Abstract
A chromosomal balanced translocation disrupting the MED13L (Mediator complex subunit13-like) gene, encoding a subunit of the Mediator complex, was previously associated with transposition of the great arteries (TGA) and intellectual disability (ID), and led to the identification of missense mutations in three patients with isolated TGA. Recently, a homozygous missense mutation in MED13L was found in two siblings with non-syndromic ID from a consanguineous family. Here, we describe for the first time, three patients with copy number changes affecting MED13L and delineate a recognizable MED13L haploinsufficiency syndrome. Using high resolution molecular karyotyping, we identified two intragenic de novo frameshift deletions, likely resulting in haploinsufficiency, in two patients with a similar phenotype of hypotonia, moderate ID, conotruncal heart defect and facial anomalies. In both, Sanger sequencing of MED13L did not reveal any pathogenic mutation and exome sequencing in one patient showed no evidence for a non-allelic second hit. A further patient with hypotonia, learning difficulties and perimembranous VSD showed a 1 Mb de novo triplication in 12q24.2, including MED13L and MAP1LC3B2. Our findings show that MED13L haploinsufficiency in contrast to the previously observed missense mutations cause a distinct syndromic phenotype. Additionally, a MED13L copy number gain results in a milder phenotype. The clinical features suggesting a neurocristopathy may be explained by animal model studies indicating involvement of the Mediator complex subunit 13 in neural crest induction.
Keywords: congenital heart defect, intellectual disability, MED13L, copy number changes, neurocristopathy
Introduction
MED13L, Mediator complex subunit13-like, is a subunit of the so-called Mediator complex that functions with DNA-binding transcription factors and RNA polymerase II for gene transcription.1 Muncke et al.2 cloned MED13L using a positional cloning approach to identify the gene on chromosome 12 interrupted by a translocation breakpoint in a patient with dextro-looped transposition of the great arteries (dTGA) and intellectual disability (ID). They designated the gene as PROSIT240 due to the protein similarity to the human thyroid hormone receptor-associated protein 240. Independently, Musante et al.3 cloned the gene by RT-PCR, 5-prime RACE of human fetal brain and lymphoblastoid cell line cDNA libraries, which they called THRAP2. Eventually, it was shown that MED13L is a component of the Mediator complex in HeLa cells.1
From the family of Mediator complex, MED12 is known to be involved in the etiology of Opitz–Kaveggia syndrome (FG syndrome, MIM no. 305450)4 and Lujan–Fryns syndrome (MIM no. 309520),5 X-linked disorders characterized by ID, hypotonia and minor anomalies such as macrocephaly or high forehead and rarely congenital heart defects. For the other members of the Mediator complex, the following disease associations have been reported so far: an 800 kb heterozygous deletion including MED13 in a patient with ID, cataract and hearing loss,6 association of infantile cerebral and cerebellar atrophy with a homozygous missense mutation in MED17,7 co-segregation of a missense mutation in MED23 with non-syndromic autosomal recessive ID8 and a homozygous missense mutation of MED25 in a family with Charcot–Marie–Tooth neuropathy.9 These findings highlight the importance of the Mediator complex in embryonic development and in particular of the nervous system. Concerning MED13L, to date, three heterozygous missense mutations in patients with dTGA, one translocation disrupting MED13L between exons 1 and 2 in a patient with ID and dTGA2 and recently, a homozygous missense mutation in two patients of a consanguineous family with non-syndromic ID were found (Table 1).10 Furthermore, a breakpoint disrupting chromosome 12 near MED13L was reported in a patient with Noonan-like phenotype with unknown contribution to the patient's phenotype.3 Here, we describe for the first time, three patients with copy number changes affecting MED13L and delineate a recognizable MED13L haploinsufficiency syndrome.
