Abstract
Recent research has suggested that the social environment can moderate the expression of genetic influences on health and that genetic influences can shape an individual’s sensitivity to the social environment. Evidence supports 4 major mechanisms: genes can influence an individual’s response to environmental stress, genes may enhance an individual’s sensitivity to both favorable and adverse environments, inherited characteristics may better fit with some environments than with others, and inherited capabilities may only become manifest in challenging or responsive environments. Further progress depends on better recognition of patterns of gene–environment interaction, improved methods of assessing the environment and its impact on genetic mechanisms, the use of appropriately designed laboratory studies, identification of heritable differences in an individual before environmental moderation occurs, and clarification of the timing of the impact of social and genetic moderation.
The term gene–environment (G×E) refers either to moderation by genetic influences of the impact of the environment on health or to conditions in which the effect of the genotype on health depends on qualities of the environment. We focus specifically on attributes of the social environment and behavioral health, while recognizing that G×E also encompasses variations in the physical environment (e.g., exposure to allergens or toxins) and a range of public health outcomes. Studies of G×E address a fundamental public health question: Do genetically influenced attributes of individuals make them especially susceptible to adverse social environments or especially responsive to favorable environments (including therapeutic interventions)? Three major research paradigms are available for estimating genetic influences: direct ascertainment of the genotype through molecular assays, twin and sibling studies, and adoption studies. We review evidence from each approach, focusing specifically on the measured social environment.
Although questions have been raised about the replicability of some G×E findings, a major question is not simply whether G×E occurs or is pervasive, but how it occurs. We build on a range of previous explorations of these mechanisms.1–4 First, using published data, we identify 4 broad classes of mechanisms by which genetic and social influences moderate each other: inherited sensitivity to stress in the social environment, differential susceptibility to either favorable or adverse social environments, the goodness of fit between individuals’ inherited dispositions and the attributes of the social environment, and the social enhancement of inherited capabilities. We then identify 2 underlying processes that cut across these 4 mechanisms: genetic variation in the sensitivity to the environment and variation in environmental sensitivity to genetic effects. Finally, we outline 5 steps for exploring specific mechanisms within these classes and link these to prevention and intervention strategies.
CLUES TO HOW GENETIC AND SOCIAL PROCESSES MODERATE EACH OTHER
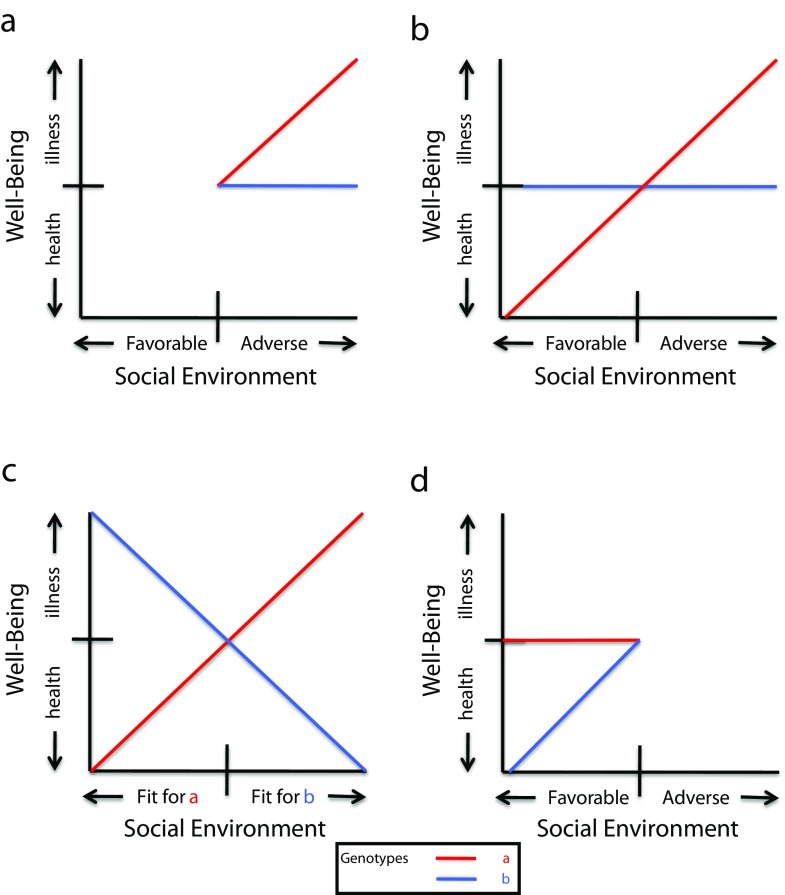
Different approaches to understanding human development have led investigators to examine different features of the linear interaction between genetic influences and the social environment. Each approach has provided clues as to how the social context and genetic influences may moderate each other’s effects on health. The 4 patterns are schematically represented in Figure 1. In each graph, variation in the environment is represented on the x-axis; variation in health is represented on the y-axis. The graph lines represent subsamples of individuals who differ by genotype as distinguished by molecular genetic assay or, for example, by an adoption study (e.g., birth parent diagnosis present or absent). As we detail later, the genotypic distinction represented in Figure 1 is not necessarily a distinction between a high or a low genetic liability for disease (i.e., a genetic main effect). Indeed, the nature of the social environment may help to determine whether a particular genotype leads to health or illness.
FIGURE 1—
Genetic and social processes' moderation of each other’s effect on psychopathology by 4 mechanisms: (a) inherited sensitivity, (b) differential susceptibility, (c) goodness of fit, and (d) social enhancement.
Inherited Stress Sensitivity
The first approach is a line of investigation that grows out of a long tradition of studying the evolution of psychopathology: identifying a diathesis or disease liability and then discovering a stressor or environmental pathogen that tips a vulnerable individual into a category of psychopathology. As applied to genetics, this intellectual format has shaped a number of reports of genetic influences on individuals’ sensitivity to adverse social environments. This line of investigation was initiated by twin and adoption studies.5–8 For example, almost 20 years ago, Kendler et al.8 showed that the effect of stressful experiences was magnified by a genetic liability for depression. The magnitude of this liability was estimated for twins without a history of depression by whether their cotwin had a history of depression. The magnitude of risk was highest when the cotwin was monozygotic and had depression and lowest when the cotwin was monozygotic and did not have a history of depression. This result has been replicated for both adults and children in a large number of studies.9 Adoption studies have provided an even more straightforward demonstration of G×E. For example, using a sample of infants adopted at birth, Leve et al.10 studied the earliest manifestation of genetic risk for externalizing behavior in adult life: the capacity of infants to self-soothe in a standard situation of frustration. Genetic risk was indexed by assessing externalizing disorders in birth mothers, controlling for their intrapartum drug use. The researchers found that failure at self-soothing occurred only when adoptive mothers were depressed. In other words, children at genetic risk were more sensitive to the stress of living with a depressed mother. More recently, the identification of specific polymorphisms11,12 that enhance individual sensitivity has raised hopes for the discovery of neurobiological mechanisms that might explain inherited stress sensitivity.
The statistical evidence supporting inherited stress sensitivity is usually represented in graphs resembling Figure 1a. Individuals with a specific genotype are uniquely sensitive to adverse environments and are more likely to fall ill when exposed to them. Broadly speaking, a graph of this kind could represent an adverse environment that, in combination with the genetic diathesis, triggers psychopathology. To date, the overwhelming number of published interactions in the form shown in Figure 1a have implied that sensitivity to adverse environments is inherited and that genetic influences on a behavioral outcome will, therefore, be most conspicuous under adverse environmental conditions. However, this same form of interaction could also imply 2 radically different underlying mechanisms: social compensation or inherited resilience. Because few published reports of these alternative mechanisms exist, we include them as subtypes of inherited stress sensitivity. We emphasize how crucial it is to interpret interactions on the basis of a clear understanding of the predictor, moderator, and criterion variables—not just on the basis of the visual form of the interaction graph.
