Abstract
Background:
This study investigated the clinical importance of linked angiogenetic biomarkers to chemotherapy, combined with the anti-vascular endothelial growth factor A (anti-VEGF-A), as a first-line treatment in patients with metastatic colorectal cancer (mCRC).
Methods:
A total of 230 patients from a randomised phase III study were included. The primary microRNA-126 (pri-miRNA-126) A24G single-nucleotide polymorphism and the mature miRNA-126 were analysed by PCR using genomic DNA (full blood) and formalin-fixed paraffin-embedded tissue sections, respectively. The epidermal growth factor-like domain 7 (EGFL7) protein was visualised and quantified using immunohistochemistry.
Results:
High tumour expression of miRNA-126 was significantly related to a longer progression-free survival. The independent prognostic value of miRNA-126 was confirmed using a Cox regression analysis (hazard ratio=0.49, 95% confidence interval=0.29–0.84, P=0.009). Although not significant, a relationship between EGFL7 expression and response rates is suggested, with EGFL7 expression at the invasive front being lower in responding patients than in the non-responders (P=0.063).
Conclusion:
The results validate the previous findings on the prognostic value of miRNA-126 in mCRC and may suggest a relationship between treatment efficacy and EGFL7 expression. As miRNA-126 may target VEGF-A as well as EGFL7, the results may provide predictive information in relation to next-generation anti-angiogenetics.
Keywords: angiogenesis, chemotherapy, colorectal neoplasms, epidermal growth factor-like domain 7, microRNA, predictive biomarkers
Each year, 1.2 million people worldwide are diagnosed with colorectal cancer (CRC), making it the third most common cancer, with an estimated annual mortality of 0.6 million (Ferlay et al, 2010). Adding anti-angiogenetics to chemotherapy improves the overall survival (OS) of patients with metastatic CRC (mCRC) (Cao et al, 2009; Wagner et al, 2009); however, the overall benefit in unselected patient cohorts is rather limited. Focusing on a decade of clinical experience with anti-angiogenetic drugs, primarily targeting the vascular endothelial growth factor A (VEGF-A), we are still left without validated biomarkers to select an anti-angiogenetic therapy for the fraction of patients responding favourably to this treatment strategy.
MicroRNAs (miRNAs) represent a group of candidates in this setting with biomarker as well as therapeutic potential. These small, non-coding RNA fragments bind to the 3′untranslated region (3′UTR) of their target transcripts, leading to an inhibition of the translational process (Esquela-Kerscher and Slack, 2006; Filipowicz et al, 2008). MicroRNA-126 is expressed in endothelial cells (ECs) with a high specificity and is considered essential to the regulation of blood vessel integrity (Fish et al, 2008; Wang et al, 2008). The VEGF-A mRNA is an miRNA-126 target, and studies have argued for a role of miRNA-126 in regulating VEGF-A-mediated signal transduction (Parker et al, 2004; Fish et al, 2008; Kuhnert et al, 2008; Wang et al, 2008). MicroRNA-126 is downregulated in several malignant tumours, (Guo et al, 2008; Li et al, 2010; Meister and Schmidt, 2010) and is often referred to as a tumour suppressor (Schepeler et al, 2012).
A single miRNA can regulate hundreds of target mRNAs, and consequently, a polymorphism affecting the biogenesis of a given miRNA may regulate the expression of multiple genes in a given molecular pathway. A study of mCRC argued for a possible predictive value of primary-miRNA (pri-miRNA) single-nucleotide polymorphisms (SNPs) with regard to first-line chemotherapy (Boni et al, 2011). Recently, one SNP in the pri-miRNA-126, A24G (rs4636297), was identified with a possible influence on the bioavailability of the mature miRNA-126 transcript (Harnprasopwat et al, 2010). The clinical importance of this SNP is largely unknown.
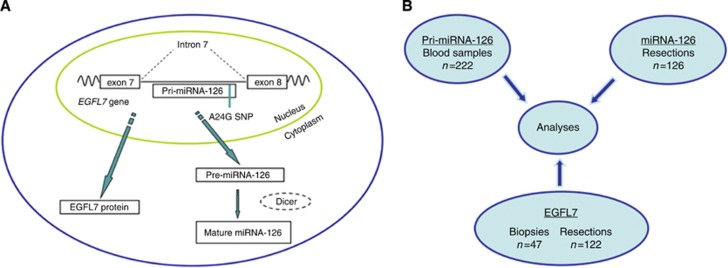
MicroRNA-126 is located within the intron 7 of the epidermal growth factor (EGF)-like domain 7 (EGFL7) gene, and EGFL7 mRNA has been identified as a potential target of miRNA-126 (Sun et al, 2010); (Figure 1A). The EGFL7 protein is important for vascular system development and is expressed postnatally in highly vascularised tissues. It is upregulated at sites of pathological angiogenesis and acts as a chemo-attractant in the recruitment of ECs (Campagnolo et al, 2005). EGFL7 is an EC-derived protein that is secreted into the extracellular matrix (ECM), where it remains in close vicinity to the ECs, regulating their spatial organisation in the vascular sprouts and guiding the migration of the sprouts through the ECM (Schmidt et al, 2007). The presence of EGFL7 is necessary for the tubulogenesis of blood vessels (Parker et al, 2004).
Figure 1.
(A) Illustration of an endothelial cell, demonstrating the interaction of the investigated parameters. (B) Diagram illustrating the investigated parameters: pri-miRNA-126 SNP; mature miRNA-126; EGFL7 protein, and sample availability. EGFL7 analyses of samples (tumour resections) from four patients were not possible as all the available tissue was used in the initial qPCR profiling of miRNA-126. Thus, immunohistochemical staining was performed on 122 resections and 47 biopsies, respectively. EGFL7 tumour was consequently scored in 169 samples, whereas EGFL7 invasive front was scored in 122 samples. Abbreviations: Pri-miRNA-126, primary microRNA-126; miRNA-126, microRNA-126; EGFL7, epidermal growth factor-like domain 7; SNP, single nucleotide polymorphism; pre-miRNA-126, precursor microRNA-126; Dicer, The RNAse III enzyme responsible for excising the miRNA duplex from the pre-miRNA hairpin.
A prognostic role of miRNA-126 in patients with CRC has been proposed (Schepeler et al, 2008; Hansen et al, 2012), and we have previously shown that miRNA-126, estimated using in situ hybridisation (ISH) in patients with mCRC, may be predictive of chemotherapy efficacy (Hansen et al, 2012). The possible predictive value in a setting of anti-VEGF-A therapy remains to be analysed. The clinical importance of EGFL7 is still sparse. An anti-EGFL7 monoclonal antibody has been developed (Genentech), and preclinical tests have suggested a synergistic effect of combining anti-VEGF-A and anti-EGFL7. This is currently being tested in the CONGO trial (ClinicalTrials.gov identifier NCT01399684). If positive, understanding the complex interaction between VEGF-A, EGFL7 and miRNA-126 will become even more important from the clinical point of view.
