Abstract
Study Objectives:
CPAP is an effective treatment for OSA that may reduce health care utilization and costs. Medicare currently reimburses the costs of long-term CPAP therapy only if the patient is adherent during a 90-day trial. If not, Medicare requires a repeat polysomnogram (PSG) and another trial which seems empirically not cost-effective. We modeled the cost-effectiveness of current Medicare policy compared to an alternative policy (clinic-only) without the adherence criterion and repeat PSG.
Design:
Cost-utility and cost-effectiveness analysis.
Setting:
U.S. Medicare Population.
Patients or Participants:
N/A.
Interventions:
N/A.
Measurements and Results:
We created a decision tree modeling (1) clinic only follow-up vs. (2) current Medicare policy. Costs were assigned based on Medicare reimbursement rates in 2012. Sensitivity analyses were conducted to test our assumptions. We estimated cumulative costs, overall adherence, and QALY gained for a 5-year time horizon from the perspective of Medicare as the payer. Current Medicare policy is more costly than the clinic-only policy but has higher net adherence and improved utility. Current Medicare policy compared to clinic-only policy costs $30,544 more per QALY.
Conclusions:
Current CMS policy promotes early identification of those more likely to adhere to CPAP therapy by requiring strict adherence standards. The policy effect is to deny coverage to those unlikely to use CPAP long-term and prevent wasted resources. Future studies are needed to measure long-term adherence in an elderly population with and without current adherence requirements to verify the cost-effectiveness of a policy change.
Citation:
Billings ME; Kapur VK. Medicare long-term CPAP coverage policy: a cost-utility analysis. J Clin Sleep Med 2013;9(10):1023-1029.
Keywords: Cost-Utility Analysis, CPAP, Medicare
Obstructive sleep apnea (OSA) is a disorder of upper airway collapse during sleep. Its health consequences include increased cardiovascular morbidity and mortality,1–5 excessive daytime sleepiness, increased risk of motor vehicle collision (MVC), and reduced health related quality of life.6 Health care utilization and costs are higher in OSA patients compared to age-, sex-, and body mass index (BMI)-matched controls; OSA severity has been associated with health care costs after adjustment for BMI.7,8
OSA is common in the Medicare population, with an estimated prevalence of 20% to 30%.5,9 Continuous positive airway pressure (CPAP), the most efficacious treatment for OSA, reduces daytime sleepiness,10 decreases the risk of cardiovascular events, and improves health related quality of life.11,12 CPAP was the 26th most common Medicare procedure code, with a total expenditure of $213 million and coverage for 2.6 million allowed services in 2009.13 Optimizing current CPAP coverage policy to yield the most efficient use of health-care resources is relevant to controlling overall health care costs and optimizing outcomes.
Long-term CPAP therapy may reduce health care utilization and costs in some patients.14 Cost-effectiveness analyses conducted in the US, UK, and Canada from the payer perspective have demonstrated that CPAP is cost-effective, with costs per quality adjusted life year (QALY) gained well below societal cutoffs (typically $50,000 per QALY in the United States).6,15–19 CPAP has been shown to be the dominant treatment (less costly, more effective) compared to lifestyle changes after 13 years in a UK study of moderate to severe OSA.15
BRIEF SUMMARY
Current Knowledge/Study Rationale: Medicare restricts long-term CPAP coverage to those demonstrating adherence during a 90-day trial period; a repeat PSG is required for a repeat trial. The study explores the cost-effectiveness of this policy compared to an alternative of only requiring clinic follow-up.
Study Impact: Current Medicare policy is more costly than a clinic-only policy but results in greater adherence with higher net utility. Identifying optimal coverage policies which promote CPAP use with adherence standards and limit wasted resources may be a useful strategy to improve health and control costs.
Despite its efficacy and cost-effectiveness, CPAP adherence is poor, with a large portion of patients not using their machines.20 Thus long-term CPAP coverage is limited by the Center for Medicare and Medicaid Services (CMS) to those who demonstrate adherence and subjective benefit during an initial 90-day trial period. Adherence is defined as CPAP use ≥ 4 hours a night, for 70% of days within a 30 consecutive day period. Durable medical equipment (DME) companies that supply and support CPAP have developed protocols to improve adherence during this trial period in order to secure Medicare payments for long-term CPAP device rental and supplies. Non-Medicare patients with private insurance companies often do not have these strict requirements; long-term CPAP is covered without documentation of adherence.
