Abstract
Purpose
Confirmatory factor analysis (CFA) was used to test the hypothesis whether adipocytokines are associated with the risk factor cluster that characterizes the metabolic syndrome (MetS).
Methods
Data from 134 nondiabetic subjects were analyzed using CFA. Insulin sensitivity (SI) was quantified using intravenous glucose tolerance tests, visceral fat area by CT scan and fasting HDL, triglycerides, monocyte chemo-attractant protein-1 (MCP-1), serum amyloid A (SAA), tumor necrosis factor-α (TNF-α), adiponectin, resistin, leptin, interleukin-6 (IL-6), C-reactive protein (CRP) and plasminogen activator inhibitor-1 (PAI-1) were measured.
Results
The basic model representing the MetS included six indicators comprising obesity, SI, lipids and hypertension, and demonstrated excellent goodness-of-fit. Using multivariate analysis, MCP-1, SAA and TNF-α were not independently associated with any of the MetS variables. Adiponectin, resistin, leptin, CRP and IL-6 were associated with at least one of the risk factors, but when added to the basic model decreased all goodness-of-fit parameters. PAI-1 was associated with all cardiometabolic factors and improved goodness-of-fit compared to the basic model.
Conclusions
Addition of PAI-1 increased the CFA model goodness-of-fit compared to the basic model, suggesting that this protein may represent an added feature of the MetS.
Keywords: metabolic syndrome, adipokines, cytokines, inflammatory proteins, factor analysis, obesity, insulin sensitivity
INTRODUCTION
The metabolic syndrome (MetS) represents the clustering of several cardiometabolic risk factors – abdominal obesity, hypertension, dyslipidemia and glucose intolerance [36] – and is associated with a greater likelihood of developing cardiovascular disease [15] and type 2 diabetes mellitus [24]. Although the clinical usefulness of the MetS has been questioned [21], the concept of a shared pathophysiology linking the risk factors seems plausible [13,33]. Both insulin resistance and intra-abdominal fat are candidates for the pathophysiologic link that unites these risk factors [12,41], but the central mechanism is still not completely understood. Adipokines, cytokines and inflammatory proteins have been proposed to be an important link between obesity and insulin resistance, and thus could be contributors to the development of the MetS [3,16]. A great deal of evidence supports the theory that adipocytokines play an important role in the pathophysiological pathways between these cardiometabolic risk factors [3,16,42,43]. However, which of these adipocytokines shows strong associations with the MetS is still unclear.
We therefore sought to assess the associations of the adipocytokines with the MetS using confirmatory factor analysis (CFA). This statistical technique permits the assessment of whether associations between observed variables can be explained by the existence of an unobserved underlying variable by goodness of fit testing of comparative models [4]. Its applications in medical research have been mainly in mental health, where complex psychiatric syndromes exists but there is no as of yet methodology to identify the underlying causal pathways, as compared to other medical disciplines (e.g., infectious diseases). With the complex and poorly understood pathophysiology of the MetS, CFA is an excellent technique to address whether adipocytokines and inflammatory proteins are part of the metabolic syndrome. We applied this technique to data from a cohort of apparently healthy, nondiabetic subjects who had measurements that included the parameters comprising the MetS as well as nine different adipokines, cytokines and inflammatory proteins: monocyte chemo-attractant protein-1 (MCP-1), serum amyloid A (SAA), tumor necrosis factor-α (TNF-α), adiponectin, resistin, leptin, interleukin-6 (IL-6), C-reactive protein (CRP) and plasminogen activator inhibitor-1 (PAI-1). Furthermore, in contrast to almost all previously available research on this topic, direct measurements of insulin sensitivity and visceral fat were available.
MATERIALS AND METHODS
Subjects
The current (secondary) data analysis was performed using data from 134 subjects who participated in a study examining the effect of insulin sensitivity on the lipoprotein responses to egg feeding [22,38]. Subjects were recruited by public advertisement from the Greater Seattle community and were eligible if they had a fasting plasma glucose <6.3 mmol/L. Subjects were excluded if they had a history of diabetes, renal or liver disease, uncontrolled thyroid disease or hypertension, coronary artery disease, anemia, LDL cholesterol >4.91 mmol/L or triglycerides >5.65 mmol/L. The Human Subjects Review Committee at the University of Washington reviewed and approved the study. All subjects provided written informed consent.
Measurements
Height, weight, blood pressure, fasting plasma levels of glucose, insulin, high density lipoprotein (HDL) cholesterol and triglycerides were measured using standard techniques as previously described [22]. Waist circumference was measured at the smallest circumference of the waist, the “natural” waistline, using a modified protocol from the NHANES III Anthropometric Measurements videotape (National Center for Health Statistics). Intra-abdominal fat (IAF) and subcutaneous fat (SQF) areas were quantified by a single slice CT scan obtained at the level of the umbilicus [22]. The insulin sensitivity index (SI) was determined using Bergman s minimal model of glucose kinetics applied to the glucose and insulin data obtained from a tolbutamide-modified frequently sampled intravenous glucose tolerance test [6,22].
The fasting plasma levels of those adipokines, cytokines and inflammatory proteins which are thought to play a role in the MetS were measured. Monocyte chemo-attractant protein 1 (MCP-1), plasminogen activator inhibitor 1 (PAI-1), resistin and tumor necrosis factor-α (TNF-α) were measured using the Human Adipokine LINCOplex Assay (Millipore Research, St Charles, MO) (intra assay variation 7.9%, 2–5%, 2–8% and 7.8% respectively, inter assay variation 18%, 5–10%, 6–13% and 16% respectively.). Adiponectin and leptin concentrations were measured by radioimmunoassay (Millipore Research) (intra assay variation 1.8–6.2% and 3.6–6.2% respectively, inter assay variation 6.9–9.3% and 3.4–8.3% respectively). Interleukin 6 (IL-6) was measured with a multiplex bead system on a Luminex analyzer (inter assay variation 7%). C-reactive protein (CRP; high-sensitivity assay) and serum amyloid A (SAA) levels were determined by nephelometric methods using Siemens reagents (Siemens Healthcare Diagnostics. Inc, Newark, DE) (intra assay variation 2.7% and 2.8–4.7% respectively, inter assay variation 3% and 4.3–6.2% respectively). Subjects with values too low to be detected by the CRP (n=7), TNF-α (n=6) and SAA (n=6) assays were excluded from the analyses. In order to reduce variability between assays of the different adipokines, cytokines and inflammatory proteins, plasma was stored at −80°C prior to being assayed and all samples for a particular measure were performed at the same time.
