Abstract
Children born very prematurely (≤32 weeks) often exhibit visual-perceptual difficulties at school-age, even in the absence of major neurological impairment. The alterations in functional brain activity that give rise to such problems, as well as the relationship between adverse neonatal experience and neurodevelopment, remain poorly understood. Repeated procedural pain-related stress during neonatal intensive care has been proposed to contribute to altered neurocognitive development in these children. Due to critical periods in the development of thalamocortical systems, the immature brain of infants born at extremely low gestational age (ELGA; ≤28 weeks) may have heightened vulnerability to neonatal pain. In a cohort of school-age children followed since birth we assessed relations between functional brain activity measured using magnetoencephalogragy (MEG), visual-perceptual abilities and cumulative neonatal pain. We demonstrated alterations in the spectral structure of spontaneous cortical oscillatory activity in ELGA children at school-age. Cumulative neonatal pain-related stress was associated with changes in background cortical rhythmicity in these children, and these alterations in spontaneous brain oscillations were negatively correlated with visual-perceptual abilities at school-age, and were not driven by potentially confounding neonatal variables. These findings provide the first evidence linking neonatal painrelated stress, the development of functional brain activity, and school-age cognitive outcome in these vulnerable children.
Keywords: Preterm, Magnetoencephalography, Neural oscillation, Neonatal pain, Cognitive outcome, Spontaneous brain activity, Visual perception, Prematurity, Spontaneous brain activity, Child development, Resting-state, Pain, Cognition, Perception, Development
1. Introduction
Children born very preterm (≤32 weeks) have an increased rate of intellectual impairment [2,7], which is associated with atypical brain development [23,40–42]. There are longstanding concerns that neonatal procedural pain may affect neurodevelopment in very preterm children [16], but empirical data are scant. In very preterm infants, greater cumulative neonatal pain-related stress has been associated with poorer cognitive and motor development [21], altered developmental trajectory of stress hormone (cortisol) expression [17,19], and impaired neonatal brain development [6]. This suggests that the impact of pain is different in the very preterm neonate due to immaturity in the developing brain and the stress and nociceptive systems [18]. Differential alterations in stress-system programming also suggest that the effects of neonatal pain are different in children born at extremely low gestational age (ELGA; 24 to 28 weeks) and at very low gestational age (VLGA; 28 to 32 weeks) [17,19]. Because of central sensitization in very preterm neonates, there are spinoff effects of repeated pain such that changes in the tactile threshold are induced, depending on prior events [18]. Thus the impact of neonatal pain in this population is more accurately conceived as pain-related stress rather than being limited to nociception. Therefore we use the term pain to denote pain-related stress.
Thalamocortical connectivity is undergoing various stages of development during the ELGA and VLGA periods [32]and is critical for how sensory information, including pain, is transmitted and processed in the neonatal brain [33]. The subplate, a transient structure critical for development of thalamocortical connectivity, reaches peak size during the ELGA period and is highly vulnerable to insult [38]. Thalamocortical interactions are critical for cortical oscillations [25], which are vital for cognition and perception [52,64]. Therefore, we compared long-term effects of pain in children born at ELGA compared to those born at VLGA. The spectral structure of cortical oscillations, expressed in power ratios among oscillations in different frequency ranges, develops throughout childhood [8,28] and is altered in at-risk children [1,9,34]. Young adults born at extremely low birth weight display an atypical ratio of low- to high-frequency power in resting brain rhythms [43], likely reflecting the development of functional brain activity as alpha- and gamma-band oscillations, which are understood to play reciprocal roles in cognition and perception [12,15,22,26, 29,30,39,51].
Very preterm children often display selective difficulties at school age in visual-perceptual abilities [eg, 4, 21, 60, 67]. We previously found alterations in the spectrum of spontaneous neuromagnetic oscillations in school-age children born very prematurely (≤32 weeks gestational age (GA)) [10] and demonstrated that such atypicalities are related to selective difficulties in visual-perceptual abilities in this population [11]. In the present study, we used magnetoencephalography (MEG) to investigate spontaneous neuromagnetic activity in schoolage ELGA, VLGA and full-term children. Among the preterm children, we examined cumulative neonatal pain (adjusted for clinical confounders) in relation to spontaneous neuromagnetic oscillations and to school-age visual-perceptual abilities. Due the distinct phases of thalamocortical development occurring in the ELGA and VLGA periods, we hypothesized that neonatal pain-related stress would impact primarily children born at ELGA.
2. Methods
2.1. Subjects
We studied 54 preterm children: 22 were born at ELGA (24 to 28 wks) (10 girls, 12 boys; mean age 7.74 years, SD = 0.39); and 32 were born at VLGA (28 to 32 wks) (21 girls, 11 boys; mean age 7.72 years, SD = 0.40). They were seen as part of a longitudinal study of the long-term effects of neonatal pain on neurocognitive development in children born very preterm [17,19,21]. Neonatal characteristics for the preterm group are provided in Table 1. Twenty-five age-matched full-term control children (17 girls, 8 boys; mean age 7.61 years, SD = 0.46) were recruited from the longitudinal study and from the community by advertisement. Informed consent was obtained from each child and parent. Exclusion criteria were major sensory, motor or cognitive impairment, current psychoactive medications (eg, Ritalin for attention deficit hyperactivity disorder) or significant brain injury (periventricular leukomalacia or grade III–IV intraventricular hemorrhage) as evidenced on neonatal cranial ultrasound [50]. As expected, ELGA infants had significantly higher scores on all neonatal risk and illness factors (eg, gestational age, illness severity, Supplemental Nutrition Assistance Program (SNAP) II, number of skin-breaking procedures, and days of mechanical ventilation, compared to VLGA infants (see Table 1). This study was approved by the Clinical Research Ethics Board of the University of British Columbia and the Research Ethics Board of the Children's and Women's Health Centre of British Columbia and conforms to the Declaration of Helsinki.
Table 1.
