Abstract

Successful treatment of a brain infection requires aspiration of the pus or excision of the abscess, followed by long-term (usually 4–8 weeks) parenteral antibiotic treatment. Local antibiotic delivery using biodegradable drug-impregnated carriers is effective in treating postoperative infections, thereby reducing the toxicity associated with parenteral antibiotic treatment and the expense involved with long-term hospitalization. We have developed vancomycin-loaded, biodegradable poly[lactic-co-glycol acid] nanofibrous membranes for the sustainable delivery of vancomycin to the brain tissue of rats by using the electrospinning technique. A high-performance liquid chromatography assay was employed to characterize the in vitro and in vivo release behaviors of pharmaceuticals from the membranes. The experimental results suggested that the biodegradable nanofibers can release high concentrations of vancomycin for more than 8 weeks in the cerebral cavity of rats. Furthermore, the membranes can cover the wall of the cavity after the removal of abscess more completely and achieve better drug delivery without inducing adverse mass effects in the brain. Histological examination also showed no inflammation reaction of the brain tissues. By adopting the biodegradable, nanofibrous drug-eluting membranes, we will be able to achieve long-term deliveries of various antibiotics in the cerebral cavity to enhance the therapeutic efficacy of cerebral infections.
Keywords: Brain abscess, cerebral infection, biodegradable, poly[lactic-co-glycol acid], nanofibers, vancomycin
Central nervous system (CNS) infection most commonly presents itself as meningitis, epidural abscess, subdural empyema, and/or brain abscess.1 Brain abscess (or cerebral abscess) is an abscess caused by inflammation and collection of infected material, coming from local (ear infection, dental abscess, infection of paranasal sinuses, infection of the mastoid air cells of the temporal bone, epidural abscess) or remote (lung, heart, kidney, etc.) infectious sources, within the brain tissue.2,3 The infection may also be introduced through a skull fracture following head trauma or surgical procedures. Multiple factors have contributed to the increasing number of diagnosed CNS infections, including prolonged lifespan, increased incidence of solid-organ transplantation, and improved diagnostic imaging modalities.2 The incidence of brain abscesses is ∼8% in developing countries, whereas in developed countries the incidence is 1–2%.2,3 The incidence of postoperative CNS infection (PCNSI) is much higher than the incidence of brain abscess. Previous studies have reported the incidence of PCNSI after neurosurgical procedures to be 5–7% and as high as 10% when antibiotic prophylaxis is not administered.1
Successful treatment of a brain abscess requires a high index of suspicion for the infection, which can have subtle presentations, and frequently requires a combination of drainage and antimicrobial therapy. The recommended duration of parenteral antibiotic therapy is prolonged, usually 4–8 weeks in accordance with the therapeutic response and neuroimaging finding.2,4 Medical treatment alone has been successful with early (illness duration <2 weeks) abscesses that are <3.0 cm in diameter in some highly selected patients.4−6 Surgical drainage followed by antimicrobial therapy is the treatment of choice for most brain abscesses. Surgical treatment can involve either aspiration or excision of the abscess. Most recent articles recommend aspiration followed by appropriate antibiotic therapy based on the sensitivity of the causative organisms.7,8 The advantages of aspiration are that it is simple, it can be used in the cerebritis stage, and it has less potential morbidity than surgical trauma does.4,9 Nevertheless, craniotomy and excision still have their importance in treating brain abscesses. Craniotomy is usually adopted for abscesses that grow after 2 weeks of antibiotic therapy or that fail to be reduced after 3–4 weeks of antibiotic treatment. It is also recommended for multiloculated abscesses and larger lesions with significant mass effects that are superficial and located in noneloquent regions of the brain.2,5,10 Indications for surgery (craniotomy) include neuroimage demonstration of intracranical large brain abscess formation in the capsule stage, the presence of a significant mass effect, high intracranical pressure, and proximity to ventricle and possibility of ventricular rupture.2,4,5 There are certain advantages of craniotomy for the excision of a brain abscess in an otherwise neurologically intact patient: the risk of repeated collection of pus is minimized; the duration of antibiotic treatment is reduced; and the expense involved in hospitalization, antibiotic treatment, and repeated imaging are saved.4,10 Craniotomy is also recommended for traumatic or postoperative brain abscesses containing foreign bodies or contaminated bone fragments.1,4,11
Vancomycin is recommended for the treatment of brain abscesses from hematogenous spread, in postoperative neurosurgical patients, following trauma, caused by methicillin-resistant Staphylococcus aureus (MRSA), and so on.1,12−14 Vancomycin should be administered intravenously over an infusion period of at least 1 h to minimize infusion-related adverse effects.15 Moreover, the administration of vancomycin requires at least 4–8 weeks of hospitalization, resulting in high expenses for hospitalization and drugs and lower quality of life for patients with CNSIs.2,4 Furthermore, long-term intravenous vancomycin infusion may cause toxic and/or other adverse side effects. Direct local delivery of antimicroorganism agents into the cerebral cavity via biodegradable devices is thus highly desired.
In the current study, we developed biodegradable vancomycin-loaded poly[lactic-co-glycol acid] (PLGA) nanofibrous membranes and evaluated their vancomycin release characteristics in the cerebral tissue of rats. To prepare the biodegradable membranes, both PLGA and vancomycin were dissolved in 1,1,1,3,3,3-hexafluoro-2-propanol (HFIP) and then electrospun into membranes with a thickness of 0.11 mm. The morphology of the electrospun nanofibers was then characterized by scanning electron microscopy (SEM). The in vitro release of vancomycin from the nanofibers was evaluated by an elution method and a high performance liquid chromatography (HPLC) assay. Biodegradable nanofibrous membranes embedded with vancomycin were then placed into the brain surface of rats after operative craniectomy was performed, and the characteristics of vancomycin release from the membranes in vivo were investigated. Histological examination of the reaction of tissue to the PLGA nanofibers was also completed.
