Abstract
Gait initiation is a transitional task involving a voluntary shift from a static, stable position to a relatively less-stable state of locomotion. During gait initiation, anticipatory postural adjustments precede stepping in order to generate forward momentum while balance is maintained. While deficits in gait initiation are frequently reported for persons with Parkinson’s disease, there is a paucity of information regarding gait initiation performance in persons with Essential Tremor. We investigated anticipatory postural adjustments and spatiotemporal characteristics of gait initiation in persons with Essential Tremor and compared them to persons with Parkinson’s disease as well as age-matched neurologically-healthy adults. Twenty-four persons with Essential Tremor, 31 persons with Parkinson’s disease, and 38 age-matched controls participated. We compared anterior-posterior and mediolateral center of pressure movements and spatiotemporal stepping characteristics during gait initiation among the three groups using Mann-Whitney U-tests with Bonferroni corrections for multiple comparisons and one-way ANOVAs. Persons with Parkinson’s disease demonstrated significantly reduced displacement and velocity of the center of pressure during early phases of anticipatory postural adjustments relative to controls. Displacement of the center of pressure was also reduced in persons with Essential Tremor, although at a later stage of the gait initiation process. Persons with Parkinson’s disease and Essential Tremor demonstrated similar reductions in step length during gait initiation when compared to controls. Persons with Parkinson’s disease and Essential Tremor exhibit different deficits in gait initiation when compared to healthy older adults. Therefore, this study provides further evidence differentiating motor control features in these movement disorders.
Keywords: gait initiation, Parkinson’s disease, Essential Tremor, anticipatory postural adjustments, motor control
Introduction
Gait initiation (GI) is a complex transitional locomotor task which requires a shift from a static, stable state to a relatively less stable, dynamic state of motion. GI is a challenging task that demands balance and postural control due to a decreasing base of support from a two leg stance to an alternating single leg stance. This is a very destabilizing period of the locomotor process, as the center of pressure (COP) separates from the center of mass (COM) and highly specific postural shifts occur to allow the body to begin forward motion1. Anticipatory postural adjustments (APAs) precede stepping during GI as a process necessary to generate momentum for efficient forward motion while the body is balanced between the two feet. Indeed, failure to generate sufficient forward momentum during GI has been shown to lead to overall poorer GI performance, as evidenced by decreased step length and decreased step velocity2.
During quiet stance, movements of the COP and COM are relatively coupled. However, as gait is initiated, APAs function to shift the COP postero-laterally toward the stepping limb while the COM moves anteriorly and towards the stance limb. Because GI requires dynamic postural control to separate the COP from the COM, the outputs of the APAs (COP and COM movements) have been used as investigative tools to evaluate dynamic postural instability3. Indeed, previous research on APAs has suggested that COP excursions during GI are diminished in pathological populations at increased risk of falling 2,4,5,6.
Parkinson’s disease (PD) is characterized by motor signs resulting from a degenerative loss of dopaminergic neurons in the substantia nigra as well as multiple motor and non-motor regions of basal ganglia2. The basal ganglia are understood to be essential in planning and initiating movement, and consequently the APAs have been shown to be diminished in persons with PD when initiating gait2,4,5. Further, previous research has demonstrated that persons with PD exhibit deficits in postural control and momentum generation during GI as evidenced by reduction of APA magnitude and restriction of the COP/COM separation when compared to their neurologically-healthy peers3,4.
Essential Tremor (ET) is a neurodegenerative movement disorder which is characterized by an involuntary shaking predominantly in the hands, forearms, and head/neck. The typical tremor is postural/action, but can be present at rest. While static postural control appears to remain relatively intact7, recent research has begun to describe a variety of locomotor deficits in ET. Earhart and colleagues recently reported decreases in cadence and walking speed in persons with severe ET, which were accompanied by impairment in dynamic stability as evidenced by reduction in double support time when compared to controls8. Moreover, persons with relatively advanced ET also demonstrated cerebellar-like deficits in dynamic stability during tandem walking tasks9. Locomotor deficits in this population have also been described more generally in a clinical setting, as a group of persons with ET composed of persons with varying degrees of severity demonstrated reduced performance on clinical measures of functional mobility whereas static balance control was unaffected10. Despite the breadth of information regarding the effects of ET on gait, mobility, and static postural control, little is known about the effects of ET on dynamic postural control during transitional locomotor periods such as GI. These periods are vitally important phases of locomotion, as it is during transitional periods when older adults are most susceptible to falling11.