Table 1. Summary of the reported patients with chromosomal abnormality or mutation affecting MED13L.
| Muncke et al (2003)2 | Muncke et al (2003)2 | Muncke et al (2003)2 | Muncke et al (2003)2 | Najmabadi et al (2011)10 | This study | This study | This study | |
|---|---|---|---|---|---|---|---|---|
| Patient 1 | Patient 2 | Patient 3 | Patient 4 | two children of a consanguineous family | Patient 1 | Patient 2 | Patient 3 | |
| Sex | F | NA | NA | NA | NA | F | F | F |
| Chromosomal abnormality/mutation | t(12;17)(q24.1;q21) de novo between E1 and E2 | Glu251 Gly maternal E6 | Arg1872 His E25 | Asp2023 Gly E28 | Arg1416 His homozygous E19 | Deletion de novo E2 | Deletion de novo E3 and E4 | Triplication de novo of the whole gene |
| Cardiac manifestations | dTGA, pVSD open foramen ovale, mild CoA | dTGA | dTGA | dTGA | — | Supracardial TAPVC, pulmonary atresia, VSD | TOF | pVSD |
| Neurologic manifestations | Microcephaly, discrete ataxia, ID, nearly absent speech | — | — | — | Mild ID | moderate ID, gross and fine motor coordination problems, hypotonia | Gross developmental delay, hypotonia | Learning difficulties, hypotonia |
Abbreviations: CoA, coarctation of the aorta; dTGA, dextro-looped transposition of the great arteries; E, exon; F, female; ID, intellectual disability; NA, not available; pVSD, perimembranous ventricular septal defect; TAPVC, total anomalous pulmonary venous connection; TOF, tetralogy of Fallot.
Patients and Methods
Patient 1
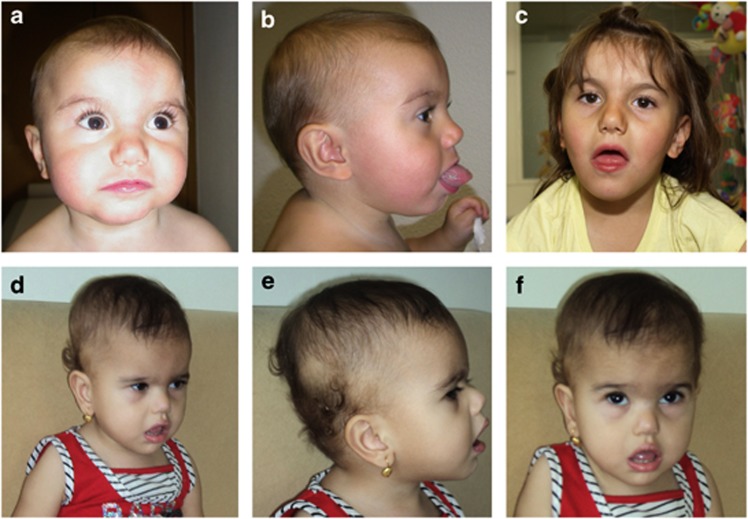
The first patient is a girl born spontaneously after 40 weeks of gestation with no complication except for oligohydramnios at the end of pregnancy. Birth weight was 2550 g (<3rd centile), length 45 cm (3rd centile) and head circumference 33 cm (3rd–10th centile). Apgar scores were 9/10/10. A 2/6 systolic murmur and macroglossia were noted at birth. At the age of 4 weeks, after the third cyanotic episode occurred while crying, a complex congenital heart defect with supracardial total anomalous pulmonary venous connection, pulmonary atresia with ventricular septal defect and multifocal pulmonary perfusion were diagnosed. Additionally, dysmorphic features including micrognathia and anteriorly positioned anus, as well as neurological anomalies such as truncal muscular hypotonia and hypertonia of the extremities and monotonous movements were noted. A cerebral MRI investigation in infancy as well as ultrasound investigations of the abdomen revealed normal results. The complex congenital heart defect could be completely corrected at the age of 9 weeks with unifocalisation of three major aortopulmonary collaterals, patch closure of ventricular septal defect, partial closure of the atrial septal defect, contegra xenograft implantation from the right ventricle to the neopulmonary artery bifurcation, anastomosis of the pulmonary vein collector with the left atrium and ligature of vertical vein. Surgery could be performed without any significant complication except for a major aortopulmonary collateral artery from the left coronary artery, which could not be unifocalized due to anatomic reasons. A surgical reoperation became necessary at the age of 9 months including a change of the right ventricle to pulmonary artery contegra xenograft using an aortic homograft and further unifocalisation of two major aortopulmonary collaterals. Post-operative course was uneventful. At 13 months of age, her weight, length and head circumference were 9.8 kg (25th–50th centile), 74 cm (10th–25th centile) and 45 cm (10th–25th centile), respectively. Physical examination revealed a broad forehead with nevus simplex and bitemporal narrowing, slightly asymmetrical face, long eyelashes and upslanting palpebral fissures, large, low set ears (5.5 cm; +4 SD), flat nasal root, bulbous nose with short alae nasi, deep philtrum, facial hypotonia with open mouth appearance and macroglossia (Figure 1). However, the patient's otherwise healthy father