Abstract
Neurodegenerative patients show often severe everyday decision making problems. Currently it is however not clear which brain atrophy regions are implicated in such decision making problems. We investigated the atrophy correlates of gambling decision making in a sample of 63 participants, including two neurodegenerative conditions (behavioural variant frontotemporal dementia — bvFTD; Alzheimer's disease — AD) as well as healthy age-matched controls. All participants were tested on the Iowa Gambling Task (IGT) and the behavioural IGT results were covaried against the T1 MRI scans of all participants to identify brain atrophy regions implicated in gambling decision making deficits. Our results showed a large variability in IGT performance for all groups with both patient groups performing especially poor on the task. Importantly, bvFTD and AD groups did not differ significantly on the behavioural performance of the IGT. However, by contrast, the atrophy gambling decision making correlates differed between bvFTD and AD, with bvFTD showing more frontal atrophy and AD showing more parietal and temporal atrophy being implicated in decision making deficits, indicating that both patient groups fail the task on different levels. Frontal (frontopolar, anterior cingulate) and parietal (retrosplenial) cortex atrophy covaried with poor performance on the IGT. Taken together, the atrophy correlates of gambling decision making show that such deficits can occur due to a failure of different neural structures, which will inform future diagnostics and treatment options to alleviate these severe everyday problems in neurodegenerative patients.
Keywords: Behavioural variant frontotemporal dementia, Alzheimer's disease, Voxel-based morphometry, Gambling decision making, Iowa Gambling Task
Highlights
► bvFTD and AD patients are both impaired in gambling decision making. ► However, atrophy correlates for gambling decision making differ between groups. ► Poor performance in decision making covaried with frontal atrophy in bvFTD. ► Poor performance in decision making covaried with parietal/temporal atrophy in AD. ► Gambling decision deficits can occur due to atrophy in different brain regions.
1. Introduction
Behavioural variant frontotemporal dementia (bvFTD) is one of the most common early onset dementias (Ratnavalli et al., 2002) with characteristic changes in behaviour and personality, such as ritualized activity, loss of empathy and social inappropriateness (Kipps et al., 2009; Rascovsky et al., 2011). These changes have been mostly attributed to atrophy in the prefrontal cortex and in particular the ventromedial prefrontal cortex (VMPFC), although other cortical and subcortical regions are also affected in bvFTD, including insula, temporal lobes, basal ganglia and thalamus (Kril and Halliday, 2004; Chow et al., 2008). The VMPFC has received in particular attention in bvFTD, as it is one of the first regions to be affected in the disease (Seeley, 2008) and has been attributed to a range of symptoms, including disinhibition (Peters et al., 2006; Hornberger et al., 2011a) and apathy (Zamboni et al., 2008). Indeed, symptoms of VMPFC dysfunction can lead to severe impairment in everyday decisions, including financial mismanagement and pathological gambling (Manes et al., 2011). Surprisingly, however, the relationship of the VMPFC dysfunction with decision making processes in bvFTD has been rarely explored so far. One reason is that to date very few established and validated decision making tests exist, with the Iowa Gambling Task (IGT) being the most commonly employed ambiguous decision making test (Gleichgerrcht et al., 2010).
The Iowa Gambling Task (IGT), a computer-administered test, allows assessment of financial decision making skills in a fairly realistic setting. The task involves choosing cards from four decks of cards labelled A, B, C and D. Every card is associated with a net reward or loss of play money. Participants complete 100 trials and are asked to try and maximise the net amount of money earned. Choosing a card from deck A or B results in a large immediate reward every time, but rewards are followed by additional large losses at infrequent intervals. Cards from decks C and D, on the other hand, are less risky. These cards lead to smaller immediate rewards, but the infrequent losses incurred are much less severe than the ones associated with cards from decks A and B. In the long term, selecting cards from decks A and B results in a net loss of money, whereas consistently choosing cards from decks C and D results in a net gain. Importantly, decisions in such a setting are made under ambiguity, i.e. the risk of the decision made is unknown to the participants as they do not know the risks associated with each deck at the beginning of the task.
Performance on the IGT has been shown to be very sensitive towards dysfunction in VMPFC regions. For example, focal lesion VMPFC patients show severely impaired performance on the IGT (Bechara et al., 1999, 2000). The somatic marker hypothesis (SMH) proposes that rewards gained or punishments incurred during the IGT result in different conscious or nonconscious somatic states. The VMPFC integrates the conflicting somatic state information associated with a specific deck of cards. If the final somatic state associated with a specific deck is positive, a person is more likely to choose a card from that deck than when the final somatic state is negative. According to the SMH, damage to the VMPFC leads to defective emotional processing, resulting in impaired decision making behaviour due to insensitivity to the future consequences of choices (Bechara et al., 2000). Thus, patients suffering from VMPFC damage would be exclusively guided by immediate prospects. Nevertheless, the VMPFC has also been implicated in response inhibition (Hornberger et al., 2011b) and set-shifting (Fellows and Farah, 2005) which are strongly associated with decision making processes (Marschner et al., 2005; Sinz et al., 2008). Poor IGT performance might thus reflect an inability to inhibit or shift previously rewarded behaviour, since in the ten first trials of this test, disadvantageous decks of cards are associated with larger rewards than advantageous ones. This suggests that failure on the IGT can be explained by several processes and not exclusively the SMH (Fellows and Farah, 2005). This notion is further supported by functional neuroimaging studies showing involvement of diverse cortical and subcortical brain regions in IGT decision making processes, including the orbitofrontal cortex (OFC), dorsolateral prefrontal cortex, parietal cortex, cingulate, striatum, amygdala and hippocampus (Ernst et al., 2002). In addition, lesion studies including patients with damage restricted to the dorsolateral prefrontal cortex also show impaired IGT performance comparable to VMPFC patients (Manes et al., 2002; Fellows and Farah, 2005).
The sensitivity of the IGT to VMPFC dysfunction has large implications for its use in bvFTD, as the IGT is so far the most commonly used decision making test of VMPFC dysfunction in these patients (Gleichgerrcht et al., 2010). Overall, very few studies have looked at IGT performance in groups of bvFTD patients. These studies found that bvFTD patients prefer cards from disadvantageous decks (Nakaaki et al., 2007; Torralva et al., 2007) and risky decision making behaviour on the IGT has even been shown in mild bvFTD patients with otherwise unaffected executive functioning (Manes et al., 2010, 2011). The sensitivity of the IGT in the detection of cognitive dysfunction in bvFTD patients has prompted suggestions to use the test as a diagnostic tool (Gleichgerrcht et al., 2010). Still, the neural correlates of IGT performance in bvFTD and whether it taps into the patients VMPFC dysfunction directly have so far not been explored.
This study set out to explore the grey matter atrophy correlates of IGT performance in a group of bvFTD patients. Based on the previous evidence we predicted that bvFTD patients would show impaired IGT performance. We further hypothesised that ventromedial prefrontal cortex atrophy would be closely related to IGT performance. Nevertheless, we also assumed that other cortical and subcortical regions would correlate with the IGT scores, replicating previous functional neuroimaging findings. Finally, we contrasted the IGT performance and neural correlates of the bvFTD group to healthy controls as well as AD patients. Our prediction was that controls would show intact IGT performance. AD patients' performance was more difficult to predict. Only two studies so far have looked at IGT performance in AD patients and their results have been mixed (Sinz et al., 2008; Torralva et al., 2000). However, we assumed that AD patients would perform better than bvFTD patients on the task, as VMPFC atrophy is less likely to be present in AD than bvFTD patients.