The aim of this translational study was to analyse the predictive value of a set of linked angiogenetic markers at the DNA (the pri-miRNA-126 SNP), RNA (mature miRNA-126) and protein (EGFL7) levels in relation to first-line chemotherapy combined with bevacizumab in patients with mCRC.
Materials and methods
Patient characteristics
The phase III Nordic ACT (I) multicentre study included 249 patients from Sweden and Denmark between October 2007 and October 2009 (ClinicalTrials.gov identifier NCT00598156). All the patients were previously untreated, had histologically verified mCRC and had received first-line chemotherapy+bevacizumab (anti-VEGF-A). The patients were initially treated with induction chemotherapy, consisting of fluorouracil and leucovorin or capecitabine in combination with either oxaliplatin (FOLFOX or XELOX, n=183) or irinotecan (FOLFIRI or XELIRI, n=66) plus bevacizumab. Six cycles were given in the case of XELOX/XELIRI and nine cycles when FLOFOX/FOLFIRI was used. One hundred and sixty-two patients were subsequently randomised to maintenance therapy consisting of bevacizumab±erlotinib (an anti-epidermal growth factor receptor (EGFR)) until progression or unacceptable toxicity. The primary reasons for exclusion after induction therapy were progressive disease, planned surgery with curative intent and adverse events. Adjuvant treatments that completed >6 months prior to study entry were allowed.
Information on patient characteristics and treatment efficacy was recorded according to good clinical practice. The study was approved by the Regional Scientific Ethical Committee and the Danish Data Protection Agency (ID VF-20060115, 2007-41-0252), and informed consent was obtained from all the patients enroled in the study.
Sampling
Samples were available from 230 of the 249 included patients (92%). Blood samples, available from 222 patients, were collected in 5–10-ml EDTA tubes and sent to Vejle Hospital to be frozen at −80 oC and stored until DNA analysis. All the histological samples followed routine formaldehyde fixation and paraffin embedding (FFPE). The available tissue samples (n=173) originated from a resected tumour in 126 (73%) patients and from a diagnostic biopsy in 47 (27%) patients. Analysis of samples (tumour resections) from four patients was not possible as all the available tissue had been used in the initial qPCR profiling of miRNA-126. Thus, immunohistochemical staining was performed in 122 resections and 47 biopsies, respectively. Tissue sections for miRNA and immunohistochemical analyses were cut adjacently from the same FFPE tissue block.
Samples available for analysis are illustrated in Figure 1B. The analyses presented in this study vary according to the contributions from the different sample sources. Thus, individual sample sizes will be presented for each analysis.
Evaluation of treatment efficacy
Response rates (RR), available from all patients, were assessed according to the Response Evaluation Criteria In Solid Tumours (RECIST 1.0) (Therasse et al, 2000). Evaluations were performed on the basis of clinical and radiologic examination, using CT scans of the chest and abdomen. Responding patients were classified as having either complete response (CR) or partial response (PR), whereas non-responding patients were classified as having either stable disease (SD) or progressive disease (PD). Response rates were used to evaluate the efficacy of the induction therapy, whereas progression-free survival (PFS) was the endpoint of the maintenance treatment. Progression-free survival was calculated from the time of randomisation (after completion of the induction therapy) until the date of the last CT scan without PD.
Pri-miRNA-126 SNP analysis
The SNP analysed in this study, pri-miRNA-126 A24G SNP (rs 4636297), is located in the EGFL7 gene in intron 7 and is the only known SNP in this region. It was chosen based on a previous publication indicating the functional importance of this SNP (Harnprasopwat et al, 2010), and because of an allele distribution allowing a meaningful comparison with the mature miRNA-126 transcript as well as the EGFL7 protein expression.
Germline DNA was isolated from the whole blood of 222 patients using the Maxwell method, according to the user manual (Promega Corporation, Madison, WI, USA) (http://www.promega.com/tbs/tm284/tm284.pdf). Genotyping was performed by PCR analysis using the ABI PRISM 7900 HT fast real-time PCR system (Applied Biosystem, Foster City, CA, USA). The primer sequences were as follows: forward 5′-CACTTCAAACTCGTACCGTGAGTAA, reverse 5′-CAGGGCTATGCCGCCTAA, and probes: 6FAM-GCGCTTTCGATGCG and VIC-CGGCGTTTTCGATG. The analysis was performed as previously described (Hansen et al, 2011). All the samples met the quality value threshold of 98%. All the blood samples were successfully processed and analysed, blinded of patient outcome, at the same institution (Vejle Hospital, Denmark).
MicroRNA-126 analysis
The qPCR profiling of miRNA-126 in 4-μm tissue sections from small diagnostic biopsies may introduce bias due to heterogeneity. Hence, it was decided to perform miRNA-126 quantification on only whole-tumour resection specimens. This was consequently performed on 126 samples and carried out using Exiqon A/S (Vedbaek, Denmark).
Initially, the RNA was extracted in accordance with an optimised Qiagen FFPE purification protocol. Afterwards, 10 ng of RNA was reverse-transcribed (RT) in 10-μl reactions using the miRCURY LNA Universal RT miRNA PCR, Polyadenylation and cDNA synthesis kit (Exiqon). cDNA was diluted by 100 × and assayed in 10-μl PCR reactions according to the miRCURY LNA protocol. MicroRNA-126, miRNA-16, miRNA-103 and negative controls were assayed using qPCR on the microRNA Ready-to-Use PCR, Pick-n-Mix panel. Amplification was performed using a LightCycler 480 Real-Time PCR System (Roche) in 384-well plates. Amplification curves were analysed using the Roche LC software, both for the determination of the crossing point (Cp) and for a melting curve analysis.
A built-in quality control would remove the reactions with several melting points, melting points not within the assay specification, an amplification efficacy of <1.6, or the reactions within 5 Cp values of the negative control reactions. Furthermore, RNA spike-in control (Sp6) was added to the RT reaction in order to evaluate both the RT reaction and the following qPCR reaction. All samples passed the quality control, and the results showed similar Cp values across all the samples, indicating successful RT-qPCR analyses. The qualities of the samples were comparable (samples were very similar in miRNA content); they were processed reproducibly in triplicates, and the mean value was used in later analyses.
The normalisation of the miRNA expression data represents an important step in data analysis, potentially influencing the final results. So far, there does not seem to be an optimal solution to this challenge. According to Chang et al (2010), several combinations of reference genes (miRNAs) have been recommended for the normalisation of RT-qPCR data in CRC. MicroRNA-16 was the single most suitable reference gene.
