Summary
Cadherin and nectin are distinct transmembrane proteins of adherens junctions. Their ectodomains mediate adhesion while their cytosolic regions couple the adhesive contact to the cytoskeleton. Both these proteins are essential for adherens junction formation and maintenance. However, some basic aspects of these proteins, such as their organization in adherence junctions, have remained open. Therefore, using super-resolution microscopy and live-imaging, we focused on the subjunctional distribution of these proteins. We showed that cadherin and nectin in the junctions of A431 cells and human keratinocytes are located in separate clusters. The size of each cluster is independent of that of the adjacent clusters and can significantly fluctuate over time. Several nectin and cadherin clusters that constitute an individual adherens junction are united by the same actin filament bundle. Surprisingly, interactions between each cluster and F-actin are not uniform since neither vinculin nor LIM domain actin-binding proteins match the boundaries of cadherin or nectin clusters. Thus, the adherens junction is not a uniform structure but a mosaic of different adhesive units with very diverse modes of interaction with the cytoskeleton. We propose that such a mosaic architecture of adherence junctions is important for the fast regulation of their dynamics.
Introduction
Classical cadherins and nectins are two families of single-pass transmembrane adhesive receptors that form an adhesive domain in adherens junctions, the major type of intercellular junctions of most vertebrate cells. Extracellular regions of these proteins mediate adhesive contacts while their intracellular regions interact with an array of cytosolic proteins known as catenins, in the case of cadherins, and with a large multidomain protein, afadin, in the case of nectins. These intracellular proteins provide structural integrity for adherens junctions and control their dynamics, connections with the cytoskeleton and signaling activities (Nelson et al., 2008; Takai et al., 2008; Harris and Tepass, 2010). Malfunctions in nectin or cadherin adhesive components of adherens junctions result in prominent developmental abnormalities (Takai et al., 2008; Harris and Tepass, 2010). For example, the afadin-knockout was shown to result in embryonic lethality associated with severe defects in the ectoderm and mesoderm (Ikeda et al., 1999; Zhadanov et al., 1999). Nectin-1 mutations lead to prominent developmental defects in humans (Sozen et al., 2001; Suzuki et al., 2000). Despite the important role of adherens junctions in tissue formation and maintenance, their structure and mechanisms of assembly are not completely understood. Specifically, little is known about how nectin and cadherin are co-recruited into adherens junctions and how they collaborate in the process of junction assembly and function.
Experiments performed mainly in Dr. Takai's laboratory revealed several molecular pathways of interactions between nectin and cadherin adhesive systems (Takai et al., 2008). One of the most direct and best studied mechanisms is the interaction of afadin with the central domain of α-catenin (Tachibana et al., 2000; Pokutta et al., 2002). In addition, nectin and cadherin adhesive systems were shown to communicate through numerous alternative mechanisms. Afadin may form a complex of yet unidentified composition with another cadherin-associated protein, p120 (Hoshino et al., 2005; Birukova et al., 2012). Evidence was presented that cadherin and nectin could interact through their ectodomains (Morita et al., 2010). Finally, both α-catenin and afadin are able to interact directly or through adapter proteins with F-actin (Takai et al., 2008). Anchorage of cadherin and nectin to the cytoskeleton can play an important role in their organization in adherens junctions.
One of the fundamental but still unanswered questions is the spatial localization of nectin and cadherin molecules in adherens junctions. This question, however, is very important since nectin incorporation into cadherin adhesive cluster can change adhesive properties of the cluster. Recent experiments showed that cadherin clustering into adherens junctions is facilitated by two types of intercadherin interactions. First, an amino-terminal extracellular cadherin domain, through strand-swap interactions, forms cadherin trans-dimer, thereby producing a molecular, but very transient, contact between adjacent cells. Cis-interactions between these trans-dimers result in the formation of a highly ordered adhesive cluster. Formation of this cluster is crucial for stabilization of the cadherin-based adhesive contact (Harrison et al., 2011). Incorporation of nectin molecules into such a cadherin cluster would likely distort its structure, thereby changing its property. Therefore, the presence of nectin in the adherens junction can, in theory, regulate junctional strength (Troyanovsky, 2012). Alternatively, nectin and cadherin may form independent clusters that are co-recruited into adherens junctions during junction assembly. In fact, this scenario, while, to our knowledge, it has never been considered, is quite possible since it would not interfere with the ordered cadherin cluster that may be structurally unfavorable.
Finally, each adherens junction is highly dynamic: it continuously exchanges its cadherin/catenin molecules and, furthermore, many of the junctions move from the site of their assembly to the subapical area of the cell-cell contact (Kametani and Takeichi, 2006; Hong et al., 2010). It is not known how the nectin component of adherens junctions behaves during these dynamic processes.
To understand these important issues, we studied nectin and cadherin in adherens junctions of different human epithelial cells including keratinocytes. This study revealed a remarkable feature of adherens junctions: their nectin and cadherin molecules are not intermixed but assembled into separate and relatively independent domains.
Results
Only a specific pool of apical and lateral adherens junctions co-recruits nectin and cadherin
Adherens junctions in A431 cells have two distinct populations. One of them, “apical” junctions, is localized at the subapical region of the lateral membranes. They are relatively immobile and apparently correspond to the zonula adherens of the polarized epithelial cells (Hong et al., 2010). The second population, spot-like “lateral” junctions, is highly dynamic. These junctions are continuously produced at the base of the lateral membrane and move in the apical direction (Kametani & Takeichi, 2006; Hong et al., 2010). These two types of junctions differ not only by their localization, but also by their composition. As has been noted for other cell types (Meng & Takeichi, 2009), nearly all apical junctions of A431 cells contained the actin-binding protein vinculin, while only a few of the lateral junctions exhibited weak, but specific vinculin staining (Fig. S1).