Sample characteristics by group (mean, SD).
| ELGAa (n = 22) | VLGAb (n = 32) | Full term (n = 25) | P value* | |
|---|---|---|---|---|
| Child age at MEG (years) mean (SD) | 7.7 (0.4) | 7.7 (0.4) | 7.6 (0.5) | 0.476 |
| Male gender, n (% male) | 12 (54.5%) | 11 (34.4%) | 8 (32.0%) | 0.226 |
| Gestational age at birth (weeks) mean (SD) | 27.1 (1.3) | 31.1 (1.3) | 39.9 (1.3) | 0.0001 |
| Birth weight (g) mean (SD) | 953 (244) | 1593 (354) | 3463 (488) | 0.0001 |
| Illness severity day 1 (SNAP-II) mean (SD) | 18 (8) | 5 (7) | – | 0.001 |
| Pain-related stress (number of skin-breaking procedures from birth to term) mean (SD) | 174 (98) | 60 (45) | – | 0.001 |
| Mechanical ventilation (days) mean (SD) | 23.45 (21.2) | 1.4 (3.2) | – | 0.0001 |
| WISC-IV Perceptual Reasoning Index (SD) | 97.45 (15.06) | 105.22 (15.64) | 111.76 (14.08) | 0.007 a < c |
| WISC-IV Processing Speed Index (SD) | 89.85 (12.14) | 99.61 (12.86) | 104.52 (17.58) | 0.004 a < c |
| Beery Perception subscore | 99.00 (16.31) | 105.97 (14.22) | 114.48 (15.70) | 0.003 a < c |
MEG, magnetoencephalography.
P value between groups in 1-way ANOVAs with post hoc Bonferroni tests.
2.2. MEG recording
Two minutes of spontaneous MEG activity was recorded from each child using a 151-channel whole-head CTF system (VSM Med-Tech, Port Coquitlam, Canada). Children were supine and viewed a “happy face” projected onto a screen positioned 40 cm above their eyes. A research assistant accompanied the children in the recording chamber in order to ensure that the child's eyes remained open and fixated on the visual stimulus. A log was kept of each child's behavior, and we did not find that the ELGA or VLGA children had more difficulty maintaining their focus. Data were digitized continuously at 1200 Hz. Fiducial coils were attached at the child's nasion and left and right preauricular points and energized at distinct high frequencies.
2.3. MEG analysis
To standardize head position relative to sensor locations between and within children, fiducial coils were localized 30 times per second. Energy emitted by the fiducial coils was removed using notch filters, and data were then aligned to a common position by performing an inverse solution, data rotation and forward solution 30 times per second [66]. Data were downsampled to 600 Hz, and ocular and nonocular artifacts were then removed using a principal components analysis–based procedure [24]. To estimate the magnitude of cortical oscillatory activity within functionally distinct frequency ranges, data were filtered into alpha (8 – 14 Hz) and gamma (30 – 60 Hz) frequency ranges and power was calculated for each frequency range at each of 151 sensors. Power was then averaged across all time points within the 2-minute recording period and subsequently averaged across all sensors to index the global (cortex-wide) oscillatory activity within each frequency range.
The ratio of gamma to alpha power was then calculated for each child. This measure was selected because it represents the normative structure of spontaneous neuromagnetic activity and is sensitive to the development of functional cortical activity during childhood and its alteration in clinical populations. Considerable evidence indicates that alpha oscillations are the dominant rhythm during a resting or idling brain state [30,51]. Gamma oscillations, conversely, characterize oscillatory brain activity relevant to active performance of cognitive tasks [12,15,22,26,29,31]. Accordingly, engagement in a particular cognitive task has been associated with reciprocal changes in alpha and gamma power [12,22,29,39], which has led to the proposal that reciprocal changes in alpha and gamma power reflect shifts between normative structures of cortical rhythmicity, reflecting spontaneous activity and task processing [27]. Aberrant alpha-gamma relationships have been linked to abnormal brain function in multiple clinical populations [35,36,44,55]. Development of the oscillatory structure of spontaneous brain activity is related to child development [1,28], including during the perinatal period that corresponds to premature birth [49]. Such ratio-based techniques have been successfully employed by previous studies of the maturation of functional brain activity during childhood [8,28] and its alteration in clinical child populations [1,9,34,43]. In particular, alterations of the ratio between high- and low-frequency oscillations has been reported in young adults born at extremely low birth weight [43].
2.4. Psychometric testing
Subsequent to MEG recording, assessment of cognitive function was carried out by a psychometrician. Because of the selective vulnerability of visual-perceptual abilities in children born very prematurely [4,20,53,54,67], together with our previous findings that altered MEG spectral structure in very preterm children is related to these abilities and not to overall intelligence [11], we focused on measures of visual-perceptual abilities in the present study. Specifically, we investigated relationships between MEG spectral structure, cumulative neonatal pain, and the Perceptual Reasoning Index and Processing Speed Index of the Wechsler Intelligence Scale for Children (WISC-IV) [65] and the Visual Perception Subscore of the Beery-Buktenica Developmental Test of Visual-Motor Integration [5].
2.5. Neonatal data
A detailed and systematic nursing- and medical-chart review was carried out by a highly experienced research nurse as described previously [21]. Variables included but were not limited to birth weight, gestational age, early illness severity (SNAP-II) on day 1, days of mechanical ventilation, daily dose of morphine and midazolam, number of surgeries, and number of skin-breaking procedures (eg, heel lance, intramuscular injection, intravenous or central line insertion). Measures were calculated from birth to term equivalent or hospital discharge, whichever came first. Cumulative neonatal pain was operationalized as the sum of all skin-breaking procedures. Cumulative exposure to morphine and midazolam was calculated as the average daily dose (ie, intravenous dose plus converted oral dose) adjusted for daily body weight, multiplied by the number of days the drug was given.
2.6. Statistical analysis
Child and demographic characteristics were analyzed using ANOVA to examine differences among the groups (ELGA, VLGA and full term). Associations between the spectral structure of spontaneous MEG oscillations and psychometric variables were analyzed using Pearson correlations. To address the central study questions, first ANOVA was carried out on the functional MEG data, with group and gender as between-child factors. Hierarchical regression was used to examine the relationship between cumulative neonatal pain and resting brain activity, controlling for medical confounders associated with the number of skin-breaking procedures in the preterm group only. To test whether greater cumulative neonatal pain predicted the gamma-alpha index, we selected clinical medical neonatal confounders a priori and simultaneously entered the neonatal confounder variables as predictors into the first block of the hierarchical regression, then entered the number of skin-breaking procedures (pain) into the second block, with the gamma-alpha index as the outcome.
3. Results
3.1. Psychometric assessment
WISC-IV Perceptual Reasoning and Processing Speed Index scores and the Perception subscore of the Beery-Buktenica Developmental Test of Visual-Motor Integration were compared across the groups using 1-way ANOVA followed by Bonferroni post hoc comparisons. The results are shown in Table 1.