2. Materials and methods
2.1. Case selection
Sixty-three participants were selected from the FRONTIER database resulting in a sample of 18 bvFTD, 17 AD patients and 28 age and education matched controls. All bvFTD patients met current consensus criteria for FTD (Neary et al., 1998; Rascovsky et al., 2007) with insidious onset, decline in social behaviour and personal conduct, emotional blunting and loss of insight. In light of the recent recognition of the phenocopy syndrome (Hornberger et al., 2008, 2009) only bvFTD patients with evidence of clear decline as reported by the caregivers and atrophy on MRI scans were included in the study. All AD patients met NINCDS–ADRDA diagnostic criteria (McKhann et al., 1984) for probable AD (see Table 1 for demographic details). Age- and education-matched healthy controls were selected from a healthy volunteer panel or were spouses/carers of patients.
Table 1.
Mean scores (standard deviation) for bvFTD, AD patients and controls on demographics and cognitive tests.
| Demographics, cognitive & behavioural tests | bvFTD | AD | Controls (Con) | F values | bvFTD vs Con | AD vs Con | bvFTD vs AD |
|---|---|---|---|---|---|---|---|
| N | 18 | 17 | 28 | ||||
| Sex (M/F) | (18/0) | (13/4) | (16/12) | ⁎⁎ | |||
| Education | 11.6 (3.3) | 13.1 (3.4) | 13.7 (2.2) | n.s. | – | – | – |
| Mean age (years) | 60.8 (7.8) | 64.5 (7.7) | 64.2 (4.4) | n.s. | – | – | – |
| Total FRS corrected | 48.31 (18.8) | 64.9 (22.8) | – | ⁎ | – | – | ⁎ |
| FRS Rasch score | − .03 (1.1) | .93 (1.4) | – | n.s. | – | – | – |
| ACE-R (max. score = 100) | 80.1 (9.7) | 76.2 (12.6) | 96.1 (2.4) | ⁎⁎⁎ | ⁎⁎⁎ | ⁎⁎⁎ | n.s. |
| CBI (max score = 180) | 74.0 (24.3) | 41.9 (30.5) | 4.8 (5.4) | ⁎⁎⁎ | ⁎⁎⁎ | ⁎⁎⁎ | ⁎⁎⁎ |
| Hayling total AB score | 31.7 (27.4) | 15.1 (14.1) | 2.0 (3.5) | ⁎⁎⁎ | ⁎⁎⁎ | ⁎ | ⁎ |
| Hayling overall scaled score | 3.1 (1.9) | 3.8 (2.1) | 6.4 (0.7) | ⁎⁎⁎ | ⁎⁎⁎ | ⁎⁎⁎ | n.s. |
| IGT | |||||||
| Original total net score | |||||||
| (C + D) − (A + B) | 1.3 (25.6) | 6.6 (26.6) | 18.9 (28.9) | n.s. | – | – | – |
| Modified total net score | |||||||
| (D − A) | 9.2 (19.2) | 11.2 (16.6) | 26.9 (15.9) | ⁎⁎ | ⁎⁎ | ⁎ | n.s. |
| Deck A | 19.7 (10.6) | 18.5 (6.3) | 12.3 (4.5) | ⁎⁎ | ⁎⁎ | ⁎ | n.s. |
| Deck B | 29.8 (12.3) | 28.2 (12.1) | 28.2 (12.9) | n.s. | – | – | – |
| Deck C | 21.5 (7.5) | 23.6 (6.4) | 20.3 (10.1) | n.s. | – | – | – |
| Deck D | 28.9 (11.4) | 29.7 (12.7) | 39.2 (13.6) | ⁎ | ⁎ | ⁎ | n.s. |
n.s. = non significant.
= p < 0.001.
= p < 0.01.
= p < 0.05.
All participants underwent general cognitive screening using the Addenbrooke's Cognitive Examination (ACE-R) (Mathuranath et al., 2000; Mioshi et al., 2006) to determine their overall cognitive functioning. The ACE-R results in a score out of 100, and includes subsections in attention, memory, language and visuo-perception. In addition, the Hayling test of inhibitory dysfunction (Burgess and Shallice, 1997) was administered in order to determine inhibitory control. The frontotemporal dementia rating scale (FRS) (Mioshi et al., 2010) was used to determine the disease severity in bvFTD and AD patients. The Cambridge Behavioural Inventory (CBI) was used as a behavioural disturbance measure with higher scores indicating more behavioural disturbance as reported by the family or carer.
2.2. Iowa Gambling Task
Patients and controls participated in the Iowa Gambling Task (Bechara et al., 2000). This computer-administered task involves making a choice between four decks of cards A, B, C and D each trial. In total, 100 trials are completed by each examinee. The trials are typically divided into five blocks of twenty cards. Participants start with a balance of $2000 and are asked to try and maximize the net reward earned during the gambling task. (If the amount of money lost exceeds the initial credit of $2000, participants have to borrow an additional amount of $2000). Selecting cards from deck A or B results in large immediate rewards, but some cards are associated with an additional large penalty. In deck A, the frequency of penalties increases after each block of cards, but the average amount of money lost per penalty stays the same. In deck B, the frequency of penalties does not change, but the amount of money lost per penalty increases. Consistently choosing cards from either deck A or deck B results in a total net loss of $3750 after 100 trials. Thus, a long-term strategy of choosing cards from deck A or B is disadvantageous and very risky. Decks C and D, on the other hand, are more conservative. Cards from deck C or D are associated with small immediate rewards. Some cards result in an additional penalty, which is much less severe than the penalties associated with cards from deck A or B. Just as in deck A, the frequency, but not the amount of punishments increases after every block of cards in deck C. Just as in deck B, the amount, but not the frequency of punishments increases after every block of cards in deck D. In contrast to decks A and B, however, each block of cards from decks C and D results in a net gain of money. Therefore, participants who persist in choosing cards from these decks end up with a net balance of $1875 after the last trial. Selecting cards from deck C or D is therefore an advantageous long-term strategy (Bechara et al., 2000).
We recorded the total number of cards chosen from each of the four decks. In addition to that, we calculated the commonly used block net score ((C + D) − (A + B)) (Torralva et al., 2007) for each of the five blocks by subtracting the number of disadvantageous choices (deck A + B) from the number of advantageous choices (C + D). An original total net score for each group was calculated by adding up block net scores. It has been suggested that the number of cards chosen from deck B and C is not as indicative of impaired decision making as the number of cards chosen from decks A and D (Bechara et al., 1994). In fact, in a number of studies, healthy subjects have been shown to prefer the disadvantageous deck B over the advantageous deck C or D (see Discussion). For this reason, we also calculated a modified total net score (deck D − A), only based on the two most distinct card decks. Positive scores for both original and modified net score indicate a dominance of advantageous deck choices, while negative scores indicate a dominance of disadvantageous deck choices.
2.3. Behavioural analysis
Data were analyzed using SPSS17.0 (SPSS Inc., Chicago, Ill., USA). Parametric demographic (age, education), neuropsychological (Iowa Gambling Task, general cognitive tests), disease severity (FRS) and behavioural (CBI) data were compared across the three groups (bvFTD, AD and controls) via one-way ANOVAs followed by Tukey post-hoc tests. A chi-squared test was used to check for significant differences in gender across all groups. In addition, a mixed factorial ANOVA (with the trial as a repeated measures factor and the diagnosis as a between subjects factor) was carried out to analyse IGT block net score data. Another mixed factorial ANOVA with performance on the IGT as an additional between subjects factor was carried out in order to compare AD and bvFTD patients who did well on the IGT with those who did not do well.