The normalisation procedure in the present analysis was optimised to correct for potential differences in samples. In this data set, the average values of miRNA-16 and miRNA-103 were chosen based on previous experience with Exiqon A/S. Thus, the miRNA-126 expression values are relative values without dimension.
EGFL7 and caldesmon immunostaining
Tissue sections were stained using antibodies against EGFL7 and caldesmon, aiming at a differentiation between the mature and immature microvessels. Four-micrometre-thick tissue sections were mounted on coated slides and dried for half an hour at 60 oC and then overnight at 37 oC. Deparaffinisation was performed in estisol for 10 min at room temperature, followed by rehydration in graded alcohol solutions (99–70%). Endogenous peroxidase was blocked by adding hydrogen peroxide (3%) for 5 min. Antigene unmasking were achieved by microwave oven heat-induced epitope retrieval, using a TEG buffer (TRIS 10 mM, EGTA 0.5 mM, Titriplex-VI, Darmstadt, Germany) at pH 9 for 10 min at 1000 W and for 15 min at 440 W. Tris-buffered saline (TBS)/Tween pH 7.6 was added for 5 min after cooling at room temperature. The anti-EGFL7 was a rabbit polyclonal antibody (ab115786, Abcam, Cambridge, UK) used in a 1 : 200 dilution and incubated for 90 min. The anti-caldesmon was a mouse monoclonal antibody (Clone h-CD, M3557, Dako, Glostrup, Denmark) used in a 1 : 50 dilution and incubated for 30 min. After washing in TBS/Tween, the visualisation was performed using Dako's EnVision G|2 Doublestain System (Rabbit/Mouse, DAB+/Permanent Red, code K5361, Dako) for 30 min. The EGFL7 was visualised using Polymer/HRP (brown) and caldesmon was visualised using Polymer/AP (red). Nuclei staining was obtained using Mayer haematoxylin solution.
The specificity of the anti-EGFL7 antibody was tested using pre-treatment with the TEG buffer at pH 9. EGFL7 Recombinant Protein, Novus H00051162-P01 4 μl and Dako Flex buffer K8007 200 μl were used along with 1 μl of the anti-EGFL7 antibody in a 1 : 200 dilution. The solution was incubated for 30 min, followed by 90 min of incubation. This resulted in only a few ECs demonstrating focal, faint staining, and no background staining or staining of tumour cells was observed.
Scoring of EGFL7 expression
EGFL7 expression
Any dark brown EGFL7-positive, caldesmon-negative, stained structure with an endothelial morphology was considered to be countable. Tissue sections were initially scanned at low power ( × 40), and five random areas in the tumour and five at the deepest invasive front were selected for quantification at × 100 power. The average scores from the five areas in the tumour and the invasive front were used for later analyses. Assessment was achieved using a point-counting graticule with 25 points (model WHN10X-H-1-3, Olympus, Ballerup, Denmark). Each point overlaying a countable structure resulted in a count, and the final estimate consequently refers to a volume fraction.
Interobserver variability
Before the scoring of all the sections by one investigator (TFH, unaware of the patient outcome), an interobserver variability test was performed between the two investigators (TFH and FBS). Interobserver variability was assessed for EGFL7 expression in the tumour and at the invasive front in a sub-sample of 17 tissue sections. Linear correlation analysis demonstrated an acceptable correlation between the EGFL7 estimates obtained at the invasive front (r=0.56, P=0.02). The EGFL7 estimates from the tumour area demonstrated a rather similar, although not significant (r=0.43, P=0.08), correlation. The results were considered to be acceptable based on the subjective nature of immunohistochemical scoring in general and the rather small sample set used in the test.
Biopsies/resections
The presented EGFL7 tumour scoring was applied to all the tissue samples (n=169), resections and diagnostic biopsies, whereas invasive front scoring was exclusively carried out in the tumour resections (n=122).
Statistics
The Wilcoxon rank sum test was used for a comparison of the median values. Fisher's exact test was used for a comparison between the categorical parameters, whereas χ2 statistics were used to test for the Hardy–Weinberg equilibrium. A linear regression analysis was used to investigate the linear association between the continuous variables and to analyse the continuous outcomes across categorical variables with more than two values, for example, miRNA-126 expression levels across genotypes AA, AG and GG. Survival functions were estimated using log rank tests and illustrated using the Kaplan–Meier method. A simple Cox regression analysis was used to estimate the hazard ratio of individual potential prognostic variables, and variables with P-values <0.1 were included in a multiple Cox regression analysis. Adjustment for multiple comparisons was not made, as the study is hypothesis-generating in several of its aspects. All patients were successfully followed-up until progression. A two-sided 0.05 significance level was used in all the statistical tests. All statistical calculations were carried out using STATA 11.2 (Stata Corp., College Station, TX, USA).