Social compensation variant.
In this variant, the same form of interaction might be attributable to the compensatory effects of a favorable environment that overrides a genetic diathesis.1 Parents may offer special encouragement to an inhibited child13 (a characteristic known to show a genetic influence), exert compensatory discipline on a child at genetic risk for externalizing behavior,14 or maintain a level of warmth and support despite heritable features in the child that might provoke hostility and harsh discipline.15 Of note, these more recent studies have made use of major advances in the statistical modeling of G×E in twin studies; in effect, they have estimated heritability at every level of a continuously measured environment.16
Inherited resilience variant.
In this variant, the genetic influence on a positive trait increases as the environment becomes more adverse, which implies that the child has inherited a capacity to offset challenges of the social context. To our knowledge, only 1 example of this form of interaction has been published: Genetic influences on reading ability increase as parental education (the environmental factor) declines.17 Pennington et al.3 termed this a “resilient interaction.”
Differential Susceptibility
More recently, a second line of research has developed, growing from a broad interest in the full range of development, both normal and pathological, and with more attunement to protective or beneficial environments. Attention to this approach was prompted by reexamination of the interaction graphs in reports of inherited stress sensitivity. In many instances, these graphs were in fact disordinal: As environments became favorable (as shown in the leftmost portion of the horizontal axis of Figure 1a), those environments with a supposed genetic diathesis to psychopathology fared better than those without. That is, some of the studies that were offered as supporting inherited sensitivity published graphs that looked somewhat like Figure 1b. The term “differential susceptibility” was developed to emphasize that individuals with a specific genotype might be more responsive to the social environment than those without the genotype, drawing strength from beneficial social contexts but also becoming more afflicted by adverse ones.
Two forms of evidence have been offered in support of this mechanism. First are studies of naturally occurring variation in environmental adversity. For example, in a sample consisting mainly of unwed mothers, women low in socioeconomic status (SES) who had a less efficient allele of the serotonin transporter gene had a greater incidence of postpartum depression than those with a high-efficiency allele. However, women with the same allele and higher SES had lower incidences of postpartum depression than those with the more efficient allele.18
Second are studies of differential sensitivity to intervention. For example, in a sample of rural African American adolescents, the short form of the serotonin transporter gene conferred increased sensitivity to the stress of having an uninvolved, nonsupportive parent, and adolescents under this condition were more likely to become substance abusers. However, these same adolescents responded more favorably when a family-strengthening intervention was provided.19,20
Goodness of Fit
A third line of investigation has been less integrated into the G×E literature, but the combination of developmental studies and psychological interventions has strongly suggested that a productive line of work lies ahead. In childhood and adolescence, positive adaptation may be less a product of either the child or the social context than of the fit between the child’s requirements or style and the capacity of a specific environment to appreciate and respond constructively to these features. Thus, a child whose temperament matches a parent’s expectations or preference for that temperament will show better adjustment than a child with a mismatch, independent of the absolute level of a particular temperament.21 Likewise, in psychotherapy, angry patients do better with less directive and less confrontational therapists, whereas more calm or collected patients do much worse under the same circumstances.22
If the goodness-of-fit mechanism were observed in G×E studies, it would appear as 2 linear functions, opposite in slope (with both slopes significantly different from zero), that must cross somewhere within the midrange of the observed environment. Preliminary genetic evidence for this mechanism is scant but comes from a range of studies. For example, the val/met COMT polymorphism has opposite effects on panic disorder in European versus Asian samples,23 which may reflect a goodness of fit between a heritable brain function and cultural demands and supports.
Of relevance is the Early Growth and Development Study (EGDS), for which we are principal investigators. This study has yielded a clear example of a goodness-of-fit interaction pattern.24 Study results have shown that structured parenting reduces behavior problems in toddlers at high genetic risk, defined by psychopathology in their birth parents, but increases behavior problems in toddlers at low genetic risk, yielding graphed findings that nearly duplicate those in Figure 1c. The implication of this study is that children do not inherit the propensity for a disorder. Rather, they inherit a need for a particular level of structure and order in their social environment. Children of birth parents high in psychopathology inherit a high need for structure. If their adoptive parents provide careful guidance through encouragement, a focus on the demands of a particular task, and clear requests for specific behaviors, these children become very well adjusted. Children of birth parents without psychopathology do not inherit such a need; in fact, their adjustment deteriorates if they receive this form of parenting.
Social Enhancement
A fourth line of work stems from systematic theoretical efforts to understand why genetic influences on adaptive behaviors may be manifest only under favorable environmental circumstances; this influential work is often termed the “bioecological model,”25,26 although we use the term “social enhancement”1 because it is more descriptive of the process.
The graphical evidence for this process is a sharply descending function as the social context declines in quality (see Figure 1d). This function would be notably steep in those with 1 genotype and flat or significantly more gradual for an alternate genotype. In the graphical notation we use here, in which low scores on the y-axis denote favorable adjustment, the pattern in Figure 1d suggests that the adjustment advantage associated with genotype B is apparent only under favorable social circumstances. As with the inherited sensitivity domain, this interaction is ordinal but opposite in form. Without methodological vigilance, the distinction between these 2 forms might reflect only a truncated range of the quality of the social context. However, studies reporting this form of interaction—mostly in the domain of intellectual aptitude and achievement—have used broadband measures of the social environment.27–30
Examples of this form of interaction are illustrative. For example, Tucker-Drob et al. 29 found a substantial genetic effect on differences in mental ability only among young children raised in upper-class strata. A common explanation for a finding of this kind is that the circumstances of lower-class life provide few opportunities for children with heritable capacities or skills to develop them; the greater opportunity in a favorable social setting allows children with these genetic endowments to flourish. An earlier study by Heath et al.27 is a second example of this mechanism. In pre–World War II Norway, educational opportunity was limited to those who were very wealthy. The genetic influences on educational attainment were nil. After World War II, when higher education became universally available, genetic influences on educational attainment were substantial. That is, the genetically influenced skills required for high educational attainment were only apparent in a social setting of universal academic opportunity.
VARIATIONS IN THE SENSITIVITY OF THE ENVIRONMENT AND OF THE INDIVIDUAL
We begin our discussion of the mechanisms underlying the graphs in Figure 1 by noting that all 4 graphs share 2 broad classes of mechanisms. First is the social compensation hypothesis. We discussed this as 1 possible interpretation of the graphical pattern depicted in Figure 1a. This hypothesis is 1 of the mechanisms that might underlie a finding that genetic influences are expressed only under adverse environments. “Social compensation” implies that an individual acts against the best interests of the family or community, in part because of genetic influences. However, the environment—perhaps more parental monitoring14 or residing in a small town with strict behavioral norms31—can counteract this inherited liability for disruptive behavior. Likewise, the proposed mechanisms of social enhancement all assume that genetic factors influence variation in effective engagement in activities requiring cognitive and linguistic skills and that these are enhanced, in reciprocal fashion, by supportive, intellectually enriched, and emotionally well-regulated environments that are attuned to a child high on measures of heritable mental ability but are not elicited in environments that are neither challenging nor responsive to a potentially competent child. We regard this as variation in the sensitivity of the environment to genetically influenced effector processes. The term “effector” is uncommon in genetics but is commonly used in anatomy to describe organs, such as muscle groups, that are designed to achieve a change in the environment. We extend the use of this term here to designate all behavioral patterns that are effective in changing the social environment. In young children, these behavioral patterns may be crying and unsoothability; in older children, they may be the selection of friends or adult mentors. All of these behaviors have been shown to be genetically influenced.7,32–36
The effector processes can be distinguished from the more common evocative gene–environment correlation. The latter is a main genetic effect on measures of the individual’s environment. For example, variations in a gene-regulating dopamine metabolism are associated with variations in sensitive maternal parenting. Other confounds controlled, one can assume that some unidentified characteristic of the child has evoked the maternal parenting style, but this characteristic does not have to be identified for a finding of this sort to exemplify evocative gene–environment correlation. Moreover, in many cases, the social environment does not moderate these evocative processes. The term “effector processes” refers to identified and measured genetically influenced behavior that alters the social environment depending on the quality of that environment. Moreover, the use of this term—as we show—implies the possibility of identifying, as a genetic main effect, these effectors early in development before they are moderated by the social environment.