3.2. Spontaneous MEG activity
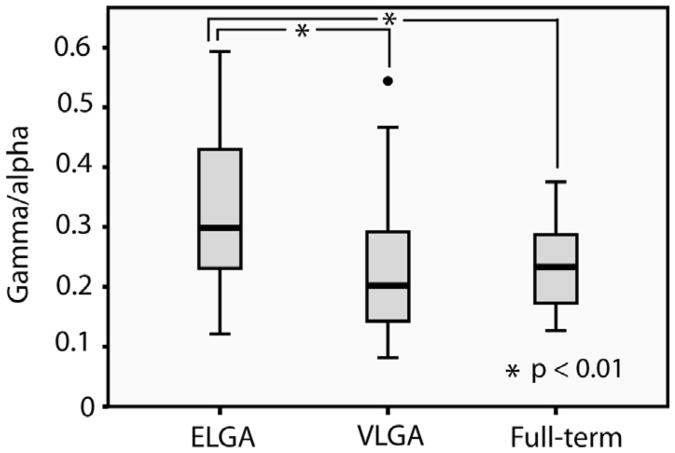
The cortex-wide ratio of spontaneous gamma- to alpha-band neuromagnetic oscillations differed significantly between the groups (F (2,78) = 5.54, P = 0.006); there was no difference by gender (F (1,78) = <1, P = 0.93), and no group × gender interaction (F (2,78) = <1, P = 0.81). Post hoc t-tests revealed that the ratio of gamma oscillations to alpha rhythms was significantly higher in the ELGA group (mean = 0.333, SD = 0.149) compared to the VLGA (mean = 0.232, SD = 0.119) and compared to the full-term controls (mean = 0.233, SD = 0.069), t(52) = 2.76, P = 0.008, 95% CI .028- .174, and t(45) = 2.882, P = 0.007, 95% CI .029-.171 respectively (Fig. 1). The VLGA and full-term groups did not differ (t[55] <1, P = 0.97).
Fig. 1.
Group differences in the spectral structure of spontaneous MEG oscillations. The dot represents an outlier.
3.3. Spectral structure of spontaneous MEG oscillations and visual- processing abilities
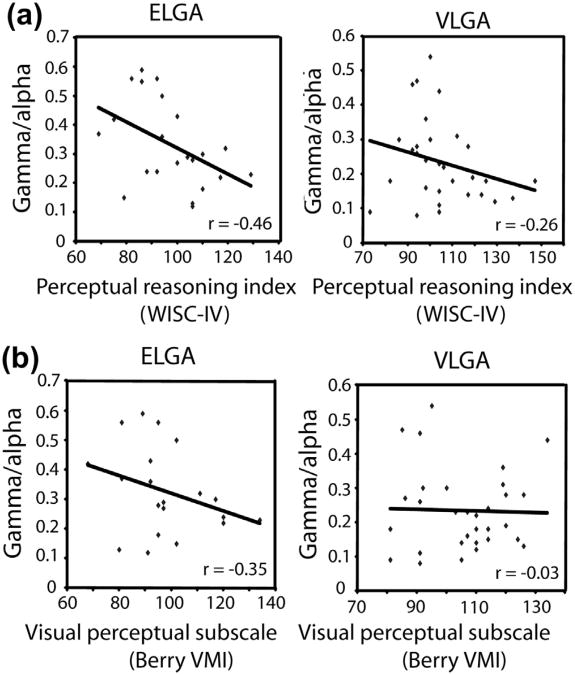
The ratio of spontaneous gamma oscillations to alpha rhythms was negatively correlated with the Perceptual Reasoning Index of the WISC-IV in ELGA children (r = −0.46, P = 0.033; Fig. 2A) but was not statistically significant for the VLGA children (r = −0.26, P = 0.155). The cortex-wide ratio of spontaneous gamma- to alpha-band was also negatively correlated with the Visual Perception Subscore of the Beery Visual-Motor Integration in children born at ELGA, but not at VLGA (r = −0.35; P = 0.127 and = −0.03, P = 0.868, respectively; Fig. 2B). The Processing Speed Index was not significantly correlated with the gamma-alpha ratio for the ELGA or VLGA children; r < 0.25. None of these measures was significantly related to MEG activity for the full-term control group (each, r < 0.25). Because of unequal sample sizes per group and the direct relationship of sample size to P value, we used the approach of Cohen that r > .30 reflects a medium effect size. In summary, visual-perceptual abilities but not speed of processing were related to the spectral architecture of background neuromagnetic rhythmicity in ELGA children but not in VLGA or full-term children.
Fig. 2.
Correlations between MEG spectral structure and visual-perceptual abilities, indexed by (a) the perceptual reasoning index of the WISC-IV; and (b) the visual perception subscore of the Beery VMI.
3.4. Neonatal pain predicts spectral structure of spontaneous MEG in ELGA children
Given that only the preterm children were exposed to neonatal intensive care, the modeling of relationships among neonatal factors was carried out only in these children. Because the ELGA group was exposed to a greater number of skin-breaking neonatal procedures, the variances differed in the ELGA and VLGA groups; further, the numbers of skin-breaking procedures were not normally distributed. Therefore, we performed a log transformation on the number of skin-breaking procedures. The variances of these log-transformed variables did not differ between the ELGA and VLGA groups (Levene's Test for Equality of Variance, P = 0.656), and the distributions were successfully normalized (Shapiro-Wilk Test of Normality for the ELGA, P = 0.472 and the VLGA, P = 0.717 groups). Moreover, cumulative morphine and days of mechanical ventilation were not normally distributed, so we applied a log transformation to these predictors as well.
