Abstract
Neurotrophins, as important regulators of neural development, function, and survival, have a therapeutic potential to repair damaged neurons. However, a controlled delivery of therapeutic molecules to injured tissue remains one of the greatest challenges facing the translation of novel drug therapeutics field. This study presents the development of an innovative protein–protein delivery technology of nerve growth factor (NGF) by an electrostatically assembled protein-based (collagen) reservoir system that can be directly injected into the injury site and provide long-term release of the therapeutic. A protein-based biomimetic hollow reservoir system was fabricated using a template method. The capability of neurotrophins to localize in these reservoir systems was confirmed by confocal images of fluorescently labeled collagen and NGF. In addition, high loading efficiency of the reservoir system was proven using ELISA. By comparing release profile from microspheres with varying cross-linking, highly cross-linked collagen spheres were chosen as they have the slowest release rate. Finally, biological activity of released NGF was assessed using rat pheochromocytoma (PC12) cell line and primary rat dorsal root ganglion (DRG) cell bioassay where cell treatment with NGF-loaded reservoirs induced significant neuronal outgrowth, similar to that seen in NGF treated controls. Data presented here highlights the potential of a high capacity reservoir-growth factor technology as a promising therapeutic treatment for neuroregenerative applications and other neurodegenerative diseases.
Keywords: Biomaterials, spheres, neural tissue engineering, neural regeneration, capsules, neurotrophins, nerve growth factor, drug delivery, dorsal root ganglia
The ability of neurotrophins to promote neuronal survival, development, synaptic plasticity, and neurotransmission have inspired researchers to use these molecules as possible therapeutic agents for damaged neurons in a variety of neurodegenerative diseases1 and acute injuries.2 However, despite their undisputed therapeutic potential, neurotrophins have a short half-life and have limited in vivo potential when used on their own.3 Therefore, a successful therapy requires a reservoir system that can protect the growth factor until it becomes delivered to the injury site. Several promising therapies have been identified to achieve this constant delivery; these include transplantation of stem cells overexpressing various neurotrophic factors4−6 or the use of viral vector delivery systems.7,8 Despite promising results obtained from in vitro and in vivo studies, translational research in these fields still falls short of clinical acceptance mainly due to poor survival of grafted cells and continuing safety issues related to viral immune response. More recently, another interesting approach using biomaterial-based particles as a neurotrophin reservoir and delivery system have been reported.9−13 Biomaterial-based particles are promising tools for controlled drug delivery system as they can overcome some limitations of cell- or gene-based therapy; however, other issues such as host response and biodegradability may arise. Therefore, to address these issues related to the use of synthetic polymers, in this study we present the development of an innovative protein–protein delivery technology of nerve growth factor (NGF) by collagen microspheres that can be directly injected into the injury site and provide long-term release of therapeutic. Collagen type I is a naturally occurring protein in mammalian tissue (approximately 30% of the whole-body protein content)14 and with its relatively high homology across species in vivo as a biomaterial has been demonstrated to have minimal immune response when implanted. In addition, due to its history in the clinic, it is widely employed in a variety of drug delivery systems.15 It has also been shown that collagen mediates neuronal adhesion to the substratum and guidance growth of neurites in vitro.16,17 Moreover, collagen has a potential to improve the survival and repair of damaged nerves,14 and is therefore readily used as a material for 3D scaffolds for nerve tissue repair.18,19 Finally, collagen is one of the three biomaterials that have progressed to clinical use as a neural scaffolding material (20)
We have developed a template method to generate a protective extracellular matrix (ECM)- based reservoir system.21 The aim of this study was to compare and to identify an optimal collagen microspheres growth factor reservoir system with minimum cytotoxicity, high loading efficiency and slow release rate of bioactive protein which can be used as a therapeutic platform for the treatment of neurodegenerative diseases. This study tests the hypothesis that a collagen-based hollow microsphere reservoir technology will provide a sustained growth factor delivery platform for multiple neurotherapeutic applications where the release profile can be modulated by the amount of cross-linking.
Results and Discussion
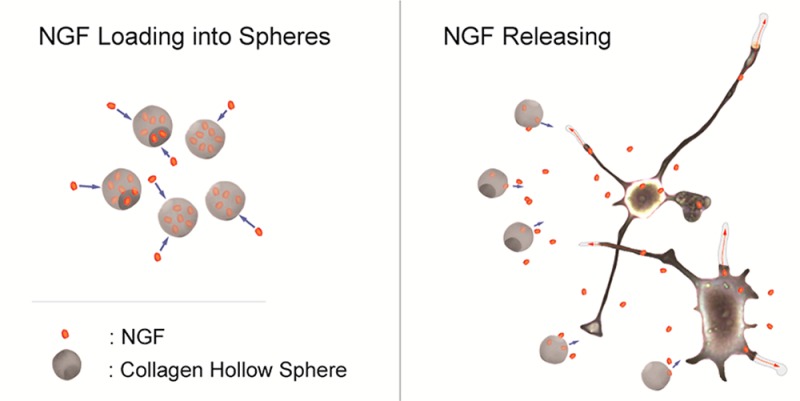
With an emphasis on spinal cord injury, for which no therapy currently exists, but also with respect to other neurodegenerative diseases, our study investigated the use of newly developed biocompatible, hollow sphere technology as a growth factor delivery platform for neuronal tissue repair. Scheme 1 illustrates the main objectives of this work that was first, to generate appropriate collagen hollow microspheres, then to load them with a model growth factor (NGF), and finally to investigate the bioactivity of released NGF and any cytotoxic effect of spheres on neuronal cells.