and younger sister were also said to have macroglossia. Developmental milestones were delayed with sitting age of 12 months, and walking age of 25 months. It was said that she spoke her first words at the age of 12 months, but further speech development was delayed. Physical therapy was initiated in infancy and later she also received speech therapy. At the age of 4 years, muscular hypotonia was still evident and she had a non-verbal IQ of 50 with a developmental age of 2.5–3 years (Snijders–Oomen Non-Verbal Intelligence Test–revised). Her formal language was delayed and she had gross and fine motor coordination problems. She could walk up and down holding onto the railings and started bicycling with support wheels. She had wide-based gait, could not stand on one leg and could not throw or catch a ball. Her receptive language was considered quite well with a good vocabulary, but her expressive language was limited to simple sentences with poor articulation. She was described as shy and cautious, but very social person with a good sense of humor.
Figure 1.
Facial features of patients 1 and 2 with MED13L haploinsufficiency. (a, b) patient 1 at 13 months and, (c) at 4.5 years of age. Note broad forehead with bitemporal narrowing, mild facial asymmetry, long eyelashes and upslanting palpebral fissures, flat nasal root, bulbous nose, deep philtrum and macroglossia. (d, e and f) patient 2 at 2 years and 9 months of age with notable similarity in facial gestalt.
Patient 2
The second patient is a girl born with delayed crying after delivery. Her birth weight was 1800 g (<3rd centile). Cyanotic tetralogy of Fallot was diagnosed at the age of 8 months. Developmental delay was first noticed when she was 10 months old. At the age of 2 years and 9 months, she had marked muscular hypotonia, was able to sit with support but could not stand. She had no speech, and could only recognize her mother and give social smile to her. On physical examination, she had a prominent occipital protuberance, a broad forehead, upslanting palpebral fissures, a flat nasal root, a bulbous nasal tip with small nares, a deep and short philtrum, large, low set ears, micrognathia, hypotonic open mouth appearance (Figure 1), bowed legs, gross developmental delay and overlapping of the fifth toe over the fourth toe.
Patient 3
The third patient is a girl born 5 days preterm with a history of transient but marked fetal hydrops during pregnancy (weeks 16–25). Her birth weight was 2200 g (<3rd centile) and the length was 47 cm (3rd centile).
Despite neonatal muscular hypotonia and frequent vomiting, she did not have neonatal feeding problems and was breastfed for more than 1 year. She could sit at the age of 8–9 months and walked without support when she was 30 months old. However, her gait was unsteady for a long time. Her language development was normal and she had good social skills. She gained bladder and bowel control at the age of 5 years.
At 6 and half years of age, her length and head circumference were 117 cm (25th centile) and 50.5 cm (10th–25th centile), respectively. Physical examination revealed a broad nasal bridge and mild pectus excavatum, only. She had satisfactory performance on most levels in her first year of school. The only cardiac malformation was a perimembranous VSD that closed spontaneously.
Microarray and confirmatory studies
DNA, extracted from peripheral blood, was analyzed with a Cytogenetics 2.7 array (Affymetrix Inc, Santa Clara, CA, USA) in patient 1, a Human Genome CGH Microarray 60 K (Agilent Technologies, Santa Clara, CA, USA) in patient 2 and an Affymetrix Genome-Wide Human SNP Array 6.0 in patient 3 according to the manufacturers' protocols. Customized MLPA was performed using synthetic probes for exon 2 of MED13L and the SALSA MLPA kit P300 Human DNA reference2 (MRC-Holland, Amsterdam, The Netherlands) in patient 1. Customized qPCR for exons 3 and 4 was performed using SYBR green in patient 2.