If initial adherence criteria are not met but CPAP treatment is still desired by the Medicare patient and clinical provider, CMS requires a repeat in-laboratory polysomnogram (PSG) to authorize another 90-day trial to continue coverage.21,22
One PSG costs CMS about half the cost of a CPAP machine purchase (13 months rental), excluding supplies. Thus, many sleep clinicians and patients question the rationale behind this policy.
Objective
We sought to evaluate the cost-effectiveness of current CMS policy, which appears empirically inefficient and costly, when compared to an alternative policy of continued CPAP coverage with only clinic visits and not a repeat PSG if adherence requirements are not met.
METHODS
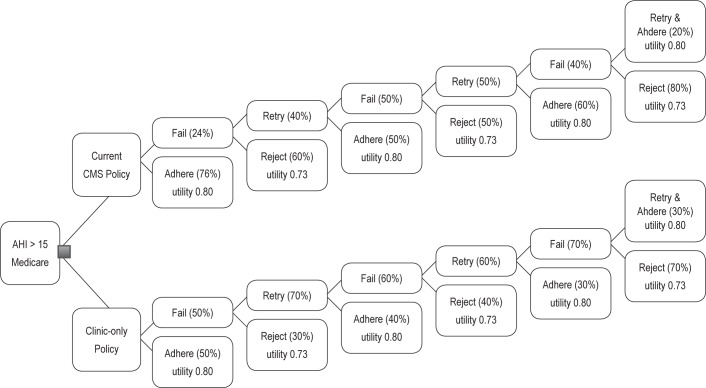
We created a decision tree to model two different policies for long-term CPAP coverage for Medicare beneficiaries with an apnea hypopnea index (AHI) ≥ 15. We chose to focus on those with AHI > 15 to eliminate CPAP-eligible subjects with mild OSA and symptoms who may have distinct adherence patterns from those with moderate or severe OSA. Medicare patients are unique, in that the entire US population at age 65 is eligible for benefits, regardless of education, income, etc. We assume that beneficiaries in both arms are similar in regard to use of supplemental private insurance. The coverage policies are: (1) clinic-only policy with an extended 13-month CPAP trial period vs. (2) current CMS policy (Figure 1). In the clinic-only policy, CPAP coverage would continue regardless of 90-day trial adherence as long as beneficiaries followed up in clinic every 3 months and desired to continue, no repeat PSG needed. After 13 months, CPAP supplies would only be covered if an adherence threshold of average use of ≥ 4 h/night for 70% of nights over 30 nights was achieved during any of the three 90 day trials.
Figure 1. Decision analysis tree.
Current CMS policy, by contrast does not cover CPAP rental fees or supply costs if initial adherence requirements are not met during the 90-day trial. To qualify for another 90-day trial of CPAP, CMS requires a repeat in-lab PSG and clinic visit.21 A maximum of three 90-day trial periods are allowed. In our model, the beneficiary could discontinue CPAP after each 3-month trial period in either arm. After 13 months of CPAP rental, CMS and the beneficiary have paid the cost of CPAP, so only supplies coverage is required.
In our base case, we assumed an initial 50% adherence rate for those in the clinic-only policy arm, based on historical controls from the literature of CPAP adherence.20,23,24 We estimated 70% would want to continue to try CPAP in this arm if they failed to meet 90-day adherence criteria, given that no repeat PSG was required. But we estimated a lower proportion of 40% would meet adherence criteria on the second attempt. For those in the CMS current policy pathway, we assumed an initial adherence rate of 76%. This was based on an informal survey of several DME companies' adherence levels among Medicare beneficiaries. We estimated that after initial failure, 40% would be willing to retry CPAP and 50% would meet adherence criteria on the second attempt, also based on DME data. Limited data were available on the proportions meeting adherence criteria on the 2nd and 3rd trials and rejecting CPAP after their second trial, so these proportions are largely estimations.
After 13 months (or 3 trial periods), the subjects in both arms would own their CPAP machines, and CMS would no longer pay DME companies a rental fee. In both arms, CMS would only cover the cost of supplies after 13 months in those meeting adherence criteria. We assumed that three 90-day trials of CPAP would require 13 months given a 2-3 week lag time between required clinic visits and repeat PSGs.
