Abstract
The development of new massive sequencing techniques has now made it possible to significantly reduce the time and costs of whole-genome sequencing (WGS). Although WGS will soon become a routine testing tool, new ethical issues have surfaced. In light of these concerns, a systematic review of papers published by expert authors on IC or specific ethical issues related to IC for WGS analysis in the clinical setting has been conducted using the Pubmed, Embase and Cochrane Library databases. Additionally, a search was conducted for international ethical guidelines for genetic studies published by scientific societies and ethical boards. Based on these documents, a minimum set of information to be provided to patients in the IC form was determined. Fourteen and seven documents from the database search and from scientific societies, respectively, were selected. A very high level of consistency between them was found regarding the recommended IC form content. Pre-test counselling and general information common to all genetic tests should be included in the IC form for WGS for diagnostic purposes, but additional information addressing specific issues on WGS are proposed, such as a plan for the ethical, clinically oriented return of incidental findings. Moreover, storage of additional information for future use should also be agreed upon with the patient in advance. Recommendations for WGS studies in the clinical setting concerning both the elements of information and the process of obtaining the IC as well as how to handle the results obtained are proposed.
Keywords: informed consent, whole genome sequencing, whole exome sequencing, incidental findings, ethical issues, clinical setting
Introduction
In 2003, the results of the Human Genome Project were presented worldwide.1, 2 The set of the exons of all the protein-coding genes is known as the exome; although this represents only 1.5% of the genome, it comprises most of the DNA variations that cause highly penetrant genetic diseases.3 Since 2004, rapid technological advances and the development of new massive parallel sequencing techniques applied to whole genome sequencing (WGS) and whole-exome sequencing (WES) analyses have made it possible to significantly reduce the time and the costs of the two processes. Currently, WGS or WES test results may be available within 7 weeks,4 costing some $10 000.3 With further cost decreases expected,4 WES and WGS analyses may soon be comparable in cost with many other clinically available tests and could become a common clinical testing tool used in routine care.3
WES and WGS began to be widely used mainly for research in 2006. It was not until 2009, however, when these types of analyses were first used for clinical diagnosis and only in select patients.4, 5, 6 The primary clinical applications of WGS are Mendelian disorders, some complex diseases, and diagnosis for stratified treatment of cancer through tumour profiling. In the near future, many other applications may be added, including tissue matching, risk prediction, and pharmacogenetics.7
When diagnosing diseases with a strong genetic component, the main advantage of these tests over single-gene sequencing or genotyping a series of known mutations is that all mutations genome-wide can be detected, patients with conditions of unknown origin can receive a definitive diagnosis, and speed of care is increased due to a reduced need for multiple tests.4, 7 WGS analysis provides a wide range of genetic data about the current health status or future risk not only concerning the patient but also their relatives and future offspring. It should be highlighted that WGS can also provide ‘incidental findings' and findings of unknown significance, bringing new ethical issues to the clinical setting.8
Although some recommendations have been recently made about the disclosure of incidental findings or additional information resulting from WGS,9 these have been broad in nature, encompassing both the clinical setting and research, and a consensus for their management is lacking. The aim of the present work is to provide specific, clinically oriented recommendations on both the elements of information to be included in the informed consent (IC) form as well as to establish some important aspects of the process of obtaining consent from patients undergoing WGS studies.
Materials and methods
The analysis was carried out in two steps. The first step, designed to determine the elements of information that would make up the basis for the eventual recommendation, consisted of a review of various types of IC forms drafted for diagnostic purposes.10, 11, 12 Once these common elements were established and compiled (‘The List'), the second step was undertaken, comparing the presence of the pieces of information found in a number of documents published by experts and scientific organisations with those included on ‘The List'. This comparison was used to come up with the final body of information recommended for inclusion in the IC forms.
To conduct the second step of the analysis, a systematic review of Pubmed-Medline, Embase, and The Cochrane Library databases was carried out on 20 June 2012. The following types of papers were included in the search: review articles, expert opinions, clinical practice guidelines, expert advice, position papers, and consensus statements. The following search strategy was used: (Personal Genome OR Personalized Genome OR individual genome OR whole-genome sequencing OR whole genome sequencing OR next generation sequencing OR next-generation sequencing) AND ((Informed Consent OR consent) OR (Ethics OR ethical issues) OR (incidental findings OR incidental results OR unexpected findings OR unexpected results) OR (disclosing genetic information OR disclosing information OR disclosing results).
