Abstract
Background:
To test the hypotheses that breast cancer patients with one to three positive lymph nodes (pN1) consist of heterogeneous prognostic subsets and that the ratio of positive nodes to total nodes dissected (lymph node ratio, LNR) might discriminate patients with a higher risk as candidates for post-mastectomy radiation therapy (PMRT).
Methods:
Using information from 7741 node-positive patients, we first identified cutoff values of the LNR using the nonparametric bootstrap method. Focusing on 3477 patients with pN1 disease, we then evaluated the clinical relevance of the LNR categorised by the estimated cutoff values (categorised LNR, cLNR).
Results:
Among 3477 patients with pN1 disease, 3059 and 418 patients were assigned into the low and intermediate cLNR groups, respectively, based on a cutoff value of 0.18. The prognostic factors associated with poor overall survival (OS) included younger age, T2 stage, negative oestrogen/progesterone receptors, high histologic grade, and intermediate cLNR. Post-mastectomy radiation therapy significantly increased OS in patients assigned to the intermediate cLNR (hazard ratio, 0.39; 95% confidence interval, 0.17–0.89; P=0.0248), whereas patients in the low cLNR group derived no additional survival benefit from PMRT.
Conclusion:
This study suggests that PMRT should be recommended for patients with pN1 disease and an intermediate cLNR.
Keywords: breast neoplasms, lymph node ratio, pN1, prognostic factor, predictive factor, post-mastectomy radiation therapy
The staging system of breast cancer allows physicians to estimate the prognosis of patients and guides treatment planning. Since the sixth edition of the American Joint Committee on Cancer (AJCC) staging system, patients with one to three positive axillary lymph nodes have been defined as having positive lymph nodes (pN1) disease (Singletary et al, 2002; Edge, 2010). Although the AJCC staging system reflects the disease state by emphasising the prognostic importance of the absolute number of affected lymph nodes (Kim et al, 2006), the rationale behind four nodes as the cutoff between pN1 and pN2 is unclear. Recently, the ratio of the number of pN1 to the number of total dissected lymph nodes (lymph node ratio, LNR) has been proposed as an alternative prognostic factor instead of the absolute number of positive nodes (Vinh-Hung et al, 2003a, 2003b; Voordeckers et al, 2004; Truong et al, 2005a; Kuru, 2006; Woodward et al, 2006; Truong et al, 2008; Vinh-Hung et al, 2009; Chagpar et al, 2011; Tausch et al, 2012).
One of the most controversial issues regarding the treatment of breast cancer is defining the indications of post-mastectomy radiation therapy (PMRT). A general consensus has been reached to advise PMRT for patients with four or more involved axillary nodes (Recht et al, 2001; Goldhirsch et al, 2009, 2011); however, the role of PMRT in patients with pN1 disease is still widely debated (Ragaz et al, 1997; Overgaard et al, 1999; Ragaz et al, 2005; Smith et al, 2005; Nielsen et al, 2006; Overgaard et al, 2007; Kim et al, 2011).
In this study, we hypothesised that patients with pN1 disease might consist of heterogeneous prognostic subsets of patients and that the LNR might be used to improve the prognostication. Furthermore, we tested LNR as a potential discriminator of patients with a higher risk who might benefit from subsequent PMRT.
Patients and methods
The Korean breast cancer registry
All data used in this study were retrieved from the Korean Breast Cancer Registry (KBCR), which has prospectively maintained an online database to store information on each patient's Korean personal identification number, age, type of surgery, primary tumour size, number of positive/dissected nodes, oestrogen receptor (ER)/progesterone receptor (PR) status, histologic grade, and details of adjuvant therapy. Nation-wide collaborative efforts from 102 general hospitals, including 41 university hospitals, have contributed to the KBCR database since 1996 (Ahn et al, 2007). Exploiting the patient's identification number as a unique identifier, we amalgamated the KBCR database with two population-based sources of information: the Korean Central Cancer Registry, which provides patient's survival data, and the Korean National Statistical Office, which publishes complete death statistics.
Cut-off values of the LNR
To define the optimal cutoff values of LNR, we first investigated the prognostic value of LNR as a continuous variable. Using multivariate Cox proportional hazards regression models, we assessed the statistical significance of the LNR adjusted for clinico-pathologic characteristics including age, tumour size, ER/PR status, histologic grade, and PMRT. As a next step, we carefully identified the upper and lower cutoff values of the LNR to classify breast cancer patients into three distinct subgroups: low, intermediate, and high. These optimal cutoff values were intended to minimise information loss by categorising the continuous variable of the LNR (categorised LNR, cLNR). All possible combinations of lower and upper cutoff values ranging from 0.001 to 0.85 with an increment of 0.001 were considered. The robustness of the estimated cutoff values was examined using the nonparametric bootstrap method (Efron, 1979) with generation of 10 000 bootstrap samples to attain the empirical sampling distributions of the estimated cutoff values.
Identification of the optimal cutoff points of the LNR was based on a population of primary breast cancer patients who had at least one positive axillary lymph node and underwent definitive mastectomy between 1998 and 2009. All of the patients received anthracycline- and taxane-based adjuvant chemotherapy according to the NCCN (National Comprehensive Cancer Network) guideline (Carlson et al, 1996). For reliable estimation of nodal involvement, patients who had fewer than 10 dissected lymph nodes were excluded. We also excluded patients who received neoadjuvant chemotherapy or breast conservation therapy, and those with ductal carcinoma in situ or stage IV disease at the time of diagnosis. As a result, a total of 7741 patients were analysed to define the optimal cutoff values of LNR.
Study population patients with pN1 disease
In this study, we intended not only to evaluate the prognostic value of the LNR but also, more importantly, to identify patients with a higher risk with pN1 disease who would benefit from PMRT after definitive initial surgery. Therefore, we restricted our study population to 3817 patients who had one to three pN1. To address the current controversy regarding PMRT, we further limited the study population to a patient cohort with T1/T2 lesions. A total of 3477 patients with pN1 disease and small tumours (T1/T2), who were treated with or without PMRT, were ultimately enrolled as our main study population. Post-mastectomy radiation therapy covered chest wall and regional lymph nodes in each patient. Irradiated volume was treated with 4 or 6-MV photons and/or electrons with median radiation dose of 50–50.4ere with 1.8–2wit per fraction. Regional lymph nodes include supraclavicular, high, and a part of low axillary lymph nodes. Internal mammary lymph nodes were included depending on physician's preference.
