Abstract
Background:
Our previous study revealed that proline-rich tyrosine kinase 2 (Pyk2) is implicated in both anchorage-independent growth and anoikis resistance in lung cancer cells. This study aims to explore the expression and clinical significance of Pyk2 and its phosphorylated forms in non-small-cell lung cancer (NSCLC).
Methods:
The mRNA and protein levels of Pyk2 or cancer stem cell markers (ALDH1a1, ABCG2 and Bmi-1) were either examined by reverse transcription–PCR or western blotting. An immunohistochemistry (IHC) assay was conducted to analyse the expression of Pyk2 and its phosphorylated forms in 128 NSCLC cases.
Results:
The levels of Pyk2 mRNA, total protein, and its phosphorylated form pY881 were higher in lung cancer lesions than in the paired noncancerous tissues. The IHC analysis showed the levels of the Pyk2 and Pyk2[pY881] proteins were highly expressed in 70 (54.7%) and 77 (60.2%) cases, respectively. Both Pyk2 and Pyk2[pY881] were independent prognostic factors for NSCLC patients. The gain and loss study of Pyk2 function revealed that Pyk2 could upregulate the expression of ALDH1a1, ABCG2 and Bmi-1 and enhance the ability of colony formation in soft agar assay in A549 and H460 cells.
Conclusion:
Both Pyk2 and phosphorylated Pyk2[pY881] are potential prognostic factors and therapeutic targets for NSCLC.
Keywords: cancer stem cell, immunohistochemistry, non-small-cell lung cancer, phosphorylated Pyk2, prognosis, Pyk2
Lung cancer remains the most common type of cancer and the leading cause of cancer deaths worldwide, representing ∼29% and 26% of all male and female cancer deaths, respectively, in 2012 (Siegel et al, 2012). Non-small-cell lung cancer (NSCLC), including adenocarcinoma (AC), squamous cell carcinoma (SCC) and large cell carcinoma (LCC), is the most common subtype, representing ∼80% of all lung cancers (Petersen and Petersen, 2001; Collins et al, 2007 ). Despite the existence of various established tumour biomarkers for early detection and new treatment modalities that include targeted therapy and immunotherapy to improve patients' therapeutic response and prognosis, the overall 5-year survival rate for NSCLC remains poor, still <15% (Siegel et al, 2012). Therefore, it is crucial to understand the underlying molecular and cellular mechanisms mediating NSCLC progression and the process of metastasis, find effective tumour biomarkers for early diagnosis and provide potential therapeutic targets for improving the current treatment efficacy and overall survival of NSCLC patients.
Proline-rich tyrosine kinase 2 (Pyk2), also known as calcium-dependent tyrosine kinase (CADTK), is a nonreceptor tyrosine kinase belonging to the FAK (focal adhesion kinase) family. The Pyk2 contains several tyrosine residues, including the major autophosphorylation site (Y402), which acts as the binding site for the SH2 domains of Src and Fyn, and the putative binding site for the SH2 domain of Grb2 (Y881) (Xiong et al, 1998). The Pyk2 is demonstrated to be involved in several cellular functions, such as cell adhesion, motility, proliferation, apoptosis and the cell cycle (Guo et al, 1998; Blaukat et al, 1999; Avraham et al, 2000; Gelman, 2003). Although one report showed that Pyk2 was expressed primarily in normal epithelial prostate tissue and benign prostatic hyperplasia but seldom in prostate cancer, acting as a tumour-suppressor gene (Stanzione et al, 2001), other reports have shown that Pyk2 is highly expressed in neuroglioma, breast cancer and hepatocellular carcinoma and has prognostic significance in hepatocellular carcinoma (Lipinski et al, 2005; Sun et al, 2007; Behmoaram et al, 2008). Our previous study has shown that Pyk2 was implicated in anchorage-independent growth and anoikis resistance in lung cancer cell lines (Zhang et al, 2010), and another study also demonstrated that Pyk2 was upregulated in NSCLC and that it modulated the activity of ERK1/2 to promote the progression of NSCLC (Zhang et al, 2008). Therefore, Pyk2 may play an important role in the development of lung cancer. However, the prognostic effect of Pyk2 expression, especially the phosphorylated forms of Pyk2 expression, in NSCLC and the molecular mechanism underlying this effect are still not fully understood.
The cancer stem cell (CSC) hypothesis suggests that a small proportion of the cells in a tumour mass, which has an indefinite proliferation and differentiation potential and give rise to phenotypically diverse cancer cells and neoplastic clones, contribute to tumour initiation, metastasis and recurrence. The limited subpopulation of CSCs in the mass of tumour cells and their unique phenotypes are considered to account for their resistance to conventional cancer therapies, resulting in tumour recurrence (Reya et al, 2001; Pardal et al, 2003). Several CSC markers, such as CD133, ABCG2, ALDH1a1, Bmi-1, SOX2, Nanog and OCT-4, have been widely used to identify the limited numbers of CSCs in lung carcinoma (Eramo et al, 2008; Bertolini et al, 2009; Jiang et al, 2009; Chiou et al, 2010; Zhang et al, 2010; Xiang et al, 2011). As a previous study showed that Pyk2 had a compensatory function in promoting FAK-null mammary CSC tumourigenicity and metastatic activity (Fan and Guan, 2011), we are interested in investigating whether Pyk2 might affect the CSC characteristics or other cellular functions of lung cancer cells.
In this study, we investigated the expression levels of Pyk2 and its two phosphorylated forms (pY402 and pY881) in primary NSCLC lesions, metastatic lymph nodes and brain lesions, and showed the positive relationship between high expression levels of Pyk2 and Pyk2[pY881] and the clinical outcomes of NSCLC using a statistical analysis. We also found for the first time that Pyk2 could upregulate the expression of ALDH1a1, ABCG2 and Bmi-1 and enhance the ability of colony formation in soft agar assay in A549 and H460 cells. Our data suggest that Pyk2 and Pyk2[pY881] may be potential prognostic factors and new therapeutic targets for the effective treatment of NSCLC.
Materials and methods
Antibodies
Mouse monoclonal antibody against ABCG2 (Cat. no. 3380) and rabbit antibodies against ALDH1a1 (Cat. no. 52492) were purchased from Abcam (Cambridge, UK). Mouse monoclonal antibody against GAPDH (sc-81545) was obtained from Santa Cruz Biotechnology (Santa Cruz, CA, USA). Mouse anti-Bmi-1 antibody (Upstate Biotechnology, Lake Placid, NY, USA), FITC, peroxidase-conjugated goat anti-rabbit IgG, goat anti-mouse IgG (Amersham Pharmacia Biotech, Piscataway, NJ, USA), rhodamine-conjugated goat anti-rabbit IgG and goat anti-mouse IgG (Jackson Laboratory, West Grove, PA, USA) secondary antibodies were used for western blotting or immunofluorescence analyses.
Rabbit polyclonal antibodies against phosphor-Pyk2[pY402] and phosphor-Pyk2[pY881] from Invitrogen (Camarillo, CA, USA) were used for the immunohistochemistry (IHC) analysis and western blotting. Rabbit polyclonal antibodies against Pyk2 from Invitrogen (Camarillo, CA, USA) were used for the IHC analysis, while rabbit polyclonal antibody against Pyk2 (Cat. no. 32571) from Abcam (Cambridge, UK) was used for western blotting and immunofluorescence analyses.
Patients and tissue samples
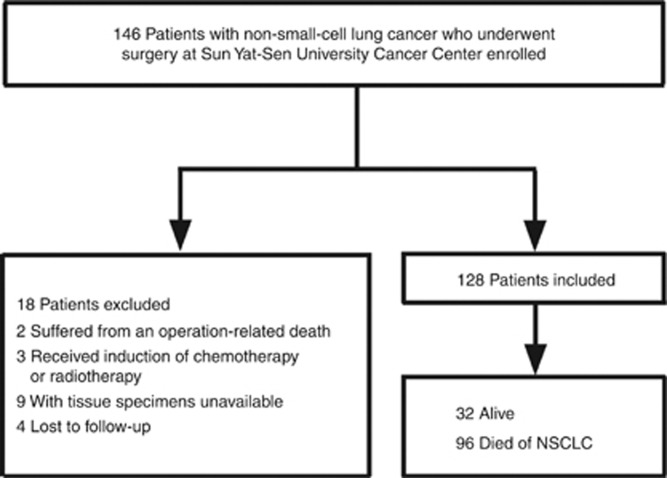
We analysed 146 consecutive patients with NSCLC who underwent surgery at Sun Yat-Sen University Cancer Center (Guangzhou, China) between June 2006 and September 2008. This study is reported according to the Reporting Recommendations for Tumor Marker Prognostic Studies (REMARK) (McShane et al, 2005). The inclusion and exclusion criteria of the study are shown in Figure 1. Briefly, two patient who suffered from an operation-related death and three patients who received induction of chemotherapy or radiation therapy were excluded. Four patients were lost to follow-up and specimens of nine patients were not available. A total of 128 patients (84 men and 44 women) were evaluated.