To facilitate the analysis, all the available documents were grouped and classified as being the work of either ‘experts' or ‘societies'. The papers belonging to the ‘experts' group were required to comply with the following selection criteria:
(a) Inclusion criteria: type of studies: WGS; objective: diagnostic purposes or research purposes with regard to future diagnostic applications; language: English or Spanish; documents specifically referring to IC or ethical issues related to IC; time frame: papers published from 2006, when WGS began to be widely used, up to the date when the search was performed.
(b) Exclusion criteria: objective: studies referring to sequencing for research purposes only; papers concerning only genome-wide association studies (GWAS), biobanks, or biorepositories; studies focused on the technical results of sequencing or genetic analysis of certain conditions, without reference to general issues or items considered in this study; papers referring to direct-to-consumer (DTC) testing only, performed in healthy people, and not medically indicated; studies referring to gene patents, carrier tests, and neonatal screening tests.
The ‘societies' group of documents consisted of international ethical guidelines for genetic studies as published by scientific societies and ethical boards. These documents included those searched and retrieved from the official websites of the following organisations: Nuffield Council on Bioethics, Human Genome Organisation (HUGO), Eurogentest, Organisation for Economic Co-operation and Development (OECD), European Society of Human Genetics (ESHG), Spanish Society of Human Genetics (AEGH), Foundation for Genomics and Population Health (PGH Foundation), American Society of Human Genetics (ASHG), and American College of Medical Genetics and Genomics (ACMG). Finally, HumGen International was also searched, as it is a reliable resource concerning ethical, legal, and social issues in human genetics.
For practical reasons, in this article, the term WGS is used indistinguishably to denote either whole-genome sequencing or WES.
The analyses for the presence of each item included in ‘the List' within every document were performed by searching for the exact term within the text. If a given word was found to be present, the exact meaning as corresponds to the one included in ‘the List' was verified in order to consider it as a positive finding. If the term was not present, an implicit reference to it was searched for in the text, and if this was not found it was considered as a negative finding. This search was performed by two of the authors independently (JMM and MM). For those cases where there was a disagreement between the two readers, an agreement was reached by consensus. The final validation of the decisions was made by a third author (CA).
Fisher's exact test was used for statistical analysis of the comparisons; values of P<0.05 were considered significant.
Results
Step 1: The 10 elements of information considered critical and which should always be included on the IC form are shown in Table 1. These elements appeared on the three IC forms reviewed, which were designed for diagnostic purposes.10, 11, 12 Additionally, ‘Explicit IC' was added as the eleventh element to indicate whether consent for WGS analysis was given explicitly.
Table 1. ‘The List': Proposed minimum elements of information to be included in all informed consent forms for WGS in the clinical setting.
| 1. | The scope of the test (Scope) |
| 2. | Brief description of the test process (Description) |
| 3. | Benefits that we expect (Benefits) |
| 4. | Possible disadvantages, risks, or complications (Risks) |
| 5. | Voluntary nature of the test (Voluntary) |
| 6. | Possibility of refusal at any time without consequences (Refusal) |
| 7. | Description of alternative diagnostic methods, if any (Alternative test) |
| 8. | Description of the measures taken to ensure confidentiality and privacy of the results at present and in the future (Confidentiality) |
| 9. | The destination of the biological samples when the study ends (storage, encryption, anonymization, or destruction) or future use of samples (Future use) |
| 10. | Management of incidental findings that may appear in the study, and the right not to know (Incidental findings) |
Abbreviaton: WGS, whole-genome sequencing.
