Abstract
Pyrrolysyl-tRNA synthetase (PylRS) is a class IIc aminoacyl-tRNA synthetase that is related to phenylalanyl-tRNA synthetase (PheRS). Genetic selection provided PylRS variants with a broad range of specificity for diverse non-canonical amino acids (ncAAs). One variant is a specific phenylalanine-incorporating enzyme. Structural models of the PylRS amino acid complex show that the small pocket size and π-interaction play an important role in specific recognition of Phe and the engineered PylRS active site resembles that of E. coli PheRS.
1. Introduction
Pyrrolysyl-tRNA synthetase (PylRS) is an aminoacyl-tRNA synthetase (aaRS) that acylates pyrrolysine (Pyl) onto its cognate tRNAPyl, a tRNA that recognizes the stop codon UAG [1]. PylRS is known to be present in about forty archaeal and bacterial species [1,2]. In the Methanosarcinaceae PylRS/tRNAPyl is a truly orthogonal synthetase/tRNA pair which is not recognized by the other aaRSs or tRNAs. Therefore it has been widely used in enzyme engineering to generate PylRS variants able to acylate non-canonical amino acids (ncAAs) including a variety of Lys, Tyr and Phe analogs [3-9]. These ncAA-tRNAs have been invaluable for co-translational insertion of ncAAs into proteins in response to the UAG codon [3-9].
Phylogenetic analyses of sequences and structures revealed PylRS to be a subclass IIc aaRS; the other enzymes of this class include PheRS and phosphoseryl-tRNA synthetase (SepRS) [10]. It appears that PylRS evolved from PheRS earlier than SepRS: it was separated before the node at which the - and -subunits of this enzyme diverged, while SepRS evolved later from -PheRS [10]. Therefore some forms of PylRS may still recognize Phe as a substrate.
The class IIc aaRS family shows similarity in the core domain. PylRS and PheRS have similarly organized hydrophobic pockets, which include aromatic residues that contribute to the substrate discrimination [10,11]. In addition, the Asn346 residue of PylRS is a ‘gate-keeper’ of the substrate binding site via hydrogen-bonding with the side chain amide oxygen of Pyl [3,4,10,12]. Since amino acid changes in the catalytic binding pocket are sufficient to endow PylRS with different substrate specificities, we reasoned that generating PylRS mutants with variations in the catalytic pocket may provide insights into the amino acid binding site of the ancestral enzymes.
In this study we investigated evolutionary aspects of Methanosarcina mazei PylRS by creating a number of variants with PheRS activity by genetic selection. The specificity of the amino acid binding pockets was analyzed with the library of ncAAs. One evolved PylRS variant showed a similar pocket structure as E. coli PheRS.
2. Materials and Methods
2.1. PyIRS mutagenesis and selection procedure
The codons at positions Ala302, Pro303, Tyr306, Asn346, Cys348 and Tyr384 of M. mazei PylRS were randomly mutated into NNS codons (N: A/G/C/U; S: G/C) as previously described [13] with some modifications. Briefly, 1 μg of the ligation products were then electroporated into E. coli TOP10 cells. Electroporated cells were recovered in 50 mL SOC medium for 15 min at 37°C. The cells were then plated on LB agar plates with 50 μg/mL kanamycin (Kan) by serial dilution. Based on the colony numbers on these plates, the library contains approximately 5.0 ± 0.7 × 108 independent transformants. DNA sequencing results of 25 PylRS variants in the library did not reveal bias at the randomization sites.
The plasmid library was introduced by electroporation into E. coli TOP10 harboring pCAM pylT (Table S1). The cells were cultivated in LB medium (1 L) supplemented with 10 μg/mL tetracycline (Tc) and 25 μg/ml Kan at 37°C for 16 hr. Approximately 109 transformants that contained the plasmid library as well as pCAM-pylT were plated on LB-agar medium with 50 μg/mL chloramphenicol (Cm), 10 μg/ml Tc, and 25 μg/ml Kan and grown at 37°C overnight. The number of colonies was calculated by counting the colonies on the LB-agar plate with 10 μg/ml Tc, and 25 μg/ml Kan, lacking Cm. Approximately 105 colonies were scraped from the plates and diluted 1:50 into fresh LB medium (20 mL) with 10 μg/mL Tc and 25 μg/mL Kan. The cells subsequently were plated on LB-agar plates with 50 µg/mL Cm, 10 µg/mL Tc and 25 µg/mL Kan for another selection for growth. After two rounds of selection, individual colonies were isolated and cultured to prepare the plasmid DNAs. The suppression activities of the clones were measured by plating the transformed cells on M9 plates supplemented with 10 μg/mL Tc, 25 μg/mL Kan, 100 μg/mL Cm and 1 mM Phe.