Scheme 1. Graphical Representation of the Fabrication of Hollow Collagen Microspheres and Protein Loading/Release.
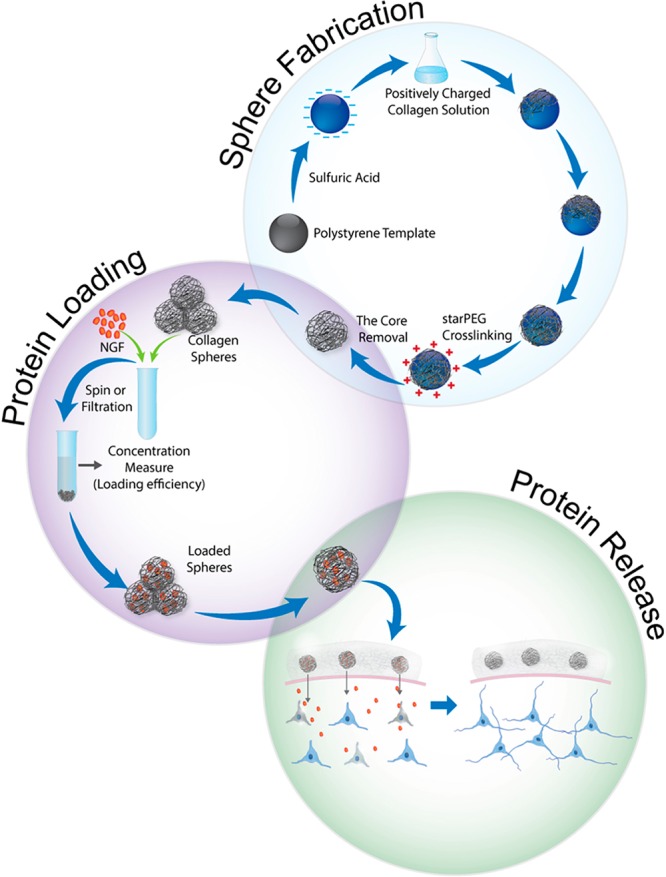
The process involves the sulfonation of a commercially available polystyrene bead of defined size, coating of these beads in a collagen solution, cross-linking of the coating, and removal of the polystyrene bead core. Spheres are incubated with loading buffer overnight to allow NGF loading into the spheres. Collagen microspheres, a protective reservoir of growth factor, are applied to neuronal cells and promote their survival and growth by sustained delivery of neurotrophins.
Fabrication and Morphological Characterization of Collagen Spheres
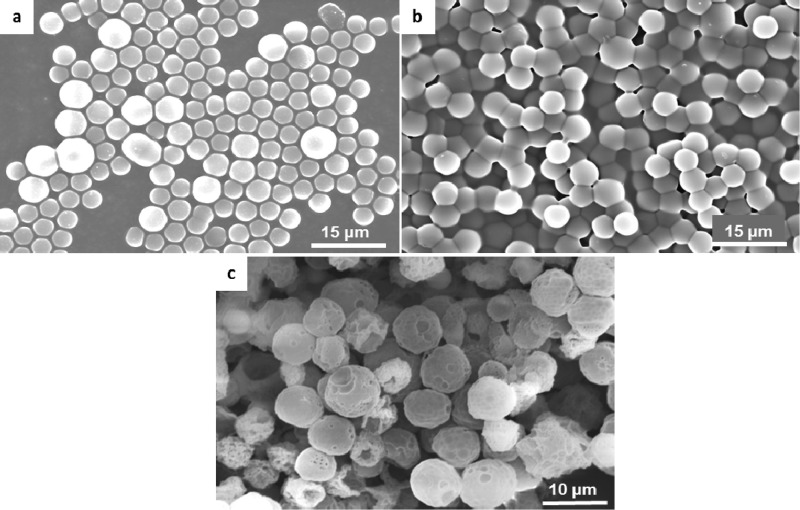
Hollow microspheres were fabricated according to the method developed in our laboratory for other natural polymers such as chitosan,22 elastin,23 and collagen.21,33 Hollow microsphere fabrication, graphically illustrated in Scheme 1, involves a negatively charged polystyrene template which is subsequently coated by positively charged collagen. Following coating, collagen is cross-linked to stabilize the matrix. This process can be controlled by the amount of the cross-linker added. Here, we have fabricated three types of spheres; highly (2×) and moderately (1×) cross-linked and non-cross-linked (0×), where a weight ratio of collagen to PEG is 2:1 and 1:1, respectively. Finally, the polystyrene template is dissolved by washing with tetrahydrofuran (THF) which generates a hollow sphere with the same shape and size as the polystyrene bead. SEM images of resulting hollow spheres as well as steps of fabrication are shown in Figure 1. To avoid sphere phagocytosis in vivo, we produced particles which were relatively larger, having a similar size, between 4 and 5 μm, where an evident hollow morphology is obtained following beads removal. The presence of hollow core is manifested by the stamp shape of the spheres due to the high vacuum in the SEM chamber.
Figure 1.
