Abstract
Polybrominated diphenyl ether (PBDE) flame retardants have been shown to disrupt thyroid hormone regulation, neurodevelopment, and reproduction in some animals. However, effects of the most heavily used PBDE, decabromodiphenyl ether (BDE-209), on thyroid functioning remain unclear. This study examined low-dose effects of BDE-209 on thyroid hormone levels and signaling in fathead minnows. Adult males received dietary exposures of BDE-209 at a low dose (~3 ng/g bw-day) and high dose (~300 ng/g bw-day) for 28 days followed by a 14-day depuration to evaluate recovery. Compared to controls, fish exposed to the low dose for 28 days experienced a 53% and 46% decline in circulating total thyroxine (TT4) and 3,5,3'-triiodothyronine (TT3), respectively, while TT4 and TT3 deficits at the high dose were 59% and 62%. Brain deiodinase activity (T4-ORD) was reduced by ~65% at both doses. BDE-209 elevated the relative mRNA expression of genes encoding deiodinases, nuclear thyroid receptors, and membrane transporters in the brain and liver in patterns that varied with time and dose, likely in compensation to hypothyroidism. Declines in the gonadal-somatic index (GSI) and increased mortality were also measured. Effects at the low dose were consistent with the high dose, suggesting non-linear relationships between BDE-209 exposures and thyroid dysfunction.
Keywords: BDE-209, deiodinase, endocrine disruption, monocarboxylate transporter, organic anion transport protein, polybrominated diphenyl ether, PTU, thyroid hormone, thyroid receptor
INTRODUCTION
The widespread use of the polybrominated diphenyl ether (PBDE) flame retardant DecaBDE has resulted in rising levels of decabromodiphenyl ether (BDE-209) in humans1–3, wild fish4–6, and other wildlife species7–9. BDE-209 is detected increasingly as the dominant PBDE in the atmosphere, sediments, soils, and indoor dust3, 10, 11. These reservoirs of contamination serve as sources of PBDE exposure as BDE-209 can undergo photolytic degradation12, microbial breakdown13, and metabolic biotransformation4, 14 to lower PBDE congeners. While DecaBDE is scheduled for phase-out in the U.S. at the end of 2013, exposures to BDE-209 and other PBDEs are expected to continue into the coming decades as products that contain them continue to be used, discarded, and recycled. Previous studies have demonstrated that exposures to lower PBDE congeners (e.g., BDE-47, BDE-99) can depress thyroid hormone levels, alter thyroid hormone transport or target tissue response capacity, and elicit phenotypic impacts such as neurodevelopmental impairments, among other adverse effects15–18. Despite the widespread use of BDE-209, however, we continue to have limited information on its potential to impair thyroid functioning. The thyroid system is substantially conserved across vertebrates, and so increasing our knowledge of BDE-209 effects on the fish thyroid can inform our understanding of effects in other species.
This study used the fathead minnow as a model to evaluate effects of low doses of BDE-209 on thyroid functioning and to further elucidate mechanisms of thyroid dysfunction. Two low dose exposures of BDE-209 were selected: (1) a higher dose of ~10 ug/g ww of food targeted to reflect a BDE-209 exposure possible from a more contaminated environment; and (2) a low dose of ~95 ng/g ww food more characteristic of background environmental levels of BDE-20919–22. The bioaccumulation of BDE-209 and its reductive metabolites was measured along with effects on circulating thyroid hormone levels, relative iodothyronine deiodinase (Dio) activity and mRNA levels in the brain and liver, and transcript abundances of genes encoding thyroid hormone receptors (trα, trβ) and several membrane bound transporters from the monocarboxylate transporter (mct) and organic anion transport protein (oatp) families. Because a limited number of studies have shown PBDE impacts on adult fish reproduction23, 24, the gonado-somatic index (GSI) was measured as an initial metric of BDE-209 effects on reproductive output.
MATERIALS AND METHODS
BDE-209 Dietary Exposures
Approximately 600 adult male fathead minnows (Pimephales promelas; 9 months old; Aquatic BioSystems, Fort Collins, CO) were distributed randomly across twelve 150-liter glass aquaria (~50 fish/tank) and assigned to the following treatments: three BDE-209 high dose tanks; three BDE-209 low dose tanks; three positive control tanks; and three negative control tanks. Fish at the high dose received dietary exposures (Omnivore Gel Diet; Aquatic Ecosystem, Inc., Apopka, FL) of BDE-209 at 10.1±0.10 μg/g wet weight (ww) of food at 3% bw/day (or ~300 ng/g bw-day). Fish at the low dose were exposed to BDE-209 at 95.3±0.41 ng/g ww of food at 3% bw/day (or ~3 ng/g bw-day). All PBDE concentrations in food were confirmed using mass spectrometry as outlined in the extraction methods below. The model anti-thyroid drug 6-propyl-2-thiouricil (PTU) was used as a positive control at 0.5 mg/g ww of food at 3% bw/day (or ~15 μg/g bw-day). BDE-209 (97% purity) and PTU were purchased from Sigma-Aldrich (St Louis, MO). Negative control fish received clean food containing cod liver oil vehicle with no BDE-209. Fish were exposed to BDE-209 and control treatments daily for 28 days followed by a 14-day depuration in which fish received clean food containing no test chemical. Fish were euthanized using MS-222 on days 0, 14, 28, and 42 (8–12 fish sampled/tank-sample day). Whole livers, brains, gonads, and plasma were dissected from all fish and preserved at −80°C for further testing. Fish carcasses were also preserved at −80°C for PBDE analysis. The sampling and tissue pooling regimen is summarized in the Supporting Information (SI; Table S1) as are the water quality conditions maintained during the study.
PBDE Extractions/Analysis
One fish carcass (dissected of visceral mass, brain, gonad, and plasma) was randomly selected for PBDE analysis across each BDE-209 treatment and control group replicate (n=3) on each sampling day. Food (BDE-209 amended and control) and fish carcasses were analyzed for a suite of 32 PBDE congeners using gas chromatography mass spectrometry operated in electron capture negative ionization mode (GC/ECNI-MS). The PBDE analytical methods are summarized in the Supporting Information and have been described previously25, 26. In addition, the Supporting Information (Table S2) reports levels of PBDEs in the BDE-209 amended and control diets.
Plasma T4 and T3 Measurements
Circulating total T4 and T3 (TT4 and TT3, respectively) were measured using a newly developed extraction method27 and liquid chromatography tandem mass spectrometry (LC/MS/MS)28. Blood was drawn from the caudal vein of euthanized fish using heparin-coated 75 mm capillary tubes, and centrifuged at 3,000 xG for five min to isolate plasma fractions. Plasma was pooled from fish (n=3; 8–12 fish/replicate). Isotopically labeled hormones, 13C12-T4 and 13C6-T3 (50 μl; 10 ng/ml; Cambridge Isotope Laboratories, Andover, MA; Accustandard, New Haven, CT), were used as internal standards to quantify levels of TT4 and TT3, respectively. Blank controls (deionized water) were extracted alongside samples and were used to correct for trace levels (~0.5%) of unlabeled hormones present as commercial impurities in the labeled standards. Method detection limits (MDLs) and intra-/inter-assay %CVs are provided in the Supporting Information.
Deiodinase Activity Assays
Brain and liver microsomes were prepared using previously published methods25, 29 by pooling tissues from six fish per replicate (n=3; six organs/replicate). Microsomes (one mg protein) were incubated with 0.64 μM of T4, and formation rates of T3, rT3, and 3,3'-diiodothyronine (T2) catalyzed by Dio enzymes were measured by LC/MS/MS using our previously published methods30. All incubations contained 900 μl of 0.1 M potassium phosphate buffer (pH 7.4), 10 mM of dithiothreitol (DTT; Sigma-Aldrich), and 100 μl of the appropriate microsomal fraction diluted to 10 mg/ml. Incubations were undertaken for 1.5 h in a water bath at 25°C. Negative controls consisted of microsomes incubated with no T4. Labeled internal standards 13C12-T4, 13C6-rT3, 13C6-T3, 13C6-3,3'-T2 (100 μl; 250 ng/ml) were added to each sample to quantify levels of T4, rT3, T3, and 3,3'-T2, respectively. Concentrations of thyroid hormones were normalized to time and protein concentration to determine deiodination rates. Blank controls containing buffer alone were used to correct for trace levels (~0.5%) of unlabeled hormones present as commercial impurities in the internal standards. MDLs are provided in the Supporting Information.
