 10.1038/cgt.2008.80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Bortolanza S, Bunuales M, Otano I, Gonzalez-Aseguinolaza G, Ortiz-de-Solorzano C, Perez D, et al. Treatment of pancreatic cancer with an oncolytic adenovirus expressing interleukin-12 in Syrian hamsters. Mol. Ther. 2009;17(4):614–622. doi: 10.1038/mt.2009.9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lichtenstein DL, Spencer JF, Doronin K, Patra D, Meyer J, Shashkova EV, et al. An acute toxicology study with INGN 007, an oncolytic adenovirus vector, in mice and permissive Syrian hamsters; comparisons with wild-type Ad5 and a replication-defective adenovirus vector. Cancer Gene Ther. 2009;16:644–654. doi: 10.1038/cgt.2009.5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ying B, Toth K, Spencer JF, Meyer J, Tollefson AE, Patra D, et al. INGN 007, an oncolytic adenovirus vector, replicates in Syrian hamsters but not mice: comparison of biodistribution studies. Cancer Gene Ther. 2009;16:625–637. doi: 10.1038/cgt.2009.6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Yu W, Fang H. Clinical trials with oncolytic adenovirus in China. Current Cancer Drug Targets. 2007;7(2):141–148. doi: 10.2174/156800907780058817. [DOI] [PubMed] [Google Scholar]
- 22.Bonadonna G, Valagussa P, Moliterni A, Zambetti M, Brambilla C. Adjuvant cyclophosphamide, methotrexate, and fluorouracil in node-positive breast cancer — the results of 20 years of follow-up. N. Engl. J. Med. 1995;332:901–906. doi: 10.1056/NEJM199504063321401. [DOI] [PubMed] [Google Scholar]
- 23.Tada K, Ito Y, Takahashi S, Lijima K, Miyagi Y, Nishimura S. Tolerability and safety of classic cyclophosphamide, methotrexate, and Fluorouracil treatment in Japanese patients with early breast cancer. Breast Cancer. 2006;13:279–83. doi: 10.2325/jbcs.13.279. [DOI] [PubMed] [Google Scholar]
- 24.de Jonge M, Huitema AD, Rodenhuis S, Beijnen JH. Clinical pharmacokinetics of cyclophosphamide. Clin. Pharmacokinet. 2005;44:1135–64. doi: 10.2165/00003088-200544110-00003. [DOI] [PubMed] [Google Scholar]
- 25.Cerullo V, Diaconu I, Kangasniemi L, Rajecki M, Escutenaire S, Koski A, et al. Immunological effects of low-dose cyclophosphamide in cancer patients treated with oncolytic adenovirus. Mol. Ther. 2011;19(9):1737–1746. doi: 10.1038/mt.2011.113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Fehr T, Sykes M. Tolerance induction in clinical transplantation. Transpl. Immunol. 2004;13:117–30. doi: 10.1016/j.trim.2004.05.009. [DOI] [PubMed] [Google Scholar]
- 27.Ferry C, Socie G. Busulfan-cyclophosphamide versus total body irradiation-cyclophosphamide as a preparative regimen before allogeneic hematopoietic stem cell transplantation for acute myeloid leukemia: what have we learned? Exp. Hematol. 2003;31:1182–1186. doi: 10.1016/j.exphem.2003.09.008. [DOI] [PubMed] [Google Scholar]
- 28.Ikeda K, Ichikawa T, Wakimoto H, J.S. S, Deisboeck TS, Finkelstein D, et al. Oncolytic virus therapy of multiple tumors in the brain requires suppression of innate and elicited antiviral responses. Nat. Med. 1999;5(8):881–887. doi: 10.1038/11320. [DOI] [PubMed] [Google Scholar]
- 29.Wakimoto H, Fulci G, Tyminski E, Chiocca EA. Altered expression of antiviral cytokine mRNAs associated with cyclophosphamide's enhancement of viral oncolysis. Gene Ther. 2004;11(214-223) doi: 10.1038/sj.gt.3302143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kambara H, Saeki Y, Chiocca EA. Cyclophosphamide allows for in vivo dose reduction of a potent oncolytic virus. Cancer Res. 2005;65(24):11255–11258. doi: 10.1158/0008-5472.CAN-05-2278. [DOI] [PubMed] [Google Scholar]
- 31.Kurozumi K, Hardcastle J, Thakur R, Yang M, Christoforidis G, Fulci G, et al. Effect of tumor microenvironment modulation on the efficacy of oncolytic virus therapy. J. Natl. Cancer Inst. 2007;99(23):1768–81. doi: 10.1093/jnci/djm229. [DOI] [PubMed] [Google Scholar]