Steps of sphere fabrication: SEM images of the coating process showing (a) 4 μm polystyrene beads, (b) collagen-coated beads, and (c) 2× collagen spheres following treatment with THF to dissolve the template.
Interaction of Functionalized Spheres with Neuronal Cells
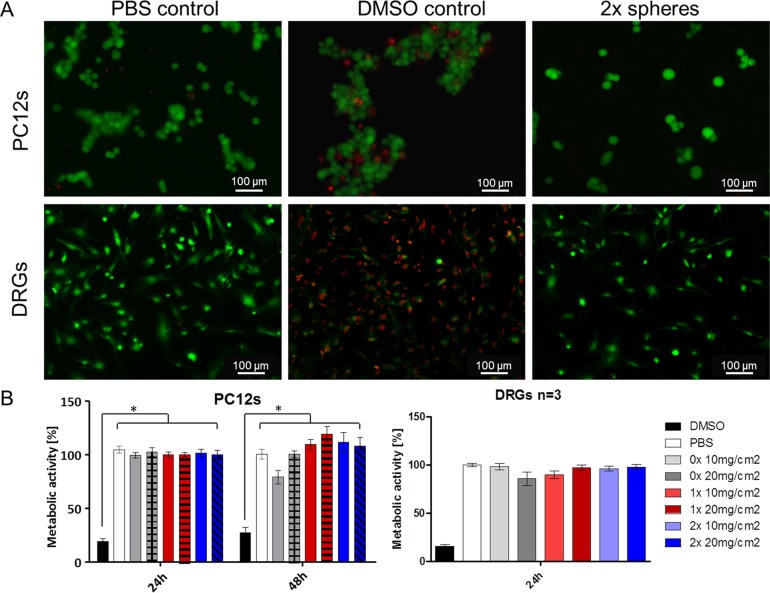
The overall goal of this study was to develop a platform delivery technology for multiple neurotherapeutic application, where microspheres will serve as a reservoir system for growth factor delivery. With this in mind, cytotoxicity of spheres to PC12 cells as well as to primary dorsal root ganglion cells (DRGs) was studied. Two doses of each type of sphere (10 and 20 mg/cm2) were applied on cells, and after 24 h cells were stained with calcein (live) and ethidium homodimer (dead). All cross-linking conditions for the spheres did not exhibit any toxicity to PC12 cells or to primary DRGs when compared to PBS and DMSO treated controls (an example of 2× spheres is presented in Figure 2A). Similarly, when analyzed with AlamarBlue assay (Figure 2B), no significant effect was seen on metabolic activity of cells treated with all types of spheres in comparison to PBS-treated controls. In contrast, following 10% DMSO treatment, metabolic activity was significantly (p < 0.01) decreased to approximately 20–30%. In addition, following treatment with microspheres, PC12 cells showed no changes in morphology within the 6 day period when compared with a nontreated control (Figure 5a, cells alone vs spheres). Therefore, one advantage of the application of collagen hollow spheres is that the spheres support the growth of both the neuronal cell line and primary sensory neurons. In contrast, most studies reported to date on neurotrophin delivery through the biodegradable spheres or other scaffolds do not include any cytotoxicity experiments to support cellular growth,24−26 or cell viability assessment following treatment with growth factor-loaded biomaterial.12,27 Thus, any potential cytotoxic effect of neurotrophin carrier/hydrogel can be masked by the presence of a beneficial growth factor.
Figure 2.
Cell–microsphere interaction. (A) Fluorescent images of PC12 cells (top panel) and DRGs (bottom panel) following 24 h treatment with PBS, 10% DMSO, and 20 mg/cm2 of 2× collagen spheres (green, live; red, dead). (B) Metabolic activity of PC12 cells and DRGs compared with PBS-treated cell control as measured by the AlamarBlue assay (p < 0.01).
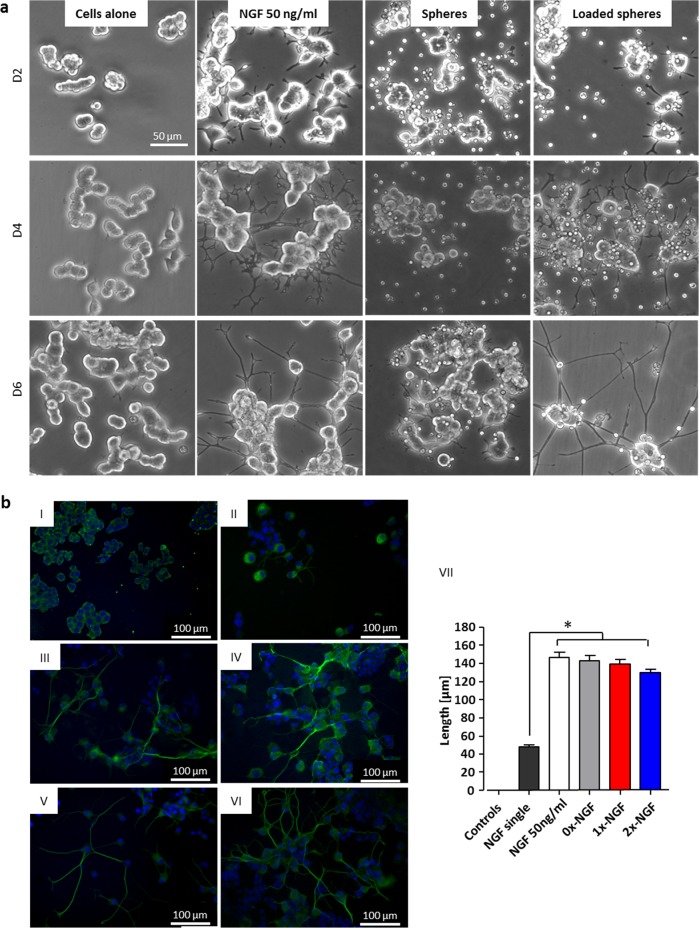
Figure 5.