Metabolic Syndrome criteria
The MetS was defined according to the updated NCEP ATP-III criteria as the presence of 3 or more of the following criteria: greater waist circumference (>102 cm for men and >88 cm for women), hypertriglyceridemia (>1.7 mmol/L), low HDL (<1.03 mmol/L for men and <1.29 mmol/L for women), hypertension (systolic pressure of ≥130mmHg and/or diastolic ≥85mmHg and/or use of antihypertensive medication) or increased fasting plasma glucose (≥5.6 mmol/L or use of glucose lowering medication) [17].
Statistical Methods and Factor Analysis
Data are presented as mean±SD. Non-normally distributed parameters were logarithmically transformed to approximate a normal distribution. Using multiple linear regression, we tested which adipocytokines or inflammatory proteins (independent variables) were independently associated with each component of the MetS (dependent variable), using a two-sided p <0.05 as the criterion for an independent association. Multiple logistic regression was used to determine which adipocytokines were independently associated with the presence of the MetS defined by the NCEP-III criteria [17]. We did this to be sure we only used those adipocytokines which were associated with the dependent variables independent of other adipocytokines. All these analyses were performed using STATA SE11 (STATA Corp., College Station, TX).
We used Structural Equation Modeling (SEM) that utilized maximum likelihood estimation in Amos 18.0 (SPSS Inc, Chicago, IL) to develop our CFA models. SEM is a statistical technique integrating CFA and path analysis [4,28]. In this approach, a hypothesized model is visually constructed by linking different observable variables (indicators) with hypothesized latent (unobservable, underlying) factors [4]. The analysis provides estimates of the strength of the relationships between the different components and goodness of fit indices that indicate the adequacy of the model [40].
We first designed the basic model. Based on previous reports we hypothesized that obesity, insulin resistance, hypertension and the lipid profile were constituents of risk factor clustering [34]. We tested multiple one- and four-factor models, based on the current literature (Figure 1) [13,27,33,36]. The best fitting model was a one factor model with six indicators: CT-measured IAF for obesity, SI for insulin sensitivity, SBP and DBP with covarying residual errors for blood pressure and TG and HDL with covarying residual errors for these lipids. Such one-factor models have been described previously [27,33,36].
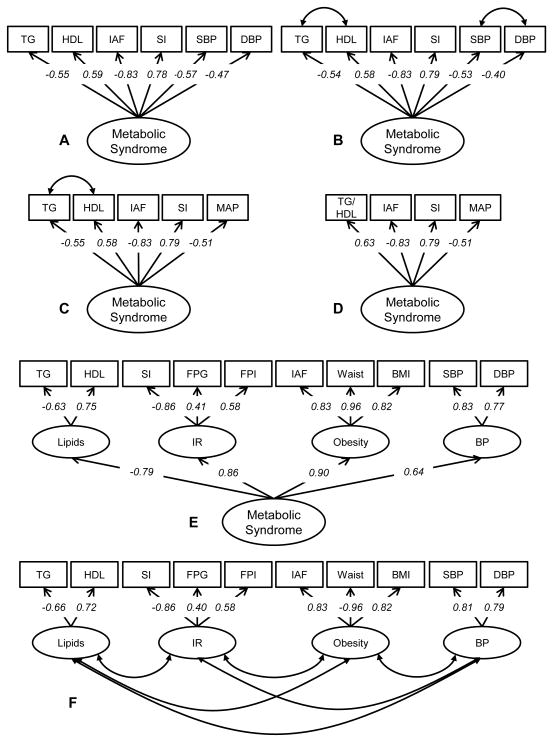
Figure 1. Basic model representing MetS.
Six different models reflecting the metabolic syndrome: (A) one-factor model with six indicators (fit statistics χ2= 52.383 (p< 0.001); SRMR= 0.1068; CFI= 0.805; and RMSEA= 0.207); (B) one-factor model with six indicators. Because of the high correlations between TG and HDL and between SBP and DBP we allowed the residual errors to covary (fit statistics χ2= 9.160 (p 0.241); SRMR= 0.0491; CFI= 0.990; and RMSEA= 0.052); (C) one-factor model with five indicators, in which SBP and DBP are replaced by MAP (fit statistics χ2= 7.958 (p 0.093); SRMR= 0.0457; CFI= 0.976; and RMSEA= 0.094); (D) one-factor model with four indicators, in which TG and HDL are replaced by a ratio of TG/HDL (fit statistics χ2= 7.869 (p 0.020); SRMR= 0.049; CFI= 0.957; and RMSEA= 0.161); (E) four-factor model with the four conditions of the MetS as separate factors, all loading on a second-order latent factor (fit statistics χ2= 62.882 (p 0.001); SRMR= 0.0581; CFI= 0.940; and RMSEA= 0.095); and (F) four-factor model with the four conditions of the MetS as separate factors, with the factors correlating to each other (fit statistics χ2= 51.246 (p 0.007); SRMR= 0.0440; CFI= 0.958; and RMSEA= 0.082). The factor loadings shown for all models are all statistically significant with p<0.001. Model B shows the best model fit, as indicated by the lowest χ2 associated p-value and SRMR and the highest CFI and RMSEA.