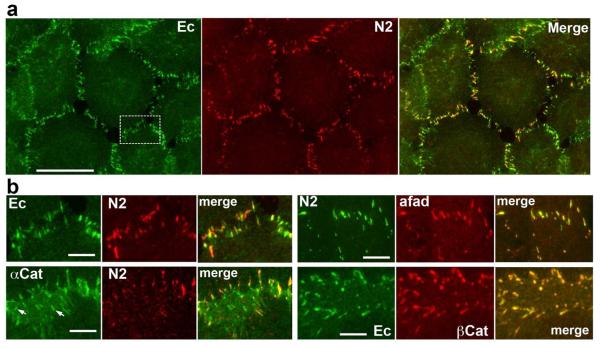
Using different commercially available anti-nectin antibodies, we determined that nectin-2 is a major one of five known nectins in A431 cells. The well-characterized intracellular nectin binding partner, afadin, was co-localized with nectin-2 at the same junctions (Fig. 1). Double staining for E-cadherin and nectin-2 revealed, in agreement with previous studies (Takai et al., 2008), that these two adhesive transmembrane receptors resided at the same junctions (Fig. 1a). Notably, only a subpopulation of adherens junctions, referred to as “complex” junctions below, contained both these adhesive proteins. Nectin-2 was not detected in many lateral junctions, especially those that were present in the central area of the lateral membrane (Fig. 1b, arrows).
Figure 1. Cadherin/catenin and nectin/afadin localization in A431 cells.
(a) A431 cells were double-stained using mouse anti-E-cadherin (Ec, green) and rabbit anti-nectin 2 (N2, red). The boxed area is zoomed in the Ec/N2 image of (b). Note that while both proteins are localized to adherens junctions, their subjunctional staining is not completely overlapped. Bar, 20 μm. (b) High magnification of the individual cell-cell contacts double stained for different junctional proteins: E-cadherin and nectin 2 (Ec and N2); α-catenin and nectin 2 (αCat and N2); nectin 2 and afadin (N2 and afad); and E-adherin and β-catenin (Ec and βCat). Note that double staining using antibodies against cadherin and nectin adhesion systems produced a mosaic staining of adherens junctions. In contrast, the stainings using antibodies against two proteins of the same adhesion system (N2 and afad or Ec and βCat) are completely overlapped.
Mosaic organization of the complex adherens junctions
Surprisingly, more detailed inspection of the cells double-stained for nectin and cadherin showed that nectin- and cadherin-derived signals did not completely match one another: in many cases the cadherin-brightest sites of the junction were the weakest for nectin staining and vice versa (Fig. 1). To substantiate that this pattern was specific and was not caused by epitope masking, we stained the cells using different combinations of antibodies specific to cadherin and nectin adhesion systems: (i) the rabbit anti-β-catenin and two different mouse anti-nectin-2 antibodies; (ii) rabbit anti-α-catenin and mouse anti-nectin-2 antibodies (Fig. 1b); (iii) rabbit anti-nectin-2 and mouse β-catenin antibodies and, finally, (iv) two different rabbit anti-afadin and mouse anti-α-catenin antibodies. In all these cases the unmatched distribution of cadherin/catenin and nectin/afadin systems was obvious. In contrast, double staining using mouse nectin- and rabbit afadin-specific antibodies or mouse E-cadherin- and rabbit β-catenin-specific antibodies generated practically indistinguishable images (Fig. 1). This observation suggested that cadherin and nectin components in the complex adherens junctions may not be intermixed but be present in distinct clusters.
To further explore this possibility, we examined the localization of E-cadherin and nectin-2 at the level of sub-diffraction resolution provided by a Nikon N-SIM microscope. This technique clearly showed that all junctions were composed of a mosaic of discernible cadherin- and nectin-enriched clusters (Fig. 2, Ec+N2 and βC+Af). The number of cadherin and nectin clusters varied from junction to junction. In addition, there was no obvious order in the cluster positioning. Some junctions consisted of just two clusters; a nectin one and a cadherin one. Others contained three or even more clusters: a nectin cluster flanked by two cadherin clusters, for example, or vice versa. Control experiments with antibodies specific to two different proteins of the same adhesive systems produced, in contrast, very similar staining. For example, localization of β- catenin exactly matched that of E-cadherin (Fig. 2, Ec+βC). Subjunctional localization of E-cadherin and α-catenin or nectin-2 and afadin was also similar, while these protein pairs showed some variability in the ratio of their fluorescence (Fig. 2, Ec+αC, and N2+Af). Another nectin protein, nectin-1, which was also detected in A431 cells, co-localized with nectin-2 in the same clusters (Fig. 2, N2+N1).
Figure 2. Sub-diffraction resolution images of cadherin and nectin clusters.