Quantitative Real-Time Reverse-Transcribed PCR
Genes encoding the following proteins were targeted for quantitative real-time PCR analysis of brain and liver tissues at each sampling day (n=6; mean ± SE): Dio enzymes [dio1 (GenBank accession no. KF042854), dio2 (KF042855), dio3 (KF042856)]; thyroid hormone receptors [trα (DQ074645); trβ (AY533142)]; MCTs [mct8 (KF053157), mct10 (KF053158)], and OATPs [oatp1c1 (KF053149), oatp1f1 (KF053150), oatp1f2 (KF053151), oatp2a1 (KF053152), oatp2b1 (KF053153), oatp3a1 (KF053154), oatp4a1 (KF053155), oatp5a1 (KF053156)]. Total RNA was extracted from livers and brains of treated and control fish from each sampling day and reverse transcribed to cDNAs using methods summarized in the Supporting Information. Primers and hydrolysis (Taqman) probes were designed to partial cDNA encoding each targeted gene and three reference genes (beta-actin form 1, β-actin-1; ribosomal protein l8, rpl8; and elongation factor1α, ef1α) (SI, Tables S5–S6). A standard curve of serially diluted total RNA (range: 0.049–75.0 ng/μl) from samples representing all treatments and sample days was assayed in triplicate, while half of samples were assayed in duplicate or individually. DNA contamination was assessed for each gene by analyzing samples that were not reverse-transcribed; no amplification was observed. B-actin-1 and rpl8 were selected as reference genes in the liver and brain, respectively, as neither BDE-209 nor PTU affected their expression. Correlation coefficients (R2) for standard curves of each gene ranged from 0.98 to 1.00. PCR efficiencies are provided in the Supporting Information (Tables S5–S6) and were calculated using the equation: efficiency = [10(−1/slope)–1]31. Relative levels of mRNA were calculated for each gene using standard curves and were expressed relative to mRNA levels of the reference gene32. Data are presented as values normalized to the negative control at each sample day.
Gonado-somatic index (GSI)
GSI values for a given replicate tank were derived using published methods33 and by taking the average GSI of 8–12 fish per replicate on each sampling day.
Statistical Analyses
For the plasma thyroid hormone analysis, the average measurement from three separate extractions was used at each replicate across treatment and sampling day. Differences in circulating thyroid hormone levels were analyzed for statistical significance within sampling day using a one-way ANOVA and Tukey's test (Graphpad Prism 6.0, La Jolla, CA). For Dio activity, differences in thyroid hormone formation rates in T4-incubated microsomes were analyzed within sampling day with a one-way ANOVA and Tukey's test. Changes in gene expression and GSIs in treated and control fish were also evaluated within sampling day using a one-way ANOVA and Tukey's test. For mortality, survival curves were analyzed using a log-rank test; statistical significance was established using a Bonferroni correction for multiple survival curve comparisons. Statistical significance was defined at the p<0.05 level.
RESULTS
Bioaccumulation/Metabolism
Accumulations of BDE-209 and several metabolites, ranging from penta- to octa-BDEs, were measured in both dose groups (See SI, Table S7). In the low dose, BDE-209 concentrations increased to 1.4±0.5 ng/g bw at sampling day 14 and then remained relatively stable with concentrations measured at 1.1±0.2 ng/g bw (100±14 ng/g lw) at sampling day 28 and 1.0±0.2 ng/g bw after the 14 day depuration. In high dose fish, BDE-209 concentrations increased from 6.1±1.0 ng/g bw at day 14 to 10±5.4 ng/g bw (2700±1200 ng/g lw) at day 28 after which levels decreased to 3.1±1.0 ng/g bw over the depuration period. BDE-154 (2,2',4,4',5,6'-hexaBDE) was the debrominated metabolite detected at the highest concentration after 28 days in both dose groups (1.5±0.1 ng/g bw and 51±7.3 ng/g bw in the low and high dose, respectively), and BDE-101 (2,2',4,5,5'-pentaBDE) was the lowest molecular weight congener detected (<0.5 ng/g bw and 6.3±0.9 ng/g bw in the low and high dose, respectively).
Plasma Thyroid Hormones
BDE-209 reduced circulating TT4 and TT3 at both doses tested over the 28-day exposure (Figure 1). By day 14, TT3 and TT4 concentrations in the low dose group were significantly reduced by 53±4.1% (1.71±0.26 ng/ml; p<0.05) and 57±6.2% (1.41±0.35 ng/ml; p<0.01), respectively, compared to negative controls (TT3=3.67±0.77 ng/ml; TT4=3.27±0.41 ng/ml). At day 28, TT3 and TT4 levels in low dose fish continued to be significantly depressed by 46±3.7% (1.62±0.19 ng/ml; p<0.05) and 53±3.6% (1.77±0.23 ng/ml; p<0.01), respectively, compared to negative controls (TT3=2.98±0.25 ng/ml; TT4=3.73±0.35 ng/ml). Over the 14-day depuration, circulating levels of thyroid hormones in low dose fish remained depressed with TT3 reduced 46±3.7% (1.62±0.19 ng/ml; p<0.05) and TT4 reduced 52±2.8% (1.42±0.14 ng/ml; p<0.01).
Figure 1.
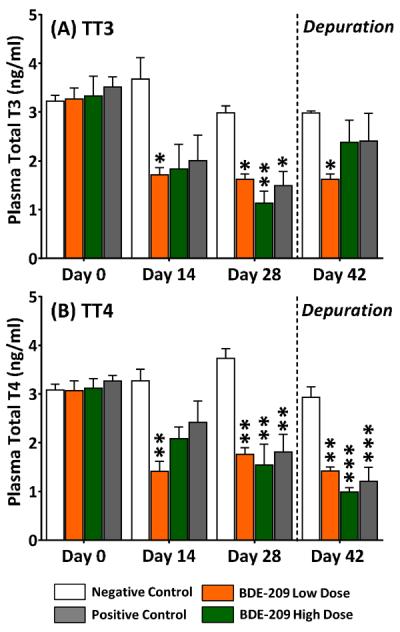
Plasma levels of (A) total T3 and (B) total T4 in adult male fathead minnows exposed to a BDE-209 low dose (95 ± 0.4 ng/g food) and high dose (10 ± 0.1 μg/g food) at 3% bw/day for 28 days followed by a 14-day depuration (n=3; mean ± SE; 8–12 fish/replicate). The model anti-thyroid agent 6-propyl-2-thiouricil (PTU) was used as a positive control (0.5 mg/g food). Data analyzed within sampling day with a one-way ANOVA and Tukey's test with statistical significance measured at the *p<0.05, **p<0.01, ***p<0.005.
At the high dose, significant (p<0.01) declines in plasma TT3 (62±8.2%; 1.13±0.43 ng/ml) and TT4 (59±11%; 1.55±0.73 ng/ml) were measured after the 28-day exposure relative to negative controls (TT3=2.98±0.25 ng/ml; TT4=3.73±0.35 ng/ml). Over the 14-day depuration, TT3 levels recovered at the high dose, but further reductions in TT4 of 66±3.0% (0.99±0.15 ng/ml) were measured. In the PTU positive control, significant (p<0.005) deficits in TT3 (50±10%; 1.49±0.50 ng/ml) and TT4 (52±10%; 1.81±0.63) were measured at sampling day 28 relative to negative controls. After the 14-day depuration, circulating TT3 levels returned to normal among PTU-treated fish but TT4 continued to be reduced by 59±10% (1.21±0.49 ng/ml).
Deiodinase Activity/mRNA Levels
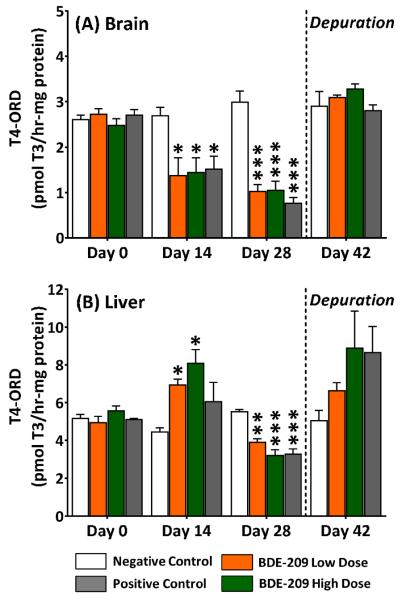
By sampling day 14, the rate of T4-ORD in the brain (Figure 2A) was reduced by 49±15% (1.37±0.39 pmol T3/hr-mg protein), 46±12% (1.44±0.32 pmol T3/hr-mg protein), and 44±11% (1.51±0.29 pmol T3/hr-mg protein) in BDE-209 low dose, high dose, and positive control fish, respectively, compared to negative controls (2.69±0.19 pmol T3/hr-mg protein). By day 28, T4-ORD in the brain had declined further by 65±6.9% and 66±5.0% (p<0.005) at the low dose (1.03±0.26 pmol T3/hr-mg protein) and high dose (1.04±0.36 pmol T3/hr-mg protein), respectively, compared to negative controls (2.99±0.43 pmol T3/hr-mg protein). T4-ORD was also substantially depressed by 74±4.3% (0.76±0.22 pmol T3/hr-mg protein; p<0.005) in the PTU positive control at day 28. After the depuration, T4-ORD in brains of BDE-209 and PTU exposed fish returned to negative control levels.