Results
Patient and sample characteristics
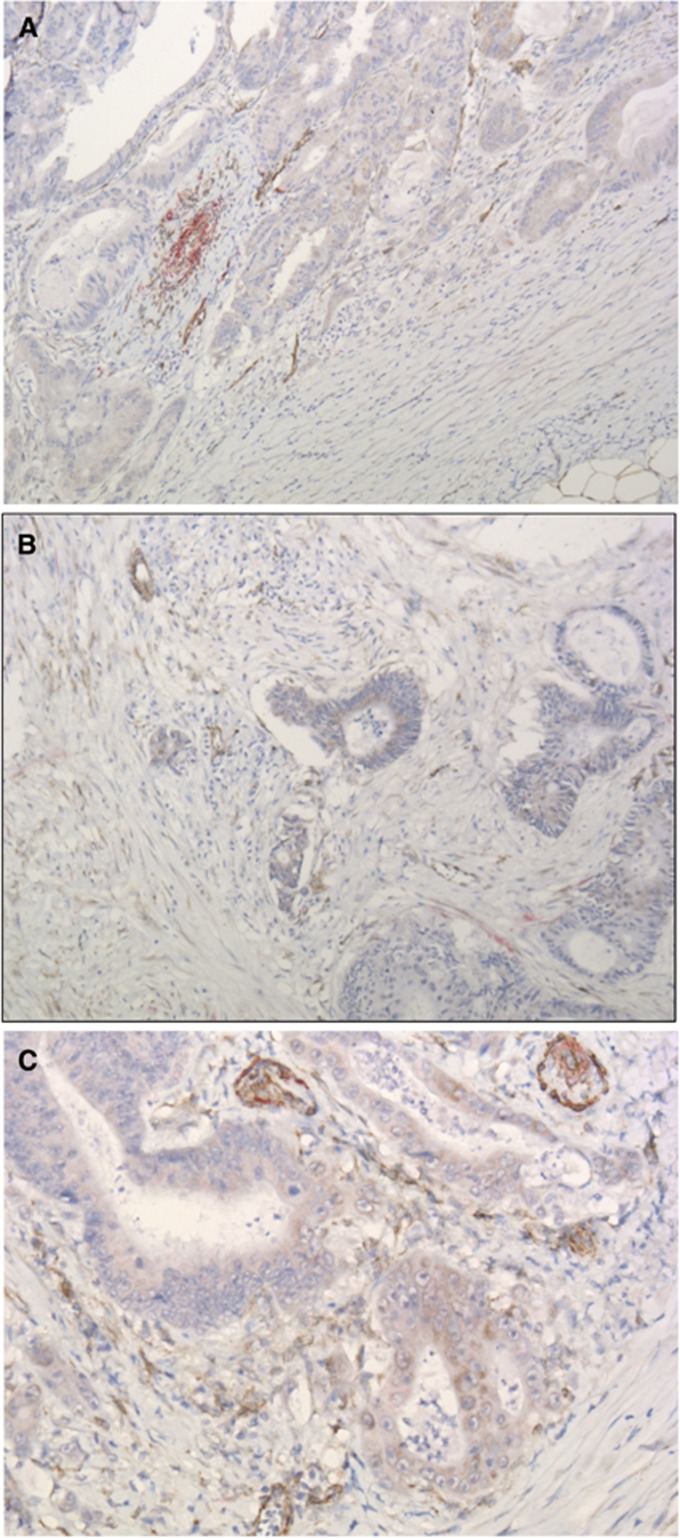
Patient characteristics are shown in Table 1. The visualisation of EGFL7 and caldesmon was successful in all the tissue sections, although the staining intensity varied between sections (Figure 2A). Unspecific background staining was detected in majority of the tissue sections, and more than half of the tissue sections showed signs of tumour cell EGFL7 expression (Figures 2B and C).
Table 1. Patient characteristics.
| |
|
Pri-miRNA-126 |
|
EGFL7 |
|||
|---|---|---|---|---|---|---|---|
| |
Number (%) |
A24G SNP (genotypes,
n=222) |
miRNA-126 (medians) |
Tumour (medians) |
Invasive front (medians) |
||
| (N=230) | AA | AG | GG | N=126 | N=169 | N=122 | |
|
Gender | |||||||
| Male | 133 | 11 | 58 | 60 | 2.32 | 0.4 | 0.4 |
| Female |
97 |
16 |
42 |
35 |
2.30 |
0.6 |
0.6 |
|
Age (years)a | |||||||
| Mean (s.d.) | 62.0 (9.3) | — | — | — | — | — | — |
| Range | 26–81 | — | — | — | — | — | — |
| >Mean | 130 | 17 | 59 | 50 | 2.42 | 0.5 | 0.5 |
| ⩽Mean |
100 |
10 |
41 |
45 |
2.20 |
0.5 |
0.4 |
|
ECOG PS | |||||||
| 0 | 159 (69) | 22 | 64 | 68 | 2.31 | 0.5 | 0.4 |
| 1–2 |
71 (31) |
5 |
36 |
27 |
2.33 |
0.5 |
0.8 |
|
Resection | |||||||
| Yes | 150 (65) | 19 | 62 | 61 | 2.32 | 0.4 | 0.4 |
| No |
80 (35) |
8 |
38 |
34 |
|
0.5 |
|
|
Localisation | |||||||
| Colon | 137 (60) | 15 | 61 | 55 | 2.32 | 0.5 | 0.6 |
| Rectum | 77 (33) | 9 | 32 | 34 | 2.26 | 0.4 | 0.4 |
| Colon+rectum (synchronous) |
16 (7) |
3 |
7 |
6 |
2.38 |
0.4 |
0.4 |
|
Metastatic Sites | |||||||
| 1 | 120 (52) | 15 | 44 | 57 | 2.33 | 0.5 | 0.5 |
| ⩾2 |
110 (48) |
12 |
56 |
38 |
2.28 |
0.5 |
0.4 |
|
Adjuvantb | |||||||
| Yes | 32 (15) | 4 | 14 | 14 | 2.19 | 0.4 | 0.4 |
| No | 187 (85) | 23 | 84 | 80 | 2.44 | 0.5 | 0.5 |
Abbreviations: ECOG PS=Eastern Cooperative Oncology Group performance status; EGFL7=epidermal growth factor-like domain 7; miRNA-126=microRNA-126; Pri-miRNA-126=primary microRNA-126; SD=standard deviation; SNP=single-nucleotide polymorphism.
The pri-miRNA-126 SNP was in Hardy–Weinberg equilibrium (P=1.0).
Age at the time of diagnosis.
Data registration incomplete.
Figure 2.
(A) Dark brown staining of endothelial-derived structures along with a larger caldesmon-positive (red staining) blood vessel. (B, C) Two examples of clusters of tumour cells with signs of cytoplasmic EGFL7 expression and unspecific background staining of the non-endothelial stromal compartment.
Correlations
The correlations between the investigated parameters are shown in Table 2. The results implied a marginally significant functional importance of the pri-miRNA-126 A24G SNP. The G-allele-containing genotypes seemed to be associated with a tendency towards lower miRNA-126 and EGFL7 invasive front expression. MicroRNA-126 expression was not correlated with EGFL7 expression, but the EGFL7-derived parameters were significantly correlated with each other.
Table 2. Associations and correlations between the investigated parameters.
|
Pri-miRNA-126 SNP genotype associations | ||||
|
Parameter |
N |
Genotypes Median, 95% CI |
Comparisons |
P-valuea |
| miRNA-126 | 15 | AA: 2.53 (2.29–2.77) | AA vs AG | 0.051 |
| 58 | AG: 2.20 (2.02–2.37) | AA vs GG | 0.180 | |
| |
46 |
GG: 2.30 (2.14–2.45) |
AG vs GG |
0.392 |
| EGFL7 tumour | 22 | AA: 0.62 (0.41–0.83) | AA vs AG | 0.276 |
| 71 | AG: 0.51 (0.42–0.60) | AA vs GG | 0.634 | |
| |
68 |
GG: 0.58 (0.47–0.68) |
AG vs GG |
0.380 |
| EGFL7 invasive front | 15 | AA: 0.67 (0.44–0.91) | AA vs AG | 0.730 |
| 55 | AG: 0.63 (0.50–0.76) | AA vs GG | 0.162 | |
| 45 | GG: 0.49 (0.37–0.61) | AG vs GG | 0.115 | |
|
Correlations |
|||
|
Comparisons |
N |
Correlation coefficient (r) |
P-value |
| miRNA-126 vs EGFL7 tumour |
118 |
0.09 |
0.348 |
| miRNA-126 vs EGFL7 invasive front |
116 |
−0.10 |
0.281 |
| EGFL7 tumour vs EGFL7 invasive front | 119 | 0.47 | <0.0001 |
Abbreviations: CI=confidence interval; EGFL7=epidermal growth factor-like domain 7; SNP= single-nucleotide polymorphism.