Therefore, the purpose of this study was to investigate APAs and spatiotemporal characteristics of GI in persons with ET. We compared these features of GI among persons with ET, PD, and neurologically-healthy older adults. As dynamic stability and gait deficits seem to be evident in persons with relatively severe ET and somewhat similar to those seen in persons with mild-to-moderate PD, we hypothesize that persons with ET will demonstrate GI deficits which are similar to but potentially less severe than those seen in persons with PD, as severe GI deficits are not commonly reported in clinical settings in ET (as is often the case in PD). ET and PD are often quite similar phenotypically, as ET is one of the most common movement disorders in the adult population, and yet approximately 30–50% of persons with ET are misdiagnosed with PD or other tremor disorders12. Thus, in this study, we aimed to further the understanding of differential motor control deficits between PD and ET.
Methods
Twenty-four participants with Essential Tremor (mean age ± SD: 68 ± 6 y, mean height: 171 ± 9 cm, mean body mass ± SD: 93 ± 21 kg, mean Fahn-Tolosa-Marin Tremor Rating Scale (TRS) 13 Motor (subscores 1–14) score ± SD: 35 ± 12, mean TRS Activities of Daily Living (subscores 15–21) score ± SD: 14 ± 5, mean TRS Total score ± SD: 49 ± 16) were referred from the Center for Movement Disorders and Neurorestoration at the University of Florida. ET participants were evaluated and diagnosed with ET by a movement disorder neurologist and were optimally treated prior to testing. Thirty-one participants with idiopathic PD (mean age ± SD: 68 ± 7 y, mean height ± SD: 170 ± 6 cm, mean body mass ± SD: 82 ± 15 kg, mean Unified Parkinson’s Disease Rating Scale (UPDRS)14 Motor score ± SD: 25 ± 7) were also recruited from the clinic and from the university community. PD participants were evaluated and diagnosis of PD was confirmed by a movement disorder neurologist using the UK Brain Bank criteria15. All PD and ET participants were tested at their self-reported best medicated state. Eight of the ET participants were taking either a beta-adrenergic antagonist, an anticonvulsant, or both while the remaining 16 ET participants were not taking any medication specifically intended to reduce tremor. All participants with PD were being treated with levodopa and, in some cases, a dopamine agonist. Thirty-eight healthy older adults (HOA; mean age ± SD: 68 ± 5 y, mean height ± SD: 167 ± 12 cm, mean body mass ± SD: 79 ± 14 kg) volunteered and were screened for neurological and musculoskeletal impairment. The HOA were enrolled from the university and the neighboring community. Before participation in the study, all subjects signed a written informed consent that was approved by the University’s Institutional Review Board.
Participants began each experimental trial standing barefoot with both feet on one force-plate (Bertec Corporation, Columbus, OH). Upon a verbal instruction of “ready”, the participants were asked to pause for several moments before volitionally initiating gait along a 12-meter walkway at their own comfortable pace. Participants were given preference in the placement and position of their feet on the force-plate, which were then restricted to that particular position for the remainder of the trials. Participants performed three GI trials with the same self-selected leg. The walkway was surrounded by an 8-camera optical motion capture system (120 Hz; Vicon Nexus, Lake Forrest, CA). Passive, retroreflective markers were placed bilaterally over the second metatarsal head, lateral malleolus, and calcaneus. Gait events (heel-offs, heel-strikes, and toe-offs) were manually labeled in Vicon Nexus based on individual marker trajectories.
We measured COP displacements and velocities during GI by assessing the pattern of COP movement using custom-written MATLAB software (MathWorks, Natick, MA). We manually distinguished two events during GI that separate the COP trace into three distinct phases as described previously16 (Figure 1). Briefly, the S1 phase begins with the onset of the lateral, posterior shift of the COP and ends with the COP positioned at the most lateral and posterior position relative to the initial stepping limb (swing limb – SW limb). The beginning of the S2 phase is defined as the onset of a lateral shift of the COP in the opposite direction toward the contralateral limb (stance limb – ST limb), and ends when the COP is in its most lateral and posterior position under the ST limb. The S3 phase is defined as the forward translation of the COP trace under the ST limb until the instance prior to heel strike of SW limb. Displacement and velocity of the COP was calculated in the anterior-posterior (AP) and mediolateral (ML) directions during the S1, S2, and S3 phases of GI.
Figure 1.