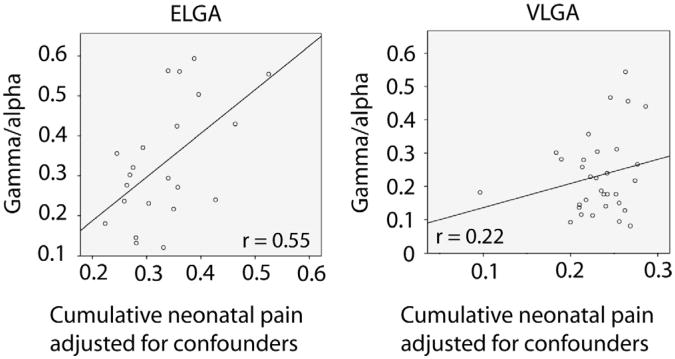
Hierarchical regression was conducted: Block 1 comprised group (ELGA or VLGA) and gender as factors; neonatal clinical confounders: number of surgeries; cumulative morphine (daily intravenous and oral doses calculated for equivalence, adjusted for daily weight); early illness severity (SNAP II on day 1; and days of mechanical ventilation. Block 2 comprised number of skin-breaking procedures (cumulative pain) and an interaction term group (ELGA or VLGA) × number of skin-breaking procedures, with spontaneous gamma-alpha MEG activity as the outcome. The results of the hierarchical regression (Table 2) showed that after controlling for the neonatal confounders, the gamma-alpha ratio differed significantly in the ELGA compared to the VLGA group (P = 0.043) and was predicted by cumulative pain (number of skin breaking procedures; P = .039), and there was a significant interaction between the groups (ELGA and VLGA) and cumulative neonatal pain (P = 0.029). No other neonatal variables were significantly associated with the gamma- to alpha-band ratio. We considered all possible interaction terms; only the interaction between the subgroup and neonatal pain was significant. The normal probability plot showed a linear pattern, indicating that the normal distribution is a good model for this dataset. R2 changed from .19 in Block 1 to .28 in Block 2 (R2 change P = 0.077). The R2 of the final fitted model was 0.28, P = 0.047. To explicate the significant interaction between groups (ELGA and VLGA) and cumulative neonatal pain, the relationship between neonatal pain (adjusted for confounders) and the gamma-alpha ratio was plotted separately for the ELGA and VLGA groups (Fig. 3). Standardized regression coefficients are provided in Table 3. With a complex regression model including both categorical and correlated continuous predictor variables, a standardized coefficient may be larger than 1 [47], as is the case in our results.
Table 2.
Results of hierarchical regression showing that the relationship between greater cumulative neonatal pain (adjusted for neonatal confounders) and the ratio of spontaneous gamma oscillations to alpha rhythms at age 7 years differs depending on the extent of prematurity at birth (extremely low gestational age or very low gestational age).
| Model | β | P value |
|---|---|---|
| Block 1a | ||
| Preterm group (ELGA, VLGA) | −.214 | .321 |
| Gender | −.029 | .843 |
| Number of surgeries | −.200 | .301 |
| Cumulative morphine (daily doses adjusted for weight) | −.042 | .900 |
| Illness severity day 1 SNAP II | .042 | .818 |
| Days of mechanical ventilation | .321 | .261 |
| Block 2b | ||
| Preterm group (ELGA, VLGA) | 3.577 | .043 |
| Gender | −.058 | .678 |
| Number of surgeries | −.280 | .150 |
| Cumulative morphine (daily doses adjusted for weight) | −.156 | .631 |
| Illness severity day 1 SNAP II | .079 | .652 |
| Days of mechanical ventilation | −.073 | .839 |
| Cumulative pain (number of skin breaking procedures) | 2.122 | .039 |
| Preterm group (ELGA, VLGA) × cumulative pain | −2.962 | .029 |
Neonatal factors associated with prematurity and cumulative pain; chart review was conducted from birth to term equivalent age.
Cumulative pain and the interaction of preterm group (ELGA, VLGA) × cumulative pain was added to the model.
Fig. 3.
Interaction effects are illustrated by showing the regression slopes for the ELGA and VLGA groups separately. Associations between neonatal pain and spontaneous MEG oscillations for the ELGA and VLGA groups, adjusted for medical confounders (number of surgeries, cumulative morphine, illness severity, and number of days on mechanical ventilation) are displayed as the plot of the residuals computed from regression analysis.
Table 3.
Pearson correlations among neonatal variables for the preterm children.
| Neonatal predictors | Skin-breaking procedures (pain-related stress) | Early illness severity (SNAP-II on day 1) | Mechanical ventilation (days) | Number of surgeries |
|---|---|---|---|---|
| Early illness severity (SNAP-II on day 1) | .44** | |||
| Mechanical ventilation (days) | .74*** | .42** | ||
| Number of surgeries | .41** | .36* | .40** | |
| Cumulative morphine | .57** | .40** | .75*** | .648*** |
P value < .01;
P value < .001;
P value < .0001.
To check for multicollinearity, Pearson correlations among the neonatal predictors were examined (Table 3). Condition indexes and the variance inflation factor (VIF) of the regression model were computed to detect multicollinearity. The maximum Condition Index for the final model was 20.03 (Condition Index <30) and VIF was 3.01 (VIF <5), which indicated the regression had insignificant multicollinearity. However, due to the relatively high bivariate correlations between days of mechanical ventilation and cumulative morphine exposure (r = 0.75; P < .0001) and number of skin-breaking procedures (r = .74; P < .0001), the hierarchical regression model was rerun excluding mechanical ventilation from the neonatal variables in Block 1. The results remained the same, with the interaction between the ELGA and VLGA groups and the number of skin-breaking procedures being statistically significant (P = .012). Therefore, we had confidence that the finding was stable. R2 improved significantly from .17 in Block 1 to R2 .28 in Block 2 (change in R2; P = 0.04); the R2 of the final fitted model was 0.28; P = 0.027.
We then reran the hierarchical regression to examine whether postnatal steroid exposure (days on dexamethasone) and postnatal benzodiazepine exposure (days on midazolam) might alter the results of the main model. Neither medication was significantly associated with the ratio of alpha- to gamma-band activity (each P > 0.192).