2.4. Imaging acquisition and voxel-based morphometry (VBM) analysis
All patient and controls underwent the same imaging protocol with whole-brain T1-weighted images using a 3T Philips MRI scanner with standard quadrature head coil (8 channels). The 3D T1-weighted sequences were acquired as follows: coronal orientation, matrix 256 × 256, 200 slices, 1 × 1 mm2 in-plane resolution, slice thickness 1 mm, and TE/TR = 2.6/5.8 ms. All scans were then visually checked for field inhomogeneity distortions and corrected for eddy current distortions. 3D T1-weighted sequences were analyzed with FSL–VBM, a voxel-based morphometry analysis (Ashburner and Friston, 2000; Good et al., 2001) which is part of the FSL software package http://www.fmrib.ox.ac.uk/fsl/fslvbm/index.html (Smith et al., 2004). First, tissue segmentation was carried out using FMRIB's Automatic Segmentation Tool (FAST) (Zhang et al., 2001) from brain extracted images. The resulting grey matter partial volume maps were then aligned to the Montreal Neurological Institute standard space (MNI152) using the nonlinear registration approach using FNIRT (Andersson et al., 2007a, 2007b), which uses a b-spline representation of the registration warp field (Rueckert et al., 1999). The registered partial volume maps were then modulated (to correct for local expansion or contraction) by dividing them by the Jacobian of the warp field. The modulated images were then smoothed with an isotropic Gaussian kernel with a standard deviation of 3 mm (FWHM: 8 mm). Finally, a voxelwise general linear model (GLM) was applied and permutation-based non-parametric testing was used to form clusters with the Threshold-Free Cluster Enhancement (TFCE) method (Smith and Nichols, 2009). Group comparisons and covariate analysis of the Hayling test score were tested for significance at p < 0.05, corrected for multiple comparisons via Family-wise Error correction across space, whereas the remaining covariate analyses were conducted at a significance level of p < 0.01, False Discovery Rate (FDR) corrected and a cluster threshold of 50 contiguous voxels for all significant atrophy clusters. The general cognitive measure (ACE-R) was entered as a covariate in the analysis to account for general cognitive impairment.
3. Results
3.1. Demographics and global cognitive functioning
Demographics and general cognitive scores can be seen in Table 1. Participant groups did not differ in terms of age (p > .1) and education (p > .05). However, there was a significant difference in gender distribution across the three groups (p < 0.01), with the patient groups (bvFTD, AD) having more male participants than the controls. The patient groups differed significantly in disease severity (Total FRS Corrected: p < 0.05) with bvFTD patients being more impaired.
For the cognitive screening test (ACE-R), both patient groups were significantly impaired in comparison to controls (p < .001) but did not differ from each other (p > .1). On the behavioural scores, bvFTD and AD patients showed significantly more behavioural disturbances than age-matched controls (p's < .001), with the bvFTD patients even significantly worse than the AD patients (p < .001).
For both Hayling scores (AB errors & overall scores) there was a significant group effect, with post-hoc analyses showing that both patient groups were impaired compared to controls (p's < .05). However, bvFTD and AD only differed significantly on the AB error score (p < .05) with bvFTD making overall more errors.
3.2. Iowa Gambling Task
In a first step, we analysed the original total net score for all participant groups (bvFTD, AD and controls), which did not differ significantly across groups (p > .1). Interestingly, however, controls achieved significantly higher modified total net scores than bvFTD (p < 0.01) and AD (p < 0.05) patients. Modified total net scores did not differ between patient groups (Table 1). Further analysis, investigating how many times cards were chosen from each deck (Table 1) showed that participant groups did not differ significantly for decks B (p > .1) and C (p > .1). However, controls chose significantly more cards from deck D (p < 0.01) and significantly less cards from deck A (p < 0.01) than AD and bvFTD patients.
As evident from Fig. 1, net scores increased from trials 1–80 for both controls and ADs, but decreased during the last 20 trials (trials 81–100). The net score of bvFTD's increased during trials 1–60, stayed stable for trials 61–80 and decreased for the final trials (81–100). Analysis for the block net scores showed only a significant difference between control and patient between trials 41–60, but not in any of the other trial blocks. A mixed factorial ANOVA analysis showed that there was no significant interaction between trial blocks and group (p > .1). There was only a statistical trend for a main effect of diagnosis (p = 0.089), and a main effect of trial block (p < 0.01).
Fig. 1.
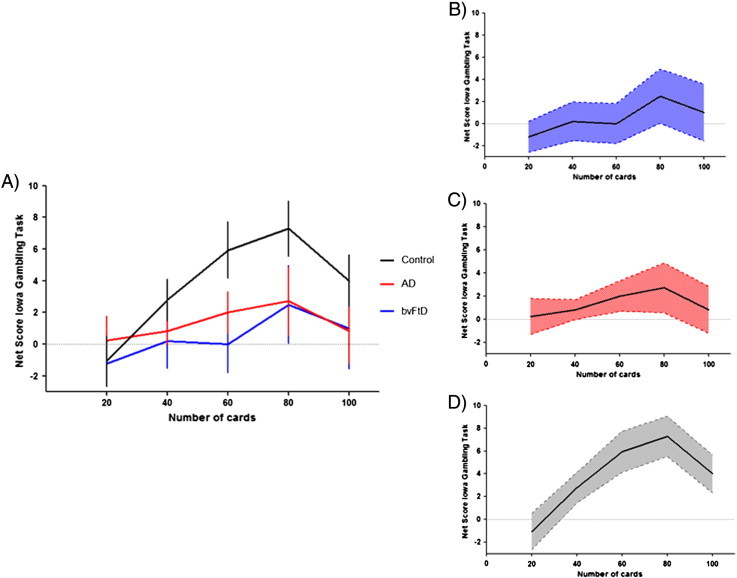
Shows the behavioural performance of all groups for the total IGT net score: A) line graph showing mean performance across trials and participant groups; error bars indicate S.E. mean; B) line graph showing mean performance across trials for bvFTD; blue shaded areas indicate 95% confidence intervals; C) line graph showing mean performance across trials for AD; red shaded areas indicate 95% confidence intervals; D) line graph showing mean performance across trials for Controls; grey shaded areas indicate 95% confidence intervals.
As evident from Fig. 1 and Table 1, patients' performance was very variable for each trial block, as indicated by the large standard deviations. We split therefore the patients into low (n = 16) vs. high (n = 19) performers based on the group mean. The results (Fig. 4A) showed that the high performing bvFTD and AD patients performed nearly at control level on the IGT, while the poor performing patients were very impaired. Not surprisingly, this results in a significant interaction between trial block vs. performance (high vs. low) (p < .05). Importantly, bvFTD and AD patients did not differ from each other for high vs. low performance respectively.
Fig. 4.
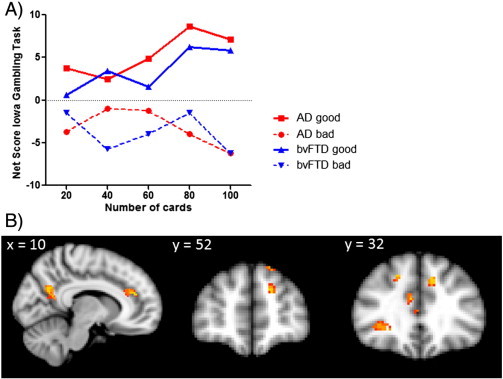
A) shows the behavioural performance of high vs. low bvFTD and AD performers for the total IGT net score across trials; B) Voxel-based morphometry analyses showing grey matter atrophy for poor versus good performers on the IGT. Clusters are overlaid on the MNI standard brain (t > 2.41). Coloured voxels show regions that were significant in the analyses for p < 0.01 FDR corrected and a cluster threshold of 50 contiguous voxels.
3.3. VBM — correlations with IGT
In a first step we entered IGT scores as covariates in the design matrix of the VBM analysis. We used uncorrected significance levels of p < 0.001 and a cluster threshold of 50 contiguous voxels for all significant atrophy clusters. As a first covariate we employed the original total net IGT score. For all participants combined, the original total net score correlated with atrophy in several prefrontal cortex regions including the orbitofrontal cortex, frontal pole and dorsolateral prefrontal cortex (Fig. 2A, Table 4). Furthermore, atrophy in the temporal cortex, parietal cortex, occipital cortex, cerebellum and hippocampus also correlated with the original total net score.
