Abstract
Background:
Epidermal growth factor receptor (EGFR) is highly expressed in adenoid cystic carcinoma (ACC). The efficacy and toxicity of cetuximab with concomitant platinum-based chemoradio- or chemotherapy in patients with locally advanced or metastatic ACC, respectively, was evaluated.
Methods:
Eligible patients (9 with locally advanced tumour and 12 with metastases) had positive tumour EGFR expression. The cetuximab loading dose (400 mg m−2) was followed by 250 mg m−2 per week. Locally advanced tumours were irradiated (mean dose 65 Gy) and treated with concomitant cisplatin (75 mg m−2, intravenously). Patients with metastases received concomitant cisplatin and 5-fluorouracil (4 × 1000 mg m−2).
Results:
For patients with locally advanced disease (median follow-up: 52 months), the median progression-free survival (PFS) was 64 months and the 2-year overall survival (OS) rate was 100%. For patients with metastases (median follow-up: 72 months), the median PFS and OS were 13 and 24 months, respectively. In both groups the objective response rate was >40%. Skin rash, in-field dermatitis, mucositis and vomiting were the most frequent grade 3/4 adverse events.
Conclusion:
In this single-arm study, the efficacy of cetuximab plus chemoradio- or chemotherapy appeared favourable as compared with historical controls. All side effects were manageable and did not hamper the treatment.
Keywords: cetuximab, adenoid cystic carcinoma, radiotherapy, chemotherapy
Adenoid cystic carcinomas (ACCs) are rare and occur mostly in salivary glands. They represent 10–20% of tumours of salivary glands and 1% of head and neck cancers. Their occurrence in other secretory glands (breast, colon, prostate, etc.) is very rare. This type of tumour is slow growing with a high potential for local recurrence (Dubergé et al, 2012). The natural history of ACC is often protracted and the patients usually live 10–20 years after the diagnosis. Approximately 50% of patients develop distant metastases. Ten per cent of metastatic patients also survive >10 years, whereas 33% of patients die within 2 years (Chau et al, 2012).
The initial therapy of these malignancies consists of surgical resection followed by radiotherapy. The role of systemic therapy is in the management of local recurrence, locally advanced and metastatic disease, which are not amenable to surgical intervention and/or radiotherapy (Hotte et al, 2005). Traditional chemotherapies are not associated with survival advantage (Laurie et al, 2011). An overall response rate between 1 and 9% was reported in a review of 11 studies using seven single-agent chemotherapies. The best results were achieved by cisplatin (Papaspyrou et al, 2011). The concurrent radiochemotherapeutic approaches where various platinum-based regimens were tested have not evolved beyond retrospective analyses; however, these options lead to sustained locoregional tumour control (Haddad et al, 2006; Samant et al, 2012). More recently, targeted therapies are expected to improve the efficacy of treatment. Unfortunately, their theoretical promise has yet to be fulfilled (Le Tourneau et al, 2011). In view of frequent epidermal growth factor receptor (EGFR) positivity in ACC (74–91%) (Vered et al, 2002; Agulnik et al, 2007; Macarenco et al, 2008; Dahse et al, 2009; Locati et al, 2009a), the EGFR antibody cetuximab seemed promising. Despite initial euphoria, the treatment results of cetuximab monotherapy failed to impress (Locati et al, 2009b). The combination of cetuximab with concomitant radiotherapy was well tolerated and resulted in high local control and treatment response rates, but the survivals were hampered by distant failure (Jensen et al, 2010). Combination of cetuximab plus chemotherapy was used very rarely (only in two case reports), resulting in objective response (De Dosso et al, 2009; Caballero et al, 2013).
Given the above data, this study aimed to evaluate the efficacy of cetuximab with concomitant platinum-based chemoradio- or chemotherapy in patients with locally advanced or distant metastatic ACC, respectively, with documented positive EGFR expression.
Patients and methods
Patient selection
Two subgroups of patients were recruited:
Cohort 1: Patients with locally advanced disease not amenable to potentially curative surgery with N0M0 status.
Cohort 2: Patients with distant metastatic lesions. In case of metastatic patients, the surgical resection or radiotherapy of primary tumour was not an exclusion criteria.
Patients ⩾18 years of age with histologically or cytologically confirmed ACC were eligible if they had IHC evidence (Ventana CONFIRM anti-EGFR (3C6) antibody) of strong (+3) or moderate (+2) EGFR-expressing tumours.
Pretreatment evaluation of patients included physical examination, clinical TNM staging, ECOG performance status assessment, computed tomography or magnetic resonance imaging of the primary site and neck, test of adequate haematologic, hepatic and renal function. All patients signed informed consent. The study was approved by the Ethics Commitee of the Institute. The trial number is EudraCT 2006-001694-23.