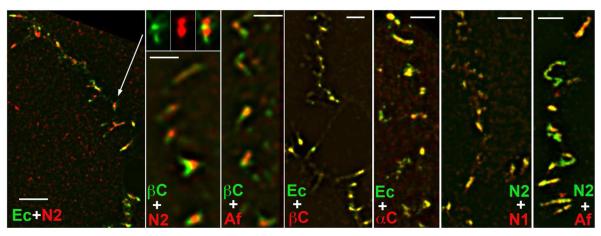
A431 cells were stained for different components of cadherin and nectin adhesion systems and imaged using a super-resolution N-SIM microscope. The left image (Ec+N2) is a low magnification (Bar, 4 μm) of the contact stained for E-cadherin (green) and nectin 2 (red). An individual adherens junction denoted by the arrow is shown in separate (green and red) channels and in combination in the next column. Note that the junction consists of two nectin and two cadherin clearly separate clusters. Similar “patchy” staining of the junctions is produced by β-catenin and nectin 2 (βC+N2) or β-catenin and afadin (βC+Af) co-staining. In contrast, E-cadherin and β-catenin (Ec+βC) or nectin 2 and nectin 1 (N2+N1) resulted in the identical images. While fluorescence for E-cadherin and α-catenin (Ec+αC) or nectin 2 and afadin (N2+Af) are not completely overlapped, these antibody pairs stained the same clusters but with variable intensities. Bars, 1.3 μm.
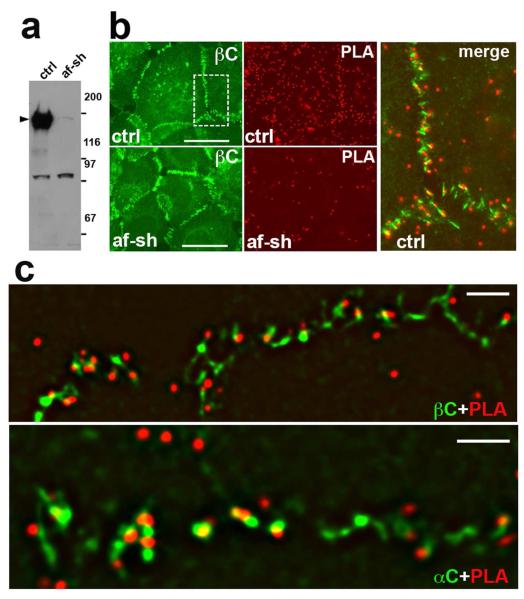
To further characterize the subcellular compartment where nectin-afadin and cadherin-catenin complexes interact, we used an in situ proximity ligation assay (PLA). The PLA fluorescent signal, an individual red fluorescent spot, is generated by the rolling cycle amplification reaction between a pair of oligonucleotide-labeled secondary antibodies (PLA probe). The reaction could efficiently proceed only when the antibodies of the probe are within 0–40 nm of each other. To exclude a possibility of masking the protein epitopes in specific subcellular compartments, several different antibodies were used. They include two different rabbit polyclonal anti-afadin antibodies and two different mouse monoclonal antibodies, against β/catenin or α/catenin. Combinations of these antibodies produced four different PLA probes, in which a rabbit antibody detected nectin and a mouse antibody detected cadherin adhesion systems. After performing the amplification reaction, the mouse antibody was additionally stained by the green anti-mouse IgG. This staining allowed us to assign the PLA signal to the particular adherens junctions. As a negative control, we produced A431 cell lines in which afadin was strongly depleted by the shRNA-based strategy (Fig. 3a). Figure 3b shows that the number of PLA signals generated in the wild-type A431 cells was significantly greater than that in the afadin-depleted cells. Importantly, the PLA signals were nearly always located outside the cadherin-containing clusters (Fig. 3c). Therefore, PLA experiments confirm that the interaction between nectin and cadherin complexes predominantly occurs either on periphery or outside of the cadherin-enriched clusters.
Figure 3. Proximal-ligation assay.
(a) Western blot analyses of control (ctrl) and afadin-depleted (af-sh) A431 cells using anti-afadin antibody. Afadin is indicated by an arrowhead. Note, that shRNA expression resulted in a nearly complete knockdown of afadin. Low molecular unspecific bands are not affected. (b) PLA (PLA, red) with control (ctrl) and afadin-depleted (af-sh) A431 cells using rabbit anti-afadin and mouse anti-β-catenin antibodies. Adherens junctions were then visualized by green anti-mouse IgG (βC, green) were co-stained with anti-mouse. An enlargement of the boxed region is shown on the right panel (merge). Bars, 20 μm. (c) the control cells stained as indicated above using either anti-β-catenin or anti-α-catenin mouse antibodies were imaged by super-resolution microscopy. Note that majority of the PLA signals (red dots) were localized outside of adherens junctions.
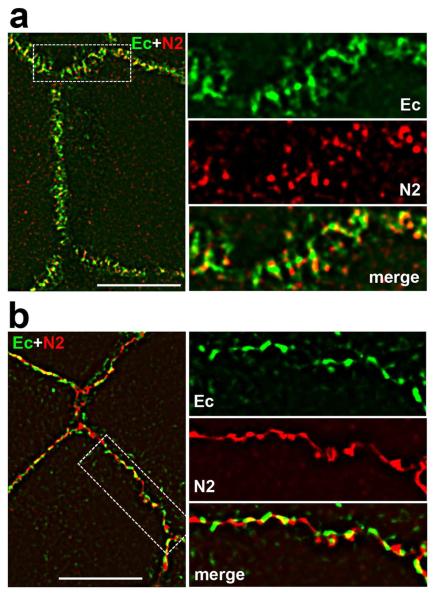
Finally, to confirm that such mosaic organization of adherens junctions was not only a feature of A431 cells, we determined the subcellular distribution of E-cadherin and nectin-2 in primary cultures of human keratinocytes and polarized colon carcinoma cells DLD1. Figure 4a shows that keratinocytes form a very complex network of adherens junctions. Separate, clearly distinct nectin and cadherin clusters were a prominent feature of this system. Both proteins, nectin-2 and E-cadherin were concentrated within apical adhesion complex of polarized cells (Fig. S2). Again, a number of closely localized but distinct cadherin and nectin clusters was resolved within this complex using super-resolution microscopy (Fig. 4b). Taken together, our immuno-morphological examination showed that complex adherens junctons consist of an array of cadherin-catenin and nectin-afadin adhesive clusters.