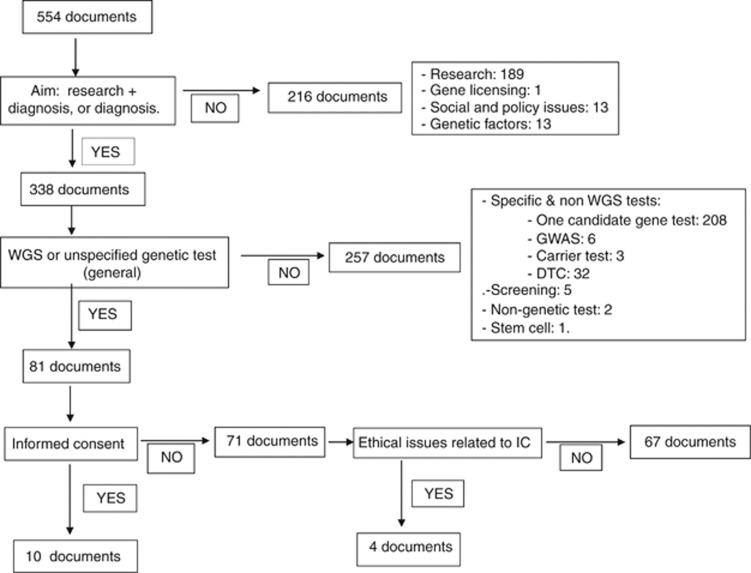
Step 2: Five hundred and fifty-four documents were found through the Pubmed-Medline and Embase searches. Fourteen documents that met the selection criteria4, 6, 8, 13, 14, 15, 16, 17, 18, 19, 20, 21, 22, 23 were selected (Figure 1). In the Cochrane search, no systematic reviews of IC in WGS were found. Hence, these 14 papers were classified under the ‘experts' group.
Figure 1.
Results of the Pubmed-Medline and Embase databases searches.
As far as the ‘societies' group of documents is concerned, seven documents issued between 2007 and 2012 were selected: OECD guidelines for quality assurance in molecular genetic testing,24 Recommendations for genetic counselling related to genetic testing,25 WMA Statement on Genetics and Medicine,26 $1000 genome,27 Next steps in the sequence: The implications of whole genome sequencing for health in the UK,7 Building on our inheritance,28 Policy statement: points to consider in the clinical application of genomic sequencing.29
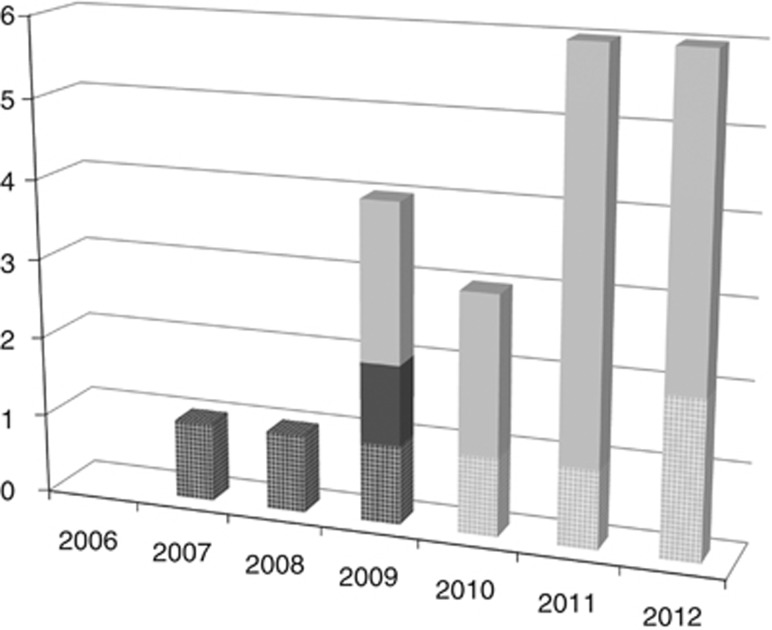
As shown in Figure 2, the publication of papers on IC in WGS tests for diagnostic purposes started in 2009, and most of these were published in 2011 and 2012. Two-thirds (n=14) have been published by experts and the rest (n=7) by scientific societies.
Figure 2.
Distribution of “papers” over time, from 2006 (when WGS began to be conducted) to June 2012. Articles about unspecific genetic tests are depicted in black. WGS studies are depicted in grey. Grid bars represent those papers carried out by societies and bold bars represent those papers published by experts.
Given that WGS for diagnostic testing was first implemented in 2009, there are no documents published before 2009 on this technique. This is why for the analysis of two elements of information, Incidental findings and Explicit IC, only the documents of four scientific societies, issued between 2009 and 2012,7, 27, 28, 29 were taken into account, and not those issued before 2009.