Study treatment
The combination of cetuximab with chemotherapy in the treatment of locally advanced and metastatic ACC patients was applied. Patients with locally advanced tumours received concomitant radiotherapy. A cetuximab loading dose (400 mg m−2) was administered 1 week before irradiation (or on the first week of treatment of metastatic patients) followed by weekly dose of 250 mg m−2 up to 5–7 times. The patients were treated with megavoltage photon beams with daily fractionation of 2 Gy per day, using three-dimensional conformal treatment planning. Radiation regimens were required to deliver the prescribed dose (66 Gy) to the planning target volume (PTV2), which included primary tumour. The mean dose was 65 Gy (range, 60–66 Gy). Elective whole neck irradiaton was not carried out, because all cases were with node-negative neck, but the first echelon nodes – when neck dissection was not performed – were treated with 50 Gy (PTV1), especially in patients with primary tumours in sites that are abounding in capillary lymphatics. During weeks 1, 3 and 5, a concomitant cisplatin (75 mg m−2 intravenously) was applied on days 1, 21 and 42. In patients with metastatic ACC, a concomitant cisplatin (75 mg m−2 intravenously) and continuous 5-fluorouracil (1000 mg m−2 per day intravenously) on days 1–4 was administered. Dose modification or treatment delay was not necessary.
Premedication before cisplatin therapy consisted of standard hydration and prophylactic antiemetic medications. Chloropyramin was administered as standard premedication of cetuximab. Premedication with H2 antagonist was optional.
Evaluation and response assessment
During the study, patients were weekly evaluated by physical examination. Comprehensive laboratory tests were performed at 21-day intervals. Subsequent clinical evaluations with repeated imaging (MRI and/or CT scan) were planned at 3-month intervals for the first 2 years after the end of the treatment or in case of metastatic patients until disease progression. After the first 2 years following the end of the treatment of locally advanced patients, the imaging tests were performed at 6-month intervals until disease progression. Tumour response was assessed according to the RECIST 1.0 criteria. Toxicity was graded according to National Cancer Institute Common Terminology Criteria for Adverse Events, version 3.0.
Statistical considerations
This was a single-institution, open-label, single-arm pilot phase II study conducted to evaluate the preliminary efficacy and toxicity of cetuximab in combination with cisplatin-based chemoradio- or chemotherapy. Planned accrual was 25 assessable patients.
The primary objective of this trial was to determine the PFS. Secondary objectives were to assess the objective response rate (CR or PR) in case of locally advanced and metastatic patients. For both subgroups, the evaluation of toxicity and overall survival (OS) were considered also as secondary objectives.
Survival was estimated by Kaplan–Meier method. Progression-free survival was considered as the time from the beginning of the treatment until disease progression or death. Living patients without known disease were censored at last follow-up.
Results
Patients and treatment
Between August 2005 and December 2012, 21 patients with ACC were enrolled onto this study. Twenty-four (86%) of 28 screened patients were positive for EGFR. Of the 24 patients, three declined participation. All enrolled patients were assessable for survival, efficacy and toxicity.
Clinicopathologic characteristics of patients are presented in Table 1. An excess of female subjects (67%) in both subgroups were present. The nodal stage was N0 for all patients in the locally advanced subgroup.
Table 1. Characteristics of patients.
| |
Locally advancedn
(%) |
Metastaticn
(%) |
|---|---|---|
| Characteristics | n=9 | n=12 |
|
Age (years) | ||
| Median | 57 | 63 |
| Range |
23–71 |
31–73 |
|
Sex | ||
| Male | 3 (33) | 4 (33) |
| Female |
6 (67) |
8 (67) |
|
Performance status | ||
| 0 | 5 (56) | 5 (42) |
| 1 |
4 (44) |
7 (58) |
|
Primary tumour localisation | ||
| Gland of palate | 4 (44) | 2 (17) |
| Lacrimal gland | 1 (11) | 1 (8) |
| Submandibular gland | — | 2 (17) |
| Maxillary sinus gland | 1 (11) | 2 (17) |
| Parotid gland | — | 2 (17) |
| Base of tongue | 1 (11) | — |
| Nasopharynx | — | 2 (17) |
| Lung | — | 1 (8) |
| Tracheal gland |
2 (22) |
— |
|
Metastasis localisation | ||
| Lung | — | 3 |
| Lymph node | — | 2a |
| Skin | — | 1 |
| Multiple |
— |
6 |
|
Histology | ||
| Cribriform | 4 (44) | — |
| Solid | 1 (11) | 1 (8) |
| Tubular | — | 1 (8) |
| Mixed | 2 (22) | 2 (17) |
| NA |
2 (22) |
8 (67) |
|
Grade | ||
| 1 | 3 (33) | 1 (8) |
| 2 | 2 (22) | 2 (17) |
| 3 | 2 (22) | 1 (8) |
| NA |
2 (22) |
8 (67) |
|
Perineural invasion | ||
| Yes | 2 (22) | 2 (17) |
| No | 5 (56) | 3 (25) |
| NA |
2 (22) |
7 (58) |
|
Tumour stage at diagnosis | ||
| T1 | — | 2 (16) |
| T2 | 1 (11)b | 4 (33) |
| T3 | 2 (22)b | 3 (25) |
| T4a | — | 1 (8) |
| T4b |
6 (67)c |
2 (17) |
|
Nodal stage at diagnosis | ||
| N0 | 9 (100) | 9 (75) |
| N1 |
— |
3 (25) |
|
Disease stage at enrollment | ||
| Stage I | 1 (11) | — |
| Stage II | 2 (22) | — |
| Stage IVA | 6 (67) | — |
| Stage IVC |
— |
12 (100) |
|
Site of progression | ||
| Lung | 1 (11) | 6 (50) |
| Local | 4 (44) | — |
| Skin | 1 (8) | |
| Dura | 1 (8) | |
| Bone | 1 (8) | |
| Orbit |
|
1 (8) |
|
EGFR status | ||
| 2+ | 6 (67) | 8 (67) |
| 3+ |
3 (33) |
4 (33) |
|
Best response | ||
| CR | 2 (22) | — |
| PR | 2 (22) | 5 (42) |
| SD ⩾18 months | 2 (22) | 5 (42) |
| SD <18 months | 3 (33) | 2 (17) |
| PD | — | — |
Abbreviations: CR=complete response; EGFR=epidermal growth factor receptor; NA=not available; PD=progressive disease; PR=partial response; SD=stable disease.