We used costs derived from the 2012 Medicare reimbursement rates (see Table 1). Costs were gathered from physician and facility fee schedules for the following the CPT codes: 95811 (split night PSG with CPAP) and 99214 (office follow-up visit).25 The costs of CPAP rental (HCPCS E0601) and CPAP supplies (A7027, A7030, A7034 masks, A7037 tubing, A7035 headgear, A7038 filter, and E0561 humidifiers) were from the 2012 Durable Medical Equipment, Prosthetics, Orthotics, and Supplies (DMEPOS) Fee Schedule.26 The Medicare reimbursement of CPAP rental for 13 months is $1,339, after which the patient owns the device for the duration of its use (5 years). The various mask and replacement part reimbursement rates were averaged. Refills of CPAP supplies were estimated to cost $264 annually: one new mask, a mask replacement part, new tubing, one filter, and one new head-gear (about a third as maximally allowed by Medicare21), as this is more typical usage pattern.27,28 We assumed none of the subjects switched to bilevel pressure device, which has a much higher monthly rental cost (HCPCS E0470/1)26 during the 5-year period.
Table 1.
Reimbursement of evaluation and treatment per Medicare 2012 fee schedules (physician,25 durable medical equipment, and supplies26)

Our main clinical outcome was the proportion using CPAP at 5 years, which was calculated from the percent meeting adherence criteria at 13 months after applying a 4% annual decline in adherence.29 We estimated the total costs for each long-term CPAP coverage policy over a 5-year time horizon with a 3% discount rate. We calculated the incremental cost effectiveness ratio (ICER) for the CMS policy compared to the clinic-only policy using the above assumptions for our base case. The ICER compares the incremental cost difference in the two policies divided by the incremental difference in the outcome of interest, which was percent adherent at 5 years. We also performed a cost-utility analysis, calculating the ICER per QALY by dividing the cost differences of the two policies by the differences in utility.
Utilities were based on results from the EQ-5D (Euroqol), the most common health related quality of life instrument used in cost-effectiveness studies of CPAP and OSA.6,15–19,28 The base case OSA utility was 0.73, which improved to 0.80 with CPAP therapy. The EQ-5D utilities values were based on 2 small studies surveying subjects with sleep apnea (AHI > 15) prior to and after CPAP therapy.6,30 These utilities were used to calculate the quality adjusted life year (QALY) gained.
Sensitivity Analysis
We performed extensive single-variable and multi-variable sensitivity analyses to evaluate the parameters of uncertainty. Primarily we varied the initial adherence proportion by +26/-20% in the clinic-only arm (30% to 76%) and +8%/-26% in the current CMS policy arm (50% to 84%). We also adjusted the proportion wanting to retry (vs. reject) CPAP after initial failure to meet adherence by ± 20%. We varied the second trial adherence rate as well as the proportion willing to do a second trial similarly. We varied costs of CPAP rental and PSG by ± 20%. We additionally substituted the costs of out-of-center sleep-testing (OCST) (CPT 95806)25 instead of in-lab PSG for retrial of CPAP in the CMS arm (not currently allowed by CMS as an option). We varied the refill rates and resulting projected annual costs from minimal (10% allowed) to near maximal allowed by Medicare. We additionally evaluated the effect of a 15% annual decline long-term adherence. Finally we substituted utilities derived from the standard gamble method among a group of OSA patients with and without CPAP therapy instead of the EQ-5D.30
Our analysis was conducted using excel spreadsheets.
RESULTS
In our base case model, current CMS policy arm yielded an overall higher adherence rate and utility. The net adherence rate after 5 years was 59% in the clinic-only policy compared to 70% in the current CMS policy (adherence difference of 11%). The overall EQ-5D utility was 0.772 in the clinic-only policy vs. 0.779 in the current CMS policy (difference of 0.007 QALY or 2.6 quality-adjusted life-days). However, the current CMS long-term CPAP coverage policy was more expensive with a cost difference of $220 per person compared to the clinic-only policy using a 3% discount rate over a 5-year time horizon. The incremental cost effectiveness ratio (ICER) was $1,999.22/percent-adherence-gained and $30,543.60 per QALY for the base case for CMS policy compared to the clinic-only policy.
Sensitivity Analyses
One-way sensitivity analyses revealed that the initial adherence rates more than CPAP or PSG costs drove the ICER. If both pathways had the same high 76% initial adherence, the clinic-only policy yielded a 3% greater adherence than the CMS policy arm for a cost of only $42 more. The resulting ICER was only $1,408/percent-adherence-gained and $21,121 per QALY for clinic-only compared to the current CMS policy. If both initial adherence rates were assumed to be low at 50%, the clinic-only policy returned a 5% greater adherence than the CMS policy while costing $9 less. In this scenario, the clinic-only policy dominated with a lower cost for a greater adherence and utility.
