Update to: European Journal of Human Genetics (2011) 19; doi:10.1038/ejhg.2010.247; published online 19 January 2011
1. DISEASE CHARACTERISTICS
1.1 Name of the disease (synonyms)
Diamond–Blackfan anemia, DBA1, 3–10 (DBA2 not confirmed), Aase–Smith syndrome, congenital hypoplastic anemia, Blackfan Diamond anemia and inherited erythroblastopenia. The recent discovery of families with clinically diagnosed X-linked DBA as a consequence of mutations in the transcription regulator GATA1 has engendered some controversy,1 but will nonetheless be categorized as DBA until agreement on the nosology of DBA is reached.
1.2 OMIM# of the disease
105650, 610629, 612527, 612528, 612561, 612562, 612563, 613308, 613309, 300835.
1.3 Name of the analyzed genes or DNA/chromosome segments
RPS19, RPS24, RPS17, RPL35A, RPL5, RPL11, RPS7, RPS10, RPS26, RPL26, GATA1.
1.4 OMIM# of the gene(s)
603474, 602412, 180472, 180468, 603634, 604175, 603658, 603632, 603701, 603704, 305371.
1.5 Mutational spectrum
In patients for whom there is a known mutation or deletion (∼70%), Diamond–Blackfan anemia results, in the vast majority of cases, from a ribosomal protein haploinsufficiency.2, 3 No racial or ethnic predilection has been identified. The relative frequency of affected ribosomal genes identified is approximately 25% RPS19; 2% RPS24; 1% RPS17; 2–4% RPL35A; 7% RPL5; 5–10% RPL11; 1% RPS7; 2–6% RPS10; and 2–6% RPS26.4, 5, 6, 7, 8, 9 A frameshift mutation in RPL26 was identified in one patient.10 Mutations resulting in haploinsufficiency or loss-of-function in all 10 genes thus far described include missense mutations, nonsense mutations, splice mutations, insertions, deletions and rearrangements.11, 12 Recently mutations in the erythroid transcription regulator GATA1, apparently not involving any disruption in ribosome biogenesis/function, have been discovered as causative in rare cases of X-linked clinical DBA.13
1.6 Analytical methods
Depending upon the nature and magnitude of the sequence change, a variety of techniques are available; for example, cytogenetics for large deletions and rearrangements, multiple ligation-dependent probe amplification for detection of large deletions and rearrangements, array comparative genomic hybridization for detection of deletions, quantitative PCR, whole-exome sequencing and whole-genome sequencing. It is estimated that approximately 10–19% of ribosomal protein-haploinsufficient DBA cases are caused by large deletions that are missed by exon sequencing.2, 14, 15
1.7 Analytical validation
The analysis from genetic tests should be performed by experienced, knowledgeable personnel, in certified diagnostic labs. Technical errors, sample mix-up and natural genetic variations may in rare cases create misinterpretations. To avoid this, analysis and validation should be performed by professionals in environments with established routines.
1.8 Estimated frequency of the disease (Incidence at birth (‘birth prevalence') or population prevalence. If known to be variable between ethnic groups, please report):
The incidence is from 5–10 per one million live births.16 There is no known ethnic predilection or exclusion.
1.9 Diagnostic setting

Comment:
In addition, genetic testing has been used in the following diagnostic settings.
E. Related stem cell transplant donor screening to be certain that the donor is not affected.
2. TEST CHARACTERISTICS
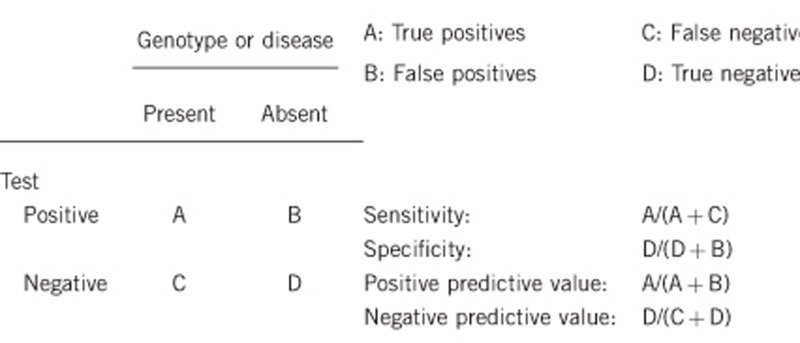
2.1 Analytical sensitivity (proportion of positive tests if the genotype is present)
Nearly 100%, within the margin of lab error (this applies to genetic testing only).