Mediastinal and mediastinal+cervical.
Refused surgery.
Unresectable.
The following surgical interventions and/or radiotherapy were applied on primary tumours of metastatic patients long before enrollment in this study: three patients were treated by primary local radiotherapy without surgical resection; nine patients having R0 (N=5) or R1 (N=4) resected tumour received adjuvant radiotherapy. The median disease-free survival after the adjuvant radiotherapy was 75 months (95% confidence interval (CI): 34–94); however, the median DFS was only 16 months (95% CI: 11–101) for patients with R1 resected tumour.
Response and survival
Of the nine patients with locally advanced tumour, four patients (44%) presented objective response (two CR and two PR) and none had PD (Table 1). Of the five patients with stable disease (SD), two patients experienced disease progression after 13 and 15 months. In one patient with SD, the progression was observed at 64 months as lung metastasis.
The objective response rate in case of metastatic patients was 42% (five cases with PR). None of these patients experienced PD. Of the seven patients with SD, five experienced progression after 2, 16, 18, 34 and 48 months. In case of patients with more than one metastatic site who responded with SD (five patients), the size of all metastatic lesions remained unchanged or some of the lesions presented minimal decrease (three patients).
The site and size (in cm) of evaluable metastases and the response for each patients with distant metastases are as follows: Patient (Pts)1– lung (1.0, 1.1, 1.2, 2.0) PR; Pts2 – skin (2.0, 2.6, 3.0, 3.0) PR; Pts3 – lung (1.2), dura (1.5) SD; Pts4 – lung (0.3, 0.4, 0.6, 1.0) SD; Pts5 – dura (3.6), bone (2.0) SD; Pts6 – lymph nodes (0.6, 0.8), lung (0.5, 1.3, 2.0, 2.4) SD; Pts7 – bone (4.1), lymph node (1.0) PR; Pts8 – lymph nodes (1.0, 1.0, 1.7) SD; Pts9 – lymph nodes (0.7, 1.0, 1.1, 1.4, 1.4) SD; Pts10 – lung (1.8, 2.0, 2.2, 3.3) SD; Pts11 – lung (1.0, 1.2, 1.2), liver (1.0, 1.3, 1.3, 2.0), kidney (2.0) SD; and Pts12 – liver (2.3, 15), kidney (9.2), lung (0.5, 1.5, 1.5) SD.
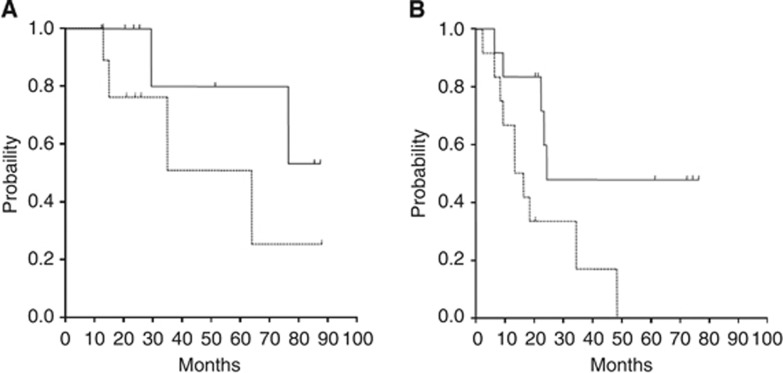
The median follow-up for patients with locally advanced disease was 52 months (95% CI: 24–89). The median PFS was 64 months (95% CI: 15–64) and the 2-year PFS rate was 76% (95% CI: 47–100). The median OS has not been reached and the 2-year OS rate was 100% (Figure 1A). To date, two patients have died without distant metastases. The median PFS of responders (CR+PR) and non-responders (SD) was 35 and 64 months, respectively.
Figure 1.