2.2. Identification of amino acids incorporated by PyIRS mutants
The plasmid pET-sfGFP (Table S1) with a UAG mutation at Ser2 position was derived from the plasmid pET-Duet1 (Invitrogen). The plasmid pET-sfGFP-pylT (Table S1) was constructed from pET-sfGFP by adding pylT, which encodes tRNAPyl. Superfolder green fluorescent protein (sfGFP) [14] was produced from cells harboring the two plasmids, pET-sfGFP-pylT and pKTS-FRS derivatives (Table S1), and then purified by Ni-NTA column chromatography. The purified proteins were analyzed by electrospray ionization mass spectrometry (ESI-MS) and LC-MS/MS for evaluation of identity and homogeneity of the amino acid at Ser2 position. All details are described in Supplementary Material.
2.3. Screening of incorporation of canonical or non-canonical amino acids (ncAAs) by sfGFP production
For the purpose of screening a library of ~300 ncAAs a colony of E. coli BL21 (DE3) cells co-transformed with pET-sfGFP-pylT and pKTS-FRS was cultured in 100 mL LB medium supplemented with 50 μg/mL Kan and 100 μg/mL ampicillin at 37°C. Then the medium was replaced with minimal medium (A600=1.0), and IPTG (1 mM) was added. Aliquots (50 μL) of the induced cell suspension were transferred into the wells of a 384-well plate containing a different ncAA (5 mM) in each well. Incubation continued for 6 hr under constant shaking. Fluorescence intensity was recorded (excitation wavelength 485 nm/20 nm; emission wavelength 528 nm/20 nm). Four wells (A1, A2, I1 and I2) without added ncAAs were the control experiments for detecting the background signals. Two wells that contained BocK (14, B3) and AlocK (15, H1) were set for control experiments detecting wild type PylRS activity. Fluorescence was recorded in every 10 min. For the canonical amino acids in this study, we used the same method as for ncAAs.
2.4. ATP-PPi exchange assay
The PylRS variants FRS1, FRS2, and FRS3 were produced from pET-FRS in BL21 (DE3) and purified as previously described [13], As reported in earlier studies [10,15], the truncated version (to overcome solubility problems) of the enzyme (residues 185-454) was used in the ATR-PPi exchange assay [13,16], The reaction mixtures were separated on PEI-cellulose plates (Merck) in 1 M urea and 1 M monopotassium phosphate. The plates were scanned in a Molecular Dynamics Storm 860 phosphorimager (Amersham Biosciences).
3. Results
3.1. Selection of PyIRS mutants that incorporate Phe at a UAG codon
Guided by the structure of the M. mazei PylRS active site [10,15], a library was constructed by making random mutations at six positions: Ala302, Pro303, Tyr306, Asn346, Cys348 and Tyr384. Selection for growth was based on read-through of UAG (codon 112, derived from Asp in the wild-type) in the type I chloramphenicol acetyltransferase gene present on pCAM-pylT. Colonies that grew on Cm LB-agar plates were chosen after two rounds of growth selection [13]. Ten colonies were picked and tested for canonical amino acid specificity by sfGFP formation (see below); 9 out of 10 PylRS variants were able to incorporate Phe. Two PylRS mutants, FRS2 and FRS3, supported growth on the selection plates containing 200 μg/mL Cm, indicating high suppression efficiency by the PylRS variants. We also confirmed their ability to grow on minimal medium agar plates supplemented with Phe (1 mM) and Cm (100 μg/mL).