Figure 4. Cadherin and nectin clusters in keratinocytes and DLD1 cells.
(a) Primary culture of human keratinocytes was cultured in high (2 mM) calcium medium for 24 h and then stained for E-cadherin (Ec, green) and nectin 2 (N2, red) and imaged using N-SIM. Enlargements of the boxed region are shown on the right panel. Note that the cell-cell junctional system of these cells consists of a mosaic of distinguished cadherin and nectin clusters. (b) The same experiment as in (a) was performed using polarized DLD1 cells. Bars, 10 μm.
The cadherin and nectin clusters are independent of one another
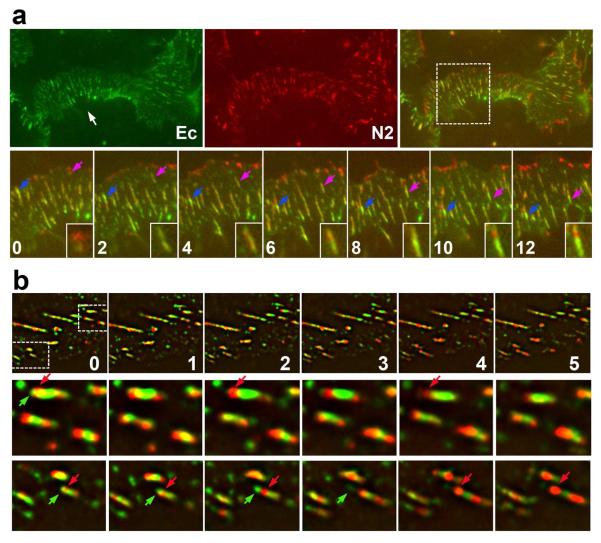
In the next set of experiments we investigated the coordination between nectin and cadherin clusters using live-cell imaging approach. To track the clusters, we co-expressed GFP-tagged cadherin and mCherry-tagged nectin-2 in cadherin-deficient A431D cells. As expected, these two proteins were co-recruited into adherens junctions (Fig. 5), which organized the cadherin-based adhesive systems consisting of apical and lateral junctions. The resulting lateral junctions, however, were significantly longer than lateral junctions in the parental A431 cells. Assembly of such enlarged junctions was apparently caused by nectin overexpression, while only the cells that expressed the lowest levels of nectin-mCherry transgene were imaged. Consistently with previous experiments (Hong et al., 2010), new lateral adherens junctions were continuously produced at the base of the lateral membrane and then flowed in the apical direction (see, for example, the movement of the junctions marked by blue and purple arrows in Fig. 5a). Notably, these junctions could increase or decrease their cadherin- and nectin-specific fluorescence intensities independently of one another. For example, immediately after formation, the junction shown in the inset of Fig. 5 mostly consisted of cadherin. After 4 min of observation, it assembled two nectin clusters, one of which became dominant over the time.
Figure 5. Time-lapse analysis of cadherin and nectin clusters.
(a) A431 cells expressing E-cadherin-GFP (green) and nectin-2-mCherry (red) were imaged using the regular wide-field microscopy. The upper micrographs show a low magnification of a single contact between two cells at an initial time point (time 0). An arrow denotes the apical contact region. Time-lapse recording of the boxed area is shown in the bottom row. Time points (in minutes) are indicated (only each second frame of the entire sequence is shown). The movement of the two individual junctions is followed by blue and purple arrows. The purple arrow junction is zoomed in the inset. Note significant changes in intensities and intra-junctional localization of cadherin and nectin over time. (b) A 5-min-long sequence showing dynamics of cadherin and nectin clusters detected by N-SIM. The upper row is a low magnification of the contact, two boxed areas are zoomed in the bottom rows. Note that the cadherin clusters denoted by green arrows disappear while the neighboring nectin clusters (red arrows) even gain in the intensities.
Super-resolution live-cell imaging confirmed and extended these observations. The 5-min-long sequence presented in Fig. 5b shows that the nectin cluster could dramatically decrease its intensity while the adjacent cadherin cluster was unchanged. Furthermore, the adherens junctions very often changed their leading clusters. In two enlarged fragments of this sequence, several junctions completely disassembled their leading cadherin cluster. The following nectin clusters, in contrast, became much more prominent. The fact that mosaic organization of adherens junctions was evident in live-cell imaging experiments confirmed that such organization is not an artifact of immunostaining.
Cadherin adhesive clusters are not affected by nectin overexpression
To further confirm that cadherin and nectin clusters were independent from each other, we, using FACS, selected A431 cells strongly overexpressing mCherry-tagged nectin. If these two adhesion molecules co-cluster, one may expect that nectin overexpression would change distribution and morphology of cadherin clusters. By contrast, we found that even the cells exhibiting the most abnormal, giant nectin clusters still produced relatively normal cadherin clusters. Interestingly, many of the cadherin clusters were located at the periphery of the giant nectin clusters (Fig. S3, insets).