By contrast, another proposed mechanism to account for inherited sensitivity to the environment (Figure 1a) or differential susceptibility (Figure 1b) focuses on genetic influences on responsiveness and attunement to the social environment. The latter hypothesis focuses on genetically influenced variation in the sensitivity of receptive processes to either adverse or favorable environments or both, termed “receptor processes.” This term is also an extension of an anatomical category and refers to a broad range of capacities of children to attend to, respond to, and interpret their social environment. Indeed, evidence exists across many species that genetic influences on behavior are mediated by genetic influences on very specific, anatomically locatable receptor mechanisms. For example, animal studies have shown that variation in sensitivity to oxygen levels influences social aggregation and that variation in genes regulating olfaction influence aggression.37 The field of imaging genetics, to which we return in the next section, is replete with examples. For example, several MRI studies have demonstrated genetic influences on the response of the amygdala to standard emotional stimuli.38–40
Mechanistic links between receptor and effector systems in G×E are likely. For example, an extensive literature has suggested that patterns of aggressive behavior in boys (a maladaptive effector system) are linked to their oversensitivity to threat (a maladaptive receptor system).41–45 However, this linkage in some domains of behavioral development highlights the need for mechanistic studies of G×E to determine whether the environmental moderator acts on either the receptor system or the effector system, or on both.
FROM GRAPHS TO MECHANISMS
The graphs shown in Figure 1 illustrate broad outlines of the 4 types of mechanisms we previously described. Further steps are necessary to provide clarity about each graph.
Recognizing Patterns in Types of Gene–Environment Interaction
Because critical comparisons among forms of interaction become central in unraveling the mechanisms underlying G×E interaction, we need to go beyond informal inspections of interaction graphs and pay attention to important features of these graphs. First, we should rigorously estimate the probability that chance alone has produced a picture that looks visually compelling. For linear interactions, slopes must be tested for their significance, with adjustments for multiple testing when several slopes are being compared.
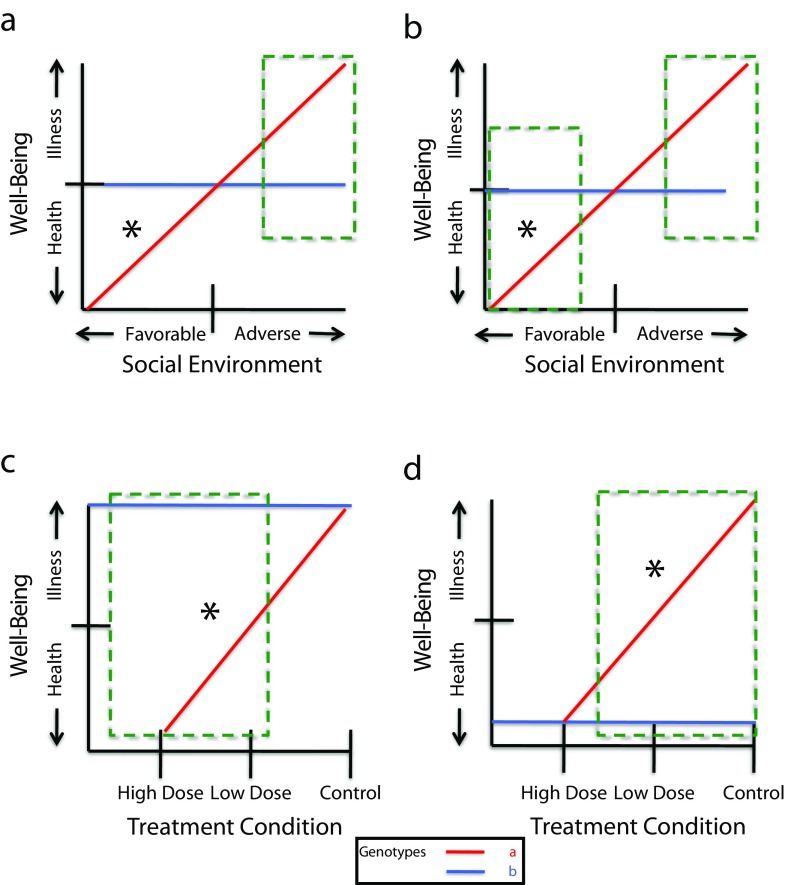
Second, regions-of-significance testing is crucial for formal comparisons of interactions46; these statistical tests indicate at which point along the x-axis the differences in slope between 2 conditions (e.g., children at high genetic risk for a mental disorder vs those at low risk) are unlikely to be a result of chance. These regions are indicated by green boxes in Figure 2. Also important is fastidious attention to problems of scaling, the inclusion of positive and negative developmental outcomes, and the selection of environmental variables that cover the full spectrum from favorable to unfavorable.
FIGURE 2—
Tests for regions of significance and statistical significance in (a) inherited sensitivity, (b) differential susceptibility, (c) inherited sensitivity to treatment effects, and (d) treatment compensation for inherited illness.
Note. An asterisk indicates slope significance, and the dashed boxes represent hypothetical regions of significance.
Third, as in all interactions, we must be certain that the predictor variable (e.g., the genotypic differences among children influencing mental illness) and the moderator variable (e.g., social class) are relatively or absolutely uncorrelated. This lack of correlation is of particular importance in G×E studies. We know, for example, from several studies that genotype is related to social class.47 This relationship is presumed to reflect a broad genetic influence on abilities required for upward mobility, on psychopathology that may lead to a downward drift, or both. If, in any particular study, the measurement of the genotype is correlated with the measurement of the moderator, we may misinterpret a statistical interaction. Rather than suggesting an environmental moderation of genetic effect, it may instead reflect (1) the impact of genotype first on the moderator (in the case of social class, this would be termed “active gene–environment correlation”) and (2) the subsequent effect of the presumed moderator on the criterion (e.g., mental illness). When modest correlations are detected, statistical correction is permissible, but power to detect an interaction is reduced substantially (for an exception in twin designs, see Purcell16). Preferred sampling procedures are those that allow testing of moderation effects free of gene–environment correlation or the use of adoption designs in which it can be shown that children are not selectively placed with adoptive families that match the characteristics of the birth parents. As a general rule, all tests of G×E must also consider the role of gene–environment correlation, a rule not explicitly examined in most published G×E studies.
Fourth, the distribution of individuals along both the x- and y-axes is crucial, because variation in these distributions alone can alter the shape and significance of linear interactions. The contrast between Figures 2a and 2b illustrates this point. For illustrative purposes, we assume that the x-axis measures the full range of an environmental variable, from very favorable to very adverse. We also assume the same for the y-axis: it runs from clinical psychopathology on the top to good mental health on the bottom. Both figures show a significant slope for genotype A and none for genotype B (as indicated by the asterisk), suggesting a disordinal interaction. However, in Figure 2a, the disordinal interaction has no region of significance to the left of the crossing lines, whereas it does in Figure 2b. Thus, a pattern resembling that in Figure 2a would favor a more detailed search for mechanisms of inherited sensitivity to stress and other adverse environments. Compare Figures 1a and 2a. A casual inspection of Figure 2a would suggest a mechanism of differential susceptibility in contrast to Figure 1a; with suitable statistical testing, they boil down to the same finding. However, Figure 2b does suggest differential susceptibility. Yet, if either of the distributions of values on the y-axis or the x-axis are skewed toward pathology, adverse environments (resulting from sampling procedures), or both, then we may not find a region of significance to the left of the intersection; we will miss the core mechanism of interaction because we have a selected sample. It is important to note that heteroskedasticity, changes in variance as a function of magnitude of a variable, and other scaling idiosyncrasies of scaling can produce G×E artifacts.