An assessment of the presence of the 11 elements of information was carried out by comparing the ‘experts' with the ‘societies' documents; statistically significant differences were found in only two items (Table 2): Description and Benefits. These two elements were mentioned by all of the seven societies, whereas Description was mentioned by four of the 14 experts and Benefits by six of 14. However, this could be due to the fact that the recommendations mentioned by the societies are before the implementation of WGS in the clinical setting.
Table 2. List of the elements of information to be included in the informed consent forms. Comparison of their presence between documents from ‘societies' and ‘experts'.
| Elements of information | EXPERTS (n=14) (%) | SOCIETIES (n=7) (%) | TOTAL (n=21)(%) | P-value |
|---|---|---|---|---|
| SCOPE | 3 (21) | 5 (71) | 8 (38) | NS |
| DESCRIPTION | 4 (29) | 7 (100) | 11 (52) | 0.0039 |
| BENEFITS | 6 (43) | 7 (100) | 13 (62) | 0.018 |
| RISKS | 8 (57) | 6 (85) | 14 (66) | NS |
| VOLUNTARY | 5 (36) | 2 (29) | 7 (33) | NS |
| REFUSAL | 1 (7) | 1 (14) | 2 (10) | NS |
| ALTERNATIVE TEST | 0 (0) | 1 (14) | 1 (5) | NS |
| CONFIDENTIALITY | 5 (36) | 5 (71) | 10 (52) | NS |
| FUTURE USE | 5 (36) | 4 (57) | 9 (43) | NS |
| INCIDENTAL FINDINGSa | 11 (79) | 4 (100) | 15 (83) | NS |
| EXPLICIT ICa | 9 (64) | 3 (75) | 12 (67) | NS |
Abbreviatons: IC, informed consent; NS, not significant.
N=4 in ‘societies' group documents (see text, in ‘Results'). Total N: 18.
Mention of Incidental findings is present in almost all the documents (83%), whereas Explicit IC, Benefits, and Risks are mentioned in two-thirds of the documents reviewed. These four items appear to be the most crucial points in the sources reviewed. Aside from these, Confidentiality and Future use were mentioned in approximately half of the documents (52 and 43%, respectively). Other elements of information such as Voluntary (33%), Refusal of the test (10%), and Alternative diagnostic tests (5%) are scarcely mentioned.
Table 3 shows the elements of information proposed for inclusion in the IC form, be they common to all types of genetic test or specific to WGS testing. The proposed content of the IC form for WGS contains elements common to any kind of genetic test: the scope, a description indicating the kind of information to be obtained, the expected benefits and risks, the existence of alternative tests if any, the voluntary nature of the test, the possibility of refusal, the future use of the data, and the confidentiality of the outcomes.
Table 3. Summary of recommended elements to be included on the Informed Consent form for WGS in the clinical setting.
| References | |
|---|---|
| Pre-test counselling | 8, 13, 14, 17, 19, 20, 24, 25, 26, 27, 29 |
| Always done by an expert | 6, 15, 18, 29 |
| Agreement of disclosure of results | 17, 25, 29 |
| Scope | 7, 15, 17, 20, 24, 25, 27, 28, 29 |
| Define or delimit scope | |
| Description | 7, 15, 17, 18, 20, 24, 25, 26, 27, 28, 29 |
| Information about the procedure, its limitations and implications | 15, 25, 26 |
| Different descriptions on the basis of the scope: affected, carrier testing, presymptomatic, newborn screening, etc | 18 |
| Ensuring understanding of clinical significance | 28, 29 |
| Benefits | 6, 7, 13, 15, 16, 17, 22, 24, 25, 26, 27, 28, 29 |
| Increased knowledge about disease risks and predispositions. Personalised lifestyle recommendations, prevention and interventions. | 13, 15, 16, 18, 26, 27 |
| More tailored drug therapy | 15 |
| Risks | 6, 7, 13, 15, 16, 17, 18, 19, 22, 24, 25, 26, 27, 29 |
| Medical risks: data of unknown significance, detection of altered genetic risks for diseases with or without treatment | 6, 13, 15, 17, 18 |
| Psychosocial risks | 13, 27 |
| Implications for reproductive decisions | 13, 15, 18, 24, 26 |
| Implications for third parties (family members): discrimination, stigmatisation, misuse (by society or by insurance companies) | 13, 18, 25, 28 |