Focusing on these patients with pN1 disease, we first evaluated the prognostic value of the cLNR adjusted for clinico-pathologic characteristics and then investigated the clinical relevance of cLNR with respect to PMRT.
Statistics
The primary end point of this study was overall survival (OS) defined as the time from the date of surgery to death of any cause. According to the Akaike Information Criteria, the independent prognostic factors significantly associated with OS were selected in stepwise manner avoiding over-parameterisation. The treatment effect of PMRT in patients with pN1 disease was assessed for each of the cLNR groups using the likelihood ratio test. Based on the log-rank test, the Kaplan–Meier OS estimates of the cLNR groups were also compared with measure, the unadjusted treatment effects of PMRT. P-values <0.05 were considered statistically significant. All statistical analyses were performed using R (http://www.r-project.org). This study was approved by the Korean Breast Cancer Registry Committee and the Institutional Review Boards of Severance Hospital.
Results
Cutoff values of LNR
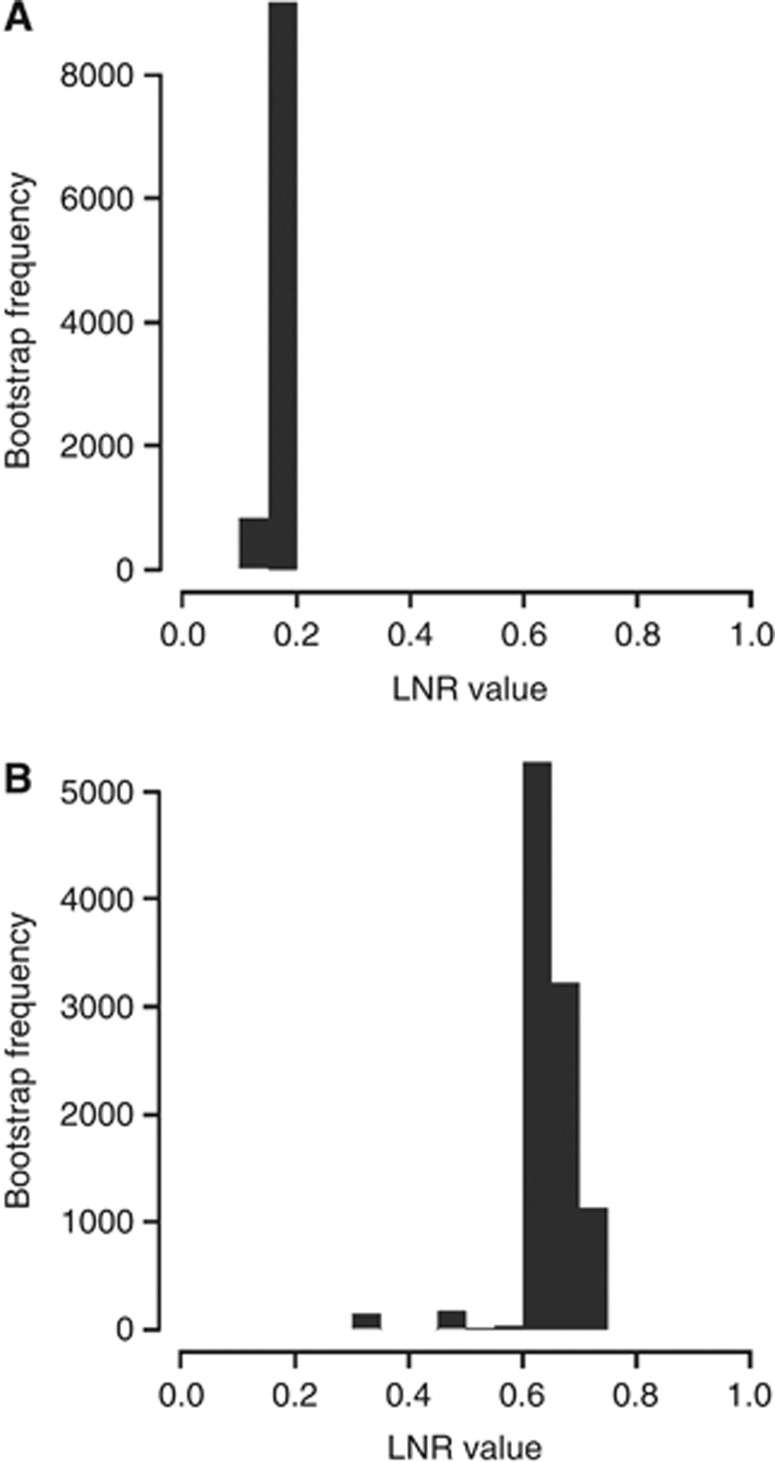
According to the multivariate analysis, the continuous variable of the LNR was the independent prognostic factor most significantly associated with OS (hazard ratio (HR), 7.57; 95% confidence interval (CI), 5.83–9.82; P<0.0001; Supplementary Table 1). On verification of the prognostic value of the LNR, the optimal lower and upper cutoff values of LNR were estimated to be 0.18 and 0.64, respectively. According to the empirical sampling distribution of the estimated cutoff values based on 10 000 bootstrap samples, the most frequent values were observed around 0.18 and 0.64, respectively (lower cutoff: mean=0.18, s.e.=0.02; upper cutoff: mean=0.64, s.e.=0.06; Figure 1).
Figure 1.
Empirical distributions of the estimated LNR cutoff values based on 10 000 bootstrap samples: (A) lower cutoff; (B) upper cutoff.
Prognostic value of the categorised LNR in patients with pN1 disease
The characteristics of the study population with pN1 disease are summarised in Table 1. Among a total of 3477 patients, 1255 (36.1%) and 2222 (63.9%) patients had T1 and T2 lesions, respectively. The mean number of dissected lymph nodes was 18.76 (s.d., 7.27). The number of patients with one, two, and three pN1 was 1699 (48.9%), 1049 (30.2%), and 729 (21.0%), respectively. As our main study population excluded patients who had fewer than 10 dissected lymph nodes, the values of the LNR ranged from 0 to 0.3. Using the lower cutoff value of 0.18, 3059 (88.0%) and 418 (12.0%) patients were assigned into the low and intermediate cLNR groups, respectively.