Figure 2.
T4-outer ring deiodination in (A) brains and (B) livers of adult male fathead minnows exposed to a BDE-209 low dose (95 ± 0.4 ng/g food) and high dose (10 ± 0.1 μg/g food) at 3% bw/day for 28 days followed by a 14-day depuration (n=3; mean ± SE; 6 organs/replicate). The model anti-thyroid agent 6-propyl-2-thiouricil (PTU) was used as a positive control (0.5 mg/g food). Data analyzed within sampling day with a one-way ANOVA and Tukey's test with statistical significance measured at the *p<0.05, **p<0.01, ***p<0.005. Note difference in y-axis scales.
In liver microsomes at sampling day 14 (Figure 2B), T4-ORD increased by 56±7.0% (6.93±0.31 pmol T3/hr-mg protein; p<0.05) at the low dose and by 81±16% (8.08±0.73 pmol T3/hr-mg protein; p<0.05) at the high dose compared to negative controls (4.46±0.22 pmol T3/hr-mg protein). In a reversal, at day 28, the rate of T4-ORD in the liver significantly declined by 29±3.3% (3.90±0.32 pmol T3/hr-mg protein; p<0.01) at the low dose and by 42±5.6% (3.20±0.53 pmol T3/hr-mg protein; p<0.005) at the high dose relative to negative controls (5.52±0.43 pmol T3/hr-mg protein). Similar to the BDE-209 high dose, rates of T4-ORD in PTU positive control fish also declined by 41±5.1% (3.27±0.49 pmol T3/hr-mg protein; p<0.005) at day 28. After the depuration, liver T4-ORD in treated animals was not significantly elevated from negative controls. No significant changes in T4-inner ring deiodination (IRD) and 3,3'-T2 production (T3-IRD/rT3-ORD) were detected.
At day 14 in BDE-209 high dose fish (Figure 3), relative dio2 mRNA levels were significantly elevated 12 times in the brain and 4.1 times in the liver compared to mRNA levels in negative controls (p<0.05). In the low dose at day 14, relative dio2 mRNA levels were significantly (p<0.01) elevated 5.3 times in the liver and 4.9 times in the brain compared to negative controls. By day 28, dio2 transcript levels in treated fish had returned to negative control levels, but relative dio1 transcripts levels in the brain were significantly (p<0.05) increased 4.3 times (high dose) and 3.8 times (low dose) that of negative controls. In addition, after the depuration, relative dio1 and dio2 transcripts were significantly (p<0.05) increased 2.9 times that of negative controls in livers of BDE-209 low dose fish. PTU had no effect on dio transcription, and no significant changes in dio3 mRNA levels were detected (SI, Figure S7).
Figure 3.
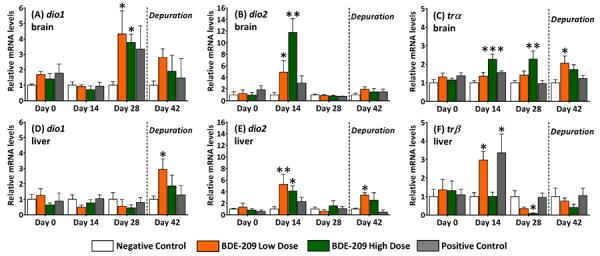
Relative mRNA expression of deiodinases (dio1, dio2) and thyroid receptors (trα, trβ) in brains and livers of adult male fathead minnows exposed to a BDE-209 low dose (95 ± 0.4 ng/g food) and high dose (10 ± 0.1 μg/g food) at 3% bw/day for 28 days followed by a 14-day depuration (n=6; mean ± SE). The model anti-thyroid agent 6-propyl-2-thiouricil (PTU) was used as a positive control (0.5 mg/g food). Statistical significance evaluated within sampling day with one-way ANOVA and Tukey's test (*p<0.05, **p<0.01, ***p<0.005).
Thyroid Hormone Receptor mRNA Expression
Relative brain trα mRNA levels were 2.3 times greater in BDE-209 high dose fish relative to negative controls on day 14 (p<0.005) and day 28 (p<0.01) of the exposure (Figure 3C). Moreover, BDE-209 caused a significant (p<0.05) increase in relative trα mRNA abundance in brains of low dose fish at the depuration. Transcription of trβ in the brain was not affected by BDE-209 (SI, Figure S7). However, in livers of the BDE-209 low dose and PTU positive control, trβ transcripts significantly (p<0.05) increased to three times that of negative controls (Figure 3F). Transcripts for trβ also significantly (p<0.05) declined at sampling day 28 in livers of BDE-209 high dose fish.
Membrane-Bound Transporter mRNA Expression
At sampling day 14, relative brain mct8 mRNA levels were about three times higher (p<0.001) in the BDE-209 high dose and twice as high (p<0.05) in the BDE-209 low dose and PTU fish than in negative controls (Figure 4A, 4B). Brain mct8 transcription returned to negative control levels at sampling day 28, but was again significantly (p<0.01) elevated about twice that of negative controls after the depuration. Relative liver mct8 transcript levels were also significantly (p<0.05) elevated in BDE-209 low dose fish at sampling day 14. No significant changes in mct10 transcription were observed (SI, Figure S7). Of the oatp isoforms tested, only oatp1c1 transcription (liver only; Figure 4C, 4D) and oatp2a1 (brain and liver; SI, Figure S8) were significantly affected by BDE-209 exposures. Relative levels of oatp1c1 increased (p<0.05) about seven times that of negative controls in livers of both BDE-209 low and high dose fish. In addition, relative oatp1c1 mRNA levels in PTU treated fish were elevated 12 times that of the negative controls. Abundances for all other oatp transcripts tested are provided in the Supporting Information (Figure S8).
Figure 4.
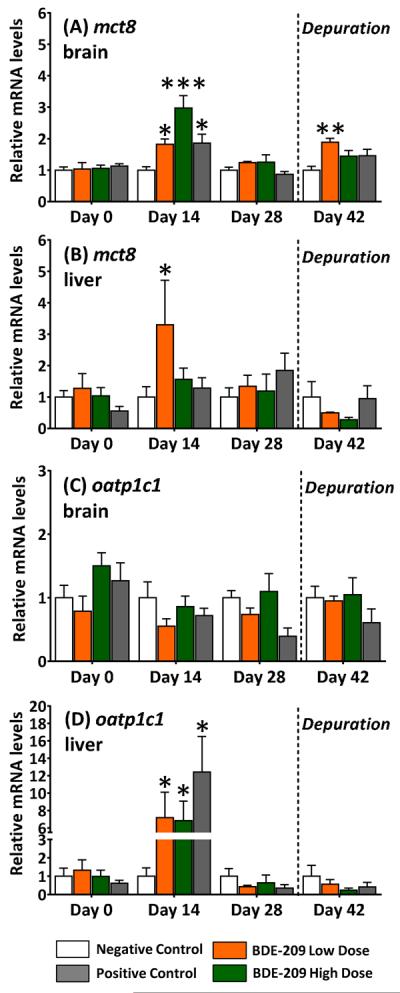
Relative mRNA expression of monocarboxylate transporter (mct8) and organic anion transport protein (oatp1c1) in brains and livers of adult male fathead minnows exposed to a BDE-209 low dose (95 ± 0.4 ng/g food) and high dose (10 ± 0.1 μg/g food) at 3% bw/day for 28 days followed by a 14-day depuration (n=6; mean ± SE). The model anti-thyroid agent 6-propyl-2-thiouricil (PTU) was used as a positive control (0.5 mg/g food). Statistical significance evaluated within sampling day with a one-way ANOVA and Tukey's test (*p<0.05, **p<0.01, ***p<0.005).
Mortality
A statistically significant increase in percent cumulative mortality was measured among both BDE 209 doses. Specifically, 13±3.1% and 12±2.9% of fish from the high and low BDE-209 treatments, respectively, died by the conclusion of the study. We observed <1% mortality in negative controls. Mortality in the PTU positive control group was increased (5.4±2.2%) but was not statistically significant. No significant changes in fish mass, fork length, or condition factor (i.e., fish mass (g)/fork length (cm) x 100) were measured.