Results and Discussion
In Vitro Release of Vancomycin from Nanofibers
With the use of appropriate processing parameters (including solvent, polymer concentration, and flow rate), electrospun nanofibrous membranes could be obtained. Figure 1 shows an SEM image of the electrospun nanofibers at 20000× magnification. The diameters of the spun PLGA nanofibers ranged from 50 to 267 nm, and the porosity of the nanofibrous membrane was high.
Figure 1.
SEM images of electrospun nanofibers.
Data on the in vitro release of vancomycin from the biodegradable nanofibers are shown in Figure 2. The nanofibrous membranes exhibited a triphasic vancomycin release profile consisting of an initial burst on day 1 (PLGA nanofibers released vancomycin at a concentration of 1800 μg/mL), a moderate-release lag phase on days 2–11 (nanofibers released vancomycin at concentrations of 13–90 μg/mL), and a second peak and accelerated drug release starting with the second week (PLGA nanofibers released vancomycin at concentrations of 180–800 μg/mL). After the electrospinning process, most of the pharmaceutical was dispersed in the bulk of the PLGA matrix; however, some drug might have been located on the surface of the nanofibers, leading to the initial burst of drug release. Following the initial burst, the drug release was controlled solely by diffusion. When the molecular weight of the polymer decreased sufficiently, polymer loss began, and drug was released along with this polymer loss. A second peak of release was thus observed in the second week. After that, the concentration gradually decreased. Therefore, the biodegradable nanofibrous membrane could elute high concentrations of vancomycin for up to 24 days.
Figure 2.
In vitro drug release studies demonstrating that vancomycin-loaded PLGA nanofibers showed sustained-release characteristics.
In Vivo Release Characteristics
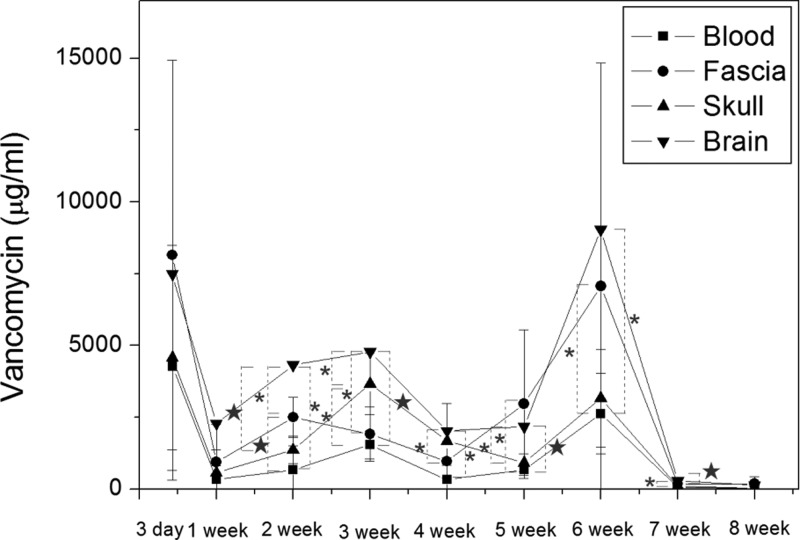
In vivo drug levels were characterized for 8 weeks by an HPLC assay. After excluding the rats that died from hypersedation (anesthesia overdose) and infection (wound, brain, peritoneal, systemic), we obtained six rats at postoperative day (POD) 3, six rats at POD 7, six rats at POD 14, six rats at POD 21, five rats at POD 28, six rats at POD 35, six rats at POD 42, five rats at POD 49, and six rats at POD 56 for analysis of the vancomycin concentration. The measured concentrations of vancomycin in different tissues are shown in Figure 3. The mean vancomycin concentration in the plasma at POD 3 was 4246.36 ± 3944.93 μg/mL, and this was the highest concentration observed in the blood. The concentration then dropped sharply and mostly remained below 1000 μg/mL (the week 1–8 mean concentration of vancomycin in the blood was 781.05 ± 885.88 μg/mL). The concentrations in the fascia, pericraniectomy skull bone, and brain tissue were 8146.96 ± 6780.78, 4570.30 ± 3914.55, and 7478.11 ± 1766.64 μg/mL, respectively, at POD 3. The concentration of vancomycin in all tissue specimens peaked at POD 3 and decreased sharply thereafter. The week 1–8 mean concentrations of vancomycin in the fascia, skull, and brain were 2086.19 ± 2254.58, 1433.2 ± 1351.45, and 3128.9 ± 2901.11 μg/mL, respectively. The concentrations of vancomycin reflect the triphasic release profile of the PLGA membranes. The concentration in the rat brain increased sharply with an initial burst that was followed by a moderate increase of drug release. A sustained and accelerated drug release was noticed at 5 weeks. The vancomycin concentrations in the brain were significantly higher than those in the blood (P < 0.05) from week 2 to week 7. The brain/blood concentration ratio was 1.75 at POD 3, 6.80 at POD 7, 6.53 at POD 14, 5.1 at POD 21, 8.52 at POD 28, 5.26 at POD 35, 6.44 at POD 42, 5.45 at POD 49, and 5.03 at the end of experiment (POD 56). The concentrations of vancomycin in the brain were relatively higher than those in the fascia and skull (except at week 5, when the concentration in the fascia was higher than the concentration in the brain; the difference was not statistically significant). All local (fascia, skull, brain) concentrations of vancomycin were much higher than the concentration in the blood.
Figure 3.
Vancomycin concentrations in different tissues of rats. The concentration in the brain was much higher than in the blood, and the difference was statistically significant from week 2 to week 7. The concentration in the brain was higher than the therapeutic concentration for more than 8 weeks. (*, P < 0.05; ★, P < 0.01).
