Abstract
Objective
Coeliac disease (CD) has been linked to gastro-oesophageal reflux disease (GORD) and eosinophilic oesophagitis (EoE), but population-based studies of the prevalence of CD in these conditions are lacking, that is, the aim of this study.
Materials and methods
An endoscopic study of 1000 randomly selected adults from the general population. CD was defined on the basis of positive serology in parallel with mucosal abnormalities of the small intestine. Any eosinophil infiltration of the oesophageal epithelium was defined as oesophageal eosinophilia and EoE was defined as having at least 15 eosinophils/high power field in biopsies from the distal oesophagus. We used Fisher’s exact test to compare the prevalence of GORD, oesophageal eosinophilia and EoE in subjects with CD vs. controls.
Results
400 subjects (40%) had gastro-oesophageal reflux symptoms (GORS), 155 (15.5%) had erosive oesophagitis, 16 (1.6%) had Barrett’s oesophagus, 48 (4.8%) had oesophageal eosinophilia and 11 (1.1%) had EoE. CD was diagnosed in 8/400 (2.0%) individuals with GORS (vs. controls: 10/600 (1.7%), p=0.81), in 3/155 (1.9%) with erosive oesophagitis (vs. 15/845 controls (1.8%), p=0.75) and in 2/48 (4.2%) individuals with oesophageal eosinophilia (controls: 16/952 (1.7%) p=0.21), but in none of those 16 with Barrett’s oesophagus (vs. 18/984 controls (1.8%), p=1.0) or of the 11 individuals with EoE (controls: 18/989 (1.8%), p=1.0).
Conclusions
This population-based study found no increased risk of CD among individuals with GORD, oesophageal eosinophilia or EoE. CD screening of individuals with GORD or EoE of individuals with CD cannot be recommended.
Keywords: Barrett’s oesophagus, coeliac disease, eosinophilic oesophagitis, erosive oesophagitis, gastro-oesophageal reflux disease
INTRODUCTION
Gastro-esophageal reflux disease (GORD) occurs in about 10–40% of the general population [1, 2], with an incidence of 3–5 per 1000 person-years [3]. Risk factors for GORD include obesity [4], smoking [1] and NSAID use [5] and patients typically report a lower quality of life [1]. In some patients, GORD may lead to erosive oesophagitis (EO) with epithelial damage [6]. A minority of these patients develop Barrett’s oesophagus (BO), and in a recent study we found that the prevalence of BO was 1.6% in the general adult population [7].
Low-grade oesophageal eosinophilia may be a manifestation of reflux disease, celiac disease (CD) and a variety of diseases [8] but eosinophilic oesophagitis (EoE) is a clinicopathologic condition characterized by symptoms related to oesophageal dysfunction and having at least 15 eosinophils/high power field (HPF, at magnification x40) in oesophageal biopsies with no other cause of oesophageal eosinophilia [8].
CD occurs in about 1–2% of the Western population [9] and is defined by ‘a chronic small intestinal immune-mediated enteropathy precipitated by exposure to dietary gluten in genetically predisposed individuals [10].
Data on CD and GORD are conflicting [11–15]. In a retrospective study of 205 patients with CD, 39 (19%) had GORD, compared to 32/400 (8%) controls [12]. Similarly both Nachman et al [11] and Lamanda et al [13] found high rates of GORD in patients with CD. However, in the largest study to date, no association was found between CD and oesophagitis (1198 adults screened for CD) or CD and reflux symptoms (5459 adults screened) [15]. In a pediatric study of 176 children with CD, and 230 control children, mucosal damage in the oesophagus was less common in the CD children than among controls [16]. However, it should be noted that a proportion of CD children in that study (22–30%) were already on a gluten-free diet (GFD) at the time of investigation [16].
Thompson et al found increased incidence of eosinophilic oesophagitis in children and adults with celiac disease [17]. It has also been suggested that eosinophilic infiltration of oesophagus could be a manifestation associated with exposure to gluten at least in a small number of children with CD and could be caused by CD itself [18].