Table 1. Comparison of clinico-pathologic factors between patients with PMRT and without PMRT.
|
All patients (n=3477) |
PMRT (n=443) |
No PMRT (n=3034) |
|
||||
|---|---|---|---|---|---|---|---|
| n | % | n | % | n | % | P-value | |
|
Age | |||||||
| ⩽35 | 300 | 8.6 | 53 | 12 | 53 | 12 | 0.0097 |
| >35 |
3177 |
91.4 |
390 |
88 |
390 |
88 |
|
|
Sex | |||||||
| Male | 20 | 0.58 | 2 | 0 | 2 | 0 | 0.9742 |
| Female |
3457 |
99.4 |
3016 |
100 |
3016 |
100 |
|
|
T stage | |||||||
| T1 | 1255 | 36.1 | 158 | 35.7 | 158 | 35.7 | 0.8823 |
| T2 |
2222 |
63.9 |
285 |
64.3 |
285 |
64.3 |
|
| No. of dissected lymph nodes |
Mean=18.76, s.d.=7.27 | Mean=18.19, s.d.=6.76 | Mean=18.84, s.d.=7.34 | 0.0597 | |||
|
No. of positive node | |||||||
| 1 | 1699 | 48.9 | 148 | 33.4 | 148 | 33.4 | <0.0001 |
| 2 | 1049 | 30.2 | 148 | 33.4 | 148 | 33.4 | |
| 3 |
729 |
21.0 |
147 |
33.2 |
147 |
33.2 |
|
|
Histologic grade | |||||||
| I | 386 | 11.1 | 47 | 10.6 | 47 | 10.6 | 0.7857 |
| II, III |
3091 |
88.9 |
396 |
89.4 |
396 |
89.4 |
|
|
Oestrogen receptor | |||||||
| Negative | 1268 | 36.5 | 167 | 37.7 | 167 | 37.7 | 0.6012 |
| Positive |
2209 |
63.5 |
276 |
62.3 |
276 |
62.3 |
|
|
Progesterone receptor | |||||||
| Negative | 1516 | 43.6 | 178 | 40.2 | 178 | 40.2 | 0.1329 |
| Positive |
1961 |
56.4 |
265 |
59.8 |
265 |
59.8 |
|
|
Endocrine therapy | |||||||
| None | 1355 | 39 | 169 | 38.1 | 169 | 38.1 | 0.7434 |
| Done |
2122 |
61 |
274 |
61.9 |
274 |
61.9 |
|
|
cLNR | |||||||
| Low (⩽0.18) | 3059 | 88 | 349 | 78.8 | 349 | 78.8 | <0.0001 |
| Intermediate (0.18–0.3) | 418 | 12 | 94 | 21.2 | 94 | 21.2 | |
Abbreviations: cLNR=categorised lymph node ratio; PMRT=post-mastectomy radiation therapy.
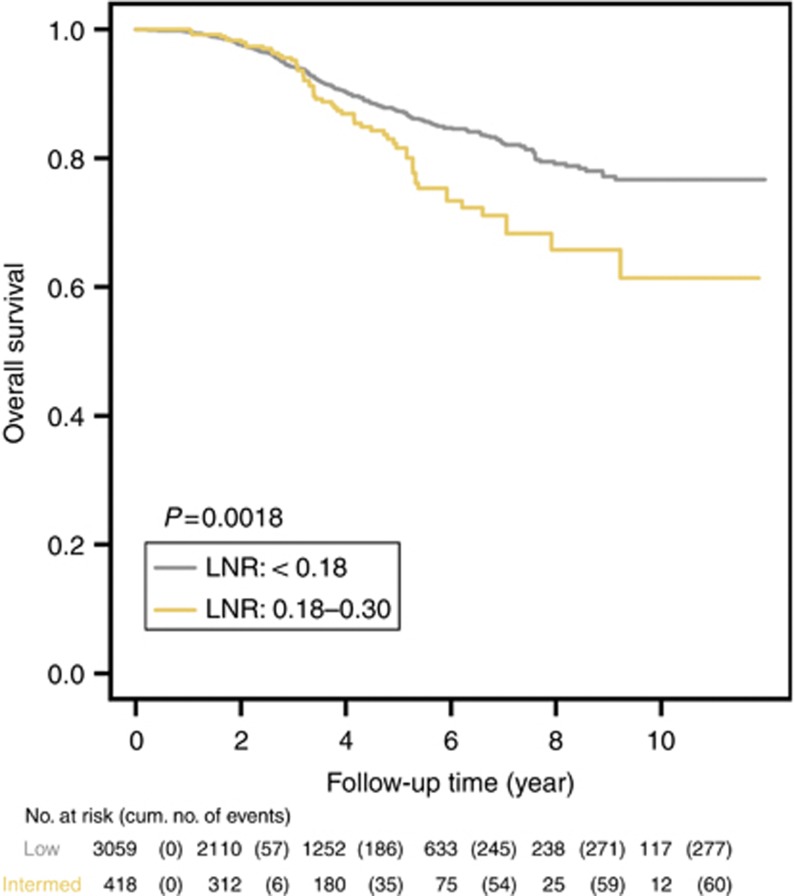
The median follow-up time was 3.3 years. Based on the multivariate analysis, younger age (<35 years), large tumour size (T2 lesion), negative ER/PR status, and high histologic grade (II and III) were significantly associated with poor OS (Table 2). The cLNR was also a significant independent prognostic factor. Compared with the low cLNR group, the adjusted HR of the intermediate cLNR group was 1.81 (95% CI, 1.34–2.45, P=0.0001; Table 2). There was a statistically significant separation between the Kaplan–Meier survival curves of the low and intermediate cLNR groups (P=0.0018) (Figure 2). The estimated survival rate at 10 years was higher in the low cLNR group (76.7%) than in the intermediate cLNR group (61.4%).