The incremental cost effectiveness ratio remained stable when varying most other assumptions including retrial rate and second trial adherence. The current CMS policy costs were consistently higher, but yielded better net adherence and utility. The CPAP supplies refill pattern over the 5 years had a large impact on the ICER: if near maximal refills were obtained, the current CMS policy was much more costly (Table 2). Varying the cost of the PSG and CPAP rental by 20% had minimal effect on the ICER as demonstrated in the tornado diagram (Figure 2). Substituting OCST instead of an in-lab PSG reduced the ICER only slightly. Using standard gamble derived utilities, where CPAP had a greater impact on quality of life, yielded a much more cost-effective ICER of $6,872 per QALY. Modeling a greater annual adherence decline resulted in an increased ICER. A 15% annual dropoff in adherence resulted in an ICER of $65,237 per QALY and much less cost-effective policy.
Table 2.
Sensitivity analyses
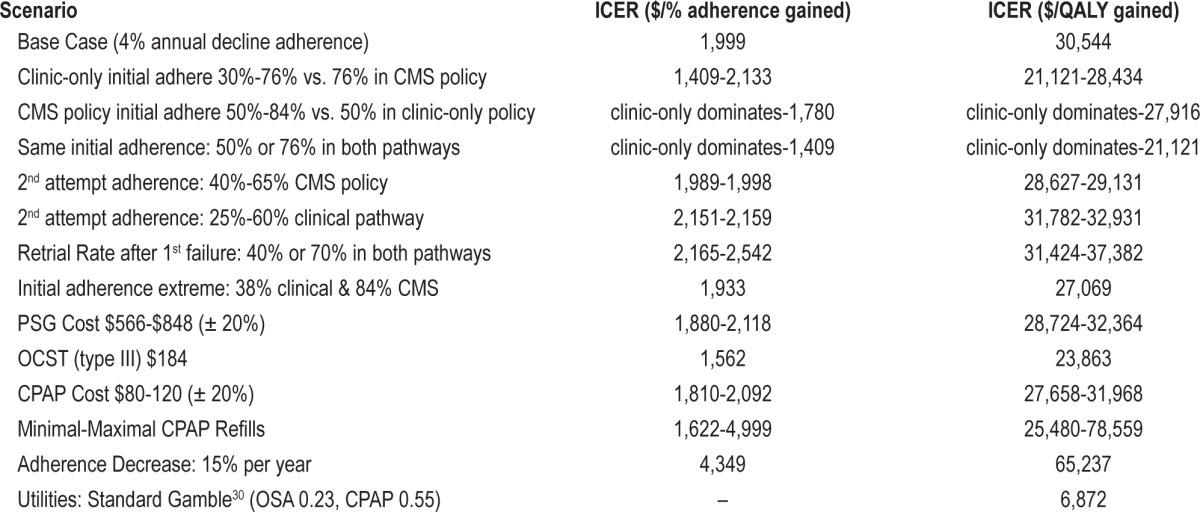
Figure 2. Plot of difference in ICER from $1,999 base case ICER (cost/%-adherence-gained) for different scenarios.
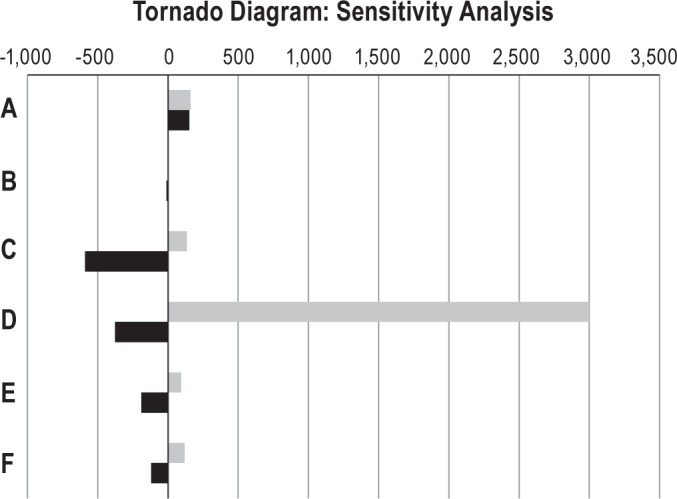
(A) 25% & 60% adherence on second CPAP trial in the CMS pathway vs. base case 40% clinic-only pathway. (B) 40% & 65% adherence on second CPAP trial in the clinic-only pathway vs. base case 50% CMS pathway. (C) 30% & 76% initial adherence in clinic-only pathway vs. base case 76% CMS pathway. (D) Minimal and maximal CPAP supply refills for both CMS and clinic-only pathways. (E) CPAP rental fees $80 & $120 (± 20%). (F) In-lab PSG fees $566 & $848 (± 20%). The black bars represent the difference in the lower range variable ICER compared to the base case. The gray bars represent the difference in the higher range variable ICER compared to the base.