Progression-free survival (dashed line) and overall survival (solid line) curves of patients with locally advanced (A) or distant metastatic (B) adenoid cystic carcinoma.
Patients with metastases were followed up for a median of 72 months (95% CI: 21–72). The median PFS was 13 months (95% CI: 9–19), whereas the median OS was 24 months (95% CI: 22–24). The 2-year survival rate was 33% and 48% for PFS and OS, respectively (Figure 1B). During follow-up five patients have died. The median PFS of responders (CR+PR) and non-responders (SD) was 8 and 19 months, respectively.
Toxicity
All patients were assessable for adverse events (Table 2). Cetuximab with chemoradiotherapy was modestly tolerated by most patients (89% of patients with locally advanced tumour experienced grade 3 or 4 toxicities). In general, toxicities were typical of those expected for concurrent cetuximab, cisplatin and radiotherapy. Skin rash, in-field dermatitis, mucositis and vomiting were the most frequent grade 3 or 4 adverse events. In metastatic patients, the cetuximab plus chemotherapy was better tolerated (50% of patients presented grade 3 or 4 adverse events). Two patients with grade 3 toxicity of vomiting due to the cisplatin refused the last or the last two cycles of chemotherapy. One patient refused the cetuximab treatment of the last two cycles because of skin rash of grade 3. Six patients had been hospitalised because of adverse events (five with locally advanced tumour and one metastatic patient).
Table 2. Toxicity of cetuximab+chemoradio- or chemotherapy.
| Locally advanced | Metastatic | |||
|---|---|---|---|---|
|
Toxicity |
All grades |
Grades 3 and 4 |
All grades |
Grades 3 and 4 |
| |
n
(%) |
n
(%) |
n
(%) |
n
(%) |
| Skin rash |
9 (100) |
6 (67) |
11 (92) |
4 (33) |
| In-field dermatitis |
7 (78) |
6 (67) |
— |
— |
| Mucositis |
8 (89) |
6 (67) |
5 (38) |
1 (8) |
| Vomiting |
9 (100) |
0 |
8 (67) |
1 (8) |
| Diarrhoea |
0 |
0 |
4 (25) |
1 (8) |
| Neutropenia |
1 (11) |
0 |
1 (8) |
0 |
| Neutropenic fever |
0 |
0 |
2 (17) |
2 (17) |
| Polineuropathy |
2 (22) |
0 |
2 (17) |
0 |
| Paronychia |
1 (11) |
0 |
2 (17) |
2 (17) |
| Haematologic |
0 |
0 |
2 (17) |
0 |
| Renal |
0 |
0 |
2 (17) |
0 |
| Ototoxicity |
0 |
0 |
2 (17) |
0 |
| Fatigue | 4 (44) | 0 | 1 (8) | 0 |
Discussion
Adenoid cystic carcinoma has a variable natural history and no systemic treatment to date has improved survival. Recent trials of targeted therapies in advanced ACC have reported few objective responses (Chau et al, 2012). This study summarises our experience with treatment of EGFR-positive locally advanced ACC patients with cetuximab and concomitant chemoradiotherapy, and with cetuximab and classical chemotherapy for metastatic ACC patients.
For non-metastatic ACC patients who have R1–2 resected or non-resected tumours, the use of radiotherapy is a standard procedure (Ko et al, 2007; Gomez et al, 2008; Shen et al, 2012). Concurrent chemoradiation (CRT) is a feasible treatment option and may lead to sustained locoregional tumour control in patients with non-resected tumour (Samant et al, 2012).
There are only few studies reporting survival of patients with locally advanced ACC treated with irradiation and chemotherapy. Haddad et al (2006) studied five patients with unresected tumour treated with platinum-based chemoradiotherapy. After a median follow-up of 36 months, four patients were alive with no evidence of disease and one patient (1 out of 5; 20%) developed distant metastasis. Gomez et al (2008) analysed inter alia four patients with inoperable disease receiving platinum-based radiochemotherapy. The median PFS was 43.2 months (3.6 years) (calculated from the given individual survivals). In a study conducted by Samant et al (2012) on 16 patients with non-resected ACC treated by concurrent chemoradiation with a median follow-up of 61 months, the median PFS was 51 months (calculated from the PFS curve) and distant metastases developed in five patients (5 out of 16; 31%) (Samant et al, 2012). In our study, the addition of cetuximab to the cisplatin-based CRT in patients with locally advanced ACC increased the median PFS up to 64 months and only one out of 9 (11%) patients developed distant metastasis after a median follow-up of 52 months. To the best of our knowledge, our study is the first trial combining targeted therapy (cetuximab) with CRT in locally advanced ACC. This combined modality markedly increased the PFS and decreased the rate of distant metastases as compared with CRT data in the literature.