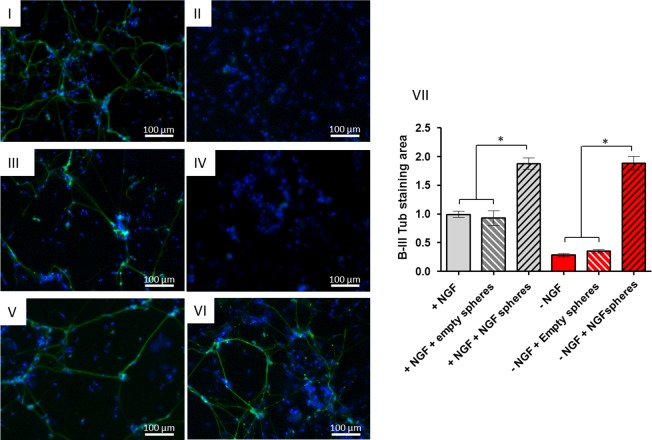
Bioactivity of released NGF assessed on PC12 cells. (a) PC12 cells alone, treated with 50 ng/mL NGF, with empty spheres and with NGF-loaded 2× spheres at D2, D4, and D6. (b) Neuronal outgrowth in PC12 cells following 6 days culture with NGF-loaded spheres (β-III tub staining (green) of differentiated cells). (I) Representative image of nontreated cells/unloaded spheres treatment, (II) single treatment with 50 ng/mL NGF, (III) constant treatment with 50 ng/mL NGF, (IV–VI) 0×, 1×, and 2× collagen spheres treatment loaded with 250 ng of NGF. (VII) Significant (p < 0.05) neuronal outgrowth was observed in constant NGF treatment and in all groups treated with NGF-loaded spheres. Data represents mean ± SD, n = 50 (neurites per group).
NGF Loading in Hollow Spheres
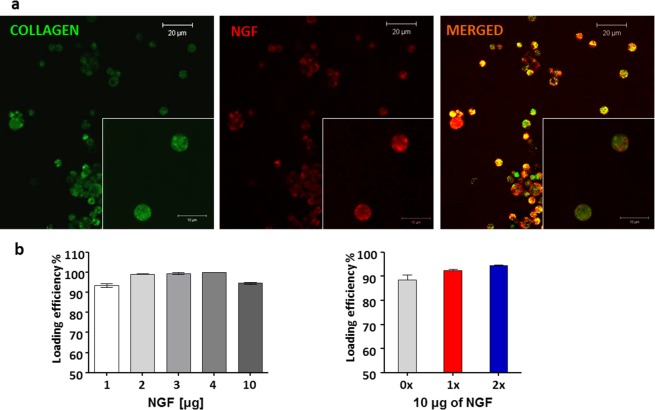
NGF was chosen as a model protein to demonstrate loading and release rates from the spheres. Fluorescent labeling of NGF was done to facilitate detection of the loading process. Then, fluorescently tagged NGF was loaded into FITC stained collagen spheres and samples were viewed under a confocal microscope. Figure 3a shows FITC stained collagen spheres, NGF-loaded spheres, and a merged image of collagen spheres loaded with NGF that confirmed growth factor interaction with microspheres. The hollow structure of spheres allows for diffusion of growth factor inside of the sphere, where it can be protected from unfavorable external environment. Post-cross-linking loading prevents protein structural damage that may occur during for example emulsion processing, lyophilization, freeze/thaw cycles and other processes involved in preparation of biodegradable nano- and microparticles.28,29 Nevertheless, confocal images of fluorescently tagged NGF and spheres showed that some portion of protein localizes also on the outer surface of the spheres (Figure 3a).
Figure 3.
Loading of NGF into collagen microspheres. (A) Confocal images of loaded spheres (green, collagen spheres; red, NGF; yellow, colocalization of NGF and collagen). (B) Loading efficiency with different doses of NGF (LH panel) and into three types of spheres (0× non-cross-linked, 1× cross-linked, and 2× highly cross-linked (RH panel). Data represents mean ± SD, n = 3.
Another advantage of our delivery platform is very high loading efficiency that has not been observed in any other neurotrophin delivery system. Using an ELISA, 90–99% loading efficiency with high loading capacity up to 10 μg of protein per mg of collagen was observed regardless of the sphere type (0×, 1×, and 2×) (Figure 3b). In contrast to previously published reports, the amounts of growth factor loaded per milligram of delivery vehicles were considerably lower and oscillated between nanogram rather than microgram25,30 or were not specified at all.27
NGF Release from Spheres
NGF in vitro release profile was investigated in a PC12 cell system as well as in an acellular system where 0.05% Tween 20–PBS, which improves NGF stability,26 was used as a release buffer. Each of the three types of collagen hollow sphere showed an ability to slowly release NGF in both the cells and no-cells systems. No significant difference was observed between 0× and 1× spheres; however, a significantly slower release rate was seen in the PC12 cells system from highly cross-linked spheres (2×) (Figure 4, left panel), for this reason 2× spheres were selected for further analysis. In the noncell system, release of therapeutic doses of growth factor was observed from all types of spheres up to 13 days with a burst release in the first four days. It is worth emphasizing that the amounts of NGF detected in the supernatants of PC12 cells were lower than those detected in n1 cell systems. This difference is due to consumption of NGF by PC12 cells.