Abbreviations = BP: blood pressure; DBP: diastolic blood pressure; FPG: fasting plasma glucose; FPI: fasting plasma insulin; HDL: high-density lipoprotein cholesterol; IAF: CT-measured intra-abdominal fat area; IR: insulin resistance; MAP: mean arterial pressure; SBP: systolic blood pressure; SI: insulin sensitivity index; TG: triglycerides.
After defining the basic model, we separately added those adipocytokines that were independently associated with at least one of the cardiometabolic risk factors from the multiple regression analysis. We then compared the goodness-of-fit of these models to the basic model. Goodness of fit indices included: chi-square (χ2), standardized root mean squared residual (RSMR), root mean square error of approximation (RMSEA) and comparative fit index (CFI) [8,18,30]. The best goodness of fit is demonstrated if the χ2 is as low as possible, the SRMR and RMSEA are small, both preferably <0.08, and the CFI is close to one. A p-value <0.05 in the χ2-test indicates that the hypothesized model does not fit the data well [18]. Different tests exist to statistically examine whether the changes in model fit parameters are significant. For nested models the chi-square can be used, for non-nested models the AIC (Akaike Information Criterion) statistics are usually compared [8]. In the current analyses the models are not nested (since the models cannot be derived from one another by restraining variances) [8], and therefore the AIC should be used. However, the AIC penalizes a model for being more complex (i.e. having more indicators) [30,37]. As such, the AIC is not optimal for the current analyses that evaluate an additional indicator. We therefore defined improvement of fit as an improvement in all reported goodness-of-fit parameters, while a worse fit was defined as a poorer fit in all parameters. An indeterminate change in parameters alludes to no change in goodness-of-fit.
RESULTS
Study Population
Characteristics of the study population are shown in Table 1. Subjects were on average middle-age and overweight, with more being female. There is wide variability of age, waist circumference and IAF area, which can partly be explained by sex differences. Differences by sex were present for a number of MetS variables (waist circumference, HDL cholesterol, blood pressure) and adipokines, cytokines and inflammatory proteins (adiponectin, leptin, PAI-1, CRP and SAA).
Table 1.
Anthropometric and metabolic characteristics.
| All Subjects (n=134) | Male (n=52) | Female (n=82) | p-value for test by sex | |
|---|---|---|---|---|
| Age (years) | 53.5 ± 10.4 | 54.0 ± 11.0 | 53.2 ± 10.0 | 0.67 |
| BMI (kg/m2)* | 25.3 [23.6 – 28.1] | 25.7 [24.8 – 28.4] | 24.9 [22.6 – 27.8] | 0.07 |
| Waist circumference (cm)* | 86.5 [77 – 95.2] | 93.8 [88.6 – 101.2] | 78.9 [73.4 – 87.2] | <0.01 |
| IAF area (cm2)* | 96.9 [45.2 – 130.9] | 121.9 [97.8 – 159.5] | 66.4 [39.9 – 112.5] | <0.01 |
| HDL cholesterol (mmol/L) | 1.4 ± 0.4 | 1.2 ± 0.3 | 1.5 ± 0.4 | <0.01 |
| Triglycerides (mmol/L)* | 1.2 [0.8 – 1.7] | 1.3 [0.8 – 1.8] | 1.1 [0.8 – 1.6] | 0.47 |
| Fasting plasma glucose (mmol/L) | 5.2 ± 0.5 | 5.3 ± 0.5 | 5.1±0.5 | 0.01 |
| Fasting plasma insulin (pmol/L)* | 63.5 [52.1 – 90.3] | 60.7 [51.0 – 86.8] | 64.9 [52.1 – 90.3] | 0.77 |
| SI (× 10−5 min− 1/[pmol/L])* | 3.27 [2.2 – 4.7] | 2.7 [1.9 – 4.1] | 3.6 [2.5 – 4.9] | 0.05 |
| Systolic blood pressure (mm Hg) | 116.2 ± 12.5 | 120 ± 11.4 | 113.7 ± 12.6 | <0.01 |
| Diastolic blood pressure (mm Hg) | 71.9 ± 7.9 | 74.5 ± 6.9 | 70.2 ± 8.1 | <0.01 |
| Mean arterial pressure (mm Hg) | 86.6 ± 8.6 | 89.7 ± 7.1 | 84.7 ± 8.9 | <0.01 |
| Adiponectin (ng/mL)* | 6.5 [4.7 – 8.1] | 5.5 [3.6 – 7.5] | 7.1 [5.5 – 8.4] | <0.01 |
| Leptin (ng/mL)* | 10 [4.9 – 18.5] | 5 [3.8 – 8] | 13.5 [9.1 – 23.4] | <0.01 |
| Resistin (ng/mL)* | 5.2 [3.8 – 6.8] | 4.9 [4.1 – 6.4] | 5.4 [3.8 – 7.2] | 0.11 |
| PAI-1 (ng/mL)* | 6.6 [4.2 – 9.3] | 7.7 [5.2 – 9.8] | 5.4 [3.8 – 9] | 0.04 |
| TNFα (ng/mL)*# | 2.2 [1.7 – 2.6] | 2.3 [1.9 – 2.7] (n=52) | 2 [1.6 – 2.6] (n=76) | 0.10 |
| MCP-1 (ng/mL)* | 76.4 [57.4 – 101.7] | 79.4 [57.9 – 108.4] | 76.1 [56.5 – 98.7] | 0.39 |
| CRP (mg/L)*# | 1.2 [0.6 – 3] | 0.8 [0.5 – 1.9] (n=49) | 1.5 [0.8 – 5.2] (n=78) | <0.01 |
| SAA (mg/L)*# | 3.2 [1.8 – 5.9] | 2.1 [1.4 – 4.3] (n=49) | 3.9 [2.4 – 7.3] (n=79) | <0.01 |
| IL-6 (pg/L)*# | 1.9 [1.4 – 3.1] | 2 [1.4 – 3] (n=50) | 1.9 [1.3 – 3.1] (n=78) | 0.74 |
Data are presented as mean ± sd for data with a normal distribution or as median [interquartile range] for data with a skewed distribution (indicated with asterisk*)
Subjects with values too low to be detected by the CRP (n=7), TNF-α (n=6), SAA (n=6) and IL-6 (n=6) assays were excluded from the analyses.