Gonado-Somatic Index
Significant declines in the GSI were measured in adult male minnows exposed to BDE-209 at all sampling time points after day 0, including after the depuration (Figure 5). At day 14, the GSI declined 42±13% (p<0.01) at the low dose and 25±9.3% (p<0.05) at the BDE-209 high dose, relative to negative controls. This significantly reduced GSI continued through the end of the 28-day exposure (decline of 23±4.9% at low dose; 31±3.8% at high dose) and extended through the depuration period (decline of 28±15% at low dose; 33±7.0% at high dose). PTU had no effect on the GSI.
Figure 5.
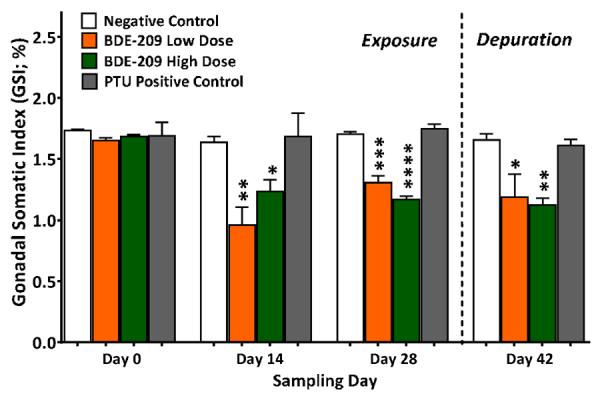
Gonado-somatic index (GSI) measured in adult male fathead exposed to a BDE-209 low dose (95 ± 0.4 ng/g food) and high dose (10 ± 0.1 μg/g food) at 3% bw/day for 28 days followed by a 14-day depuration minnows (n=3; mean ± SE; 8–12 fish/replicate). The model anti-thyroid agent 6-propyl-2-thiouricil (PTU) was used as a positive control (0.5 mg/g food). Statistical significance evaluated with a one-way ANOVA and Tukey's test within sampling day (*p<0.01, **p<0.01, ***p<0.005).
DISCUSSION
Bioaccumulation/Metabolism
The BDE-209 bioaccumulation measured here at the low dose (100±14 ng/g lw) and high dose (2700±1200 ng/g lw) is consistent with levels measured in human serum1–3, 34, 35, wild fish, and other wild species4–6, 9, 36 (SI, Table S8). Penta- to octaBDE debrominated metabolites measured in adult minnows were identical to metabolites detected in juvenile fathead minnows exposed to BDE-209 25. Based on the metabolites detected, a pathway of sequential reductive debromination can be proposed (SI, Figure S2) that supports our previous observations of preferential debromination by cleavage of bromine from meta-substituted positions14, 25, 37.
Reduced Plasma Thyroid Hormones
The significant deficits in circulating thyroid hormones after the 28-day exposure were consistent with reductions induced by the PTU positive control. PTU is a model anti-thyroid drug that acts primarily at the central hypothalamic-pituitary-thyroid (HPT) axis to reduce T4 by inhibiting thyroid peroxidase iodination of tyrosine residues in thyroglobulin in thyroid follicles. While this is the first study to examine BDE-209 effects on circulating thyroid hormone levels in adult fish, data here are consistent with studies showing that PBDEs, including BDE-209, can elicit reductions in plasma thyroid hormone levels in other species at various life-stages23, 38–41.
Altered Deiodinase Activity and mRNA Levels
The highly altered brain T4-ORD in adult minnows suggests that thyroid regulation in the brain may be particularly sensitive to BDE-209. T4-ORD reductions in brain microsomes of adult minnows (65±6.9%; Figure 2A) were consistent with declines in T4-ORD (~74%) measured in juvenile fathead minnows receiving equivalent dietary treatments25. In contrast to the brain, an increase in T4-ORD was measured in the liver at day 14. This dichotomy suggests tissue-specific differences in regulatory responses to systemic thyroid hormone reductions caused by BDE-209. The elevated T4-ORD in the liver may be attributable to a compensatory response of this tissue to reductions in circulating thyroid hormones. Alternatively, reductions in Dio activity in brain microsomes of treated fish may demonstrate the inability of the brain to compensate locally to depressed levels of hormone. By day 28, T4-ORD was significantly reduced in both the brain and liver, suggesting that any compensatory responses were transient.
BDE-209 effects on T4-ORD were consistent with effects measured in the PTU positive control. In addition to mediating thyroid hormone production at the central HPT, PTU acts in mammalian peripheral tissue by inhibiting Dio1 and is therefore an effective compound to delineate relative Dio activity profiles42. However, in fishes, PTU effects on peripheral Dio activity are less clear with some studies suggesting that Dio1 in some species may be resistant to PTU43, 44. Unlike in BDE-209 treated fish, the PTU positive control treatment had no effect on dio1 mRNA expression in the brain or liver, suggesting that its effects on minnows were mediated at the central HPT rather than by inhibiting Dio1, while BDE-209 effects appear to be mediated both centrally and in peripheral tissues.
The transient upregulation of dio1 and dio2 in response to BDE-209 provides evidence of localized responses of peripheral liver and brain tissues to depressed plasma thyroid hormones. In vertebrates, Dio1 and Dio2 catalyze the T4-ORD pathway to produce the genomically active T3 hormone. This study is one of only a few that has targeted PBDE-induced changes in relative abundances of dio mRNA transcription. Consistent with our findings, the relative mRNA expression of dio1 and dio2 was increased in zebrafish larvae exposed aqueously to BDE-20945 and the commercial PentaBDE mixture46. An increase in dio2 transcripts was also measured in livers of larval Chinese rare minnow (Gobiocypris rarus) exposed aqueously to BDE-209, although in contrast to our results, a decrease in dio2 transcripts was reported in brains of adult rare minnows47. Finally, the elevated dio1 and dio2 mRNA levels in BDE-209 exposed minnows were consistent with studies in which methimazole-induced hypothyroidism increased, while exogenous thyroid hormone decreased, relative dio1 and dio2 mRNA levels in livers and brains of fishes48–51. Thus, transcriptional regulation of Dios appears to be an important compensatory pathway in teleost responses to BDE-209 and pharmacologically-induced hypothyroidism.
Compensatory Responses to BDE-209
The functional and biochemical properties of Dios can provide insights into measured differences in apparent compensatory responses of upregulated dio mRNA expression in BDE-209 exposed minnows. In particular, Dio2 has demonstrated substantial physiological plasticity in vertebrates, making it a sensitive regulator of T4-ORD and intracellular T3 homeostasis. It has been shown to be highly sensitive to thyroid hormone with a short half-life in mammals of ~40 min52. The early upregulation of dio2 mRNA expression measured at day 14 in livers and brains of BDE-209 treated fish may be attributable to the rapid homeostatic behavior of Dio2 in response to depressed plasma thyroid hormones. Notably, T4-ORD activity was not increased in brains of BDE-209 treated fish, suggesting that this transcriptional response did not translate to a detectable increase in Dio activity in the adult male minnow brain, although protein levels were not measured.
The absence of a response of brain Dio activity may in part be related to tissue differences in Dio expression. Absolute levels of dio2 transcript were six times lower in the brain than liver of adult minnows, suggesting that Dio2 activity may likewise be low. Early studies have raised questions about whether dio2 is expressed in brains of piscivores as only negligible T4-ORD activity has been measured in the fish brain53, 54. In accordance with our results, more recent studies using quantitative PCR techniques have localized dio2 transcripts to the fish brain49, 55, 56. Limited evidence also suggests that the transcriptional response of dio2 in the brain may be more sensitive to systemic thyroid hormone changes than dio2 in the liver49. Although not known at this time, dio2 transcriptional responses to BDE-209 may vary with tissue type and could be linked to divergent functional roles of Dio2 in these tissues or to differences in sensitivity to T3.
It is also possible that compensation to prolonged thyroid hormone depression might vary or be more efficient in some tissues than others. The upregulation of dio1 transcription in the minnow brain at day 28 appears indicative of such regulatory variation under longer periods of hypothyroidism. For instance, no change in relative dio1 mRNA abundance was observed in brains or livers of parrotfish subjected to experimentally elevated T3 or depressed T4 (by methimazole) for three days49. In contrast, dio1 mRNA transcripts became elevated in two species of tilapia after a 90-day methimazole treatment50. Irrespective of the mechanism, compensatory responses of enzymes involved in peripheral thyroid hormone metabolism appear to change over time as BDE-209 exposures continue chronically.