2.2 Analytical specificity (proportion of negative tests if the genotype is not present)
Nearly 100%, within the margin of lab error (this applies to genetic testing only).
2.3 Clinical sensitivity (proportion of positive tests if the disease is present)
The clinical sensitivity can be dependent on variable factors such as age or family history. In such cases, a general statement should be given, even if a quantification can only be made case by case.
Currently, ∼70% of clinically diagnosed cases can be identified by mutation analysis, multiple ligation-dependent probe amplification,15, 17 or array based screening for copy number variations (eg, comparative genomic hybridization).
2.4 Clinical specificity (proportion of negative tests if the disease is not present)
The clinical specificity can be dependent on variable factors such as age or family history. In such cases, a general statement should be given, even if quantification can only be made case by case.
Nearly 100%. False positives due to technical or clerical errors are possible. If the disease, in the broadest sense, is not present, the test will generally be negative. The penetrance and expressivity of DBA is quite variable; thus, there are instances, even in multiplex families, where a genetically affected individual has subtle or no evidence of clinical disease. Therefore, the definition of what is disease is critical.18 In this instance, a clinically unaffected individual could have a positive test. Clinicians, patients/families and geneticists may define ‘presence of disease' quite differently.
2.5 Positive clinical predictive value (life time risk to develop the disease if the test is positive)
Again, the definition of what constitutes disease (see 2.4) is critical. There are not enough data to fully predict the ‘natural history' in non-expressing (clinically unaffected) individuals with known DBA-causing mutations or deletions that result in ribosomal protein haploinsufficiency. The majority of such individuals do have some evidence of disease although these manifestations can be subtle. A minority of patients with known genotypes have no discernible clinical or hematological evidence of DBA. X-linked DBA, as a consequence of a mutation in GATA1, is too rare to allow for the prediction of probable phenotypes although the presence of significant congenital anomalies seems unlikely.
2.6 Negative clinical predictive value (probability not to develop the disease if the test is negative)
Assume an increased risk based on family history for a non-affected person. Allelic and locus heterogeneity may need to be considered.
Index case in that family had been tested:
If the proband is positive for a mutation, virtually 100%.
Index case in that family had not been tested:
A non-affected first-degree relative to an index case (non-tested) has an indeterminate risk, as only 70% of the patients have a known DBA-associated ribosomal gene mutation or deletion.
3. CLINICAL UTILITY
3.1 (Differential) diagnostics: The tested person is clinically affected
(To be answered if in 1.9 ‘A' was marked)
3.1.1 Can a diagnosis be made other than through a genetic test?
3.1.2 Describe the burden of alternative diagnostic methods to the patient
A clinical diagnosis is based on a history, physical examination, laboratory tests that require venipuncture and a bone marrow aspirate that may be performed under local or general (deep conscious sedation) anesthesia or deep conscious sedation. There is a minimal but incremental risk for anesthesia and the aspirate, which include pain, bleeding and rarely (∼1:50 000) death. Occasionally, ultrasound or radiological imaging is required to determine the presence of certain congenital anomalies supportive of the diagnosis of DBA.
3.1.3 How is the cost effectiveness of alternative diagnostic methods to be judged?
There are no studies available to determine the cost effectiveness of any diagnostic approach to DBA. Costs will differ depending upon the laboratory fees and imaging required. The consequence of missing the diagnosis or failing to determine the presence or absence of particular hematological or non-hematological manifestations of DBA may be significant.