Table 2. Multivariate analysis of overall survival of breast cancer patients with pN1 disease.
| Variable | Hazard ratio | 95% CI | P-value |
|---|---|---|---|
| Age, ⩾36 |
0.55 |
0.41–0.74 |
<0.0001 |
| Tumour size, T2 |
1.31 |
1.02–1.67 |
0.0336 |
| Oestrogen receptor, positive |
0.68 |
0.53–0.89 |
0.0042 |
| Progesterone receptor, positive |
0.54 |
0.42–0.71 |
<0.0001 |
| Histologic grade, II and III |
1.65 |
1.03–2.63 |
0.0380 |
| cLNR, intermediate |
1.81 |
1.34–2.45 |
0.0001 |
| cLNR, low: PMRT, yes |
1.25 |
0.86–1.82 |
0.2415 |
| cLNR, intermediate: PMRT, yes | 0.39 | 0.17–0.89 | 0.0248 |
Abbreviations: CI=confidence interval; cLNR=categorised lymph node ratio; PMRT=post-mastectomy radiation therapy; pN1=positive lymph nodes.
Figure 2.
Kaplan–Meier OS estimates of breast cancer patients with pN1 disease according to the cLNR risk groups.
Predictive value of categorised LNR for efficacy of PMRT in patients with pN1 disease
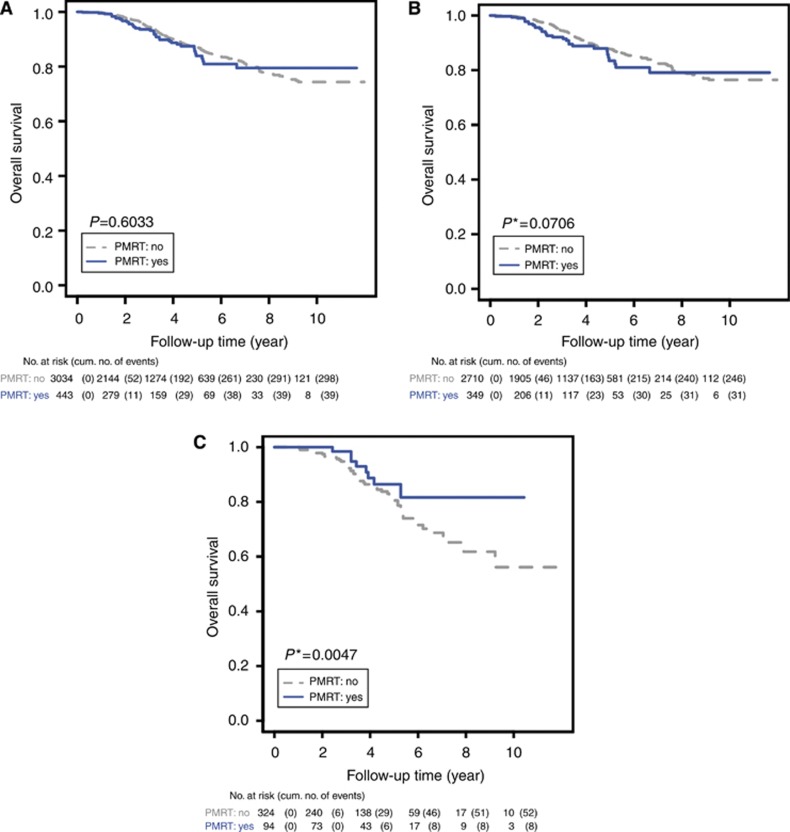
The study population included 443 (12.7%) patients who received PMRT after mastectomy. As indicated by the interaction terms between PMRT and the cLNR groups, there was a significant benefit of PMRT for the intermediate cLNR group (likelihood ratio test, P=0.0248; Table 2). In contrast, there was no apparent benefit of PMRT for patients with a low cLNR (likelihood ratio test, P=0.2415). For patients with an intermediate cLNR, PMRT significantly reduced relative risk (HR, 0.39; 95% CI, 0.17–0.89) and absolute risk (25.48% s.e., 15.36% Supplementary Figure 1).
As expected, for the intermediate cLNR group PMRT greatly increased the estimated survival rate at 10 years from 56.13% to 81.61% (Table 3, Figure 3C), and Kaplan–Meier survival curves according to PMRT or no PMRT indicated a statistically significant difference (P=0.0047). In contrast, there was no significant increase in the estimated survival rates at 10 years for patients with PMRT in the low cLNR group (76.47% vs 79.10; Table 3, Figure 3B) and in the whole study population with pN1 disease (74.35% vs 79.48% Table 3, Figure 3A). Consistent with these findings, PMRT did not have a significant effect on the Kaplan–Meier survival curves for the low pLNR group (P=0.0706) or for all patients with pN1 disease (P=0.6033).
Table 3. Kaplan–Meier overall survival estimates at 10 years for patients with pN1 disease classified by the cLNR risk groups.
| |
|
PMRT: no |
PMRT: yes |
||||
|---|---|---|---|---|---|---|---|
| Group | No. of patients | 10-Year OS (%) | 95% CI | No. of Patients | 10-Year OS (%) | 95% CI | No. of Patients |
| All patients |
3477 |
74.35 |
70.93–77.93 |
3034 |
79.48 |
73.11–86.40 |
443 |
| Low cLNR |
3059 |
76.47 |
73.01–80.10 |
2710 |
79.10 |
71.81–87.14 |
349 |
| IntermediatecLNR | 418 | 56.13 | 43.40–72.58 | 324 | 81.61 | 69.78–95.45 | 94 |
Abbreviations: CI=confidence interval; cLNR=categorised lymph node ratio; OS=overall survival; PMRT=post-mastectomy radiation therapy; pN1=positive lymph nodes.
Figure 3.
Kaplan–Meier OS estimates of breast cancer patients with pN1 disease according to PMRT: (A) all patients with pN1 disease; (B) patients with the low cLNR; and (C) patients with the intermediate cLNR. *Indicates P-value adjusted for multiple comparisons using Tukey's procedure.