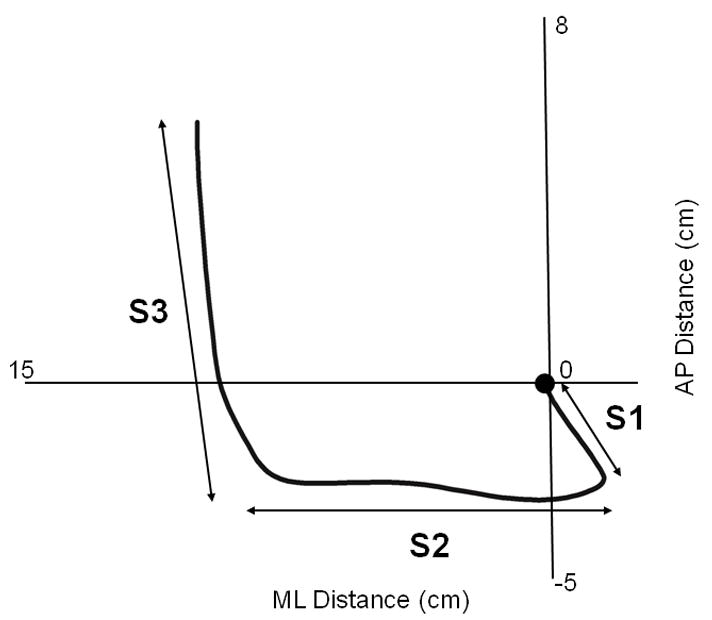
A representative center of pressure movement pattern indicating the S1, S2, and S3 phases during gait initiation in a healthy older adult.
We also measured spatiotemporal gait parameters during GI including: a) the length of the first SW limb step, which was defined as the displacement of the heel marker from static position to first heel-strike; b) time of the first SW limb step, defined as the time between the first heel-off and first heel-strike of the SW limb; and c) the velocity of the first SW limb, which was measured as the SW step length divided by SW step time. Step length, step time, and step velocity were also measured for the first ST limb step using similar methods. Thus, we ultimately calculated the following outcome measures: SW and ST step length, SW and ST step time, and SW and ST step velocity.
Analysis
As the distribution of all COP variables violated assumptions of normality, mean differences for all COP variables were compared among the HOA, ET, and PD groups using Mann-Whitney U-tests with Bonferroni corrections for multiple comparisons. One-way ANOVAs were conducted to compare age, mass, height, initial stance width, and mean spatiotemporal variables among groups. Level of significance was set at α=.05 for both the Mann-Whitney U-tests (prior to Bonferroni correction) and the one-way ANOVAs.
Results
There were no significant differences in age, height, or initial stance width among groups. The ET group had significantly more body mass than PD and HOA.
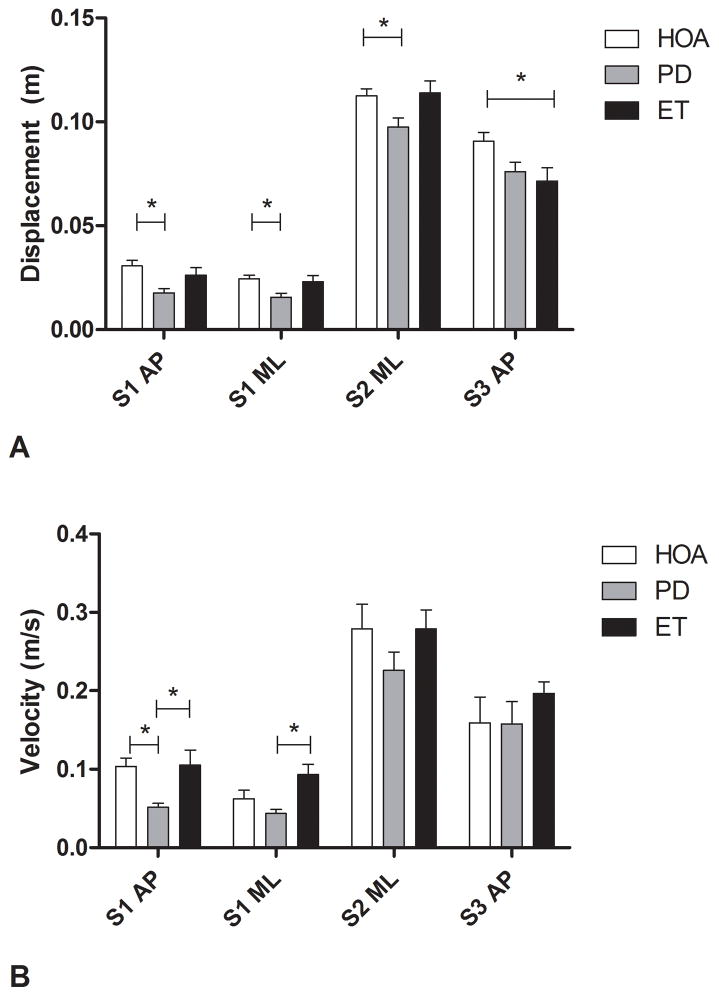
Mean COP trajectories during GI for ET, PD, and HOA are shown in Figure 2. Persons with ET had a significantly diminished S3 AP displacement when compared to HOA (mean ± SD: 7.15 ± 3.13 cm vs. 9.07 ± 2.58 cm, p= 0.012). Persons with PD had a significantly diminished S1 AP displacement (1.77 ± 1.17 vs. 3.07 ± 1.62 cm, p< 0.001), S1 ML displacement (1.56 ± 1.07 vs. 2.44 ± 1.14 cm, p= 0.001), S2 ML displacement (9.75 ± 2.40 vs. 11.25 ± 2.13 cm, p= 0.012), and S1 AP velocity (5.14 ± 2.92 vs. 10.35 ± 6.57 cm/s, p < 0.001) when compared to HOA. When comparing persons with ET to those with PD, PD participants had significantly decreased S1 AP (5.14 ± 2.92 vs. 10.52 ± 9.49 cm/s, p= 0.004) and S1 ML (4.36 ± 2.91 vs. 9.31 ± 6.38 cm/s. p= 0.001) velocities than those with ET (Figure 3).