Figure 1.
Flowchart of this study.
The age of the patients ranged from 33 to 82 years, and the mean age at the time of surgery was 59.4 years. Postsurgical pathologic stages of all the NSCLC patients were determined based on the seventh edition of the Union for International Cancer Control (UICC) Staging System for Lung Cancer. Histologically, 37 had squamous carcinoma and 91 patients had adenocarcinoma. Of the total 128 patients, 39, 34, 30 and 25 had stage I, II, III and IV tumours, respectively. The postsurgical survival time was calculated from the date of surgery to the time of cancer-related death or the most recent follow-up if the patient was alive. The median follow-up interval was 37.0 months (range: 3–70 months). The 5-year cumulative survival rate for all patients was 16%.
Tissue samples that had been histologically and clinically proofed were obtained from the archives of the Department of Sample Resources, Sun Yat-sen University Cancer Center. There were 15 paired freshly frozen lung carcinoma tissues and noncancerous tissues adjacent to cancer lesions from 15 NSCLC patients; 10 paraffin-embedded, noncancerous human lung tissues; and 128 paraffin-embedded NSCLC tissue samples. Consent was obtained from the patients, and the Institute Research Ethics Committee of Sun Yat-Sen University Cancer Center approved the clinical samples for research purposes.
Real-time RT–PCR analysis
The expression level of Pyk2 mRNA was detected using SYBR Green real-time reverse transcription–PCR (RT–PCR) (Invitrogen, Carlsbad, CA, USA). The total RNA was extracted from the paired carcinoma and noncancerous tissues of 15 NSCLC patients using TRIzol reagent (Life Technologies, Grand Island, NY, USA). Reactions were performed in triplicate in three independent experiments. Real-time PCR was conducted with a CFX connect Real-Time PCR Detection System (185-5200) (Invitrogen, Carlsbad, CA, USA). The housekeeping gene GAPDH was used as an internal control to normalise the variable expression levels of Pyk2. The sequences of the primers sequences are listed in Supplementary Table 1.
Western blotting
Western blotting analysis was performed as previously described (Zhang et al, 2010). The membrane was incubated at 4 °C overnight with primary antibodies (as labelled in the figures) followed by incubation with horseradish peroxidase-conjugated goat anti-mouse or anti-rabbit IgG secondary antibody (1 : 3000) at room temperature for 1 h. The membranes were probed with mouse anti-GAPDH monoclonal antibody (1 : 4000) to confirm the equal loading of the samples. The signals were detected by enhanced chemiluminescence (ECL; Amersham Pharmacia Biotech).
Immunohistochemistry
Immunohistochemistry was performed as previously described (Liao et al, 2007). Where relevant, sections were incubated overnight at 4 °C with rabbit anti-Pyk2 (1 : 200; Invitrogen, Carlsbad, CA, USA), rabbit anti-Pyk2[pY402] (1 : 100; Invitrogen, Carlsbad, CA, USA) or rabbit anti-Pyk2[pY881] (1 : 50; Invitrogen, Carlsbad, CA, USA). The degree of the immunostaining of the paraffin-embedded sections was evaluated and scored independently by two pathologists. The intensity of staining and the proportion of positively stained tumour cells were used as the criteria of evaluation. The tumour cell proportion was scored as follows: 0 (no positive tumour cells), 1 (⩽30% positive tumour cells), 2 (31–50% positive tumour cells), 3 (51–75% positive tumour cells) and 4 (⩾76% positive tumour cells). Staining intensity was graded according to the following criteria: 0 (no staining), 1 (weak staining, light yellow), 2 (moderate staining, yellowish brown) and 3 (intense staining, brown). The staining index was calculated by multiplying the above two scores to yield a final score of 0, 1, 2, 3, 4, 6, 9 or 12. The tumours were finally determined to be of low expression (score ⩽3) or high expression (score ⩾4).
RT–PCR and plasmid construction
The total RNA extracts from the normal lung cell lines were prepared using TRIzol reagent (Life Technologies) according to the manufacturer's instructions. The RNA was then treated with RNA-free DNase, and 2.5 μg total RNA was used for cDNA synthesis with random hexamers. The primers used for the amplification of Pyk2 are listed in Supplementary Table 1.
The full length of homo Pyk2 was subcloned into the vector pBabe. The pSuper-retro-constructs containing the Pyk2 short hairpin RNA (shRNA) were created by cloning the following 19-nt Pyk2-specific RNAi target sequences into a pSuper-retro construct: Pyk2 shRNA 1: 5′-GCTTCTATAGCAACAGCTT-3′ Pyk2 shRNA 2: 5′-GGTCCTGAATCGTATTCTT-3′.
Cell culture and establishment of Pyk2 stably overexpressing and knockdown cell lines
Two poorly differentiated lung cancer cell lines, human lung adenocarcinoma cells (A549) and large cell human lung carcinoma cells (NCI-H460), were obtained from the American Type Cell Culture Collection (ATCC, Rockville, MD, USA). The cells were cultured in RPMI-1640 medium (Life Technologies, Carlsbad, CA, USA) supplemented with 10% fetal bovine serum (FBS), 100 IU ml−1 streptomycin and 100 μg ml−1 penicillin in a humidified 5% CO2 incubator at 37 °C.
To establish stable cell lines, recombinant retroviruses expressing the vector pBabe, pBabe subcloned with Pyk2, pRETRO-SUPER and pRETRO-SUPER subcloned with Pyk2 shRNA 1 or Pyk2 shRNA 2 were generated and used to infect A549 and H460 cells as previously described (Brummelkamp et al, 2002; Kong et al, 2010; Zhang et al, 2010). The A549 and H460 cell populations expressing the desired plasmids were selected with 2 μg ml−1 of puromycin (Sigma-Aldrich, St Louis, MO, USA) for 2 days. The successful overexpression and knockdown of Pyk2 were verified by western blotting.
Immunofluorescence analysis
Cells were plated for immunofluorescence on coverslips as described previously (Song et al, 2006). Briefly, the cells were incubated overnight at 4 °C with primary antibodies against Pyk2, ABCG2, ALDH1a1 or Bmi-1 and then incubated in the dark for 30 min at room temperature with secondary goat antibodies against rabbit or mouse IgG (Invitrogen, Carlsbad, CA, USA). The coverslips were counterstained with DAPI and examined using an Olympus confocal imaging system (Olympus FV100, Olympus, Japan).
Anchorage-independent growth assay
Six-well plates were covered with a layer of 0.5% agar in medium supplemented with 20% FBS. Cells were prepared in 0.33% agar and seeded in triplicate, with a total number of 5 × 103 cells in each well. The plates were incubated at 37 °C in a humid atmosphere of 5% CO2 for 2 weeks with medium added every 3 days. At least three independent experiments were performed. Colonies were photographed between 10 and 14 days at an original magnification of × 40 under a phase contrast microscope to count the colonies >50 μm in diameter.
Statistical analysis
The SPSS 16.0 statistical software package (SPSS Inc., Chicago, IL, USA) was used for all statistical analyses. The differences among variables were evaluated by χ2 analysis or two-tailed Student's t-tests. The data were all presented as the mean±s.e.m. unless otherwise indicated. The χ2 analysis and Fisher's exact tests were performed to analyse the correlations between Pyk2, Pyk2[pY881] expression and clinicopathologic characteristics. Bivariate correlations between the study's variables were evaluated using Spearman's rank correlation coefficients.
The postsurgical survival time was calculated from the date of surgery to the time of cancer-related death or the most recent follow-up if the patient was alive. Survival curves were plotted using the Kaplan–Meier method and compared using the log-rank test to determine significance. The survival data of the prognostic factors were evaluated by univariate and multivariate Cox regression models. The P-values of ⩽0.05 in all cases were considered to indicate a statistically significant result.