N reflects the number of patient samples with complete data for the individual analyses.
P-values obtained by linear regression.
Response rates
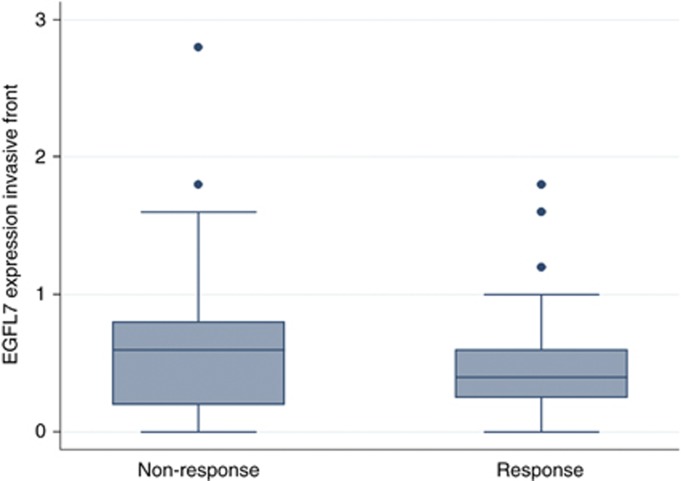
The relationship between the investigated parameters and RR is shown in Table 3. The relationship between low EGFL7 expression at the invasive front and response to the induction chemotherapy, combined with anti-VEGF-A, showed a marginal statistical significance (P=0.063, Figure 3). Using the median EGFL7 score at the invasive front (0.5) as the cutoff, the positive predictive value of a score of ⩽0.5 was 62% (40/65), whereas the negative predictive value of a score of ⩾0.6 was 65% (37/57). None of the other investigated parameters were associated with RR.
Table 3. Relations between the investigated parameters and response rates.
| |
Response rates |
|
|
|---|---|---|---|
| Parameter | Response (N), median | Non-response (N), median | P-value |
|
Pri-miRNA-126 genotypes |
(105) |
(117) |
|
| AA | 12 | 15 | — |
| AG | 51 | 49 | 0.61a |
| GG |
42 |
53 |
— |
|
miRNA-126 |
(62) |
(64) |
|
| miRNA-126 |
2.32 |
2.29 |
0.65b |
|
EGFL7 expression |
(84) |
(85) |
|
| Tumour |
0.4 |
0.6 |
0.28b |
|
EGFL7 expression |
(60) |
(62) |
|
| Invasive front | 0.4 | 0.6 | 0.06b |
Abbreviations: EGFL7=epidermal growth factor-like domain 7; miRNA-126=microRNA-126; Pri-miRNA-126=primary microRNA-126.
Fischer's exact test.
Wilcoxon rank sum test.
Figure 3.
Box plot illustrating the distribution of EGFL7 expression at the invasive front according to response rates (n=122, P=0.06). Boxes indicate the range from lower to upper quartile values, with the line inside the box representing the median. The vertical lines mark the highest and lowest value observed within a distance of 1.5 times the inter-quartile range from the bottom and the top of the boxes, respectively. Dots are outliers.
Survival
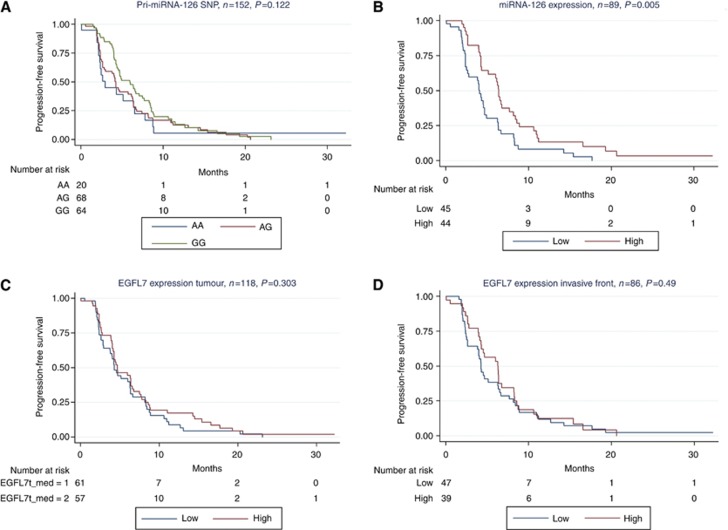
The miRNA-126 expression was the only investigated parameter significantly related to PFS (P=0.005, Figure 4). Patients with high miRNA-126 expression levels had a longer PFS on maintenance therapy. The same relationship was seen when patients were stratified according to maintenance treatment (data not shown).
Figure 4.
Progression-free survival curves according to (A) pri-miRNA-126 SNP genotypes (green=GG, red=AG, blue=AA); (B) miRNA-126 expression levels (red=above median, blue=below median); (C) EGFL7 expression in the tumour (red=above median, blue=below median); (D) EGFL7 expression at the invasive tumour front (red=above median, blue=below median). N reflects the number of patients randomised to maintenance treatment (patients with progressive disease were excluded per protocol) and thus differs from the base-line numbers presented in Table 1.
Variables demonstrating P-values <0.1 in the simple Cox model (Table 4) were included in the Cox regression model. An independent prognostic value was demonstrated in the multiple regression analysis only by miRNA-126 expression (HR=0.49, 95% CI=0.29–0.84, P=0.009).