| Voluntary | 6, 17, 18, 22, 23, 25, 26 |
| Possibility of refusal at any time | 18, 25 |
| Description of alternative diagnostic methods | 25 |
| Privacy and confidentiality | 4, 7, 13, 18, 22, 23, 24, 25, 26, 28 |
| Respect of privacy, keeping confidentiality of the test results | 7 |
| Whether data will be recorded within electronic medical records or not | 4, 13, 18, 23, 25 |
| Who will have access to the data and under what circumstances | 17, 28 |
| To inform relatives or not Include patients' data in databases | 18 |
| Storage and future uses of test results | 7, 13, 17, 18, 20, 22, 24, 27, 28 |
| Storage in the medical file or in a patient record for future analysis | 28 |
| Research (special IC is needed) | 17, 18, 20, 24, 27, 28 |
| Destruction or recontacting patients, reinterpreting results | 13, 18, 20, 24, 28 28 |
| Management of incidental findings that may appear in the analysis | 7, 8, 13, 14, 15, 16, 17, 18, 19, 20, 21, 22, 23, 27, 28, 29 |
| Returning of Incidental findings in a clinically oriented manner (‘categorisation'). Also depending on the type of disease and adult/child | 14, 20, 21, 22, 23, 28 |
| Need to state standards for what is reportable | 29 |
| Right not to know | 13, 14, 18, 20, 23, 27, 29 |
| Specific IC | 4, 7, 13, 14, 15, 17, 18, 20, 23, 27, 28 |
Abbreviation: IC, informed consent.
However, there are some facts that arise from WGS that must be taken into consideration and addressed in the IC form, namely, pre-test counselling and management of incidental findings (Table 3). Pre-test counselling is strongly recommended before WGS due not only to the sheer amount of information revealed by WGS but, more important, also to the different types of data representing differing risks and repercussions for the patient.8, 13, 14, 17, 19, 20, 24, 25, 26, 27, 28, 29 The immediate benefit of WGS in clinical practice is the rapid genetic diagnosis of the disease, especially in rare and genetically heterogeneous diseases. Other possible benefits include additional knowledge of the risks and susceptibility to complex disorders and the potential for designing tailored drug therapies based on pharmacogenetics.15 However, patients should be warned of the large number of genetic variants that will be observed under WGS testing, known as ‘incidental findings'. These incidental findings or ‘additional information' may be of uncertain or unknown significance and may contain undesired information for the patient, his/her relatives, and other parties.7, 8, 13, 14, 15, 16, 17, 18, 19, 20, 21, 22, 23, 27, 28, 29
Berg et al,20 have recently proposed to classify this genetic information into categories related to their risk and clinical utility in order to facilitate the IC process and patient decision-making, suggesting a scalable approach to the process of returning the incidental results identified.20 In Table 4, a variation on the categorisation published by Berg et al,20 is proposed, comprising six different levels of genetic information according to the present or future effect of the variant, their actionability, carrier status, and penetrance. Additionally, it is suggested that information with clinical utility related with the current diagnosis (Group 1) or otherwise clinically manageable disorders affecting the patient or his/her offspring (Groups 2A, 3A, and 4) must always be disclosed to the patient. On the other hand, the possible disclosure of other types of information (Groups 2B, 3B, 5, and 6) should be discussed and agreed upon in advance with the patient during the IC process. In the case of minors, we strongly suggest that information about the risks of non-preventable and untreatable future diseases be postponed until the individual reaches the legal age for consent and may decide for him or herself.6, 7, 14, 18, 22, 27
Table 4. Categorisation of the possible genetic information obtained from WGS.