Results
Expression of Pyk2, Pyk2[pY402] and Pyk2[pY881] was upregulated in NSCLC tissues by real-time RT–PCR and western blotting analysis
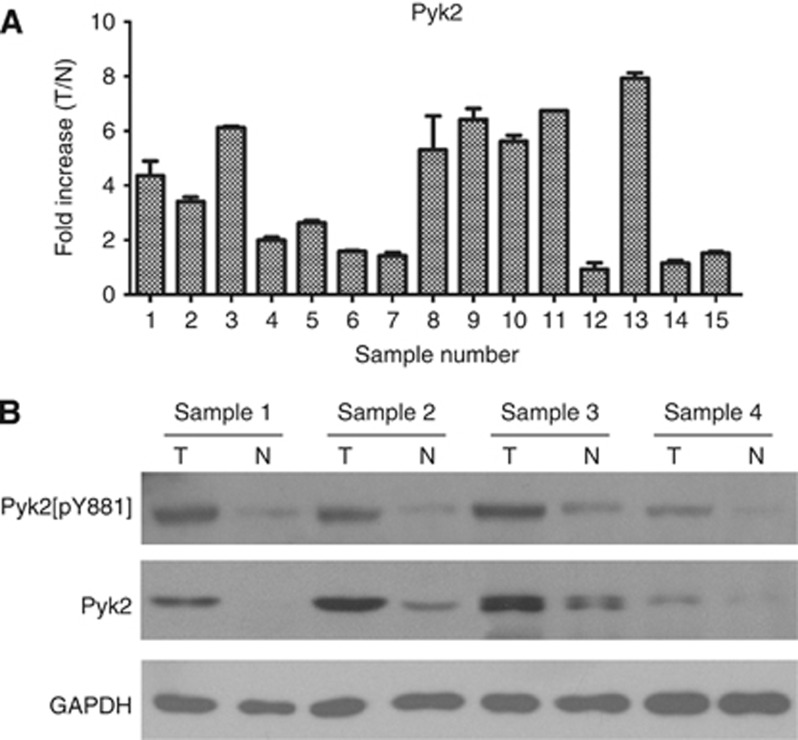
To determine the mRNA and protein expression levels of Pyk2 in NSCLC lesions, real-time RT–PCR analysis and western blotting analysis were conducted in paired fresh frozen NSCLC tissues and adjacent noncancerous tissues from the same patient. Both the mRNA and protein levels of Pyk2 were found to be differentially overexpressed between the human NSCLC samples and the paired normal tissues adjacent to the tumours (Figure 2A and B). The protein levels of phosphorylated Pyk2 (pY402 and pY881) were also upregulated in cancerous tissue compared with noncancerous tissue using western blotting analysis (Figure 2B and Supplementary Figure 1A); however, only weak pY402 expression was observed in NSCLC tissues.
Figure 2.
Expression of Pyk2 and Pyk2[pY881] is elevated in fresh frozen primary NSCLC tissues compared with noncancerous tissues adjacent to cancer lesions. (A) Relative expression of Pyk2 mRNA in paired fresh frozen primary NSCLC tissues (T) and noncancerous tissues (N) from the same patient by real-time RT–PCR analysis. As an internal control, GAPDH was used. Reactions were performed in triplicate in three independent experiments. (B) Expression of Pyk2 and Pyk2[pY881] protein in paired fresh frozen primary NSCLC tissues (T) and noncancerous tissues (N) from the same patient by western blotting analysis. As an internal reference, GAPDH was used.
Overexpression of Pyk2 and Pyk2[pY881] in NSCLC tissue samples by immunohistochemistry
To further examine whether Pyk2, Pyk2[pY402] and Pyk2[pY881] protein upregulation are linked to the clinical outcomes of NSCLC, the following samples were applied to immunohistochemical staining: 10 archived noncancerous human lung tissue samples; 128 paraffin-embedded, archived NSCLC tissues; 47 cases of matched metastatic mediastinal or supraclavicular lymph nodes from NSCLC patients; and 13 cases of matched brain metastases derived from late-stage NSCLC patients. The immunohistochemical results are summarised in Table 1.
Table 1. Correlation between Pyk2, Pyk2[pY881] expression and clinicopathologic characteristics of NSCLC patients.
| Pyk2 expression: no. of patients (%) | Pyk2[pY881] expression: no. of patients (%) | |||||
|---|---|---|---|---|---|---|
|
Characteristics |
Lowa(58) |
Higha
(70) |
P-valueb |
Lowc
(51) |
Highc
(77) |
P-valueb |
|
Sex | ||||||
| Male | 38 (45.2) | 46 (54.8) | 0.981 | 30 (35.7) | 54 (64.3) | 0.187 |
| Female |
20 (45.5) |
24 (54.5) |
|
21 (47.7) |
23 (52.3) |
|
|
Age, years | ||||||
| ≦60 | 29 (42.6) | 39 (57.4) | 0.519 | 26 (38.2) | 42 (61.8) | 0.692 |
| >60 |
29 (48.3) |
31 (51.7) |
|
25 (41.7) |
35 (58.3) |
|
|
Smoking status | ||||||
| No | 29 (47.5) | 32 (52.5) | 0.629 | 28 (45.9) | 33 (54.1) | 0.182 |
| Yes |
29 (43.3) |
38 (56.7) |
|
23 (34.3) |
44 (65.7) |
|
|
CEAd | ||||||
| − | 30 (49.2) | 31 (50.8) | 0.381 | 28 (45.9) | 33 (54.1) | 0.102 |
| + |
20 (40.8) |
29 (59.2) |
|
15 (30.6) |
34 (69.4) |
|
|
NSEe | ||||||
| − | 27 (45.8) | 32 (54.2) | 0.376 | 25 (42.4) | 34 (57.6) | 0.361 |
| + |
10 (35.7) |
18 (64.3) |
|
9 (32.1) |
19 (67.9) |
|
|
EGFRf | ||||||
| − | 2 (14.3) | 12 (85.7) | 0.201 | 8 (57.1) | 6 (42.9) | 0.288 |
| + |
14 (37.8) |
23 (62.2) |
|
15 (40.5) |
22 (42.9) |
|
|
Histology | ||||||
| Adenocarcinoma | 43 (47.3) | 48 (52.7) | 0.489 | 33 (36.3) | 58 (63.7) | 0.194 |
| Squamous carcinoma |
15 (40.5) |
22 (59.5) |
|
18 (48.6) |
19 (51.4) |
|
|
Differentiation | ||||||
| Well–moderate | 31 (50.0) | 31 (50.0) | 0.302 | 33 (53.2) | 29 (46.8) | 0.003 |
| Poor |
27 (40.9) |
39 (59.1) |
|
18 (27.3) |
48 (72.7) |
|
|
T stageg | ||||||
| T1 | 17 (53.1) | 15 (46.9) | 0.236 | 19 (59.4) | 13 (40.6) | 0.033 |
| T2 | 34 (46.6) | 39 (53.4) | 24 (32.9) | 49 (67.1) | ||
| T3–T4 |
7 (30.4) |
16 (69.6) |
|
8 (34.8) |
15 (65.2) |
|
|
N stageg | ||||||
| N0 | 36 (55.4) | 29 (44.6) | 0.020 | 34 (52.3) | 31 (47.7) | 0.003 |
| N1–N3 |
22 (34.9) |
41 (65.1) |
|
17 (27.0) |
46 (73.0) |
|
|
M stageg | ||||||
| M0 | 55 (53.4) | 48 (46.6) | <0.001 | 47 (45.6) | 56 (54.7) | 0.007 |
| M1 |
3 (12.0) |
22 (88.0) |
|
4 (16.0) |
21 (84.0) |
|
|
Clinical stageg | ||||||
| I | 24 (61.5) | 15 (38.5) | 0.001 | 24 (61.5) | 15 (38.5) | 0.003 |
| II | 19 (55.9) | 15 (44.1) | 12 (35.3) | 22 (64.7) | ||
| III | 12 (40.0) | 18 (60.0) | 11 (36.7) | 19 (63.3) | ||
| IV | 3 (12.0) | 22 (88.0) | 4 (16.0) | 21 (84.0) | ||
Abbreviations: CEA=carcinoembryonic antigen; EGFR=epidermal growth factor receptor; NSCLC=non-small-cell lung cancer; NSE=neuron-specific enolase; Pyk2=proline-rich tyrosine kinase 2.
Pyk2 low expression: score ⩽3; high expression: score ⩾4.
Two-sided P-values were calculated by Pearson's χ2 test or continuity correction to evaluate the significance of correlations. Bold print indicates statistical significance.
Pyk2[pY881] low expression: score ⩽3; high expression: score ⩾4.
CEA: −, normal (0–5 ng ml−1); +, elevated (>5 ng ml−1); 18 patients' CEA unknown.
NSE: −, normal (0–15.2 ng ml−1); +, elevated (>15.2 ng ml−1); 41 patients' NSE unknown.
EGFR: −, no EGFR mutation and amplification; +, EGFR mutation or amplification; 77 patients' EGFR unknown.
Tumour size, lymph node involvement, distant metastasis and clinical stage were classified or reclassified according to the seventh edition of the International Union Against Cancer (UICC) Staging system for Lung Cancer.