DISCUSSION
According to our model, current CMS policy for long-term CPAP coverage yields greater overall CPAP adherence rates and improves quality of life but at a higher cost. This is based on the premise in our model of higher initial adherence with current CMS policy than with the clinic only policy. Current CMS policy appears to be designed to identify and incentivize CPAP adherence through strict initial adherence requirements and mandated in-lab PSG for those failing to meet requirements and desiring to continue CPAP. The impact of this, we assume in our model, is to increase the proportion of early adherence and continue CPAP coverage only in subjects likely to be adherent. Data we collected from local DME companies show higher CPAP adherence rates in the 90-day trial period than reported in observational studies and trials. Our model suggests that CMS policy leads to about an 11% difference in net long-term adherence rates given our stated assumptions.
The current CMS policy was consistently more costly than the clinic-only policy of continued CPAP coverage without repeat PSG. We calculated an incremental cost of about $2,000 per percent-adherence-gained over 5 years. The incremental cost of one quality of life adjusted year, as assessed by the EQ-5D, was below societal thresholds at $30,000/QALY. If utilities were derived from the standard gamble method instead, the ICER was much lower at $6,872 per QALY. If the impact of CPAP on quality of life is greater than assessed by the EQ-5D and better reflected by the standard gamble derived utilities, the current CMS policy would be more cost-effective.
Sensitivity analyses demonstrated that the current CMS policy initial adherence rates and refill patterns were the primary determinants of our ICER. If the policy did not result in differing initial adherence rates, the current CMS policy would be costlier without improving adherence. The rates we used in our base case for the CMS policy had real-world data behind them from our local DME surveys but may not represent nationwide adherence rates. The parameters of greater uncertainty (the initial adherence and retrial rates in the clinic-only pathway) had less of an impact on the ICER. The second and third trial adherence levels and re-retrial rates were the most speculative with the least data. But varying these values had little impact on the ICER, with only minor resulting changes in adherence, utility, and overall costs.
The other sensitivity analyses yielded more surprising results. Varying the cost by 20% of the PSG and CPAP rental, which may have been projected drivers of cost, impacted the ICER minimally. CPAP refill patterns had the largest impact on the ICER, making the current CMS policy not cost-effective if maximal refills were routinely obtained. But literature suggests that minimal refill rates are more the norm.27 A larger decline in long-term adherence, which is likely more consistent with real-world observations, suggests that the current CMS policy is even less cost-effective with higher ICERs. The base model assumed only a 4% decline in adherence which may falsely elevate the incremental benefit in adherence and utility over 5-years. If adherence declines precipitously over the years, the current CMS policy is not cost-effective.
This analysis has several limitations. Adherence rates in the proposed clinic-only pathway among Medicare patients without current adherence requirements are unknown; we used adherence rates from CPAP trials that typically included participants younger than 65. These adherence rates may not generalize to an elderly Medicare population with more comorbidities. If initial adherence rates did not differ between the CMS policy, the clinic-only policy would yield greater adherence at a much lower additional cost. Additionally, many patients may switch to a more costly bilevel device, which would also impact our ICER. The use of the EQ-5D to estimate utility in the OSA and CPAP cost-effectiveness literature is widespread. However, the instrument has a ceiling effect and is not designed to be sensitive to the subjective effects of OSA and the benefits of CPAP. The EQ-5D values also come from studies of younger, healthier OSA populations with sleepiness.6,30 The impact of OSA in these subjects likely differs from elderly Medicare subjects with more comorbidities who may not be sleepy. The correct assessment of the impact of CPAP on quality of life may dramatically change the cost-effectiveness of these policies.