Potential weaknesses of earlier studies include ascertainment of GORD individuals through highly specialized centers (with the exception of the Collin et al study [15]), and lack of appropriate population-based controls. Because of limitations in earlier studies on CD and GORD, and conflicting data [11–15] we set out to determine the risk of CD in a large population-based sample of individuals with GORD, oesophageal eosinophilia and EoE according to symptom questionnaires and upper endoscopy. We specifically examined the prevalence of CD in patients with self-reported gastroesophageal reflux symptoms (GORS), erosive oesophagitis confirmed through endoscopy and BE, any oesophageal eosinophilia and EoE confirmed through histology.
METHODS
The study area consisted of two neighboring communities, Kalix and Haparanda (the “Kalixanda study”). The gender and age distribution of the study area was similar to that of the Swedish national average when the study started in 1998. In short, we contacted every seventh adult (N. = 3000, mean age 50.4 years) randomly selected from the target population consisting of 21,610 individuals aged 20–80 years as of September 1998 [19].
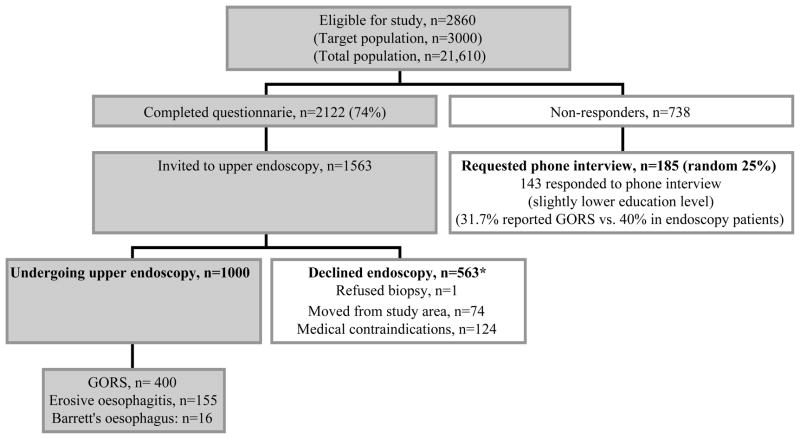
For logistic reasons, the inclusion time was 2.5 years and 140 individuals had emigrated or were otherwise not eligible for the study. Hence, through postal contact we invited 2860 eligible individuals (Figure 1), and asked them to respond to the Abdominal Symptom Questionnaire (ASQ, enclosed in the invitation). The ASQ contained a list of 24 gastrointestinal symptoms, and study participants were asked to confirm or reject the presence of any troublesome symptom during the last 3 months. In total, some 2122 individuals completed the ASQ (response rate 74%)(Figure 1) [19].
Figure 1. Flow chart of study participants, the population-based Kalixanda study.
*Other reasons for non-participation included e.g. ischemic and other heart disease, mental/cerebral disorders, previous upper GI surgery, current malignancy, pregnancy and other severe disorders (for details, see Aro et al. [19]).
Following the study plan we carried out an upper endoscopy in one third of the original 3000, i.e. 1000 individuals (corresponding to 4.6% of the target population). At time of endoscopy, the study participants were asked to once again fill in an extended ASQ and also to report consumption of aspirin, non-steroidal anti-inflammatory drugs, antacids, H2 receptor antagonists, and proton pump inhibitors (PPI) used during the last 3 months. They were also asked about tobacco (smoking or snuff) and alcohol use. Further details of the ASQ have been presented elsewhere [19, 20].
In order to include 1000 individuals with upper endoscopy we contacted 1563 individuals who had responded to the ASQ of whom 198 had a medical contraindication or had moved (Figure 1). The response rate for those eligible was hence 73% (n=1001/1365) [19]. Endoscopy participants (average age 53.5 years, 51% women) were significantly older than the study population, but had the same gender distribution. The higher average age was due lower participation rates in the youngest age stratum. The age and gender distribution for individuals undergoing upper endoscopy did however not differ from that of the 2122 ASQ responders.