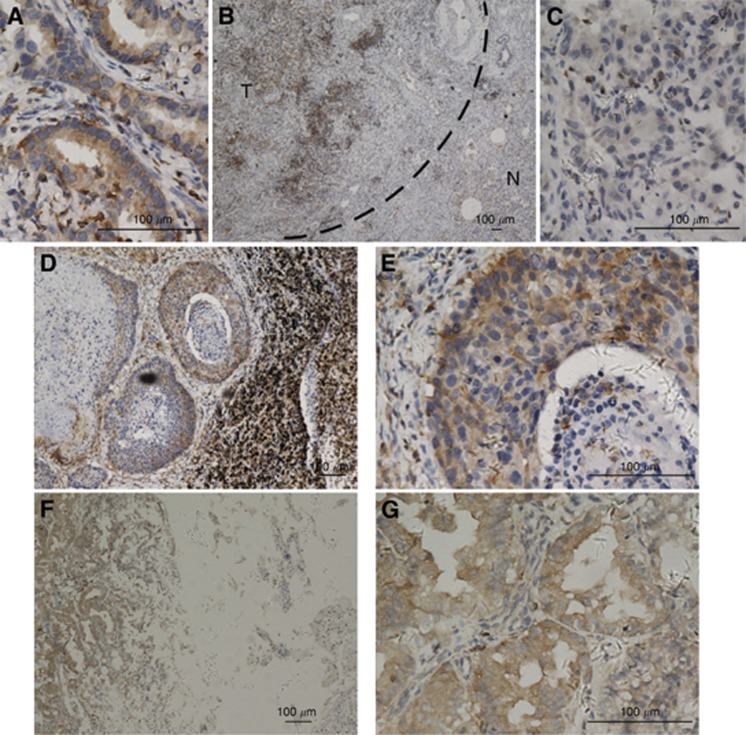
As shown in Figure 3, Pyk2 was found to be overexpressed in the NSCLC lesions, metastatic lymph nodes and brain lesions (Figure 3A, B, D–G). In contrast, Pyk2 was either undetectable or only marginally detectable in the noncancerous lung tissues of the adjacent section regions (Figure 3B and C). Pyk2 protein was detected in 124 of 128 (97%) human NSCLC cases, and strong cytoplasmic staining of Pyk2 protein was detected in 70 (54.7%) tumours (Table 1).
Figure 3.
Expression of Pyk2 in different tissues by immunohistochemical staining. (A) As can be observed, Pyk2 shows cytoplasmic staining in primary lung cancer lesions ( × 400). (B, C) Protein expression of Pyk2 is increased in primary lung cancer lesions compared with the adjacent noncancerous tissues (B × 40; C × 400). (D, E) The Pyk2 shows strong cytoplasmic staining in the corresponding metastatic lymph nodes of squamous carcinoma (D × 100; E × 400) and (F, G) metastatic brain lesions of adenocarcinoma (F × 100; G × 400).
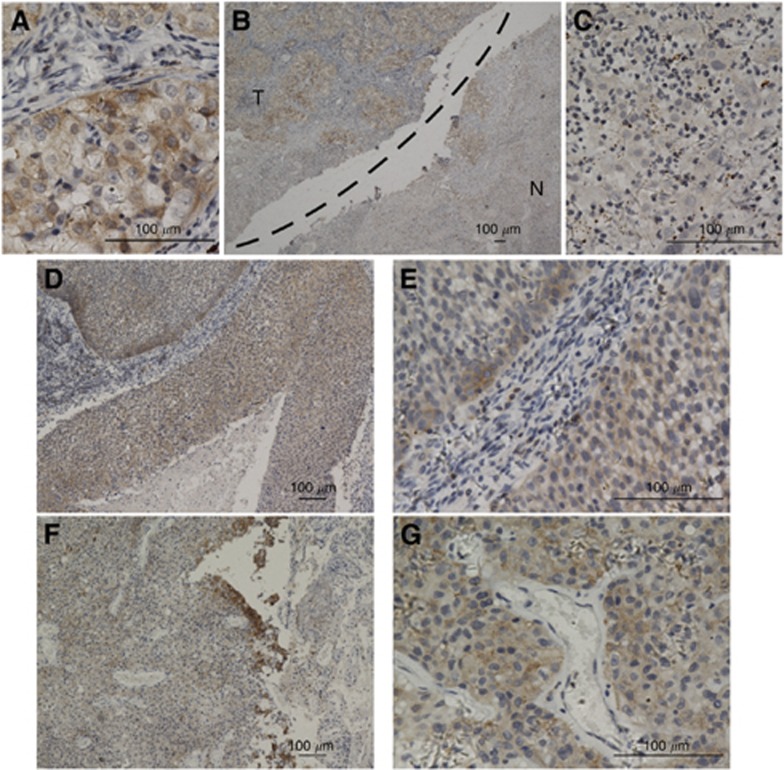
Similar to Pyk2, as shown in Figure 4, Pyk2[pY881] was found to be overexpressed in primary NSCLC lesions, metastatic lymph nodes and brain lesions (Figure 4A, B, D–G), whereas in the noncancerous lung tissues, Pyk2[pY881] was either undetectable or only marginally detectable (Figures 4B and C). Pyk2[pY881] protein, also primarily localised in the cytoplasm, was detected in 118 of the 128 (92%) human NSCLC cases. A high expression of Pyk2[pY881] protein was detected in 77 (60.2%) tumours (Table 1).
Figure 4.
The Pyk2[pY881] staining in different tissues by immunohistochemical staining. (A) As can be observed, Pyk2[pY881] shows cytoplasmic staining in primary lung carcinoma lesions ( × 400). (B, C) Protein expression of Pyk2[pY881] is much higher in primary lung cancer lesions than in the adjacent noncancerous tissues (B × 40; C × 400). (D, E) The Pyk2[pY881] shows strong cytoplasmic staining in the corresponding metastatic lymph nodes of squamous carcinoma (D × 100; E × 400) and (F, G) metastatic brain lesions of adenocarcinoma (F × 100; G × 400).
Pyk2[pY402] protein, however, was either undetectable or only marginally detectable in the human primary NSCLC lesions and metastatic lesions (Supplementary Figure 1B).
As a whole, these observations suggest that high levels of both Pyk2 and phosphorylated Pyk2[pY881] protein expression, but not Pyk2[pY402] protein expression, may play a role in the clinical development and progression of NSCLC.
Relationship between the clinical data and the Pyk2 and Pyk2[pY881] expression levels
The immunohistochemical staining results of the Pyk2 and Pyk2[pY881] expression levels were statistically analysed to determine their relationships with the clinical features of the NSCLC patients. As shown in Table 1, Pyk2 expression was strongly correlated with the N stage (P=0.020), M stage (P<0.001) and clinical stage (P=0.001) of NSCLC patients, whereas it was not associated with age, gender, smoking status, carcinoembryonic antigen (CEA), neuron-specific enolase (NSE), epidermal growth factor receptor (EGFR) (mutation or amplification), histology, differentiation (P=0.302) or T stage (P=0.236). Phosphorylated Pyk2[pY881] protein expression was strongly correlated with the differentiation (P=0.003), T stage (P=0.033), N stage (P=0.003), M stage (P=0.007) and clinical stage (P=0.003) of the NSCLC patients.
Spearman's correlation analysis was further used to confirm the correlation between the Pyk2 and Pyk2[pY881] protein expression and clinicopathologic features. As shown in Supplementary Table 2, the Spearman correlations of the Pyk2 expression level to the N stage, M stage and clinical stage were 0.231 (P=0.009), 0.343 (P<0.001), and 0.342 (P<0.001), respectively, whereas correlations of the Pyk2[pY881] expression level to the differentiation, T stage, N stage, M stage and clinical stage were 0.298 (P=0.001), 0.166 (P=0.041), 0.250 (P=0.004), 0.252 (P=0.004) and 0.310 (P<0.001), respectively.
Taken together, our data revealed that the Pyk2 protein expression level increases concurrently with NSCLC clinical metastasis, although it may not increase in the early tumour development stage. The expression of Pyk2[pY881] not only increases throughout the NSCLC progression and metastasis stages but is also associated with poor differentiation in the cases included in the present study.
Comparison of the Pyk2 and Pyk2[pY881] expression levels in primary NSCLC lesions and metastatic lymph nodes
The expression levels of Pyk2 and Pyk2[pY881] in the matched metastatic lymph nodes of 47 NSCLC patients were also evaluated, and the expression levels between the primary NSCLC lesions and metastatic LNs were compared. As shown in Supplementary Table 3, there was a statistically significant difference between the two lesion sites for the expression level of Pyk2[pY881] (P=0.028), with a higher Pyk2[pY881] expression level in the metastatic LNs than in the primary lesions, whereas no statistically significant differences were found for Pyk2 expression. This result may imply that Pyk2[pY881] is a more sensitive marker in metastasised lesions than in primary lesions.
Pyk2 and Pyk2[pY881] are associated with a poor prognosis
After revealing the correlation of Pyk2 and Pyk2[pY881] expression with clinicopathologic characteristics, we intended to investigate the relationship between these two protein expression levels and patients' survival. The effects of clinicopathologic characteristics, including sex, age, smoking status, histology, differentiation, T stage, N stage, M stage and clinical stage, in conjunction with these two protein expression levels, on patient survival, were evaluated using Kaplan–Meier analysis and log-rank tests.