3.1.4 Will disease management be influenced by the result of a genetic test?

3.2 Predictive Setting: The tested person is clinically unaffected but carries an increased risk based on family history
(To be answered if in 1.9 ‘B' was marked)
3.2.1 Will the result of a genetic test influence lifestyle and prevention?
Yes.
If the test result is positive (please describe):
In a family, either multiplex or identified through an affected proband, a positive test in a family member will impart the burden of the disease. In the case of X-linked inheritance, the burden of the carrier state falls to the female carriers. This individual with an autosomal dominant mutation may be variably affected, and in whom a clinical diagnosis is obvious, a positive test will just confirm the diagnosis. When there is no, or only minimal, evidence of the disease, a positive genetic test will confirm that the individual may transmit the mutation to the offspring. Reproductive options can be pursued that may decrease the likelihood of having an affected child.22, 23 The positive individual would also be disqualified as a stem cell transplant donor, regardless of histocompatibility, even if the individual was clinically unaffected.19
Mildly affected or unaffected carriers of a DBA-associated ribosomopathy should be informed about subclinical and clinical expression of the disease (eg, subnormal hemoglobin levels), recurrence risks in next generation and complications associated with pregnancies. A regular check of the hemoglobin levels may be recommended.
If the test result is negative (please describe):
In a family, either multiplex or identified through an affected proband, a negative test will relieve the burden of the disease from the negative individual, remove the necessity for any reproductive options related to the transmission of the autosomal dominant or X-linked disorder and allow the individual to be a stem cell transplant donor based on histocompatibility.
3.2.2 Which options in view of lifestyle and prevention does a person at-risk have if no genetic test has been done (please describe)?
For clinically affected individuals, genetic testing provides the advantage of confirmation of diagnosis as other marrow-failure syndromes may share significant clinical overlap. The identification of a specific gene involved has no advantage to the individual, as no relevant genotype–phenotype correlations have been established. However, in the absence of testing reproductive options cannot be established for the prevention of transmission of the disease to the next generation.
3.3 Genetic risk assessment in family members of a diseased person
(To be answered if in 1.9 ‘C' was marked)
3.3.1 Does the result of a genetic test resolve the genetic situation in that family?
Yes, risk assessment is preferably performed on an individual basis and related to the family structure, clinical investigations and a combination of biochemical and genetic test results.
If a DBA-associated ribosomal protein gene mutation or deletion has been found in the family (a mutation or deletion has only been identified in ∼70% of families).
3.3.2 Can a genetic test in the index patient save genetic or other tests in family members?
Yes, if it is positive for a known DBA-associated ribosomal protein gene or GATA1 mutation or deletion, only that gene needs to be tested.
3.3.3 Does a positive genetic test result in the index patient enable a predictive test in a family member?
Yes.
3.4 Prenatal diagnosis
(To be answered if in 1.9 ‘D' was marked)
3.4.1 Does a positive genetic test result in the index patient enable a prenatal diagnosis?
Yes, in almost all instances. There is a small possibility of gonadal mosaicism in which a child with a positive test is born to parents with a negative test.24
4. IF APPLICABLE, FURTHER CONSEQUENCES OF TESTING
Please assume that the result of a genetic test has no immediate medical consequences. Is there any evidence that a genetic test is nevertheless useful for the patient or his/her relatives? (Please describe)
Yes, prenatal testing may be offered if a genetic marker is identified in an affected family member. Genetic testing of the parents or other relatives of an affected child will allow reproductive choices that may reduce or eliminate the possibility of vertical transmission of DBA. As well, positive genetic testing helps remove any doubt about the diagnosis. Genetic testing may in some cases also be combined with human leukocyte antigen typing to have a matched sibling for stem cell transplantation.