Complex adherens junctions are anchored to actin bundles
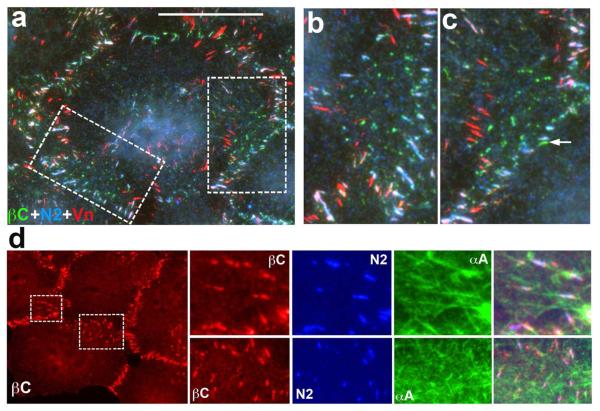
The general subcellular distribution of vinculin-containing and complex adherens junctions appeared similar: they both were predominantly located at the subapical areas of the lateral membranes. Using triple staining for nectin, cadherin, and vinculin, we studied the relationship between these three junctional components. This immunostaining demonstrated that nearly all complex apical adherens junctions were vinculin-positive (Fig. 6). Very few apical adherens junctions that were free of nectin were also free of vinculin (arrow in Fig. 6c). The lateral, cadherin-only-positive adherens junctions also did not exhibit vinculin staining.
Figure 6. Adherens junctions and the actin cytoskeleton.
Triple immunofluorescence microscopy of A431 cells using goat anti-β-catenin (green) in comparison with mouse anti-vinculin (red) and rabbit anti-nectin 2 (blue). Low magnification image (a) shows general distribution of the stained structures. Bar, 20 μm. Two selected cell-cell contacts that are shown at higher magnifications in (b) and (c) are indicated by broken lines. Note that nearly all apical junctions, except one that contains only β-catenin (arrow), incorporate all three proteins. The junctions, which are in the middle of lateral membrane, contain only cadherin/β-catenin complex but neither nectin nor vinculin. (d) triple staining using goat anti-β-catenin (βC, red), mouse anti-nectin 2 (N2, blue) and rabbit anti-α-actinin (αA, green). The low-resolution image shows only β-catenin staining. Note that the nectin/cadherin-positive junctions associate with α-actinin-enriched actin bundles.
Higher magnifications of these triple-stained images (Fig. 6b,c) also showed that vinculin localization precisely matched neither cadherin nor nectin clusters in the junctions. To verify this surprising observation we studied the subjunctional distribution of vinculin using super-resolution microscopy. Indeed, neither β-catenin-vinculin nor afadin-vinculin co-staining revealed complete co-localization of these proteins in adherens junctions (Fig. S4a,b).
Such surprising disorder in subjunctional localization of vinculin and cadherin prompted us to investigate the association between adherens junctions and the actin cytoskeleton in more detail. To reveal the relationships between actin filaments bundles and cadherin- and nectin-enriched adherens junctions, we triple-stained A431 cells using an antibody against the F-actin-bundling protein, α-actinin, in combination with anti-nectin and anti-cadherin staining. Figure 6d shows that the complex junctions interact with the prominent α-actinin-containing F-actin bundles. Super-resolution microscopy strongly confirmed this observation: double staining for F-actin and cadherin demonstrated that the majority of the junctions were underlined by the dense actin-filament bundles (Fig. S4c). Only a few spot-like lateral junctions, which apparently lacked vinculin, did not show obvious anchorage to F-actin (arrow in Fig. S4c).
In addition to vinculin, adherens junctions are known to recruit the actin-binding proteins from the zyxin subfamily of LIM domain proteins (Kadrmas and Beckrle, 2004). In our attempt to identify the actin-binding proteins that would be precisely co-localized either with cadherin or nectin domains of complex adherens junctions, we studied the subjunctional localization of five different zyxin-like proteins we identified in adherens junctions of A431 cells (LPP, ajuba, migfilin, TRIP6, and WTIP). Similar to vinculin, none of these proteins showed precise co-localization with any of two (nectin or cadherin) clusters of adherens junctions (Fig. S4d). Therefore, both cadherin and nectin clusters have very diverse modes of their interactions with the actin cytoskeleton.
Discussion
In this work we identified a previously overlooked structural feature of adherens junctions: they consist of a mosaic of cadherin and nectin-enriched clusters. These clusters are to a large extent independent of one another since they can gain or loose size and even be assembled or disassembled in complete disregard to the behavior of the adjacent clusters. Such independence of the clusters within an individual adherens junction is apparently based on their self-assembling properties. The molecular details of cadherin cluster self-assembly were elucidated in recent structural studies (Troyanovsky, 2012; Brasch et al., 2012). While this process has been studied less in case of nectin, the fact that this protein is able to form junctions in cadherin-deficient cells supports its cadherin independence (Miyahara et al., 2000; Harrison et al., 2012).