Multivariate analysis
Using multiple linear regression, we examined which adipokines, cytokines and inflammatory proteins were independently associated with the different dependent variables (Table 2). PAI-1 and adiponectin showed associations with four of the cardiometabolic risk factors. IL-6, resistin, leptin, CRP, and adiponectin showed at least one independent association, while MCP-1, SAA and TNF-α were not independently associated with any of the risk factors. Interestingly, none of the adipokines, cytokines and inflammatory proteins showed an association with subjects who met the NCEP ATP-III based definition of the MetS (data not shown).
Table 2.
Multiple linear regression of adipokines, cytokines and inflammatory proteins with each of the cardiometabolic risk factors.
| Dependent Variables | Independent Variables | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| IL-6 | MCP-1 | CRP | SAA | TNF-α | PAI-1 | Resistin | Leptin | Adiponectin | |
| TG | −0.08 | 0.12 | 0.34* | −0.22 | −0.08 | 0.24* | −0.25* | 0.24* | −0.21* |
| HDL | −0.06 | 0.11 | −0.11 | 0.21 | −0.15 | −0.15 | 0.11 | 0.06 | 0.52‡ |
| IAF | 0.14 | −0.03 | 0.27* | −0.14 | 0.13 | 0.41‡ | −0.18 | 0.07 | −0.20* |
| SI | −0.17* | 0.09 | −0.13 | 0.16 | −0.09 | −0.34‡ | 0.19* | −0.33‡ | 0.24† |
| SBP | 0.15 | 0.04 | −0.08 | 0.23 | −0.09 | 0.19 | 0.02 | −0.06 | −0.09 |
| DBP | 0.15 | 0.02 | −0.08 | 0.15 | −0.13 | 0.23* | 0.08 | −0.04 | −0.04 |
Regression coefficients are per 1-sd increase.
p<0.05;
p<0.01;
p<0.001.
Confirmatory Factor Analysis
Figure 1 illustrates different basic models representing the MetS, using sophisticated measurements to represent insulin sensitivity and anthropometric measures of central obesity. The first model includes six indicators (TG, HDL, SI, IAF, SBP and DBP) loading on a single Metabolic Syndrome factor (Figure 1A). This model shows a poor fit, as indicated by the low p-value (<0.001), low CFI (0.805), high RMSEA (0.207) and high SRMR (0.1068). Since TG and HDL, and SBP and DBP tend to cluster together, we allowed the residual errors to covary (Figure 1B). This increased model fit notably, with a low χ2 (9.160; p=0.241) and SRMR (0.0491), a high CFI (0.990) and a low RMSEA (0.052). We then tested a model in which SBP and DBP were replaced by mean arterial pressure (MAP), but this decreased model fit, albeit slightly (Figure 1C). Replacing TG and HDL with the ratio of TG/HDL decreased model fit even further (Figure 1D). In two four-factor models, different factors represent the conditions of the MetS: SI, fasting insulin and glucose load on a factor to represent the insulin resistance-factor, IAF, BMI and Waist load on the obesity-factor, HDL and TG represent the lipids-factor and SBP and DBP load on a factor representing blood pressure (Figures 1E and 1F). In the first model the four factors load on a second-order latent factor representing the MetS (Figure 1E). In the second model, the four factors are correlated (Figure 1F). Both four-factor models have a poor model fit. As indicated before, the one-factor, six indicator model with covarying residual errors showed best goodness-of fit indices (Figure 1B). Interestingly, both SI and IAF showed strong factor loadings (>0.75) with the latent factor underlying the MetS.
We then individually and separately added the different adipocytokines found to have independent associations with the cardiometabolic risk factors in the multivariate models (Table 3). All but resistin showed significant factor loadings (estimated by R-score) with the metabolic syndrome -factor. Adiponectin, leptin, CRP, IL-6 and resistin led to worse goodness-of-fit parameters, indicating a poorer model. When PAI-1 was added to the model, the χ2-associated p-value and CFI increased, while SRMR and RMSEA decreased, all implying an improvement of the model.
Table 3.
Confirmatory factor analysis adding the different adipokines, cytokines and inflammatory proteins found to have independent associations with the cardiometabolic risk factors in the multivariate models
| Extra Variable | Estimates | Model Statistics | ||||||
|---|---|---|---|---|---|---|---|---|
| R-score | p-value | χ2 | dF | p-value | SRMR | CFI | RMSEA | |
| None | - | - | 9.160 | 7 | 0.241 | 0.0491 | 0.990 | 0.052 |
| Adiponectin | 0.823 | <0.001 | 52.209 | 12 | 0.000 | 0.0899 | 0.859 | 0.172 |
| Leptin | −0.389 | 0.034 | 38.951 | 12 | 0.000 | 0.0775 | 0.893 | 0.141 |
| CRP | −0.565 | 0.003 | 23.636 | 12 | 0.023 | 0.0627 | 0.952 | 0.093 |
| IL-6 | −0.356 | 0.048 | 15.972 | 12 | 0.193 | 0.0582 | 0.983 | 0.054 |
| Resistin | −0.060 | 0.722 | 15.560 | 12 | 0.212 | 0.0621 | 0.984 | 0.051 |
| PAI-1 | −0.899 | <0.001 | 12.926 | 12 | 0.374 | 0.0453 | 0.996 | 0.026 |
Legend: Addition of adipocytokines to the basic model (extra variable: none) leads to a change in model fit. An improved model fit is represented by an increased χ2-associated p-value, a lower SRMS, higher CFI and lower RMSEA. Only PAI-1 increases all model-fit parameters. The adipocytokines are ordered from worst fit to best fit.