Figure 4.
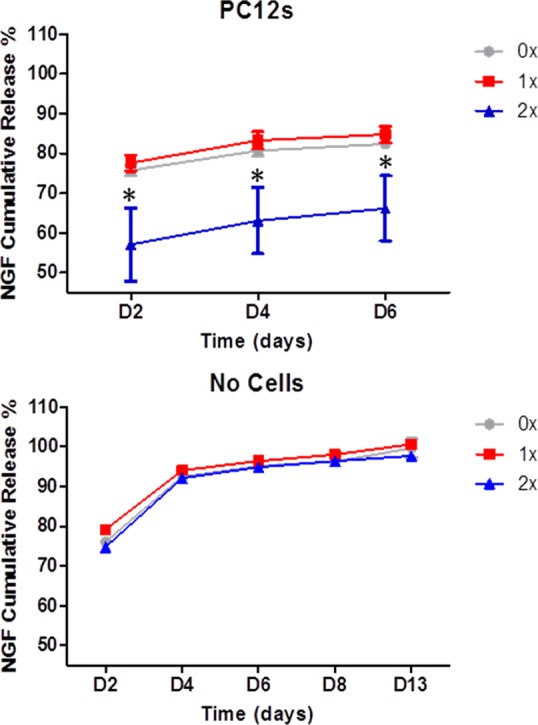
Release profile of NGF from collagen spheres measured by ELISA. In the presence of PC12 cells, a slower release was observed from highly cross-linked spheres (2×) (top panel). A therapeutic dose of NGF was released from spheres up to 13 days post loading (bottom panel). Data represents mean ± SD, n = 3.
The release profile of growth factors from biomaterial devices has been monitored in the range of hours,24 days,26 and weeks;10 however, the 13 days duration limit of testing was selected based on the instability of NGF upon extended incubation times (31)
Although not investigated in this work, embedding of NGF-loaded spheres within collagen hydrogel can further slowdown protein release.32 Therefore, growth factor-loaded spheres may be directly injected into injury site or, as illustrated in Scheme 1, spheres can be embedded within hydrogel and then placed directly into a lesion site in injured spinal cord. The direct delivery of spheres to injured central nervous system tissue overcomes the challenge of crossing the blood-brain barrier.
Bioactivity of Released NGF
Finally, it was shown that released NGF promotes neuronal cell differentiation. First, bioactivity of released NGF was evaluated using a neuronal outgrowth assay in PC12 cells, which have the ability to differentiate into neuronal cells in the presence of NGF. 2× collagen spheres were loaded with NGF and applied to nondifferentiated PC12 cells which were then cultured for 6 days. Cells treated with NGF-loaded spheres exhibited similar morphology to NGF-treated positive controls. In contrast, PC12 cells treated with unloaded spheres did not differentiate and maintained round cell body shapes similar to untreated cells (Figure 5a). This experiment gave similar results when repeated using non-crosslinked spheres (see Supporting Information Figure 1). For quantitative assessment of this phenomenon, βIII tubulin stained cells treated for 6 days with three types of microsphere (0×, 1×, and 2×) were analyzed. Significant neuronal outgrowth (p < 0.05) was observed in all groups treated with NGF-loaded spheres and in constant NGF treatment in comparison to single NGF-treated control (Figure 5b). Cells treated with empty spheres and untreated control did not exhibit any neurites. Bioactivity of released NGF was also assessed on embryonic rat DRGs which were seeded in medium with or without NGF and then treated with NGF-loaded or unloaded 2X collagen spheres. Measurements of βIII tubulin staining areas following 36 h of treatment reveal a significant difference (p < 0.05) between cells treated with NGF-loaded and unloaded spheres cultured in medium with or without NGF (Figure 6).
Figure 6.
NGF released from highly cross-linked collagen spheres promotes neurite growth in embryonic DRGs. Neurite outgrowth was visualized by β-III tub staining (green). (I, III, V) DRGs cultured in medium with 10 ng/mL of NGF and treated for 36 h with PBS, unloaded and 250 ng NGF-loaded spheres, respectively. (II, IV, VI) DRGs cultured in medium without NGF and treated for 36 h with PBS, unloaded and 250 ng NGF-loaded spheres, respectively. (VII) Measurements of βIII tubulin staining area show a significant difference (p < 0.05) between cells treated with NGF-loaded and unloaded spheres cultured in medium with or without NGF. Data represents mean ± SD, n = 10.
All together these data show that NGF loading into collagen hollow spheres and subsequent release does not influence growth factor activity.