Thyroid Receptors
BDE-209 affected the relative expression of tr transcripts in a tissue-specific manner (Figure 3C, 3F). Two genetically distinct nuclear receptors TRα and TRβ have been identified in fathead minnows with additional subtypes characterized in teleosts57–59. Similar to results here, BDE-209 increased trα and trβ mRNA expression in zebrafish larvae45. Aqueous exposures to the commercial PentaBDE mixture, in contrast, had no effect on relative tr mRNA abundance in zebrafish larvae46. However, thyroid receptor expressional regulation by both thyroid hormones and endocrine disrupting chemicals can vary with fish life-stage57–60. The only other study to date that has examined PBDE impacts on tr gene expression in adult fish was conducted with BDE-4723. In this study, trα transcripts were elevated significantly (p<0.005) in brains of female, but not male, fathead minnows exposed to BDE-47, while trβ transcripts were depressed (p<0.05) in brains of both sexes. Thus, in addition to age-related influences, there appear to be congener specific differences in PBDE effects on thyroid receptor expression patterns.
The elevated relative tr mRNA levels measured in BDE-209 and PTU dosed fish also reveals an apparent contradiction with studies in hyperthyroid fish. Specifically, an increase in relative trα and trβ transcript levels has been measured in brains and livers of adult fathead minnows treated with T358. TRs themselves contain thyroid response elements and tr transcription can be auto-induced by T360, 61. Based on the ability of T3 to induce transcription of its own receptors, a decrease in tr mRNA expression might be predicted in BDE-209 and PTU treated fish given the hypothyroidism observed. However, thyroid hormone regulation of TRs appears to be condition dependent. Both hypothyroidism (by thyroidectomy) and hyperthyroidism (T3-induced) have been shown to increase TR expression in the adult rat brain62. Other evidence suggests that tr transcription can vary within the same tissue63 and over time as observed in rat brain cell cultures dosed with BDE-9964. Thus, our findings of BDE-209 induced elevations in trα mRNA abundance in the adult minnow brain at day 14 with altered trβ mRNA transcription in the liver might indicate alternative mechanisms of peripheral responses to BDE-209 (and PTU) that have yet to be fully described.
Membrane Bound Transporters
This study is the first to detect impacts of PBDEs on the expression of plasma membrane transporters of thyroid hormones in fish. In both fish and mammals, mct8 has been structurally and functionally characterized as a specific and active transporter of T4 and T365, 66. OATPs mediate the cellular uptake of a range of amphipathic organic molecules, including thyroid hormones and xenobiotics. In humans, OATP1C1 has been found to have relative narrow substrate specificity with a high affinity for transporting T4 (Km = 90 nM) and rT3 (Km = 130 nM)67. In fishes, however, the diversity of OATPs has only recently been explored in zebrafish68 and fathead minnows69. The observed increases in mct8 and oatp1c1 transcript levels in BDE-209 exposed minnows may be characteristic of an upregulation in the expression of these transporters to compensate for the reduced availability of circulating thyroid hormones. This idea is supported by recent findings that T3-treated (hyperthyroid) adult male fathead minnows exhibited reduced mct8 and oatp1c1 mRNA levels in the brain and liver69. Neither BDE-209 nor PTU increased brain oatp1c1 mRNA transcripts, despite the substantial hypothyroid status of these fish and the known localization of oatp1c1 to the fathead minnow brain, ultimately raising further questions about the capacity of the fish brain to maintain homeostasis in cellular thyroid hormone levels under conditions of BDE-209 induced hypothyroidism.
Mechanisms of Thyroid Disruption
Biological effects of BDE-209 on thyroid hormone signaling in the adult minnow proceeded through multiple pathways that involved: declines in circulating thyroid hormones; disrupted T4-ORD in peripheral brain and liver tissues; and altered transcription of genes involved in thyroid hormone production, transport, and genomic signaling. Fish exposed to BDE-209 at both the low and high dose experienced profound deficits in plasma T4 and T3 as well as reduced T4-ORD activity in the liver and brain after a 28-day exposure. While T4-ORD activity recovered after the depuration, circulating T4 (both doses) and T3 (low dose only) remained depressed for at least 14 days after the BDE-209 exposure ceased. Brains of adult minnows appeared particularly sensitive to BDE-209 based on the severely reduced T4-ORD measured in these tissues after 28 days of BDE-209 exposure. Several genes encoding proteins with key functions in thyroid signaling, including dio1, dio2, trα, trβ, mct8, and oatp1c1 showed increased expression in BDE-209 exposed fish, although these increases appeared to be transient, compensatory responses to BDE-209 induced hypothyroidism.
Mortality
The increased mortality in this study is not a commonly evaluated endpoint for PBDEs and has not been observed in previous in vivo fish studies conducted in our laboratory25, 70. However, consistent with our results, a significant increase in mortality (~44%) was measured in adult zebrafish exposed to 1-μM concentrations of BDE-209 for five months, although mortality was also elevated in negative controls (~38%)71. Fathead minnow adult males exposed to BDE-47 by the diet have likewise shown reduced body condition factors and erratic swimming behaviors, although declines in survival were not reported24. No significant changes in body condition were detected in the present study, suggesting that body wasting was not occurring. It is notable, however, that minnows exposed to both doses of BDE-209 displayed greater frequencies of aggressive/territorial behaviors (e.g., fighting, chasing, head-butting) than did negative control and PTU-exposed fish. Thus, while unanticipated, it is possible that BDE-209 mediated shifts in behavior that could have indirectly contributed to increased mortality by increasing physiological stress from social interactions.
Reduced GSI
Few studies to date have evaluated BDE-209 effects on fish reproduction. Consistent with results here, BDE-209 studies in zebrafish have reported altered expression of spermatogenesis genes47 and decreased GSIs with reduced sperm counts71. Adult fathead minnows exposed orally to BDE-47 have also been shown to have decreased mature spermatozoa23, 24 and reduced spawning due to male infertility24. Studies in young laboratory rodents have also shown that PBDEs can elicit anti-androgenic effects that impair reproductive development72–74. It is notable that in the current study PTU had no effect on the GSI even though thyroid hormones have been shown to influence reproductive functioning75–77. This difference suggests that BDE-209 may be impacting adult male reproduction by non-thyroidal mechanisms of action.
Low Dose Effects
The BDE-209 low dose (~3 ng/g bw-day) elicited impacts on thyroid signaling and reductions in the GSI at similar levels to the high dose (~300 ng/g bw-day). Doses tested in this study were generally less than those administered in rodent studies conducted to date with BDE-20938–40. Further study is needed to determine whether non-monotonic dose-responses are occurring in fish exposed to BDE-209 as have been detected with other endocrine disruptors78. In addition, identification of the congeners driving the thyroid disruption and reduced GSI (i.e., parent BDE-209 and/or its metabolites) was beyond the scope of this study but merits further investigation. The thyroid system is well-conserved across vertebrate taxa, and our findings that BDE-209 exposure at low doses can impact thyroid hormone homeostasis and signaling at several levels point to a need to further evaluate the potential for BDE-209 induced thyroid dysfunction in humans and wildlife.
Supplementary Material
Acknowledgements
This study was supported by a National Institute of Environmental Health Sciences research grant (R01-ES016099) and U.S. EPA STAR graduate fellowship (FP-917145010). Findings and conclusions in this article are those of the authors and do not necessarily represent the views of the NIEHS or EPA.
Footnotes
Author Contributions
The manuscript was written through contributions of all authors. All authors have given approval to the final version of the manuscript.
The authors declare no competing financial interests.
Supporting Information
Descriptions of PBDE analytical methods, isolation/sequencing of partial cDNAs, quantitative PCR methods, primer/hydrolysis probe sequences, PBDE bioaccumulation/metabolism, MDLs and intra-/inter-assay %CVs of plasma thyroid hormone/Dio activity, and relative mRNA transcript levels of dio3, mct10, and oatps. This material is available free of charge via the Internet at http://pubs.acs.org.