Discussion
The presence of axillary lymph node metastasis is the single most important prognostic factor in patients with breast cancer (Carter et al, 1989; Fitzgibbons et al, 2000; Vinh-Hung et al, 2003a), and the prognostic importance of the total number of pN1 has long been recognised (Carter et al, 1989; Fisher et al, 1993; Recht et al, 1999; Fitzgibbons et al, 2000; Edge, 2010). The latest AJCC staging system defined patients with one to three positive axillary lymph nodes as pN1 disease (Singletary et al, 2002; Edge, 2010), while patients with more than four positive axillary lymph nodes are classified as pN2 or pN3. Although patients with pN2 or pN3 disease have a clearly worse prognosis than those with pN1 disease (Axelsson et al, 1992; Kim et al, 2006), the biologic rationale for four nodes as the threshold between pN1 and pN2 is unclear.
We hypothesised that patients with pN1 disease might comprise different prognostic subsets of patients, mainly for the following two reasons: first, in past analysis of the SEER (Surveillance, Epidemiology and End Results) database, (Vinh-Hung et al 2003a) reported that the relative mortality hazard continues to increase with each involved node without any obvious cutoff point. Second, the current staging system of breast cancer is based merely on the number of pN1 without considering the total number of lymph nodes dissected. In cases where only a few lymph nodes are examined, there is the potential possibility of downstaging the axilla (Vinh-Hung et al, 2009; Danko et al, 2010).
The LNR has been proposed as an alternative measure to improve the prognostication system in patients with node-positive disease (Vinh-Hung et al, 2003a, 2003b; Voordeckers et al, 2004; Truong et al, 2005a; Kuru, 2006; Woodward et al, 2006; Truong et al, 2008; Vinh-Hung et al, 2009; Danko et al, 2010; Chagpar et al, 2011; Tausch et al, 2012). Compared with the number-based staging system, the ratio-based staging system exploits additional information on the total number of lymph nodes dissected. However, the use of additional information does not rule out the issue of misclassification. For example, if the total number of lymph nodes removed is very small, a high LNR may result from insufficient dissection of the axillary lymph node. In this case, the ratio-based staging system may result in upstaging of patients.
For reliable estimation of nodal involvement by the LNR, it would be desirable to define a minimum number of dissected lymph nodes. Previous observational studies have shown that sampling fewer than 10 axillary nodes more than doubles the risk of subsequent loco-regional failure (Recht et al, 1999; Katz et al, 2000, 2001; Woodward et al, 2003), and that the estimation of nodal involvement is more reliable when at least 10 nodes are excised (Fisher et al, 2002). The AJCC requires that a minimum of six axillary lymph nodes be removed and examined whereas other authorities recommend that at least 10 lymph nodes be examined (National Institutes of Health, 1991; The Steering Committee, 1998). To ensure that patients in this study received appropriate axillary dissection, we restricted the study population to patients who had a minimum of 10 dissected lymph nodes.
We initially aimed to evaluate the clinical value of the LNR among patients with pN1 disease. As a preliminary step, we confirmed the prognostic value of the LNR as a continuous variable for all node-positive patients using multivariate Cox regression analysis (Supplementary Table 1). Subsequently, we categorised the LNR by lower and upper cutoff values of 0.18 and 0.64, respectively, and all node-positive patients were assigned into low, intermediate, and high cLNR groups, respectively. As expected, there were significant separations among the Kaplan–Meier OS estimates for the three cLNR groups (P<0.0001; Supplementary Figure 2).
Next, we evaluated the clinical value of the cLNR, focusing on patients with pN1 disease. For patients with pN1 disease, the LNR ranged from 0 to 0.3 because only patients with at least 10 dissected nodes were included in our study population. As a result, all of the patients with one positive node were assigned into the low cLNR group, while patients having two and three positive nodes were assigned into low and intermediate cLNR groups according to the total number of dissected nodes. By multivariate analysis, we demonstrated that the cLNR further classified patients with pN1 disease into two heterogeneous prognostic subsets, with patients in the intermediate cLNR group having a clearly worse prognosis than those in the low cLNR group in terms of OS.
After ascertaining that the cLNR was indeed significantly associated with OS, we further investigated the treatment effects of PMRT in patients with pN1 disease. A clear survival benefit of PMRT was observed in patients assigned to the intermediate cLNR group, in which PMRT was associated with a 61% reduction in overall mortality (Table 2). Truong et al (2005b) also suggested that the percentage of node positive (>25% of removed nodes) were statistically significant independent factors associated with greater loco-regional recurrence, meriting consideration of PMRT in patients with pN1 and T1/T2 disease.
Currently, the general consensus is that PMRT is indicated for patients with four or more involved axillary nodes. However, there is no agreement on whether all patients with pN1 disease should be recommended for PMRT (Recht et al, 2001; Goldhirsch et al, 2009, 2011). The previous retrospective study using the SEER database reported no remarkable survival benefit of PMRT for patients with pN1 disease (Smith et al, 2005). However, according to the latest analysis of the Early Breast Cancer Trialists' Collaboration Group, PMRT reduced local recurrence risk and mortality in patients with node-positive disease regardless of the degree of nodal involvement (Clarke et al, 2005). More importantly, recent randomised prospective trials demonstrated improved survival after PMRT in all node-positive women (Ragaz et al, 1997; Overgaard et al, 1999; Ragaz et al, 2005; Nielsen et al, 2006), and the survival increment was at least equally beneficial in patients with pN1 disease (Overgaard et al, 2007). Despite the level 1 evidence of the above randomised trials supporting the benefits of PMRT, it should be noted that these randomised trials have been criticised for their less extensive axillary surgery and relatively high risk of loco-regional recurrence (Overgaard et al, 1997, 1999; Recht et al, 1999; Katz et al, 2000; Woodward et al, 2003; Ragaz et al, 2005; Nielsen et al, 2006). As such, we believe that interpretation of the benefit of PMRT should be limited to patients with fewer than 10 dissected lymph nodes. The ongoing randomised MRC/EORTC SUPREMO trial is designed to evaluate the results of chest wall irradiation in the management of the patients who underwent MRM with pN1 disease. It may provide us better information regarding the role of PMRT in this patient group (Kunkler et al, 2008).