Three endoscopists (2 from primary care, 1 from secondary care) carried out the upper endoscopies. They were all blinded to the ASQ responses and the medical history of the study participants. To secure a high internal validity of the endoscopies, we arranged for an expert endoscopist and a Professor of Gastrointestinal Surgery to review the macroscopic findings according to standardized classification systems such as the Los Angeles classification. An external reviewer (Professor of Gastroenterology) also carried out a test session regarding esophageal findings on video, and found a high concordance rate between the study endoscopists [2, 19].
Definitions of gastrointestinal disease
GORS was defined as “having troublesome reflux symptoms (heartburn and/or acid regurgitation) during the last three months” in line with the Montreal definition of GORD [6], EE as “having a break in the esophageal mucosa” according to the Los Angeles classification [21] (for details see Ronkainen et al [7]), and BE as “suspected columnar-lined oesophagus histologically confirmed by the presence of specialized intestinal metaplasia” [7].
CD was defined on the basis of positive serology in parallel with mucosal abnormalities of the small intestine (≥25 intraepithelial lymphocytes/100 enterocytes and/or villous atrophy). Two biopsies were taken from the bulb, and two from the distal part of the duodenum. Sixteen subjects (1.6%) had serologic and histologic evidence of gluten-sensitive enteropathy and another two had a previous diagnosis of CD, 1.8% in total [9]. Of the 16 individuals with newly diagnosed CD, all were positive for DQ2 [9].
Any eosinophil infiltration of the oesophageal epithelium was defined as oesophageal eosinophilia and EoE was defined as having at least 15 eosinophils/high power field (HPF, at magnification x40) in biopsies from the distal oesophagus [22].
All histopathology was examined by two experienced gastrointestinal pathologists. Sections were stained with H&E. H. pylori infection was histologically detected by means of Warthin-Starry silver staining. Further details on the definitions in this paper have been published elsewhere [2, 7, 9], [22].
Statistics
Because of less than 5 expected events in some categories (e.g. the number of CD patients in individuals with BE) we used Fisher’s exact test to calculate p-values. Statistical significance was set to 0.05. All statistical analyses were performed through the Intercooled STATA program 8.0 [23].
Ethics
The study was approved by the ethics committees of Umeå University, Sweden, and the Mayo Clinic and conducted in accordance with the revised Declaration of Helsinki.
RESULTS
Of the 1000 adults undergoing endoscopy with biopsy, 400 (40%) had GORS, 155 (15.5%) had EO, 16 (1.6%) had Barrett’s oesophagus, 48 (4.8%) had oesophageal eosinophilia and 11 (1.1%) had EoE. The mean age of patients with esophageal disease was similar (GORS: 52.4 years; EO: 52.7 years; Barrett’s oesophagus: 55.7 years and EoE: 55.6 years), while the mean age of those with CD was 53.5 years (standard deviation, SD=12.4 years). Of 18 individuals with CD, 8 (44%) were women. The percentage of subjects with GORS who were women was 54.7%, which was higher than EO (30.3%) and Barrett’s oesophagus (43.8%). Additional data on characteristics of study participants according to CD status are given in Table 1. Of the 155 individuals with EO, 63.2% reported having GORS. Of the 400 patients with GORS, 127 (31.8%) had macroscopic signs of EO. Some 2.6% of individuals with EO had BO [7]. Additional details including grading of EO according to the Los Angeles classification, the relationship between short and long BO and EoE have been published elsewhere [2, 7, 9, 22].
Table 1.