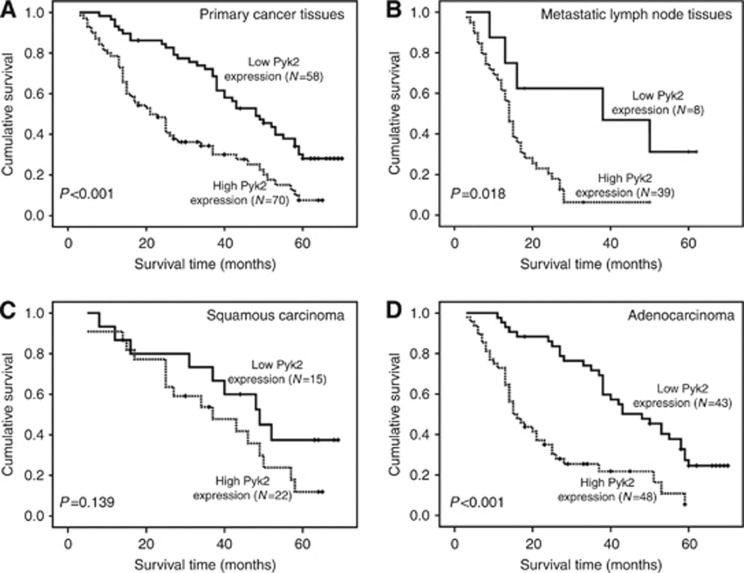
As shown in Figure 5A, the length of survival time showed a statistically significant difference between the patients with high expression levels and the patients with low expression levels of Pyk2 (P<0.001), with the low Pyk2 expression group having a better survival. The cumulative 5-year survival rate was 26.0% in the low Pyk2 expression group, whereas it was only 6.0% in the high Pyk2 expression group. In light of the potential involvement of Pyk2 in the advanced NSCLC progression stage, we further analysed the patients' survival in terms of Pyk2 expression in the 47 matched metastatic lymph node tissues. As shown in Figure 5B, examination of the metastatic lymph nodes tissues revealed that patients with a high Pyk2 expression had a poor overall survival compared with patients expressing low Pyk2 (P=0.018). Furthermore, Pyk2 expression levels were still strongly correlated with patients' survival even after stratifying the patients based on their clinicopathologic features (Figure 5C and D and Supplementary Figure 2). Moreover, patients with a high Pyk2 expression had poorer overall survival regardless of tumour differentiation, T stage and N stage. In addition, the overall survival showed a statistically significant difference between the high and low Pyk2 expression groups in the adenocarcinoma, M0 stage and late clinical stage (III+IV) subgroups.
Figure 5.
Influence of Pyk2 expression on overall survival of NSCLC patients. (A) Overall survival curves show that patients with high Pyk2 expression have short survival times by analysing 128 primary NSCLC tissues (P<0.001). (B) Patients with high Pyk2 expression show poor overall survival based on an analysis of 47 matched metastatic lymph node tissues (P=0.018). (C, D) In the squamous carcinoma subtype, the patients' overall survival demonstrates no statistically significant difference between the high and low Pyk2 expression groups (P=0.139). In the adenocarcinoma subtype, patients with high Pyk2 expression show short overall survival times (P<0.001). The Pyk2 low expression: score ⩽3; high expression: score ⩾4.
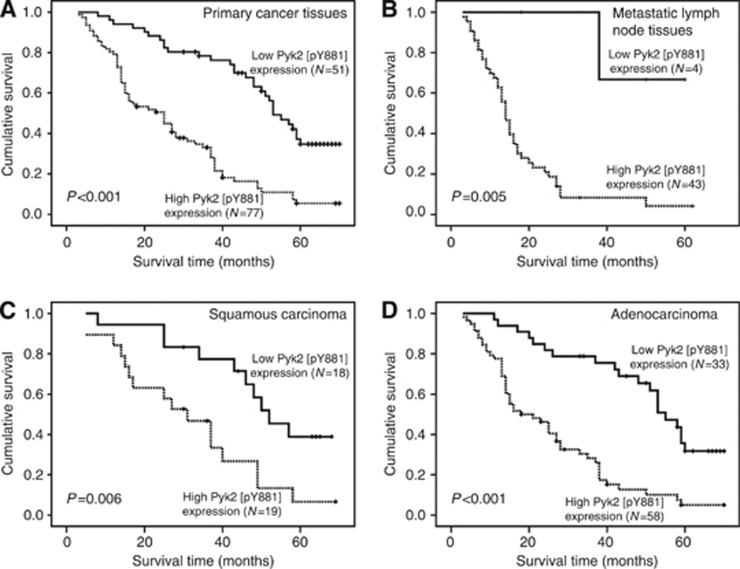
In Figure 6A, the length of the survival time was significantly different between patients with high and low expression levels of Pyk2[pY881] (P<0.001), with the high Pyk2[pY881] expression group having worse survival. The cumulative 5-year survival rate was 32.0% in the low Pyk2[pY881] expression group, whereas it was only 5.0% in the high Pyk2[pY881] expression group. Furthermore, the patients' survival in terms of the Pyk2[pY881] expression in the 47 corresponding metastatic lymph node tissues was analysed. As shown in Figure 6B, an examination of the metastatic lymph nodes tissues revealed that patients with high Pyk2[pY881] expression had poor overall survival compared with patients expressing low Pyk2[pY881] (P=0.005). In addition, the Pyk2[pY881] expression levels were still strongly correlated with patients' survival even after stratifying the patients based on their clinicopathologic features (Figure 6C and D and Supplementary Figure 3). In particular, patients with high Pyk2[pY881] expression have poorer overall survival regardless of tumour histology, differentiation, T, N and M stage and clinical stage. Moreover, the overall survival showed a statistically significant difference between the high and low Pyk2[pY881] expression groups in the EGFR wild-type subgroup.
Figure 6.
Impact of Pyk2[pY881] expression on overall survival of NSCLC patients. (A) Overall survival curves show that patients with high Pyk2[pY881] expression have short survival time by analysing 128 primary NSCLC tissues (P<0.001). (B) Patients with high Pyk2[pY881] expression show poor overall survival by analysing 47 matched metastatic lymph node tissues (P=0.005). (C, D) In the squamous carcinoma subtype, patients with high Pyk2[pY881] expression have poor overall survival (P=0.006). In the adenocarcinoma subtype, patients with high Pyk2[pY881] expression show short overall survival time (P<0.001). The Pyk2[pY881] low expression: score ⩽3; high expression: score ⩾4.
Univariate and multivariate analyses were conducted to determine whether the Pyk2 and Pyk2[pY881] expression levels are independent prognostic factors of NSCLC patients' outcomes. Both the Pyk2 and Pyk2[pY881] expression levels in the primary NSCLC tissues, as well as M and clinical stage, were recognised as independent prognostic factors when the Pyk2 and Pyk2[pY881] expression levels were analysed apart (Table 2). The relative risks of Pyk2 and Pyk2[pY881] for the NSCLC patients' prognosis were 2.089 (95% CI: 1.346–3.241) and 3.086 (95% CI: 1.941–4.907), respectively. Taken together, our data suggest that Pyk2 and Pyk2[pY881] may represent novel and potentially useful biomarkers for the prognosis of NSCLC patients.
Table 2. Univariate and multivariate analyses of different prognostic parameters in 128 patients with NSCLCs.
| Univariate analysis | Multivariate analysisa | Multivariate analysisb | Multivariate analysisc | |||||
|---|---|---|---|---|---|---|---|---|
|
Variables |
HR (95% CI) |
P-value |
HR (95% CI) |
P-value |
HR (95% CI) |
P-value |
HR (95% CI) |
P-value |
|
Sex | ||||||||
| Male | 1.000 | |||||||
| Female |
0.990 (0.650–1.510) |
0.964 |
|
|
|
|
|
|
|
Age, years | ||||||||
| ≦60 | 1.000 | |||||||
| >60 |
0.831(0.555–1.243) |
0.367 |
|
|
|
|
|
|
|
Smoking status | ||||||||
| No | 1.000 | |||||||
| Yes |
0.945 (0.633–1.411) |
0.782 |
|
|
|
|
|
|
|
CEAd | ||||||||
| − | 1.000 | |||||||
|
+ |
0.951 (0.613–1.477) |
0.824 |
|
|
|
|
|
|
|
NSEe | ||||||||
| − | 1.000 | |||||||
|
+ |
1.267 (0.750–2.139) |
0.377 |
|
|
|
|
|
|
|
EGFRf | ||||||||
| − | 1.000 | |||||||
|
+ |
0.833 (0.420–1.650) |
0.600 |
|
|
|
|
|
|
|
Histology | ||||||||
| Adenocarcinoma | 1.000 | |||||||
| Squamous carcinoma |
0.747 (0.476–1.173) |
0.205 |
|
|
|
|
|
|
|
Differentiation | ||||||||
| Well–moderate | 1.000 | 1.000 | 1.000 | 1.000 | ||||
| Poor |
1.511 (1.009–2.261) |
0.045 |
1.455 (0.943–2.245) |
0. 090 |
1.068 (0.680–1.676) |
0.776 |
1.093 (0.705–1.693) |
0.691 |
|
T stage | ||||||||
| T1 | 1.000 | |||||||
| T2 | 1.067 (0.652–1.747) | 0.795 | ||||||
| T3–T4 |
1.130 (0.819–1.559) |
0.456 |
|
|
|
|
|
|
|
N stage | ||||||||
| N0 | 1.000 | 1.000 | 1.000 | 1.000 | ||||
| N1–N3 |
2.212 (1.473–3.320) |
<0.001 |
1.516 (0.938–2.448) |
0.089 |
1.494 (0.923–2.419) |
0.102 |
1.480 (0.908–2.412) |
0.116 |
|
M stage | ||||||||
| M0 | 1.000 | 1.000 | 1.000 | 1.000 | ||||
| M1 |
4.855 (2.928–8.051) |
<0.001 |
2.099 (1.162–3.793) |
0.014 |
1.832 (1.026–3.272) |
0.041 |
1.892 (1.054–3.399) |
0.033 |
|
Clinical stage | ||||||||
| I–II | 1.000 | 1.000 | 1.000 | 1.000 | ||||
| III–IV |
3.468 (2.283–5.269) |
<0.001 |
2.709 (1.630–4.502) |
<0.001 |
2.668 (1.591–4.473) |
<0.001 |
2.690 (1.617–4.474) |
<0.001 |
|
Pyk2 expression | ||||||||
| Lowg | 1.000 | 1.000 | 1.000 | |||||
| Highg |
2.423 (1.594–3.685) |
<0.001 |
2.089 (1.346–3.241) |
0.001 |
|
|
1.480 (0.908–2.412) |
0.116 |
|
Pyk2[pY881] expression | ||||||||
| Lowh | 1.000 | 1.000 | 1.000 | |||||
| Highh | 3.505 (2.231–5.505) | <0.001 | 3.086 (1.941–4.907) | <0.001 | 3.223 (2.017–5.150) | <0.001 | ||
Abbreviations: CEA=carcinoembryonic antigen; CI=confidence interval; EGFR=epidermal growth factor receptor; HR=hazard ratio; NSCLC=non-small-cell lung cancer; NSE=neuron-specific enolase; Pyk2=proline-rich tyrosine kinase 2. Bold values indicates statistical significance.