- 32.Qiao J, Wang H, Kottke T, White C, Twigger K, Diaz RM, et al. Cyclophosphamide facilitates antitumor efficacy against subcutaneous tumors following intravenous delivery of reovirus. Clin. Cancer Res. 2008;14(1):259–269. doi: 10.1158/1078-0432.CCR-07-1510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Lun XQ, Jang J, Tang N, Deng H, Head R, Bell JC, et al. Efficacy of systemically administered oncolytic vaccinia virotherapy for malignant gliomas is enhanced by combination therapy with rapamycin and cyclophosphamide. Clin. Cancer Res. 2009;15(8):2777–2788. doi: 10.1158/1078-0432.CCR-08-2342. [DOI] [PubMed] [Google Scholar]
- 34.Fulci G, Breymann L, Gianni D, Kurozomi K, Rhee SS, Yu J, et al. Cyclophosphamide enhances glioma virotherapy inhibiting innate immune responses. Proc. Natl. Acad. Sci. U. S. A. 2006;103(34):12873–12878. doi: 10.1073/pnas.0605496103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Thomas MA, Spencer JF, Wold WSM. Use of the Syrian hamster as an animal model for oncolytic adenovirus vectors. In: Tollefson AE, Wold WSM, editors. Methods in Molecular Medicine. 2nd edn. Vol. 1. Humana Press; Totowa: 2007. pp. 169–183. [DOI] [PubMed] [Google Scholar]
- 36.Lichtenstein DL, Toth K, Doronin K, Tollefson AE, Wold WSM. Functions and mechanisms of action of the adenovirus E3 proteins. Int. Rev. Immunol. 2004;23:75–111. doi: 10.1080/08830180490265556. [DOI] [PubMed] [Google Scholar]
- 37.Harter ML, Shanmugam G, Wold WS, Green M. Detection of adenovirus type 2-induced early polypeptides using cycloheximide pretreatment to enhance viral protein synthesis. J. Virol. 1976;19(1):232–242. doi: 10.1128/jvi.19.1.232-242.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Diaconu I, Cerullo V, Escutenaire S, Kanerva A, Bauerschmitz GJ, Hernandez-Alcoceba R, et al. Human adenovirus replication in immunocompetent Syrian hamsters can be attenuated with chlorpromazine or cidofovir. Journal of Gene Medicine. 2010;12(5):435–445. doi: 10.1002/jgm.1453. [DOI] [PubMed] [Google Scholar]
- 39.Guse K, Dias JD, Bauerschmitz G, Hakkarainen T, Aavik E, Ranki T, et al. Luciferase imaging for evaluation of oncolytic adenovirus replication in vivo. Gene Ther. 2007;14:902–911. doi: 10.1038/sj.gt.3302949. [DOI] [PubMed] [Google Scholar]
- 40.Ignowski JM, Schaffer DV. Kinetic analysis and modeling of Firefly luciferase as a quantitative reporter gene in live mammalian cells. Biotechnol. Bioeng. 2004;86(7):827–834. doi: 10.1002/bit.20059. [DOI] [PubMed] [Google Scholar]
- 41.Sauthoff H, Hu J, Maca C, Goldman M, Heitner S, Yee H, et al. Intratumoral spread of wild-type adenovirus is limited after local injection of human xenograft tumors: virus persists and spreads systemically at late time points. Hum. Gene Ther. 2003;14(5):425–433. doi: 10.1089/104303403321467199. [DOI] [PubMed] [Google Scholar]
- 42.Yun CO. Overcoming the extracellular matrix barrier to improve intratumoral spread and therapeutic potential of oncolytic virotherapy. Curr Opin Mol Ther. 2008;10(4):356–61. [PubMed] [Google Scholar]
- 43.Wein LM, Wu JT, Kirn DH. Validation and analysis of a mathematical model of a replication-competent oncolytic virus for cancer treatment: implications for virus design and delivery. Cancer Res. 2003;63(6):1317–1324. [PubMed] [Google Scholar]
- 44.Currier MA, Adams LC, Mahller YY, Cripe TP. Widespread intratumoral virus distribution with fractionated injection enables local control of large human rhabdomyosarcoma xenografts by oncolytic herpes simplex viruses. Cancer Gene Ther. 2005;12:407–416. doi: 10.1038/sj.cgt.7700799. [DOI] [PubMed] [Google Scholar]
- 45.Hallden G, Hill R, Wang Y, Anand A, Liu TC, Lemoine NR, et al. Novel immunocompetent murine tumor models for the assessment of replication-competent oncolytic adenovirus efficacy. Mol. Ther. 2003;8(3):412–424. doi: 10.1016/s1525-0016(03)00199-0. [DOI] [PubMed] [Google Scholar]
- 46.Harrison D, Sauthoff H, Heitner S, Jagirdar J, Rom WN, Hay JG. Wild-type adenovirus decreases tumor xenograft growth, but despite viral persistence complete tumor responses are rarely achieved--deletion of the viral E1b-19-kD gene increases the viral oncolytic effect. Hum. Gene Ther. 2001;12(10):1323–1332. doi: 10.1089/104303401750270977. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.