The concentrations of vancomycin in different layers of ipsilateral and contralateral brain tissue are shown in Figure 4. As expected, the superficial layer (i.e., layer 1) achieved a higher concentration during the first few weeks (weeks 1–3) and remained the most concentrated one during the majority of the study period, while the deepest layer (layer 5) exhibited lower concentrations of vancomycin. The differences reached statistical significance at week 1 (P = 0.019), week 2 (P = 0.38), and week 3 (P = 0.21). The difference was pronounced at the beginning (week 1) and at the end (week 6). On the other hand, the drug concentration in the contralateral brain tissue (sampled about 2 mm from the central fissure of the brain) was low at the beginning and gradually reached the concentration level between layer 2 (1.5–3 mm deep) and layer 3 (3–4.5 mm deep).
Figure 4.
Concentrations of vancomycin in different layers of ipsilateral and contralateral brain tissues. (*, P < 0.05; ★, P < 0.01).
Histological Study of Postimplanted Brain Tissue
Figure 5 shows the gross wound condition and gradual degradation of the vancomycin-loaded biodegradable PLGA nanofibrous membrane. The scalp wounds and brain tissues were clear, and no infection (exudate, pus, or granulation formation) were noticed grossly. The biodegradable PLGA/vancomycin nanofibrous membranes degraded gradually after operative implantation and were almost completely diminished by 8 weeks.
Figure 5.
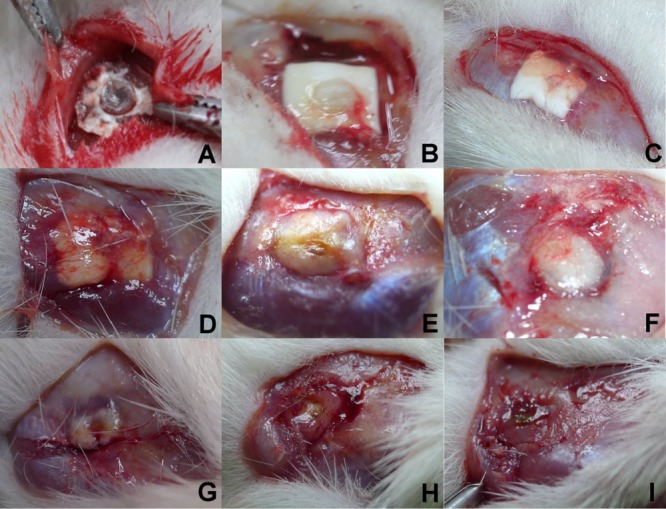
Gross wound conditions: (A) 3 days; (B) 1 week; (C) 2 weeks; (D) 3 weeks; (E) 4 weeks; (F) 5 weeks; (G) 6 weeks; (H) 7 weeks; (I) 8 weeks. The scalp wound and brain tissue were quite clear, and no infection (exudate, pus, or granulation formation) was noticed during the whole experimental period. By week 8, the PLGA/vancomycin nanomembranes were nearly completely dissolved.
The coronal sections of the ipsilateral brain tissue at various time points (POD 3 and weeks 1, 2, 3, 4, 5, 6, 7, and 8) are revealed in Figure 6. No inflammation (which is achieved by the increased movement of plasma and leukocytes, especially granulocytes, from the blood into the injured tissues), tissue necrosis, or dura mater thickness was observed in the histological examination.
Figure 6.

Histological examination of the brain tissue at different time points: (A) 3 days; (B) 1 week; (C) 2 weeks; (D) 3 weeks; (E) 4 weeks; (F) 5 weeks; (G) 6 weeks; (H) 7 weeks; (I) 8 weeks. No inflammation (which is achieved by the increased movement of plasma and leukocytes, especially granulocytes, from the blood into the injured tissues), tissue necrosis, or dura mater thickness was observed in the histological examination.
Discussion
Despite remarkable advances in antimicrobial therapy, neurosurgical techniques, and neuroradiological diagnosis, brain abscess and CNS infection remain challenging, and their mortality rate is still high.16,17 Bacterial infection in surgery, especial in CNS surgery, can be devastating and is associated with significant morbidity and poor functional outcomes. Although there has been considerable progress in reducing the incidence of infection after CNS surgery, it remains a potentially devastating complication. Previous studies involving a minimum of 1000 intracranial neurosurgical procedures have reported that the incidence of PCNSI after neurosurgical procedures is 5–10%.1,11,18 Traditionally, the management of postoperative infection requires surgical debridement, removal of all necrotic tissue and implants, and administration of systemic antibiotics. Nevertheless, the antibiotic treatment may be inadequate or ineffective in patients with poorly vascularized infected tissues.