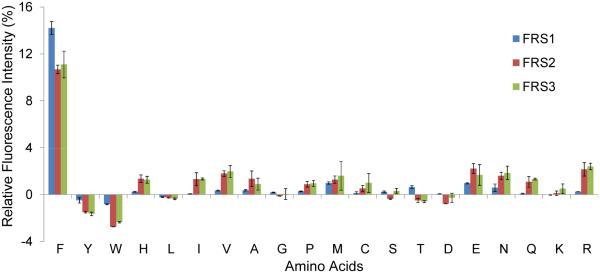
DNA sequencing revealed the amino acids at the mutation sites of FRS2 and FRS3 (Table 1). Earlier two M. mazei PylRS mutants able to charge Phe were reported [3]. When grown in LB medium one mutant in particular, the FRS1 Asn346Ala/Cys348Leu variant, showed good specificity for Phe as judged by mass spectrometric analysis of the reporter protein [3]. Since FRS1 had not been characterized further, we included it in our study of FRS2 and FRS3. We first tested the substrate specificity of these enzymes by screening for amino acid incorporation against all canonical amino acids in response to the UAG codon in position 2 of the sfGFP (sfGFP-UAG2) gene [4]. The experiment, performed in minimal medium supplemented with the individual canonical amino acids (1 mM), measured the sfGFP production level by fluorescence intensity. Addition of Phe led to the highest sfGFP production by all three PylRS variants (Fig. 1); the relative fluorescence was 14% (FRS1) or 11% (FRS2 or FRS3) compared to the value of wild type sfGFP (with Ser in position 2).
Table 1.
Sequences of PylRS variants that acylate tRNAPyl with Phe
| WT | A302 | P303 | Y306 | N346 | C348 | Y384 | Reference |
|---|---|---|---|---|---|---|---|
| FRS1 | A | P | Y | A | L | Y | [3] |
| FRS2 | L | P | M | S | L | L | This work |
| FRS3 | F | P | L | T | F | L | This work |
Figure 1.
Relative fluorescence emission of sfGFP in twenty natural amino acids with FRS variants. The y-axis units are the percentages of fluorescence intensity from sfGFP with Ser2TAG mutation compared to the fluorescence intensity of wild type sfGFP. The fluorescence intensity was measured by excitation wavelength in 485 nm/20 nm and emission wavelength 528 nm/20 nm. Each fluorescence intensity was presented after subtraction with the value from the control experiment without adding the amino acid.
To better characterize the efficiency of Phe incorporation, the PheRS activity of these PylRS variants was determined in the ATP-PPi exchange assay [13]. For the assay, we used the truncated catalytic domain of PylRS as described in Materials and Methods. All FRS variants showed similar Km and kcat values (Table 2). Their Km values for Phe are similar to each other (14-23 mM), but ~500 fold higher than the Km value of E. coli PheRS (40 μM) or of Methanosarcina barkeri PylRS (55 μM) for their cognate substrates Phe and Pyl, respectively [17,18]. The lower substrate affinity of FRS1, FRS2, and FRS3 is in line with the Km values for N -acetyllysine (8-35 mM) by N -acetyllysyl-tRNA synthetases, another set of engineered PylRS variants [13]. The kcat values of FRS1, FRS2 and FRS3 were lower than that of wild-type M. barkeri PylRS but comparable to it [18], even though E. coli PheRS had a substantially better value [17]. Taken together, the relatively low catalytic activities of the FRS enzymes can be explained by poor binding of the amino acid substrate Phe. In addition, these data suggest that mutagenesis of more than six amino acids is required to create a high affinity binding site for an acceptable aaRS substrate analog.
Table 2.
ATP-PPi exchange kinetics of PylRS variants with Phea
| Enzyme | Km (mM) | kcat (min−1) | Reference |
|---|---|---|---|
| FRS1 | 21.0 ± 3.3 | 2.2 ± 0.2 | This work |
| FRS2 | 14.0 ± 1.5 | 3.7 ± 0.1 | This work |
| FRS3 | 22.8 ± 3.8 | 4.5 ± 0.3 | This work |
| M. barkeri PylRS | 0.055 ± 0.005 | 6.3 ± 0.7 | [18] |
| E. coli PheRS | 0.040 | 8280 | [17] |
The values were calculated from three independent experiments.