DISCUSSION
We used CFA to examine whether adipokines, cytokines and inflammatory proteins share the same underlying factor as the MetS. PAI-1 improved the model fit and thus appears to be more correlated with the latent factor that underlies the MetS than the other markers assessed. These findings confirm that inflammation is an important feature of the MetS. Also observed were very high factor loadings between the latent MetS factor and both SI and IAF, suggesting that these both play a key role in the syndrome. However, other adipocytokines, such as adiponectin and CRP, that have previously been suggested as important in the MetS [11,16,44], did not improve the model fit.
As with any latent variable model, it is not possible to characterize the identified underlying MetS feature from this analysis, but our results do argue for a single process that links the observed features. This underlying factor represents the variance which all MetS components have in common. This can be either something clinical (e.g. all components of the MetS are associated with developing cardiovascular disease [15,24]) or pathophysiological (the reason why the components of the MetS tend to cluster together). Our finding that PAI-1 shares the underlying factor with the MetS is therefore not surprising, considering what is currently known regarding this marker and the syndrome [1]. We also show that PAI-1 is strongly associated with visceral obesity and insulin resistance [1] but the novel finding of this analysis is that PAI-1 is associated with the common factor underlying the observed features of the MetS. This implies that this adipocytokine shares variance in common with the well accepted features of the MetS. This commonality might possibly be pathophysiological (sharing the same causes). While many studies have shown the association of inflammation and the observed features of the MetS syndrome, here we have shown that PAI-1 can be the measurable marker for inflammation associated with the underlying pathophysiology of the metabolic syndrome.
PAI-1 is an important mediator in the hemostatic pathway [19] and has been shown to be an acute-phase protein [35]. It is produced by adipocytes, macrophages in fat, vascular endothelium and/or hepatocytes [5]. The secretion of PAI-1 is increased by insulin, angiotensin II, IL-6, free fatty acids and very-low-density-lipoproteins [2,31,32,35]. Therefore, increased levels of PAI-1 may be a consequence of the metabolic derangement(s) in the MetS. However, decreasing PAI-1 levels or antagonizing the effects of this cytokine prevents the development of obesity and atherosclerosis in animal models [10,25]. In humans, PAI-1 is associated with development of atherosclerosis and myocardial infarction [23,39]. Moreover, PAI-1 interferes with insulin signaling and thus may contribute to insulin resistance and glucose intolerance [1]. It has been shown that PAI-1 predicts the development of type 2 diabetes independent of the observed features of the MetS [14]. Therefore, PAI-1 may be playing a role in the MetS both as a cause and consequence.
Interestingly, we showed that adiponectin, CRP, leptin, IL-6, and resistin decreased the goodness-of-fit compared to the basic model. Therefore, we believe that adiponectin, CRP, leptin, resistin and IL-6 are associated with the observed features of the MetS, but not its underlying factor (which could represent the pathophysiology). Especially for adiponectin and CRP, this is an interesting finding, since these adipocytokines have been considered main candidates for linking together the cardiometabolic risk factors that characterize the MetS [3,9,11,16,44].
In the current analyses we started with developing a basic model representing the MetS, thereby comparing different one- and four-factor models. Controversy exists which model is best. Some previous studies show that four-factor models are superior [13], while others show a better fit for one-factor models [27,33,36]. Interestingly, in many four-factor models, a second-order latent factor has been added, thereby converging the four factors to one underlying factor. This suggests that only one factor underlies the MetS [36]. In our healthy sample, the one-factor model was superior to the four-factor model. It must be noted that we included CT-derived IAF area and intravenous glucose tolerance test derived S, which distinguishes our models from others in the literature that employ indirect measurements of visceral adiposity and insulin sensitivity.
Although we recognize that our data may be mistakenly viewed as largely confirmatory, we must emphasize that our employed measurement techniques are novel compared to the existing literature. Our approach provides greater validity to the conclusions that can be made about the likelihood of observed metabolic syndrome features being related to the underlying syndrome as opposed to a correlate of another observed feature of the syndrome. Unique to our study is that we did not use the standard diagnostic criteria for the MetS (e.g. the NCEP ATP-III or WHO-criteria). Kahn et al have suggested that these criteria are arbitrary, with components and cut-off values not based on experimental data [20]. CFA allowed us to use continuous variables, bypassing the stated limitations of MetS definitions. Thus, it provides the additional advantage over exploratory factor analysis of permitting testing of specific model structures for the metabolic syndrome, and an assessment of whether observed features are an intrinsic characteristic of a defined structure. Moreover, we used direct measurements of two underlying conditions thought to be causally linked to the MetS: intra-abdominal fat and insulin sensitivity. Therefore our results are not likely to be biased by the limitations of cut-off values or misclassification due to use of surrogate measures of visceral fat or insulin resistance such as waist circumference or HOMA-IR. Interestingly, both IAF and SI showed strong, independent associations with the common factor, indicating that both central obesity and insulin resistance define MetS, rather than only one of them [12,41].
Two other studies using CFA to link new variables to the MetS should be mentioned. Boronat et al. showed that PAI-1 and HbA1c are linked to the “metabolic syndrome”-factor, using an approach similar to that we employed. They focused on cardiovascular risk markers (CRP, fibrinogen, lipoprotein(a), HbA1c, PAI-1, von Willebrand factor and homocysteine), and only entered those variables independently associated with the MetS defined by the International Diabetes Federation and not with its individual features as we did [7]. They found PAI-1 showed a strong and significant association with the common “metabolic syndrome”-factor. In another study by Marsland et al., it was shown that an extra “inflammatory factor” comprising IL-6 and CRP increased the fit of the basic model [26]. Unfortunately, PAI-1 was not measured in this study. These studies support our finding that inflammation is an important condition in the MetS, and that PAI-1 might be the best marker to measure. Our study is novel however in the sophisticated techniques we have used to measure visceral fat and insulin sensitivity, the different biological markers employed, and because we avoided using arbitrary cut-off point definitions. Moreover, we added the independent adipocytokines separately to the basic model to test the goodness-of-fit, thereby increasing the sensitivity of finding associations with individual parameters as opposed to having considered these measurements as a group.