| Group 1: Genetic findings useful for the current diagnosis of the disease that which initially led to the analysis. |
| Group 2: Any clinically relevant genetic findings, which may have immediate benefits for the patient related to present diseases or clinical conditions. |
| Group 2A: Diseases for which possible treatment is available (eg, cardiovascular diseases predisposing to sudden cardiac death). |
| Group 2B: Diseases with no treatment available (eg, Charcot–Marie–Tooth type 1A). |
| Group 3: Genetic mutations related to high risks for future Mendelian diseases. |
| Group 3A: Information about risks of preventable or treatable diseases (eg, Lynch syndrome or BRCA1/2). |
| Group 3B: Information about risks of non-preventable, non-treatable future diseases (eg, Huntington disease). |
| Group 4: Information about carrier status of mutations for an X-linked or an autosomal recessive disorder impacting reproductive life decisions (eg, Tay–Sachs disease, cystic fibrosis). |
| Group 5: Information of variable risk for future diseases. |
| Genetic traits that may be translated into high predisposition for certain complex diseases (eg, ApoE4 and Alzheimer's disease). |
| Most pharmacogenetic variants (eg, β-Blockers and β1-adrenergic receptor). |
| Group 6: Information of unknown significance. |
Discussion
New advances in genetics and genomics, and the possibility of routinely using WGS as a diagnostic tool in clinical settings in the very near future, are issues of both medical and social interest. These novel applications will provide patients and healthcare professionals with a wealth of new information, potentially altering a number of current clinical practices. However, some ethical issues of this type of analysis should be considered before its widespread use is instituted, the most critical matters being IC and the disclosure of results. The aim of this work was to propose a minimum body of information to be included in the IC form, as a part of the IC process, and also to design a category-based model for disclosure of WGS results as previously agreed upon between the clinician and the patient.
Although the search for documents with relevance to this work has been extensive thanks to the use of the most important databases, it has limitations in that databases do not host all articles on a given subject and also due to the fact that the search was limited to documents written in English or Spanish. However, it is believed that the most relevant documents have been reviewed and analysed as, to minimise possible losses of documents, the search was conducted by two authors (JMM and MM) independently.
Due to the large number of variants that may be discovered as well as the variety of their nature, each variant should be stratified according to its predicted effects on the health of the individual and its reliability on its expected outcome for health.20, 30 A similar method of categorising the information, which arises from WGS according to clinical relevance, has been proposed by other authors working exclusively within the realm of research.31, 32, 33
To date, only a few documents have specifically focused on this subject. However, since 2011, the interest in this topic has grown, leading to a significant increase in the number of international publications surrounding this debate.
Although different authors8, 21 have stated that no consensus exists on specific ethical issues for WGS, this analysis shows a reasonably high level of consistency on the 11 elements of information considered.
Some authors7, 8, 13, 22 have written in favour of narrowing down the scope of WGS analysis by using filters to obtain a more targeted analysis. However, some situations will arise in which the origin of the problem is unknown, thus requiring a broad approach in an attempt to establish a proper diagnosis. Here it is proposed that the clinician and the patient reach an agreement in advance as to what additional information the patient wishes to know, thus making analysis more or less filtered.
The recent paper by Christenhusz et al,9 reviews the different opinions available when dealing with the issue of disclosing incidental findings of WGS in both research and clinical settings. The majority of the papers reviewed by these authors, independently of their position on the matter, concluded that the disclosure of incidental findings must be done with caution.9 According to Facio et al,34 patients are willing to receive their WGS results, showing highest interest when dealing with a condition that may be treated or prevented and for carrier status (equivalent for the proposed groups 1, 2A, 3A, and 4 in Table 4).
The last issue to be agreed upon with the patient is what should be done with the information he/she does not wish to know. This information can be destroyed, stored in the patient's medical file, or saved in a record that will be handed over to the patient. The two latter approaches leave open the possibility of disclosing and discussing the information with the patient at a later date if he/she should have a change of mind. Sharp17 has argued that the clinician has the duty to re-contact the patient to inform him/her about changes in the interpretation of genetic information in situations where new scientific knowledge becomes available. However, no consensus has been reached on this to date. It has been suggested that the responsibility of re-contacting is on the side of patients and their families.13 Accordingly, it is recommended that clinicians come to an agreement with patients as to the possibility of there being future contact in order to update him or her on the interpretation of the results obtained.
In conclusion, the documents reviewed reveal that there is some general agreement on the use and contents of the IC form in WGS in the clinical setting. However, for the process to be useful for clinicians and patients, an extensive societal debate on this topic should be held in order to define a policy. Dialogue and education along these lines will serve to increase society's knowledge about this technology in particular and about clinical genetics as a whole. By doing so, experts, societies, and patients alike will be able to make decisions based on well-designed guidelines as to both the elements of information to be included on IC forms and the correct handling of the information obtained. Within the context of this process, the approaches recommended here could aid in this pursuit.