4. Discussion
We provide the first evidence linking neonatal pain-related stress, functional brain activity, and visual-perceptual abilities at school age in children born at ELGA. Our findings underscore the influence of cumulative neonatal procedural pain on the long-term neurodevelopment of extremely preterm children and illuminate the relationships among specific aspects of adverse neonatal experience, the altered development of functional brain activity, and the variability in selective developmental difficulties in domains such as visual-perceptual ability observed in these children, even in the absence of neurological impairment. It is important to note that our findings diverged depending on relative maturity at birth, in that spontaneous neuromagnetic activity was atypical only in the ELGA children and was associated with neonatal pain-related stress and school-age visual-perceptual abilities only in this group. These findings provide new vistas onto how critical periods in the development of specific brain systems underlying particular functional domains, such as visual-perceptual abilities, may establish neonatal windows of heightened vulnerability to factors such as neonatal procedural pain-related stress. We found that exposure to morphine analgesia did not ameliorate associations of neonatal pain with functional brain activity, which is important information for guiding optimal clinical care of ELGA infants. This indicates that current pharmacological strategies to control neonatal pain may not be effective in mitigating the long-term influence of procedural pain on selective developmental difficulties prevalent among very preterm children. The use of hierarchical regression as the statistical approach provided the advantage of investigating the relationship among neonatal skin-breaking procedures, MEG activity and school-age outcome, independent of the effects of multiple medical confounders associated with prematurity. Using this approach, we demonstrated that the observed effects were not driven by confounding variables, such as early illness severity, mechanical ventilation, surgery, or postnatal morphine exposure. Our findings of relationships among cumulative neonatal pain, functional brain activity, and school-age cognitive outcome extend recent reports that pain [6] and stress [58] are associated with altered structural and functional brain development during the neonatal period, and that neonatal procedural pain is related to altered development of very preterm children in the first 2 years of life [17,19].
The discovery that the spectral architecture of spontaneous brain oscillations is altered and is related to cumulative neonatal pain-related stress and visual-perceptual abilities in ELGA but not VLGA children may relate to the distinct phases of development occurring within thalamocortical systems within the 24- to 28-week and the 29- to 32-week gestational periods [32]. This has implications on how painful stimuli are processed within the developing central nervous system [33]. Brain responses reflecting the processing of nociceptive stimuli are evident in the neonatal brain [57] and undergo dramatic maturation during the developmental epoch corresponding to very preterm birth [14]. The importance of this developmental period to the maturation of oscillatory brain activity is evidenced by progressive changes in the spectral composition of EEG recordings throughout the preterm period [48,49], and hemodynamic investigations of functional connectivity (fcMRI) have uncovered altered thalamocortical interactions in preterm infants [59]. Recent MRI findings from our research group concerning a different cohort of very preterm infants also suggests that early procedural pain, rather than pain throughout the NICU course, may be associated with altered brain development [6].
Altered structural thalamocortical connectivity has also been reported in very preterm children [3,13]. The generation of cortical oscillations, including those in the alpha and gamma frequency ranges, depend critically on thalamocortical interplay [25,36], and reduction in resting alpha power coincident with increased prevalence of gamma rhythms is associated with multiple pathological conditions that have been attributed to alterations in thalamocortical connectivity [35,44,55]. Such aberrations in the normative constitution of background cortical rhythmicity likely reflect disturbances in basic oscillatory mechanisms underlying the generation of cognition and perception [36,52]. The reciprocal relationship between gamma- and alpha-band oscillations plays a special role in the orchestration of cognitive and perceptual dynamics because alpha oscillations are relevant to an inhibited cortical state [20,27] and characterize idling or resting brain activity [51], whereas local gamma rhythms (>30 Hz) have been linked to active recruitment of brain regions and information processing [15,26,31]. It has been demonstrated that young adults born at extremely low birth weight express an altered ratio of low-frequency to high-frequency brain oscillations [43]. We have previously demonstrated altered expression of resting alpha oscillations in very preterm children (≤32 weeks GA) [10] and have shown that this atypical spectral structure of functional cortical oscillations is related to selective problems in visual-perceptual abilities in this group, rather than to general intelligence [11]. Our findings in the present study suggest that neonatal pain-related stress may be one factor that impacts the development of cortical oscillatory activity, which depends critically on thalamocortical mechanisms [25] and plays a critical role in the generation of cognition and perception [25,52,64].
Synchronization of oscillatory activity has been purported to reflect functional coupling relevant to the formation of task-dependent networks supporting cognition, perception and motor control [62,63], and the disturbance of such mechanisms has been linked to various neurological and neuropsychiatric conditions [56]. Oscillatory mechanisms of long-range connectivity show maturation throughout childhood [61], and alterations in inter-regional oscillatory communication have been identified in conditions affecting childhood cognitive development [37,46]. We previously demonstrated altered long-range alpha-band MEG synchronization during visual short-term memory retention in very preterm children (≤32 weeks GA), which included children born at both ELGA and VLGA, and showed that these atypicalities were correlated with visual-perceptual ability in the very preterm group [11]. Such results, taken together with the findings of the present study, indicate that changes in the expression of oscillatory brain activity, both during task performance and at rest, may play an important role in understanding the biological basis for the considerable variability in functional outcome in preterm children who escape brain injury and major intellectual, sensory and motor impairment [45,60]. Moreover, the results presented here demonstrate how the development of specific aspects of brain activity relevant to cortical activation and the formation of functional networks can be traced to particular attributes of adverse neonatal experience during developmental windows of heightened vulnerability. The finding that the structure of spontaneous oscillatory brain activity (gamma-to-alpha ratio) is not correlated with visual-perceptual abilities in full-term children indicates that these observed alterations in functional brain activity are related to developmental factors specific to prematurity, rather than to typical development. Here we demonstrated that global patterns altered spontaneous MEG activity in ELGA children and revealed relationships between cumulative neonatal pain-related stress and visual-perceptual abilities, thus providing the foundation for future research that investigates changes in oscillatory structure within specific brain systems and outcome in other functional domains.
We have presented the first evidence linking neonatal pain-related stress, functional brain activity, and school-age visual perceptual abilities in children born very prematurely. Our study was very conservative, in that we excluded children with major neonatal brain injury or major sensory, motor or cognitive impairment, as well as children on medications for attention deficit hyperactivity disorder. We also demonstrated that associations between spontaneous neuromagnetic activity, neonatal pain, and visual-perceptual abilities are specific to children born at ELGA. This discovery of the selective sensitivity of developing systems underlying functional brain oscillations during the ELGA maturational window to repeated procedural pain, together with this elucidation of relationships between development of cortical rhythmicity and school-age cognitive outcome in very preterm children who escape major impairment, reflects an important step toward mapping the effects of clinical care in the NICU on the neurodevelopment in these children.
Acknowledgments
This study was funded by grant RO1 HD039783 from the Kennedy Shriver Institute of Child Health and Human Development (NICHD/NIH) to REG. We thank Gisela Gosse for coordinating the study, Dr Rollin Brant for assistance with the statistical analysis and Katia Jitlina and Amanda Degenhardt for assistance with data collection. REG holds a Senior Scientist award with the Child and Family Research Institute. UR holds a Leadership Chair in Cognitive Neuroscience in Early Childhood Health and Development supported by the BC Leading Edge Endowment Fund (BC LEEF).