Pyk2[pY881] excluded.
Pyk2 excluded.
Pyk2 and Pyk2[pY881] included.
CEA: −, normal (0–5 ng ml−1); +, elevated (>5 ng ml−1); 18 patients' CEA unknown.
NSE: −, normal (0–15.2 ng ml−1); +, elevated (>15.2 ng ml−1); 41 patients' NSE unknown.
EGFR: −, no EGFR mutation and amplification; +, EGFR mutation or amplification; 77 patients' EGFR unknown.
Pyk2 low expression: score ⩽3; high expression: score ⩾4.
Pyk2[pY881] low expression: score ⩽3; high expression: score ⩾4.
Pyk2 upregulates cancer stem cell marker expression and enhances transforming ability of NSCLC cells
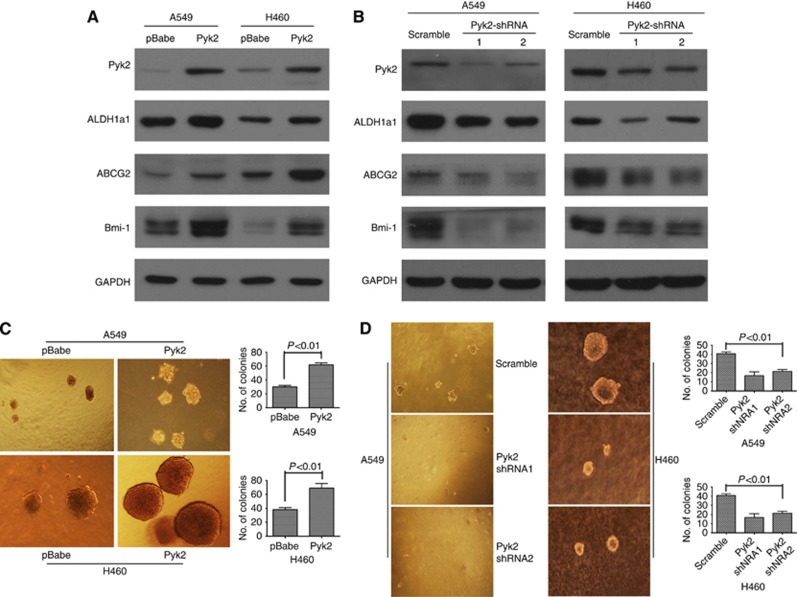
As Pyk2 is a potent prognostic marker for NSCLC patients, we investigated its molecular mechanism in NSCLC cell lines. Interestingly, as shown in Figure 7A and B, CSC markers, such as ALDH1a1, ABCG2 and Bmi-1, were found to be upregulated at the protein level in Pyk2 stably overexpressing A549 and H460 cell lines. Furthermore, the protein levels of the CSC markers ALDH1a1, ABCG2 and Bmi-1 were downregulated in Pyk2 knockdown in the A549 and H460 cell lines. Immunofluorescence staining further confirmed this phenomenon (Supplementary Figure 4). The above experiments suggest that Pyk2 can induce CSC marker expression in NSCLC cells.
Figure 7.
The Pyk2 upregulates CSC marker expression and transforming ability in NSCLC cells. (A) Overexpression of Pyk2 upregulated the expression of the CSC markers ALDH1a1, ABCG2 and Bmi1 at the protein level in A549 and H460 cells. (B) Knockdown of Pyk2 downregulated the expression of the CSC markers ALDH1a1, ABCG2 and Bmi1 at the protein level in A549 and H460 cells. (C) Anchorage-independent growth in soft agar of A549 and H460 cells with or without exogenous Pyk2 expression. (D) Anchorage-independent growth in soft agar of A549 and H460 cells with Pyk2 shRNA or scrambled shRNA. Three independent experiments in triplicate were performed. Magnification: × 40. Error bars represent the s.e.m.
We next used an anchorage-independent growth assay in soft agar to investigate the effect of Pyk2 on the transforming ability of NSCLC cells. As shown in Figure 7C and D, the knockdown of endogenous Pyk2 protein expression in the A549 and H460 cells caused a significant inhibition of their anchorage-independent growth, as indicated by the reduction of the colony size and colony number on soft agar (P<0.05), whereas the overexpression of endogenous Pyk2 in A549 and H460 cells caused significant promotion of their anchorage-independent growth. Therefore, these data indicate that Pyk2 can enhance the malignant transformation of NSCLC cells.
Discussion
This study revealed that Pyk2 protein and phosphorylated Pyk2[pY881] protein were upregulated in primary NSCLC lesions and metastatic lymph nodes in comparison with normal lung tissues. Importantly, Pyk2 and Pyk2[pY881] protein expression levels were found to significantly correlate with the prognosis of NSCLC and were independent prognostic factors of NSCLC patients. The gain and loss study of Pyk2 function revealed that Pyk2 could upregulate the expression of ALDH1a1, ABCG2 and Bmi-1 and enhanced the ability of colony formation in soft agar assay in A549 and H460 cells. Our study suggests that Pyk2 and Pyk2[pY881] may represent novel predictive markers for the clinical outcomes of NSCLC.
Proline-rich tyrosine kinase 2 is a nonreceptor tyrosine kinase that plays different roles in the intracellular signalling pathways that regulate a number of biological processes, such as cell proliferation, differentiation, adhesion and migration, that have been shown to correlate with tumour development and aggression. A previous report has shown that the Pyk2 protein expression level was upregulated in NSCLC and that the higher expression and activation of Pyk2 might induce the modulation of ERK1/2 activity, leading to the progression of NSCLC (Zhang et al, 2008). Our previous study has also shown that Pyk2 played a critical role in anchorage-independent growth and anoikis resistance in lung carcinoma cell lines (Zhang et al, 2010). In this study, we confirmed that Pyk2 was upregulated in NSCLC and further investigated the relationship between the expression levels of Pyk2 protein in cancer tissues and patients' clinical outcomes, showing for the first time that Pyk2 is an independent predictive factor for the clinical prognosis of NSCLC. Our results were supported by a previous study that patients with higher levels of Pyk2 in hepatocellular carcinoma tissues had larger tumour size, advanced Edmonson grading and poor prognosis (Sun et al, 2007).
In addition, we investigated whether the phosphorylated forms of Pyk2 play important roles in NSCLC progression. We thus chose Pyk2[pY402] and Pyk2[pY881] as two representative Pyk2 phosphorylation sites. However, Pyk2[pY402] protein expression in NSCLC tissues was low by both western blotting and immunohistochemistry assays, suggesting that the Pyk2[pY402] expression level in NSCLC tissues might be sufficiently low to escape detection with IHC analysis. The possibility that the negative result might be related to the valence of the rabbit anti-Pyk2[pY402] antibody also cannot be ruled out. Further research is needed to confirm our conjecture. Interestingly, a high level of Pyk2[pY881] protein expression in NSCLC tissues was closely associated with poorer differentiation; higher T, N and M stages; advanced clinical stage; and shorter survival times of the patients. Based on the multivariate analyses, Pyk2[pY881] was shown to be an independent predictive factor for the clinical outcomes of NSCLC. This study is the first to reveal that the phosphorylated form of Pyk2 is related to the clinical outcomes of cancer patients. Previous studies have shown that Pyk2 phosphorylated on Tyr881 provides a binding site for Grb2 to form the SHPS-1 signalling complex, thus leading to Erk1/2 (MAP kinase) activation and cell proliferation in response to IGF-I (Shen et al, 2010). It appears that Pyk2 phosphorylated on Tyr881 may be critical for its function in cell migration (Keogh et al, 2002). Our former study has preliminarily shown that cell aggregation induces increased levels of the tyrosine phosphorylation of Pyk2 and promotes tumour cell anchorage-independent growth (Zhang et al, 2010). This study further verifies that high expression levels of phosphorylated Pyk2[pY881] protein in lung carcinoma tissues are correlated with tumour differentiation and the prognosis of NSCLC patients. The underlying molecular mechanisms by which Pyk2[pY881] promotes NSCLC carcinogenesis and progression will be further investigated.