The major limitation of our study is that the sample size was relatively small and may have limited our ability to detect important associations. However, with a case-to-parameter ratio of 8:1 (recommended >5:1) and case-to-variable ratio of 14:1 (recommended >10:1) our study sample should be sufficient for conducting a valid factor analysis according to published criteria [29]. The relatively small sample size unfortunately did not allow us to investigate the differences between gender, as is suggested by table 1. As with any observational research conducted on a selected sample of subjects, it is not clear whether these results will generalize to persons with dissimilar characteristics than seen in our population.
In conclusion, the utility of CFA has enabled us to build a model comprising central obesity, insulin sensitivity, hypertension and lipid disturbances, united by a latent metabolic syndrome -factor as a structure explaining the observed features. Addition of adipokines, cytokines and inflammatory proteins led to the conclusion that levels of PAI-1 share the common pathway leading to the MetS. These results directly implicate markers of inflammation in the MetS. Based on our observations, the case could be made for including PAI-1 in the criteria of the MetS if confirmed in further research.
Acknowledgments
This work was supported by the Medical Research Service of the Department of Veterans Affairs, a grant in aid from the Egg Nutrition Center administered through the US Department of Agriculture, the Nutrition Obesity Research Center at the University of Washington (DK-035816), the Diabetes Research Center at the University of Washington (DK-017047), the General Clinical Research Center (RR-000037), and a generous gift from the Robert B. McMillen Family Trust. J.B.M. was supported by a Career Development Award from the American Diabetes Association and by NIDDK DK-080140. M.M.S and P.W. were medical students participating in an exchange program between Vrije Universiteit University Medical Centre, Amsterdam, The Netherlands, and the University of Washington, Seattle, Washington. P.W. received funding from the Dutch Diabetes Foundation. VA Puget Sound Health Care System provided support for the involvement of Drs. Boyko, Kahn and Utzschneider in this study.
Abbreviations
- AIC
Akaike information criterion
- CFA
confirmatory factor analysis
- CFI
comparative fit index
- CRP
C-reactive protein
- CT
computerized tomography
- IAF
intra-abdominal fat
- IL-6
interleukin 6
- MCP-1
monocyte chemo-attractant protein 1
- MetS
metabolic syndrome
- PAI-1
plasminogen activator inhibitor 1
- RMSEA
root mean square error of approximation
- SAA
serum amyloid A
- SEM
Structural Equation Modeling
- SI
insulin sensitivity index
- SQF
subcutaneous fat
- SRMR
standardized root mean squared residual
- TG
triglycerides
Footnotes
AUTHOR CONTRIBUTIONS
MMS, PW, KMU, JBM, EJB researched the data. MMS, KMU, EJB and SEK wrote the manuscript. PW, KMU, JT, FG, MF, DBC, KAM, AC, RHK, JBM, EJB and SEK contributed to the discussion and reviewed/edited the manuscript.
References
- 1.Alessi MC, Juhan-Vague I. PAI-1 and the metabolic syndrome: links, causes, and consequences. Arterioscler Thromb Vasc Biol. 2006;26(10):2200–2207. doi: 10.1161/01.ATV.0000242905.41404.68. [DOI] [PubMed] [Google Scholar]
- 2.Alessi MC, Juhan-Vague I, Kooistra T, Declerck PJ, Collen D. Insulin stimulates the synthesis of plasminogen activator inhibitor 1 by the human hepatocellular cell line Hep G2. Thromb Haemost. 1988;60(3):491–494. [PubMed] [Google Scholar]
- 3.Arner P. Insulin resistance in type 2 diabetes -- role of the adipokines. Curr Mol Med. 2005;5(3):333–339. doi: 10.2174/1566524053766022. [DOI] [PubMed] [Google Scholar]
- 4.Babyak MA, Green SB. Confirmatory factor analysis: an introduction for psychosomatic medicine researchers. Psychosom Med. 2010;72(6):587–597. doi: 10.1097/PSY.0b013e3181de3f8a. [DOI] [PubMed] [Google Scholar]
- 5.Bastelica D, Morange P, Berthet B, Borghi H, Lacroix O, Grino M, et al. Stromal cells are the main plasminogen activator inhibitor-1-producing cells in human fat: evidence of differences between visceral and subcutaneous deposits. Arterioscler Thromb Vasc Biol. 2002;22(1):173–178. doi: 10.1161/hq0102.101552. [DOI] [PubMed] [Google Scholar]
- 6.Beard JC, Bergman RN, Ward WK, Porte D., Jr The insulin sensitivity index in nondiabetic man. Correlation between clamp-derived and IVGTT-derived values. Diabetes. 1986;35(3):362–369. doi: 10.2337/diab.35.3.362. [DOI] [PubMed] [Google Scholar]
- 7.Boronat M, Saavedra P, Varillas VF, Novoa FJ. Use of confirmatory factor analysis for the identification of new components of the metabolic syndrome: the role of plasminogen activator inhibitor-1 and Haemoglobin A1c. Nutr Metab Cardiovasc Dis. 2009;19(4):271–276. doi: 10.1016/j.numecd.2008.07.007. [DOI] [PubMed] [Google Scholar]