Among various antimicroorganisms, vancomycin is a commonly used antibiotic and a concentration-independent killer of Gram-positive pathogens. Vancomycin should be administered intravenously over an infusion period of at least 1 h to minimize infusion-related adverse side effects.15 Local antibiotic delivery using antibiotic-impregnated biodegradable and/or nonbiodegradable carriers has been proposed to provide sustained release of antibiotics to infected areas and to substitute for intravenous antibiotics infusion. Stone et al. (19) proposed that antibiotic-loaded poly(methyl methacrylate) (PMMA) beads may be a useful adjunct in the contemporary surgical management of vascular surgical site infection associated with prosthetic grafts. Other studies have reported that vancomycin is effective even in low doses when used in PMMA cement spacers for osseous space maintenance and infection control.20−22 The concentration of antibiotics eluted from the cement is much greater than the concentration achieved by systemic therapy. However, the nondegradability of PMMA drug delivery systems (i.e., the persistence of the carriers after delivery) limits their clinical application, especially in the CNS system. The development of biodegradable carriers is seen as theoretically advantageous because of the potential reduction in the risk of secondary infection and elimination of the need for another surgery to remove the implant.21,23 The duration of antibiotic treatments is reduced, and the expenses involved in hospitalization, antibiotic treatment, and repeated imaging examinations are saved. Biodegradable antibiotic delivery systems are thus highly desired for the prevention and management of surgical infections.24,25
Vancomycin treatment failure is not uncommon; even methicillin-resistant S. aureus (MRSA) strains can be fully susceptible to vancomycin [minimum inhibitory concentration (MIC) ≤ 2 μg/mL], but a reduction in the efficacy of vancomycin against MRSA strains with a high vancomycin MIC (1–2 μg/mL) has been described in observational studies.26 Pharmacodynamic studies have shown that the area under the concentration–time curve (AUC)/MIC ratio is the most useful pharmacodynamic parameter to predict vancomycin effectiveness and suggested a target AUC/MIC ratio of 400 or greater to eradicate S. aureus.15,27−29 In addition, trough serum concentration monitoring is the most accurate and practical method to monitor the serum vancomycin level.15,27,29
The results of our study showed that the PLGA nanofibers eluted highly effective concentrations of vancomycin (week 1–6 mean concentration = 4102.62 ± 1766.64 μg/mL, range from 2021.99 to 9032.23 μg/mL) with an AUC/MIC ratio of at least 1000–4500 in the first 6 weeks after implantation. In studies examining the penetration of vancomycin into the cerebrospinal fluid (CSF) of patients with uninflamed meninges, fairly low concentrations of 0–3.45 mg/L have been demonstrated, with corresponding CSF/serum ratios of 0–0.18. As expected, inflamed meninges improve the penetration of vancomycin into the CNS, with reported concentrations of 6.4–11.1 mg/L and CSF/serum ratios of 0.36–0.48.30,31 The vancomycin concentration in the local (treatment) area is much lower than the concentration in the blood via intravenous vancomycin infusion. In contrast, in this study the vancomycin concentration in the blood was much lower than the concentration in the brain: in weeks 1–6 the mean drug concentrations were 4102.61 ± 2768.93 in the brain and 1026.34 ± 766.64 μg/mL (range from 335.92 to 2625.49 μg/mL) in the blood, respectively. The corresponding brain/serum ratios were 5.10–8.52, which are much greater than the ratios in systemic vancomycin infusion (0.36–0.48). In weeks 7 and 8, the concentrations of vancomycin dropped sharply to 286.83 and 128.73 μg/mL in the brain and 64.50 and 25.85 μg/mL in the blood, respectively. The concentrations in all tissues (fascia, skull, brain, and blood) were much higher than the therapeutic concentration. As in the brain tissue, the concentrations in the fascia (mean 2086.19 ± 2254.58 μg/mL, range from 177.00 to 7061.11 μg/mL) and skull (mean 1433.27 ± 1351.45 μg/mL, range from 34.46 to 3658.58 μg/mL) achieved AUC/MIC ratios much greater than 400. The PLGA nanofibers can release high concentrations of vancomycin for the treatment of post-traumatic or non-post-traumatic subdural or epidural infection, such as subdural empyema (abscess) and epidural abscess. Release of antibiotics at a concentration significantly greater than the MIC has great potential for rapid bacterial clearing.22,32 Despite the fact that increasing the trough concentration to 15–20 mg/L resulted in a highly desirable increased AUC/MIC ratio of >400, it is currently not supported by any clinical trials,27,33 mainly because higher trough concentrations increase the potential for toxicity.27 Furthermore, the in vivo vancomycin concentrations released from PLGA nanofibers were much greater than the vitro concentrations. For all pharmaceuticals, the in vivo environment seems to provide a lower metabolic rate than the in vitro environment. This might explain why the drug concentration during in vivo release is greater than that during in vitro release. It has been reported that drug transport across the blood–brain barrier (BBB) by nanoparticles appears to be due to receptor-mediated interactions with brain capillary endothelial cells that is facilitated by certain plasma apo-lipoproteins adsorbed by nanoparticles in the blood.34,35 Although no biodegradable membranes have been applied in brain surgery to date, nanoscaled fibers, particularly those with a size of ∼100 nm, have been proven to be taken up by cells at rates that are 15–250 times faster than for micrometer-sized fibers.36 Furthermore, the results of our previous study suggested inferior cell viability in the nanofibrous drug-eluting membrane group at 3 and 7 days compared to the regular membrane (no drug loading) group. The possible influence of released pharmaceuticals on cell proliferation, however, gradually diminished at 14 and 21 days.37 Despite the fact that no rats died or showed any associated adverse effects at high concentrations of vancomycin, a more detailed study will be needed in the future to identify the optimal drug dosage (or the optimal size of the membrane) to achieve effective antimicrobial effects at the local area while remaining lower than the toxic level in the plasma.