Fifth, for the pattern of interaction to serve as a guide for probing the mechanism of interaction between genes and environment, it must be replicable. Problems of nonreplication plague all of science. One example is the astonishing series of publications and correspondence in Nature on the irreproducibility of preclinical cancer research.48,49 Many authors regard nonreplication as a crisis in the behavioral sciences as well (see, for example, the special section in the November 2012 issue of Perspectives on Psychological Science50). Recently, research on the social moderation of effects of specific polymorphisms has been caught in the crosshairs of this concern. One of the original publications in this series,12 reporting on the moderation of the effects of stress on depression by a polymorphism of the serotonin transporter gene, has stimulated wide interest and many attempts at replication and extension. Two strategies have been used to evaluate these follow-up studies. The first has used a broad range of studies, including those with neurobiological assessments of human participants and animal subjects. These studies seek to delineate the biological mechanisms by which a single polymorphism (or a small number of polymorphisms) can have such a notable impact on an organism’s sensitivity to stress.2 A second approach to appraisal has focused on just those studies that have assessed depression as the criterion variable and that have used measures of environmental stress that were roughly comparable to those in the original report. Three meta-analyses of this narrower band of follow-up work have concluded that the original finding is not reproducible.51–53 The Karg et al.53 review reported that the original Caspi et al.2 finding was reproducible by including studies that used a broader range of environmental stressors, such as medical illness, hurricane exposure, and caring for a spouse with dementia. More recently, Duncan and Keller,52 in an analysis of all published articles on the interaction of a specific polymorphism (the serotonin transporter and other genes) and environmental stress, pointed out that many published studies in this domain are underpowered, and thus their results may be spurious. They concluded that this literature is biased because positive findings are more likely to be published than negative, nonconfirming findings. Although publication bias is of deep concern, our own view is that the measured G×E studies are worth pursuing when they lead to replicable mechanistic studies on the interplay of heritable neurobiological processes and the social environment.
Additional statistical cautions have been published and widely ignored. For example, Eaves7 showed how the special peril of a combination of dichotomized variables (such as the presence or absence of a diagnosis) and the use of log linear regressions can produce G×E as a computational artifact when underlying genetic and environmental liabilities are strictly additive.
Figures 2c and 2d illustrate another important contrast using a randomized trial of a behavioral intervention. Figure 2c illustrates patients with 2 different genotypes. In the control condition, both show psychopathology, but only the patient with genotype A improves in the treatment condition. This graph illustrates differential sensitivity to an intervention. When individuals with genotype A are exposed to adverse social environments and also show the pattern of inherited sensitivity (Figure 1a), then the cumulative data from both studies argue for a mechanism of differential susceptibility—for better or worse. However, Figure 2d illustrates a different pattern. Only genotype A shows serious psychopathology in the control condition, but no difference is shown in the treatment condition. Here, the intervention in effect provides social compensation (the compensation here is provided by the therapy) for the behavioral problems associated with genotype A. Figures 2c and 2d would lead to very distinctive follow-up searches for the specific mechanisms involved: Figure 2c might initiate a search for biological mechanisms selectively responsive not only to the intervention but also to the adverse circumstances that—in interaction with genotype A—initially influenced the pathology. By contrast, Figure 2d highlights the nature of a genetically influenced deficit and the social or psychological mechanisms involved in suppressing this genetic influence. The pattern shown in Figure 2d is nearly identical to that obtained in the study by Brody et al.,20 which examined the preventive intervention with African American youths. The intervention study, however, did not have a low-dose condition, which we used in Figure 2d for illustration. Thus, although Brody et al.20 is frequently cited as supporting the concepts of differential susceptibility, whether it does in the absence of the low-dose group is not clear.
All of these contrasts, although beyond our review scope, assume that additional confounding circumstances, widely recognized precautions in the G×E field, have been ruled out. These confounds include passive gene–environment correlation, stratification artifacts in molecular genetic studies, and the possibility that the environmental variable in the interaction equation actually reflects an unmeasured genetic influence (gene × gene interaction).
Adequate Assessment of the Environmental Moderator
Attention to the form and mode of a G×E interaction is also crucial for a careful investigation of the social context that serves a moderating role. Thus far, the short and long alleles of the serotonin transporter gene enhance sensitivity to an astonishing variety of noxious experiences across the life span, from child abuse and bullying during childhood to severe medical illness during late adulthood.53 Scant attention has been paid to what all of these environmental circumstances have in common except that they are stressful. Indeed, a basic tenet of the diathesis-stress model is that virtually any stress may tip an individual into illness. The nature of the illness (i.e., the specificity in the process) is carried by the diathesis, which in this case is the genotype (in some cases, a single polymorphism or several or broader genotypic differences detected in twin and adoption studies). Therefore, a crucial step in this line of analysis is to discover the common factor among this array of circumstances. Does each environmental circumstance trigger the expression of genetic risk through a common effect on biological stress systems, or are there subsets of triggering mechanisms?
For other forms of interaction, however, a different strategy is required. For example, when the form of interaction suggests that the social environment enhances a specific ability, we need to know exactly how this enhancement occurs to develop a mechanistic understanding and inform prevention efforts. Clarifying a social enhancement mechanism, as suggested by graphical results shown in Figure 1d, will require longitudinal studies focusing on reciprocal relationships between nascent child capabilities and responses of parents, teachers, and others. These are the proximal processes originally underscored in the formulation of the social enhancement (or bioecological) model.25,26
Increased Precision in Laboratory Modeling
G×E in human development is complex. Studies that depend on distribution of polymorphisms as they exist in study samples and on natural variation in environmental adversity or support are plagued, as we have noted, by potentially confounding factors. Thus, laboratory experiments can play a significant role in detailing G×E mechanisms because they allow researchers to have full control of environmental variation and, in some cases, genetic variation. This level of control helps both to eliminate confounds and to strengthen mechanistic inferences.
Three approaches to experimental control of the environment have been offered. First, in silico experiments with cultured cells have provided clues as to how neurotransmitters or hormones may differentially activate gene variants.54 Second, animal studies permit not only direct analysis of brain tissue but also precise control of rearing conditions.55,56 Third, laboratory settings provide the opportunity to precisely control social stimuli, as in the imaging genetic studies described in the next section.57,58 The form and overall process of interaction has a profound effect on how laboratory experiments are designed in the search for mechanistic explanations. For example, the form and process underlying the inherited sensitivity mechanism in the interaction between serotonin transporter gene variants and stress has shaped the design of imaging genetic studies, an example of studies in laboratory settings. These studies have been designed to study the impact of allelic variation of this gene on the response of neural systems to noxious stimuli under the assumption that the short form (and the long form) of the serotonin transporter gene enhances sensitivity only to noxious environments. However, several recent reports have emphasized that this allelic variation does not always function in the inherited sensitivity mode. For a number of developmental outcomes, the differential sensitivity form of interaction may better fit the data.18,59,60 If substantiated, these findings would lead to redesigned laboratory experiments: a search for both neural and behavioral intermediate phenotypes favoring sensitivity both to pleasure and to adversity. Thus, the integration of precise laboratory designs with knowledge gained about the form and process of an interaction can help to clarify underlying mechanisms and may therefore help to guide subsequent intervention development.