Exome sequencing
Whole-exome sequencing on genomic DNA of patient 1 was performed using the SureSelect XT HumanAllExon 50 Mb Kit (Agilent Technologies) with 75-bp forward reads on a SOLiD 5500xl System (ABI/Life Technologies). The average depth of coverage was 40 × and about 80% of the targeted bases were assessed by ≥5 independent sequence reads; only non-silent exonic and splice site variants in genes known to cause developmental disorders (according to HGMD and OMIM) were considered.
Mutation analysis
All exons of MED13L and candidate nucleotide variants from exome sequencing were analyzed after PCR amplification from the patient's DNA by Sanger sequencing using an ABI Genetic Analyzer 3730 (Applied Biosystems, Foster City, CA, USA).
Results
In patient 1, chromosome studies showed a normal 46,XX karyotype and FISH-testing of the 22q11.2 DiGeorge region revealed no microdeletion. Copy number profiling with the 2.7 array revealed only 10 rare CNVs, including a 17 kb deletion encompassing exon 2 of MED13L (Supplementary Figure 1A) ([g.(115,155,757_115,158,648)_115,175,505_115,176,337)del NCBI Build 36.1]). No CNV including MED13L exons was observed among our 820 control individuals. Deletion of exon 2 was confirmed as de novo by customized MLPA and in silico analysis determined that the deletion is out of frame.
Considering the possibility of a second hit in this patient, whole-exome sequencing was performed with the evaluation of mutations located in the known disease-causing genes related to congenital anomalies and/or ID. A missense heterozygous mutation (c.1468 G>A, p.G490S) within the ATRX gene (ENSG00000085224) was detected, but turned out to be maternally inherited and was predicted as benign by PolyPhen-2 classification. In spite of the limitations in the exome sequencing data, deletion of MED13L is the most likely cause of this patient's phenotype.
In patient 2, by means of Agilent 60 K Microarray, a 115 kb deletion encompassing exons 3 and 4 of MED13L was detected, which is out of frame too (g.( 114,906,957_114,960,998)_( 115,075,825_115,116,716) del NCBI Build 36.1). The deletion was confirmed as de novo by customized qPCR. Sanger sequencing of all exons and flanking introns of MED13L in patients 1 and 2 revealed no pathogenic mutation.
In patient 3, the 6.0 array in the patient and her parents showed a 1 Mb de novo triplication in 12q24.2 spanning from 114 508 443 to 115 527 724 (Homosapiens, build 36/hg18), which includes the whole MED13L gene, several non-protein-coding RNA genes and microtubule-associated protein 1 light chain 3 beta-2 gene (MAP1LC3B2) (Supplementary Figure 1B) ([g.(114507371_114,508,443)_( 115,527,724_115528576)tri NCBI Build 36.1]).
Discussion
We report the first three patients with copy number variants of MED13L, two intragenic out-of-frame de novo deletions, and a 1 Mb triplication of the whole gene and the flanking regions (Figure 2). The two patients with out-of-frame deletions showed a distinct phenotype with similar facial dysmorphism, conotruncal congenital heart defect, hypotonia and moderate developmental delay (Figure 1). Facial anomalies included upslanting palprebral fissures, flat nasal root with bulbous tip, deep philtrum, micrognathia, large, low set ears and broad forehead. As both deletions are out-of-frame, it is likely that they disrupt the affected allele completely, resulting in nonsense-mediated mRNA decay of the transcript. Following the recent report of a homozygous missense mutation of MED13L in a family with non syndromic ID,10 which suggests a recessive inheritance pattern, the gene was sequenced in patients 1 and 2 but revealed no pathogenic mutation. Furthermore, considering the two hit hypothesis, exome sequencing was performed in patient 1. However, it did not reveal any strong finding possibly contributing to the patient's phenotype. Absence of an obvious second hit in patient 1 in addition to the phenotypic similarity to the second patient with intragenic deletion strongly suggests that the phenotype in these patients is indeed caused by MED13L haploinsufficiency. The third patient with the triplication had a milder phenotype with mild hypotonia, a clinically insignificant cardiac defect and mild developmental delay only. Thus, like in the cases with 22q11.2 duplication or triplication, overexpression of the gene seems to lead to a much milder phenotype than deletions, yet with an overlapping clinical spectrum.11 On the other hand, the triplication of our patient in addition to MED13L encompasses several non-protein-coding RNA genes and the gene MAP1LC3B2 with unknown function. A larger de novo duplication of 2.3 Mb spanning 12q24.21q24.23 containing 16 genes together with MED13L has been reported in a girl with syndromic ID without cardiac abnormality.12 However, several of these affected genes are expressed in the brain and/or are involved in embryogenesis and thus, it is likely that a combination effect of them is contributing to their patient's phenotype. Of note, there are two further de novo CNVs affecting MED13L in the DECIPHER database, including a gain of 60 kbp within exons 5–28 in a female patient with ID, developmental delay, auricular tags and macrostomia (DECIPHER no. 255109), and the other one is a 120 kbp de novo loss encompassing exons 3 and 4 in a male patient with ID and developmental delay (DECIPHER no. 257915). If the intragenic duplication would be in tandem, though, it would be unlikely to cause gain of function but may rather disrupt the gene.