Poly(lactic-co-glycolic acid) (PLGA) has been widely studied as a therapeutic delivery vehicle because it is biodegradable and biocompatible.38,39 The degradability of PLGA within an organism varies depending on the monomer composition [i.e., the polylactide (PLA)/polyglycolide (PGA) ratio]. When PLA/PGA = 50:50, the polymer exhibits the highest degradation rate and can be completely degraded in approximately ∼2 months.40 The effective therapeutic period of PLGA/vancomycin is sufficient for the treatment of brain abscesses (usually 4–8 weeks).2,4 In addition, since vancomycin cannot inhibit all specimens of bacterials, especially Gram-negative bacterials, our previous study suggested that multi-drug-loaded nanofibers may be used for the treatment of CNS infections.41
Overall, the postoperative wounds showed no exudate accumulation or gramulation tissue formation during the whole degradation process of the PLGA nanofibrous membranes. The PLGA plug and membrane were completely degraded by week 8. In the histology study, no obvious tissue infiltration by mononuclear cells (including macrophages, lymphocytes, eosinophils, mast cells, and plasma cells) was observed. Degradation of PLGA polymers occurs by means of autocatalytic hydrolysis of the ester bond into oligomers of lactic acid and glycolic acid, which ultimately degrade in the Krebs cycle into CO2 and H2O.35,42,43 The good biocompatibility of PLGA and its degradation through natural pathways provided the impetus for the preparation of drug-eluting nanofibers from this material. Furthermore, the nanofibrous membrane used was thin (about 0.1 mm) and did not induce a mass effect (i.e., a large mass at the epidural space that could potentially cause nerve compression) after postoperative implantation into the brain tissue. Since the implantation of the assembled PLGA plug and PLGA/vancomycin nanomembrane is readily available during the operation, it is easy to insert the nanofibrous membrane at the end of the operation. These results suggest that electrospun, vancomycin-eluting PLGA nanofibers would be a good candidate for a sustainable drug delivery vehicle for the treatment of CNS infections including brain abscess, epidural abscess, and subdural empyema after surgical debridement.
Conclusions
We have developed antibiotic-loaded biodegradable poly[lactic-co-glycol acid] (PLGA) nanofibrous membranes for sustainable delivery of vancomycin to the brain tissue of rats. The experimental results suggested that the biodegradable nanofibers release high concentrations of vancomycin for more than 8 weeks in the cerebral cavity of rats. Furthermore, the membranes can cover the wall of the cavity more completely after the removal of the abscess and achieve better drug delivery without inducing adverse mass effects in the brain. Histological examination also showed no obvious inflammation reaction of the brain tissues. Vancomycin-eluting PLGA nanofibers would be a good candidate for a sustainable drug delivery vehicle in the cerebral cavity to enhance the therapeutic efficacy of cerebral infections.
Methods
Preparation of PLGA/Vancomycin Nanofibers
The PLGA used in this study was a commercially available material (Resomer RG 503, Boehringer Ingelheim, Germany) with a lactide/glycolide ratio of 50:50. The drug used was commercial-grade vancomycin hydrochloride (Sigma-Aldrich, Saint Louis, MO, U.S.A.).
The drug-eluting nanofibrous membranes were prepared using an electrospinning process.41 The electrospinning setup utilized in this study consisted of an adjustable high-voltage direct-current (DC) power supply, a syringe pump and needle (internal diameter 0.42 mm), a ground electrode, and an aluminum sheet. The needle was connected to the high-voltage power supply, which generated positive DC voltage and current/power up to 35 kV and 4.16 mA/125 W, respectively. To prepare the nanofibers, PLGA (1250 mg) and vancomycin (250 mg) were first dissolved in 5 mL of HFIP (Sigma-Aldrich). The solution was then delivered and electrospun using a syringe pump with a volumetric flow rate of 1.8 mL/h. The distance between the needle tip and the ground electrode was 12 cm, and the positive voltage applied to the polymer solutions was 17 kV. All of the electrospinning experiments were carried out at room temperature. The electrospun nanofibers were collected as a membrane on the aluminum sheet, and the thickness of the membrane was measured to be 0.11 mm.
To prevent migration of the nanofibrous membranes in the cerebral tissue, biodegradable plugs made of PLGA polymers were also fabricated using a compression sintering method. The PLGA powder was first compression-molded (44) to form plugs with a length of 5 mm and a diameter of 1.4 mm. The compressed plugs with the mold were then placed in an oven for sintering at 70 °C, and a sintering time of 30 min was used to attain isothermal sintering of the materials. Figure 7 shows a photograph of the nanofibrous membrane and the plug.
Figure 7.

Photograph of the nanofibrous membrane and the plug.
SEM Observations
The morphology of the electrospun nanofibers was observed on a scanning electron microscope (Hitachi S-3000N) after gold coating. The average diameter and diameter distribution were obtained by analyzing SEM images using a commercial image analysis program (Optimas, version 5.22).
In Vitro Release Behavior of Vancomycin
The characteristics of vancomycin release from the nanofibrous membrane were determined using the in vitro elution method. Samples with an area of 10 mm × 10 mm cut from the electrospun membranes were placed in glass test tubes (one sample per test tube, total number = 3) with 1 mL of phosphate-buffered solution (0.15 mol/L, pH 7.4) in each. The glass test tubes were incubated at 37 °C for 24 h, after which the eluent was collected and analyzed. Fresh phosphate-buffered solution (1 mL) was added for the following 24 h period, and this procedure was repeated for 30 days. Drug concentrations in the eluents were determined using an HPLC assay (Hitachi L-2200 multisolvent delivery system).
Surgical Procedure and Animal Care
Sixty Wistar rats, each weighing 200–300 g, were anesthetized by intraperitoneal injection of 6% chloral hydrate (0.6 mL/kg of body weight). The rats were randomly subdivided into nine groups (3 days and 1, 2, 3, 4, 5, 6, 7, and 8 weeks) with 6–7 rats in each group. After shaving and sterilization, a scalp incision about 1.5 cm long was made lateral to midline between the ear and the eye. Dissection of the temporalis muscle and periosteum by scalpel (Figure 8A) and a small craniectomy (about 1 cm × 1 cm) was made using an electric burr (Figure 8B,C). After local hemostasis, the biodegradable PLGA/vancomycin nanofibrous membrane with a size of 8 mm × 8 mm assembled with the PLGA plug (having a length of 5 mm and a diameter of 1.4 mm) was inserted into the brain tissue (Figure 8D). The scalp wound was sutured with 3-0 nylon. After surgery, the rats were housed randomly, with three or four rats in one cage. Rats exhibiting any intraoperative brain injury or infection (including scalp, skull bone, or brain tissue) were excluded from the study. All of the animal procedures received institutional approval, and the care for all of the studied animals was in accordance with the regulations of the National Institute of Health of the Republic of China (Taiwan) under the supervision of a licensed veterinarian.