Figure 2.
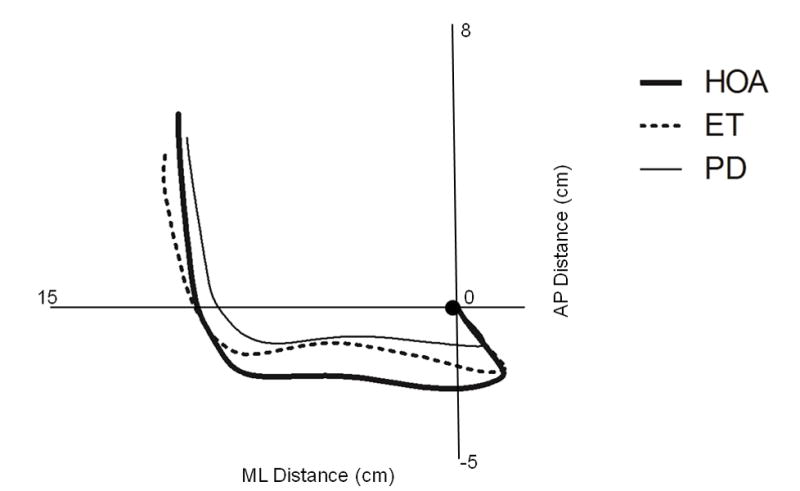
Mean center of pressure movement patterns during gait initiation in persons with Essential Tremor (ET), Parkinson’s disease (PD), and healthy older adults (HOA).
Figure 3.
Center of pressure displacements (A) and velocities (B) during the S1, S2 and S3 phases of gait initiation in healthy older adults (HOA), persons with Parkinson’s disease (PD), and persons with Essential Tremor (ET). AP, anterior-posterior; ML, mediolateral. Error bars indicate standard error.
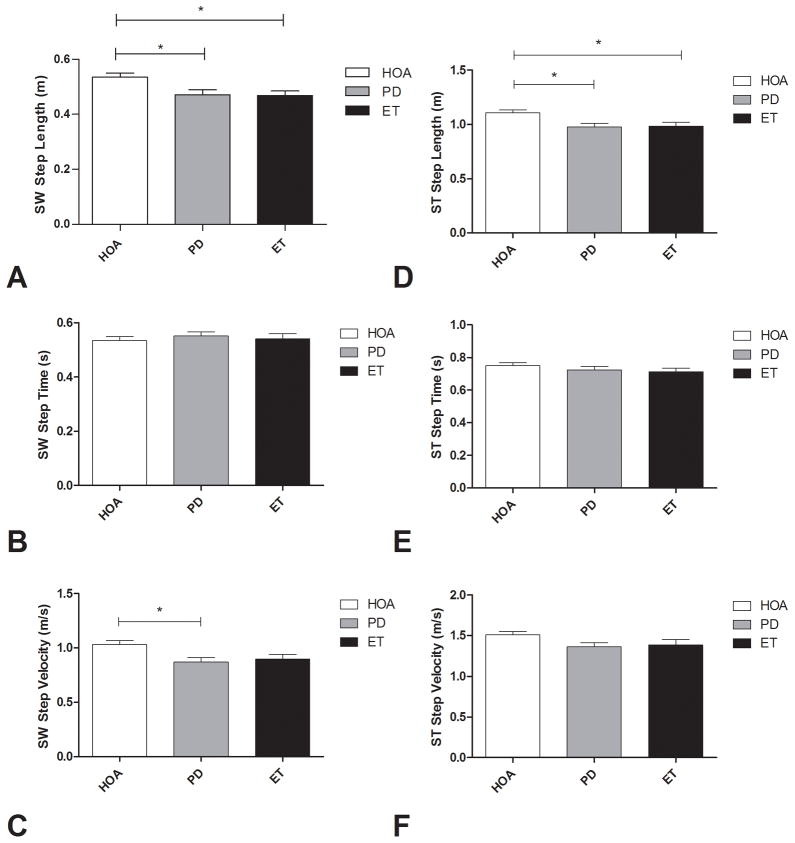
Persons with ET had a significantly diminished SW step length (0.47 ± 0.08 vs. 0.54 ± 0.09 m, p=.018) and ST step length (0.98 ± 0.18 vs. 1.11 ± 0.15 m, p=.024) when compared to HOAs. Persons with PD had a significantly diminished SW step length (0.47 ± 0.11 vs. 0.54 ± 0.09 m, p= .014), ST step length (0.98 ± 0.20 vs. 1.11 ± 0.15 m, p=.007), and SW step velocity (0.95 ± 0.26 vs. 1.04 ± 0.59 m/s, p=.010) when compared to HOAs. There were no significant differences in any spatiotemporal GI measures when comparing persons with ET to those with PD (Figure 4).