According to results of this study, we suggest that the indications of PMRT in patients with pN1 disease are as follows: first, among patients with at least 10 dissected lymph nodes, PMRT should be recommended for patients with two and three positive nodes, who would be classified into an intermediate cLNR group. Second, an additional survival benefit of PMRT is less clear for patients with one positive lymph node and at least 10 dissected lymph nodes.
The inherent limitation of this retrospective study is that patients were assigned to PMRT without randomisation, and it is therefore possible that unrecognised biases might influence our results. Nevertheless, most of the clinico-pathologic characteristics such as T stage, histologic grade, ER/PR, and number of dissected node were well-balanced between the two groups of patients with or without PMRT (Table 1). However, we did find that patients with three positive nodes and younger age were more likely to receive PMRT and consequently the patients who were treated with PMRT might have been biased toward having a worse prognosis. This limitation of our study was in part balanced by several strengths. First, LNR research to date has been limited because there is no clear consensus about the cutoff points and many researchers have therefore used their own criteria (Voordeckers et al, 2004; Truong et al, 2005a; Kuru, 2006; Truong et al, 2008; Danko et al, 2010; Chagpar et al, 2011; Tausch et al, 2012). However, we objectively determined robust cutoff values using a nonparametric resampling method that does not require any predefined assumptions or distributional specifications. Second, this study not only evaluated the prognostic value of the cLNR, but also tested the clinical benefit of PMRT in patients with pN1 disease. To the best of our knowledge, there has been no previous report explicitly using cLNR as a predictive marker for PMRT, especially focusing on patients with pN1 disease. Third, the data analysed in this study were retrieved from the nation-wide KBCR. Therefore, we could minimise the selection bias often created by sampling from a single institution. Finally, all of the patients included in this study were treated with systemic adjuvant chemotherapy, thus avoiding biases related to systemic treatment that might affect evaluation of the benefit of PMRT.
In conclusion, our study showed that the cLNR enhanced risk stratification in patients with pN1 disease and that patients with intermediate cLNR had a worse prognosis than those with low cLNR. The cLNR also identified a subgroup of patients with pN1 disease who would respond better to PMRT. Provided appropriate axillary dissection is satisfied as a prerequisite, PMRT should be recommended for patients with two or three positive nodes who belong to the intermediate cLNR group.
The authors declare no conflict of interest.
Footnotes
Supplementary Information accompanies this paper on British Journal of Cancer website (http://www.nature.com/bjc)
Supplementary Material
References
- Ahn SH, Son BH, Kim SW, Kim SI, Jeong J, Ko SS, Han W. Poor outcome of hormone receptor-positive breast cancer at very young age is due to tamoxifen resistance: nationwide survival data in Korea—a report from the Korean Breast Cancer Society. J Clin Oncol. 2007;25:2360–2368. doi: 10.1200/JCO.2006.10.3754. [DOI] [PubMed] [Google Scholar]
- Axelsson CK, Mouridsen HT, Zedeler K. Axillary dissection of level I and II lymph nodes is important in breast cancer classification. The Danish Breast Cancer Cooperative Group (DBCG) Eur J Cancer. 1992;28A:1415–1418. doi: 10.1016/0959-8049(92)90534-9. [DOI] [PubMed] [Google Scholar]
- Carlson RW, Goldstein LJ, Gradishar WJ, Lichter AS, McCormick B, Moe RE, Theriault RL. NCCN Breast Cancer Practice Guidelines. The National Comprehensive Cancer Network. Oncology (Williston Park) 1996;10:47–75. [PubMed] [Google Scholar]
- Carter CL, Allen C, Henson DE. Relation of tumor size, lymph node status, and survival in 24,740 breast cancer cases. Cancer. 1989;63:181–187. doi: 10.1002/1097-0142(19890101)63:1<181::aid-cncr2820630129>3.0.co;2-h. [DOI] [PubMed] [Google Scholar]
- Chagpar AB, Camp RL, Rimm DL. Lymph node ratio should be considered for incorporation into staging for breast cancer. Ann Surg Oncol. 2011;18:3143–3148. doi: 10.1245/s10434-011-2012-9. [DOI] [PubMed] [Google Scholar]
- Clarke M, Collins R, Darby S, Davies C, Elphinstone P, Evans E, Godwin J, Gray R, Hicks C, James S, MacKinnon E, McGale P, McHugh T, Peto R, Taylor C, Wang Y. Effects of radiotherapy and of differences in the extent of surgery for early breast cancer on local recurrence and 15-year survival: an overview of the randomised trials. Lancet. 2005;366:2087–2106. doi: 10.1016/S0140-6736(05)67887-7. [DOI] [PubMed] [Google Scholar]
- Danko ME, Bennett KM, Zhai J, Marks JR, Olson JA., Jr. Improved staging in node-positive breast cancer patients using lymph node ratio: results in 1 788 patients with long-term follow-up. J Am Coll Surg. 2010;210 (797-805 e791:805–797. doi: 10.1016/j.jamcollsurg.2010.02.045. [DOI] [PubMed] [Google Scholar]
- Edge SB, American Joint Committee on Cancer. American Cancer Society 2010AJCC Cancer Staging Handbook: From the AJCC Cancer Staging Manual7th (edn).Springer: New York; [DOI] [PubMed] [Google Scholar]