Recovering the Early-Appearing Genetic Main Effects
To understand the developmental mechanisms influencing environmental moderation of genetic influences, we must recover the genetic main effects that preceded the interaction in development. All the mechanisms suggested by Figure 1a–1d propose an initial genetic main effect that is subsequently moderated by favorable or adverse environments. From a statistical point of view, the goodness-of-fit completely disordinal interaction can be obtained without a gene or environment main effect. However, in the Leve et al.24 study, a proposed underlying mechanism is that children inherit varying levels of need for structure in the social environment. Because the hypothesized need has exactly the opposite effect on the criterion variable depending on parental style, a completely disordinal interaction results. Thus, even in this case in which neither a genetic nor an environmental main effect at the time of ascertainment is required to produce a disordinal interaction, the hypothesis states that at a prior time in development such a main effect should be detectable. The distinction between genetic influences on effector versus receptor processes will be crucial. For example, consider a G×E finding suggesting that the social environment is compensating for the child’s inherited inability to regulate his or her own behavior. To probe this possibility, one would need to identify the nature of this child’s dysregulation before the environment has a chance to moderate it.
Likewise, when the form of interaction suggests special receptor sensitivity, favorable or adverse receptor sensitivity should be detectable before either noxious or favorable environments have moderated it into poor or highly successful adaptation. Imaging genetic studies are designed to do just that. For example, Meyer-Lindenburg et al.61 sought an explanation for the role of the MAOA-L allele in the evolution of externalizing disorders in men under conditions of social adversity. They found that men with the MAOA-L allele showed excessive left amygdala response to fearful and angry faces, decreased responses in the orbitofrontal cortex and other cortical areas, and decreased connectivity between the orbitofrontal cortex and the amygdala. These are genetic main effects on a receptor system, in this case the frontal–amygdala system, that is presumably upstream in a causal flow that may lead to externalizing disorders as the downstream clinical outcome of interest. In this example, a genetic main effect on externalizing disorders may never be apparent because its effect on the downstream criterion (i.e., externalizing) is manifest only by its capacity to moderate the impact of an adverse environment (i.e., parental abuse).
However, prior experiments of this kind have been heuristic. They are generally done with nonclinical samples that are not followed to determine the actual role of these genetically influenced receptor sensitivities in the evolution of psychopathology as a consequence of later-appearing external stress. In subsequent work, when embedded in a developmentally sensitive design, using knowledge of the timing of the evolution of genetic expression, environmental influence, and behavioral syndromes, these techniques may play a powerful role in delineating genetic main effects in critical receptor systems before they are moderated by favorable or adverse social environments. Developmental studies of this kind could uncover that environmental effects will be apparent early on and that genes might be expressed later in development to moderate these influences.
For interactions suggesting environmental moderation of effector systems, researchers will have to look at genetic main effects on patterns of active behavior that, subsequently in development, are moderated by the environment. One place to look is the rapidly growing literature of evocative gene–environment correlations.62 Of special interest are heritable characteristics of children that evoke specific reactions from parents. In many cases, these evoked parental patterns go on to exacerbate externalizing maladjustment in the child.33,63–66 Preliminary evidence has suggested that some parents can resist these provocations and, as a consequence, the heritable and potentially maladaptive effector patterns (the inherited tendency to provoke caretakers) are curbed by parents who avoid counteraggression and instead behave with warmth.15
Timing of Gene–Environment Interaction
The foregoing discussion emphasized that a mechanistic explanation of G×E requires one to identify not only how G×E occurs but also when. Some G×E may occur in the intrapartum period.67–70 Questions about the timing of G×E are part of a larger interest in developmental science in critical periods (i.e., stages in the life span in which influences on development are restricted) and sensitive periods (i.e., when influences are more likely, but not exclusively, to occur). Preliminary evidence has now suggested 2 domains of questions about timing: one focusing on timing of biological mechanisms intervening between a gene variant and effector or receptor process and one focusing on social influences in G×E processes.
Malleability of biological mechanisms.
As noted, the short and long variants of the serotonin transporter gene may confer a lifelong sensitivity to adverse experience. Preliminary evidence has suggested that the impact of some of these adverse consequences may be reversible by behavioral intervention in the short term.20 However, current evidence has suggested that those individuals may remain especially sensitive to subsequent stressful circumstances.53 Twin research has suggested that genes influencing personality, cognitive process, and psychopathology become activated during the toddler and adolescent periods, whereas genes influencing adult functioning have a sustained effect across the rest of the life span,63 although this approach cannot identify the specific genes involved.
Once specific genes are identified, practically oriented research might focus on the neural circuits they regulate as significant targets for long-term preventive efforts. Thus, prevention researchers will want to know whether these systems are malleable and whether their adverse effects can be reversed for an extended period of time. From a practical perspective, the success of using the serotonin transporter gene variants in G×E studies engenders a paradoxical challenge. Many reports on the role of variants of this gene in enhancing sensitivity to stress have reflected the central role serotonin plays in the central nervous system. A variety of neural circuits have been linked to the serotonin transporter gene and may be involved in G×E processes: a fear circuit centered on the amygdala and moderated by the prefrontal and anterior cingulate cortex,40,41,71 a pain perception circuit originating in the caudal brain stem,72 and the hypothalamic–pituitary–adrenal axis.73 These neural circuits might have specific effects in moderating different social stressors, and research on the malleability of each might be a crucial component of programmatic prevention research.
Timing of social influences.
Relatively few studies have been done regarding the timing of social influences on G×E processes. An illustrative study74 has suggested a relatively restricted time period during which stressful circumstances may enhance the genetic impact on the development of panic disorder. Stressful experiences in childhood and adolescence, but not adulthood, enhance the genetic influence on adult anxiety responses to CO2, an important intermediate phenotype in the development of panic disorder.74 Findings such as these raise questions for all observed G×E findings:
When in development does the interaction occur (in this example, before age 18 years)?
If the interaction occurs only or primarily in an earlier period, do its effects on later development persist across time (in this example, they persist for at least 10 years into adult life) and, if persistent, for how long and by what mechanism?
Even if the interaction occurs in an earlier, sensitive period, can its effects be reversed by naturally occurring circumstances or intervention later in development?
Assessing the timing of environmental influences in human development has proved difficult. Longitudinal studies are effective in ensuring that the hypothesized risk precedes in time the illness or condition under study. However, many environmental risk factors from insecure attachment to maternal depression to low SES extend across time, making it difficult to distinguish between a persistent effect of early experience and the cumulative effect of adversity. Considerable advances in the crucial question of timing have come from newly emerging on–off designs that capitalize on environmental circumstances that have a precisely known time of onset (on designs), a precisely known time of cessation (off designs), or, much less frequently, both. Cochlear implantation is probably the best known design enabling researchers to specify the impact of a child’s first hearing spoken language at various ages,75 but it has not yet been used in G×E studies. Placement in foster care or adoption after residence in an orphanage can be used as an off design when the research question asks at what age an early deficit occasioned by severe deprivation can no longer be reversed.76
The prospective adoption design, with suitable attention to potential confounds, is a powerful on–off design that has special promise in exploring the timing and specific mechanisms of G×E. When a child is adopted at birth, the adoption design can more clearly distinguish between prenatal and postnatal maternal influences on child development if, as in the case of the EGDS, no correlation exists between the characteristics of birth and rearing parents that might come about as a result of selective versus random placement of the child.77 Thus, birth-mother influences are off at time of placement. The onset of fetal exposure to maternal psychopathology or substance use can be determined with reasonable accuracy in such a design. For example, the EGDS has clarified the relative role of prenatal and postnatal depression on suppressing normal cortisol activity in preschool-aged children.78 The companion adoption design, in which children are placed for adoption at conception (in vitro fertilization of an ovum from a donor) draws an even firmer boundary at the on position: It more sharply delimits the effects of fetal exposure from a priori genetic transmission of maternal characteristics at conception.79
We should note 2 other major strengths that the prospective adoption design has for investigating the mechanisms of G×E. First, it has an excellent chance of identifying the early-appearing genetic main effects that may precede moderation by the social environment. This is particularly the case when one wants to inquire about intermediate phenotypes that link genes to a disease. For example, one can ask, what is the earliest appearance of genetic liability for externalizing disorders in adults? The EGDS showed that, by 9 months, infants of birth mothers with externalizing disorders showed abnormal attentional patterns that might be exaggerated by depressive symptoms in the rearing parent.10 Second, the adoption design is a uniquely powerful design for determining the role of infant and toddler effector processes in these early indicators of genetic liability. These roles can be inferred by a simple correlation between birth parent characteristics and the pattern of responses of the rearing parent to the child (once confounds such as the openness of the adoption are ruled out).