Charasteristics of participants, the population-based Kalixanda study.
| Characteristics | Coeliac disease (%) | No Coeliac disease (%) | P-value |
|---|---|---|---|
| Total | 18 (1.8) | 982 | - |
| Symptoms | |||
| Diarrhea | 3/15 (20)* | 214/858 (24.9)* | 1.00 |
| Weight loss | 1/18 (6) | 21/972 (2.2)* | 0.34 |
| Underweight | 0/18 (0) | 8/972 (0.8)* | 1.00 |
| Alcohol consumption > 50g/week | 5/18 (28) | 254/982 (25.9) | 0.79 |
| Smoking | 5/18 (28) | 187/982 (18.4) | 0.35 |
| Helicobacter pylori + | 5/18 (28) | 334/982 (34.0) | 0.80 |
| Use of medication in the last 3 months before biopsy | |||
| Any GI medication | 3/18 (17) | 187/982 (19.0) | 1.00 |
| Proton Pump inhibitor | 1/18 (6) | 48/982 (4.9) | 0.60 |
| H2-blocker | 0/18 (0) | 31/982 (3.2) | 1.00 |
| NSAID | 2/18 (11) | 67/982 (6.8) | 0.36 |
| ASA | 1/18 (6) | 106/982 (10.8) | 0.71 |
Data available in a subset of study participants.
GORS and CD
CD was diagnosed in 8/400 (2.0%) individuals with GORS (controls: 10/600 (1.7%), p=0.81). There was also a lack of association between GORD-related medication and CD (Table 1).
EO and CD
We found no increased prevalence of CD in patients with EO (p=0.75). Three out of 155 (1.9%) patients with EO had CD, compared to 15/845 (1.8%) controls without EO. (One of the three CD patients with EO had reflux symptoms). There was no association between “EO without GORS” and CD (p=0.24).
BO and CD
There was no case of CD in individuals with BO (0/16) (vs. controls: 18/984 (1.8%), p=1.0). Neither did we find any increase in the risk of CD in patients with specialized intestinal metaplasia (1/60 had CD; vs. 18/940 controls, p=1.0).
EoE and CD
CD was diagnosed in 2/48 (4.2%) individuals with oesophageal eosinophilia (controls: 16/952 (1.7%), p=0.21), but in none of the 11 individuals with EoE (controls: 18/989 (1.8%), p=1.0).
DISCUSSION
In this population-based cohort study of randomly selected adults undergoing upper endoscopy, we found no association between gastroesophageal reflux symptoms, erosive oesophagitis, Barrett’s oesophagus, eosinophilic oesophagitis and coeliac disease (CD). The lack of association between CD and GORD is further supported by the lack of association between GORD-related medication and CD (Table 1).
Comparison with earlier literature
In an Argentinean setting Nachman et al found that 30% of CD patients had substantial GORD symptoms, compared to only 5.7% of controls [11]. However, the proportions of individuals with CD and controls taking proton-pump inhibitors were similar [11], potentially suggesting that while any heartburn may be more common in CD (or will lead to an investigation for CD), severe heartburn necessitating treatment (and potentially causing tissue damage) is not [11]. Of note is also the low prevalence of heartburn among the controls in their study since GORD is usually seen in about 10–20% of the general population [1]. Most Argentinean patients with CD in the Nachman et al study had classical CD [11], and in another study, esophageal abnormalities were more common in those CD patients who had steatorrhea [14]. In our study some 20% of CD patients indicated diarrhea, and 1/18 (6%) reported weight loss prior to diagnosis.
Our study shared several of the characteristics of a Finnish study that also found a null relationship between CD and GORD [15]. In both Sweden and Finland are the awareness and prevalence of CD high, and CD is often investigated in a primary health care setting. In fact many participants of our study were Finnish-speaking since our study region borders to Finland. Inhabitants in the two countries also share the same HLA risk associations for CD with a great preponderance of DQ2 [24], that may explain the high (~2%) prevalence of CD reported in recent studies [9, 25]. In contrast to the Finnish study [15], all endoscopies in our study were performed by endoscopists who were blinded to the patient’s symptoms so as to assure an objective grading of esophageal findings. The average age of our study participants was 4 years above non-responders, due to a lower response rate in younger age categories. The Kalixanda area is characterized by a slightly lower socioeconomic status than the Swedish average, and responders had slightly higher education than non-responders [19]. However, differences in socio-economic status between communities in Sweden are small by international standards and this is unlikely to affect the relationship between esophageal disorders and CD since upper endoscopy was performed independently of socioeconomic status. Notably, a recent study from Sweden found only very small differences in socioeconomic status between CD patients and matched controls [26].