Our analyses would be strengthened by additional real-world data on adherence and retrial success rates in a Medicare population with and without the current adherence requirements. Additionally, a more accurate evaluation of the impact of CPAP on the Medicare OSA population's quality of life would improve the validity of this study. Prior to undertaking a cost-effectiveness analysis with a longer time horizon, these limitations need to be addressed. An analysis that incorporates future costs and utilities from differences in health care utilization, different rates of cardiovascular events, and motor vehicle accidents from untreated OSA, as incorporated into other cost-effectiveness studies,15,16,19,28 is unlikely to be accurate, given the uncertainty of long-term adherence patterns. But fully accounting for the downstream costs and evaluating the societal costs would be an important next step in assessing the cost-effectiveness of current CMS long-term coverage policy. It is possible that current CMS policy with its resulting higher adherence may be even more cost-effective if downstream costs of untreated OSA are incorporated.
Current CMS CPAP coverage policy compared to our alternative yields a benefit in quality of life below societal cutoffs of $50,000 per QALY. It is more costly with its structured incentives but yields a greater net adherence and quality of life compared to the clinic-only coverage policy. The required additional testing, the added co-pay and inconvenience of an overnight PSG, and the threat of loss of CPAP coverage incentivize the patient to meet adherence requirements. It also provides incentives to the DME companies to assist beneficiaries during that 90-day trial to meet CMS requirements and lock in CMS coverage for CPAP and related supplies. Physicians may have less financial incentive as they may benefit from the fees associated with an additional (potentially clinically unnecessary) PSG, but they may also strive harder in their patients' interest to help them meet thresholds to ensure long-term CPAP use and continued therapy for OSA.
By contrast, the clinic-only policy we propose without strict adherence requirements does not provide incentives to demonstrate adherence. It encourages continued trials of CPAP even if the initial 90-day experience is poor without the hassle of another PSG. Beneficiaries may keep machines sitting in their closets at Medicare's expense for the duration of the 13-month trial. Without incentive, DME companies may no longer work with beneficiaries as aggressively to help them utilize their CPAP and solve problems limiting adherence. Adherence rates may suffer with minimal cost savings and potential increased downstream costs from lack of OSA treatment. So surprisingly, requiring adherence and the additional burden of a repeat PSG may be a cost-effective long-term coverage strategy. However, different incentive strategies to improve adherence, such as cost-sharing of CPAP or reduced co-pays with greater adherence, may have a similar effect with less cost to CMS and without the inconvenience and waste of an unneeded PSG. CMS policy makers should consider alternatives to incentivize CPAP use for the patient, DME companies, physicians, and sleep centers without wasting resources.
DISCLOSURE STATEMENT
This was not an industry supported study. The authors have indicated no financial conflicts of interest. This material is based upon work supported in part by the Department of Veterans Affairs, Veterans Health Administration, Office of Research and Development and Health Services Research and Development (HSR&D). Dr. Billings was supported by a HSR&D MD Postdoctoral fellowship: TPM# 61-038. The study's contents are solely the responsibility of the authors. The views expressed in this article are those of the authors and do not necessarily reflect the position or policy of the Department of Veterans Affairs, the United States Government or funding agencies. The authors had full access to all of the data in the study and take responsibility for the integrity of the data and the accuracy of the data analysis.