Conclusions
In conclusion, collagen hollow microspheres generated using a template method can be loaded with a large amount of NGF with very high loading efficiency. The release of bioactive NGF can be tailored by the amount of cross-linker, and the release of therapeutic dose can be achieved over a period of 13 days. In addition, cytotoxicity testing did not show any negative effects on viability or metabolic activity of both the neuronal cell line and primary rat neurons. The collagen hollow microspheres developed have a potential for use as a reservoir for neurotrophic factors for multiple neurotherapeutic applications.
Methods
All materials and reagents used in this study were purchased from Sigma Aldrich Ireland Ltd. (Dublin, Ireland) unless otherwise stated. Collagen type I and pentaerythritol poly(ethylene glycol) ether octasuccinimidyl glutarate (8a15kSG PEG) were supplied by Covidien (North Haven, CT).
Morphological Characterization of Collagen Hollow Spheres
Following preparation, morphology of collagen spheres as well as of polystyrene beads was evaluated using scanning electron microscopy (SEM) (Hitachi S-4700 field emission microscope operating with a beam voltage of 15 kV). A drop of sample was placed on carbon tabs mounted on the SEM specimen stubs and then dried and gold-coated using an Emitech K550 coating system (Derby, England, U.K.).
Cytotoxicity of Microspheres
The toxicity of three types of collagen spheres (0×, 1×, and 2x) was evaluated on PC12 cells (DMEM, 10% horse serum, and 5% fetal bovine serum, 0.7% L glutamine, 1% pen/strep) as well as on primary DRGs. Cells were seeded in 24-well plates for live/dead assay (Molecular Probes) or in 96-well plates in order to evaluate metabolic activity by AlamarBlue assay (Invitrogen, Dublin, Ireland). Two doses of each type of sphere (10 and 20 mg/cm2) were exposed to cells, and after 24 h cells were stained with calcein (live) and ethidium homodimer (dead). Metabolic activity was measured following 24 or 48 h. Nontreated cells and 10% DMSO-treated cells were used as controls.
Fluorescent Labeling of NGF and Collagen
NGF (2.5s mouse, Alomone Laboratories, Jerusalem, Israel) which is commonly available and relatively inexpensive was chosen as a model protein to demonstrate loading and release rates from the spheres. Fluorescently labeled NGF was used to facilitate detection of loading process. DyLight594 Microscale Antibody Labeling Kit (Thermo Scientific, Rockford, IL) was used to fluorescently tag NGF. One milligram of collagen spheres was incubated with 1 mg/mL FITC (Invitrogen) for 4 h on a shaker at room temperature (RT) and then washed twice with 80% ethanol and distilled water each.
Loading of Microsphere Reservoirs with Growth Factors
One milligram of spheres was mixed with 250 μL of PBS–0.05% Tween solutions (loading buffer) containing various amounts of NGF (1, 2, 3, 4, and 10 μg), and spheres were incubated on a shaker at RT overnight to allow proteins to translocate into spheres. Samples were then spun down for 5 min at 12 000 rpm to separate loaded spheres from the loading buffer, and supernatant was collected for ELISA (R&D Systems, Abingdon, U.K.) analysis. Based on the concentration of NGF in the supernatant, the loading efficiency was calculated. Fluorescently labeled NGF was also loaded into FITC-labeled collagen microspheres before being mounted and examined under inverted confocal microscope.
Release Study
The release profile of NGF was characterized in PBS–0.05%Tween 20 (release buffer) at 37 °C (noncell release system). Twenty-five micrograms of loaded microspheres was incubated with 500 μL of the release buffer, and at various time points microspheres were spun down and supernatant was collected and replaced. The collected supernatant was frozen at −20 °C until ELISA was conducted.
Bioactivity of Released NGF
In parallel, a noncell system, loaded microspheres were added to PC12 cells seeded on coverslips in 24-well plate (25 μg per 1 well) and cells were incubated at 37 °C. Every second day, cell morphology was evaluated under inverted light microscopy and supernatants were collected for ELISA analysis (cell release system). At the final time point, supernatants were collected and cells were fixed with ice-cold methanol and stored at −20 °C before immunocytochemical staining for neurofilaments. The bioactivity of released NGF was also confirmed on primary DRGs in a similar manner, except that cell morphology was evaluated every 12 h.
Immunocytochemistry
Cells fixed on coverslips were washed twice in PBS for 5 min at RT. The samples were then incubated in blocking buffer (2% BSA in PBS) for 1 h. To determine neuronal morphology, a neuronal specific βIII tubulin monoclonal antibody (Millipore) was used at 1:200 dilution in blocking buffer. Following overnight incubation with primary antibody at 4 °C, coverslips were washed twice with PBS and secondary fluorescent antibody Alexa Fluor 488 donkey anti-mouse (Invitrogen) was used for 1 h at RT. Coverslips were washed twice in PBS and incubated with DAPI–PBS solution (1 μg/mL) for 5 min. After washing in PBS, coverslips were mounted on slides with Vectashield (Labkem, Ireland) and stored at 4 °C before image analysis. Imaging was done using an inverted fluorescent microscope (Olympus IX81, Mason Technologies, Dublin, Ireland).
Analysis of Neurite Length and Area
Neurite outgrowth from differentiated PC12 and DRG cells was determined from images stained with βIII tubulin using ImageJ software (MosaicJ, Philippe Thevenaz) where the area of positively stained DRG cells was measured, or individual PC12 cells neurites were traced manually (Neuron J plugin).