- 8.Brown TA. Confirmatory Factor Analysis for Applied Research. New York, NY: The Guilford Press; 2006. [Google Scholar]
- 9.Cnop M, Havel PJ, Utzschneider KM, Carr DB, Sinha MK, Boyko EJ, et al. Relationship of adiponectin to body fat distribution, insulin sensitivity and plasma lipoproteins: evidence for independent roles of age and sex. Diabetologia. 2003;46(4):459–469. doi: 10.1007/s00125-003-1074-z. [DOI] [PubMed] [Google Scholar]
- 10.Crandall DL, Quinet EM, El AS, Hreha AL, Leik CE, Savio DA, et al. Modulation of adipose tissue development by pharmacological inhibition of PAI-1. Arterioscler Thromb Vasc Biol. 2006;26(10):2209–2215. doi: 10.1161/01.ATV.0000235605.51400.9d. [DOI] [PubMed] [Google Scholar]
- 11.Devaraj S, Singh U, Jialal I. Human C-reactive protein and the metabolic syndrome. Curr Opin Lipidol. 2009;20(3):182–189. doi: 10.1097/MOL.0b013e32832ac03e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Donahue RP, Bean JA, Donahue RD, Goldberg RB, Prineas RJ. Does insulin resistance unite the separate components of the insulin resistance syndrome? Evidence from the Miami Community Health Study. Arterioscler Thromb Vasc Biol. 1997;17(11):2413–2417. doi: 10.1161/01.atv.17.11.2413. [DOI] [PubMed] [Google Scholar]
- 13.Ferguson TF, Funkhouser E, Roseman J. Factor analysis of metabolic syndrome components in the Coronary Artery Risk Development in Young Adults (CARDIA) study: examination of factors by race-sex groups and across time. Ann Epidemiol. 2010;20(3):194–200. doi: 10.1016/j.annepidem.2009.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Festa A, D’Agostino R, Jr, Tracy RP, Haffner SM. Elevated levels of acute-phase proteins and plasminogen activator inhibitor-1 predict the development of type 2 diabetes: the insulin resistance atherosclerosis study. Diabetes. 2002;51(4):1131–1137. doi: 10.2337/diabetes.51.4.1131. [DOI] [PubMed] [Google Scholar]
- 15.Gami AS, Witt BJ, Howard DE, Erwin PJ, Gami LA, Somers VK, et al. Metabolic syndrome and risk of incident cardiovascular events and death: a systematic review and meta-analysis of longitudinal studies. J Am Coll Cardiol. 2007;49(4):403–414. doi: 10.1016/j.jacc.2006.09.032. [DOI] [PubMed] [Google Scholar]
- 16.Gil-Campos M, Canete RR, Gil A. Adiponectin, the missing link in insulin resistance and obesity. Clin Nutr. 2004;23(5):963–974. doi: 10.1016/j.clnu.2004.04.010. [DOI] [PubMed] [Google Scholar]
- 17.Grundy SM, Cleeman JI, Daniels SR, Donato KA, Eckel RH, Franklin BA, et al. Diagnosis and management of the metabolic syndrome: an American Heart Association/National Heart, Lung, and Blood Institute Scientific Statement. Circulation. 2005;112(17):2735–2752. doi: 10.1161/CIRCULATIONAHA.105.169404. [DOI] [PubMed] [Google Scholar]
- 18.Hooper D, Coughlan J, Mullen MR. Structural Equation Modelling: Guidelines for Determining Model Fit. The Electronic Journal of Business Research Methods. 2008;6(1):53–60. [Google Scholar]
- 19.Juhan-Vague I, Alessi MC, Mavri A, Morange PE. Plasminogen activator inhibitor-1, inflammation, obesity, insulin resistance and vascular risk. J Thromb Haemost. 2003;1(7):1575–1579. doi: 10.1046/j.1538-7836.2003.00279.x. [DOI] [PubMed] [Google Scholar]
- 20.Kahn R. Metabolic syndrome: is it a syndrome? Does it matter? Circulation. 2007;115(13):1806–1810. doi: 10.1161/CIRCULATIONAHA.106.658336. [DOI] [PubMed] [Google Scholar]
- 21.Kahn R, Buse J, Ferrannini E, Stern M. The metabolic syndrome: time for a critical appraisal: joint statement from the American Diabetes Association and the European Association for the Study of Diabetes. Diabetes Care. 2005;28(9):2289–2304. doi: 10.2337/diacare.28.9.2289. [DOI] [PubMed] [Google Scholar]
- 22.Knopp RH, Retzlaff B, Fish B, Walden C, Wallick S, Anderson M, et al. Effects of insulin resistance and obesity on lipoproteins and sensitivity to egg feeding. Arterioscler Thromb Vasc Biol. 2003;23(8):1437–1443. doi: 10.1161/01.ATV.0000082461.77557.C7. [DOI] [PubMed] [Google Scholar]
- 23.Kohler HP, Grant PJ. Plasminogen-activator inhibitor type 1 and coronary artery disease. N Engl J Med. 2000;342(24):1792–1801. doi: 10.1056/NEJM200006153422406. [DOI] [PubMed] [Google Scholar]
- 24.Lorenzo C, Okoloise M, Williams K, Stern MP, Haffner SM. The metabolic syndrome as predictor of type 2 diabetes: the San Antonio heart study. Diabetes Care. 2003;26(11):3153–3159. doi: 10.2337/diacare.26.11.3153. [DOI] [PubMed] [Google Scholar]
- 25.Ma LJ, Mao SL, Taylor KL, Kanjanabuch T, Guan Y, Zhang Y, et al. Prevention of obesity and insulin resistance in mice lacking plasminogen activator inhibitor 1. Diabetes. 2004;53(2):336–346. doi: 10.2337/diabetes.53.2.336. [DOI] [PubMed] [Google Scholar]