There are some concerns regarding the side effects of triple combination (cetuximab, cisplatin and radiotherapy) because one study for patients with locoregionally advanced squamous cell carcinomas of head and neck using concurrent cetuximab, cisplatin and radiotherapy was closed for significant adverse events (Pfister et al, 2006); however, in other two studies for similar lesions using cetuximab and cisplatin-based CRT, the toxicity was manageable (Kuhnt et al, 2010; Merlano et al, 2011). It needs to be mentioned that in the latter studies, cisplatin was administered weekly (lower dose), whereas in the first trial every 3 weeks (higher dose). Interestingly, in this latter trial, the efficacy (objective response and survival) was more enhanced. In our study, cisplatin was given every 2 weeks, and besides encouraging efficacy, the toxicity was manageable, although the tumour type was ACC and not squamous cell carcinoma.
The combination of radiotherapy with cetuximab in advanced/recurrent ACC patients was the aim of two studies reporting acceptable toxicity and promising response and local control rates; however; the OS rate remained relatively low (Jensen et al, 2010; Zwicker et al, 2011). Further studies are neccessary to evaluate the efficacy of this combined treatment (Jensen et al, 2011).
Chemotherapy might have palliative benefit for a small proportion of patients with metastatic/recurrent ACC. Objective responses were uncommon (13% of 141 patients) in eight trials of single-agent chemotherapeutic drugs. The combination chemotherapy used in 17 trials showed objective response in 24.5% of 143 patients, of whom the majority had distant metastases. If combination chemotherapy is chosen, available data support cisplatin combined with anthracycline (Laurie et al, 2011). In one trial reported by Hill et al (1997), the combination of cisplatin with 5-fluorouracil was administered to 11 metastatic ACC patients, with three partial responses (27% objective response). In our study, the use of cetuximab in metastatic patients concomitant with the combination of cisplatin and 5-fluorouracil increased the objective response rate by ∼60% as compared with the investigation of Hill et al (1997). The presence of cetuximab also increased the median PFS in this study as compared with the Hill's study (13 vs 9 months) and the OS was doubled (24 vs 12 months).
In the case of metastatic ACC patients, single-agent targeted therapy or combined targeted chemotherapy have already been studied. Bortezomib, a proteosome inhibitor, was investigated in patients with metastatic ACC. No objective responses were reported with bortezomib monotherapy, whereas after bortezomib plus doxorubicin 14% of patients presented partial response (Argiris et al, 2011). Imatinib, an inhibitor of protein tyrosine kinase of ABL, PDGFR and c-kit receptor, used as monotherapy seemed to be ineffective in the treatment of metastatic ACC (Hotte et al, 2005; Lin et al, 2005; Ochel et al, 2005; Guigay et al, 2007; Pfeffer et al, 2007), excepting some responses in small case series (Alcedo et al, 2004; Faivre et al, 2005). Combination of imatinib with cisplatin provided objective response in 29% of patients with locally advanced or metastatic ACC. The median progression-free survival and OS was 15 and 35 months, respectively (Ghosal et al, 2011). Sunitinib, a multitargeted tyrosine kinase inhibitor, in a phase II study showed no objective responses in patients with recurrent and/or metastatic ACC (Chau et al, 2012). The combination of sunitinib with chemotherapy has not yet been studied. A phase II study of the anti-EGFR inhibitor cetuximab monotherapy did not report any major objective response and the time to progression was only 6 months of recurrent or metastatic ACC (Locati et al, 2009b). On the basis of two case reports, the addition of cetuximab to chemotherapy (FOLFOX6 or paclitaxel) resulted in a partial response and relatively long-lasting SD (>1 year) in patients with metastatic ACC, previously treated with chemotherapy without success (De Dosso et al, 2009; Caballero et al, 2013). Similar to these case reports in our phase II study, the combination of cetuximab with cisplatin showed an objective response rate >40% and 13 and 24 months median PFS and OS, respectively, in patients with metastatic ACC. Other anti-EGFR therapies (gefitinib, lapatinib) were also used in the treatment of metastatic ACC, but the treatments failed to achieve any objective responses (Glisson et al, 2005; Agulnik et al, 2007). Combination of these targeted therapies with classical chemotherapies might be worthy to test in further studies. Other targeted therapies (dasatinib, dovitinib, vorinostat, axitinib, etc.) are investigated in ongoing clinical trials of recurrent/locally advanced/metastatic ACC. To the best of our knowledge, our study is the first trial combining cetuximab with chemotherapy in metastatic ACC. This modality markedly increased survival as compared with data in the literature, which are reporting results of other targeted agents plus chemotherapy or other targeted agents as monotherapies.
The unexpected PFS data of responders vs non-responders in both cohorts of our study may be interpreted with caution because of the low number of patients; however, on the other hand, the biologic therapies in contrast to the cytotoxic CRTs exert cytostatic effect, which may benefit outcomes with long-lasting SD. Even for ‘non-responding' (SD) patients, the PFSs in both cohorts of our study proved to be longer compared with the historical data.
The relatively good result of our trial may be explained by the recently identified importance of EGFR inhibition in preventing tumour growth and metastatic potential of ACC (Huang et al, 2013; Jia et al, 2013); however, the low rate of grade 3 tumours and perineural invasion may also contribute to the final outcome.