Fig. 2.
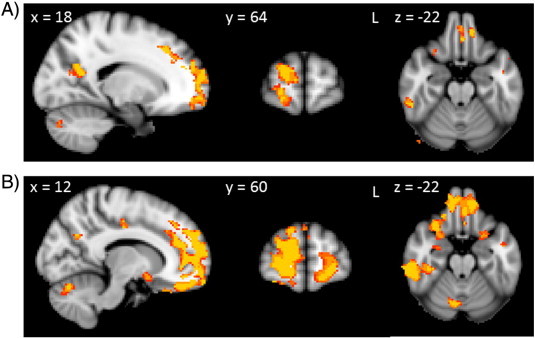
Voxel-based morphometry analyses showing grey matter atrophy covarying with the original IGT net score (A) and the modified IGT net score (B). Clusters are overlaid on the MNI standard brain (t > 2.41). Coloured voxels show regions that were significant in the analyses for p < 0.01 FDR corrected and a cluster threshold of 50 contiguous voxels.
Table 4.
Bad versus good IGT performers.
| Regions | Hemisphere |
MNI coordinates |
Number of voxels | T score |
||
|---|---|---|---|---|---|---|
| (L/R/B) | X | Y | Z | (peak voxel) | ||
| Bad versus good mean net score | ||||||
| Precuneous cortex | B | 16 | − 60 | 12 | 318 | 3.1 |
| Cingulate gyrus, anterior division | R | 2 | 34 | 8 | 135 | 3.1 |
| Orbitofrontal cortex | R | 26 | 28 | − 6 | 115 | 3.1 |
| Frontal pole | B | − 16 | 52 | 24 | 110 | 3.1 |
In a second analysis, we used the modified total net score as a covariate in the VBM analysis (Fig. 2B, Table 4). Regions of atrophy correlated with the modified total net score overlapped with regions of atrophy related to the original total net score. Interestingly, however, more widespread regions of atrophy were correlated with the modified total net score than with the original total net score. Compared to the original total net score, the modified total net score correlated with additional cortical regions, such as the anterior cingulate and subcortical regions, including the amygdala and the nucleus accumbens (Fig. 2B, Table 4). As the modified total net score seems to be a more sensitive measure, we decided to use the modified total net score in a VBM analysis of bvFTD and AD patients, separately. In bvFTD patients, the modified total net score correlated with atrophy in the prefrontal cortex, occipital cortex and cerebellum. AD patients' modified total net scores, on the other hand, correlated with atrophy in the parietal and temporal cortex (Fig. 3, Table 3). A similar analysis of the original total score did not reveal any significant results for bvFTD and AD separately.
Fig. 3.
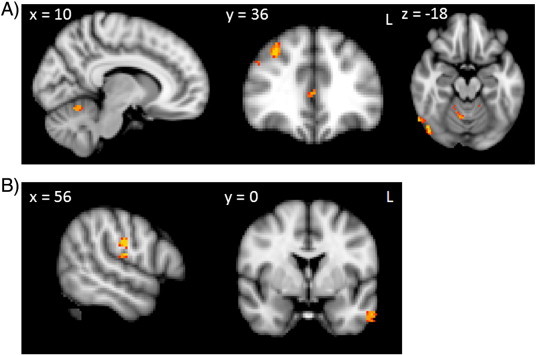
Voxel-based morphometry analyses showing grey matter atrophy covarying with the modified IGT net score for A) bvFTD and B) AD. Clusters are overlaid on the MNI standard brain (t > 2.41). Coloured voxels show regions that were significant in the analyses for p < 0.01 FDR corrected and a cluster threshold of 50 contiguous voxels.
Table 3.
Modified total net score.
| Regions | Hemisphere (L/R/B) | MNI coordinates |
Number of voxels | T score |
||
|---|---|---|---|---|---|---|
| X | Y | Z | (peak voxel) | |||
| All groups combined | ||||||
| Temporal pole/orbitofrontal cortex/frontal pole/anterior cingulate/dorsolateral prefrontal cortex | B | 42 | 20 | − 40 | 11,318 | 2.98 |
| Parietal operculum cortex | B | − 50 | − 24 | 16 | 1954 | 2.98 |
| Insula | B | − 32 | 18 | − 8 | 874 | 2.98 |
| Cerebellum | B | 38 | − 52 | − 60 | 467 | 2.98 |
| Supramarginal gyrus | B | 38 | − 32 | 34 | 307 | 2.98 |
| Precuneous | B | − 12 | − 58 | 20 | 97 | 2.98 |
| Hippocampus | B | − 28 | − 38 | 0 | 90 | 2.98 |
| Superior parietal lobe | R | 28 | − 40 | 46 | 80 | 2.98 |
| Occipital pole | R | 16 | − 96 | − 10 | 65 | 2.98 |
| Superior temporal gyrus | L | − 48 | − 4 | − 20 | 65 | 2.98 |
| Cingulate, posterior division | R | 12 | − 16 | 38 | 59 | 2.98 |
| Putamen | B | − 22 | 8 | 10 | 40 | 2.98 |
| Amygdala | R | 16 | − 6 | − 10 | 30 | 2.74 |
| bvFTD | ||||||
| Middle frontal gyrus/frontal pole | R | 26 | 26 | 36 | 104 | 3.36 |
| Lateral occipital cortex | R | 48 | − 76 | − 20 | 81 | 3.36 |
| Cerebellum | R | 8 | − 62 | − 16 | 55 | 3.36 |
| AD | ||||||
| Central opercular cortex/postcentral gyrus | R | 60 | − 18 | 14 | 98 | 3.32 |
| Middle temporal gyrus, anterior division | L | − 58 | 0 | − 30 | 56 | 3.32 |
Finally, a comparison of high and low performing patients showed significantly more atrophy in the lateral orbitofrontal cortex, frontal poles, cingulate and precuneous cortex for patients who did not do well on the IGT (Fig. 4B, Table 2). Separate analyses for bvFTD and AD did not reveal any significant results for bad vs. good IGT performers.
Table 2.
IGT total net score.
| Regions | Hemisphere |
MNI coordinates |
Number of voxels | T score |
||
|---|---|---|---|---|---|---|
| (L/R/B) | X | Y | Z | (peak voxel) | ||
| Orbitofrontal cortex | B | 0 | 34 | − 24 | 4090 | 2.98 |
| Parietal operculum cortex | L | − 50 | − 32 | 20 | 848 | 2.98 |
| Precuneous cortex | B | − 10 | − 66 | 16 | 502 | 2.98 |
| Central opercular cortex | R | 56 | − 16 | 14 | 434 | 2.98 |
| Insula | R | 32 | 12 | 14 | 211 | 2.98 |
| Supramarginal gyrus | B | 38 | − 30 | 34 | 196 | 2.98 |
| Precentral gyrus | L | − 38 | 8 | 22 | 169 | 2.98 |
| Planum polare | L | − 44 | − 4 | − 20 | 133 | 2.98 |
| Inferior temporal gyrus, temporooccipital part | R | 56 | − 40 | − 24 | 110 | 2.98 |
| Cingulate, posterior division | R | 16 | − 22 | 40 | 92 | 2.74 |
| Frontal pole | B | − 12 | 42 | − 28 | 91 | 2.98 |
| Cerebellum | B | 16 | − 80 | − 36 | 90 | 2.74 |
| Hippocampus | R | 40 | − 30 | − 10 | 68 | 2.98 |
| Middle frontal gyrus | R | 32 | − 4 | 42 | 68 | 2.98 |
| Inferior temporal gyrus, posterior division | L | − 60 | − 22 | − 34 | 67 | 2.98 |
| Occipital fusiform gyrus | L | − 26 | − 72 | − 4 | 64 | 2.98 |
3.4. VBM — comparison of Hayling and IGT atrophy covariate regions
In a final analysis, we explored whether atrophy covariates of the modified net score of the IGT overlapped with the atrophy covariates on a commonly used disinhibition test, the Hayling test of inhibitory dysfunction. As expected, ventromedial atrophy, including orbitofrontal cortex as well as anterior cingulate correlated with the Hayling score (Fig. 5, blue areas). Atrophy patterns showed some overlap between the disinhibition and decision making measures. Nevertheless, some regions within the ventromedial prefrontal cortex correlated with the modified total net score, but not with the Hayling test score (Fig. 5).