Figure 8.
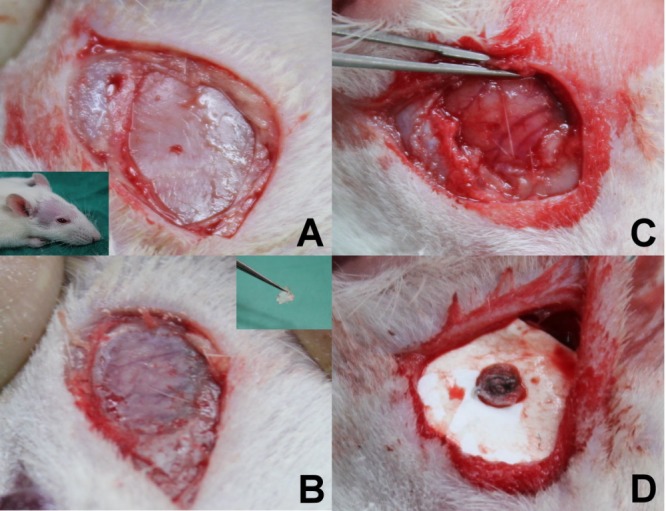
Surgical procedures: (A) A small incision (about 1.5 cm) was made at the postorbital region, and periosteum tissue was dissected. (B) An electric burr was used to drill skull bone (about 1 cm × 1 cm), and then the thin skull bone was removed using a needle holder. (C) A small craniectomy (1 cm × 1 cm) and adequate hemostasis were performed. (D) The PLGA/vancomycin nanomembrane and PLGA plug were assembled and inserted into the brain cortex, after which the wound was closured.
Pharmacokinetics of Vancomycin
Rats were given overdosed anesthesia (chloral hydrate) intraperitoneally (1.2 mL/kg of body weight). Blood samples were collected by cardiac puncture using syringes. Scalp fascia and pericraniectomy skull bone were sampled at about 0.05 g each. Ipsilateral brain tissue (covered by the PLGA/vancomycin membrane) was also extirpated. The wedge-shaped brain tissue (with a size of 8 mm × 8 mm and a thickness of 8–10 mm) at the brain surface was sliced into five different layers (i.e., layers 1–5 from the surface beneath the membrane down to the center of brain), each with a thickness of about 1.5 mm, using a rodent brain slicer (Zivic Instruments). About 0.05 g of brain tissue in each layer was sampled. Contralateral brain tissues were sampled at least 2 mm away from the central fissure and about 0.05 g of brain tissue was sampled. Specimens were collected at 3 days and 1, 2, 3, 4, 5, 6, 7, and 8 weeks and centrifuged, and the plasma was collected and stored at −80 °C until analysis. The vancomycin concentrations in the buffer for the elution studies were determined by HPLC assays conducted on a Hitachi L-2200 multisolvent delivery system. A SYMMETRY C18, 4.6 cm × 150 mm HPLC column (Waters) was used for the separation of the antibiotics. The mobile phase contained 0.01 mol of ammonium formate (Sigma-Aldrich) and methanol (Sigma-Aldrich) (20/80 v/v). The absorbance was monitored at 210 nm, and the flow rate was 1.0 mL/min. All of the samples were assayed in triplicate, and sample dilutions were performed to bring the unknown concentrations into the range of the assay standard curve. A calibration curve was made for each set of measurements (correlation coefficient >0.99). The elution product could be specifically identified and quantified with high sensitivity using the HPLC system. The brain tissue of at least one rat at each time point was extirpated for histology analysis.
Statistical Analysis
Data collected from the samples were analyzed by a paired sample t test using commercially available SPSS software (version 12.0; SPSS Inc., Chicago, IL, U.S.A.). Data are expressed as means ± standard deviations, and the threshold for significance was established at a P value of less than 0.05.
Author Contributions
S.-J.L. conceptualized the experiments, designed and directed the experiments, and edited the manuscript. Y.-Y.T. designed and performed the pharmacological research and statistical analyses and wrote the manuscript. Y.-C.K. oversaw research activities and experimental design, carried out pharmacological evaluation, and created figures. J.-Y.L. was involved in processing nanofibrous membranes and assisting with animal experiments. W.-A.C. was involved in processing nanofibrous membranes and conception of the project.
Chang Gung Memorial Hospital provided financial supports of this research.
The authors declare no competing financial interest.