- 26.Marsland AL, McCaffery JM, Muldoon MF, Manuck SB. Systemic inflammation and the metabolic syndrome among middle-aged community volunteers. Metabolism. 2010;59(12):1801–1808. doi: 10.1016/j.metabol.2010.05.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Martinez-Vizcaino V, Martinez MS, Aguilar FS, Martinez SS, Gutierrez RF, Lopez MS, et al. Validity of a single-factor model underlying the metabolic syndrome in children: a confirmatory factor analysis. Diabetes Care. 2010;33(6):1370–1372. doi: 10.2337/dc09-2049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Mueller RO, Hancock GR. Best Practices in Structural Equation Modeling. In: Osborne JW, editor. Best Practices in Quantitative Methods. Thousand Oaks, CA: SAGE Publications; 2007. pp. 488–508. [Google Scholar]
- 29.Mundfrom DJ, Shaw DG, Ke TL. Minimum Sample Size Recommendations for Conducting Factor Analyses. Int J Test. 2005;5(2):159–168. [Google Scholar]
- 30.Newsom J. Some Clarifications and Recommendations on Fit Indices. 2011 Nov 30; http://www.upa.pdx.edu/IOA/newsom/semclass/
- 31.Nilsson L, Banfi C, Diczfalusy U, Tremoli E, Hamsten A, Eriksson P. Unsaturated fatty acids increase plasminogen activator inhibitor-1 expression in endothelial cells. Arterioscler Thromb Vasc Biol. 1998;18(11):1679–1685. doi: 10.1161/01.atv.18.11.1679. [DOI] [PubMed] [Google Scholar]
- 32.Nilsson L, Gafvels M, Musakka L, Ensler K, Strickland DK, Angelin B, et al. VLDL activation of plasminogen activator inhibitor-1 (PAI-1) expression: involvement of the VLDL receptor. J Lipid Res. 1999;40(5):913–919. [PubMed] [Google Scholar]
- 33.Pladevall M, Singal B, Williams LK, Brotons C, Guyer H, Sadurni J, et al. A single factor underlies the metabolic syndrome: a confirmatory factor analysis. Diabetes Care. 2006;29(1):113–122. doi: 10.2337/diacare.29.1.113. [DOI] [PubMed] [Google Scholar]
- 34.Reaven GM. Banting lecture 1988. Role of insulin resistance in human disease. Diabetes. 1988;37(12):1595–1607. doi: 10.2337/diab.37.12.1595. [DOI] [PubMed] [Google Scholar]
- 35.Rega G, Kaun C, Weiss TW, Demyanets S, Zorn G, Kastl SP, et al. Inflammatory cytokines interleukin-6 and oncostatin m induce plasminogen activator inhibitor-1 in human adipose tissue. Circulation. 2005;111(15):1938–1945. doi: 10.1161/01.CIR.0000161823.55935.BE. [DOI] [PubMed] [Google Scholar]
- 36.Shen BJ, Todaro JF, Niaura R, McCaffery JM, Zhang J, Spiro A, III, et al. Are metabolic risk factors one unified syndrome? Modeling the structure of the metabolic syndrome X. Am J Epidemiol. 2003;157(8):701–711. doi: 10.1093/aje/kwg045. [DOI] [PubMed] [Google Scholar]
- 37.Tanaka JS. Multifaceted conceptions of fit in structural equation models. In: Bollen KA, Long JS, editors. Testing structural equation models. Newbury Park, CA: Sage; 1993. [Google Scholar]
- 38.Tannock LR, O’Brien KD, Knopp RH, Retzlaff B, Fish B, Wener MH, et al. Cholesterol feeding increases C-reactive protein and serum amyloid A levels in lean insulin-sensitive subjects. Circulation. 2005;111(23):3058–3062. doi: 10.1161/CIRCULATIONAHA.104.506188. [DOI] [PubMed] [Google Scholar]
- 39.Thogersen AM, Jansson JH, Boman K, Nilsson TK, Weinehall L, Huhtasaari F, et al. High plasminogen activator inhibitor and tissue plasminogen activator levels in plasma precede a first acute myocardial infarction in both men and women: evidence for the fibrinolytic system as an independent primary risk factor. Circulation. 1998;98(21):2241–2247. doi: 10.1161/01.cir.98.21.2241. [DOI] [PubMed] [Google Scholar]
- 40.Thompson B. Exploratory and Confirmatory Factor Analysis. Washington DC: American Psychological Association; 2004. [Google Scholar]
- 41.Tong J, Boyko EJ, Utzschneider KM, McNeely MJ, Hayashi T, Carr DB, et al. Intra-abdominal fat accumulation predicts the development of the metabolic syndrome in non-diabetic Japanese-Americans. Diabetologia. 2007;50(6):1156–1160. doi: 10.1007/s00125-007-0651-y. [DOI] [PubMed] [Google Scholar]
- 42.Vettor R, Milan G, Rossato M, Federspil G. Review article: adipocytokines and insulin resistance. Aliment Pharmacol Ther. 2005;(Suppl 2):3–10. doi: 10.1111/j.1365-2036.2005.02587.x. [DOI] [PubMed] [Google Scholar]
- 43.Yudkin JS, Juhan-Vague I, Hawe E, Humphries SE, Di MG, Margaglione M, et al. Low-grade inflammation may play a role in the etiology of the metabolic syndrome in patients with coronary heart disease: the HIFMECH study. Metabolism. 2004;53(7):852–857. doi: 10.1016/j.metabol.2004.02.004. [DOI] [PubMed] [Google Scholar]
- 44.Yudkin JS, Stehouwer CD, Emeis JJ, Coppack SW. C-reactive protein in healthy subjects: associations with obesity, insulin resistance, and endothelial dysfunction: a potential role for cytokines originating from adipose tissue? Arterioscler Thromb Vasc Biol. 1999;19(4):972–978. doi: 10.1161/01.atv.19.4.972. [DOI] [PubMed] [Google Scholar]