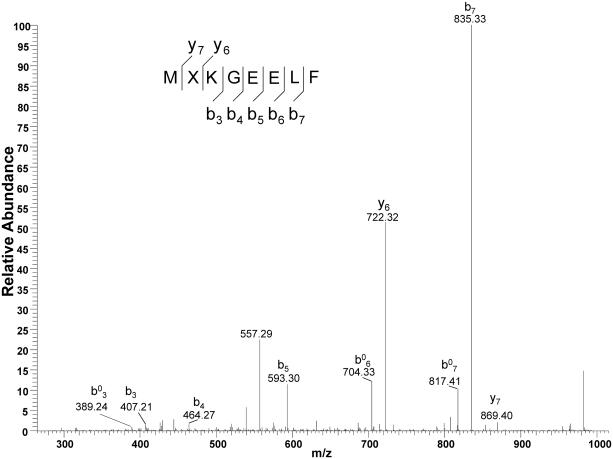
We confirmed the extent of Phe incorporation at position 2 of sfGFP (sfGFP-F2) brought about by FRS2 and FRS3 and growth in M9 medium. The molecular mass (determined by ESI-MS) of the produced sfGFP-F2 agreed well with its calculated molecular mass (Fig. S2). In addition, LC-MS/MS analysis identified y and b fragments that contain Phe at position 2 of the protein by FRS3-tRNAPyl pair (Fig. 2) or FRS2-tRNAPyl pair (Fig. S3).
Figure 2.
Mass spectrometric analysis of sfGFP-F2 that was produced using FRS3-tRNAPyl pair in M9 medium. The LC-MS/MS spectra of the MXKGEELF (X denotes F) fragment from the full-length GFP-F2 protein. b0 stands for b-H2O.
3.2. The FRS variant enzymes exhibited different substrate specificity
In order to determine the substrate range that these FRS enzymes may recognize, we decided to test their ability to utilize individual compounds from a large ncAA collection. For this reason we developed a high-throughput assay based on UAG suppression of the sfGFP-UAG2 gene. E. coli BL21 (DE3) cells co-transformed with pKTS-FRS and pET-sfGFP-pylT were cultivated in M9 medium supplemented with different ncAAs (5 mM) in 384-well plates. After 6 hr incubation production of the resulting sfGFP variants was determined by in vivo fluorescence measurements. The results were quantified using a calibration curve established with purified wild-type sfGFP (Fig. S4).
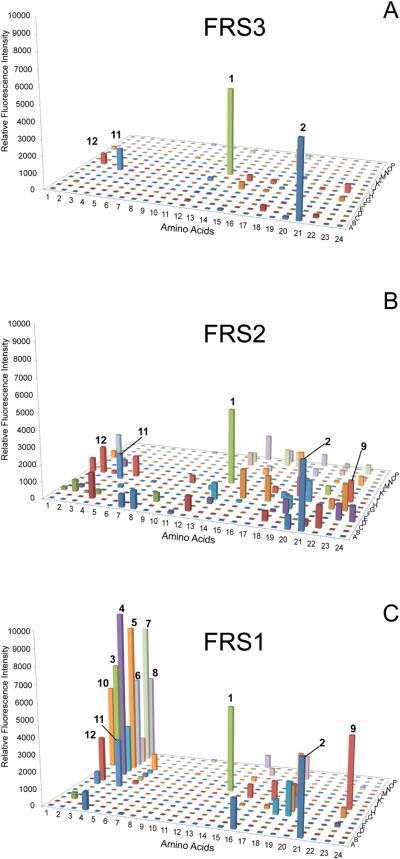
In agreement with our biochemical results (Table 2) all FRS enzymes incorporated Phe well into sfGFP as indicated by the high fluorescence signal for Phe (Fig. 3, 1, well I13) Interestingly, 2-thienyl-alanine (Fig. 3, 2, well A21) also gave high signals with all three enzymes. The data show that FRS3 is the enzyme with highest Phe (1) specificity, as only 2, 11, and 12 gave appreciable fluorescence signals (Fig. 3A). The profile of FRS2 indicated that 1 and 2 are the favorable substrates, however, many other ncAAs gave major signals (Fig. 3B). On the other hand, FRS1 (Fig. 3C) has a distinctive preference for meta- and ortho-substituted Phe analogs (ncAA 3–12, see Scheme S1); some of them exhibited higher fluorescence intensity than Phe suggesting that they are better substrates for FRS1.