REFERENCES
- 1.Bi XH, Thomas GO, Jones KC, Qu WY, Sheng GY, Martin FL, Fu JM. Exposure of electronics dismantling workers to polybrominated diphenyl ethers, polychlorinated biphenyls, and organochlorine pesticides in South China. Environ Sci Technol. 2007;41(16):5647–5653. doi: 10.1021/es070346a. [DOI] [PubMed] [Google Scholar]
- 2.Lunder S, Hovander L, Athanassiadis I, Bergman A. Significantly Higher Polybrominated Diphenyl Ether Levels in Young US Children than in Their Mothers. Environ Sci Technol. 2010;44(13):5256–5262. doi: 10.1021/es1009357. [DOI] [PubMed] [Google Scholar]
- 3.Stapleton HM, Eagle S, Sjodin A, Webster TF. Serum PBDEs in a North Carolina Toddler Cohort: Associations with Handwipes, House Dust, and Socioeconomic Variables. Environ Health Perspect. 2012;120(7):1049–1054. doi: 10.1289/ehp.1104802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.La Guardia MJ, Hale RC, Harvey E. Evidence of debromination of decabromodiphenyl ether (BDE-209) in biota from a wastewater receiving stream. Environ Sci Technol. 2007;41(19):6663–6670. doi: 10.1021/es070728g. [DOI] [PubMed] [Google Scholar]
- 5.Liu YP, Li JG, Zhao YF, Wen S, Huang FF, Wu YN. Polybrominated diphenyl ethers (PBDEs) and indicator polychlorinated biphenyls (PCBs) in marine fish from four areas of China. Chemosphere. 2011;83(2):168–174. doi: 10.1016/j.chemosphere.2010.12.045. [DOI] [PubMed] [Google Scholar]
- 6.Johnson-Restrepo B, Kannan K, Addink R, Adams DH. Polybrominated diphenyl ethers and polychlorinated biphenyls in a marine foodweb of coastal Florida. Environ Sci Technol. 2005;39(21):8243–8250. doi: 10.1021/es051551y. [DOI] [PubMed] [Google Scholar]
- 7.Chen D, Letcher RJ, Burgess NM, Champoux L, Elliott JE, Hebert CE, Martin P, Wayland M, Weseloh DVC, Wilson L. Flame retardants in eggs of four gull species (Laridae) from breeding sites spanning Atlantic to Pacific Canada. Environ Pollut. 2012;168:1–9. doi: 10.1016/j.envpol.2012.03.040. [DOI] [PubMed] [Google Scholar]
- 8.Gauthier LT, Hebert CE, Weseloh DVC, Letcher RJ. Dramatic changes in the temporal trends of polybrominated diphenyl ethers (PBDEs) in herring gull eggs from the Laurentian Great Lakes: 1982–2006. Environ Sci Technol. 2008;42(5):1524–1530. doi: 10.1021/es702382k. [DOI] [PubMed] [Google Scholar]
- 9.Shaw SD, Berger ML, Weijs L, Covaci A. Tissue-specific accumulation of polybrominated diphenyl ethers (PBDEs) including Deca-BDE and hexabromocyclododecanes (HBCDs) in harbor seals from the northwest Atlantic. Environ Int. 2012;44:1–6. doi: 10.1016/j.envint.2012.01.001. [DOI] [PubMed] [Google Scholar]
- 10.Klosterhaus SL, Stapleton HM, La Guardia MJ, Greig DJ. Brominated and chlorinated flame retardants in San Francisco Bay sediments and wildlife. Environ Int. 2012;47:56–65. doi: 10.1016/j.envint.2012.06.005. [DOI] [PubMed] [Google Scholar]
- 11.Wang JX, Lin ZK, Lin KF, Wang CY, Zhang W, Cui CY, Lin JD, Dong QX, Huang CJ. Polybrominated diphenyl ethers in water, sediment, soil, and biological samples from different industrial areas in Zhejiang, China. J Hazard Mater. 2011;197:211–219. doi: 10.1016/j.jhazmat.2011.09.078. [DOI] [PubMed] [Google Scholar]
- 12.Stapleton HM, Dodder NG. Photodegradation of decabromodiphenyl ether in house dust by natural sunlight. Environ Toxicol Chem. 2008;27(2):306–312. doi: 10.1897/07-301R.1. [DOI] [PubMed] [Google Scholar]
- 13.Gerecke AC, Hartmann PC, Heeb NV, Kohler HPE, Giger W, Schmid P, Zennegg M, Kohler M. Anaerobic degradation of decabromodiphenyl ether. Environ Sci Technol. 2005;39(4):1078–1083. doi: 10.1021/es048634j. [DOI] [PubMed] [Google Scholar]
- 14.Stapleton HM, Alaee M, Letcher RJ, Baker JE. Debromination of the flame retardant decabromodiphenyl ether by juvenile carp (Cyprinus carpio) following dietary exposure. Environ Sci Technol. 2004;38(1):112–119. doi: 10.1021/es034746j. [DOI] [PubMed] [Google Scholar]
- 15.Eriksson P, Viberg H, Jakobsson E, Orn U, Fredriksson A. A brominated flame retardant, 2,2 ',4,4 ',5-pentabromodiphenyl ether: Uptake, retention, and induction of neurobehavioral alterations in mice during a critical phase of neonatal brain development. Toxicological Sciences. 2002;67(1):98–103. doi: 10.1093/toxsci/67.1.98. [DOI] [PubMed] [Google Scholar]
- 16.Kuriyama SN, Wanner A, Fidalgo-Neto AA, Talsness CE, Koerner W, Chahoud I. Developmental exposure to low-dose PBDE-99: Tissue distribution and thyroid hormone levels. Toxicology. 2007;242(1–3):80–90. doi: 10.1016/j.tox.2007.09.011. [DOI] [PubMed] [Google Scholar]
- 17.Lema SC, Schultz IR, Scholz NL, Incardona JP, Swanson P. Neural defects and cardiac arrhythmia in fish larvae following embryonic exposure to 2,2 ',4,4 '-tetrabromodiphenyl ether (PBDE 47) Aquat Toxicol. 2007;82(4):296–307. doi: 10.1016/j.aquatox.2007.03.002. [DOI] [PubMed] [Google Scholar]
- 18.Szabo DT, Richardson VM, Ross DG, Diliberto JJ, Kodavanti PRS, Birnbaum LS. Effects of Perinatal PBDE Exposure on Hepatic Phase I, Phase II, Phase III, and Deiodinase 1 Gene Expression Involved in Thyroid Hormone Metabolism in Male Rat Pups. Toxicol Sci. 2009;107(1):27–39. doi: 10.1093/toxsci/kfn230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hale RC, La Guardia MJ, Harvey E, Gaylor MO, Mainor TM. Brominated flame retardant concentrations and trends in abiotic media. Chemosphere. 2006;64(2):181–186. doi: 10.1016/j.chemosphere.2005.12.006. [DOI] [PubMed] [Google Scholar]
- 20.Law RJ, Herzke D. Current levels and trends of brominated flame retardants in the environment. In: Barcelo D, Kostianoy AG, editors. The Handbook of Environmental Chemistry; Brominated Flame Retardants. Vol. 16. Springer Publishing Services; Heidelberg, Germany: 2011. pp. 123–141. [Google Scholar]
- 21.Peng XZ, Tang CM, Yu YY, Tan JH, Huang QX, Wu JP, Chen SJ, Mai BX. Concentrations, transport, fate, and releases of polybrominated diphenyl ethers in sewage treatment plants in the Pearl River Delta, South China. Environ Int. 2009;35(2):303–309. doi: 10.1016/j.envint.2008.07.021. [DOI] [PubMed] [Google Scholar]
- 22.Shaw SD, Kannan K. Polybrominated Diphenyl Ethers in Marine Ecosystems of the American Continents: Foresight from Current Knowledge. Rev Env Health. 2009;24(3):157–229. doi: 10.1515/reveh.2009.24.3.157. [DOI] [PubMed] [Google Scholar]
- 23.Lema SC, Dickey JT, Schultz IR, Swanson P. Dietary Exposure to 2,2 ',4,4 '-Tetrabromodiphenyl Ether (PBDE-47) Alters Thyroid Status and Thyroid Hormone-Regulated Gene Transcription in the Pituitary and Brain. Environ Health Perspect. 2008;116(12):1694–1699. doi: 10.1289/ehp.11570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Muirhead EK, Skillman D, Hook SE, Schultz IR. Oral exposure of PBDE-47 in fish: Toxicokinetics and reproductive effects in Japanese medaka (Oryzias latipes) and fathead minnows (Pimephales promelas) Environ Sci Technol. 2006;40(2):523–528. doi: 10.1021/es0513178. [DOI] [PubMed] [Google Scholar]
- 25.Noyes PD, Hinton DE, Stapleton HM. Accumulation and Debromination of Decabromodiphenyl Ether (BDE-209) in Juvenile Fathead Minnows (Pimephales promelas) Induces Thyroid Disruption and Liver Alterations. Toxicol Sci. 2011;122(2):265–274. doi: 10.1093/toxsci/kfr105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Stapleton HM, Kelly SM, Allen JG, McClean MD, Webster TF. Measurement of polybrominated diphenyl ethers on hand wipes: Estimating exposure from hand-to-mouth contact. Environ Sci Technol. 2008;42(9):3329–3334. doi: 10.1021/es7029625. [DOI] [PubMed] [Google Scholar]