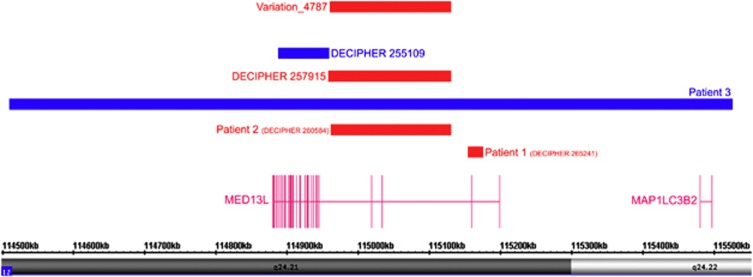
Figure 2.
Copy number variants of MED13L in our patients. Patient 1 (DECIPHER no. 265241) with a de novo deletion of exon 2 (red bar), patient 2 (DECIPHER no. 260584) with a de novo deletion encompassing exons 3 and 4 (red bar) and patient 3 with a de novo triplication of the region 114 508 443–115 527 724 (Homosapiens, build 36/hg18) (blue bar), which includes the whole MED13L gene, several non-protein-coding RNA genes and MAP1LC3B2. Patients 255 109 and 257 915 are from the DECIPHER database with 60 kbp de novo gain within exons 5–28 of MED13L and 120 kbp de novo loss within its exons 3 and 4, respectively (https://decipher.sanger.ac.uk). Variation_4787 from the Database of Genomic Variants (build 36) (http://projects.tcag.ca/variation, build 36) is shown in the upper part. Variation_4787, a 173 kbp deletion observed in 6 out of 95 controls using BAC Array CGH, is likely a false-positive finding due to a misbehaving BAC. This variation was not observed in our 820 control individuals.
Notably, a variant deletion of exons 3 and 4 was reported in 6 out of 95 controls using BAC Array CGH by Wong et al. 2007,13 which is likely a false-positive finding, as we did not observe it in our 820 control individuals (Variation_4787; Figure 2).
Our findings suggest that abnormal MED13L dosage can affect both cardiac and neurological development, although both DECIPHER entries not available for this study may indicate reduced penetrance for heart defects. As some heterozygous missense mutations apparently result in isolated heart defects2 while one homozygous missense mutation was reported to cause non-syndromic autosomal recessive ID,10 these mutations may affect tissue-specific distinct functions of the protein. In fact, according to Scansite (http://scansite.mit.edu) and pfam (http://pfam.sanger.ac.uk/) predictions, the missense mutations observed in isolated dTGA patients are located in the N- or C-terminal Mediator complex subunit 13 domains, whereas the homozygous ID mutation does not overlap with any special domain or motif.