In conclusion, the addition of cetuximab to chemoradiation or chemotherapy has improved treatment efficacy in locally advanced and in metastatic ACC patients. Side effects like severe skin toxicity and mucositis were manageable and did not hamper the treatment course. Considering the natural history of ACC with slow progression, longer follow-up will be needed for better evaluation of the treatment effect including accurate imaging. Further clinical trials based on collaborative multicentric efforts can help in selecting the optimal targeted therapy-based strategy in ACC.
The authors declare no conflict of interest.
Footnotes
This work is published under the standard license to publish agreement. After 12 months the work will become freely available and the license terms will switch to a Creative Commons Attribution-NonCommercial-Share Alike 3.0 Unported License.
References
- Agulnik M, Cohen EW, Cohen RB, Chen EX, Vokes EE, Hotte SJ, Winquist E, Laurie S, Hayes DN, Dancey JE, Brown S, Pond GR, Lorimer I, Daneshmand M, Ho J, Tsao MS, Siu LL. Phase II study of lapatinib in recurrent or metastatic epidermal growth factor receptor and/or erbB2 expressing adenoid cystic carcinoma and non adenoid cystic carcinoma malignant tumors of the salivary glands. J Clin Oncol. 2007;25:3978–3984. doi: 10.1200/JCO.2007.11.8612. [DOI] [PubMed] [Google Scholar]
- Alcedo JC, Fábrega JM, Arosemena JR, Urrutia A. Imatinib mesylate as treatment for adenoid cystic carcinoma of the salivary glands: report of two successfully treated cases. Head Neck. 2004;26:829–831. doi: 10.1002/hed.20094. [DOI] [PubMed] [Google Scholar]
- Argiris A, Ghebremichael M, Burtness B, Axelrod RS, Deconti RC, Forastiere AA. A phase 2 trial of bortezomib followed by the addition of doxorubicin at progression in patients with recurrent or metastatic adenoid cystic carcinoma of the head and neck: a trial of the Eastern Cooperative Oncology Group (E1303) Cancer. 2011;117:3374–3382. doi: 10.1002/cncr.25852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caballero M, Sosa EA, Tagliapietra A, Grau JJ. Metastatic adenoid cystic carcinoma of the salivary gland responding to cetuximab plus weekly paclitaxel after no response to weekly paclitaxel alone. Head Neck. 2013;35:E52–E54. doi: 10.1002/hed.21870. [DOI] [PubMed] [Google Scholar]
- Chau NG, Hotte SJ, Chen EX, Chin SF, Turner S, Wang L, Siu LL. A phase II study of sunitinib in recurrent and/or metastatic adenoid cystic carcinoma (ACC) of the salivary glands: current progress and challenges in evaluating molecularly targeted agents in ACC. Ann Oncol. 2012;23:1562–1570. doi: 10.1093/annonc/mdr522. [DOI] [PubMed] [Google Scholar]
- De Dosso S, Mazzucchelli L, Ghielmini M, Saletti P. Response to oxaliplatin with cetuximab in minor salivary gland adenoid cystic carcinoma. Tumori. 2009;95:378–381. doi: 10.1177/030089160909500319. [DOI] [PubMed] [Google Scholar]
- Dahse R, Driemel O, Schwarz S, Kromeyer-Hauschild K, Berndt A, Kosmehl H. KRAS status and epidermal growth factor receptor expression as determinants for anti-EGFR therapies in salivary gland carcinomas. Oral Oncol. 2009;45:826–829. doi: 10.1016/j.oraloncology.2009.01.013. [DOI] [PubMed] [Google Scholar]
- Dubergé T, Bénézery K, Resbeut M, Azria D, Minsat M, Ellis S, Teissier E, Zaccariotto A, Champetier C, Cowen D. Adenoid cystic carcinoma of the head and neck: a retrospective series of 169 cases. Cancer Radiother. 2012;16:247–256. doi: 10.1016/j.canrad.2012.02.003. [DOI] [PubMed] [Google Scholar]
- Faivre S, Raymond E, Casiraghi O, Temam S, Berthaud P.2005Imatinib mesylate can induce objective response in progressing, highly expressing KIT adenoid cystic carcinoma of the salivary glands J Clin Oncol 236271–6273.author reply 6273–6274. [DOI] [PubMed] [Google Scholar]
- Ghosal N, Mais K, Shenjere P, Julyan P, Hastings D, Ward T, Ryder WD, Bruce I, Homer J, Slevin NJ. Phase II study of cisplatin and imatinib in advanced salivary adenoid cystic carcinoma. Br J Oral Maxillofac Surg. 2011;49:510–515. doi: 10.1016/j.bjoms.2010.09.013. [DOI] [PubMed] [Google Scholar]