ABBREVIATIONS
- AHI
apnea hypopnea index
- BMI
body mass index
- CMS
Centers for Medicare and Medicaid Services
- CPAP
continuous positive airway pressure
- DME
durable medical equipment
- ICER
incremental cost-effectiveness ratio
- MVC
motor vehicle collision
- OSA
obstructive sleep apnea
- OCST
out-of-center sleep testing
- PSG
polysomnogram
- QALY
quality adjusted life year
REFERENCES
- 1.Marin JM, Carrizo SJ, Vicente E, Agusti AG. Long-term cardiovascular outcomes in men with obstructive sleep apnoea-hypopnoea with or without treatment with continuous positive airway pressure: an observational study. Lancet. 2005;365:1046–53. doi: 10.1016/S0140-6736(05)71141-7. [DOI] [PubMed] [Google Scholar]
- 2.Shahar E, Whitney CW, Redline S, et al. Sleep-disordered breathing and cardiovascular disease: cross-sectional results of the Sleep Heart Health Study. Am J Respir Crit Care Med. 2001;163:19–25. doi: 10.1164/ajrccm.163.1.2001008. [DOI] [PubMed] [Google Scholar]
- 3.Young T, Peppard P. Sleep-disordered breathing and cardiovascular disease: epidemiologic evidence for a relationship. Sleep. 2000;23(Suppl 4):S122–6. [PubMed] [Google Scholar]
- 4.Yaggi HK, Concato J, Kernan WN, Lichtman JH, Brass LM, Mohsenin V. Obstructive sleep apnea as a risk factor for stroke and death. N Engl J Med. 2005;353:2034–41. doi: 10.1056/NEJMoa043104. [DOI] [PubMed] [Google Scholar]
- 5.Young T, Shahar E, Nieto FJ, et al. Predictors of sleep-disordered breathing in community-dwelling adults: the Sleep Heart Health Study. Arch Intern Med. 2002;162:893–900. doi: 10.1001/archinte.162.8.893. [DOI] [PubMed] [Google Scholar]
- 6.Mar J, Rueda JR, Duran-Cantolla J, Schechter C, Chilcott J. The cost-effectiveness of nCPAP treatment in patients with moderate-to-severe obstructive sleep apnoea. Eur Respir J. 2003;21:515–22. doi: 10.1183/09031936.03.00040903. [DOI] [PubMed] [Google Scholar]
- 7.Banno K, Ramsey C, Walld R, Kryger MH. Expenditure on health care in obese women with and without sleep apnea. Sleep. 2009;32:247–52. doi: 10.1093/sleep/32.2.247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kapur V, Blough DK, Sandblom RE, et al. The medical cost of undiagnosed sleep apnea. Sleep. 1999;22:749–55. doi: 10.1093/sleep/22.6.749. [DOI] [PubMed] [Google Scholar]
- 9.Bixler EO, Vgontzas AN, Ten Have T, Tyson K, Kales A. Effects of age on sleep apnea in men: prevalence and severity. Am J Respir Crit Care Med. 1998;157:144–8. doi: 10.1164/ajrccm.157.1.9706079. [DOI] [PubMed] [Google Scholar]
- 10.Engleman HM, Martin SE, Deary IJ, Douglas NJ. Effect of continuous positive airway pressure treatment on daytime function in sleep apnoea/hypopnea syndrome. Lancet. 1994;343:572–5. doi: 10.1016/s0140-6736(94)91522-9. [DOI] [PubMed] [Google Scholar]
- 11.Giles TL, Lasserson TJ, Smith BH, White J, Wright J, Cates CJ. Continuous positive airways pressure for obstructive sleep apnoea in adults. Cochrane Database Syst Rev. 2006;3:CD001106. doi: 10.1002/14651858.CD001106.pub3. [DOI] [PubMed] [Google Scholar]
- 12.Martinez-Garcia MA, Soler-Cataluna JJ, Ejarque-Martinez L, et al. Continuous positive airway pressure treatment reduces mortality in patients with ischemic stroke and obstructive sleep apnea: a 5-year follow-up study. Am J Respir Crit Care Med. 2009;180:36–41. doi: 10.1164/rccm.200808-1341OC. [DOI] [PubMed] [Google Scholar]
- 13.Centers for Medicare and Medicaid Services. Top 200 Level 11 Current Procedural Terminology (HCPCS/CPT) Codes CY 2009. [Accessed December 22 2012]. https://www.cms.gov/Research-Statistics-Data-and-Systems/Statistics-Trends-and-Reports/MedicareFeeforSvcPartsAB/Downloads/Level2CHARG09.pdf.