The complex phenotype observed in MED13L haploinsufficiency is in line with the contribution of different Mediator complex subunits to transcriptional activation of developmentally regulated genes in model organisms,14, 15, 16 which can have remarkably gene-specific, and even tissue-specific functions.17 mRNA expression of MED13L in human has been detected in fetal brain and heart, and adult tissues including skeletal muscle, brain (especially, cerebellum), heart and aorta, kidney and peripheral blood leukocytes.2, 3 Animal studies imply a specific role for MED13, similar in size and ∼50% identical to MED13L, in regulating transcription of Wnt and Notch target genes, and Hh signal transduction,18, 19, 20 which are involved in neural crest induction.21 Neural crest cell migration has an important role in the development of the heart, the nervous system and the facial mesenchyme, which could explain the syndromic signs of MED13L haploinsufficiency and the clinical overlap with DiGeorge syndrome.22 Of note, a pathogenic role for neural crest cells was also suspected for Lujan-Fryns Syndrome with the involvement of MED12, another member of the Mediator complex.23
In addition, MED13L and other subunits of the Mediator complex were recently shown to have a role in Rb/E2F-induced growth inhibition.24 So far, no increased tumor susceptibility was observed in patients with MED13L defects, however, that may be due to the limited number of patients known and their young age.
In conclusion, our findings show that MED13L haploinsufficiency in contrast to the previously observed missense mutations cause a distinct syndromic phenotype. In addition, like in other haploinsufficiency syndromes, a MED13L copy number gain results in a milder phenotype.
Acknowledgments
We sincerely thank the affected individuals and their families for participation and their permission to publish the results. We are grateful to Dr Cédric Le Caignec (Service de Génétique Médicale, Nantes, France) and Dr Björn Menten (Centrum Medische Genetica, Ghent, Belgium) for accepting the citation of their DECIPHER Consortium cases (numbers 255109 and 257915, respectively) in our discussion. We also thank the DECIPHER Consortium. This research was supported by grants from the Swiss National Science Foundation (SNF 320030_135669) and the Forschungskredit of the University of Zurich, grant number 54220201.
The authors declare no conflict of interest.
Footnotes
Supplementary Information accompanies the paper on European Journal of Human Genetics website (http://www.nature.com/ejhg)
Supplementary Material
References
- Sato S, Tomomori-Sato C, Parmely TJ, et al. A set of consensus mammalian mediator subunits identified by multidimensional protein identification technology. Mol Cell. 2004;14:685–691. doi: 10.1016/j.molcel.2004.05.006. [DOI] [PubMed] [Google Scholar]
- Muncke N, Jung C, Rudiger H, et al. Missense mutations and gene interruption in PROSIT240, a novel TRAP240-like gene, in patients with congenital heart defect (transposition of the great arteries) Circulation. 2003;108:2843–2850. doi: 10.1161/01.CIR.0000103684.77636.CD. [DOI] [PubMed] [Google Scholar]
- Musante L, Bartsch O, Ropers HH, Kalscheuer VM. cDNA cloning and characterization of the human THRAP2 gene which maps to chromosome 12q24, and its mouse ortholog Thrap2. Gene. 2004;332:119–127. doi: 10.1016/j.gene.2004.02.044. [DOI] [PubMed] [Google Scholar]
- Risheg H, Graham JM, Clark RD, et al. A recurrent mutation in MED12 leading to R961W causes Opitz-Kaveggia syndrome. Nat Genet. 2007;39:451–453. doi: 10.1038/ng1992. [DOI] [PubMed] [Google Scholar]
- Schwartz CE, Tarpey PS, Lubs HA, et al. The original Lujan syndrome family has a novel missense mutation (p.N1007S) in the MED12 gene. J Med Genet. 2007;44:472–477. doi: 10.1136/jmg.2006.048637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boutry-Kryza N, Labalme A, Till M, et al. An 800 kb deletion at 17q23.2 including the MED13 (THRAP1) gene, revealed by aCGH in a patient with a SMC 17p. Am J Med Genet A. 2012;158A:400–405. doi: 10.1002/ajmg.a.34222. [DOI] [PubMed] [Google Scholar]