- 27.Noyes PD. Polybrominated Diphenyl Ether (PBDE) Flame Retardants: Accumulation, Metabolism, and Disrupted Thyroid Regulation in Early and Adult Life Stages of Fish. Duke University; Durham: 2013. [Google Scholar]
- 28.Wang DL, Stapleton HM. Analysis of thyroid hormones in serum by liquid chromatography-tandem mass spectrometry. Anal. Bioanal. Chem. 2010;397(5):1831–1839. doi: 10.1007/s00216-010-3705-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Noyes PD, Kelly SM, Mitchelmore CL, Stapleton HM. Characterizing the in vitro hepatic biotransformation of the flame retardant BDE 99 by common carp. Aquat Toxicol. 2010;97(2):142–150. doi: 10.1016/j.aquatox.2009.12.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Butt CM, Wang DL, Stapleton HM. Halogenated Phenolic Contaminants Inhibit the In Vitro Activity of the Thyroid-Regulating Deiodinases in Human Liver. Toxicol Sci. 2011;124(2):339–347. doi: 10.1093/toxsci/kfr117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Rasmussen R. Quantification on the LightCycler. In: Meuer S WC, Nakagawara K, editors. Rapid cycle real-time PCR, methods and applications. Springer Press; Heidelberg: 2000. pp. 21–34. [Google Scholar]
- 32.Pfaffl MW. A new mathematical model for relative quantification in real-time RT-PCR. Nucleic Acids Res. 2001;29(9) doi: 10.1093/nar/29.9.e45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Ankley GT, Jensen KM, Kahl MD, Korte JJ, Makynen EA. Description and evaluation of a short-term reproduction test with the fathead minnow (Pimephales promelas) Environ Toxicol Chem. 2001;20(6):1276–1290. [PubMed] [Google Scholar]
- 34.Fischer D, Hooper K, Athanasiadou M, Athanassiadis I, Bergman A. Children show highest levels of polybrominated diphenyl ethers in a California family of four: A case study. Environ Health Perspect. 2006;114(10):1581–1584. doi: 10.1289/ehp.8554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Qu WY, Bi XH, Sheng GY, Lu SY, Fu H, Yuan J, Li LP. Exposure to polybrominated diphenyl ethers among workers at an electronic waste dismantling region in Guangdong, China. Environ Int. 2007;33(8):1029–1034. doi: 10.1016/j.envint.2007.05.009. [DOI] [PubMed] [Google Scholar]
- 36.Chen D, Mai BX, Song J, Sun QH, Luo Y, Luo XJ, Zeng EY, Hale RC. Polybrominated diphenyl ethers in birds of prey from Northern China. Environ Sci Technol. 2007;41(6):1828–1833. doi: 10.1021/es062045r. [DOI] [PubMed] [Google Scholar]
- 37.Roberts S, Noyes PD, Gallagher EP, Stapleton HM. Species-Specific Differences and Structure-Activity Relationships in the Debromination of PBDE Congeners in Three Fish Species. Environ. Sci. Technol. 2011;45(5):1999–2005. doi: 10.1021/es103934x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Fujimoto H, Woo GH, Inoue K, Takahashi M, Hirose M, Nishikawa A, Shibutani M. Impaired oligodendroglial development by decabromodiphenyl ether in rat offspring after maternal exposure from mid-gestation through lactation. Reprod. Toxicol. 2011;31(1):86–94. doi: 10.1016/j.reprotox.2010.09.003. [DOI] [PubMed] [Google Scholar]
- 39.Rice DC, Reeve EA, Herlihy A, Zoeller RT, Thompson WD, Markowski VP. Developmental delays and locomotor activity in the C57BL6/J mouse following neonatal exposure to the fully-brominated PBDE, decabromodiphenyl ether. Neurotoxicol Teratol. 2007;29(4):511–520. doi: 10.1016/j.ntt.2007.03.061. [DOI] [PubMed] [Google Scholar]
- 40.Tseng LH, Li MH, Tsai SS, Lee CW, Pan MH, Yao WJ, Hsu PC. Developmental exposure to decabromodiphenyl ether (PBDE 209): Effects on thyroid hormone and hepatic enzyme activity in male mouse offspring. Chemosphere. 2008;70(4):640–647. doi: 10.1016/j.chemosphere.2007.06.078. [DOI] [PubMed] [Google Scholar]
- 41.Tomy GT, Palace VP, Halldorson T, Braekevelt E, Danell R, Wautier K, Evans B, Brinkworth L, Fisk AT. Bioaccumulation, biotransformation, and biochemical effects of brominated diphenyl ethers in juvenile lake trout (Salvelinus namaycush) Environ Sci Technol. 2004;38(5):1496–1504. doi: 10.1021/es035070v. [DOI] [PubMed] [Google Scholar]
- 42.Visser TJ, Kaplan MM, Leonard JL, Larsen PR. Evidence for 2 pathways of iodothyronine 5'-deiodination in rat pituitary that differ in kinetics, propylthiouracil sensitivity, and response to hypothyroidism. J Clin Invest. 1983;71(4):992–1002. doi: 10.1172/JCI110854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Orozco A, Linser P, Valverde-R C. Kinetic characterization of outer-ring deiodinase activity (ORD) in the liver, gill and retina of the killifish Fundulus heteroclitus. Comp Biochem Phys B. 2000;126(3):283–290. doi: 10.1016/s0305-0491(00)00186-3. [DOI] [PubMed] [Google Scholar]
- 44.Sanders JP, VanderGeyten S, Kaptein E, Darras VM, Kuhn ER, Leonard JL, Visser TJ. Characterization of a propylthiouracil-insensitive type I iodothyronine deiodinase. Endocrinology. 1997;138(12):5153–5160. doi: 10.1210/endo.138.12.5581. [DOI] [PubMed] [Google Scholar]
- 45.Chen Q, Yu LQ, Yang LH, Zhou BS. Bioconcentration and metabolism of decabromodiphenyl ether (BDE-209) result in thyroid endocrine disruption in zebrafish larvae. Aquat Toxicol. 2012;110:141–148. doi: 10.1016/j.aquatox.2012.01.008. [DOI] [PubMed] [Google Scholar]
- 46.Yu LQ, Deng J, Shi XJ, Liu CS, Yu K, Zhou BS. Exposure to DE-71 alters thyroid hormone levels and gene transcription in the hypothalamic-pituitary-thyroid axis of zebrafish larvae. Aquat Toxicol. 2010;97(3):226–233. doi: 10.1016/j.aquatox.2009.10.022. [DOI] [PubMed] [Google Scholar]
- 47.Li W, Zhu L, Zha J, Wang Z. Effects of decabromodiphenyl ether (BDE-209) on mRNA transcription of thyroid hormone pathway and spermatogenesis associated genes in Chinese rare minnow (Gobiocypris rarus) Environ Toxicol. 2011 doi: 10.1002/tox.20767. [DOI] [PubMed] [Google Scholar]
- 48.Garcia GC, Jeziorski MC, Valverde-R C, Orozco A. Effects of iodothyronines on the hepatic outer-ring deiodinating pathway in killifish. Gen Comp Endocr. 2004;135(2):201–209. doi: 10.1016/j.ygcen.2003.09.010. [DOI] [PubMed] [Google Scholar]
- 49.Johnson KM, Lema SC. Tissue-specific thyroid hormone regulation of gene transcripts encoding iodothyronine deiodinases and thyroid hormone receptors in striped parrotfish (Scarus iseri) Gen Comp Endocr. 2011;172(3):505–517. doi: 10.1016/j.ygcen.2011.04.022. [DOI] [PubMed] [Google Scholar]
- 50.Van der Geyten S, Toguyeni A, Baroiller JF, Fauconneau B, Fostier A, Sanders JP, Visser TJ, Kuhn ER, Darras VM. Hypothyroidism induces type I iodothyronine deiodinase expression in tilapia liver. Gen Comp Endocr. 2001;124(3):333–342. doi: 10.1006/gcen.2001.7722. [DOI] [PubMed] [Google Scholar]
- 51.Walpita CN, Van der Geyten S, Rurangwa E, Darras VM. The effect of 3,5,3'-triiodothyronine supplementation on zebrafish (Danio rerio) embryonic development and expression of iodothyronine deiodinases and thyroid hormone receptors. Gen Comp Endocr. 2007;152(2–3):206–214. doi: 10.1016/j.ygcen.2007.02.020. [DOI] [PubMed] [Google Scholar]
- 52.St Germain DL. The effects and interactions of substrates, inhibitors, and the cellular thiol-disulfide balance on the regulation of type-II iodothyronine 5'-deiodinase. Endocrinology. 1988;122(5):1860–1868. doi: 10.1210/endo-122-5-1860. [DOI] [PubMed] [Google Scholar]