Apparently, a key structure unifying the cadherin and nectin clusters into a discernible adherens junction is a junction-associated actin filament bundle. In this respect, the actin cytoskeleton, which has been regarded as a structure supporting cadherin cluster stability, might have an additional role – it may hold individual clusters together and coordinate their motility and internal dynamics. Our data also suggests that the mechanisms of cadherin and nectin anchorage to F-actin are diverse even within a single cadherin or nectin cluster. At least, none of the actin-associated proteins, such as vinculin or five different zyxin family members, showed exact co-localization with the individual clusters. This observation is surprising since cadherin/catenin complex binds vinculin (Rangarajan & Izard, 2012; Choi et al., 2012), while nectin intracellular tail was shown to interact with some zyxin-like protein members (Call et al., 2011). The only protein that matched cadherin clusters was the direct intracellular cadherin partner, β/catenin, which is unable to interact with actin on its own. Even another member of the cadherin-catenin complex, α-catenin, which is a bona-fide actin-binding protein, showed surprising cluster-to-cluster staining variability. Therefore, the modes of cadherin and nectin interactions with actin filaments are likely to be controlled by the local sub-cluster processes that might include the tiny differences in the mechanical forces within the single cluster and short-range signal-transduction pathways. Interestingly, the absolute majority of nectin-containing junctions recruit vinculin. It may be possible, therefore, that nectin and vinculin recruitment into the junctions have tight relationship.
Mosaic architecture of adherens junctions raises several interesting questions. If cadherin and nectin clusters can be assembled independently, how are they co-assembled? Indeed, tracking adherens junctions in live cells documents that adherens junctions might incorporate both nectin and cadherin clusters from the moment of their formation (see Fig. 5 insets). The simplest mechanism of such co-assembly is that actin filaments may form a local structure, which instructs both cadherin and nectin where they should form clusters. For example, just by pushing adjacent membranes toward one another, the actin cytoskeleton may be able to maintain, for a few seconds, an intercellular distance that is the same as in adherens junctions. Such a site would immediately recruit both cadherin and nectin through a diffusion-trap mechanism (Wu et al., 2010). The differences in extracellular binding events would segregate nectin and cadherin clusters. By this scenario, the actin cytoskeleton underlining the site of adhesion provides a foundation for the simultaneous assembly of both clusters. One may propose, however, much more complex relationships between nectin and cadherin clusters. For example, interactions between α-catenin and afadin that may occur along the periphery of a nectin cluster can nucleate the formation of a new cadherin cluster. In turn, this new cadherin cluster might increase the local nectin concentration, thereby promoting formation of a new nectin cluster. This mechanism might be responsible for the previously observed positive effect of nectin junctions on cadherin junction assembly. In general, the functional interplay between nectin and cadherin clusters in adherens junctions is a very important subject for future studies.
Another interesting aspect of the junctional mosaic organization is that it might structurally organize adherens junction dynamics. First, since the size of the individual cluster is much smaller than the individual junction, their coordinated disassembly would increase the plasticity of cell-cell adhesion. Furthermore, the boundary between cadherin and nectin clusters might demarcate the site of cadherin or nectin endocytosis. Finally, since nectin adhesion is apparently weaker than the cadherin one (Takai et al., 2008), the growth of the nectin cluster concurrently with a decrease of the cadherin cluster would diminish junctional strength. Interestingly, our observation that none of the actin-binding proteins in the junctions demarcate individual clusters suggests that the actin filament organization in adherens junctions is regulated by much more complex mechanisms than just by the type of transmembrane adhesive receptor.
In conclusion, our examination of cadherin and nectin subjunctional distribution discovered a high level of complexity in adherens junction architecture. This complexity is likely to be important to junction assembly and the regulation of junctional strength and dynamics.
Material and Methods
Cell Culture, Antibodies, and Plasmids
Transfection and growth of human A431 and A431D cells were done as described (Hong et al., 2010). The plasmids encoding human E-cadherin tagged by GFP and mouse nectin-2 tagged with mCherry were described (Hong et al., 2010; Harrison et al., 2012). After co-transfection and antibiotic selection, the cells were sorted for transgenes expression by FACS and only moderate-expressing cells were used. Primary human foreskin keratinocytes were provided by the Northwestern University Skin Disease Research Center Keratinocyte Core Facility and propagated in low calcium as previously described (Lin et al., 2010). To generate afadin-depleted A431 cells, the cells were transduced with lentiviruses encoding afadin-specific shRNAs (V2LHS250765 and V2LHS250765, Thermo Fisher Scientific, Waltman, MA) and infected cells were selected with puromycine treatment (5 μ/ml).
The following antibodies were used: mouse anti-E-cadherin (Zymed Laboratories, South San Francisco, CA); mouse anti-vinculin and rabbit anti-afadin (Sigma, St. Louis, MO); mouse anti-β-catenin (BD, Franklin Lakes, NJ); goat antibodies specific to β-catenin, α-catenin, and WTIP; mouse antibodies specific to nectin-2 (clone B-C12 and clone R2.525), migfilin (clone G7), TRIP6 (clone F5), and rabbit anti-ajuba (all from Santa Cruz Inc Dallas, TX); rabbit polyclonal anti-afadin, rabbit mAB anti-nectin-2, goat anti-LPP, and mouse anti-α-actinin (abCAM, Cambidge, MA). AlexaFluor555 phalloidin was purchased from Invitrogen (Grand Island, NY).