- Efron B. Bootstrap methods: another look at the jackknife. Ann Stat. 1979;7:1–26. [Google Scholar]
- Fisher B, Jeong JH, Anderson S, Bryant J, Fisher ER, Wolmark N. Twenty-five-year follow-up of a randomized trial comparing radical mastectomy, total mastectomy, and total mastectomy followed by irradiation. N Engl J Med. 2002;347:567–575. doi: 10.1056/NEJMoa020128. [DOI] [PubMed] [Google Scholar]
- Fisher ER, Costantino J, Fisher B, Redmond C. Pathologic findings from the National Surgical Adjuvant Breast Project (Protocol 4). Discriminants for 15-year survival. National Surgical Adjuvant Breast and Bowel Project Investigators. Cancer. 1993;71:2141–2150. doi: 10.1002/1097-0142(19930315)71:6+<2141::aid-cncr2820711603>3.0.co;2-f. [DOI] [PubMed] [Google Scholar]
- Fitzgibbons PL, Page DL, Weaver D, Thor AD, Allred DC, Clark GM, Ruby SG, O'Malley F, Simpson JF, Connolly JL, Hayes DF, Edge SB, Lichter A, Schnitt SJ. Prognostic factors in breast cancer. College of American Pathologists Consensus Statement 1999. Arch Pathol Lab Med. 2000;124:966–978. doi: 10.5858/2000-124-0966-PFIBC. [DOI] [PubMed] [Google Scholar]
- Goldhirsch A, Ingle JN, Gelber RD, Coates AS, Thurlimann B, Senn HJ. Thresholds for therapies: highlights of the St Gallen International Expert Consensus on the primary therapy of early breast cancer 2009. Ann Oncol. 2009;20:1319–1329. doi: 10.1093/annonc/mdp322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldhirsch A, Wood WC, Coates AS, Gelber RD, Thurlimann B, Senn HJ. Strategies for subtypes—dealing with the diversity of breast cancer: highlights of the St. Gallen International Expert Consensus on the Primary Therapy of Early Breast Cancer 2011. Ann Oncol. 2011;22:1736–1747. doi: 10.1093/annonc/mdr304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Katz A, Buchholz TA, Thames H, Smith CD, McNeese MD, Theriault R, Singletary SE, Strom EA. Recursive partitioning analysis of locoregional recurrence patterns following mastectomy: implications for adjuvant irradiation. Int J Radiat Oncol Biol Phys. 2001;50:397–403. doi: 10.1016/s0360-3016(01)01465-1. [DOI] [PubMed] [Google Scholar]
- Katz A, Strom EA, Buchholz TA, Thames HD, Smith CD, Jhingran A, Hortobagyi G, Buzdar AU, Theriault R, Singletary SE, McNeese MD. Locoregional recurrence patterns after mastectomy and doxorubicin-based chemotherapy: implications for postoperative irradiation. J Clin Oncol. 2000;18:2817–2827. doi: 10.1200/JCO.2000.18.15.2817. [DOI] [PubMed] [Google Scholar]
- Kim SI, Park BW, Lee KS. Comparison of stage-specific outcome of breast cancer based on 5th and 6th AJCC staging system. J Surg Oncol. 2006;93:221–227. doi: 10.1002/jso.20513. [DOI] [PubMed] [Google Scholar]
- Kim SI, Park S, Park HS, Kim YB, Suh CO, Park BW. Comparison of treatment outcome between breast-conservation surgery with radiation and total mastectomy without radiation in patients with one to three positive axillary lymph nodes. Int J Radiat Oncol Biol Phys. 2011;80:1446–1452. doi: 10.1016/j.ijrobp.2010.04.051. [DOI] [PubMed] [Google Scholar]
- Kunkler IH, Canney P, van Tienhoven G, Russell NS, Mrc Eortc Supremo Trial Management Group Elucidating the role of chest wall irradiation in 'intermediate-risk' breast cancer: the MRC/EORTC SUPREMO trial. Clin Oncol (R Coll Radiol) 2008;20:31–34. doi: 10.1016/j.clon.2007.10.004. [DOI] [PubMed] [Google Scholar]
- Kuru B. Prognostic significance of total number of nodes removed, negative nodes removed, and ratio of positive nodes to removed nodes in node positive breast carcinoma. Eur J Surg Oncol. 2006;32:1082–1088. doi: 10.1016/j.ejso.2006.06.005. [DOI] [PubMed] [Google Scholar]
- National Institutes of Health NIH consensus conference. Treatment of early-stage breast cancer. JAMA. 1991;265:391–395. [PubMed] [Google Scholar]
- Nielsen HM, Overgaard M, Grau C, Jensen AR, Overgaard J. Loco-regional recurrence after mastectomy in high-risk breast cancer—risk and prognosis. An analysis of patients from the DBCG 82 b&c randomization trials. Radiother Oncol. 2006;79:147–155. doi: 10.1016/j.radonc.2006.04.006. [DOI] [PubMed] [Google Scholar]
- Overgaard M, Hansen PS, Overgaard J, Rose C, Andersson M, Bach F, Kjaer M, Gadeberg CC, Mouridsen HT, Jensen MB, Zedeler K. Postoperative radiotherapy in high-risk premenopausal women with breast cancer who receive adjuvant chemotherapy. Danish Breast Cancer Cooperative Group 82b Trial. N Engl J Med. 1997;337:949–955. doi: 10.1056/NEJM199710023371401. [DOI] [PubMed] [Google Scholar]
- Overgaard M, Jensen MB, Overgaard J, Hansen PS, Rose C, Andersson M, Kamby C, Kjaer M, Gadeberg CC, Rasmussen BB, Blichert-Toft M, Mouridsen HT. Postoperative radiotherapy in high-risk postmenopausal breast-cancer patients given adjuvant tamoxifen: Danish Breast Cancer Cooperative Group DBCG 82c randomised trial. Lancet. 1999;353:1641–1648. doi: 10.1016/S0140-6736(98)09201-0. [DOI] [PubMed] [Google Scholar]
- Overgaard M, Nielsen HM, Overgaard J. Is the benefit of postmastectomy irradiation limited to patients with four or more positive nodes, as recommended in international consensus reports? A subgroup analysis of the DBCG 82 b&c randomized trials. Radiother Oncol. 2007;82:247–253. doi: 10.1016/j.radonc.2007.02.001. [DOI] [PubMed] [Google Scholar]