- Kaufmann R, Straussberg R, Mandel H, et al. Infantile cerebral and cerebellar atrophy is associated with a mutation in the MED17 subunit of the transcription preinitiation Mediator complex. Am J Hum Genet. 2010;87:667–670. doi: 10.1016/j.ajhg.2010.09.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hashimoto S, Boissel S, Zarhrate M, et al. MED23 mutation links intellectual disability to dysregulation of immediate early gene expression. Science. 2011;333:1161–1163. doi: 10.1126/science.1206638. [DOI] [PubMed] [Google Scholar]
- Leal A, Huehne K, Bauer F, et al. Identification of the variant Ala335Val of MED25 as responsible for CMT2B2: molecular data, functional studies of the SH3 recognition motif and correlation between wild-type MED25 and PMP22 RNA levels in CMT1A animal models. Neurogenetics. 2009;10:275–287. doi: 10.1007/s10048-009-0183-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Najmabadi H, Hu H, Garshasbi M, et al. Deep sequencing reveals 50 novel genes for recessive cognitive disorders. Nature. 2011;478:57–63. doi: 10.1038/nature10423. [DOI] [PubMed] [Google Scholar]
- Yobb TM, Somerville MJ, Willatt L, et al. Microduplication and triplication of 22q11.2: a highly variable syndrome. Am J Hum Genet. 2005;76:865–876. doi: 10.1086/429841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruiter M, Koolen DA, Pfundt R, et al. A novel 2.3 Mb microduplication of 12q24.21q24.23 detected by genome-wide tiling-path resolution array comparative genomic hybridization in a girl with syndromic mental retardation. Clin Dysmorphol. 2006;15:133–137. doi: 10.1097/01.mcd.0000220605.94413.bb. [DOI] [PubMed] [Google Scholar]
- Wong KK, deLeeuw RJ, Dosanjh NS, et al. A comprehensive analysis of common copy-number variations in the human genome. Am J Hum Genet. 2007;80:91–104. doi: 10.1086/510560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang JC, Walker A, Blackwell TK, Yamamoto KR. The Caenorhabditis elegans ortholog of TRAP240, CeTRAP240/let-19, selectively modulates gene expression and is essential for embryogenesis. J Biol Chem. 2004;279:29270–29277. doi: 10.1074/jbc.M401242200. [DOI] [PubMed] [Google Scholar]
- Lim J, Lee OK, Hsu YC, Singh A, Choi KW. Drosophila TRAP230/240 are essential coactivators for Atonal in retinal neurogenesis. Dev Biol. 2007;308:322–330. doi: 10.1016/j.ydbio.2007.05.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ito M, Okano HJ, Darnell RB, Roeder RG. The TRAP100 component of the TRAP/Mediator complex is essential in broad transcriptional events and development. EMBO J. 2002;21:3464–3475. doi: 10.1093/emboj/cdf348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Conaway RC, Conaway JW. Function and regulation of the Mediator complex. Curr Opin Genet Dev. 2011;21:225–230. doi: 10.1016/j.gde.2011.01.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoda A, Kouike H, Okano H, Sawa H. Components of the transcriptional Mediator complex are required for asymmetric cell division in C. elegans. Development. 2005;132:1885–1893. doi: 10.1242/dev.01776. [DOI] [PubMed] [Google Scholar]
- Janody F, Treisman JE. Requirements for Mediator complex subunits distinguish three classes of notch target genes at the Drosophila wing margin. Dev Dyn. 2011;240:2051–2059. doi: 10.1002/dvdy.22705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Janody F, Martirosyan Z, Benlali A, Treisman JE. Two subunits of the Drosophila mediator complex act together to control cell affinity. Development. 2003;130:3691–3701. doi: 10.1242/dev.00607. [DOI] [PubMed] [Google Scholar]
- Stuhlmiller TJ, Garcia-Castro MI. Current perspectives of the signaling pathways directing neural crest induction. Cel Mol Life Sci. 2012;69:3715–3737. doi: 10.1007/s00018-012-0991-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Calmont A, Ivins S, Van Bueren KL, et al. Tbx1 controls cardiac neural crest cell migration during arch artery development by regulating Gbx2 expression in the pharyngeal ectoderm. Development. 2009;136:3173–3183. doi: 10.1242/dev.028902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin AE, Pober BR, Mullen MP, Slavotinek AM. Cardiovascular malformations in Fryns syndrome: is there a pathogenic role for neural crest cells. Am J Med Genet A. 2005;139:186–193. doi: 10.1002/ajmg.a.31023. [DOI] [PubMed] [Google Scholar]
- Angus SP, Nevins JR. A role for Mediator complex subunit MED13L in Rb/E2F-induced growth arrest. Oncogene. 2012;31:4709–4717. doi: 10.1038/onc.2011.622. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.