Table 4. Cox regression analysis (n=89 in the multiple analysis).
| |
Simple analysis |
Multiple analysis |
||||
|---|---|---|---|---|---|---|
| HR | 95% CI | P-value | HR | 95% CI | P-value | |
|
Gender | ||||||
| Female | 1 | — | — | — | — | — |
| Male |
0.94 |
0.65–1.35 |
0.735 |
— |
— |
— |
|
Age | ||||||
| <Mean | 1 | — | — | — | — | — |
| >Mean |
1.04 |
0.72–1.48 |
0.850 |
— |
— |
— |
|
Resection | ||||||
| No | 1 | — | — | — | — | — |
| Yes |
0.88 |
0.61–1.28 |
0.513 |
— |
— |
— |
|
Localisation | ||||||
| Colon | 1 | — | — | — | — | — |
| Rectum |
0.94 |
0.64–1.39 |
0.773 |
— |
— |
— |
|
Adjuvant chemotherapy | ||||||
| No | 1 | — | — | 1 | — | — |
| Yes |
1.54 |
0.98–2.41 |
0.059 |
1.47 |
0.81–2.68 |
0.205 |
|
Metastatic sites | ||||||
| 1 | 1 | — | — | — | — | — |
| ⩾2 |
1.22 |
0.87–1.73 |
0.252 |
— |
— |
— |
|
Performance status | ||||||
| 0 | 1 | — | — | 1 | — | — |
| 1–2 |
1.85 |
1.25–2.74 |
0.002 |
1.57 |
0.88–2.81 |
0.124 |
|
Pri-miRNA-126 SNP | ||||||
| AA+AG | 1 | — | — | — | — | — |
| GG |
0.81 |
0.51–1.29 |
0.374 |
— |
— |
— |
|
miRNA-126 | ||||||
| <Median | 1 | 1 | ||||
| >Median |
0.52 |
0.33–0.82 |
0.005 |
0.49 |
0.29–0.84 |
0.009 |
|
EGFL7 tumour | ||||||
| <Median | 1 | — | — | — | — | — |
| >Median |
0.81 |
0.55–1.21 |
0.303 |
— |
— |
— |
|
EGFL7 invasive front | ||||||
| <Median | 1 | — | — | — | — | — |
| >Median | 0.85 | 0.54–1.35 | 0.490 | — | — | — |
Abbreviations: CI=confidence interval; EGFL7=epidermal growth factor-like domain 7; HR=hazard ratio; SNP=single-nucleotide polymorphism.
N=89 refers to the number of patients with complete data to be included in the multiple analysis.
Discussion
The present results may indicate a functional importance of the pri-miRNA-126 SNP and validate previous results regarding the prognostic value of miRNA-126 in patients with mCRC. The clinical significance of EGFL7 in CRC tissue is more speculative, suggesting a possible link between EGFL7 expression and RR to chemotherapy combined with bevacizumab.
The staining of EGFL7 resulted in varying degrees of unspecific background staining. This may partly be due to the polyclonal nature of the antibody. The test of the antibody specificity suggested a rather high specificity towards the EGFL7 protein in that almost all staining was blocked. A lower than expected frequency of neo-angiogenesis might also indicate that not all ECs were visualised. This hypothesis was investigated in adjacent tissue sections stained with CD34 in five cases with the visualisation of an increased number of ECs compared with the anti-EGFL7 staining (not shown). The shedding of the EGFL7 protein to the ECM to support the sprouting angiogenetic process will lead to a non-EC-specific expression at the tissue level, which may offer an additional explanation as to the background staining. Furthermore, it is possible that only high-EGFL7-expressing ECs were visualised, and hence, anti-EGFL7 may identify blood vessels with a high sprouting potential. EGFL7 expression was detected in cancer cells too, as also reported by Wu et al (2009) in a study of patients with hepatocellular carcinoma and by Li et al (2011) in a study on squamous cell carcinoma. One explanation for these findings could be the unspecific binding of the antibody. Other explanations could be the autocrine stimulation of tumour cells by the ECs in the case of hypoxia, in which the expression of EGFL7 is upregulated. Furthermore, a role of EGFL7 in Notch signalling seems possible (Nichol and Stuhlmann, 2012), and the endocytosed complexes of the EGFL7/Notch receptor may explain the detection of EGFL7 in the cytoplasm of the CRC cells. Future studies will hopefully clarify the optimal antibody and scoring technique for the interpretation of EGFL7 expression.
The present results may point to a functional importance of the pri-miRNA-126 SNP. Patients with the AA genotype demonstrated a tendency towards a higher expression of mature miRNA-126 in the tumours, although the difference was only marginally significant. This is in accordance with the initial report by Harnprasopwat et al (2010). As far as we know, the current pri-miRNA-126 SNP has not been reported in patients with CRC previously, but Yang et al (2011) presented a similar genotype distribution in patients with breast cancer.
The miRNA-126 expression was not correlated with the EGFL7-derived parameters, which is in accordance with previous findings (Diaz et al, 2008). Post-transcriptional inhibition of the EGFL7 mRNA by miRNA-126 might be one explanation. Differences in methodology and tumour heterogeneity may represent other explanations. Furthermore, it is likely that miRNA-126 and EGFL7 transcriptions are regulated by different stimuli (Sun et al, 2010).
EGFL7 expression at the invasive front may be lower among the responding patients, but this could not be concluded in the present study. An inverse correlation between miRNA-126 and EGFL7 could be expected a priori. The miRNA-126 may target the EGFL7 mRNA. MicroRNA-126 is downregulated in malignant tissue, whereas EGFL7 is upregulated in relation to carcinogenesis, supporting the sprouting process of immature microvessels. In contrast, miRNA-126 primarily governs the biological characteristics of more mature blood vessels with higher vascular integrity. We have previously demonstrated a relationship between the high expression of miRNA-126 in the primary tumour and the response to chemotherapy, and based on these findings, the present relationship between EGFL7 and RR might not be surprising. According to Delfortrie et al (2011), tumours with high EGFL7 expression are characterised by impaired blood vessel integrity, representing an obstacle to the delivery of chemotherapy to the tumour cells caused by a diminished pressure gradient, which is in accordance with the present results. The possible relationship between EGFL7 expression and RR was only seen regarding EGFL7 expression at the invasive front. This area characterises the growth potential of the tumour, and it might also be exposed to a higher degree to chemotherapy on account of higher interstitial pressure in the central parts of the tumour.
The lack of correlation between miRNA-126 expression and RR in the present study might be explained by differences in the methodology and the treatment. The miRNA-126 expression was estimated using the ISH supplied by an image-guided quantification in the first study (Hansen et al, 2012) and RT-qPCR in the present study. Furthermore, the two treatment regimens differ at one, perhaps very crucial, point, namely the addition of anti-VEGF-A. It is likely that this addition may alter the predictive value of an angiogenic marker such as miRNA-126. We observed no difference in the relative miRNA-126 expression between responders and non-responders (2.32 vs 2.29). However, a subgroup analysis demonstrated a lower miRNA-126 expression in patients with PD (1.91), although the statistical power of this analysis was low (only nine patients).
The present findings on the prognostic value of miRNA-126 in patients with mCRC validate the previous results (Hansen et al, 2012). An independent prognostic value favouring patients with higher levels of miRNA-126 in their primary tumour was documented. The prognostic value of different miRNAs, including miRNA-126, was analysed by Díaz et al (2008) in a cohort of 110 patients with stage I through IV colon cancer. Only 48 patients were at stage III or IV, and no prognostic value of miRNA-126 was reported. This divergence, compared with our results, may be explained by the rather heterogeneous cohort of patients in the Díaz study and the rather limited number of patients with stage IV disease. MicroRNA-126 may act as an inhibitor of the nesting process of metastatic cells and may thereby inhibit the growth potential of the metastatic disease (Tavazoie et al, 2008; Png et al, 2011).