- Ragaz J, Jackson SM, Le N, Plenderleith IH, Spinelli JJ, Basco VE, Wilson KS, Knowling MA, Coppin CM, Paradis M, Coldman AJ, Olivotto IA. Adjuvant radiotherapy and chemotherapy in node-positive premenopausal women with breast cancer. N Engl J Med. 1997;337:956–962. doi: 10.1056/NEJM199710023371402. [DOI] [PubMed] [Google Scholar]
- Ragaz J, Olivotto IA, Spinelli JJ, Phillips N, Jackson SM, Wilson KS, Knowling MA, Coppin CM, Weir L, Gelmon K, Le N, Durand R, Coldman AJ, Manji M. Locoregional radiation therapy in patients with high-risk breast cancer receiving adjuvant chemotherapy: 20-year results of the British Columbia randomized trial. J Natl Cancer Inst. 2005;97:116–126. doi: 10.1093/jnci/djh297. [DOI] [PubMed] [Google Scholar]
- Recht A, Edge SB, Solin LJ, Robinson DS, Estabrook A, Fine RE, Fleming GF, Formenti S, Hudis C, Kirshner JJ, Krause DA, Kuske RR, Langer AS, Sledge GW, Jr, Whelan TJ, Pfister DG. Postmastectomy radiotherapy: clinical practice guidelines of the American Society of Clinical Oncology. J Clin Oncol. 2001;19:1539–1569. doi: 10.1200/JCO.2001.19.5.1539. [DOI] [PubMed] [Google Scholar]
- Recht A, Gray R, Davidson NE, Fowble BL, Solin LJ, Cummings FJ, Falkson G, Falkson HC, SGt Taylor, Tormey DC. Locoregional failure 10 years after mastectomy and adjuvant chemotherapy with or without tamoxifen without irradiation: experience of the Eastern Cooperative Oncology Group. J Clin Oncol. 1999;17:1689–1700. doi: 10.1200/JCO.1999.17.6.1689. [DOI] [PubMed] [Google Scholar]
- Singletary SE, Allred C, Ashley P, Bassett LW, Berry D, Bland KI, Borgen PI, Clark G, Edge SB, Hayes DF, Hughes LL, Hutter RV, Morrow M, Page DL, Recht A, Theriault RL, Thor A, Weaver DL, Wieand HS, Greene FL. Revision of the American Joint Committee on Cancer staging system for breast cancer. J Clin Oncol. 2002;20:3628–3636. doi: 10.1200/JCO.2002.02.026. [DOI] [PubMed] [Google Scholar]
- Smith BD, Smith GL, Haffty BG. Postmastectomy radiation and mortality in women with T1-2 node-positive breast cancer. J Clin Oncol. 2005;23:1409–1419. doi: 10.1200/JCO.2005.05.100. [DOI] [PubMed] [Google Scholar]
- Tausch C, Taucher S, Dubsky P, Seifert M, Reitsamer R, Kwasny W, Jakesz R, Fitzal F, Filipcic L, Fridrik M, Greil R, Gnant M. Prognostic value of number of removed lymph nodes, number of involved lymph nodes, and lymph node ratio in 7502 breast cancer patients enrolled onto trials of the Austrian Breast and Colorectal Cancer Study Group (ABCSG) Ann Surg Oncol. 2012;19:1808–1817. doi: 10.1245/s10434-011-2189-y. [DOI] [PubMed] [Google Scholar]
- The Steering Committee The Steering Committee on Clinical Practice Guidelines for the Care and Treatment of Breast Cancer. CMAJ. 1998;158:S1–S2. [PubMed] [Google Scholar]
- Truong PT, Berthelet E, Lee J, Kader HA, Olivotto IA. The prognostic significance of the percentage of positive/dissected axillary lymph nodes in breast cancer recurrence and survival in patients with one to three positive axillary lymph nodes. Cancer. 2005a;103:2006–2014. doi: 10.1002/cncr.20969. [DOI] [PubMed] [Google Scholar]
- Truong PT, Olivotto IA, Kader HA, Panades M, Speers CH, Berthelet E. Selecting breast cancer patients with T1-T2 tumors and one to three positive axillary nodes at high postmastectomy locoregional recurrence risk for adjuvant radiotherapy. Int J Radiat Oncol Biol Phys. 2005b;61:1337–1347. doi: 10.1016/j.ijrobp.2004.08.009. [DOI] [PubMed] [Google Scholar]
- Truong PT, Vinh-Hung V, Cserni G, Woodward WA, Tai P, Vlastos G. The number of positive nodes and the ratio of positive to excised nodes are significant predictors of survival in women with micrometastatic node-positive breast cancer. Eur J Cancer. 2008;44:1670–1677. doi: 10.1016/j.ejca.2008.05.011. [DOI] [PubMed] [Google Scholar]
- Vinh-Hung V, Burzykowski T, Cserni G, Voordeckers M, Van De Steene J, Storme G. Functional form of the effect of the numbers of axillary nodes on survival in early breast cancer. Int J Oncol. 2003a;22:697–704. [PubMed] [Google Scholar]
- Vinh-Hung V, Cserni G, Burzykowski T, van de Steene J, Voordeckers M, Storme G. Effect of the number of uninvolved nodes on survival in early breast cancer. Oncol Rep. 2003b;10:363–368. [PubMed] [Google Scholar]
- Vinh-Hung V, Verkooijen HM, Fioretta G, Neyroud-Caspar I, Rapiti E, Vlastos G, Deglise C, Usel M, Lutz JM, Bouchardy C. Lymph node ratio as an alternative to pN staging in node-positive breast cancer. J Clin Oncol. 2009;27:1062–1068. doi: 10.1200/JCO.2008.18.6965. [DOI] [PubMed] [Google Scholar]
- Voordeckers M, Vinh-Hung V, Van de Steene J, Lamote J, Storme G. The lymph node ratio as prognostic factor in node-positive breast cancer. Radiother Oncol. 2004;70:225–230. doi: 10.1016/j.radonc.2003.10.015. [DOI] [PubMed] [Google Scholar]
- Woodward WA, Strom EA, Tucker SL, Katz A, McNeese MD, Perkins GH, Buzdar AU, Hortobagyi GN, Hunt KK, Sahin A, Meric F, Sneige N, Buchholz TA. Locoregional recurrence after doxorubicin-based chemotherapy and postmastectomy: Implications for breast cancer patients with early-stage disease and predictors for recurrence after postmastectomy radiation. Int J Radiat Oncol Biol Phys. 2003;57:336–344. doi: 10.1016/s0360-3016(03)00593-5. [DOI] [PubMed] [Google Scholar]
- Woodward WA, Vinh-Hung V, Ueno NT, Cheng YC, Royce M, Tai P, Vlastos G, Wallace AM, Hortobagyi GN, Nieto Y. Prognostic value of nodal ratios in node-positive breast cancer. J Clin Oncol. 2006;24:2910–2916. doi: 10.1200/JCO.2005.03.1526. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.