CONCLUSIONS
In this article, we expand on recent calls to explore how mechanisms of G×E can be used to inform preventive and therapeutic interventions. Beginning with careful attention to the shape of the interaction, findings from existing research have suggested several clear steps for elucidating mechanisms that will be useful targets for intervention. These steps include laboratory models of G×E processes; careful, prospective developmental studies that identify genetic main effects on receptor or effector systems before they are moderated by the social environment; and strong research designs to elucidate the timing of G×E processes.
Acknowledgments
This project was supported by the National Institute of Child Health and Human Development and National Institute on Drug Abuse, National Institutes of Health (NIH), US Public Health Service (PHS), and Office of Behavioral and Social Science Research (grant R01 HD042608); the National Institute of Mental Health, NIH, PHS (grant R01 MH092118); and the National Institute on Drug Abuse and National Institute of Mental Health, NIH, PHS, and the Office of Behavioral and Social Science Research (grant R01 DA020585).
Note. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Human Participant Protection
This article is a review; however, we reference our own published work, for which we received institutional review board approval from the Oregon Social Learning Center, the George Washington University, and the Pennsylvania State University. All participants provided informed consent.
References
- 1.Shanahan MJ, Hofer SM. Social context in gene–environment interactions: retrospect and prospect. J Gerontol B Psychol Sci Soc Sci. 2005;60(spec issue 1):65–76. doi: 10.1093/geronb/60.special_issue_1.65. [DOI] [PubMed] [Google Scholar]
- 2.Caspi A, Hariri AR, Holmes A et al. Genetic sensitivity to the environment: the case of the serotonin transporter gene and its implications for studying complex diseases and traits. Am J Psychiatry. 2010;167(5):509–527. doi: 10.1176/appi.ajp.2010.09101452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Pennington BF, McGrath LM, Rosenberg J et al. Gene x environment interactions in reading disability and attention-deficit/hyperactivity disorder. Dev Psychol. 2009;45(1):77–89. doi: 10.1037/a0014549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Dick DM. Gene-environment interaction in psychological traits and disorders. Annu Rev Clin Psychol. 2011;7:383–409. doi: 10.1146/annurev-clinpsy-032210-104518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Cadoret RJ, Yates WR, Troughton E et al. Genetic-environmental interaction in the genesis of aggressivity and conduct disorders. Arch Gen Psychiatry. 1995;52(11):916–924. doi: 10.1001/archpsyc.1995.03950230030006. [DOI] [PubMed] [Google Scholar]
- 6.Cadoret RJ, Winokur G, Langbehn D, Troughton E. Depression spectrum disease, I: the role of gene-environment interaction. Am J Psychiatry. 1996;153(7):892–899. doi: 10.1176/ajp.153.7.892. [DOI] [PubMed] [Google Scholar]
- 7.Eaves LJ. Genotype x environment interaction in psychopathology: fact or artifact? Twin Res Hum Genet. 2006;9(1):1–8. doi: 10.1375/183242706776403073. [DOI] [PubMed] [Google Scholar]
- 8.Kendler KS, Kessler RC, Walters EE et al. Stressful life events, genetic liability, and onset of an episode of major depression in women. Am J Psychiatry. 1995;152(6):833–842. doi: 10.1176/ajp.152.6.833. [DOI] [PubMed] [Google Scholar]
- 9.Vendlinski MK, Lemery-Chalfant K, Essex MJ, Goldsmith H. Genetic risk by experience interaction for childhood internalizing problems: converging evidence across multiple methods. J Child Psychol Psychiatry. 2011;52(5):607–618. doi: 10.1111/j.1469-7610.2010.02343.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Leve LD, Kerr DC, Shaw D et al. Infant pathways to externalizing behavior: evidence of genotype x environment interaction. Child Dev. 2010;81(1):340–356. doi: 10.1111/j.1467-8624.2009.01398.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Champoux M, Bennett A, Shannon C et al. Serotonin transporter gene polymorphism, differential early rearing, and behavior in rhesus monkey neonates. Mol Psychiatry. 2002;7(10):1058–1063. doi: 10.1038/sj.mp.4001157. [DOI] [PubMed] [Google Scholar]
- 12.Caspi A, Sugden K, Moffitt TE et al. Influence of life stress on depression: moderation by a polymorphism in the 5-HTT gene. Science. 2003;301(5631):386–389. doi: 10.1126/science.1083968. [DOI] [PubMed] [Google Scholar]
- 13.Arcus D, Gardner S, Anderson C. Miami, FL: May 1992. Infant reactivity, maternal style, and the development of inhibited and uninhibited behavioral profiles. Paper presented at: 8th Biennial Meeting of the International Society for Infant Studies. [Google Scholar]
- 14.Dick DM, Viken R, Purcell S et al. Parental monitoring moderates the importance of genetic and environmental influences on adolescent smoking. J Abnorm Psychol. 2007;116(1):213–218. doi: 10.1037/0021-843X.116.1.213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Feinberg ME, Button TM, Neiderhiser JM et al. Parenting and adolescent antisocial behavior and depression: evidence of genotype x parenting environment interaction. Arch Gen Psychiatry. 2007;64(4):457–465. doi: 10.1001/archpsyc.64.4.457. [DOI] [PubMed] [Google Scholar]
- 16.Purcell S. Variance components models for gene environment interaction in twin analysis. Twin Res. 2002;5(6):554–571. doi: 10.1375/136905202762342026. [DOI] [PubMed] [Google Scholar]
- 17.Friend A, DeFries JC, Olson RK et al. Heritability of high reading ability and its interaction with parental education. Behav Genet. 2009;39(4):427–436. doi: 10.1007/s10519-009-9263-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Mitchell C, Notterman D, Brooks-Gunn J et al. Role of mother’s genes and environment in postpartum depression. Proc Natl Acad Sci USA. 2011;108(20):8189–8193. doi: 10.1073/pnas.1014129108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Brody GH, Beach SR, Steven RH et al. Parenting moderates a genetic vulnerability factor in longitudinal increases in youths’ substance use. J Consult Clin Psychol. 2009;77(1):1–11. doi: 10.1037/a0012996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Brody GH, Gene H, Beach SR et al. Prevention effects moderate the association of 5-HTTLPR and youth risk behavior initiation: gene x environment hypotheses tested via a randomized prevention design. Child Dev. 2009;80(3):645–661. doi: 10.1111/j.1467-8624.2009.01288.x. [DOI] [PubMed] [Google Scholar]
- 21.Talwar R, Nitz K, Lerner RM. Relations among early adolescent temperament, parent and peer demands, and adjustment: a test of the goodness of fit model. J Adolesc. 1990;13(3):279–298. doi: 10.1016/0140-1971(90)90019-4. [DOI] [PubMed] [Google Scholar]
- 22.Karno MP, Longabaugh R. What do we know? Process analysis and the search for a better understanding of Project MATCH’S anger-by-treatment matching effect. J Stud Alcohol. 2004;65(4):501–512. doi: 10.15288/jsa.2004.65.501. [DOI] [PubMed] [Google Scholar]