Cuomo et al reported that 14/15 patients with CD had a pathologic pH recording [12]. However, these 15 patients constituted less than half of their CD patients (n=39) so it is unclear to what extent they were representative of the average CD patient [12]. We did not measure esophageal pH but on the other hand we required mucosal damage for two of our three main exposures (GORD and BE, but not GORS), thereby avoiding subjective grading of symptoms (by the endoscopist or the patient). In addition, the endoscopists of our study were unaware of the patients’ CD status when they performed the endoscopies and when they biopsied patients. That most of our patients with GORD suffered from GORS rather than EE and BE is consistent with earlier research [27].
Research on CD and BE is scarce and mostly limited to single cases reports [11], with the exception of one study on CD and BE in a hospital setting [28]. That study found a 3.9-fold increased risk of BE in patients with CD [28]. This excess risk contrasts with our null findings, the reason probably being that our study was general population-based while their study [28] was based on patients seeking healthcare. Also, not all underwent biopsy as the diagnosis of BE was made on endoscopy and only confirmed by biopsy.
Quaglietta et al reported an association between CD and EoE in children and Thompson et al found an association between CD and EoE in both pediatric and adult populations in 1439 cases of CD in comparison to population-based incidence rates [17, 29]. This is in contrast with our findings and probably explained by the fact that our study was general population-based and their study was done in a specialized clinic at a tertiary care referral institution.
Strengths and limitations
Among the strengths of our paper is the detailed examination of all study participants (everyone had an upper endoscopy with biopsy and responded to a questionnaire). Our study design also avoided selection bias, since symptoms were not a criterion for study inclusion. In other studies [11], patients may have been diagnosed due to reflux, or contacted health care due to complaints related to esophageal disease.
The large number (n=1000) of study participants allowed us to study the association between CD and even rare esophageal diseases in a population-based setting. CD was diagnosed based on both histopathology findings and serology data [9]. We used a symptom questionnaire (ASQ) that has been extensively validated [20].
Several mechanisms have been suggested to explain the association between CD and EE seen in some other studies. These include delayed gastric emptying that could predispose to gastroesophageal reflux, but also abnormal esophageal motility per se [14]. Lucendo has listed other potential causes of GORD in patients with CD including increased hormone levels that might affect the lower esophageal sphincter (LES) pressure [18]. We did not measure LES pressure. When Cuomo et al measured LES pressure, it was non-significantly lower in CD patients than in controls, but numbers were small (15 patients with CD) [12].
There are also reasons to believe that GORD should be less common in CD. It is also possible that CD complicated by severe malnutrition may result in hypochlorhydria [30]. Obesity, predisposes to reflux [31], but is negatively associated with having a CD diagnosis [32]. And in our recent study of CD prevalence in the Kalixanda region, positive CD serology was negatively associated with BMI>25 (OR=0.34) [9]. Another important GORD risk factors is smoking [1], which in many studies has been less common among patients with CD [33]. Interestingly enough, our study on the prevalence of CD in Kalixanda found a negative relationship between increased intraepithelial count (>25) and having dyspepsia in the last 3 months before endoscopy (OR=0.43) [9].
This study has some limitations. Since this was a screening study for CD we did not evaluate the importance of GFD in patients with both CD and GORD. Initiation of GFD will usually improve GORD symptoms in CD patients [11, 12, 14, 15, 34], but degree of dietary adherence does not seem to influence GORD severity in those with GFD [11]. Neither did we examine if discontinuation of PPIs in patients with both CD and GORD lead to relapse [11]. Also, we had distal biopsies from the esophagus only which may cause uncertainty for the diagnosis of EOE because the condition may be patchy [8].
In conclusion, this population-based study of 1000 individuals undergoing upper endoscopy found no association between GORS, EE, BE, oesophageal eosinophilia, EoE and CD. These findings confirm earlier Finnish data where individuals with CD from a primary care setting were at no increased risk of GORD. While all endoscopists need to be aware of CD whenever they are undertaking endoscopy, screening for CD in individuals with GORD or screening for EoE in individuals with CD cannot be routinely recommended.