Fig. 5.
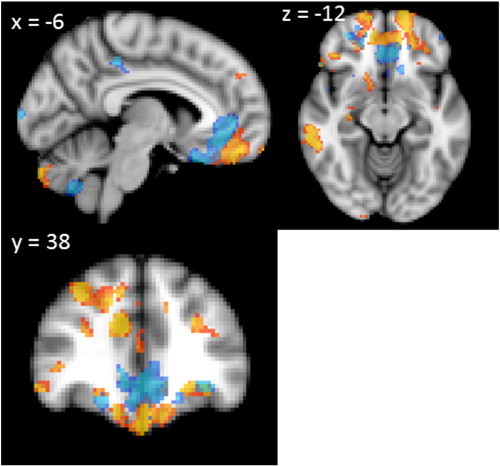
Voxel-based morphometry analyses showing grey matter atrophy covarying with the AB error score on the Hayling test of inhibitory function (blue) and the modified IGT net score (orange-yellow). Clusters are overlaid on the MNI standard brain (t > 2.41). Coloured voxels show regions that were significant in the analyses for p < 0.01 FDR corrected and a cluster threshold of 50 contiguous voxels.
4. Discussion
Our results show that participants' performance on the IGT was highly variable, with some patients performing well, while others were severely impaired. Imaging analysis showed that poor performance on the IGT is related to atrophy in several cortical and subcortical brain regions, in particular frontal brain regions. Different atrophy patterns were associated with impaired decision making behaviour in AD and bvFTD patients. Surprisingly, although the control group showed a higher IGT performance than both patient groups, this trend did not reach significance.
Previous studies in bvFTD patients showed a clear preference for disadvantageous decks on the IGT (Manes et al., 2010, 2011; Torralva et al., 2007). Studies looking at IGT performance in AD patients yielded more mixed results. Sinz et al. (2008) showed that AD patients neither demonstrated a preference for disadvantageous nor advantageous decks, switching frequently between decks. On the other hand, Torralva et al. (2000) found that AD patients showed a slight preference for disadvantageous decks. In keeping with these findings, we found that both AD and bvFTD patients showed poor decision making behaviour on the IGT. Although patients' performance was clearly impaired, AD patients showed an increasing preference for advantageous decks throughout the task and bvFTD patients also demonstrated a preference for advantageous decks towards the end of the task, resulting in a positive net score (Fig. 1), which contrasts with the findings of previous studies outlined above. To our knowledge, this study is the first to contrast AD and bvFTD patients' performance on the IGT directly, which revealed no significant performance differences between the patient groups. Furthermore, in contrast to previous studies, we did not find a significant difference between the performance of patients and controls. Interestingly, there was great individual variability of the performance among each group of participants, with a proportion of controls seemingly impaired on the task, while some patients performed well, which could explain why the patient groups did not differ for most of the IGT scores from the controls. Importantly, there are numerous previous reports of great variability in risk taking behaviour on the IGT observed in neurologically healthy individuals, showing that IGT performance can depend on trait anxiety (Miu et al., 2008), emotionality (Peters and Slovic, 2000), education level (Evans et al., 2004) as well as conscious awareness of task rules (Maia and McClelland, 2004). For example, Denburg and colleagues found that only between 37.5% and 52.5% of healthy elderly participants showed a clear preference for advantageous over disadvantageous decks of cards on the IGT. Premature prefrontal cortex ageing and pre-clinical forms of degenerative disease have been offered as possible explanations for these variable results in healthy controls (Denburg et al., 2005, 2006). However, unlike our patients, the controls in our sample did not show impairment on the Hayling Test, which taps into similar cortical regions as the IGT (Hornberger et al., 2011a) and did not show behavioural dysfunction on the behavioural questionnaire (CBI). Thus, it seems unlikely that premature prefrontal cortex ageing and pre-clinical forms of degenerative disease explain poor IGT performance in our study.
There is also increasing evidence to suggest that decision making behaviour in neurologically healthy participants may not primarily be guided by the advantageousness or disadvantageousness of a deck of cards, as proposed by SMH, but rather by its frequency of gains and losses. Decks B and D are associated with a high ratio of wins to losses, whereas gains are less frequent in decks A and C. Studies have shown that most healthy participants prefer the disadvantageous, high-frequency-gain deck B to the advantageous low-frequency-gain deck C (Caroselli et al., 2006; Lin et al., 2007; Wilder et al., 1998). Similarly, participants can show a clear preference for the disadvantageous high-frequency-gain deck B over the advantageous high-frequency-gain deck D (Caroselli et al., 2006). These results suggest that many healthy decision makers apply a “win-stay, lose-shift” strategy on the IGT (Lin et al., 2007) and that their behaviour is not driven only by long-term outcomes expectancies.
Considering these findings, it is not surprising that the total number of card selections from decks B and C was similar in our patients and controls. In our study, controls chose significantly fewer cards from deck A and significantly more cards from deck D than patients, indicating better decision making. It is questionable whether the resulting difference in net scores, which did not reach significance, adequately reflects differences in decision-making behaviour between control and patient groups. For this reason, we used not only the original total net score in our analysis, but also a modified version of total net score, which compares the number of cards chosen from deck D with the number of cards chosen from deck A. Using the modified total net score, results revealed significant differences between control participants and patient groups, although the difference between AD and bvFTD patients remained not significant. Notably, the modified net score seemed to be the most sensitive measure of impairment. Crucially, since AD and bvFTD patients' performance did not differ, our results call into question the validity of the IGT as a diagnostic test for bvFTD.
A similar complex picture emerged from our imaging analyses. Regions of atrophy associated with the original total net score and modified total net score were very similar. We found that IGT performance covaried with atrophy in numerous regions, including the orbitofrontal and dorsolateral prefrontal cortex, frontal pole, cingulate, parietal and temporal cortex, cerebellum, putamen, amygdala and hippocampus. These findings dovetail with results of functional neuroimaging studies showing that there are activations in multiple cortical and subcortical brain regions during IGT performance (Ernst et al., 2002; Bolla et al., 2004; Lin et al., 2008). Importantly, in keeping with affective theories of decision-making, such as the SMH, atrophy in the orbitofrontal cortex and amygdala correlated with IGT scores. The amygdala (Gupta et al., 2011) and the orbitofrontal cortex (Rangel and Hare, 2010) are likely to play a vital role in the emotional appraisal of different decks of cards, guiding decision making. In addition, atrophy in the orbitofrontal cortex is associated with disinhibited behaviour (Hornberger et al., 2011b), which has been suggested as a possible cause for poor IGT performance. In order to find out if, and to what extent, response inhibition deficits might influence IGT performance, we projected regions of atrophy associated with the Hayling test score onto regions of atrophy associated with the modified net score (Fig. 5). Only parts of these brain regions overlapped, indicating that disinhibited behaviour does not fully account for poor IGT performance.