Acknowledgments
This work was supported by the EuroGentest2 (Unit 2: ‘Genetic testing as part of health care'), a Coordination Action under FP7 (grant agreement number 261469), the European Society of Human Genetics and grants from the Daniella Maria Arturi Foundation (JML, AV), Pediatric Cancer Foundation (JML), National Institutes of Health R01 HL 079571-08 (JML, AV), The Feinstein Institute for Medical Research General Clinical Research Center M01 RR018535 (JML, AV), Telethon (ID), DBA Foundation (ID, AV, JML), Italian Ministry of University and Research (ID), Gruppo di Sostegno DBA Italia (ID), Cariplo 2011-554 (ID) and the Swedish Research Council (ND).
The authors declare no conflict of interest.
References
- Weiss MJ, Mason PJ, Bessler M. What's in a name. J Clin Invest. 2012;122:2246–2349. doi: 10.1172/JCI63989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gazda HT, Zhong R, Long L, et al. RNA and protein evidence for haplo-insufficiency in Diamond-Blackfan anaemia patients with RPS19 mutations. Br J Haematol. 2004;127:105–113. doi: 10.1111/j.1365-2141.2004.05152.x. [DOI] [PubMed] [Google Scholar]
- Farrar JE, Vlachos A, Atsidaftos E, et al. Ribosomal protein gene deletions in Diamond Blackfan anemia. Blood. 2011;118:6943–6951. doi: 10.1182/blood-2011-08-375170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Draptchinskaia N, Gustavsson P, Andersson B, et al. The gene encoding ribosomal protein S19 is mutated in Diamond-Blackfan anaemia. Nat Genet. 1999;21:169–175. doi: 10.1038/5951. [DOI] [PubMed] [Google Scholar]
- Gazda HT, Grabowska A, Merida-Long LB, et al. Ribosomal protein S24 gene is mutated in Diamond-Blackfan anemia. Am J Hum Genet. 2006;79:1110–1118. doi: 10.1086/510020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cmejla R, Cmejlova J, Handrkova H, Petrak J, Pospisilova D. Ribosomal protein S17 gene (RPS17) is mutated in Diamond-Blackfan anemia. Hum Mutat. 2007;28:1178–1182. doi: 10.1002/humu.20608. [DOI] [PubMed] [Google Scholar]
- Farrar JE, Nater M, Caywood E, et al. Abnormalities of the large ribosomal subunit protein, Rpl35a, in Diamond-Blackfan anemia. Blood. 2008;112:1582–1592. doi: 10.1182/blood-2008-02-140012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gazda HT, Sheen MR, Vlachos A, et al. Ribosomal protein L5 and L11 mutations are associated with cleft palate and abnormal thumbs in Diamond-Blackfan anemia patients. Am J Hum Genet. 2008;83:769–780. doi: 10.1016/j.ajhg.2008.11.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doherty L, Sheen MR, Vlachos A, et al. Ribosomal protein genes RPS10 and RPS26 are commonly mutated in Diamond-Blackfan anemia. Am J Hum Genet. 2010;86:222–228. doi: 10.1016/j.ajhg.2009.12.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gazda HT, Preti M, Sheen MR, et al. Frameshift mutation in p53 regulator RPL26 is associated with multiple physical abnormalities and a specific pre-ribosomal RNA processing defect in Diamond-Blackfan anemia. Hum Mutat. 2012;33:1037–1044. doi: 10.1002/humu.22081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boria I, Quarello P, Avondo F, et al. A new database for ribosomal protein genes which are mutated in Diamond-Blackfan Anemia. Hum Mutat. 2008;29:E263–E270. doi: 10.1002/humu.20864. [DOI] [PubMed] [Google Scholar]
- Campagnoli MF, Ramenghi U, Armiraglio M, et al. Fribourg S, Gleizes PE, Loreni F, Dianzani I. RPS19 mutations in patients with Diamond-Blackfan anemia. Hum Mutat. 2008;29:911–920. doi: 10.1002/humu.20752. [DOI] [PubMed] [Google Scholar]