Acknowledgments
Grant Support (Funding):
JFL was supported by grants from The Swedish Society of Medicine, the Swedish Research Council – Medicine (522-2A09-195), the Swedish Coeliac Society and the Fulbright Commission.
PA the Finnish Medical Foundation, Vappu and Oskari Yliperttula’s Foundation (Finland), AstraZeneca R&D (Sweden),
JAM was supported by DK 57892
JR The Swedish Society of Medicine, the Finnish Medical Foundation, Vappu and Oskari Yliperttula’s Foundation (Finland), AstraZeneca R&D (Sweden), Orion Research Foundation (Finland).
Independence (role of the sponsors): None of the funders had any role in the design and conduct of the study; collection, management, analysis, and interpretation of the data; and preparation, review, or approval of the manuscript.
Glossary
- CD
Coeliac disease
- EoE
Eosinophilic oesophagitis
- VA
Villous atrophy
Footnotes
Details of ethics approval: The study was approved by the ethics committees of Umeå University, Sweden, and the Mayo Clinic and conducted in accordance with the revised Declaration of Helsinki
Disclosure / Conflict of interest declaration: None
Guarantor: JR had full access to all the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analyses.
Writing Assistance: None.
References
- 1.Eslick GD, Talley NJ. Gastroesophageal reflux disease (GERD): risk factors, and impact on quality of life-a population-based study. J Clin Gastroenterol. 2009;43:111–7. doi: 10.1097/MCG.0b013e31815ea27b. [DOI] [PubMed] [Google Scholar]
- 2.Ronkainen J, Aro P, Storskrubb T, Johansson SE, Lind T, Bolling-Sternevald E, et al. High prevalence of gastroesophageal reflux symptoms and esophagitis with or without symptoms in the general adult Swedish population: a Kalixanda study report. Scand J Gastroenterol. 2005;40:275–85. doi: 10.1080/00365520510011579. [DOI] [PubMed] [Google Scholar]
- 3.Ruigomez A, Garcia Rodriguez LA, Wallander MA, Johansson S, Dent J. Comparison of gastro-oesophageal reflux disease and heartburn diagnoses in UK primary care. Curr Med Res Opin. 2006;22:1661–8. doi: 10.1185/030079906X120986. [DOI] [PubMed] [Google Scholar]
- 4.Ayazi S, Hagen JA, Chan LS, DeMeester SR, Lin MW, Ayazi A, et al. Obesity and gastroesophageal reflux: quantifying the association between body mass index, esophageal acid exposure, and lower esophageal sphincter status in a large series of patients with reflux symptoms. J Gastrointest Surg. 2009;13:1440–7. doi: 10.1007/s11605-009-0930-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kotzan J, Wade W, Yu HH. Assessing NSAID prescription use as a predisposing factor for gastroesophageal reflux disease in a Medicaid population. Pharm Res. 2001;18:1367–72. doi: 10.1023/a:1013010616496. [DOI] [PubMed] [Google Scholar]
- 6.Vakil N, van Zanten SV, Kahrilas P, Dent J, Jones R. The Montreal definition and classification of gastroesophageal reflux disease: a global evidence-based consensus. Am J Gastroenterol. 2006;101:1900–20. doi: 10.1111/j.1572-0241.2006.00630.x. quiz 43. [DOI] [PubMed] [Google Scholar]
- 7.Ronkainen J, Aro P, Storskrubb T, Johansson SE, Lind T, Bolling-Sternevald E, et al. Prevalence of Barrett’s esophagus in the general population: an endoscopic study. Gastroenterology. 2005;129:1825–31. doi: 10.1053/j.gastro.2005.08.053. [DOI] [PubMed] [Google Scholar]