Footnotes
Conflict of interest statement: The authors have no conflicts of interest to declare.
References
- 1.Ahn H, Prichep L, John ER, Baird M, Trepetin M, Kaye H. Developmental equations reflect brain dysfunctions. Science. 1980;210:1255–8. doi: 10.1126/science.7434027. [DOI] [PubMed] [Google Scholar]
- 2.Allen MC. Neurodevelopmental outcomes of preterm infants. Curr Opin Neurobiol. 2008;21:123–8. doi: 10.1097/WCO.0b013e3282f88bb4. [DOI] [PubMed] [Google Scholar]
- 3.Anjari M, Srinivasan L, Allsop JM, Hajnal JV, Rutherford MA, Edwards AD, Counsell SJ. Diffusion tensor imaging with tract-based spatial statistics reveals local white matter abnormalities in preterm infants. NeuroImage. 2007;35:1021–7. doi: 10.1016/j.neuroimage.2007.01.035. [DOI] [PubMed] [Google Scholar]
- 4.Atkinson J, Braddick O. Visual and visuocognitive development in children born very prematurely. Prog Brain Res. 2007;164:123–49. doi: 10.1016/S0079-6123(07)64007-2. [DOI] [PubMed] [Google Scholar]
- 5.Beery KE, Buktenica NA, Beery NA. Berry-Buktenica Developmental Test of Visual-Motor Integration. 5th. Minneapolis, MN: Psychological Corporation; 2004. [Google Scholar]
- 6.Brummelte S, Grunau RE, Chau V, Poskitt KJ, Brant R, Vinall J, Gover A, Synnes AR, Miller SP. Procedural pain and brain development in premature newborns. Ann Neurol. 2012;71:385–96. doi: 10.1002/ana.22267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Behrman RE, StithButler A, editors. Institute of medicine committee on understanding premature birth and assuring healthy outcomes, board on health sciences outcomes Premature birth: causes, consequences and prevention. Washington, DC: The National Academies Press; 2007. [Google Scholar]
- 8.Clarke AR, Barry RJ, McCarthy R, Selikowitz M. Age and sex effects in the EEG: development of the normal child. Clin Neurophysiol. 2001;112:806–14. doi: 10.1016/s1388-2457(01)00488-6. [DOI] [PubMed] [Google Scholar]
- 9.Clarke AR, Barry RJ, McCarthy R, Selikowitz M. EEG-defined subtypes of children with attention-deficit hyperactivity disorder. Clin Neurophysiol. 2001;112:2098–105. doi: 10.1016/s1388-2457(01)00668-x. [DOI] [PubMed] [Google Scholar]
- 10.Doesburg SM, Ribary U, Herdman AT, Moiseev A, Cheung T, Miller SP, Poskitt KJ, Weinberg H, Whitfield MF, Synnes A, Grunau RE. Magnetoencephalography reveals slowing of peak oscillatory frequency in children born very preterm. Pediatr Res. 2011;70:171–5. doi: 10.1203/PDR.0b013e3182225a9e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Doesburg SM, Ribary U, Herdman AT, Miller SP, Poskitt KJ, Moiseev A, Whitfield MF, Synnes A, Grunau RE. Altered long-range alpha-band synchronization during visual short-term memory retention in children born very preterm. NeuroImage. 2011;54:2330–9. doi: 10.1016/j.neuroimage.2010.10.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Doesburg SM, Roggeveen AB, Kitajo K, Ward LM. Large-scale gamma-band phase synchronization and selective attention. Cereb Cortex. 2008;18:386–96. doi: 10.1093/cercor/bhm073. [DOI] [PubMed] [Google Scholar]
- 13.Dudink J, Maarten L, van Pul C, Buijs J. Fractional anisotropy in white matter tracts of very-low birth-weight infants. Pediatr Radiol. 2007;37:1216–23. doi: 10.1007/s00247-007-0626-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Fabrizi L, Slater R, Worley A, Meek J, Boyd S, Olhede S, Fitzgerald M. A shift in sensory processing that enables the developing brain to discriminate touch from pain. Curr Biol. 2011;21:1552–8. doi: 10.1016/j.cub.2011.08.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Fries P, Nikolić D, Singer W. The gamma cycle. Trends Neurosci. 2007;30:309–16. doi: 10.1016/j.tins.2007.05.005. [DOI] [PubMed] [Google Scholar]
- 16.Grunau RE. Early pain in preterm infants: a model of long-term effects. Clin Perinatol. 2002;29:373–94. doi: 10.1016/s0095-5108(02)00012-x. [DOI] [PubMed] [Google Scholar]
- 17.Grunau RE, Haley DW, Whitfield MF, Weinberg J, Yu W, Thiessen P. Altered basal cortisol levels at 3, 6, 8 and 18 months in infants born at extremely low gestational age. J Pediatr. 2007;150:151–6. doi: 10.1016/j.jpeds.2006.10.053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Grunau RE, Holsti L, Peters JWB. Long-term consequences of pain in human neonates. Arch Sem Fet Neonat Med. 2006;11:268–75. doi: 10.1016/j.siny.2006.02.007. [DOI] [PubMed] [Google Scholar]
- 19.Grunau RE, Weinberg J, Whitfield MF. Neonatal procedural pain and preterm infant cortisol response to novelty at 8 months. Pediatrics. 2004;114:e77–84. doi: 10.1542/peds.114.1.e77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Grunau RE, Whitfield MF, Davis C. Pattern of learning disabilities in children with extremely low birth weight and broadly normal intelligence. Arch Pediatr Adolesc Med. 2002;156:615–20. doi: 10.1001/archpedi.156.6.615. [DOI] [PubMed] [Google Scholar]
- 21.Grunau RE, Whitfield MF, Petrie-Thomas J, Synnes AR, Cepeda IL, Keidar A, Rogers M, Mackay M, Hubber-Richard P, Johannesen D. Neonatal pain, parenting stress and interaction, in relation to cognitive and motor development at 8 and 18 months in preterm infants. PAIN®. 2009;143:138–46. doi: 10.1016/j.pain.2009.02.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Haegens S, Osipova D, Oostenveld R, Jensen O. Somatosensory working memory performance in humans depends on both engagement and disengagement of regions in a distributed network. Hum Brain Mapp. 2007;31:26–35. doi: 10.1002/hbm.20842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hart AR, Whitby EW, Griffiths PD, Smith MF. Magnetic resonance imaging and developmental outcome following preterm birth: review of current evidence. Dev Med Child Neurol. 2008;50:655–63. doi: 10.1111/j.1469-8749.2008.03050.x. [DOI] [PubMed] [Google Scholar]