- Glisson BS, Blumenschein G, Francisco M, Erasmus J, Zinner R, Kies M. Phase II trial of gefitinib in patients with incurable salivary gland cancer. J Clin Oncol. 2005;23 (Suppl. Abstr:5532. [Google Scholar]
- Gomez DR, Hoppe BS, Wolden SL, Zhung JE, Patel SG, Kraus DH, Shah JP, Ghossein RA, Lee NY. Outcomes and prognostic variables in adenoid cystic carcinoma of the head and neck: a recent experience. Int J Radiat Oncol Biol Phys. 2008;70:1365–1372. doi: 10.1016/j.ijrobp.2007.08.008. [DOI] [PubMed] [Google Scholar]
- Guigay J, Bidault F, Temam S, Janot F, Raymond E, Faivre S. Antitumor activity of imatinib in progressive, highly expressing KIT adenoid cystic carcinoma of the salivary glands: a phase II study. J Clin Oncol. 2007;25 (Suppl. Abstr:6086. [Google Scholar]
- Haddad RI, Posner MR, Busse PM, Norris CM, Goguen LA, Wirth LJ, Blinder R, Krane JF, Tishler RB. Chemoradiotherapy for adenoid cystic carcinoma: preliminary results of an organ sparing approach. Am J Clin Oncol. 2006;29:153–157. doi: 10.1097/01.coc.0000203756.36866.17. [DOI] [PubMed] [Google Scholar]
- Hill ME, Constenla DO, A'Hern RP, Henk JM, Rhys-Evans P, Breach N, Archer D, Gore ME. Cisplatin and 5-fluorouracil for symptom control in advanced salivary adenoid cystic carcinoma. Oral Oncol. 1997;33:275–278. doi: 10.1016/s0964-1955(97)00026-2. [DOI] [PubMed] [Google Scholar]
- Hotte SJ, Winquist EW, Lamont E, MacKenzie M, Vokes E, Chen EX, Brown S, Pond GR, Murgo A, Siu LL. Imatinib mesylate in patients with adenoid cystic cancers of the salivary glands expressing c-kit: a Princess Margaret Hospital phase II consortium study. J Clin Oncol. 2005;23:585–590. doi: 10.1200/JCO.2005.06.125. [DOI] [PubMed] [Google Scholar]
- Huang Y, Yu T, Fu X, Chen J, Liu Y, Xia Y, Zhang Z, Li L. EGFR inhibition prevents in vitro tumor growth of salivary adenoid cystic carcinoma. BMC Cell Biol. 2013;14:13. doi: 10.1186/1471-2121-14-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jensen AD, Krauss J, Weichert W, Debus J, Münter MW. RadioImmunotherapy for adenoid cystic carcinoma: a single-institution series of combined treatment with cetuximab. Radiat Oncol. 2010;5:102. doi: 10.1186/1748-717X-5-102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jensen AD, Nikoghosyan A, Hinke A, Debus J, Münter MW. Combined treatment of adenoid cystic carcinoma with cetuximab and IMRT plus C12 heavy ion boost: ACCEPT (ACC, Erbitux® and particle therapy) BMC Cancer. 2011;11:70. doi: 10.1186/1471-2407-11-70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jia J, Zhang W, Liu JY, Chen G, Liu H, Zhong HY, Liu B, Cai Y, Zhang JL, Zhao YF. Epithelial mesenchimal transition is required for aquisition of anoikis resistance and metastatic potential in adenoid cystic carcinoma. PLoS One. 2013;7:e51549. doi: 10.1371/journal.pone.0051549. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- Ko YH, Lee MA, Hong YS, Lee KS, Jung CK, Kim YS, Sun DI, Kim BS, Kim MS, Kang JH. Prognostic factors affecting the clinical outcome of adenoid cystic carcinoma of the head and neck. Jpn J Clin Oncol. 2007;37:805–811. doi: 10.1093/jjco/hym119. [DOI] [PubMed] [Google Scholar]
- Kuhnt T, Sandner A, Wendt T, Engenhart-Cabillic R, Lammering G, Flentje M, Grabenbauer G, Schreiber A, Pirnasch A, Dunst J. Phase I trial of dose-escalated cisplatin with concomitant cetuximab and hyperfractionated-accelerated radiotherapy in locally advanced squamous cell carcinoma of the head and neck. Ann Oncol. 2010;21:2284–2289. doi: 10.1093/annonc/mdq216. [DOI] [PubMed] [Google Scholar]
- Laurie SA, Ho AL, Fury MG, Sherman E, Pfitser DG. Systemic therapy in the management of metastatic or locally recurrent adenoid cystic carcinoma of the salivary glands: a systematic review. Lancet Oncol. 2011;12:815–824. doi: 10.1016/S1470-2045(10)70245-X. [DOI] [PubMed] [Google Scholar]