- 23.Domschke K, Deckert J, O’Donovan MC, Glatt SJ. Meta-analysis of COMT val158met in panic disorder: ethnic heterogeneity and gender specificity. Am J Med Genet B Neuropsychiatr Genet. 2007;144B(5):667–673. doi: 10.1002/ajmg.b.30494. [DOI] [PubMed] [Google Scholar]
- 24.Leve LD, Harold GT, Ge X et al. Structured parenting of toddlers at high versus low genetic risk: two pathways to child problems. J Am Acad Child Adolesc Psychiatry. 2009;48(11):1102–1109. doi: 10.1097/CHI.0b013e3181b8bfc0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Bronfenbrenner U, Ceci SJ. Heredity, environment and the question “how?” A first approximation. In: Plomin R, McClearn GE, editors. Nature, Nurture and Psychology. Washington, DC: American Psychological Association; 1993. pp. 313–324. [Google Scholar]
- 26.Bronfenbrenner U, Ceci SJ. Nature-nuture reconceptualized in developmental perspective: a bioecological model. Psychol Rev. 1994;101(4):568–586. doi: 10.1037/0033-295x.101.4.568. [DOI] [PubMed] [Google Scholar]
- 27.Heath AC, Berg K, Eaves LJ et al. Education policy and the heritability of educational attainment. Nature. 1985;314(6013):734–736. doi: 10.1038/314734a0. [DOI] [PubMed] [Google Scholar]
- 28.Rowe DC, Jacobson KC, Van den Oord EJCG. Genetic and environmental influences on vocabulary IQ: parental education level as moderator. Child Dev. 1999;70(5):1151–1162. doi: 10.1111/1467-8624.00084. [DOI] [PubMed] [Google Scholar]
- 29.Tucker-Drob EM, Rhemtulla M, Harden KP, Turkheimer E, Fask D. Emergence of a gene-by-socioeconomic status interaction on infant mental ability between 10 months and 2 years. Psychol Sci. 2011;22(1):125–133. doi: 10.1177/0956797610392926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Turkheimer E, Haley A, Waldron M et al. Socioeconomic status modifies heritability of IQ in young children. Psychol Sci. 2003;14(6):623–628. doi: 10.1046/j.0956-7976.2003.psci_1475.x. [DOI] [PubMed] [Google Scholar]
- 31.Legrand LN, Keyes M, McGue M et al. Rural environments reduce the genetic influence on adolescent substance use and rule-breaking behavior. Psychol Med. 2008;38(9):1341–1350. doi: 10.1017/S0033291707001596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Goldsmith HH, Lemery KS, Buss KA, Campos JJ. Genetic analyses of focal aspects of infant temperament. Dev Psychol. 1999;35(4):972–985. [PubMed] [Google Scholar]
- 33.Narusyte J, Andershed A-K, Neiderhiser JM, Lichtenstein P. Aggression as a mediator of genetic contributions to the association between negative parent-child relationships and adolescent antisocial behavior. Eur Child Adolesc Psychiatry. 2007;16(2):128–137. doi: 10.1007/s00787-006-0582-z. [DOI] [PubMed] [Google Scholar]
- 34.Shanahan MJ, Erickson LD, Vaisey S, Smolen A. Helping relationships and genetic propensities: A combinatoric study of DRD2, mentoring, and educational continuation. Twin Res Hum Genet. 2007;10(2):285–298. doi: 10.1375/twin.10.2.285. [DOI] [PubMed] [Google Scholar]
- 35.Harakeh Z, Neiderhiser JM, Spotts EL et al. Genetic factors contribute to the association between peers and young adults smoking: univariate and multivariate behavioral genetic analyses. Addict Behav. 2008;33(9):1113–1122. doi: 10.1016/j.addbeh.2008.02.017. [DOI] [PubMed] [Google Scholar]
- 36.Manke B, McGuire S, Reiss D et al. Genetic contributions to adolescents’ extrafamilial social interactions: teachers, best friends, and peers. Soc Dev. 1995;4(3):238–256. [Google Scholar]
- 37.Bendesky A, Bargmann CI. Genetic contributions to behavioural diversity at the gene-environment interface. Nat Rev Genet. 2011;12(12):809–820. doi: 10.1038/nrg3065. [DOI] [PubMed] [Google Scholar]
- 38.Bäckman L, Nyberg L, Lindenberger U, Li SC, Farde L. The correlative triad among aging, dopamine, and cognition: current status and future prospects. Neurosci Biobehav Rev. 2006;30(6):791–807. doi: 10.1016/j.neubiorev.2006.06.005. [DOI] [PubMed] [Google Scholar]
- 39.Hariri AR, Drabant EM, Munoz KE et al. A susceptibility gene for affective disorders and the response of the human amygdala. Arch Gen Psychiatry. 2005;62(2):146–152. doi: 10.1001/archpsyc.62.2.146. [DOI] [PubMed] [Google Scholar]
- 40.Hariri AR, Mattay VS, Tessitore A et al. Serotonin transporter genetic variation and the response of the human amygdala. Science. 2002;297(5580):400–403. doi: 10.1126/science.1071829. [DOI] [PubMed] [Google Scholar]
- 41.Crick NR, Dodge KA. Social information-processing mechanisms on reactive and proactive aggression. Child Dev. 1996;67(3):993–1002. [PubMed] [Google Scholar]
- 42.Crozier JC, Dodge KA, Fontaine RG et al. Social information processing and cardiac predictors of adolescent antisocial behavior. J Abnorm Psychol. 2008;117(2):253–267. doi: 10.1037/0021-843X.117.2.253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Dodge KA, Crick NR. Social information processing bases of aggressive behavior in children. Pers Soc Psychol Bull. 1990;16(1):8–22. [Google Scholar]
- 44.Fite JE, Bates JE, Holtzworth-Munroe A et al. Social information processing mediates the intergenerational transmission of aggressiveness in romantic relationships. J Fam Psychol. 2008;22(3):367–376. doi: 10.1037/0893-3200.22.3.367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Fontaine RG, Yang C, Dodge KA et al. Development of response evaluation and decision (RED) and antisocial behavior in childhood and adolescence. Dev Psychol. 2009;45(2):447–459. doi: 10.1037/a0014142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Preacher KJ, Curran PJ, Bauer DJ. Computational tools for probing interactions in multiple linear regression, multilevel modeling, and latent curve analysis. J Educ Behav Stat. 2006;31(4):437–448. [Google Scholar]
- 47.Lichtenstein P, Harris JR, Pedersen NL, McClearn G. Socioeconomic status and physical health, how are they related? An empirical study based on twins reared apart and twins reared together. Soc Sci Med. 1993;36(4):441–450. doi: 10.1016/0277-9536(93)90406-t. [DOI] [PubMed] [Google Scholar]
- 48.Editorial note. Nature. 2012;485(7396):41. doi: 10.1038/485041d. [DOI] [PubMed] [Google Scholar]
- 49.Begley CG, Ellis LM. Drug development: Raise standards for preclinical cancer research. Nature. 2012;483(7391):531–533. doi: 10.1038/483531a. [DOI] [PubMed] [Google Scholar]
- 50.Pashler H, Wagenmakers E- J. Special section on replicability in psychological science: a crisis of confidence? Perspect Psychol Sci. 2012;7(6):528–530. doi: 10.1177/1745691612465253. [DOI] [PubMed] [Google Scholar]
- 51.Munafò MR, Durrant C, Lewis G, Flint J. Gene x environment interactions at the serotonin transporter locus. Biol Psychiatry. 2009;65(3):211–219. doi: 10.1016/j.biopsych.2008.06.009. [DOI] [PubMed] [Google Scholar]