Figure 4.
Swing (SW - A, B, C) and stance (ST - D, E, F) limb step length, step time, and step velocity during gait initiation in healthy older adults (HOA), persons with Parkinson’s disease (PD), and persons with Essential Tremor (ET). Error bars indicate standard error.
Discussion
The ability to effectively utilize APAs is essential for maximizing forward momentum generation during GI. While previous research has described gait and static postural control in ET, this is the first study to assess the effects of ET on dynamic postural control mechanisms underlying GI and subsequent spatiotemporal parameters of initial stepping. Further, this study compared dynamic postural control in persons with ET and PD which are two movement disorders with some motor and non-motor feature overlap17. We observed that persons with ET had diminished forward movement of the COP under the stance limb as the swing leg moved out and in front in preparation for ground contact. Moreover, persons with ET took shorter steps in comparison to healthy older adults. We also observed that movement of the COP during the APA period was significantly slower in both the forward and lateral (towards the swing limb) directions in persons with PD when compared to persons with ET; however, the magnitude of the movement was similar. Though the observed COP patterns were altered in different ways in persons with PD and ET, similar impairments in stepping during GI were observed between the two groups relative to controls.
The initial posterolateral shift in the COP during GI serves two primary purposes: the COP shifts posteriorly to help generate forward momentum and laterally toward the swing limb to propel the COM toward the stance limb18. Persons with PD have previously been shown to reduce the magnitude of this posterolateral shift3, presumably to decrease the distance between the COP and COM during GI3. The S3 phase is typically unaffected in PD, as it has been suggested that COP velocity during S3 may actually be increased in PD in an attempt to recover some of the forward momentum dissipated during the diminished S1 phase19. In sum, these kinetic patterns have been found to result in slower, shorter steps; indeed, the results of the current study are similar in magnitude to previously-published results in PD and HOA2 and generally support these findings. On the contrary, the diminished AP shift during S3 in persons with ET may be a compensatory mechanism to increase stability by reducing the forward movement of the COP while in single support on the stance limb. The results of this study show that this has significant functional implications, as the reduction in AP movement of the COP during S3 accompanied shorter steps in ET compared to HOA.
GI disturbances in persons with PD may result from dysfunction of basal ganglia regions and supplementary motor area as well as ascending and descending projections derived from the pedunculopontine nucleus (PPN). The basal ganglia are important in early motor planning and execution, and research has shown strong correlations between degeneration of dopaminergic neurons within the substantia nigra and the clinical manifestation of PD20. However, since dopaminergic treatment does not completely resolve PD motor deficits21, the PPN likely plays a role in locomotor difficulties in PD22. Indeed, it has been observed in animal models that the PPN exerts a modulatory influence over basal ganglia activity during GI23,24,25. These mechanisms in humans, however, are beyond the scope of the current study and should be investigated in future GI research.
Our results suggest that persons with ET also exhibit GI disturbances despite documented lesser dopaminergic dysfunction when compared to PD26. Though the basal ganglia remain relatively unaffected, previous research has shown that the cerebellum and brainstem are affected in ET27, 28. While there is mounting evidence that these two structures play an important role in ET etiology, the precise functional, biochemical, or morphological cause is still unknown and highly controversial27,29,30. The implication of cerebellar dysfunction in ET has been supported by the manifestation of a multitude of typical cerebellar spectrum motor deficits in persons with ET, such as tandem walking difficulties, gait ataxia, and intention tremor9,31,32. Further, while the cerebellum has primarily been thought to influence movement coordination and adaptation33, several other studies have indeed reported implications of the cerebellum in various movement initiation tasks34,35. For instance, GI deficits have also been previously observed in persons with cerebellar disease. Timmann and Horak found that persons with cerebellar ataxia maintained temporal control of APAs, but initiated gait with shorter, slower steps33. Indeed, these results are very similar to our findings in ET, which further suggest similarities in motor control patterns between persons with ET and those with cerebellar deficits. Timmann and Horak also noted the similarity of GI performance in cerebellar patients to persons with PD, and they postulated that the short and slow steps observed in cerebellar patients may have resulted as a compensatory mechanism for gait ataxia, as opposed to the centrally-induced bradykinesia observed in PD33. As previously mentioned, our findings support this idea in that the diminished S3 displacement in ET may be compensatory for instability during single support whereas the diminished S1 phase in PD likely serves reduce COM movement. We suggest that this may also be the case in persons with ET affected by ataxia32,36, although steady-state gait analysis was beyond the scope of this study.