It has been reported that Pyk2 is induced and involved in monocyte differentiation (Park et al, 2008). In prostate cancer, Pyk2 seemed to have a role in the tumourigenesis, differentiation and cancer invasion, and Pyk2 could be a marker of prostate cell differentiation (Picascia et al, 2002). However, more reports have shown that Pyk2 is highly expressed in neuroglioma, breast cancer and hepatocellular carcinoma (Lipinski et al, 2005; Sun et al, 2007; Behmoaram et al, 2008). Until now, the correlation between the phosphorylated Pyk2 protein and the differentiation status in tumour cells has not been studied. Our study showed that high level of phosphorylated Pyk2[pY881] expression, but not Pyk2 expression, was strongly correlated with poor differentiation in lung cancer (P=0.003 and P=0.302, respectively). Consistently, phosphorylated Pyk2[pY881] was induced during epithelial–mesenchymal transition (EMT) in mouse mammary NMuMG cells (Nakamura et al, 2001). As EMT plays a major role during cancer invasion and metastases, it is worthwhile to further investigate whether phosphorylated Pyk2[pY881] could also induce EMT in lung cancer in the future.
A variety of G-protein-coupled receptors (GPCRs) are coupled with the mitogen-activated protein (MAP) kinase signalling cascade by the implication of the EGFR and the nonreceptor protein tyrosine kinases Src and Pyk2 (Della et al, 1997; Luttrell et al, 1999; Hackel et al, 1999; Carpenter, 1999). A previous study also reveals that Pyk2 activation triggers the Src family kinase (SFK)/EGFR signalling pathway and cell motility after wounding sheets of epithelial cells (Block et al, 2010). These findings suggest that there is an underlying interrelationship between EGFR and Pyk2. However, a statistical relationship between the mutation status of EGFR and the expression level of Pyk2 or Pyk2[pY881] was not detected by a χ2 analysis. This result may be ascribed to the limited number of the 51 NSCLC cases we analysed by roughly categorising the EGFR site mutation and amplification as an EGFR mutation subtype (37 patients). Owing to our limited tissue samples, the overall survival analyses were not significantly different between the high and low Pyk2 expression groups in squamous carcinoma (37 patients), EGFR wild-type (14 patients), EGFR mutation-type (37 patients) and M1 stage (25 patients) subgroups, and there was no statistically significant difference in the overall survival between the high and low Pyk2[pY881] expression groups in the EGFR mutation-type (37 patients) subgroup. Thus, a larger number of NSCLC cases is needed to yield a conclusive result. Besides, this is just a retrospective study, and a validation group as well as an independent group is needed to further confirm our study results.
Finally, our gain and loss study of Pyk2 function revealed that Pyk2 could upregulate the expression of ALDH1a1, ABCG2 and Bmi-1 and enhance the ability of colony formation in soft agar assay in A549 and H460 cells. This is supported by a former study that Pyk2 has a compensatory function in promoting FAK-null mammary CSC tumourigenicity and metastatic activity with the PI3K/Akt signalling pathway as a major mediator (Fan and Guan, 2011). This study represents the first time that a direct association between Pyk2 and CSC marker expression has been revealed. Further study is needed to investigate the interaction between Pyk2 and potential CSC signalling pathways. Considering the results of this study and of our previous study on Pyk2 in lung cancer (Zhang et al, 2010), we conclude that Pyk2 induces the progression of NSCLC partially by the molecular mechanisms of enhancing the tumour-transforming ability, increasing CSC marker expression and inducing anchorage-independent growth and anoikis resistance.
Conclusions
We have demonstrated for the first time that Pyk2 is an independent predictive factor for the clinical prognosis of NSCLC and has a direct interaction with CSC marker expression. Pyk2 protein and its phosphorylated form (Pyk2[pY881]) were upregulated in primary NSCLC lesions and metastatic lymph nodes in comparison with those in normal lung tissues. The Pyk2 can upregulate CSC marker expression and enhance the transforming ability of NSCLC cells. Thus, Pyk2 and Pyk2[pY881] are potential prognostic factors and therapeutic targets for the effective treatment of NSCLC.
Acknowledgments
This work was supported by a grant from the National Scientific Foundation of China (No. 81071932).
The authors declare no conflict of interest.
Footnotes
Supplementary Information accompanies this paper on British Journal of Cancer website (http://www.nature.com/bjc)
This work is published under the standard license to publish agreement. After 12 months the work will become freely available and the license terms will switch to a Creative Commons Attribution-NonCommercial-Share Alike 3.0 Unported License.
Supplementary Material
References
- Avraham H, Park SY, Schinkmann K, Avraham S. RAFTK/Pyk2-mediated cellular signalling. Cell Signal. 2000;12:123–133. doi: 10.1016/s0898-6568(99)00076-5. [DOI] [PubMed] [Google Scholar]
- Behmoaram E, Bijian K, Jie S, XU Y, Darnel A, Bismar TA, Alaoui-Jamali MA. Focal adhesion kinase-related proline-rich tyrosine kinase 2 and focal adhesion kinase are co-overexpressed in early-stage and invasive ErbB-2-positive breast cancer and cooperate for breast cancer cell tumorigenesis and invasiveness. Am J Pathol. 2008;173:1540–1550. doi: 10.2353/ajpath.2008.080292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bertolini G, Roz L, Perego P, Tortoreto M, Fontanella E, Gatti L, Pratesi G, Fabbri A, Andriani F, Tinelli S, Roz E, Caserini R, Lo VS, Camerini T, Mariani L, Delia D, Calabro E, Pastorino U, Sozzi G. Highly tumorigenic lung cancer CD133+ cells display stem-like features and are spared by cisplatin treatment. Proc Natl Acad Sci USA. 2009;106:16281–16286. doi: 10.1073/pnas.0905653106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blaukat A, Ivankovic-Dikic I, Gronroos E, Dolfi F, Tokiwa G, Vuori K, Dikic I. Adaptor proteins Grb2 and Crk couple Pyk2 with activation of specific mitogen-activated protein kinase cascades. J Biol Chem. 1999;274:14893–14901. doi: 10.1074/jbc.274.21.14893. [DOI] [PubMed] [Google Scholar]
- Block ER, Tolino MA, Klarlund JK. Pyk2 activation triggers epidermal growth factor receptor signaling and cell motility after wounding sheets of epithelial cells. J Biol Chem. 2010;285:13372–13379. doi: 10.1074/jbc.M109.083089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brummelkamp TR, Bernards R, Agami R. Stable suppression of tumorigenicity by virus-mediated RNA interference. Cancer Cell. 2002;2:243–247. doi: 10.1016/s1535-6108(02)00122-8. [DOI] [PubMed] [Google Scholar]
- Carpenter G. Employment of the epidermal growth factor receptor in growth factor-independent signaling pathways. J Cell Biol. 1999;146:697–702. doi: 10.1083/jcb.146.4.697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chiou SH, Wang ML, Chou YT, Chen CJ, Hong CF, Hsieh WJ, Chang HT, Chen YS, Lin TW, Hsu HS, Wu CW. Coexpression of Oct4 and Nanog enhances malignancy in lung adenocarcinoma by inducing cancer stem cell-like properties and epithelial-mesenchymal transdifferentiation. Cancer Res. 2010;70:10433–10444. doi: 10.1158/0008-5472.CAN-10-2638. [DOI] [PubMed] [Google Scholar]
- Collins LG, Haines C, Perkel R, Enck RE. Lung cancer: diagnosis and management. Am Fam Physician. 2007;75:56–63. [PubMed] [Google Scholar]