Figure 3.
Range of substrate specificity of FRS enzymes. Suppression of the sfGFP-UAG2 gene by the library of ncAA-tRNAPyl was measured by fluorescence intensity. Four wells (A1, A2, I1 and I2) were set as control experiments without adding any ncAA to detect the background signals. (A) The substrate specificity profile of FRS3. (B) The substrate specificity profile of FRS2. (C) The substrate specificity profile of FRS1 The ncAA chemical structures are given in Scheme S1 and Table S2.
4. Discussion
Amino acid specificity of evolved FRS enzymes
The quantitative read-through of the UAG codon in the superfolder GFP (sfGFP-UAG2) reporter gene by ncAA-tRNAPyl species from a large library of ncAAs provided a rapid scan of the substrate spectrum of our FRS mutants. sfGFP is an ideal reporter as it is a robust, thermostable, easily produced [14], and ncAA incorporation is readily verified by mass spectrometry (Fig. 2). This will be an important tool for characterizing the specificity range of aaRS enzymes in the future. The experiment revealed that FRS3 has the highest specificity for Phe among the FRS enzymes (Fig. 3).
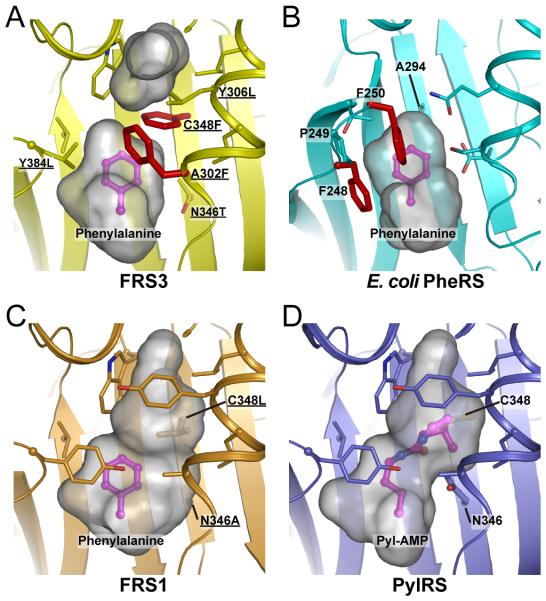
The active site of the FRS enzymes
To understand the different substrate specificities, we constructed structural models of the substrate binding sites (Fig. 4) based on the structures of PylRS and PylRS-derived O-methyl tyrosyl-tRNA synthetase (PDB ID: 2ZIM [10], 3QTC [12]). The model of FRS3 (Fig. 4A) shows a small binding pocket, similar in size to that of E. coli PheRS (Fig. 4B) [11]. Out of our library of ~300 ncAAs, only the side chain structures of Phe (1) and three additional ncAAs (2, 11, 12) are accommodated by the compact binding pocket in FRS3. In the wild-type PylRS position 348 is occupied by Cys; a mutation to Phe in FRS3 reduces the size of the pocket. Compared with wild-type PylRS, the Asn346Ala mutation allows binding of Phe analogs to FRS1 (Figs. 4C and 4D). The larger pocket accommodates in addition to 2 also the meta-substituted Phe analogs 3-8. Interestingly, the four ortho-substituted Phe analogs (9-12) were also incorporated; this is the first report that PylRS variants can expand the genetic code with compounds 9-12.
Figure 4.
The comparison of FRS3, FRS1, PylRS and E. coli PheRS structures. Structural models of FRS3 and FRS1 with Phe were generated by geometry-minimization using PHENIX [20] based on the PylRS and PylRS-derived O-methyl-tyrosyl-tRNA synthetase structures (PDB ID: 2ZIM [10] and 3QTC [12], respectively). The gray surface model indicated the volume and shape of the substrate-binding pocket; the Phe substrate (in magenta) and residues surrounding the substrate-binding pocket are shown as sticks. The residues that were chosen for the library are underlined. All figures were prepared using Pymol software (the PyMOL Molecular Graphics System, Schrödinger, LLC). (A) FRS3: Ala302Phe and Cys348Phe are located close to the substrate Phe (red sticks). (B) The co-crystal structure of E. coli PheRS with Phe (PDB ID: 3PCO [11]). Phe248 and Phe250 are conserved in bacterial PheRSs and contribute to the substrate recognition by π-interactions (red sticks). (C) FRS1: The arrangement of Ala302 and Asn346Ala can form a larger pocket for Phe analogs. (D) The co-crystal structure of PylRS with Pyl-AMP.