Limitations
Eight participants with ET were taking ET related medication at the time of testing, while the remaining subjects were not taking medication. Medications to treat ET are not entirely effective in eliminating the symptoms, and many persons with ET do not significantly benefit32,36. Indeed, results Mann-Whitney U-tests for the COP variables and independent samples t-tests for the spatiotemporal variables did not indicate significant differences in any GI measures between the ET participants taking medication and those not taking medication included in this study. Our sample of persons with ET is composed largely of persons with relatively severe ET while our population of persons with PD is comprised mostly of persons with mild-to-moderate PD. Accordingly, further research should focus on the pathophysiological underpinnings which contribute to the GI deficits observed between the two groups for a better understanding of how and why each disease affects GI. Another limitation was that we were unable to calculate COP-COM characteristics due to lack of full body marker placement during the trials. All GI trials were performed at self-selected speeds with self-selected positioning of the feet, and thus the ability of persons with ET and PD to modulate speed of GI and effects of wide versus narrow initial stances were not investigated.
Conclusion
GI is a complex transitory phase that challenges dynamic and postural stability and balance. In this study, we investigated APA and spatiotemporal features of GI in persons with PD, ET, and age-matched healthy controls. We found that APA and spatiotemporal parameters were affected in both the ET and PD groups, as both persons with ET and PD took shorter steps and demonstrated reduced APAs when compared to healthy older controls. Through kinetic and kinematic analysis, this study suggests that APA deficits not only exist during GI in persons with PD, as previously understood, but also affect persons with ET.
Highligts.
We compare gait initiation in Parkinson’s disease, Essential Tremor, and controls.
Gait initiation is impaired in both Parkinson’s disease and Essential Tremor.
Impairments differ between Parkinson’s disease and Essential Tremor.
These differences may provide insight into pathophysiology of Essential Tremor.
Acknowledgments
This work was supported in part by NIH grants R03HD054594, 1R21AG033284-01A2, and the UF National Parkinson’s Foundation Center of Excellence.
Footnotes
Conflict of Interest:
The authors declare that there are no relevant conflicts of interest.
Financial disclosures:
Kristina Fernandez has no relevant financial disclosures.
Ryan Roemmich is funded by a University of Florida College of Health and Human Performance Pre-doctoral Fellowship.
Dr. Stegemöller is funded by NIH Grants R21 AG033284-01A2 (postdoctoral associate), T32 DC008768-05 (postdoctoral fellow), and the University of Florida.
Shinichi Amano is funded by a University of Florida College of Health and Human Performance Graduate Teaching Assistantship.
Dr. Thompson has no relevant financial disclosures.
Dr. Okun serves as a consultant for the National Parkinson Foundation, and has received research grants from NIH, NPF, the Michael J. Fox Foundation, the Parkinson Alliance, Smallwood Foundation, and the UF Foundation. Dr. Okun has previously received honoraria, but in the past >24 months has received no support from industry including travel. Dr. Okun has received royalties for publications with Demos, Manson, and Cambridge (movement disorders books). Dr. Okun has participated in CME activities on movement disorders sponsored by the USF CME office, PeerView, and by Vanderbilt University. The institution and not Dr. Okun receives grants from Medtronic and ANS/St. Jude, and the PI has no financial interest in these grants. Dr. Okun has participated as a site PI and/or co-I for several NIH, foundation, and industry sponsored trials over the years but has not received honoraria.