Acknowledgments
We are grateful to Oliver Shaw for his editorial assistance.
The authors declare no conflict of interest.
Web Resources
Nuffield Council on Bioethics ( http://www.nuffieldbioethics.org/)The Spanish Committee on Bioethics (http://www.comitedebioetica.es/) Human Genome Organisation (http://www.hugo-international.org/) Eurogentest (http://www.eurogentest.org) The organisation for economic co-operation and development (http://www.oecd.org) Spanish Society of Human Genetics (http://www.aegh.org/) Foundation for Genomics and Population Health (http://www.phgfoundation.org/) European Society of Human Genetics (https://www.eshg.org) British Society of Human Genetics (http://www.bshg.org.uk/) Swiss Society of Medical Genetics (http://www.sgmg.ch/view_page_professional.php) American Society of Human Genetics (http://www.ashg.org/) American College of Medical Genetics (http://www.acmg.net) HumGen International (http://www.humgen.org )
References
- Human Genome Project Information , http://www.ornl.gov/sci/techresources/Human_Genome/home.shtml (Accessed 20 June 2012).
- National Human Genome Research Institute , http://www.genome.gov/11006943 (Accessed 20 June 2012).
- Biesecker LG. Opportunities and challenges for the integration of massively parallel genomic sequencing into clinical practice: lessons from the ClinSeq project. Genet Med. 2012;14:393–398. doi: 10.1038/gim.2011.78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Welch JS, Westerlvet P, Ding L, et al. Use of whole-genome sequencing to diagnose a cryptic fusion oncogene JAMA 20113151577–1584.(*). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lunshof JE, Bobe J, Aach J, et al. Personal genomes in progress: from the Human Genome Project to the Personal Genome Project. Dialogues Clin Neurosci. 2010;12:47–60. doi: 10.31887/DCNS.2010.12.1/jlunshof. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haga SB, Terry SF.Ensuring the safe use of genomic medicine in children Clin Pediatr 200948703–708.(*). [DOI] [PubMed] [Google Scholar]
- Foundation for Genomics and Population Health Next steps in the sequence, the implications of whole genome sequencing for health in the UK PHG Foundation: Cambridge, MA; 2011 , http://www.phgfoundation.org/reports/10364/ (Accessed 20 June 2012). [Google Scholar]
- Ku Ch-S, Wu M, Cooper DN, et al. Technological advances in DNA sequence enrichment and sequencing for germline genetic diagnosis Expert Rev. Mol. Diagn 201212159–173.(*). [DOI] [PubMed] [Google Scholar]
- Christenhusz GM, Decriendt K, Dierickx K.To tell or not to tell? A systematic review of ethical reflections on incidental findings arising in genetics contexts Eur J Hum Genet 2012. e-pub ahead of print, 27 June 2012doi: 10.1038/ejhg.2012.130 [DOI] [PMC free article] [PubMed]
- Asociación Española de Genética Humana Consentimiento informado para la realización de pruebas diagnósticas http://aegh.org/images/cidiagnostico.pdf (Accessed 20 June 2012).
- British Society for Human Genetics Consent and Confidentiality in Genetic Practice: Guidance on Genetic Testing and Sharing Genetic InformationReport of the Joint Committee on Medical Genetics 2006 http://www.bshg.org.uk/documents/official_docs/consent_pages_web.pdf (Accessed 20 June 2012).
- Swiss Society of Medical Genetics Informed choice in diagnostic genetic testing 2007 , http://www.sgmg.ch/user_files/images/Einverst%C3%A4ndniserkl%C3%A4rung%20SGMG%20erg%C3%A4nzt%20E%202007.pdf (accessed 20 June 2012).