Other prefrontal regions were also involved in the performance of the IGT, in particular frontopolar, dorsolateral prefrontal cortex and anterior cingulate regions. Overall, the role of the frontopolar cortex is still little understood. However, there is evidence to suggest that the frontopolar cortex affects exploratory behaviour in ambiguous decision making tasks (Daw et al., 2006), which would explain its involvement in the IGT. In addition, the frontal pole region seems to be involved in the processing of goal-tree sequences, allowing individuals to keep in mind specific goals while processing subgoals or responding to new environmental demands (Koechlin et al., 1999). A similar function is usually attributed to the dorsolateral prefrontal cortex, which has been implicated in working memory tasks (Goldman-Rakic, 1992). A comparison of participants who performed well on the IGT with those who did not revealed significant differences in damage to the prefrontal cortex, including the anterior cingulate. The anterior cingulate seems to be involved in reinforcement-guided action selection, mediating between previous experience and voluntary choice (Rushworth et al., 2007; Sul et al., 2010).
Although the IGT is more seen as a prefrontal cortex dependent task, other atrophy regions also correlate with IGT performance. Neither on a behavioural, nor on a neural level do our results therefore support the assumption that the IGT specifically taps into VMPFC dysfunction. For example, poor performance on the IGT was also associated with atrophy in the parietal cortex. It is currently not clear why the parietal cortex is involved in this task. However, the parietal cortex, similar to the prefrontal cortex, is involved in a multitude of functions that could affect IGT performance, such as attentional processes (Le et al., 1998; Perry and Zeki, 2000; Wojciulik and Kanwisher, 1999), response inhibition (de Zubicaray et al., 2000; Garavan et al., 1999), working memory (LaBar et al., 1999), task switching (Sohn et al., 2000), numerical comparisons (Pesenti et al., 2000) and the coding of probability (Platt and Glimcher, 1999). Interestingly, the parietal cortex and in particular the posterior cingulate have been implicated in episodic memory processes (Daselaar et al., 2003; Desgranges et al., 1998), which could explain why they were implicated in the IGT performance. Behavioural studies suggest that explicit knowledge of the task rules is important to perform on the task (Gupta et al., 2009; Gutbrod et al., 2006; Maia and McClelland, 2004) and therefore remembering which decks are advantageous and which are not. Thus, impairment of episodic memory processes should affect performance on the IGT, which is in particular relevant for the AD patient group, which shows substantial episodic memory impairment. Along these lines, temporal lobe atrophy, including hippocampal atrophy, was also significantly associated with IGT performance. This ties in nicely with previous findings showing that hippocampal atrophy is associated with explicit knowledge of task rule (Giovagnoli et al., 2001). In addition, the putamen has been implicated in rule-based task learning (Ell et al., 2006). These findings are in keeping with reports suggesting involvement of the declarative memory system in complex decision making tasks like the IGT (Gupta et al., 2009).
More surprising was the fact that cerebellar atrophy was also associated with the IGT task performance. Just like the anterior cingulate, the cerebellum has been implicated in error based learning (Doya, 2000). Although the cerebellum has mainly been associated with motor functions, there is evidence to suggest that damage to the cerebellum also adversely affects error based learning in cognitive tasks (Fiez et al., 1992) as well as more general cognitive processes (Baumann and Mattingley, 2012). Thus, it is possible that cerebellar atrophy impacts on cognitive control processes during the IGT. Alternatively, correlation of cerebellar atrophy with IGT performance might simply reflect the task's hand-eye coordination demands, which have not been systematically investigated in neurodegenerative conditions such as bvFTD and AD.
Interestingly, separate analyses of atrophy regions associated with IGT performance in AD and bvFTD patients suggest that decision making impairment results from damage to different brain regions in the two patient groups. In bvFTD patients, performance improvement was related to atrophy in prefrontal cortex regions, occipital cortex and cerebellum, whereas AD patients' performance improvement was associated with atrophy in the parietal and temporal cortex. These results suggest that there are different underlying reasons for poor IGT performance in AD and bvFTD patients.
Taken together, the behavioural results of our study call into question the specificity, though not the sensitivity of the IGT as a diagnostic test to discriminate neurodegeneration conditions. Our imaging results corroborate this notion by showing that multiple brain regions involved in multiple operations are involved in the IGT performance. Crucially, some of the regions are more commonly affected in bvFTD (OFC), while others are more commonly impaired in AD (precuneous). Thus, employment of the IGT as a test to discriminate these two conditions on VMPFC dysfunction is questionable and there is an urgent need for more specific VMPFC diagnostic tests in the future.
Acknowledgements
We would like to thank the participants and their families. Data for the clinical study was obtained from FRONTIER clinic at Neuroscience Research Australia, which is funded by an Australian Research Council Centre of Excellence grant and National Health and Medical Research Council of Australia grants. We wish to thank the staff of FRONTIER for their assistance, in particular David Foxe, Sharon Savage and Felicity Leslie. This work was supported by the Australian Research Council [DP110104202 to M.H., FF0776229 to J.R.H.].
Footnotes
This is an open-access article distributed under the terms of the Creative Commons Attribution-NonCommercial-ShareAlike License, which permits non-commercial use, distribution, and reproduction in any medium, provided the original author and source are credited.
References
- Andersson J.L.R., Jenkinson M., Smith S. 2007. Non-Linear Registration aka Spatial Normalisation. [Google Scholar]
- Andersson J.L.R., Jenkinson M., Smith S. 2007. Non-linear Optimisation. [Google Scholar]
- Ashburner J., Friston K.J. Voxel-based morphometry—the methods. NeuroImage. 2000;11:805–821. doi: 10.1006/nimg.2000.0582. [DOI] [PubMed] [Google Scholar]
- Baumann O., Mattingley J.B. Functional topography of primary emotion processing in the human cerebellum. NeuroImage. 2012;61:805–811. doi: 10.1016/j.neuroimage.2012.03.044. [DOI] [PubMed] [Google Scholar]
- Bechara A. Insensitivity to future consequences following damage to human prefrontal cortex. Cognition. 1994;50:7–15. doi: 10.1016/0010-0277(94)90018-3. [DOI] [PubMed] [Google Scholar]
- Bechara A. Different contributions of the human amygdala and ventromedial prefrontal cortex to decision-making. Journal of Neuroscience. 1999;19:5473–5481. doi: 10.1523/JNEUROSCI.19-13-05473.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bechara A., Tranel D., Damasio H. Characterization of the decision-making deficit of patients with ventromedial prefrontal cortex lesions. Brain. 2000;123(Pt 11):2189–2202. doi: 10.1093/brain/123.11.2189. [DOI] [PubMed] [Google Scholar]