Dr. Hass is funded by NIH grants R03HD054594, 1R21AG033284-01A2 and UF National Parkinson’s Foundation Center of Excellence.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Jian Y, Winter DA, Ishac MC, Gilchrist L. Trajectory of the body COG and COP during initiation and termination of gait. Gait Posture. 1992;1:9–22. [Google Scholar]
- 2.Halliday SE, Winter DA, Frank JS, Patla AE, Prince F. The initiation of gait in young, elderly, and Parkinson’s disease subjects. Gait Posture. 1998 Apr 8;:8–14. doi: 10.1016/s0966-6362(98)00020-4. [DOI] [PubMed] [Google Scholar]
- 3.Hass CJ, Waddell DE, Fleming RP, Juncos JL, Gregor RJ. Gait initiation and dynamic balance control in Parkinson’s disease. Arch Phys Med Rehabil. 2005 Nov;86(11):2172–6. doi: 10.1016/j.apmr.2005.05.013. [DOI] [PubMed] [Google Scholar]
- 4.Martin M, Shinberg M, Kuchibhatla M, Ray L, Carollo JJ, Schenkman ML. Gait initiation in community-dwelling adults with Parkinson disease: comparison with older and younger adults without the disease. Phys Ther. 2002 Jun;82(6):566–77. [PubMed] [Google Scholar]
- 5.Schmit JM, Riley MA, Dalvi A, et al. Deterministic center of pressure patterns characterize postural instability in Parkinson’s disease. Exp Brain Res. 2006 Jan;168(3):357–67. doi: 10.1007/s00221-005-0094-y. [DOI] [PubMed] [Google Scholar]
- 6.Krishnan V, Kanekar N, Aruin AS. Anticipatory postural adjustments in individuals with multiple sclerosis. Neurosci Lett. 2012 Jan;506( 2):256–60. doi: 10.1016/j.neulet.2011.11.018. [DOI] [PubMed] [Google Scholar]
- 7.Bove M, Marinelli L, Avanzino L, Marchese R, Abbruzzese G. Posturographic analysis of balance control in patients with essential tremor. Mov Disord. 2006 Feb;21(2):192–8. doi: 10.1002/mds.20696. [DOI] [PubMed] [Google Scholar]
- 8.Earhart GM, Clark BR, Tabbal SD, Perlmutter JS. Gait and balance in essential tremor: variable effects of bilateral thalamic stimulation. Mov Disord. 2009 Feb;24( 3):386–91. doi: 10.1002/mds.22356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Stolze H, Petersen G, Raethjen J, Wenzelburger R, Deuschl G. The gait disorder of advanced essential tremor. Brain. 2001 Nov;124( Pt 11):2278–86. doi: 10.1093/brain/124.11.2278. [DOI] [PubMed] [Google Scholar]
- 10.Parisi SL, Heroux ME, Culham EG, Norman KE. Functional mobility and postural control in essential tremor. Arch Phys Med Rehabil. 2006 Oct;87(10):1357–64. doi: 10.1016/j.apmr.2006.07.255. [DOI] [PubMed] [Google Scholar]
- 11.Tinetti ME, Doucette JT, Claus EB. The contribution of predisposing and situational risk factors to serious fall injuries. J Am Geriatr Soc. 1995 Nov;43(11):1207–13. doi: 10.1111/j.1532-5415.1995.tb07395.x. [DOI] [PubMed] [Google Scholar]
- 12.Jain S, Lo SE, Louis ED. Common misdiagnosis of a common neurological disorder: how are we misdiagnosing essential tremor? Arch Neurol. 2006 Aug;63( 8):1100–4. doi: 10.1001/archneur.63.8.1100. [DOI] [PubMed] [Google Scholar]
- 13.Stacy MA, Elble RJ, Ondo WG, Wu SC, Hulihan J TRS study group. Assessment of interrater and intrarater reliability of the Fahn-Tolosa-Marin Tremor Rating Scale in essential tremor. Mov Disord. 2007 Apr;22(6):833–8. doi: 10.1002/mds.21412. [DOI] [PubMed] [Google Scholar]
- 14.Movement Disorder Society Task Force on Rating Scales for Parkinson’s Disease. The Unified Parkinson’s Disease Rating Scale (UPDRS): status and recommendations. Mov Disord. 2003 Jul;18(7):738–50. doi: 10.1002/mds.10473. [DOI] [PubMed] [Google Scholar]
- 15.Hughes AJ, Daniel SE, Kilford L, Lees AJ. Accuracy of clinical diagnosis of idiopathic Parkinson’s disease. A clinico-pathological study of 100 cases. JNNP. 1992;55:181–184. doi: 10.1136/jnnp.55.3.181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hass CJ, Gregor RJ, Waddell DE, et al. The Influence of Tai Chi Training on the Center of Pressure Trajectory During Gait Initiation in Older Adults. Arch Phys Med Rehabil. 2004 Oct;(85):1593–8. doi: 10.1016/j.apmr.2004.01.020. [DOI] [PubMed] [Google Scholar]
- 17.Shahed J, Jankovic J. Exploring the relationship between essential tremor and Parkinson’s disease. Parkinsonism Relat Disord. 2007 Mar;13(2):67–76. doi: 10.1016/j.parkreldis.2006.05.033. [DOI] [PubMed] [Google Scholar]