- 52.Duncan LE, Keller MC. A critical review of the first 10 years of candidate gene-by-environment interaction research in psychiatry. Am J Psychiatry. 2011;168(10):1041–1049. doi: 10.1176/appi.ajp.2011.11020191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Karg K, Burmeister M, Shedden K, Sen S. The serotonin transporter promoter variant (5-HTTLPR), stress, and depression meta-analysis revisited: evidence of genetic moderation. Arch Gen Psychiatry. 2011;68(5):444–454. doi: 10.1001/archgenpsychiatry.2010.189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Cole SW, Arevalo JM, Takahashi R et al. Computational identification of gene-social environment interaction at the human IL6 locus. Proc Natl Acad Sci USA. 2010;107(12):5681–5686. doi: 10.1073/pnas.0911515107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Barr CS, Newman TK, Becker ML et al. Serotonin transporter gene variation is associated with alcohol sensitivity in rhesus macaques exposed to early-life stress. Alcohol Clin Exp Res. 2003;27(5):812–817. doi: 10.1097/01.ALC.0000067976.62827.ED. [DOI] [PubMed] [Google Scholar]
- 56.Newman TK, Syagailo YV, Barr CS et al. Monoamine oxidase a gene promoter variation and rearing experience influences aggressive behavior in rhesus monkeys. Biol Psychiatry. 2005;57(2):167–172. doi: 10.1016/j.biopsych.2004.10.012. [DOI] [PubMed] [Google Scholar]
- 57.Way BM, Taylor SE. The serotonin transporter promoter polymorphism is associated with cortisol response to psychosocial stress. Biol Psychiatry. 2010;67(5):487–492. doi: 10.1016/j.biopsych.2009.10.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Way BM, Taylor SE. A polymorphism in the serotonin transporter gene moderates cardiovascular reactivity to psychosocial stress. Psychosom Med. 2011;73(4):310–317. doi: 10.1097/PSY.0b013e31821195ed. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Pluess M, Belsky J, Way BM, Taylor SE. 5-HTTLPR moderates effects of current life events on neuroticism: differential susceptibility to environmental influences. Prog Neuropsychopharmacol Biol Psychiatry. 2010;34(6):1070–1074. doi: 10.1016/j.pnpbp.2010.05.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Schoebi D, Way BM, Karney BR, Bradbury TN. Genetic moderation of sensitivity to positive and negative affect in marriage. Emotion. 2012;12(2):208–212. doi: 10.1037/a0026067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Meyer-Lindenberg A, Buckholtz JW, Kolachana B et al. Neural mechanisms of genetic risk for impulsivity and violence in humans. Proc Natl Acad Sci USA. 2006;103(16):6269–6274. doi: 10.1073/pnas.0511311103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Reiss D, Leve LD. Genetic expression outside the skin: clues to mechanisms of genotype x environment interaction. Dev Psychopathol. 2007;19(4):1005–1027. doi: 10.1017/S0954579407000508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Neiderhiser JM, Reiss D, Hetherington EM, Plomin R. Relationships between parenting and adolescent adjustment over time: genetic and environmental contributions. Dev Psychol. 1999;35(3):680–692. doi: 10.1037//0012-1649.35.3.680. [DOI] [PubMed] [Google Scholar]
- 64.Burt SA, McGue M, Krueger RF, Iacono WG. How are parent-child conflict and childhood externalizing symptoms related over time? Results from a genetically informative cross-lagged study. Dev Psychopathol. 2005;17(1):145–165. doi: 10.1017/S095457940505008X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Larsson H, Viding E, Rijsdijk FV, Plomin R. Relationships between parental negativity and childhood antisocial behavior over time: a bidirectional effects model in a longitudinal genetically informative design. J Abnorm Child Psychol. 2008;36(5):633–645. doi: 10.1007/s10802-007-9151-2. [DOI] [PubMed] [Google Scholar]
- 66.Neiderhiser JM, Marceau K, Reiss D. Four factors for the initiation of substance use by young adulthood: a 10-year follow-up twin and sibling study of marital conflict, monitoring, siblings, and peers. Dev Psychopathol. 2013;25(1):133–149. doi: 10.1017/S0954579412000958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Pausová Z, Paus T, Sedova L, Berube J. Prenatal exposure to nicotine modifies kidney weight and blood pressure in genetically susceptible rats: a case of gene-environment interaction. Kidney Int. 2003;64(3):829–835. doi: 10.1046/j.1523-1755.2003.00172.x. [DOI] [PubMed] [Google Scholar]
- 68.Pluess M, Velders FP, Belsky J et al. Serotonin transporter polymorphism moderates effects of prenatal maternal anxiety on infant negative emotionality. Biol Psychiatry. 2011;69(6):520–525. doi: 10.1016/j.biopsych.2010.10.006. [DOI] [PubMed] [Google Scholar]
- 69.Wang X, Dow-Edwards D, Anderson V et al. Discrete opioid gene expression impairment in the human fetal brain associated with maternal marijuana use. Pharmacogenomics J. 2006;6(4):255–264. doi: 10.1038/sj.tpj.6500375. [DOI] [PubMed] [Google Scholar]
- 70.Wang X, Zuckerman B, Pearson C et al. Maternal cigarette smoking, metabolic gene polymorphism, and infant birth weight. JAMA. 2002;287(2):195–202. doi: 10.1001/jama.287.2.195. [DOI] [PubMed] [Google Scholar]
- 71.Pezawas L, Meyer-Lindenberg A, Drabant EM et al. 5-HTTLPR polymorphism impacts human cingulate-amygdala interactions: a genetic susceptibility mechanism for depression. Nat Neurosci. 2005;8(6):828–834. doi: 10.1038/nn1463. [DOI] [PubMed] [Google Scholar]
- 72.Lindstedt F, Berrebi J, Greayer E et al. Conditioned pain modulation is associated with common polymorphisms in the serotonin transporter gene. PLoS ONE. 2011;6(3):e18252. doi: 10.1371/journal.pone.0018252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Mueller A, Brocke B, Fries E et al. The role of the serotonin transporter polymorphism for the endocrine stress response in newborns. Psychoneuroendocrinology. 2010;35(2):289–296. doi: 10.1016/j.psyneuen.2009.07.002. [DOI] [PubMed] [Google Scholar]
- 74.Spatola CA, Scaini S, Pesenti-Gritti P et al. Gene-environment interactions in panic disorder and CO sensitivity: effects of events occurring early in life. Am J Med Genet B Neuropsychiatr Genet. 2011;156B(1):79–88. doi: 10.1002/ajmg.b.31144. [DOI] [PubMed] [Google Scholar]
- 75.Dettman S, Dowell R. Language acquisition and critical periods for children using cochlear implants. In: Markark M, editor. The Oxford Handbook of Deaf Studies, Language, and Education. Vol 2. New York, NY: Oxford University Press; 2010. pp. 331–342. [Google Scholar]
- 76.Nelson CA, Zeanah CH, Fox NA et al. Cognitive recovery in socially deprived young children: the Bucharest Early Intervention Project. Science. 2007;318(5858):1937–1940. doi: 10.1126/science.1143921. [DOI] [PubMed] [Google Scholar]
- 77.Leve LD, Neiderhiser JM, Shaw DS, Ganiban J, Natsuaki MN, Reiss D. The Early Growth and Development Study: a prospective adoption study from birth through middle childhood. Twin Res Hum Genet. 2013;16(1):412–423. doi: 10.1017/thg.2012.126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Laurent HK, Leve LD, Neiderhiser JM et al. Effects of prenatal and postnatal parent depressive symptoms on adopted child HPA regulation: Independent and moderated influences. Dev Psychol. 2013;49(5):876–886. doi: 10.1037/a0028800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Harold GT, Shelton KH, Rice FJ et al. Disentangling genetic and environmental influences on children’s development: Introducing a novel methodology. Acta Psychol Sin. 2008;40(10):1124–1134. [Google Scholar]