- Della RG, van Biesen T, Daaka Y, Luttrell DK, Luttrell LM, Lefkowitz RJ. Ras-dependent mitogen-activated protein kinase activation by G protein-coupled receptors. Convergence of Gi- and Gq-mediated pathways on calcium/calmodulin, Pyk2, and Src kinase. J Biol Chem. 1997;272:19125–19132. doi: 10.1074/jbc.272.31.19125. [DOI] [PubMed] [Google Scholar]
- Eramo A, Lotti F, Sette G, Pilozzi E, Biffoni M, Di Virgilio A, Conticello C, Ruco L, Peschle C, De Maria R. Identification and expansion of the tumorigenic lung cancer stem cell population. Cell Death Differ. 2008;15:504–514. doi: 10.1038/sj.cdd.4402283. [DOI] [PubMed] [Google Scholar]
- Fan H, Guan JL. Compensatory function of Pyk2 protein in the promotion of focal adhesion kinase (FAK)-null mammary cancer stem cell tumorigenicity and metastatic activity. J Biol Chem. 2011;286:18573–18582. doi: 10.1074/jbc.M110.200717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gelman IH. Pyk 2 FAKs, any two FAKs. Cell Biol Int. 2003;27:507–510. doi: 10.1016/s1065-6995(03)00078-7. [DOI] [PubMed] [Google Scholar]
- Guo C, Zheng C, Martin-Padura I, Bian ZC, Guan JL. Differential stimulation of proline-rich tyrosine kinase 2 and mitogen-activated protein kinase by sphingosine 1-phosphate. Eur J Biochem. 1998;257:403–408. doi: 10.1046/j.1432-1327.1998.2570403.x. [DOI] [PubMed] [Google Scholar]
- Hackel PO, Zwick E, Prenzel N, Ullrich A. Epidermal growth factor receptors: critical mediators of multiple receptor pathways. Curr Opin Cell Biol. 1999;11:184–189. doi: 10.1016/s0955-0674(99)80024-6. [DOI] [PubMed] [Google Scholar]
- Jiang F, Qiu Q, Khanna A, Todd NW, Deepak J, Xing L, Wang H, Liu Z, Su Y, Stass SA, Katz RL. Aldehyde dehydrogenase 1 is a tumor stem cell-associated marker in lung cancer. Mol Cancer Res. 2009;7:330–338. doi: 10.1158/1541-7786.MCR-08-0393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keogh RJ, Houliston RA, Wheeler-Jones CP. Human endothelial Pyk2 is expressed in two isoforms and associates with paxillin and p130Cas. Biochem Biophys Res Commun. 2002;290:1470–1477. doi: 10.1006/bbrc.2002.6350. [DOI] [PubMed] [Google Scholar]
- Kong QL, Hu LJ, Cao JY, Huang YJ, Xu LH, Liang Y, Xiong D, Guan S, Guo BH, Mai HQ, Chen QY, Zhang X, Li MZ, Shao JY, Qian CN, Xia YF, Song LB, Zeng YX, Zeng MS. Epstein-Barr virus-encoded LMP2A induces an epithelial-mesenchymal transition and increases the number of side population stem-like cancer cells in nasopharyngeal carcinoma. PLoS Pathog. 2010;6:e1000940. doi: 10.1371/journal.ppat.1000940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liao WT, Song LB, Zhang HZ, Zhang X, Zhang L, Liu WL, Feng Y, Guo BH, Mai HQ, Cao SM, Li MZ, Qin HD, Zeng YX, Zeng MS. Centromere protein H is a novel prognostic marker for nasopharyngeal carcinoma progression and overall patient survival. Clin Cancer Res. 2007;13:508–514. doi: 10.1158/1078-0432.CCR-06-1512. [DOI] [PubMed] [Google Scholar]
- Lipinski CA, Tran NL, Menashi E, Rohl C, Kloss J, Bay RC, Berens ME, Loftus JC. The tyrosine kinase pyk2 promotes migration and invasion of glioma cells. Neoplasia. 2005;7:435–445. doi: 10.1593/neo.04712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luttrell LM, Daaka Y, Lefkowitz RJ. Regulation of tyrosine kinase cascades by G-protein-coupled receptors. Curr Opin Cell Biol. 1999;11:177–183. doi: 10.1016/s0955-0674(99)80023-4. [DOI] [PubMed] [Google Scholar]
- Mcshane LM, Altman DG, Sauerbrei W, Taube SE, Gion M, Clark GM. Reporting recommendations for tumor marker prognostic studies (REMARK) J Natl Cancer Inst. 2005;97:1180–1184. doi: 10.1093/jnci/dji237. [DOI] [PubMed] [Google Scholar]
- Nakamura K, Yano H, Schaefer E, Sabe H. Different modes and qualities of tyrosine phosphorylation of Fak and Pyk2 during epithelial-mesenchymal transdifferentiation and cell migration: analysis of specific phosphorylation events using site-directed antibodies. Oncogene. 2001;20:2626–2635. doi: 10.1038/sj.onc.1204359. [DOI] [PubMed] [Google Scholar]
- Pardal R, Clarke MF, Morrison SJ. Applying the principles of stem-cell biology to cancer. Nat Rev Cancer. 2003;3:895–902. doi: 10.1038/nrc1232. [DOI] [PubMed] [Google Scholar]
- Park MH, Park SY, Kim Y. Induction of proline-rich tyrosine kinase2 (Pyk2) through C/EBPbeta is involved in PMA-induced monocyte differentiation. FEBS Lett. 2008;582:415–422. doi: 10.1016/j.febslet.2008.01.001. [DOI] [PubMed] [Google Scholar]
- Petersen I, Petersen S. Towards a genetic-based classification of human lung cancer. Anal Cell Pathol. 2001;22:111–121. doi: 10.1155/2001/374304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Picascia A, Stanzione R, Chieffi P, Kisslinger A, Dikic I, Tramontano D. Proline-rich tyrosine kinase 2 regulates proliferation and differentiation of prostate cells. Mol Cell Endocrinol. 2002;186:81–87. doi: 10.1016/s0303-7207(01)00667-0. [DOI] [PubMed] [Google Scholar]
- Reya T, Morrison SJ, Clarke MF, Weissman IL. Stem cells, cancer, and cancer stem cells. Nature. 2001;414:105–111. doi: 10.1038/35102167. [DOI] [PubMed] [Google Scholar]
- Shen X, Xi G, Radhakrishnan Y, Clemmons DR. Recruitment of Pyk2 to SHPS-1 signaling complex is required for IGF-I-dependent mitogenic signaling in vascular smooth muscle cells. Cell Mol Life Sci. 2010;67:3893–3903. doi: 10.1007/s00018-010-0411-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siegel R, Naishadham D, Jemal A. Cancer statistics, 2012. CA Cancer J Clin. 2012;62:10–29. doi: 10.3322/caac.20138. [DOI] [PubMed] [Google Scholar]
- Song LB, Zeng MS, Liao WT, Zhang L, Mo HY, Liu WL, Shao JY, Wu QL, LI MZ, Xia YF, Fu LW, Huang WL, Dimri GP, Band V, Zeng YX. Bmi-1 is a novel molecular marker of nasopharyngeal carcinoma progression and immortalizes primary human nasopharyngeal epithelial cells. Cancer Res. 2006;66:6225–6232. doi: 10.1158/0008-5472.CAN-06-0094. [DOI] [PubMed] [Google Scholar]
- Stanzione R, Picascia A, Chieffi P, Imbimbo C, Palmieri A, Mirone V, Staibano S, Franco R, De Rosa G, Schlessinger J, Tramontano D. Variations of proline-rich kinase Pyk2 expression correlate with prostate cancer progression. Lab Invest. 2001;81:51–59. doi: 10.1038/labinvest.3780211. [DOI] [PubMed] [Google Scholar]
- Sun CK, Ng KT, Sun BS, Ho JW, Lee TK, Ng I, Poon RT, Lo CM, Liu CL, Man K, Fan ST. The significance of proline-rich tyrosine kinase2 (Pyk2) on hepatocellular carcinoma progression and recurrence. Br J Cancer. 2007;97:50–57. doi: 10.1038/sj.bjc.6603827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xiang R, Liao D, Cheng T, Zhou H, Shi Q, Chuang TS, Markowitz D, Reisfeld RA, Luo Y. Downregulation of transcription factor SOX2 in cancer stem cells suppresses growth and metastasis of lung cancer. Br J Cancer. 2011;104:1410–1417. doi: 10.1038/bjc.2011.94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xiong WC, Macklem M, Parsons JT. Expression and characterization of splice variants of PYK2, a focal adhesion kinase-related protein. J Cell Sci. 1998;111 (Pt 14:1981–1991. doi: 10.1242/jcs.111.14.1981. [DOI] [PubMed] [Google Scholar]
- Zhang S, Qiu X, Gu Y, Wang E. Up-regulation of proline-rich tyrosine kinase 2 in non-small cell lung cancer. Lung Cancer. 2008;62:295–301. doi: 10.1016/j.lungcan.2008.05.008. [DOI] [PubMed] [Google Scholar]
- Zhang X, Xu LH, Yu Q. Cell aggregation induces phosphorylation of PECAM-1 and Pyk2 and promotes tumor cell anchorage-independent growth. Mol Cancer. 2010;9:7. doi: 10.1186/1476-4598-9-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang XY, Dong QG, Huang JS, Huang AM, Shi CL, Jin B, Sha HF, Feng JX, Geng Q, Zhou J, Xu HL, Han BH. The expression of stem cell-related indicators as a prognostic factor in human lung adenocarcinoma. J Surg Oncol. 2010;102:856–862. doi: 10.1002/jso.21718. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.