- 8.Liacouras CA, Furuta GT, Hirano I, Atkins D, Attwood SE, Bonis PA, et al. Eosinophilic esophagitis: updated consensus recommendations for children and adults. J Allergy Clin Immunol. 2011;128:3–20. e6. doi: 10.1016/j.jaci.2011.02.040. quiz 1–2. [DOI] [PubMed] [Google Scholar]
- 9.Walker MM, Murray JA, Ronkainen J, Aro P, Storskrubb T, D’Amato M, et al. Detection of Celiac Disease and Lymphocytic Enteropathy by Parallel Serology and Histopathology in a Population-Based Study. Gastroenterology. 2010;139:112–9. doi: 10.1053/j.gastro.2010.04.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ludvigsson JF, Leffler DA, Bai JC, Biagi F, Fasano A, Green PH, et al. The Oslo definitions for coeliac disease and related terms. Gut. 2012 doi: 10.1136/gutjnl-2011-301346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Nachman F, Vazquez H, Gonzalez A, Andrenacci P, Compagni L, Reyes H, et al. Gastroesophageal reflux symptoms in patients with celiac disease and the effects of a gluten-free diet. Clin Gastroenterol Hepatol. 2011;9:214–9. doi: 10.1016/j.cgh.2010.06.017. [DOI] [PubMed] [Google Scholar]
- 12.Cuomo A, Romano M, Rocco A, Budillon G, Del Vecchio Blanco C, Nardone G. Reflux oesophagitis in adult coeliac disease: beneficial effect of a gluten free diet. Gut. 2003;52:514–7. doi: 10.1136/gut.52.4.514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lamanda R, Panarese A, De Stefano S. Coeliac disease and gastroesophageal non-erosive reflux_ prevalence and effect of gluten-free diet. Dig Liver Dis. 2009;41:S90. [Google Scholar]
- 14.Iovino P, Ciacci C, Sabbatini F, Acioli DM, D’Argenio G, Mazzacca G. Esophageal impairment in adult celiac disease with steatorrhea. Am J Gastroenterol. 1998;93:1243–9. doi: 10.1111/j.1572-0241.1998.00403.x. [DOI] [PubMed] [Google Scholar]
- 15.Collin P, Mustalahti K, Kyronpalo S, Rasmussen M, Pehkonen E, Kaukinen K. Should we screen reflux oesophagitis patients for coeliac disease? Eur J Gastroenterol Hepatol. 2004;16:917–20. doi: 10.1097/00042737-200409000-00017. [DOI] [PubMed] [Google Scholar]
- 16.Oderda G, Forni M, Morra I, Tavassoli K, Pellegrino P, Ansaldi N. Endoscopic and histologic findings in the upper gastrointestinal tract of children with coeliac disease. J Pediatr Gastroenterol Nutr. 1993;16:172–7. doi: 10.1097/00005176-199302000-00013. [DOI] [PubMed] [Google Scholar]
- 17.Thompson JS, Lebwohl B, Reilly NR, Talley NJ, Bhagat G, Green PH. Increased incidence of eosinophilic esophagitis in children and adults with celiac disease. J Clin Gastroenterol. 2012;46:e6–e11. doi: 10.1097/MCG.0b013e318221aefd. [DOI] [PubMed] [Google Scholar]
- 18.Lucendo AJ. Esophageal manifestations of celiac disease. Dis Esophagus. 2011;24:470–5. doi: 10.1111/j.1442-2050.2011.01190.x. [DOI] [PubMed] [Google Scholar]
- 19.Aro P, Ronkainen J, Storskrubb T, Bolling-Sternevald E, Carlsson R, Johansson SE, et al. Valid symptom reporting at upper endoscopy in a random sample of the Swedish adult general population: the Kalixanda study. Scand J Gastroenterol. 2004;39:1280–8. doi: 10.1080/00365520410008141. [DOI] [PubMed] [Google Scholar]
- 20.Agreus L, Svardsudd K, Nyren O, Tibblin G. Reproducibility and validity of a postal questionnaire. The abdominal symptom study. Scand J Prim Health Care. 1993;11:252–62. doi: 10.3109/02813439308994840. [DOI] [PubMed] [Google Scholar]