Immunofluorescence and Live-Cell Imaging
Live-cell imaging and regular wide-field immunofluorescence microscopy were performed essentially as described earlier (Troyanovsky et al., 2006; Hong et al., 2010). Cells grown on glass coverslips were fixed and permeabilized either with methanol-acetone (Figs 2–4, 3) or with 3% formaldehyde-1% Triton X-100 (Fig. S4c). For better detection of vinculin and LIM-domain proteins in sites of adhesion, cells were fixed in 1 mg/ml solution of cysteine-specific cross-linker BM[PEO]3 with following cell permeabilization using 0.1% Triton X-100 (Figs 6a–c, S1, and S4a,b,d). Such treatment completely removed cytosolic pool of the proteins. For live-cell imaging, cell suspension (~1×105 cells) was plated into a homemade chamber built on cover glass. The next day, the culture media was replaced with imaging media (L-15 plus 10% FBS) and the chamber was imaged by the Eclipse Ti-E microscope (Nikon, Melville, NY) at 37°C controlled with Nikon's NIS-Elements software. The microscope was equipped with an incubator chamber, a CoolSNAP HQ2 camera (Photometrics, Tucson, AZ), Plan Apo 60×/1.40 and Plan Apo VC 100×/1.40 lenses and halogen and mercury light sources. Time-lapse images were taken in both FITC and TRITC channels in one sec intervals using halogen light that minimized phototoxicity and photobleaching. All images were saved as Tiff files and processed using ImageJ software (National Institutes of Health). Subdiffraction resolution images were taken using a Nikon N-SIM system available at the Imaging Center of Northwestern University. To reconstruct the sub-resolution structures, the images were analytically processed using Nikon element Version 4 software.
In situ PLA
Cells were fixed with methanol /acetone and incubated for 30 min with the rabbit anti-afadin polyclonal antibody (two different antibodies, which produced identical results, were tested) and mouse monoclonal anti-β-catenin or anti-α-catenin antibodies. Ligation and amplification were performed as described in the manufacturer's protocol (Duolink In Situ PLA; Olink Bioscience) using the following reagents: PLA probe anti–rabbit plus, PLA probe anti–mouse minus, and detection reagent Red. After PLA, the samples were incubated for additional 30 min with FITC-labeled donkey antibody against mouse IgG.
Supplementary Material
ACKNOWLEDGMENTS
We are grateful to Mr. P. Hoover (SDRC, Northwestern University) for providing human keratinocytes and Dr. J.K. Wahl (University of Nebraska) for providing A431D cells. This work was supported by Grants AR44016 and AR057992 from the National Institutes of Health.
Footnotes
CONFLICT OF INTEREST The authors state no conflict of interest.
Online supplemental material. Fig. S1 shows double staining of A431 cells for β-catenin and vinculin. Fig. S2 shows immunolocalization of nectin-2 and E-cadherin in polarized DLD-1 cells. Fig. S3 shows double-label immunofluorescence microscopy of A431cells overexpressing recombinant mCherry-tagged nectin-2. Fig. S4 presents characterization of the actin cytoskeleton associated with adherens junctions.
References
- Birukova AA, Fu P, Wu T, Dubrovskyi O, Sarich N, Poroyko V, Birukov KG. Afadin controls p120-catenin-ZO-1 interactions leading to endothelial barrier enhancement by oxidized phospholipids. J Cell Physiol. 2012;227:1883–1890. doi: 10.1002/jcp.22916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brasch J, Harrison OJ, Honig B, Shapiro L. Thinking outside the cell: how cadherins drive adhesion. Trends Cell Biol. 2012;22:299–310. doi: 10.1016/j.tcb.2012.03.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Call GS, Brereton D, Bullard JT, Chung JY, Meacham KL, Morrell DJ, Reeder DJ, Schuler JT, Slade AD, Hansen MD. A zyxin-nectin interaction facilitates zyxin localization to cell-cell adhesions. Biochem Biophys Res Commun. 2011;415:485–489. doi: 10.1016/j.bbrc.2011.10.099. [DOI] [PubMed] [Google Scholar]
- Choi HJ, Pokutta S, Cadwell GW, Bobkov AA, Bankston LA, Liddington RC, Weis WI. αE-catenin is an autoinhibited molecule that coactivates vinculin. Proc Natl Acad Sci U S A. 2012;109:8576–8581. doi: 10.1073/pnas.1203906109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harris TJ, Tepass U. Adherens junctions: from molecules to morphogenesis. Nat Rev Mol Cell Biol. 2010;11:502–514. doi: 10.1038/nrm2927. [DOI] [PubMed] [Google Scholar]
- Harrison OJ, Jin X, Hong S, Bahna F, Ahlsen G, Brasch J, Wu Y, Vendome J, Felsovalyi K, Hampton CM, Troyanovsky RB, Ben-Shaul A, Frank J, Troyanovsky SM, Shapiro L, Honig B. The extracellular architecture of adherens junctions revealed by crystal structures of type I cadherins. Structure. 2011;19:244–256. doi: 10.1016/j.str.2010.11.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hong S, Troyanovsky RB, Troyanovsky SM. Spontaneous assembly and active disassembly balance adherens junction homeostasis. Proc Natl Acad Sci U S A. 2010;107:3528–3533. doi: 10.1073/pnas.0911027107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hong S, Troyanovsky RB, Troyanovsky SM. Cadherin exits the junction by switching its adhesive bond. J Cell Biol. 2011;192:1073–1083. doi: 10.1083/jcb.201006113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoshino T, Sakisaka T, Baba T, Yamada T, Kimura T, Takai Y. Regulation of E-cadherin endocytosis by nectin through afadin, Rap1, and p120ctn. J Biol Chem. 2005;280:24095–24103. doi: 10.1074/jbc.M414447200. [DOI] [PubMed] [Google Scholar]