In the FRS3 model there are two Phe residues (Ala302Phe and Cys348Phe) located close to the Phe substrate (Fig. 4A). These Phe residues may participate in stabilizing the substrate through π-interactions of the rings as is the case for E. coli PheRS (Fig. 4B) [11]. The conserved Phe-Pro-Phe motif in bacterial PheRSs is considered an element for recognition of the substrate [19]. It appears that Ala302Phe and Cys348Phe restrict the rotation of Phe by π-interactions and form a tight-fitting pocket. We suggest that the smaller size of the binding pocket and the Phe residues close to the substrate contribute to the restricted substrate specificity of FRS3.
Supplementary Material
Highlights.
Molecular evolution of PylRS reveals the ancestral PheRS activity.
Superfolder GFP reporter enables rapid analysis of PylRS substrate specificity.
The pocket size is critical to Phe recognition and rejection of Phe analogs.
Acknowledgements
This work was supported by the National Institute for General Medical Sciences (GM22854) and the Defense Advanced Research Projects Agency (contract N66001-12-C-4020). A.N. is a Japan Society for the Promotion of Science Postdoctoral Fellow for Research Abroad. We thank T.T. Lam, K. Wilczak, and E. Z. Voss, W.M. Keck Foundation Biotechnology Resource Laboratory, Yale University, for the high-resolution mass spectrometry data. We are grateful to K. Yamashita for advice on structural models, and thank J. Ling and P. O’Donoghue for critical discussions and helpful insights.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Gaston MA, Jiang R, Krzycki JA. Functional context, biosynthesis, and genetic encoding of pyrrolysine. Curr Opin Microbiol. 2011;14:342–349. doi: 10.1016/j.mib.2011.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Yuan J, O'Donoghue P, Ambrogelly A, Gundllapalli S, Sherrer RL, Palioura S, Simonovic M, Söll D. Distinct genetic code expansion strategies for selenocysteine and pyrrolysine are reflected in different aminoacyl-tRNA formation systems. FEBS Lett. 2010;584:342–349. doi: 10.1016/j.febslet.2009.11.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Wang YS, Russell WK, Wang Z, Wan W, Dodd LE, Pai PJ, Russell DH, Liu WR. The de novo engineering of pyrrolysyl-tRNA synthetase for genetic incorporation of L-phenylalanine and its derivatives. Mol Biosyst. 2011;7:714–717. doi: 10.1039/c0mb00217h. [DOI] [PubMed] [Google Scholar]
- 4.Wang YS, Fang X, Wallace AL, Wu B, Liu WR. A rationally designed pyrrolysyl-tRNA synthetase mutant with a broad substrate spectrum. J Am Chem Soc. 2012;134:2950–2953. doi: 10.1021/ja211972x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Wang YS, Fang X, Chen HY, Wu B, Wang ZU, Hilty C, Liu WR. Genetic incorporation of twelve meta-substituted phenylalanine derivatives using a single pyrrolysyl-tRNA synthetase mutant. ACS Chem Biol. 2013;8:405–415. doi: 10.1021/cb300512r. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hoesl MG, Budisa N. Recent advances in genetic code engineering in Escherichia coli. Curr Opin Biotechnol. 2012;23:751–757. doi: 10.1016/j.copbio.2011.12.027. [DOI] [PubMed] [Google Scholar]
- 7.Wan W, Huang Y, Wang Z, Russell WK, Pai PJ, Russell DH, Liu WR. A facile system for genetic incorporation of two different noncanonical amino acids into one protein in Escherichia coli. Angew. Chem. Int. Ed. Engl. 2010;49:3211–3214. doi: 10.1002/anie.201000465. [DOI] [PubMed] [Google Scholar]