- 14.Peker Y, Hedner J, Johansson A, Bende M. Reduced hospitalization with cardiovascular and pulmonary disease in obstructive sleep apnea patients on nasal CPAP treatment. Sleep. 1997;20:645–53. doi: 10.1093/sleep/20.8.645. [DOI] [PubMed] [Google Scholar]
- 15.Guest JF, Helter MT, Morga A, Stradling JR. Cost-effectiveness of using continuous positive airway pressure in the treatment of severe obstructive sleep apnoea/hypopnoea syndrome in the UK. Thorax. 2008;63:860–5. doi: 10.1136/thx.2007.086454. [DOI] [PubMed] [Google Scholar]
- 16.Tan MC, Ayas NT, Mulgrew A, et al. Cost-effectiveness of continuous positive airway pressure therapy in patients with obstructive sleep apnea-hypopnea in British Columbia. Can Respir J. 2008;15:159–65. doi: 10.1155/2008/719231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.McDaid C, Griffin S, Weatherly H, et al. Continuous positive airway pressure devices for the treatment of obstructive sleep apnoea-hypopnoea syndrome: a systematic review and economic analysis. Health Technol Assess. 2009;13:iii–iv. xi–xiv, 1–119, 143–274. doi: 10.3310/hta13040. [DOI] [PubMed] [Google Scholar]
- 18.Weatherly HL, Griffin SC, McDaid C, et al. An economic analysis of continuous positive airway pressure for the treatment of obstructive sleep apnea-hypopnea syndrome. Int J Technol Assess Health Care. 2009;25:26–34. doi: 10.1017/S0266462309090047. [DOI] [PubMed] [Google Scholar]
- 19.Ayas NT, FitzGerald JM, Fleetham JA, et al. Cost-effectiveness of continuous positive airway pressure therapy for moderate to severe obstructive sleep apnea/hypopnea. Arch Intern Med. 2006;166:977–84. doi: 10.1001/archinte.166.9.977. [DOI] [PubMed] [Google Scholar]
- 20.Weaver TE, Grunstein RR. Adherence to continuous positive airway pressure therapy: the challenge to effective treatment. Proc Am Thorac Soc. 2008;5:173–8. doi: 10.1513/pats.200708-119MG. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Noridian Healthcare Solutions, LLC. Local coverage determination (LCD) for positive airway pressure (PAP) devices for the treatment of obstructive sleep apnea (L171) 2012. Nov 4, [Accessed January 16 2013]. https://www.noridianmedicare.com/dme/coverage/docs/lcds/current_lcds/positive_airway_pressure_pap_devices_for_the_treatment_of_obstructive_sleep_apnea.htm.
- 22.Centers for Medicare and Medicaid Services. Positive Airway Pressure (PAP) Devices. 2010. Dec, [Accessed January 16 2013]. http://www.cms.gov/Outreach-and-Education/Medicare-Learning-Network-MLN/MLNProducts/downloads/PAP_DocCvg_Factsheet_ICN905064.pdf.
- 23.Sawyer AM, Gooneratne NS, Marcus CL, Ofer D, Richards KC, Weaver TE. A systematic review of CPAP adherence across age groups: clinical and empiric insights for developing CPAP adherence interventions. Sleep Med Rev. 2011;15:343–56. doi: 10.1016/j.smrv.2011.01.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Rosen CL, Auckley D, Benca R, et al. A multi-site randomized trial of portable sleep studies and positive airway pressure autotitration versus laboratory-based polysomnography for the diagnosis and treatment of obstructive sleep apnea: The HomePAP Study. Sleep. 2012;35:757–67. doi: 10.5665/sleep.1870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Centers for Medicare and Medicaid Services. Physician Fee Schedule Search, Year 2012B. [Accessed January 16 2013]. http://www.cms.gov/apps/physician-fee-schedule/search/search-criteria.aspx.
- 26.Centers for Medicare and Medicaid Services. Durable Medical Equipment, Prosthetics/Orthotics & Supplies Fee Schedule. [Accessed January 16 2013]. http://www.cms.gov/DMEPOSFeeSched/01_overview.asp.
- 27.Patel N, Sam A, Valentin A, Quan SF, Parthasarathy S. Refill rates of accessories for positive airway pressure therapy as a surrogate measure of long-term adherence. J Clin Sleep Med. 2012;8:169–75. doi: 10.5664/jcsm.1772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Pietzsch JB, Garner A, Cipriano LE, Linehan JH. An integrated health- economic analysis of diagnostic and therapeutic strategies in the treatment of moderate-to-severe obstructive sleep apnea. Sleep. 2011;34:695–709. doi: 10.5665/SLEEP.1030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.McArdle N, Devereux G, Heidarnejad H, Engleman HM, Mackay TW, Douglas NJ. Long-term use of CPAP therapy for sleep apnea/hypopnea syndrome. Am J Respir Crit Care Med. 1999;159:1108–14. doi: 10.1164/ajrccm.159.4.9807111. [DOI] [PubMed] [Google Scholar]
- 30.Chakravorty I, Cayton RM, Szczepura A. Health utilities in evaluating intervention in the sleep apnoea/hypopnoea syndrome. Eur Respir J. 2002;20:1233–8. doi: 10.1183/09031936.00.00014401. [DOI] [PubMed] [Google Scholar]