- Bolla K.I. Sex-related differences in a gambling task and its neurological correlates. Cerebral Cortex. 2004;14:1226–1232. doi: 10.1093/cercor/bhh083. [DOI] [PubMed] [Google Scholar]
- Burgess P., Shallice T. T.V.T Company; Thurston Suffolk: 1997. The Hayling and Brixton Tests. [Google Scholar]
- Caroselli J.S. The simulated gambling paradigm applied to young adults: an examination of university students' performance. Applied Neuropsychology. 2006;13:203–212. doi: 10.1207/s15324826an1304_1. [DOI] [PubMed] [Google Scholar]
- Chow T.W. Magnetic resonance imaging in frontotemporal dementia shows subcortical atrophy. Dementia and Geriatric Cognitive Disorders. 2008;26:79–88. doi: 10.1159/000144028. [DOI] [PubMed] [Google Scholar]
- Daselaar S.M. Neuroanatomical correlates of episodic encoding and retrieval in young and elderly subjects. Brain. 2003;126:43–56. doi: 10.1093/brain/awg005. [DOI] [PubMed] [Google Scholar]
- Daw N.D. Cortical substrates for exploratory decisions in humans. Nature. 2006;441:876–879. doi: 10.1038/nature04766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Zubicaray G.I. Motor response suppression and the prepotent tendency to respond: a parametric fMRI study. Neuropsychologia. 2000;38:1280–1291. doi: 10.1016/s0028-3932(00)00033-6. [DOI] [PubMed] [Google Scholar]
- Denburg N.L., Tranel D., Bechara A. The ability to decide advantageously declines prematurely in some normal older persons. Neuropsychologia. 2005;43:1099–1106. doi: 10.1016/j.neuropsychologia.2004.09.012. [DOI] [PubMed] [Google Scholar]
- Denburg N.L. Psychophysiological anticipation of positive outcomes promotes advantageous decision-making in normal older persons. International Journal of Psychophysiology. 2006;61:19–25. doi: 10.1016/j.ijpsycho.2005.10.021. [DOI] [PubMed] [Google Scholar]
- Desgranges B., Baron J.C., Eustache F. The functional neuroanatomy of episodic memory: the role of the frontal lobes, the hippocampal formation, and other areas. NeuroImage. 1998;8:198–213. doi: 10.1006/nimg.1998.0359. [DOI] [PubMed] [Google Scholar]
- Doya K. Complementary roles of basal ganglia and cerebellum in learning and motor control. Current Opinion in Neurobiology. 2000;10:732–739. doi: 10.1016/s0959-4388(00)00153-7. [DOI] [PubMed] [Google Scholar]
- Ell S.W., Marchant N.L., Ivry R.B. Focal putamen lesions impair learning in rule-based, but not information-integration categorization tasks. Neuropsychologia. 2006;44:1737–1751. doi: 10.1016/j.neuropsychologia.2006.03.018. [DOI] [PubMed] [Google Scholar]
- Ernst M. Decision-making in a risk-taking task: a PET study. Neuropsychopharmacology. 2002;26:682–691. doi: 10.1016/S0893-133X(01)00414-6. [DOI] [PubMed] [Google Scholar]
- Evans C.E., Kemish K., Turnbull O.H. Paradoxical effects of education on the Iowa Gambling Task. Brain and Cognition. 2004;54:240–244. doi: 10.1016/j.bandc.2004.02.022. [DOI] [PubMed] [Google Scholar]
- Fellows L.K., Farah M.J. Different underlying impairments in decision-making following ventromedial and dorsolateral frontal lobe damage in humans. Cerebral Cortex. 2005;15:58–63. doi: 10.1093/cercor/bhh108. [DOI] [PubMed] [Google Scholar]
- Fiez J.A. Impaired non-motor learning and error detection associated with cerebellar damage. A single case study. Brain. 1992;115(Pt 1):155–178. doi: 10.1093/brain/115.1.155. [DOI] [PubMed] [Google Scholar]
- Garavan H., Ross T.J., Stein E.A. Right hemispheric dominance of inhibitory control: an event-related functional MRI study. Proceedings of the National Academy of Sciences of the United States of America. 1999;96:8301–8306. doi: 10.1073/pnas.96.14.8301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giovagnoli A.R., Erbetta A., Bugiani O. Preserved semantic access in global amnesia and hippocampal damage. The Clinical Neuropsychologist. 2001;15:508–515. doi: 10.1076/clin.15.4.508.1889. [DOI] [PubMed] [Google Scholar]
- Gleichgerrcht E. Decision-making cognition in neurodegenerative diseases. Nature Reviews. Neurology. 2010;6:611–623. doi: 10.1038/nrneurol.2010.148. [DOI] [PubMed] [Google Scholar]
- Goldman-Rakic P.S. Working memory and the mind. Scientific American. 1992;267:110–117. doi: 10.1038/scientificamerican0992-110. [DOI] [PubMed] [Google Scholar]
- Good C.D. A voxel-based morphometric study of ageing in 465 normal adult human brains. NeuroImage. 2001;14:21–36. doi: 10.1006/nimg.2001.0786. [DOI] [PubMed] [Google Scholar]
- Gupta R. Declarative memory is critical for sustained advantageous complex decision-making. Neuropsychologia. 2009;47:1686–1693. doi: 10.1016/j.neuropsychologia.2009.02.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gupta R. The amygdala and decision-making. Neuropsychologia. 2011;49:760–766. doi: 10.1016/j.neuropsychologia.2010.09.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gutbrod K. Decision-making in amnesia: do advantageous decisions require conscious knowledge of previous behavioural choices? Neuropsychologia. 2006;44:1315–1324. doi: 10.1016/j.neuropsychologia.2006.01.014. [DOI] [PubMed] [Google Scholar]
- Hornberger M. Executive function in progressive and nonprogressive behavioral variant frontotemporal dementia. Neurology. 2008;71:1481–1488. doi: 10.1212/01.wnl.0000334299.72023.c8. [DOI] [PubMed] [Google Scholar]
- Hornberger M. Can progressive and non-progressive behavioral variant frontotemporal dementia be distinguished at presentation? Journal of Neurology, Neurosurgery, and Psychiatry. 2009;80:591–593. doi: 10.1136/jnnp.2008.163873. [DOI] [PubMed] [Google Scholar]
- Hornberger M., Geng J., Hodges J.R. Convergent grey and white matter evidence of orbitofrontal cortex changes related to disinhibition in behavioural variant frontotemporal dementia. Brain. 2011;134:2502–2512. doi: 10.1093/brain/awr173. [DOI] [PubMed] [Google Scholar]
- Hornberger M., Geng J., Hodges J.R. Convergent grey and white matter evidence of orbitofrontal cortex changes related to disinhibition in behavioural variant frontotemporal dementia. Brain. 2011;134:2502–2512. doi: 10.1093/brain/awr173. [DOI] [PubMed] [Google Scholar]
- Kipps C.M. Combined magnetic resonance imaging and positron emission tomography brain imaging in behavioural variant frontotemporal degeneration: refining the clinical phenotype. Brain. 2009;132:2566–2578. doi: 10.1093/brain/awp077. [DOI] [PubMed] [Google Scholar]
- Koechlin E. The role of the anterior prefrontal cortex in human cognition. Nature. 1999;399:148–151. doi: 10.1038/20178. [DOI] [PubMed] [Google Scholar]
- Kril J.J., Halliday G.M. Clinicopathological staging of frontotemporal dementia severity: correlation with regional atrophy. Dementia and Geriatric Cognitive Disorders. 2004;17:311–315. doi: 10.1159/000077161. [DOI] [PubMed] [Google Scholar]
- LaBar K.S. Neuroanatomic overlap of working memory and spatial attention networks: a functional MRI comparison within subjects. NeuroImage. 1999;10:695–704. doi: 10.1006/nimg.1999.0503. [DOI] [PubMed] [Google Scholar]
- Le T.H., Pardo J.V., Hu X. 4T-fMRI study of nonspatial shifting of selective attention: cerebellar and parietal contributions. Journal of Neurophysiology. 1998;79:1535–1548. doi: 10.1152/jn.1998.79.3.1535. [DOI] [PubMed] [Google Scholar]
- Lin C.H. Is deck B a disadvantageous deck in the Iowa Gambling Task? Behavioral and Brain Functions. 2007;3:16. doi: 10.1186/1744-9081-3-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin C.H. Brain maps of Iowa gambling task. BMC Neuroscience. 2008;9:72. doi: 10.1186/1471-2202-9-72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maia T.V., McClelland J.L. A reexamination of the evidence for the somatic marker hypothesis: what participants really know in the Iowa gambling task. Proceedings of the National Academy of Sciences of the United States of America. 2004;101:16075–16080. doi: 10.1073/pnas.0406666101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manes F. Decision-making processes following damage to the prefrontal cortex. Brain. 2002;125:624–639. doi: 10.1093/brain/awf049. [DOI] [PubMed] [Google Scholar]