- Ali-Khan SE, Daar AS, Shuman C, Ray PN, Scherer SW.Whole genome scanning: resolving clinical diagnosis and management amidst complex data Pediatr Res 200966357–363.(*). [DOI] [PubMed] [Google Scholar]
- Netzer C, Klein C, Kohlhase J, et al. New challenges for informed consent through whole genome array testing J Med Genet 200946495–496.(*). [DOI] [PubMed] [Google Scholar]
- Ormond KE, Wheeler MT, Hudgins L, et al. Challenges in the Clinical application of whole-genome sequencing Lancet 20103751749–1751.(*). [DOI] [PubMed] [Google Scholar]
- Ransohoff DF, Khoury MJ.Personal genomics: information can be harmful Eur J Clin Invest 20104064–68.(*). [DOI] [PubMed] [Google Scholar]
- Sharp RR.Downsizing genomic medicine: Approaching the ethical complexity of whole-genome sequencing by starting small Genet Med 201113191–194.(*). [DOI] [PubMed] [Google Scholar]
- Sijmons RH, Van Langen IM, Sijmons JG.A clinical perspective on ethical issues in genetic testing Account Res 201118148–162.(*). [DOI] [PubMed] [Google Scholar]
- Raffan E, Semple RK.Next generation sequencing—implications for clinical practice British Med Bull 20119953–71.(*). [DOI] [PubMed] [Google Scholar]
- Berg JS, Khoury MJ, Evans JP.Deploying whole genome sequencing in clinical practice and public health: Meeting the challenge one bin at a time Genet Med 2011113499–504.(*). [DOI] [PubMed] [Google Scholar]
- Green RC, Berg JS, Berry GT, et al. Exploring concordance and discordance for return of incidental findings from clinical sequencing Genet Med 201214405–410.(*). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hastings R, De Wert G, Fowler B, et al. The changing landscape of genetic testing and its impact on clinical and laboratory services and research in Europe Eur J Hum Genet 201220911–916.(*). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thorogood A, Knoppers BM, Dondorp WJ.Whole-genome sequencing and the physician Clin Genet 201281511–513.(*). [DOI] [PubMed] [Google Scholar]
- Organisation for Economic Co-operation and Development OECD guidelines for quality assurance in molecular genetic testing 2007 , http://www.oecd.org/dataoecd/43/6/38839788.pdf (Accessed 20 June 2012).
- Eurogentest Recommendations for genetic counselling related to genetic testing 2008 , http://www.eurogentest.org/web/info/public/unit3/final_recommendations _genetic_counselling.xhtml (Accessed 20 June 2012).
- WMA Statement on Genetics and Medicine Adopted by the 56th WMA General Assembly, Santiago, Chile, October 2005 and amended by the 60th WMA General Assembly, New Delhi, India, October2009 , http://www.wma.net/en/30publications/10policies/g11/ (Accessed 20 June, 2012).
- Dondorp WJ, de Wert GM.The ‘thousand-dollar genome': an ethical exploration, Monitoring Report Ethics and Health, 2010/2. The Hague: Centre for Ethics and Health2010 , http://www.gezondheidsraad.nl/en/publications/thousand-dollar-genome-ethical-exploration (Accessed 20 June 2012).
- Human Genomes Strategy Group Building on our inheritance,Genomic technology in healthcare. A report by the Human Genomics Strategy GroupJanuary2012 , http://www.dh.gov.uk/en/Publicationsandstatistics/Publications/ PublicationsPolicyAndGuidance/DH_132369 (Accessed 20 June 2012).
- American College of Medical Genetics and Genomics (ACMG) Policy Statement, Points to Consider in the Clinical Application of Genomic SequencingMarch2012 , http://www.acmg.net/StaticContent/PPG/Clinical_Application_of_ Genomic_Sequencing.pdf (Accessed 20 June 2012).
- Dimmock D. A personal perspective on returning secondary results of clinical genome sequencing. Genome Med. 2012;4:54. doi: 10.1186/gm353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bunnik EM, Schermer MH, Janssens AC. The role of disease characteristics in the ethical debate on personal genome testing. BMC Med Genom. 2012;5:4. doi: 10.1186/1755-8794-5-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Macarthur DG. Challenges in clinical genomics. Genom Med. 2012;4:43. doi: 10.1186/gm342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Samuels ME, Orr A, Guernsey DL, et al. Is gene discovery research or diagnosis. Genet Med. 2008;10:385–390. doi: 10.1097/GIM.0b013e3181770172. [DOI] [PubMed] [Google Scholar]
- Facio FM, Eidem H, Fisher T, et al. Intentions to receive individual results from whole-genome sequencing among participants in the ClinSeq study Eur J Hum Genet 2012. e-pub ahead of print, 15 August 2012doi: 10.1038/ejhg.2012.179 [DOI] [PMC free article] [PubMed]