Statistical Analysis
All statistical analyses were performed using GraphPad Prism version 5 (GraphPad Software, Inc.). Data were compared using one-way analysis of variance (ANOVA) followed by multiple comparison procedures (Tukey’s test). Values were considered as significantly different with a p < 0.05.
Acknowledgments
The authors acknowledge the facilities and scientific and technical assistance of the Centre for Microscopy and Imaging at the National University of Ireland Galway (www.imaging.nuigalway.ie), a facility that is cofunded by the Irish Government’s Programme for Research in Third Level Institutions, Cycles 4 and 5, National Development Plan 2007-2013.
Glossary
Abbreviations
- NGF
nerve growth factor
- PC12
rat pheochromocytoma
- DRG
dorsal root ganglia
Supporting Information Available
Description of sphere synthesis, primary DRGs preparation, the bioactivity of released NGF assessed on PC12 cells following treatment with NGF-loaded noncross-linked (0×) collagen spheres (SI Figure 1). This material is available free of charge via the Internet at http://pubs.acs.org.
Author Contributions
H.K. and B.B. contributed equally.
Funding for this work was provided by Covidien LLC and the Industrial Development Agency of Ireland.
The authors declare no competing financial interest.
Supplementary Material
References
- Weissmiller A. M.; Wu C. (2012) Current advances in using neurotrophic factors to treat neurodegenerative disorders. Transl. Neurodegener. 1, 1–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hollis E. R. 2nd; Tuszynski M. H. (2011) Neurotrophins: potential therapeutic tools for the treatment of spinal cord injury. Neurotherapeutics 8, 694–703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCall J.; Weidner N.; Blesch A. (2012) Neurotrophic factors in combinatorial approaches for spinal cord regeneration. Cell Tissue Res. 349, 27–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moloney T. C.; Rooney G. E.; Barry F. P.; Howard L.; Dowd E. (2010) Potential of rat bone marrow-derived mesenchymal stem cells as vehicles for delivery of neurotrophins to the Parkinsonian rat brain. Brain Res. 1359, 33–43. [DOI] [PubMed] [Google Scholar]
- Tuszynski M. H.; Grill R.; Jones L. L.; McKay H. M.; Blesch A. (2002) Spontaneous and augmented growth of axons in the primate spinal cord: effects of local injury and nerve growth factor-secreting cell grafts. J. Comp. Neurol. 449, 88–101. [DOI] [PubMed] [Google Scholar]
- Wang J. M.; Zeng Y. S.; Wu J. L.; Li Y.; Teng Y. D. (2011) Cograft of neural stem cells and schwann cells overexpressing TrkC and neurotrophin-3 respectively after rat spinal cord transection. Biomaterials 32, 7454–7468. [DOI] [PubMed] [Google Scholar]
- Bonner J. F.; Blesch A.; Neuhuber B.; Fischer I. (2010) Promoting directional axon growth from neural progenitors grafted into the injured spinal cord. J. Neurosci. Res. 88, 1182–1192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lim S. T.; Airavaara M.; Harvey B. K. (2010) Viral vectors for neurotrophic factor delivery: a gene therapy approach for neurodegenerative diseases of the CNS. Pharmacol. Res. 61, 14–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Boer R.; Knight A. M.; Borntraeger A.; Hebert-Blouin M. N.; Spinner R. J.; Malessy M. J.; Yaszemski M. J.; Windebank A. J. (2011) Rat sciatic nerve repair with a poly-lactic-co-glycolic acid scaffold and nerve growth factor releasing microspheres. Microsurgery 31, 293–302. [DOI] [PubMed] [Google Scholar]
- de Boer R.; Knight A. M.; Spinner R. J.; Malessy M. J.; Yaszemski M. J.; Windebank A. J. (2010) In vitro and in vivo release of nerve growth factor from biodegradable poly-lactic-co-glycolic-acid microspheres. J. Biomed. Mater. Res., Part A 95, 1067–1073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xiong Y.; Zhu J. X.; Fang Z. Y.; Zeng C. G.; Zhang C.; Qi G. L.; Li M. H.; Zhang W.; Quan D. P.; Wan J. (2012) Coseeded Schwann cells myelinate neurites from differentiated neural stem cells in neurotrophin-3-loaded PLGA carriers. Int. J. Nanomed. 7, 1977–1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang Z.; Duan H.; Mo L.; Qiao H.; Li X. (2010) The effect of the dosage of NT-3/chitosan carriers on the proliferation and differentiation of neural stem cells. Biomaterials 31, 4846–4854. [DOI] [PubMed] [Google Scholar]
- Zhao W.; Zhang C.; Jin C.; Zhang Z.; Kong D.; Xu W.; Xiu Y. (2011) Periurethral injection of autologous adipose-derived stem cells with controlled-release nerve growth factor for the treatment of stress urinary incontinence in a rat model. Eur. Urol. 59, 155–163. [DOI] [PubMed] [Google Scholar]