- Le Tourneau C, Razak AR, Levy C, Calugaru V, Galatoire O, Dendale R, Desjardins L, Gan HK. Role of chemotherapy and molecularly targeted agents in the treatment of adenoid cystic carcinoma of the lacrimal gland. Br J Ophthalmol. 2011;95:1483–1489. doi: 10.1136/bjo.2010.192351. [DOI] [PubMed] [Google Scholar]
- Lin CH, Yen RF, Jeng YM, Tzen CY, Hsu C, Hong RL. Unexpected rapid progression of metastatic adenoid cystic carcinoma during treatment with imatinib mesylate. Head Neck. 2005;27:1022–1027. doi: 10.1002/hed.20274. [DOI] [PubMed] [Google Scholar]
- Locati LD, Perrone F, Losa M, Mela M, Casieri P, Orsenigo M, Cortelazzi B, Negri T, Tamborini E, Quattrone P, Bossi P, Rinaldi G, Bergamini C, Calderone RG, Liberatoscioli C, Licitra L. Treatment relevant target immunophenotyping of 139 salivary gland carcinomas (SGCs) Oral Oncol. 2009a;45:986–990. doi: 10.1016/j.oraloncology.2009.05.635. [DOI] [PubMed] [Google Scholar]
- Locati LD, Bossi P, Perrone F, Potepan P, Crippa F, Mariani L, Casieri P, Orsenigo M, Losa M, Bergamini C, Liberatoscioli C, Quattrone P, Calderone RG, Rinaldi G, Pilotti S, Licitra L. Cetuximab in recurrent and/or metastatic salivary gland carcinomas: a phase II study. Oral Oncol. 2009b;45:574–578. doi: 10.1016/j.oraloncology.2008.07.010. [DOI] [PubMed] [Google Scholar]
- Macarenco RS, Uphoff TS, Gilmer HF, Jenkins RB, Thibodeau SN, Lewis JE, Molina JR, Yang P, Aubry MC. Salivary gland-type lung carcinomas. An EGFR immunohistochemical, molecular genetic, and mutational analysis study. Mod Pathol. 2008;21:1168–1175. doi: 10.1038/modpathol.2008.113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Merlano M, Russi E, Benasso M, Corvò R, Colantonio I, Vigna-Taglianti R, Vigo V, Bacigalupo A, Numico G, Crosetto N, Gasco M, Lo Nigro C, Vitiello R, Violante S, Garrone O. Cisplatin-based chemoradiation plus cetuximab in locally advanced head and neck cancer: a phase II clinical study. Ann Oncol. 2011;22:712–717. doi: 10.1093/annonc/mdq412. [DOI] [PubMed] [Google Scholar]
- Ochel HJ, Gademann G, Rocken C, Wordehoff H. Effects of imatinib mesylate on adenoid cystic carcinomas. Anticancer Res. 2005;25:3659–3664. [PubMed] [Google Scholar]
- Papaspyrou G, Hoch S, Rinaldo A, Rodrigo JP, Takes RP, van Herpen C, Werner JA, Ferlito A. Chemotherapy and targeted therapy in adenoid cystic carcinoma of the head and neck: a review. Head Neck. 2011;33:905–911. doi: 10.1002/hed.21458. [DOI] [PubMed] [Google Scholar]
- Pfeffer MR, Talmi Y, Catane R, Symon Z, Yosepovitch A, Levitt M. A phase II study of Imatinib for advanced adenoid cystic carcinoma of head and neck salivary glands. Oral Oncol. 2007;43:33–36. doi: 10.1016/j.oraloncology.2005.12.026. [DOI] [PubMed] [Google Scholar]
- Pfister DG, Su YB, Kraus DH, Wolden SL, Lis E, Aliff TB, Zahalsky AJ, Lake S, Needle MN, Shaha AR, Shah JP, Zelefsky MJ. Concurrent cetuximab, cisplatin, and concomitant boost radiotherapy for locoregionally advanced, squamous cell head and neck cancer: a pilot phase II study of a new combined-modality paradigm. J Clin Oncol. 2006;24:1072–1078. doi: 10.1200/JCO.2004.00.1792. [DOI] [PubMed] [Google Scholar]
- Samant S, van den Brekel MW, Kies MS, Wan J, Robbins KT, Rosenthal DI, Rasch C, Weber RS. Concurrent chemoradiation for adenoid cystic carcinoma of the head and neck. Head Neck. 2012;34:1263–1268. doi: 10.1002/hed.21905. [DOI] [PubMed] [Google Scholar]
- Shen C, Xu T, Huang C, Hu C, He S. Treatment outcomes and prognostic features in adenoid cystic carcinoma originated from the head and neck. Oral Oncol. 2012;48:445–449. doi: 10.1016/j.oraloncology.2011.12.002. [DOI] [PubMed] [Google Scholar]
- Vered M, Braunstein E, Buchner A. Immunohistochemical study of epidermal growth factor receptor in adenoid cystic carcinoma of salivary gland origin. Head Neck. 2002;24:632–636. doi: 10.1002/hed.10104. [DOI] [PubMed] [Google Scholar]
- Zwicker F, Roeder F, Thieke C, Timke C, Münter MW, Huber PE, Debus J. IMRT reirradiation with concurrent cetuximab immunotherapy in recurrent head and neck cancer. Strahlenther Onkol. 2011;187:32–38. doi: 10.1007/s00066-010-2149-7. [DOI] [PubMed] [Google Scholar]