- 24.Herdman AT, Cheyne D. A practical guide to MEG and beam forming. In: Handy TC, editor. Brain signal analysis: advances in neuroelectric and neuromagnetic methods. Cambridge, MA: MIT Press; 2009. pp. 99–140. [Google Scholar]
- 25.Hughes SW, Crunelli V. Thalamic mechanisms of EEG alpha rhythms and their pathological implications. Neuroscientist. 2005;11:357–72. doi: 10.1177/1073858405277450. [DOI] [PubMed] [Google Scholar]
- 26.Jensen O, Kaiser J, Lachaux JP. Human gamma-frequency oscillations associated with attention and memory. Trends Neurosci. 2007;30:317–24. doi: 10.1016/j.tins.2007.05.001. [DOI] [PubMed] [Google Scholar]
- 27.Jensen O, Mazaheri A. Shaping functional architecture by oscillatory brain activity: gating by inhibition. Front Hum Neurosci. 2010;4:186. doi: 10.3389/fnhum.2010.00186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.John ER, Ahn H, Prichep L, Trepetin M, Brown D, Kaye H. Developmental equations for the electroencephalogram. Science. 1980;210:1255–8. doi: 10.1126/science.7434026. [DOI] [PubMed] [Google Scholar]
- 29.Jerbi K, Ossandón T, Hamamé CM, Senova S, Dalal SS, Jung J, Minotti L, Bertrand O, Berthoz A, Kahane P, Lachaux JP. Task-related gamma-band dynamics from an intracerebral perspective: review and implications for surface EEG and MEG. Hum Brain Mapp. 2009;30:1758–71. doi: 10.1002/hbm.20750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Klimesch W, Sauseng P, Hanslmayr S. EEG alpha oscillations: the inhibition timing hypothesis. Brian Res Rev. 2007;53:63–88. doi: 10.1016/j.brainresrev.2006.06.003. [DOI] [PubMed] [Google Scholar]
- 31.Kaiser J, Lutzenberger W. Human gamma-band activity: a window to cognitive processing. Neuroreport. 2005;16:207–11. doi: 10.1097/00001756-200502280-00001. [DOI] [PubMed] [Google Scholar]
- 32.Kostovic I, Jovanov-Miloševic N. Development of cerebral connections during the first 20-45 weeks' gestation. Sem Fetal Neonat Med. 2006;11:415–22. doi: 10.1016/j.siny.2006.07.001. [DOI] [PubMed] [Google Scholar]
- 33.Kostović I, Judas M. The development of the subplate and thalamocortical connections in the human fetal brain. Acta Paediatr. 2010;99:1119–27. doi: 10.1111/j.1651-2227.2010.01811.x. [DOI] [PubMed] [Google Scholar]
- 34.Kulandaivel K, Holmes GL. Power spectral analysis in infants with seizures: relationship to development. Epilepsy Behav. 2011;20:700–5. doi: 10.1016/j.yebeh.2011.02.021. [DOI] [PubMed] [Google Scholar]
- 35.Llinás RR, Ribary U, Jeanmonod D, Kronberg E, Mitra PP. Thalamocortical dysrhythmia: a neurological and neuropsychiatric syndrome characterized by magnetoencephalography. Proc Natl Acad Sci USA. 1999;96:15222–7. doi: 10.1073/pnas.96.26.15222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Llinás R, Urbano FJ, Leznik E, Ramírez RR, van Marle HJ. Rhythmic and dysrhythmic thalamocortical dynamics: GABA systems and the edge effect. Trends Neurosci. 2005;28:325–33. doi: 10.1016/j.tins.2005.04.006. [DOI] [PubMed] [Google Scholar]
- 37.Mazaheri A, Coffey-Corina S, Mangun GR, Bekker EM. Functional disconnection of frontal cortex and visual cortex in attention-deficit/hyperactivity disorder. Biol Psychiatry. 2010;67:617–23. doi: 10.1016/j.biopsych.2009.11.022. [DOI] [PubMed] [Google Scholar]
- 38.McQuillen PS, Ferriero DM. Selective vulnerability in the developing central nervous system. Paediatr Neurol. 2004;30:227–35. doi: 10.1016/j.pediatrneurol.2003.10.001. [DOI] [PubMed] [Google Scholar]
- 39.Meeuwissen EB, Takashima A, Fernández G, Jensen O. Increase in posterior alpha activity during rehearsal predicts long-term memory formation of word sequences. Front Hum Neurosci. 2011;4:186. doi: 10.1002/hbm.21167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Ment LR, Hirtz D, Hüppi PS. Imaging biomarkers of outcome in the developing preterm brain. Lancet Neurol. 2009;8:1042–55. doi: 10.1016/S1474-4422(09)70257-1. [DOI] [PubMed] [Google Scholar]
- 41.Mento G, Bisiachi PS. Neurocognitive development in preterm infants: insights from different approaches. Lancet Neurol. 2012;8:1042–55. doi: 10.1016/j.neubiorev.2011.08.008. [DOI] [PubMed] [Google Scholar]
- 42.Miller SP, Ferriero DM. From selective vulnerability to connectivity: insights from newborn brain imaging. Trends Neurosci. 2009;32:496–505. doi: 10.1016/j.tins.2009.05.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Miskovic V, Schmidt LA, Boyle M, Saigal S. Regional electroencephalogram (EEG) spectral power and hemispheric coherence in young adults born at extremely low birth weight. Clin Neurophysiol. 2009;120:231–8. doi: 10.1016/j.clinph.2008.11.004. [DOI] [PubMed] [Google Scholar]
- 44.Moazami-Goudarzi M, Sarnthein J, Michels L, Moukhtieva R, Jeanmonod D. Enhanced frontal low and high frequency power synchronization in the resting EEG of parkinsonian patients. NeuroImage. 2008;41:985–97. doi: 10.1016/j.neuroimage.2008.03.032. [DOI] [PubMed] [Google Scholar]