References
- McClelland S. III; Hall W. A. (2007) Postoperative central nervous system infection: Incidence and associated factors in 2111 neurosurgical procedures. Clin. Infect. Dis. 45(1), 55–59. [DOI] [PubMed] [Google Scholar]
- Moorthy R. K.; Rajshekhar V. (2008) Management of brain abscess: An overview. Neurosurg. Focus 24(6), E3. [DOI] [PubMed] [Google Scholar]
- Bernardini G. L. (2004) Diagnosis and management of brain abscess and subdural empyema. Curr. Neurol. Neurosci. Rep. 4(6), 448–456. [DOI] [PubMed] [Google Scholar]
- Cavusoglu H.; Kaya R. A.; Turkmenoglu O. N.; Colak I.; Aydin Y. (2008) Brain abscess: Analysis of results in a series of 51 patients with a combined surgical and medical approach during an 11-year period. Neurosurg. Focus 24(6), E9. [DOI] [PubMed] [Google Scholar]
- Mampalam T. J.; Rosenblum M. L. (1988) Trends in the management of bacterial brain abscesses: A review of 102 cases over 17 years. Neurosurgery 23(4), 451–458. [DOI] [PubMed] [Google Scholar]
- Rosenblum M. L.; Hoff J. T.; Norman D.; Edwards M. S.; Berg B. O. (1980) Nonoperative treatment of brain abscesses in selected high-risk patients. J. Neurosurg. 52(2), 217–225. [DOI] [PubMed] [Google Scholar]
- Duma C. M.; Kondziolka D.; Lunsford L. D. (1992) Image-guided stereotactic management of non-AIDS-related cerebral infection. Neurosurg. Clin. North Am. 3(2), 291–302. [PubMed] [Google Scholar]
- Kutlay M.; Colak A.; Yildiz S.; Demircan N.; Akin O. N. (2005) Stereotactic aspiration and antibiotic treatment combined with hyperbaric oxygen therapy in the management of bacterial brain abscesses. Neurosurgery 57(6), 1140–1146. [PubMed] [Google Scholar]
- Stephanov S. (1988) Surgical treatment of brain abscess. Neurosurgery 22(4), 724–730. [DOI] [PubMed] [Google Scholar]
- Su T. M.; Lan C. M.; Tsai Y. D.; Lee T. C.; Lu C. H.; Chang W. N. (2008) Multiloculated pyogenic brain abscess: Experience in 25 patients. Neurosurgery 62(Suppl. 2), 556–561. [DOI] [PubMed] [Google Scholar]
- Korinek A. M.; Golmard J. L.; Elcheick A.; Bismuth R.; van Effenterre R.; Coriat P.; Puybasset L. (2005) Risk factors for neurosurgical site infections after craniotomy: A critical reappraisal of antibiotic prophylaxis on 4,578 patients. Br. J. Neurosurg. 19(2), 155–162. [DOI] [PubMed] [Google Scholar]
- Tunkel A. R.; Hartman B. J.; Kaplan S. L.; Kaufman B. A.; Roos K. L.; Scheld W. M.; Whitley R. J. (2004) Practice guidelines for the management of bacterial meningitis. Clin. Infect. Dis. 39(9), 1267–1284. [DOI] [PubMed] [Google Scholar]
- Liu C.; Bayer A.; Cosgrove S. E.; Daum R. S.; Fridkin S. K.; Gorwitz R. J.; Kaplan S. L.; Karchmer A. W.; Levine D. P.; Murray B. E.; Rybak M. J.; Talan D. A.; Chambers H. F. (2011) Clinical practice guidelines by the Infectious Diseases Society of America for the treatment of methicillin-resistant Staphylococcus aureus infections in adults and children. Clin. Infect. Dis. 52(3), e18–e55. [DOI] [PubMed] [Google Scholar]
- Wang Q.; Shi Z.; Wang J.; Shi G.; Wang S.; Zhou J. (2008) Postoperatively administered vancomycin reaches therapeutic concentration in the cerebral spinal fluid of neurosurgical patients. Surg. Neurol. 69(2), 126–129. [DOI] [PubMed] [Google Scholar]
- (1988) (ASHP) therapeutic position statement on the preferential use of metronidazole for the treatment of Clostridium difficile-associated disease. Am. J. Health Syst. Pharm. 55(13), 1407–1411. [DOI] [PubMed] [Google Scholar]
- Yang S. Y. (1981) Brain abscess: A review of 400 cases. J. Neurosurg. 55(5), 794–799. [DOI] [PubMed] [Google Scholar]
- Tseng J. H.; Tseng M. Y. (2006) Brain abscess in 142 patients: Factors influencing outcome and mortality. Surg. Neurol. 65(6), 557–562. [DOI] [PubMed] [Google Scholar]
- Korinek A. M.; Baugnon T.; Golmard J. L.; van Effenterre R.; Coriat P.; Puybasset L. (2006) Risk factors for adult nosocomial meningitis after craniotomy: Role of antibiotic prophylaxis. Neurosurgery 59(1), 126–133. [DOI] [PubMed] [Google Scholar]
- Stone P. A.; Armstrong P. A.; Bandyk D. F.; Brumberg R. S.; Flaherty S. K.; Back M. R.; Johnson B. L.; Shames M. L. (2006) Use of antibiotic-loaded polymethylmethacrylate beads for the treatment of extracavitary prosthetic vascular graft infections. J. Vasc. Surg. 44(4), 757–761. [DOI] [PubMed] [Google Scholar]
- Xie Z.; Liu X.; Jia W.; Zhang C.; Huang W.; Wang J. (2009) Treatment of osteomyelitis and repair of bone defect by degradable bioactive borate glass releasing vancomycin. J. Controlled Release 139(2), 118–126. [DOI] [PubMed] [Google Scholar]
- Kanellakopoulou K.; Giamarellos-Bourboulis E. J. (2000) Carrier systems for the local delivery of antibiotics in bone infections. Drugs 59(6), 1223–1232. [DOI] [PubMed] [Google Scholar]
- Shi M.; Kretlow J. D.; Nguyen A.; Young S.; Baggett L. S.; Wong M. E.; Kasper F. K.; Mikos A. G. (2010) Antibiotic-releasing porous polymethylmethacrylate constructs for osseous space maintenance and infection control. Biomaterials 31(14), 4146–4156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- El-Husseiny M.; Patel S.; MacFarlane R. J.; Haddad F. S. (2011) Biodegradable antibiotic delivery systems. J. Bone Joint Surg. Br. 93(2), 151–157. [DOI] [PubMed] [Google Scholar]