- Sankaran VG, Ghazvinian R, Do R, et al. Exome sequencing identifies GATA1 mutations resulting in Diamond-Blackfan anemia. J Clin Invest. 2012;122:2439–2443. doi: 10.1172/JCI63597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boria I, Garelli E, Gazda HT, et al. The ribosomal basis of Diamond-Blackfan anemia: mutation and database update. Hum Mutat. 2010;31:1269–1279. doi: 10.1002/humu.21383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quarello P, Garelli E, Brusco A, et al. High frequency of ribosomal protein gene deletions in Italian Diamond Blackfan anemia patients detected by Multiplex Ligation-dependent Probe Amplification (MLPA) assay. Haematologica. 2012;97:1813–1817. doi: 10.3324/haematol.2012.062281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Willig TN, Niemeyer CM, Leblanc T, et al. Identification of new prognosis factors from the clinical and epidemiologic analysis of a registry of 229 Diamond-Blackfan anemia patients. DBA group of Société d'Hématologie et d'Immunologie Pédiatrique (SHIP), Gesellshaft für Pädiatrische Onkologie und Hämatologie (GPOH) and the European Society for Pediatric Hematology and Immunology (ESPHI) Pediatr Res. 1999;46:553–561. doi: 10.1203/00006450-199911000-00011. [DOI] [PubMed] [Google Scholar]
- Quarello P, Garelli E, Brusco A, et al. Multiplex ligation-dependent probe amplification enhances molecular diagnosis of Diamond-Blackfan anemia due to RPS19 deficiency. Haematologica. 2008;93:1748–1750. doi: 10.3324/haematol.13423. [DOI] [PubMed] [Google Scholar]
- Vlachos A, Ball S, Dahl N, et al. Diagnosing and treating Diamond Blackfan anaemia: results of an international clinical consensus conference. Br J Haematol. 2008;142:859–876. doi: 10.1111/j.1365-2141.2008.07269.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Orfali RF, Wynn RF, Stevens RF, Chopra R, Ball SE. Failure of red cell production following allogenic BMT for Diamond Blackfan anaemia (DBA) illustrates functional significance of high erythrocyte adenosine deaminase (eADA) activity in the donor. Blood. 1999;94:414a. [Google Scholar]
- Vlachos A, Federman N, Reyes-Haley C, Abramson J, Lipton JM. Hematopoietic stem cell transplantation for Diamond Blackfan anemia: a report from the Diamond Blackfan Anemia Registry. Bone Marrow Transplant. 2001;27:381–386. doi: 10.1038/sj.bmt.1702784. [DOI] [PubMed] [Google Scholar]
- Quarello P, Garelli E, Carando A, et al. Diamond-Blackfan anemia: genotype-phenotype correlations in Italian patients with RPL5 and RPL11 mutations. Haematologica. 2010;95:206–213. doi: 10.3324/haematol.2009.011783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Samuel GN, Strong KA, Kerridge I, Jordens CF, Ankeny RA, Shaw PJ. Establishing the role of pre-implantation genetic diagnosis with human leucocyte antigen typing: what place do "saviour siblings" have in paediatric transplantation. Arch Dis Child. 2009;94:317–320. doi: 10.1136/adc.2008.138529. [DOI] [PubMed] [Google Scholar]
- Kuliev A, Rechitsky S, Tur-Kaspa I, Verlinsky Y. Preimplantation genetics: improving access to stem cell therapy. Ann N Y Acad Sci. 2005;1054:223–227. doi: 10.1196/annals.1345.028. [DOI] [PubMed] [Google Scholar]
- Cmejla R, Blafkova J, Stopka T, et al. Ribosomal protein S19 gene mutations in patients with diamond-blackfan anemia and identification of ribosomal protein S19 pseudogenes. Blood Cells Mol Dis. 2000;26:124–132. doi: 10.1006/bcmd.2000.0286. [DOI] [PubMed] [Google Scholar]