Why was the EGFL7 expression, with a possible relationship to RR, not related to PFS? There seems to be a rather good correlation between the two effect parameters, RR and PFS, in first-line therapy, when the same treatment is given non-stop until progression (Louvet et al, 2001). This was, however, not the case in the present study. First of all, patient selection was performed by randomisation, excluding patients with PD and rendering the patient cohorts on induction treatment and maintenance treatment non-identical. Second, the treatment differs in that no chemotherapy is given during the maintenance period, and third, randomisation was performed between two different maintenance treatments. Hence, a correlation between RR and PFS cannot be taken for granted in this situation. One could speculate that high levels of EGFL7 would be related to a high degree of neo-angiogenesis and a high sprouting potential of immature and leaky blood vessels, representing an obstacle to the delivery of chemotherapy to the malignant cells under the induction treatment. These biological characteristics might be of less importance during the chemotherapy-free maintenance period, when treatment alone with anti-VEGF-A (±anti-EGFR) might actually be of benefit to the high-EGFL7-expressing tumours.
Although statistically significant results are presented, they do not immediately justify individualised treatment decisions; however, they add to the increasing clinical knowledge about anti-angiogenetics. The results might be of particular importance to future treatment strategies, including both anti-VEGF-A and anti-EGFL7 treatment of mCRC.
In conclusion, this study provides clinical data indicating a relationship between miRNA-126 and the clinical outcome of mCRC patients treated with chemotherapy combined with anti-VEGF-A, whereas the impact of EGFL7 is more speculative.
Acknowledgments
We are very thankful for the technical assistance provided by Birgit Roed Sørensen and Stine Jørgensen and for the linguistic editing provided by Karin Larsen. We thank Boye Schnack Nielsen for assistance with testing the specificity of the EGFL7 antibody. This study was supported by Roche, The Research Council of Lillebaelt Hospital, The Cancer Foundation and The Danish Council for Independent Research.
René dePont Christensen received an honorarium from Roche. The remaining authors declare no conflict of interest.
Footnotes
Disclaimer
None of the sponsors had any influence on any part of the study.
This work is published under the standard license to publish agreement. After 12 months the work will become freely available and the license terms will switch to a Creative Commons Attribution-NonCommercial-Share Alike 3.0 Unported License.
References
- Boni V, Zarate R, Villa JC, Bandres E, Gomez MA, Maiello E, Garcia-Foncillas J, Aranda E. Role of primary miRNA polymorphic variants in metastatic colon cancer patients treated with 5-fluorouracil and irinotecan. Pharmacogenomics J. 2011;11:429–436. doi: 10.1038/tpj.2010.58. [DOI] [PubMed] [Google Scholar]
- Campagnolo L, Leahy A, Chitnis S, Koschnick S, Fitch MJ, Fallon JT, Loskutoff D, Taubman MB, Stuhlmann H. EGFL7 is a chemoattractant for endothelial cells and is up-regulated in angiogenesis and arterial injury. Am J Pathol. 2005;167:275–284. doi: 10.1016/S0002-9440(10)62972-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cao Y, Tan A, Gao F, Liu L, Liao C, Mo Z. A meta-analysis of randomized controlled trials comparing chemotherapy plus bevacizumab with chemotherapy alone in metastatic colorectal cancer. Int J Colorectal Dis. 2009;24:677–685. doi: 10.1007/s00384-009-0655-9. [DOI] [PubMed] [Google Scholar]
- Chang KH, Mestdagh P, Vandesompele J, Kerin MJ, Miller N. MicroRNA expression profiling to identify and validate reference genes for relative quantification in colorectal cancer. BMC Cancer. 2010;10:173. doi: 10.1186/1471-2407-10-173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Delfortrie S, Pinte S, Mattot V, Samson C, Villain G, Caetano B, Lauridant-Philippin G, Baranzelli MC, Bonneterre J, Trottein F, Faveeuw C, Soncin F. Egfl7 promotes tumor escape from immunity by repressing endothelial cell activation. Cancer Res. 2011;71:7176–7186. doi: 10.1158/0008-5472.CAN-11-1301. [DOI] [PubMed] [Google Scholar]
- Diaz R, Silva J, Garcia JM, Lorenzo Y, Garcia V, Pena C, Rodriguez R, Munoz C, Garcia F, Bonilla F, Dominguez G. Deregulated expression of miR-106a predicts survival in human colon cancer patients. Genes Chromosomes Cancer. 2008;47:794–802. doi: 10.1002/gcc.20580. [DOI] [PubMed] [Google Scholar]
- Esquela-Kerscher A, Slack FJ. Oncomirs - microRNAs with a role in cancer. Nat Rev Cancer. 2006;6:259–269. doi: 10.1038/nrc1840. [DOI] [PubMed] [Google Scholar]
- Ferlay J, Shin HR, Bray F, Forman D, Mathers C, Parkin DM. Estimates of worldwide burden of cancer in 2008: GLOBOCAN 2008. Int J Cancer. 2010;127:2893–2917. doi: 10.1002/ijc.25516. [DOI] [PubMed] [Google Scholar]
- Filipowicz W, Bhattacharyya SN, Sonenberg N. Mechanisms of post-transcriptional regulation by microRNAs: are the answers in sight. Nat Rev Genet. 2008;9:102–114. doi: 10.1038/nrg2290. [DOI] [PubMed] [Google Scholar]
- Fish JE, Santoro MM, Morton SU, Yu S, Yeh RF, Wythe JD, Ivey KN, Bruneau BG, Stainier DY, Srivastava D. miR-126 regulates angiogenic signaling and vascular integrity. Dev Cell. 2008;15:272–284. doi: 10.1016/j.devcel.2008.07.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guo C, Sah JF, Beard L, Willson JKV, Markowitz SD, Guda K. The noncoding RNA, miR-126, suppresses the growth of neoplastic cells by targeting phosphatidylinositol 3-kinase signalling and is frequently lost in colon cancers. Genes Chromosomes Cancer. 2008;47:939–946. doi: 10.1002/gcc.20596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hansen TF, Christensen Rd, Andersen RF, Spindler KL, Johnsson A, Jakobsen A. The predictive value of single nucleotide polymorphisms in the VEGF system to the efficacy of first-line treatment with bevacizumab plus chemotherapy in patients with metastatic colorectal cancer: Results from the Nordic ACT trial. Int J Colorectal Dis. 2011;27 (6:715–720. doi: 10.1007/s00384-011-1382-6. [DOI] [PubMed] [Google Scholar]