- 18.Polcyn AF, Lipsitz LA, Kerrigan DC, Collins JJ. Age-related changes in the initiation of gait: degradation of central mechanisms for momentum generation. Arch Phys Med Rehabil. 1998 Dec;79(12):1582–9. doi: 10.1016/s0003-9993(98)90425-7. [DOI] [PubMed] [Google Scholar]
- 19.Hass CJ, Waddell DE, Wolf SL, Juncos JL, Gregor RJ. Gait initiation in older adults with postural instability. Clin Biomech. 2008 Jul;23(6):743–53. doi: 10.1016/j.clinbiomech.2008.02.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Gelb DJ, Oliver E, Gilman S. Diagnostic criteria for Parkinson disease. Arch Neurol. 1999;56:33e9. doi: 10.1001/archneur.56.1.33. [DOI] [PubMed] [Google Scholar]
- 21.Zetusky WJ, Jankovic J, Pirozzolo FJ. The heterogeneity of Parkinson’s disease: clinical and prognosticimplications. Neurology. 1985 Apr;35(4):522–526. doi: 10.1212/wnl.35.4.522. [DOI] [PubMed] [Google Scholar]
- 22.Takakusaki K, Tomita N, Yano M. Substrates for normal gait and pathophysiology of gait disturbances with respect to the basal ganglia dysfunction. J Neurol. 2008 Aug;255( Suppl 4):19–29. doi: 10.1007/s00415-008-4004-7. [DOI] [PubMed] [Google Scholar]
- 23.Lee MS, Rinne JO, Marsden CD. The pedunculopontine nucleus: its role in the genesis of movement disorders. Yonsei Med J. 2000 Apr;41(2):167–184. doi: 10.3349/ymj.2000.41.2.167. [DOI] [PubMed] [Google Scholar]
- 24.Garcia-Rill E, Skinner RD, Fitzgerald JA. Chemical activation of the mesencephalic locomotor region. Brain Res. 1985 Mar 18;330(1):43–54. doi: 10.1016/0006-8993(85)90006-x. [DOI] [PubMed] [Google Scholar]
- 25.Skinner RD, Garcia-Rill E. The mesencephalic locomotor region (MLR) in the rat. Brain Res. 1984 Dec 10;323(2):385–389. doi: 10.1016/0006-8993(84)90319-6. [DOI] [PubMed] [Google Scholar]
- 26.Isaias IU, Marotta G, Hirano S, et al. Imaging essential tremor. Mov Disord. 2010 Apr;25(6):679–86. doi: 10.1002/mds.22870. [DOI] [PubMed] [Google Scholar]
- 27.Louis ED, Faust PL, Vonsattel JP, et al. Neuropathological changes in essential tremor: 33 cases compared with 21 controls. Brain. 2007 Dec;130(Pt 12):3297–307. doi: 10.1093/brain/awm266. [DOI] [PubMed] [Google Scholar]
- 28.Jenkins IH, Bain PG, Coeebatvh JG, et al. A positron emission tomography study of essential tremor: Evidence for over activity of cerebellar connections. Ann Neurol. 1993;34:82–90. doi: 10.1002/ana.410340115. [DOI] [PubMed] [Google Scholar]
- 29.Rajput AH, Robinson CA, Rajput ML, Robinson SL, Rajput A. Essential tremor is not dependent upon cerebellar Purkinje cell loss. Parkinsonism Relat Disord. 2012 Feb; doi: 10.1016/j.parkreldis.2012.01.013. [DOI] [PubMed] [Google Scholar]
- 30.LaRoia H, Louis ED. Association between essential tremor and other neurodegenerative diseases: what is the epidemiological evidence? Neuroepidemiology. 2011;37(1):1–10. doi: 10.1159/000328866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kronenbuerger M, Konczak J, Ziegler W, et al. Balance and motor speech impairment in essential tremor. Cerebellum. 2009 Sep;8(3):389–98. doi: 10.1007/s12311-009-0111-y. [DOI] [PubMed] [Google Scholar]
- 32.Louis ED. Essential tremor. Lancet Neurol. 2005 Feb;4(2):100–10. doi: 10.1016/S1474-4422(05)00991-9. [DOI] [PubMed] [Google Scholar]
- 33.Timmann D, Horak FB. Perturbed step initiation in cerebellar subjects: 2. Modification of anticipatory postural adjustments. Exp Brain Res. 2001 Nov;141(1):110–20. doi: 10.1007/s002210100858. [DOI] [PubMed] [Google Scholar]
- 34.Kassavetis P, Hoffland BS, Saifee TA, et al. Cerebellar brain inhibition is decreased in active and surround muscles at the onset of voluntary movement. Exp Brain Res. 2011 Mar;209(3):437–42. doi: 10.1007/s00221-011-2575-5. [DOI] [PubMed] [Google Scholar]
- 35.Boecker H, Jankowski J, Ditter P, Scheef L. A role of the basal ganglia and midbrain nuclei for initiation of motor sequences. Neuroimage. 2008 Feb;39(3):1356–69. doi: 10.1016/j.neuroimage.2007.09.069. [DOI] [PubMed] [Google Scholar]
- 36.Deuschl G, Elble R. Essential tremor – Neurodegenerative or nondegenerativdisease towards a working definition of ET. Mov Disord. 2009;24:2033–41. doi: 10.1002/mds.22755. [DOI] [PubMed] [Google Scholar]