- 21.Lundell LR, Dent J, Bennett JR, Blum AL, Armstrong D, Galmiche JP, et al. Endoscopic assessment of oesophagitis: clinical and functional correlates and further validation of the Los Angeles classification. Gut. 1999;45:172–80. doi: 10.1136/gut.45.2.172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ronkainen J, Talley NJ, Aro P, Storskrubb T, Johansson SE, Lind T, et al. Prevalence of oesophageal eosinophils and eosinophilic oesophagitis in adults: the population-based Kalixanda study. Gut. 2007;56:615–20. doi: 10.1136/gut.2006.107714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.STATA 8.0. Texas: 2003. [Google Scholar]
- 24.Maki M, Mustalahti K, Kokkonen J, Kulmala P, Haapalahti M, Karttunen T, et al. Prevalence of Celiac disease among children in Finland. N Engl J Med. 2003;348:2517–24. doi: 10.1056/NEJMoa021687. [DOI] [PubMed] [Google Scholar]
- 25.Vilppula A, Kaukinen K, Luostarinen L, Krekela I, Patrikainen H, Valve R, et al. Increasing prevalence and high incidence of celiac disease in elderly people: a population-based study. BMC Gastroenterol. 2009;9:49. doi: 10.1186/1471-230X-9-49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Olen O, Bihagen E, Rasmussen F, Ludvigsson JF. Socioeconomic position and education in patients with coeliac disease. Dig Liver Dis. 2012 doi: 10.1016/j.dld.2012.01.006. [DOI] [PubMed] [Google Scholar]
- 27.DeVault KR, Castell DO. Updated guidelines for the diagnosis and treatment of gastroesophageal reflux disease. Am J Gastroenterol. 2005;100:190–200. doi: 10.1111/j.1572-0241.2005.41217.x. [DOI] [PubMed] [Google Scholar]
- 28.Maieron R, Elli L, Marino M, Floriani I, Minerva F, Avellini C, et al. Celiac disease and intestinal metaplasia of the esophagus (Barrett’s esophagus) Dig Dis Sci. 2005;50:126–9. doi: 10.1007/s10620-005-1289-6. [DOI] [PubMed] [Google Scholar]
- 29.Quaglietta L, Coccorullo P, Miele E, Pascarella F, Troncone R, Staiano A. Eosinophilic oesophagitis and coeliac disease: is there an association? Aliment Pharmacol Ther. 2007;26:487–93. doi: 10.1111/j.1365-2036.2007.03388.x. [DOI] [PubMed] [Google Scholar]
- 30.Viteri FE, Schneider RE. Gastrointestinal alterations in protein-calorie malnutrition. Med Clin North Am. 1974;58:1487–505. doi: 10.1016/s0025-7125(16)32085-5. [DOI] [PubMed] [Google Scholar]
- 31.Jacobson BC, Somers SC, Fuchs CS, Kelly CP, Camargo CA., Jr Body-mass index and symptoms of gastroesophageal reflux in women. N Engl J Med. 2006;354:2340–8. doi: 10.1056/NEJMoa054391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Olen O, Montgomery SM, Marcus C, Ekbom A, Ludvigsson JF. Coeliac disease and body mass index: A study of two Swedish general population-based registers. Scand J Gastroenterol. 2009;44:1198–206. doi: 10.1080/00365520903132013. [DOI] [PubMed] [Google Scholar]
- 33.Snook JA, Dwyer L, Lee-Elliott C, Khan S, Wheeler DW, Nicholas DS. Adult coeliac disease and cigarette smoking [see comments] Gut. 1996;39:60–2. doi: 10.1136/gut.39.1.60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Usai P, Manca R, Cuomo R, Lai MA, Russo L, Boi MF. Effect of gluten-free diet on preventing recurrence of gastroesophageal reflux disease-related symptoms in adult celiac patients with nonerosive reflux disease. J Gastroenterol Hepatol. 2008;23:1368–72. doi: 10.1111/j.1440-1746.2008.05507.x. [DOI] [PubMed] [Google Scholar]