- Liu S. J.; Ueng S. W.-N.; Lin S. S.; Chan E. C. (2002) In vivo release of vancomycin from biodegradable beads. J. Biomed. Mater. Res. 63(6), 807–813. [DOI] [PubMed] [Google Scholar]
- Le Ray A. M.; Chiffoleau S.; Iooss P.; Grimandi G.; Gouyette A.; Daculsi G.; Merle C. (2003) Vancomycin encapsulation in biodegradable poly(ε-caprolactone) microparticles for bone implantation. Influence of the formulation process on size, drug loading, in vitro release and cytocompatibility. Biomaterials 24(3), 443–449. [DOI] [PubMed] [Google Scholar]
- Soriano A.; Marco F.; Martinez J. A.; Pisos E.; Almela M.; Dimova V. P.; Alamo D.; Ortega M.; Lopez J.; Mensa J. (2008) Influence of vancomycin minimum inhibitory concentration on the treatment of methicillin-resistant Staphylococcus aureus bacteremia. Clin. Infect. Dis. 46(2), 193–200. [DOI] [PubMed] [Google Scholar]
- Rybak M. J.; Lomaestro B. M.; Rotschafer J. C.; Moellering R. C. Jr.; Craig W. A.; Billeter M.; Dalovisio J. R.; Levine D. P. (2009) Therapeutic monitoring of vancomycin in adults: Summary of consensus recommendations from the American Society of Health-System Pharmacists, the Infectious Diseases Society of America, and the Society of Infectious Diseases Pharmacists. Pharmacotherapy 29(11), 1275–1279. [DOI] [PubMed] [Google Scholar]
- Patel N.; Pai M. P.; Rodvold K. A.; Lomaestro B.; Drusano G. L.; Lodise T. P. (2011) Vancomycin: We can’t get there from here. Clin. Infect. Dis. 52(8), 969–974. [DOI] [PubMed] [Google Scholar]
- Nicasio A. M.; Bulitta J. B.; Lodise T. P.; D’Hondt R. E.; Kulawy R.; Louie A.; Drusano G. L. (2012) Evaluation of once-daily vancomycin against methicillin-resistant Staphylococcus aureus in a hollow-fiber infection model. Antimicrob. Agents Chemother. 56(2), 682–686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rybak M. J. (2006) The pharmacokinetic and pharmacodynamic properties of vancomycin. Clin. Infect. Dis. 42(Suppl. 1), S35–S39. [DOI] [PubMed] [Google Scholar]
- Albanese J.; Leone M.; Bruguerolle B.; Ayem M. L.; Lacarelle B.; Martin C. (2000) Cerebrospinal fluid penetration and pharmacokinetics of vancomycin administered by continuous infusion to mechanically ventilated patients in an intensive care unit. Antimicrob. Agents Chemother. 44(5), 1356–1358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sakoulas G.; Moise-Broder P. A.; Schentag J.; Forrest A.; Moellering R. C. Jr.; Eliopoulos G. M. (2004) Relationship of MIC and bactericidal activity to efficacy of vancomycin for treatment of methicillin-resistant Staphylococcus aureus bacteremia. J. Clin. Microbiol. 42(6), 2398–2402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lodise T. P.; Patel N.; Lomaestro B. M.; Rodvold K. A.; Drusano G. L. (2009) Relationship between initial vancomycin concentration–time profile and nephrotoxicity among hospitalized patients. Clin. Infect. Dis. 49(4), 507–514. [DOI] [PubMed] [Google Scholar]
- Kreuter J.; Gelperina S. (2008) Use of nanoparticles for cerebral cancer. Tumori 94(2), 271–277. [DOI] [PubMed] [Google Scholar]
- Olivier J. C. (2005) Drug transport to brain with targeted nanoparticles. NeuroRx 2(1), 108–119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Decuzzi P.; Pasqualini R.; Arap W.; Ferrari M. (2009) Intravascular delivery of particulate systems: Does geometry really matter?. Pharm. Res. 26(1), 235–243. [DOI] [PubMed] [Google Scholar]
- Chen D. W.; Hsu Y.-H.; Liao J.-Y.; Liu S. J.; Chen J.-K.; Ueng S. W.-N. (2012) Sustainable release of vancomycin, gentamicin and lidocaine from novel electrospun sandwich-structured PLGA/collagen nanofibrous membranes. Int. J. Pharm. 430(1–2), 335–341. [DOI] [PubMed] [Google Scholar]
- Fournier E.; Passirani C.; Montero-Menei C. N.; Benoit J. P. (2003) Biocompatibility of implantable synthetic polymeric drug carriers: Focus on brain biocompatibility. Biomaterials 24(19), 3311–3331. [DOI] [PubMed] [Google Scholar]
- Wu L.; Ding J. (2004) In vitro degradation of three-dimensional porous poly(d,l-lactide-co-glycolide) scaffolds for tissue engineering. Biomaterials 25(27), 5821–5830. [DOI] [PubMed] [Google Scholar]
- Mi F. L.; Lin Y. M.; Wu Y. B.; Shyu S. S.; Tsai Y. H. (2002) Chitin/PLGA blend microspheres as a biodegradable drug-delivery system: Phase-separation, degradation and release behavior. Biomaterials 23(15), 3257–3267. [DOI] [PubMed] [Google Scholar]
- Chen D. W.; Liao J. Y.; Liu S. J.; Chan E. C. (2012) Novel biodegradable sandwich-structured nanofibrous drug-eluting membranes for repair of infected wounds: An in vitro and in vivo study. Int. J. Nanomed. 7, 763–771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beduneau A.; Saulnier P.; Benoit J. P. (2007) Active targeting of brain tumors using nanocarriers. Biomaterials 28(33), 4947–4967. [DOI] [PubMed] [Google Scholar]
- Barbu E.; Molnar E.; Tsibouklis J.; Gorecki D. C. (2009) The potential for nanoparticle-based drug delivery to the brain: Overcoming the blood–brain barrier. Expert Opin. Drug Delivery 6(6), 553–565. [DOI] [PubMed] [Google Scholar]
- Liu S. J.; Kau Y. C.; Liaw C. W.; Peng Y. J. (2009) In vitro elution of vancomycin/amikacin/steroid from solvent-free biodegradable scleral plugs. Int. J. Pharm. 370(1–2), 75–80. [DOI] [PubMed] [Google Scholar]