- Ikeda W, Nakanishi H, Miyoshi J, Mandai K, Ishizaki H, Tanaka M, Togawa A, Takahashi K, Nishioka H, Yoshida H, Mizoguchi A, Nishikawa S, Takai Y. Afadin: A key molecule essential for structural organization of cell-cell junctions ofpolarized epithelia during embryogenesis. J Cell Biol. 1999;146:1117–1132. doi: 10.1083/jcb.146.5.1117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kadrmas JL, Beckerle MC. The LIM domain: from the cytoskeleton to the nucleus. Nat Rev Mol Cell Biol. 2004;5:920–931. doi: 10.1038/nrm1499. [DOI] [PubMed] [Google Scholar]
- Kametani Y, Takeichi M. Basal-to-apical cadherin flow at cell junctions. Nat Cell Biol. 2007;9:92–98. doi: 10.1038/ncb1520. [DOI] [PubMed] [Google Scholar]
- Lin S, Gordon K, Kaplan N, Getsios S. Ligand targeting of EphA2 enhances keratinocyte adhesion and differentiation via desmoglein 1. Mol Biol Cell. 2010;21:3902–3914. doi: 10.1091/mbc.E10-03-0242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meng W, Takeichi M. Adherens junction: molecular architecture and regulation. Cold Spring Harb Perspect Biol. 2009;1:a002899. doi: 10.1101/cshperspect.a002899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miyahara M, Nakanishi H, Takahashi K, Satoh-Horikawa K, Tachibana K, Takai Y. Interaction of nectin with afadin is necessary for its clustering at cell-cell contact sites but not for its cis dimerization or trans interaction. J Biol Chem. 2000;275:613–618. doi: 10.1074/jbc.275.1.613. [DOI] [PubMed] [Google Scholar]
- Morita H, Nandadasa S, Yamamoto TS, Terasaka-Iioka C, Wylie C, Ueno N. Nectin-2 and N-cadherin interact through extracellular domains and induce apical accumulation of F-actin in apical constriction of Xenopus neural tube morphogenesis. Development. 2010;137:1315–1325. doi: 10.1242/dev.043190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nelson WJ. Regulation of cell-cell adhesion by the cadherin-catenin complex. Biochem Soc Trans. 2008;36:149–155. doi: 10.1042/BST0360149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pokutta S, Drees F, Takai Y, Nelson WJ, Weis WI. Biochemical and structural definition of the l-afadin- and actin-binding sites of alpha-catenin. J Biol Chem. 2002;277:18868–18874. doi: 10.1074/jbc.M201463200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rangarajan ES, Izard T. The cytoskeletal protein α-catenin unfurls upon binding to vinculin. J Biol Chem. 2012;287:18492–18499. doi: 10.1074/jbc.M112.351023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sözen MA, Suzuki K, Tolarova MM, Bustos T, Fernández Iglesias JE, Spritz RA. Mutation of PVRL1 is associated with sporadic, non-syndromic cleft lip-palate in northern Venezuela. Nat Genet. 2001;29:141–142. doi: 10.1038/ng740. [DOI] [PubMed] [Google Scholar]
- Suzuki K, Hu D, Bustos T, Zlotogora J, Richieri-Costa A, Helms JA, Spritz RA. Mutations of PVRL1, encoding a cell-cell adhesion molecule-herpesvirus receptor, in cleft lip-palate-ectodermal dysplasia. Nat Genet. 2000;25:427–430. doi: 10.1038/78119. [DOI] [PubMed] [Google Scholar]
- Takai Y, Ikeda W, Ogita H, Rikitake Y. The immunoglobulin-like cell adhesion molecule nectin and its associated protein afadin. Annu Rev Cell Dev Biol. 2008;24:309–342. doi: 10.1146/annurev.cellbio.24.110707.175339. [DOI] [PubMed] [Google Scholar]
- Tachibana K, Nakanishi H, Mandai K, Ozaki K, Ikeda W, Yamamoto Y, Nagafuchi A, Tsukita S, Takai Y. Two cell adhesion molecules, nectin and cadherin, interact through their cytoplasmic domain-associated proteins. J Cell Biol. 2000;150:1161–1176. doi: 10.1083/jcb.150.5.1161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Troyanovsky RB, Sokolov EP, Troyanovsky SM. Endocytosis of cadherin from intracellular junctions is the driving force for cadherin adhesive dimer disassembly. Mol Biol Cell. 2006;17:3484–3493. doi: 10.1091/mbc.E06-03-0190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Troyanovsky S. Adherens junction assembly. Subcell Biochem. 2012;60:89–108. doi: 10.1007/978-94-007-4186-7_5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu Y, Jin X, Harrison O, Shapiro L, Honig BH, Ben-Shaul A. Cooperativity between trans and cis interactions in cadherin-mediated junction formation. Proc Natl Acad Sci U S A. 2010;107:17592–17597. doi: 10.1073/pnas.1011247107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhadanov AB, Provance DW, Jr, Speer CA, Coffin JD, Goss D, Blixt JA, Reichert CM, Mercer JA. Absence of the tight junctional protein AF-6 disrupts epithelial cell-cell junctions and cell polarity during mouse development. Curr Biol. 1999;9:880–888. doi: 10.1016/s0960-9822(99)80392-3. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.