- Alovskaya A., Alekseeva T., Phillips J. B., King V., and Brown R. (2007) Fibronectin, Collagen, Fibrin-Components of Extracellular Matrix for Nerve regeneration. In Topics in Tissue Engineering; Ashammakhi N., Reis R., and E Chiellini E., Eds.; Vol 3, 2007, pp 1–26, http://www.oulu.fi/spareparts/ebook_topics_in_t_e_vol3/cover.html (Accessed July 4, 2013). [Google Scholar]
- Friess W. (1998) Collagen--biomaterial for drug delivery. Eur. J. Pharm. Biopharm. 45, 113–136. [DOI] [PubMed] [Google Scholar]
- Tomaselli K. J.; Damsky C. H.; Reichardt L. F. (1987) Interactions of a neuronal cell line (PC12) with laminin, collagen IV, and fibronectin: identification of integrin-related glycoproteins involved in attachment and process outgrowth. J. Cell Biol. 105, 2347–58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turner D. C.; Flier L. A.; Carbonetto S. (1989) Identification of a cell-surface protein involved in PC12 cell-substratum adhesion and neurite outgrowth on laminin and collagen. J. Neurosci. 9, 3287–3296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choi B. H.; Zhu S. J.; Kim S. H.; Kim B. Y.; Huh J. H.; Lee S. H.; Jung J. H. (2005) Nerve repair using a vein graft filled with collagen gel. J. Reconstr. Microsurg. 21, 267–272. [DOI] [PubMed] [Google Scholar]
- Khaing Z. Z.; Schmidt C. E. (2012) Advances in natural biomaterials for nerve tissue repair. Neurosci. Lett. 519, 103–114. [DOI] [PubMed] [Google Scholar]
- Angius D.; Wang H.; Spinner R. J.; Gutierrez-Cotto Y.; Yaszemski M. J.; Windebank A. J. (2012) A systematic review of animal models used to study nerve regeneration in tissue-engineered scaffolds. Biomaterials 33, 8034–8039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Browne S.; Fontana G.; Rodriguez B. J.; Pandit A. (2012) A protective extracellular matrix-based gene delivery reservoir fabricated by electrostatic charge manipulation. Mol. Pharmaceutics 9, 3099–2106. [DOI] [PubMed] [Google Scholar]
- Rethore G.; Mathew A.; Naik H.; Pandit A. (2009) Preparation of chitosan/polyglutamic acid spheres based on the use of polystyrene template as a nonviral gene carrier. Tissue Eng., Part C 15, 605–613. [DOI] [PubMed] [Google Scholar]
- Dash B. C.; Mahor S.; Carroll O.; Mathew A.; Wang W.; Woodhouse K. A.; Pandit A. (2011) Tunable elastin-like polypeptide hollow sphere as a high payload and controlled delivery gene depot. J. Controlled Release 152, 382–92. [DOI] [PubMed] [Google Scholar]
- Doleski S.; Yao L.; Pandit A.; Elvira C. (2010) NGF release from thermo-responsive collagen-polyNIPAam polymer networks supports neuronal cell growth and differentiation. J. Biomed. Mater. Res., Part A 94, 457–465. [DOI] [PubMed] [Google Scholar]
- Habraken W. J.; Boerman O. C.; Wolke J. G.; Mikos A. G.; Jansen J. A. (2009) In vitro growth factor release from injectable calcium phosphate cements containing gelatin microspheres. J. Biomed. Mater. Res., Part A 91, 614–622. [DOI] [PubMed] [Google Scholar]
- Pfister L. A.; Alther E.; Papaloizos M.; Merkle H. P.; Gander B. (2008) Controlled nerve growth factor release from multi-ply alginate/chitosan-based nerve conduits. Eur. J. Pharm. Biopharm. 69, 563–572. [DOI] [PubMed] [Google Scholar]
- Burdick J. A.; Ward M.; Liang E.; Young M. J.; Langer R. (2006) Stimulation of neurite outgrowth by neurotrophins delivered from degradable hydrogels. Biomaterials 27, 452–459. [DOI] [PubMed] [Google Scholar]
- Bilati U.; Allemann E.; Doelker E. (2005) Strategic approaches for overcoming peptide and protein instability within biodegradable nano- and microparticles. Eur. J. Pharm. Biopharm. 59, 375–388. [DOI] [PubMed] [Google Scholar]
- Stanwick J. C.; Baumann M. D.; Shoichet M. S. (2012) Enhanced neurotrophin-3 bioactivity and release from a nanoparticle-loaded composite hydrogel. J. Controlled Release 160, 666–675. [DOI] [PubMed] [Google Scholar]
- Impellitteri N. A.; Toepke M. W.; Lan Levengood S. K.; Murphy W. L. (2012) Specific VEGF sequestering and release using peptide-functionalized hydrogel microspheres. Biomaterials 33, 3475–3484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eng M.; Ling V.; Briggs J. A.; Souza K.; Canova-Davis E.; Powell M. F.; De Young L. R. (1997) Formulation development and primary degradation pathways for recombinant human nerve growth factor. Anal. Chem. 69, 4184–90. [DOI] [PubMed] [Google Scholar]
- Pakulska M. M., Ballios B. G., and Shoichet M. S. (2012) Injectable hydrogels for central nervous system therapy. Biomed. Mater. published online Mar 29, 2012. DOI: 10.1088/1748-6041/7/2/024101. [DOI] [PubMed] [Google Scholar]
- Helary C.; Browne S.; Mathew A.; Wang W.; Pandit A. (2012) Transfection of macrophages by collagen hollow spheres loaded with polyplexes: a step towards modulating inflammation. Acta Biomater. 8, 4208–4214. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.