- Manes F.F. Frontotemporal dementia presenting as pathological gambling. Nature Reviews. Neurology. 2010;6:347–352. doi: 10.1038/nrneurol.2010.34. [DOI] [PubMed] [Google Scholar]
- Manes F. Decision-making in frontotemporal dementia: clinical, theoretical and legal implications. Dementia and Geriatric Cognitive Disorders. 2011;32:11–17. doi: 10.1159/000329912. [DOI] [PubMed] [Google Scholar]
- Marschner A. Reward-based decision-making and aging. Brain Research Bulletin. 2005;67:382–390. doi: 10.1016/j.brainresbull.2005.06.010. [DOI] [PubMed] [Google Scholar]
- Mathuranath P.S. A brief cognitive test battery to differentiate Alzheimer's disease and frontotemporal dementia. Neurology. 2000;55:1613–1620. doi: 10.1212/01.wnl.0000434309.85312.19. [DOI] [PubMed] [Google Scholar]
- McKhann G. Clinical diagnosis of Alzheimer's disease: report of the NINCDS–ADRDA Work Group under the auspices of Department of Health and Human Services Task Force on Alzheimer's Disease. Neurology. 1984;34:939–944. doi: 10.1212/wnl.34.7.939. [DOI] [PubMed] [Google Scholar]
- Mioshi E. The Addenbrooke's Cognitive Examination Revised (ACE-R): a brief cognitive test battery for dementia screening. International Journal of Geriatric Psychiatry. 2006;21:1078–1085. doi: 10.1002/gps.1610. [DOI] [PubMed] [Google Scholar]
- Mioshi E. Clinical staging and disease progression in frontotemporal dementia. Neurology. 2010;74:1591–1597. doi: 10.1212/WNL.0b013e3181e04070. [DOI] [PubMed] [Google Scholar]
- Miu A.C., Heilman R.M., Houser D. Anxiety impairs decision-making: psychophysiological evidence from an Iowa gambling task. Biological Psychology. 2008;77:353–358. doi: 10.1016/j.biopsycho.2007.11.010. [DOI] [PubMed] [Google Scholar]
- Nakaaki S. Impairment of decision-making cognition in a case of frontotemporal lobar degeneration (FTLD) presenting with pathologic gambling and hoarding as the initial symptoms. Cognitive and Behavioral Neurology. 2007;20:121–125. doi: 10.1097/WNN.0b013e31804c6ff7. [DOI] [PubMed] [Google Scholar]
- Neary D. Frontotemporal lobar degeneration: a consensus on clinical diagnostic criteria. Neurology. 1998;51:1546–1554. doi: 10.1212/wnl.51.6.1546. [DOI] [PubMed] [Google Scholar]
- Perry R.J., Zeki S. The neurology of saccades and covert shifts in spatial attention: an event-related fMRI study. Brain. 2000;123(Pt 11):2273–2288. doi: 10.1093/brain/123.11.2273. [DOI] [PubMed] [Google Scholar]
- Pesenti M. Neuroanatomical substrates of arabic number processing, numerical comparison, and simple addition: a PET study. Journal of Cognitive Neuroscience. 2000;12:461–479. doi: 10.1162/089892900562273. [DOI] [PubMed] [Google Scholar]
- Peters E., Slovic P. The springs of action: affective and analytical information processing in choice. Personality and Social Psychology Bulletin. 2000;26:1465–1475. [Google Scholar]
- Peters F. Orbitofrontal dysfunction related to both apathy and disinhibition in frontotemporal dementia. Dementia and Geriatric Cognitive Disorders. 2006;21:373–379. doi: 10.1159/000091898. [DOI] [PubMed] [Google Scholar]
- Platt M.L., Glimcher P.W. Neural correlates of decision variables in parietal cortex. Nature. 1999;400:233–238. doi: 10.1038/22268. [DOI] [PubMed] [Google Scholar]
- Rangel A., Hare T. Neural computations associated with goal-directed choice. Current Opinion in Neurobiology. 2010;20:262–270. doi: 10.1016/j.conb.2010.03.001. [DOI] [PubMed] [Google Scholar]
- Rascovsky K. Diagnostic criteria for the behavioral variant of frontotemporal dementia (bvFTD): current limitations and future directions. Alzheimer Disease and Associated Disorders. 2007;21:S14–S18. doi: 10.1097/WAD.0b013e31815c3445. [DOI] [PubMed] [Google Scholar]
- Rascovsky K. Sensitivity of revised diagnostic criteria for the behavioural variant of frontotemporal dementia. Brain. 2011;134:2456–2477. doi: 10.1093/brain/awr179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ratnavalli E. The prevalence of frontotemporal dementia. Neurology. 2002;58:1615–1621. doi: 10.1212/wnl.58.11.1615. [DOI] [PubMed] [Google Scholar]
- Rueckert D. Nonrigid registration using free-form deformations: application to breast MR images. IEEE Transactions on Medical Imaging. 1999;18:712–721. doi: 10.1109/42.796284. [DOI] [PubMed] [Google Scholar]
- Rushworth M.F. Contrasting roles for cingulate and orbitofrontal cortex in decisions and social behaviour. Trends in Cognitive Sciences. 2007;11:168–176. doi: 10.1016/j.tics.2007.01.004. [DOI] [PubMed] [Google Scholar]
- Seeley W.W. Selective functional, regional, and neuronal vulnerability in frontotemporal dementia. Current Opinion in Neurology. 2008;21:701–707. doi: 10.1097/WCO.0b013e3283168e2d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sinz H. Impact of ambiguity and risk on decision making in mild Alzheimer's disease. Neuropsychologia. 2008;46:2043–2055. doi: 10.1016/j.neuropsychologia.2008.02.002. [DOI] [PubMed] [Google Scholar]
- Smith S.M., Nichols T.E. Threshold-free cluster enhancement: addressing problems of smoothing, threshold dependence and localisation in cluster inference. NeuroImage. 2009;44:83–98. doi: 10.1016/j.neuroimage.2008.03.061. [DOI] [PubMed] [Google Scholar]
- Smith S.M. Advances in functional and structural MR image analysis and implementation as FSL. NeuroImage. 2004;23(Suppl. 1):S208–S219. doi: 10.1016/j.neuroimage.2004.07.051. [DOI] [PubMed] [Google Scholar]
- Sohn M.H. The role of prefrontal cortex and posterior parietal cortex in task switching. Proceedings of the National Academy of Sciences of the United States of America. 2000;97:13448–13453. doi: 10.1073/pnas.240460497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sul J.H. Distinct roles of rodent orbitofrontal and medial prefrontal cortex in decision making. Neuron. 2010;66:449–460. doi: 10.1016/j.neuron.2010.03.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Torralva T. Impairments of social cognition and decision making in Alzheimer's disease. International Psychogeriatrics. 2000;12:359–368. doi: 10.1017/s1041610200006463. [DOI] [PubMed] [Google Scholar]
- Torralva T. The relationship between affective decision-making and theory of mind in the frontal variant of fronto-temporal dementia. Neuropsychologia. 2007;45:342–349. doi: 10.1016/j.neuropsychologia.2006.05.031. [DOI] [PubMed] [Google Scholar]
- Wilder K.E., Weinberger D.R., Goldberg T.E. Operant conditioning and the orbitofrontal cortex in schizophrenic patients: unexpected evidence for intact functioning. Schizophrenia Research. 1998;30:169–174. doi: 10.1016/s0920-9964(97)00135-7. [DOI] [PubMed] [Google Scholar]
- Wojciulik E., Kanwisher N. The generality of parietal involvement in visual attention. Neuron. 1999;23:747–764. doi: 10.1016/s0896-6273(01)80033-7. [DOI] [PubMed] [Google Scholar]
- Zamboni G. Apathy and disinhibition in frontotemporal dementia: insights into their neural correlates. Neurology. 2008;71:736–742. doi: 10.1212/01.wnl.0000324920.96835.95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Y., Brady M., Smith S. Segmentation of brain MR images through a hidden Markov random field model and the expectation-maximization algorithm. IEEE Transactions on Medical Imaging. 2001;20:45–57. doi: 10.1109/42.906424. [DOI] [PubMed] [Google Scholar]