- Hansen TF, Soerensen FB, Lindebjerg J, Jakobsen A. The predictive value of microRNA-126 in relation to first line treatment with capecitabine and oxaliplatin in patients with metastatic colorectal cancer. BMC Cancer. 2012;12:83. doi: 10.1186/1471-2407-12-83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harnprasopwat R, Ha D, Toyoshima T, Lodish H, Tojo A, Kotani A. Alteration of processing induced by a single nucleotide polymorphism in pri-miR-126. Biochem Biophys Res Commun. 2010;399:117–122. doi: 10.1016/j.bbrc.2010.07.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuhnert F, Mancuso MR, Hampton J, Stankunas K, Asano T, Chen CZ, Kuo CJ. Attribution of vascular phenotypes of the murine Egfl7 locus to the microRNA miR-126. Development. 2008;135:3989–3993. doi: 10.1242/dev.029736. [DOI] [PubMed] [Google Scholar]
- Li JJ, Yang XM, Wang SH, Tang QL. Prognostic role of epidermal growth factor-like domain 7 protein expression in laryngeal squamous cell carcinoma. J Laryngol Otol. 2011;125:1152–1157. doi: 10.1017/S0022215111002441. [DOI] [PubMed] [Google Scholar]
- Li XM, Wang AM, Zhang J, Yi H. Down-regulation of miR-126 expression in colorectal cancer and its clinical significance. Med Oncol. 2010;28:1054–1057. doi: 10.1007/s12032-010-9637-6. [DOI] [PubMed] [Google Scholar]
- Louvet C, De GA, Tournigand C, Artru P, Maindrault-Goebel F, Krulik M. Correlation between progression free survival and response rate in patients with metastatic colorectal carcinoma. Cancer. 2001;91:2033–2038. [PubMed] [Google Scholar]
- Meister J, Schmidt MH. miR-126 and miR-126*: new players in cancer. Sci World J. 2010;10:2090–2100. doi: 10.1100/tsw.2010.198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nichol D, Stuhlmann H. EGFL7: a unique angiogenic signalling factor in vascular development and disease. Blood. 2012;119:1345–1352. doi: 10.1182/blood-2011-10-322446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parker LH, Schmidt M, Jin SW, Gray AM, Beis D, Pham T, Frantz G, Palmieri S, Hillan K, Stainier DY, De Sauvage FJ, Ye W. The endothelial-cell-derived secreted factor Egfl7 regulates vascular tube formation. Nature. 2004;428:754–758. doi: 10.1038/nature02416. [DOI] [PubMed] [Google Scholar]
- Png KJ, Halberg N, Yoshida M, Tavazoie SF. A microRNA regulon that mediates endothelial recruitment and metastasis by cancer cells. Nature. 2011;481:190–194. doi: 10.1038/nature10661. [DOI] [PubMed] [Google Scholar]
- Schepeler T, Holm A, Halvey P, Nordentoft I, Lamy P, Riising EM, Christensen LL, Thorsen K, Liebler DC, Helin K, Ørntoft TF, Andersen CL. Attenuation of the beta-catenin/TCF4 complex in colorectal cancer cells induces several growth-suppressive microRNAs that target cancer promoting genes. Oncogene. 2012;31:2750–2760. doi: 10.1038/onc.2011.453. [DOI] [PubMed] [Google Scholar]
- Schepeler T, Reinert JT, Ostenfeld MS, Christensen LL, Silahtaroglu AN, Dyrskjot L, Wiuf C, Sorensen FJ, Kruhoffer M, Laurberg S, Kauppinen S, Orntoft TF, Andersen CL. Diagnostic and prognostic microRNAs in stage II colon cancer. Cancer Res. 2008;68:6416–6424. doi: 10.1158/0008-5472.CAN-07-6110. [DOI] [PubMed] [Google Scholar]
- Schmidt M, Paes K, De MA, Smyczek T, Yang S, Gray A, French D, Kasman I, Klumperman J, Rice DS, Ye W. EGFL7 regulates the collective migration of endothelial cells by restricting their spatial distribution. Development. 2007;134:2913–2923. doi: 10.1242/dev.002576. [DOI] [PubMed] [Google Scholar]
- Sun Y, Bai Y, Zhang F, Wang Y, Guo Y, Guo L. miR-126 inhibits non-small cell lung cancer cells proliferation by targeting EGFL7. Biochem Biophys Res Commun. 2010;391:1483–1489. doi: 10.1016/j.bbrc.2009.12.098. [DOI] [PubMed] [Google Scholar]
- Tavazoie SF, Alarcon C, Oskarsson T, Padua D, Wang Q, Bos PD, Gerald WL, Massague J. Endogenous human microRNAs that suppress breast cancer metastasis. Nature. 2008;451:147–152. doi: 10.1038/nature06487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Therasse P, Arbuck SG, Eisenhauer EA, Wanders J, Kaplan RS, Rubinstein L, Verweij J, Van GM, van Oosterom AT, Christian MC, Gwyther SG. New guidelines to evaluate the response to treatment in solid tumors. European Organization for Research and Treatment of Cancer, National Cancer Institute of the United States, National Cancer Institute of Canada. J Natl Cancer Inst. 2000;92:205–216. doi: 10.1093/jnci/92.3.205. [DOI] [PubMed] [Google Scholar]
- Wagner AD, Arnold D, Grothey AA, Haerting J, Unverzagt S. Anti-angiogenic therapies for metastatic colorectal cancer. Cochrane Database Syst Rev. 2009;3:CD005392. doi: 10.1002/14651858.CD005392.pub3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang S, Aurora AB, Johnson BA, Qi X, McAnally J, Hill JA, Richardson JA, Bassel-Duby R, Olson EN. The endothelial-specific microRNA miR-126 governs vascular integrity and angiogenesis. Dev Cell. 2008;15:261–271. doi: 10.1016/j.devcel.2008.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu F, Yang LY, Li YF, Ou DP, Chen DP, Fan C. Novel role for epidermal growth factor-like domain 7 in metastasis of human hepatocellular carcinoma. Hepatology. 2009;50:1839–1850. doi: 10.1002/hep.23197. [DOI] [PubMed] [Google Scholar]
- Yang R, Dick M, Marme F, Schneeweiss A, Langheinz A, Hemminki K, Sutter C, Bugert P, Wappenschmidt B, Varon R, Schott S, Weber BH, Niederacher D, Arnold N, Meindl A, Bartram CR, Schmutzler RK, Muller H, Arndt V, Brenner H, Sohn C, Burwinkel B. Genetic variants within miR-126 and miR-335 are not associated with breast cancer risk. Breast Cancer Res Treat. 2011;127:549–554. doi: 10.1007/s10549-010-1244-x. [DOI] [PubMed] [Google Scholar]