- 45.Mulder H, Pichford NJ, Haggar MS, Marlow N. Development of executive function and attention in preterm children: a systematic review. Dev Neuropsychol. 2009;34:393–421. doi: 10.1080/87565640902964524. [DOI] [PubMed] [Google Scholar]
- 46.Murias M, Webb SJ, Greenson J, Dawson G. Resting state cortical connectivity reflected in EEG coherence in individuals with autism. Biol Psychiatry. 2007;62:270–3. doi: 10.1016/j.biopsych.2006.11.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Nichols DP. Using categorical variables in regression. SPSS Keywords. 1995;56 Available at < http://www.ats.ucla.edu/stat/spss/library/catreg.htm>; 1995. [Google Scholar]
- 48.Okumura A, Kubota T, Toyota N, Kidokoro H, Maruyama K, Kato T, Hayakawa F, Watanabe K. Amplitude spectral analysis of maturational changes in delta waves in preterm infants. Brain Dev. 2003;25:406–10. doi: 10.1016/s0387-7604(03)00027-5. [DOI] [PubMed] [Google Scholar]
- 49.Okamura A, Kubota T, Tsuji T, Hayakawa F, Watanabe K. Amplitude spectral analysis of theta/alpha/beta waves in preterm infants. Pediatr Neurol. 2006;34:30–4. doi: 10.1016/j.pediatrneurol.2005.06.005. [DOI] [PubMed] [Google Scholar]
- 50.Papile LA, Burstein J, Burstein R, Koffler H. Incidence and evolution of subependymal and intraventricular hemorrhage: a study of infants with birth weights less than 1500 grams. J Pediatr. 1978;92:529–34. doi: 10.1016/s0022-3476(78)80282-0. [DOI] [PubMed] [Google Scholar]
- 51.Pfurtscheller G, Stancak A, Neuper C. Event-related synchronization (ERS) in the alpha-band—an electrophysiological correlate of cortical idling: a review. Int J Psychophysiol. 1996;24:39–46. doi: 10.1016/s0167-8760(96)00066-9. [DOI] [PubMed] [Google Scholar]
- 52.Ribary U. Dynamics of thalamo-cortical network oscillations and human perception. Prog Brain Res. 2005;150:127–42. doi: 10.1016/S0079-6123(05)50010-4. [DOI] [PubMed] [Google Scholar]
- 53.Rickards AL, Kelly EA, Doyle LW, Callanan C. Cognition, academic progress, behavior and self concept at 14 years of age in very low birth weight children. J Dev Behav Pediatr. 2001;22:11–8. doi: 10.1097/00004703-200102000-00002. [DOI] [PubMed] [Google Scholar]
- 54.Roberts G, Anderson PJ, De Luca C, Doyle LW. Changes in neurodevelopmental outcome at age eight in geographic cohorts of children born at 22–27 weeks gestational age during the 1990s. Arch Dis Child Fetal Neonatal Ed. 2010;95:90–4. doi: 10.1136/adc.2009.165480. [DOI] [PubMed] [Google Scholar]
- 55.Sarnthein J, Stern J, Aufenberg C, Rousson V, Jeanmonod D. Increased EEG power and slowed dominant frequency in patients with neurogenic pain. Brain. 2006;129:55–64. doi: 10.1093/brain/awh631. [DOI] [PubMed] [Google Scholar]
- 56.Schnitzler A, Gross J. Normal and pathological oscillatory communication in the human brain. Nat Rev Neurosci. 2005;6:285–96. doi: 10.1038/nrn1650. [DOI] [PubMed] [Google Scholar]
- 57.Slater R, Worley A, Fabrizi L, Roberts S, Meek J, Boyd S. Evoked potentials generated by noxious stimulation in the human infant brain. Eur J Pain. 2010;70:541–9. doi: 10.1016/j.ejpain.2009.05.005. [DOI] [PubMed] [Google Scholar]
- 58.Smith GC, Gutovich J, Smyser C, Pineda R, Newnham C, Tjong TH, Vavasseur C, Wallendorf M, Niel J, Inder T. Neonatal intensive care unit stress is associated with brain development in preterm infants. Ann Neurol. 2011;70:541–9. doi: 10.1002/ana.22545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Smyser C, Inter TE, Shimony JS, Hill JE, Degnan AJ, Snyder AZ, Neil JJ. Longitudinal analysis of neural network development in preterm infants. Cereb Cortex. 2010;20:2852–62. doi: 10.1093/cercor/bhq035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Taylor HG, Minich NM, Klein N, Hack M. Longitudinal outcomes of very low birth weight: neuropsychological findings. J Int Neuropsychol Soc. 2004;10:149–63. doi: 10.1017/S1355617704102038. [DOI] [PubMed] [Google Scholar]
- 61.Uhlhaas PJ, Roux F, Rodriguez E, Rotarska-Jagiela A, Singer W. Neural synchrony and the development of cortical networks. Trends Cogn Sci. 2010;14:72–80. doi: 10.1016/j.tics.2009.12.002. [DOI] [PubMed] [Google Scholar]
- 62.Uhlhaas PJ, Pipa G, Lima B, Melloni L, Neuenschwander S, Nikolic D, Singer W. Neural synchrony in cortical networks: history, concept and current status. Front Integr Neurosci. 2009;3:17. doi: 10.3389/neuro.07.017.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Varela F, Lachaux JP, Rodriguez E, Martinerie J. The brainweb: phase synchronization and large scale integration. Nat Rev Neurosci. 2001;2:229–39. doi: 10.1038/35067550. [DOI] [PubMed] [Google Scholar]
- 64.Ward LM. Synchronous neural oscillations and cognitive processes. Trends Cogn Sci. 2003;7:553–9. doi: 10.1016/j.tics.2003.10.012. [DOI] [PubMed] [Google Scholar]
- 65.Wechsler D. Wechsler intelligence scales for children. 4th. San Antonio, TX: Psychological Corporation; 2003. WISC-IV. [Google Scholar]
- 66.Wilson H, Moiseev A, Podin S, Quraan M. Continuous head localization and data correction in MEG; International Congress Series 1300; Washington DC. 2007. pp. 623–626. [Google Scholar]
- 67.Vicari S, Caravale B, Carlesimo GA, Casadei AM, Allemand F. Spatial working memory deficits in children at ages 3–4 who were low birth weight, preterm infants. Neuropsychology. 2004;18:673–8. doi: 10.1037/0894-4105.18.4.673. [DOI] [PubMed] [Google Scholar]