- 53.Frith SD, Eales JG. Thyroid hormone deiodination pathways in brain and liver of rainbow trout, Oncorhynchus mykiss. Gen Comp Endocr. 1996;101(3):323–332. doi: 10.1006/gcen.1996.0035. [DOI] [PubMed] [Google Scholar]
- 54.Mol KA, Van der Geyten S, Burel C, Kuhn ER, Boujard T, Darras VM. Comparative study of iodothyronine outer ring and inner ring deiodinase activities in five teleostean fishes. Fish Physiol Biochem. 1998;18(3):253–266. [Google Scholar]
- 55.Sutija M, Longhurst TJ, Joss JMP. Deiodinase type II and tissue specific mRNA alternative splicing in the Australian lungfish, Neoceratodus forsteri. Gen Comp Endocr. 2003;132(3):409–417. doi: 10.1016/s0016-6480(03)00115-1. [DOI] [PubMed] [Google Scholar]
- 56.Wambiji N, Park Y-J, Kim S-J, Hur S-P, Takeuchi Y, Takemura A. Expression of type II iodothyronine deiodinase gene in the brain of a tropical spinefoot, Siganus guttatus. Comp Biochem Phys A. 2011;160(4):447–452. doi: 10.1016/j.cbpa.2011.03.023. [DOI] [PubMed] [Google Scholar]
- 57.Filby AL, Tyler CR. Cloning and characterization of cDNAs for hormones and/or receptors of growth hormone, insulin like-growth factor-I, thyroid hormone, and corticosteroid and the gender-, tissue-, and developmental-specific expression of their mRNA transcripts in fathead minnow (Pimephales promelas) Gen Comp Endocr. 2007;150(1):151–163. doi: 10.1016/j.ygcen.2006.07.014. [DOI] [PubMed] [Google Scholar]
- 58.Lema SC, Dickey JT, Schultz IR, Swanson P. Thyroid hormone regulation of mRNAs encoding thyrotropin beta-subunit, glycoprotein alpha-subunit, and thyroid hormone receptors alpha and beta in brain, pituitary gland, liver, and gonads of an adult teleost, Pimephales promelas. J Endocrinol. 2009;202(1):43–54. doi: 10.1677/JOE-08-0472. [DOI] [PubMed] [Google Scholar]
- 59.Nelson ER, Habibi HR. Thyroid receptor subtypes: Structure and function in fish. Gen Comp Endocr. 2009;161(1):90–96. doi: 10.1016/j.ygcen.2008.09.006. [DOI] [PubMed] [Google Scholar]
- 60.Liu YW, Lo LJ, Chan WK. Temporal expression and T3 induction of thyroid hormone receptors alpha 1 and beta 1 during early embryonic and larval development in zebrafish, Danio rerio. Mol Cell Endocr. 2000;159(1–2):187–195. doi: 10.1016/s0303-7207(99)00193-8. [DOI] [PubMed] [Google Scholar]
- 61.Manchado M, Infante C, Rebordinos L, Canavate JP. Molecular characterization, gene expression and transcriptional regulation of thyroid hormone receptors in Senegalese sole. Gen Comp Endocr. 2009;160(2):139–147. doi: 10.1016/j.ygcen.2008.11.001. [DOI] [PubMed] [Google Scholar]
- 62.Hamada S, Yoshimasa Y. Increases in brain nuclear triiodothyronine receptors associated with increased triiodothyronine in hyperthyroid and hypothyroid rats. Endocrinology. 1983;112(1):207–211. doi: 10.1210/endo-112-1-207. [DOI] [PubMed] [Google Scholar]
- 63.Constantinou C, Margarity M, Valcana T. Region-specific effects of hypothyroidism on the relative expression of thyroid hormone receptors in adult rat brain. Mol Cell Biochem. 2005;278(1–2):93–100. doi: 10.1007/s11010-005-6934-z. [DOI] [PubMed] [Google Scholar]
- 64.Blanco J, Mulero M, Lopez M, Domingo JL, Sanchez DJ. BDE-99 deregulates BDNF, Bcl-2 and the mRNA expression of thyroid receptor isoforms in rat cerebellar granular neurons. Toxicology. 2011;290(2–3):305–311. doi: 10.1016/j.tox.2011.10.010. [DOI] [PubMed] [Google Scholar]
- 65.Arjona FJ, de Vrieze E, Visser TJ, Flik G, Klaren PHM. Identification and Functional Characterization of Zebrafish Solute Carrier Slc16a2 (Mct8) as a Thyroid Hormone Membrane Transporter. Endocrinology. 2011;152(12):5065–5073. doi: 10.1210/en.2011-1166. [DOI] [PubMed] [Google Scholar]
- 66.Friesema ECH, Ganguly S, Abdalla A, Fox JEM, Halestrap AP, Visser TJ. Identification of monocarboxylate transporter 8 as a specific thyroid hormone transporter. J Biol Chem. 2003;278(41):40128–40135. doi: 10.1074/jbc.M300909200. [DOI] [PubMed] [Google Scholar]
- 67.Pizzagalli F, Hagenbuch B, Stieger B, Klenk U, Folkers G, Meier PJ. Identification of a novel human organic anion transporting polypeptide as a high affinity thyroxine transporter. Mol Endocrinol. 2002;16(10):2283–2296. doi: 10.1210/me.2001-0309. [DOI] [PubMed] [Google Scholar]
- 68.Popovic M, Zaja R, Smital T. Organic anion transporting polypeptides (OATP) in zebrafish (Danio rerio): Phylogenetic analysis and tissue distribution. Comp Biochem Phys A. 2010;155(3):327–335. doi: 10.1016/j.cbpa.2009.11.011. [DOI] [PubMed] [Google Scholar]
- 69.Muzzio AM, Noyes PD, Stapleton HM, Lema SC. The organic anion transporting protein (OATP) family in a teleost fish model. Integr Comp Biol. 2013;53(Suppl. 1):E340. [Google Scholar]
- 70.Stapleton HM, Brazil B, Holbrook RD, Mitchelmore CL, Benedict R, Konstantinov A, Potter D. In vivo and in vitro debromination of decabromodiphenyl ether (BDE 209) by juvenile rainbow trout and common carp. Environ Sci Technol. 2006;40(15):4653–4658. doi: 10.1021/es060573x. [DOI] [PubMed] [Google Scholar]
- 71.He JH, Yang DR, Wang CY, Liu W, Liao JH, Xu T, Bai CL, Chen JF, Lin KF, Huang CJ, Dong QX. Chronic zebrafish low dose decabrominated diphenyl ether (BDE-209) exposure affected parental gonad development and locomotion in F1 offspring. Ecotoxicology. 2011;20(8):1813–1822. doi: 10.1007/s10646-011-0720-3. [DOI] [PubMed] [Google Scholar]
- 72.Kuriyama SN, Talsness CE, Grote K, Chahoud I. Developmental exposure to low-dose PBDE-99: Effects on male fertility and neurobehavior in rat offspring. Environ Health Perspect. 2005;113(2):149–154. doi: 10.1289/ehp.7421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Stoker TE, Cooper RL, Lambright CS, Wilson VS, Furr J, Gray LE. In vivo and in vitro anti-androgenic effects of DE-71, a commercial polybrominated diphenyl ether (PBDE) mixture. Toxicol Appl Pharm. 2005;207(1):78–88. doi: 10.1016/j.taap.2005.05.010. [DOI] [PubMed] [Google Scholar]
- 74.Tseng LH, Lee CW, Pan MH, Tsai SS, Li MH, Chen JR, Lay JJ, Hsu PC. Postnatal exposure of the male mouse to 2,2',3,3',4,4',5,5',6,6'-decabrominated diphenyl ether: Decreased epididymal sperm functions without alterations in DNA content and histology in testis. Toxicology. 2006;224(1–2):33–43. doi: 10.1016/j.tox.2006.04.003. [DOI] [PubMed] [Google Scholar]
- 75.Cyr DG, Eales JG. Interrelationships between thyroidal and reproductive endocrine systems in fish. Rev Fish Biol Fisher. 1996;6(2):165–200. [Google Scholar]
- 76.Habibi HR, Nelson ER, Allan ERO. New insights into thyroid hormone function and modulation of reproduction in goldfish. Gen Comp Endocr. 2012;175(1):19–26. doi: 10.1016/j.ygcen.2011.11.003. [DOI] [PubMed] [Google Scholar]
- 77.Liu CS, Zhang XW, Deng J, Hecker M, Al-Khedhairy A, Giesy JP, Zhou BS. Effects of Prochloraz or Propylthiouracil on the Cross-Talk between the HPG, HPA, and HPT Axes in Zebrafish. Environ Sci Technol. 2010;45(2):769–775. doi: 10.1021/es102659p. [DOI] [PubMed] [Google Scholar]
- 78.Vandenberg LN, Colborn T, Hayes TB, Heindel JJ, Jacobs DR, Lee DH, Shioda T, Soto AM, vom Saal FS, Welshons WV, Zoeller RT, Myers JP. Hormones and Endocrine-Disrupting Chemicals: Low-Dose Effects and Nonmonotonic Dose Responses. Endocr Rev. 2012;33(3):378–455. doi: 10.1210/er.2011-1050. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.