- 8.Davis L, Chin JW. Designer proteins: applications of genetic code expansion in cell biology. Nat Rev Mol Cell Biol. 2012;13:168–182. doi: 10.1038/nrm3286. [DOI] [PubMed] [Google Scholar]
- 9.Liu WR, Wang YS, Wan W. Synthesis of proteins with defined posttranslational modifications using the genetic noncanonical amino acid incorporation approach. Mol Biosyst. 2011;7:38–47. doi: 10.1039/c0mb00216j. [DOI] [PubMed] [Google Scholar]
- 10.Kavran JM, Gundllapalli S, O'Donoghue P, Englert M, Söll D, Steitz TA. Structure of pyrrolysyl-tRNA synthetase, an archaeal enzyme for genetic code innovation. Proc Natl Acad Sci U S A. 2007;104:11268–11273. doi: 10.1073/pnas.0704769104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Mermershtain I, Finarov I, Klipcan L, Kessler N, Rozenberg H, Safro MG. Idiosyncrasy and identity in the prokaryotic Phe-system: crystal structure of E. coli phenylalanyl-tRNA synthetase complexed with phenylalanine and AMP. Protein Sci. 2011;20:160–167. doi: 10.1002/pro.549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Takimoto JK, Dellas N, Noel JP, Wang L. Stereochemical basis for engineered pyrrolysyl-tRNA synthetase and the efficient in vivo incorporation of structurally divergent non-native amino acids. ACS Chem Biol. 2011;6:733–743. doi: 10.1021/cb200057a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Umehara T, Kim J, Lee S, Guo LT, Söll D, Park HS. N-acetyl lysyl-tRNA synthetases evolved by a CcdB-based selection possess N-acetyl lysine specificity in vitro and in vivo. FEBS Lett. 2012;586:729–733. doi: 10.1016/j.febslet.2012.01.029. [DOI] [PubMed] [Google Scholar]
- 14.Pédelacq JD, Cabantous S, Tran T, Terwilliger TC, Waldo GS. Engineering and characterization of a superfolder green fluorescent protein. Nat Biotechnol. 2006;24:79–88. doi: 10.1038/nbt1172. [DOI] [PubMed] [Google Scholar]
- 15.Yanagisawa T, Ishii R, Fukunaga R, Kobayashi T, Sakamoto K, Yokoyama S. Crystallographic studies on multiple conformational states of active-site loops in pyrrolysyl-tRNA synthetase. J Mol Biol. 2008;378:634–652. doi: 10.1016/j.jmb.2008.02.045. [DOI] [PubMed] [Google Scholar]
- 16.Herring S, Ambrogelly A, Polycarpo CR, Söll D. Recognition of pyrrolysine tRNA by the Desulfitobacterium hafniense pyrrolysyl-tRNA synthetase. Nucleic Acids Res. 2007;35:1270–1278. doi: 10.1093/nar/gkl1151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ibba M, Kast P, Hennecke H. Substrate specificity is determined by amino acid binding pocket size in Escherichia coli phenylalanyl-tRNA synthetase. Biochemistry. 1994;33:7107–7112. doi: 10.1021/bi00189a013. [DOI] [PubMed] [Google Scholar]
- 18.Li WT, Mahapatra A, Longstaff DG, Bechtel J, Zhao G, Kang PT, Chan MK, Krzycki JA. Specificity of pyrrolysyl-tRNA synthetase for pyrrolysine and pyrrolysine analogs. J Mol Biol. 2009;385:1156–1164. doi: 10.1016/j.jmb.2008.11.032. [DOI] [PubMed] [Google Scholar]
- 19.Moor N, Lavrik O, Favre A, Safro M. Prokaryotic and eukaryotic tetrameric phenylalanyl-tRNA synthetases display conservation of the binding mode of the tRNA(Phe) CCA end. Biochemistry. 2003;42:10697–10708. doi: 10.1021/bi034732q. [DOI] [PubMed] [Google Scholar]
- 20.Adams PD, Afonine PV, Bunkoczi G, Chen VB, Davis IW, Echols N, Headd JJ, Hung LW, Kapral GJ, Grosse-Kunstleve RW, McCoy AJ, Moriarty NW, Oeffner R, Read RJ, Richardson DC, Richardson JS, Terwilliger TC, Zwart PH. PHENIX: a comprehensive Python-based system for macromolecular structure solution. Acta Crystallogr D Biol Crystallogr. 2010;66:213–221. doi: 10.1107/S0907444909052925. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.